Abstract
Background:
This meta-analysis was conducted to compare the therapeutic effect and safety of subthreshold micropulse laser (SML) vs photodynamic therapy (PDT) in treatment of chronic central serous chorioretinopathy (cCSC).
Methods:
PubMed, EMBASE, and the Cochrane Library were searched for all relevant studies published up to August 17, 2020. Data of interest were analyzed by STATA (version 14.0) software.
Results:
Four randomized clinical trials (RCTs) and 5 retrospective studies with 790 eyes were included in this meta-analysis after study selection. The results showed that SML significantly improved the best-corrected visual acuity (BCVA) compared with PDT at 6 to 8 weeks, 6 months, and 7 to 8 months in patients with cCSC (weighted mean difference (WMD) = −0.15, 95% confidence intervals (CI): −0.23 to −0.07, P < .01; WMD = −2.83, 95% CI: −4.79 to −0.87, P < .01; and WMD = −2.61, 95% CI: −4.23 to −1.24, P = .026, respectively). There was also a statistically significant difference between SML and PDT groups in the differences in the complete resolution of subretinal fluid (SRF) (risk radios = 0.388, 95% CI: 0.307 to 0.491, P < .01). There were no significant differences between the SML and PDT in the overall effect with central macular thickness (CMT), adverse events, complete resolution of SRF and treatment response.
Conclusions:
Based on the available evidence, this meta-analysis demonstrated that SML may be considered as a competitive alternative to PDT for treating cCSC, and as the first-line treatment of cCSC.
Keywords: chronic central serous chorioretinopathy, meta-analysis, photodynamic therapy, subthreshold micropulse laser
1. Introduction
Central serous chorioretinopathy (CSC) is a relatively common early-onset eye disease, characterized by an accumulation of leaked serous fluid under the retina, causing a detachment of the neuroretina.[1] The disease affects mainly young males between 20 to 50 years of age, with type A of personality, who are often exposed to prolonged stress.[2] The effects on the retina are usually self-limited since spontaneous resolution occurs in most patients. However, about 20% of the disease becomes chronic characterized by the long-term persistence of subretinal fluid (SRF), which can result in atrophy of the retinal pigment epithelium (RPE), cystoid retinal degeneration, choroidal neovascularization (CNV), and permanent vision loss.[1–5]
The main treatment options currently in usage for chronic CSC (cCSC) are photodynamic therapy (PDT), subthreshold micropulse laser (SML) treatment, and treatment with mineralocorticoid antagonists such as eplerenone.[6–20] To date, there is no international consensus on the optimal treatment protocol of cCSC. PDT and SML can be used for near-concave and sub-concave lesions, which are widely used in the treatment of cCSC. PDT has been used to treat cCSC effectively.[21] It presumably causes a transient ischemia and long-term choroidal vascular remodeling, with a reduction in choroidal congestion, leading to a decrease in choroidal hyperpermeability and consequently reduced extravascular leakage.[22] However, adverse events associated with PDT include choroidal non-perfusion, retinal pigment epithelium (RPE) atrophy and CNV.[23,24] Another promising treatment option is subthreshold micropulse laser (SML) treatment without any ophthalmoscopically visible laser burns. The subthreshold laser energy affects almost exclusively the RPE, with limited damage to the overlying neural retina.[25] SML delivers laser energy as a train of repetitive short diode pulses, with an “on” time and an interpulse “off” time with a sublethal cellular thermal effect.[26] Although both treatments have high reported anatomic success rates (i.e., the complete resolution of SRF),[7–9,11–13] there is currently no consensus with respect to which intervention may be more effective. To the best of our knowledge, there has been no meta-analysis of randomized controlled trials (RCTs) or retrospective studies comparing the outcomes of SML vs PDT in patients with cCSC. Therefore, we undertook a meta-analysis of all available RCTs or retrospective studies to assess the efficacy of these 2 treatments for cCSC.
2. Materials and methods
2.1. Data sources and literature searches
We searched PubMed, EMBASE, and the Cochrane Library to yield relevant studies from their inception to August 17, 2020, using Medical Subject Headings and free words combined with central serous retinopathy, photochemotherapy, subthreshold diode-laser micropulse. Only studies published in English were included.
2.2. Eligibility criteria
Comparative studies (i.e., randomized clinical trials (RCTs), and retrospective study) were included if they met the following criteria:
-
1.
Population: participants with chronic CSC with visual impairment history lasting at least 3 months,
-
2.
Intervention: at least 2 comparators of interest (micropulse laser treatment and PDT treatment),
-
3.
available full-text,
-
4.
the study reported at least 1 outcome of interest, including the mean change in BCVA, any adverse effects, the mean change of the subfoveal central thickness(SFCT) and the mean change of the central macular thickness (CMT) from baseline to at least 1month,
-
5.
publication as an article in a peer-reviewed journal.
This literature screening was performed by 2 authors (ZZ.W. and JS.A.) independently, and any discrepancies were resolved via discussions.
2.3. Data collection and quality assessment
Two editors (ZZ.W. and JS.A.) screened titles and abstracts to identify potentially eligible articles independently and in duplicate, and then they checked the full text to determine the final inclusions. When more than 1 report used data from the same study, we included only the latest report to avoid duplicate counting of the data. For the included studies, both reviewers independently extracted data regarding study characteristics (author, study design, country, sample size, intervention and control, mean symptom duration and follow-up period), patient characteristics (sex, age, mean change in the best-corrected visual acuity (BCVA) and the subfoveal central thickness (SFCT) and CMT), and outcomes of interest. We assessed the quality of RCTs for the following 4 aspects according to Modified Jaded Scale: Randomization, allocation concealment, lost to follow-up and blinding. For observational studies, we applied the Newcastle-Ottawa Scale, which included 8 items within 3 domains to evaluate the bias in patient selection, comparability, and outcome assessments.
2.4. Statistical analysis
Data analyses were performed using STATA (version 14.0; Stata Corp) software. For continuous variables (e.g., BCVA), the WMD was measured, outcome was reported with a 95% CI. P < .05 was considered statistically significant on the test for overall effect. In terms of dichotomous data, we calculated risk radios and 95% CIs to express the strength of association. The I2 statistic was calculated to assess heterogeneity between studies (P < .05 was considered representative of significant statistical heterogeneity). If there was heterogeneity between studies, a random-effects model was applied to the data. Alternatively, a fixed effects model was used for pooling the data. A subanalysis was performed by evaluating the heterogeneity between the different follow-up time (1 month–12 months). The extent of heterogeneity was statistically quantified by I2 statistics across studies. We performed a sensitivity analysis by excluding studies with significantly different characteristics. In addition, Egger linear regression test were used to quantitatively assess publication bias (P < .05 was considered representative of significant statistical publication bias).
3. Results
3.1. Overall characteristics of selected trials and quality assessment
A total of 52 studies were yielded from PubMed, Cochrane, and EMBASE databases after 36 duplicate articles are removed. Thirty eight articles were removed after the title and abstract review because they were not observational studies or their topics and results did not meet our requirements, leaving 14 studies included for full-length article review. After that, 5 Conference articles were excluded. Hence, 4 RCTs and 5 retrospective studies were included in this meta-analysis. A flow diagram of the search procedure and results is provided in Figure 1. One study had 3 treatment groups (SML, PDT, and control group).[14] In total, there were 790 eyes included in this meta-analysis. Of note, 378 eyes were included in the SML group, and 412 eyes were included in the PDT group. In all the included studies, no statistical significant differences in the outcomes were reported between the SML groups and PDT groups at baseline. The characteristics of the studies included and quality scores are summarized in Table 1.
Figure 1.

Flow diagram of the study selection process.
Table 1.
Characteristics of the included studies.
| Literature | Study design | Country | Interventions | Sample size (SML/PDT)(eye, n) | Mean Age(Y)(SML/PDT) | Sex (male/ female) | Follow-up period (m) | Mean symptom duration (SML/PDT) | Jadad | NOS |
| Hom et al, 2020[7] | RCT | China | SML(577-nm)/ half- dose PDT | 18/15 | 53.17 ± 10.48/50.93 ± 11.0 | 15/4:11/4 | 6 | 6.7 ± 2.9 | 6 | – |
| van Rijssen et al, 2020[6] | Retrospective study | Leiden University Medical Center, Leiden, Netherlands | SML/half-dose PDT | 29/29 | 48 ± 7.6/ 47 ± 8.1 | 26/3:24/5 | 6–8 | – | – | 7 |
| van Rijssen et al, 2019[8] | RCT | Germany, United Kingdom, Leiden and the Netherlands, and France | SML(810-nm)/ half- dose PDT | 79/79 | 48.76 ± 8.6 | 128:30 | 7–8 | 7.36 ± 4.4 | 3 | – |
| van Dijk et al, 2018[9] | RCT | France, Germany, Netherlands, United Kingdom | SML(810-nm)/ half- dose PDT | 80/80 | 48.6 ± 8.3/48.9 ± 8.9 | 69/11:60/20 | 7–8 | 6(3.76–11)/6 (4-9.75) | 4 | - |
| Roca et al, 2018[10] | Retrospective study | - | SML(577-nm)/ half- dose PDT | 92/67 | 44.0 ± 10/47.2 ± 10.8 | 61/31:56/11 | 12 | 9.5 ± 9.5/15.8 ± 14.6 | – | 7 |
| Cyprian et al, 2018[11] | Retrospective study | India | SML/ half- dose PDT | 23/22 | 48.9 ± 7.5/50.1 ± 7.5 | 32:7 | 6 | 43.7 ± 46.4/ 39.0 ± 35.5 | – | 6 |
| Scholz et al, 2016[12] | Retrospective study | Germany | SML(577-nm)/ half- dose PDT | 42/58 | 49 ± 8.6/ 53 ± 9.5 | 33/9:38/20 | 1.5 | 46.8 ± 50.4/31.2 ± 39.6 | – | 6 |
| Ozmert et al, 2016[13] | Retrospective study | Turkey | SML(577-nm)/ half- dose PDT | 18/15 | 44.7 ± 9.5/52.7 ± 11.2 | 22:8 | 12 | 18.8 ± 13.5/13.0 ± 9.1 | – | 6 |
| Kretz et al, 2015[14] | RCT | USA | SML(810-nm)/ half- dose PDT | 20/24 | 46.9 ± 7.62/46.6 ± 7.91 | 14/6:20/4 | 4 | 11.5 ± 10.9/12.8 ± 11.3 | 5 | – |
3.2. Effects on best-corrected visual acuity
BCVA is one of the most important methods to evaluate treatment efficacy by functional measurement. Two studies involving 192 eyes compared SML with PDT in terms of mean change in BCVA (logarithm of the minimum angle of resolution [logMAR]) at 1 months and 3 studies (237 eyes) at 3 months and 6 months from baseline. No significant difference was found in BCVA (logMAR) between the SML and PDT groups at 1 months and 3 months after the initial treatment (WMD = −0.06, 95% CI: −0.20 to 0.07, P = .374, and WMD = −0.09, 95% CI: −0.22 to 0.04, P = .183, respectively) (Fig. 2). The pooled results revealed that SML treatment seemed to be superior to PDT in terms of mean change in logMAR BCVA at 6 months after treatment (WMD = −0.15, 95% CI: −0.23 to −0.07, P < .01) (Fig. 2). Another 2 studies involving 223 eyes compared SML with PDT in terms of mean change in BCVA (Early Treatment Diabetic Retinopathy Study letters) at 6 to 8 weeks and 188 eyes included at 7 to 8 months, and the pooled results revealed that SML significantly increased BCVA (Early Treatment Diabetic Retinopathy Study letters) compared with PDT at 6 to 8 weeks and 7 to 8 months (WMD = −2.83, 95% CI: −4.79 to −0.87, P < .01; and WMD = −2.61, 95% CI: −4.23 to −1.24, P = .026, respectively), with no heterogeneity identified (Fig. 3).
Figure 2.

Forest plot of mean change from baseline in best-corrected visual acuity (logMAR BCVA) in eyes with chronic central serous chorioretinopathy (cCSC) treated with subthreshold micropulse laser (SML) and photodynamic therapy (PDT). Follow-up examinations occurred 1 month, 3 months, and 6 months after initiating therapy. Dots show the estimated mean difference and error bars indicated 95% confidence intervals (CI). Values to the left of the vertical line indicate a BCVA advantage for the SML group and values to the right indicate a BCVA advantage for the PDT group.
Figure 3.

Forest plot of mean change from baseline in best-corrected visual acuity (BCVA, ETDRS letters) in eyes with chronic central serous chorioretinopathy (cCSC) treated with subthreshold micropulse laser (SML) and photodynamic therapy (PDT). Follow-up examinations occurred during the first 6 to 8 weeks and 7 to 8 months after treatment. Dots show the estimated mean difference and error bars indicated 95% confidence intervals (CI). Values to the left of the vertical line indicate a BCVA advantage for the SML group and values to the right indicate a BCVA advantage for the PDT group.
3.3. Effects on central macular thickness
Three studies involving 303 eyes compared SML with PDT in terms of mean change in CMT at 1 to 2 months after the initial treatment, 4 studies (281 eyes) reported results at 3 to 4 months, 3 studies (237 eyes) at 6 months, and 2 studies (192 eyes) at 12 months. As with CMT, the pooled results showed that both treatments were efficacious in reducing CMT at all follow-up time points. There were no significant difference between the 2 treatments in the mean change of CMT at any time after treatment (WMD = −25.14, 95% CI: −79.008 to 28.734, P = .360; WMD = 2.881, 95% CI: −35.069 to 40.832, P = .882; WMD = −19.87, 95% CI: −62.169 to 22.431, P = .357; and WMD = −9.834, 95% CI: −105.947 to 86.278, P = .841, respectively), with no heterogeneity identified (Fig. 4). Egger linear regression test indicated no publication bias for any of the parameters.
Figure 4.

Forest plot of mean change from baseline in central macular thickness (CMT) in eyes with chronic central serous chorioretinopathy (cCSC) treated with subthreshold micropulse laser (SML) and photodynamic therapy (PDT). Follow-up examinations occurred during the first 1 to 2 months, 3 to 4 months, 6 months and 12 months after initiating therapy. Dots show the estimated mean difference and error bars indicated 95% confidence intervals (CI). Values to the left of the vertical line indicate a central macular thickness (CMT) advantage for the SML group and values to the right indicate a central macular thickness (CMT) advantage for the PDT group.
3.4. Effects on SFCT
Two studies involving 217 eyes compared SML with PDT in terms of mean decrease in SFCT during 1 to 2 months after treatment, 2 studies (204 eyes) at 3 months and 6 months, and 2 studies (217 eyes) after 6 months treatment. Overall, the SFCT in both treatment groups diminished significantly over time. Nevertheless, PDT seemed to be superior to SML in terms of mean change in SFCT at 1 to 2 months and over 6 months treatment (WMD = 88.17, 95% CI: 55.68 to 117.65, P < .01; WMD = 69.80, 95% CI: 43.80 to 95.79, P < .01 respectively), and it showed the same trend at 3 months and 6 months with no significant difference was found in BCVA between the SML and PDT groups (WMD = 35.75, 95% CI: −7.53 to 79.03, P = .105, and WMD = 14.79, 95% CI: −27.49 to 57.07, P = .493, respectively) (Fig. 5). Egger linear regression test indicated no publication bias for any of the parameters.
Figure 5.

Forest plot of mean change from baseline in subfoveal choroidal thickness (SFCT) in eyes with chronic central serous chorioretinopathy (cCSC) treated with subthreshold micropulse laser (SML) and photodynamic therapy (PDT). Follow-up examinations occurred during the first 1 to 2 months, 3 months, 6 months, and over 6 months after initiating therapy. Dots show the estimated mean difference and error bars indicated 95% confidence intervals (CI). Values to the left of the vertical line indicate a subfoveal choroidal thickness (SFCT) advantage for the SML group and values to the right indicate a subfoveal choroidal thickness (SFCT) advantage for the PDT group.
3.5. Effects on treatment response
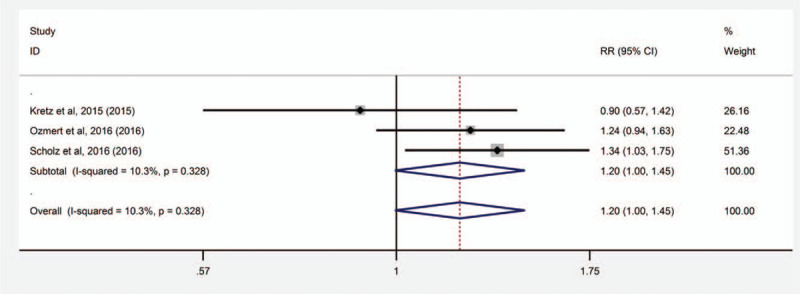
Three studies involving 122 eyes compared SML with PDT in terms of the treatment response after treatment. All pooled results show high treatment response with both types of treatment. SML seemed to be superior to PDT in terms of the treatment response after treatment. There were no significant difference between SML and PDT in the treatment response after treatment (RR = 1.203, 95% CI: 1.996 to 1.452, P = .055) (Fig. 6).
Figure 6.

Forest plot of the incidence of complete resolution of SRF in eyes with chronic central serous chorioretinopathy (cCSC) treated with subthreshold micropulse laser (SML) and photodynamic therapy (PDT). Follow-up examinations occurred during the 2 time periods less than or equal to 6 months and over 6 months after treatment. Dots show the estimated mean difference and error bars indicated 95% confidence intervals (CI). Values to the left of the vertical line indicate a lower incidence of complete resolution of SRF for the SML group and values to the right indicate a lower incidence of complete resolution of SRF for the PDT group.
3.6. Effects on complete resolution of SRF
Five studies involving 497 eyes compared SML with PDT in terms of the complete resolution of SRF during the period less than or equal to 6 months, and 3 studies (304 eyes) at the time of over 6 months after treatment. There was no significant difference in terms of the complete resolution of SRF during the 2 periods after treatment. (RR = 0.719, 95% CI: 0.328 to 1.577, P = .411; RR = 0.661, 95% CI: 0.414 to 1.055, P = .107, respectively) (Fig. 7).
Figure 7.
Forest plot of the treatment response in eyes with chronic central serous chorioretinopathy (cCSC) treated with subthreshold micropulse laser (SML) and photodynamic therapy (PDT). Dots show the estimated mean difference and error bars indicated 95% confidence intervals (CI). Values to the left of the vertical line indicate a higher treatment response for the SML group and values to the right indicate a higher treatment response for the PDT group.
3.7. Adverse events
Three studies involving 122 eyes compared SML with PDT in terms of the incidence of adverse events after treatment. Overall, all results demonstrated low incidence of adverse events with both types of treatment. SML seemed to be superior to PDT with fewer adverse events after treatment. However, there were no significant difference between SML and PDT in the incidence of adverse events after treatment (RR = 0.62, 95% CI: 0.27 to 1.46, P = .274) (Fig. 8).
Figure 8.

Forest plot of the incidence of adverse events in eyes with chronic central serous chorioretinopathy (cCSC) treated with subthreshold micropulse laser (SML) and photodynamic therapy (PDT). Dots show the estimated mean difference and error bars indicated 95% confidence intervals (CI). Values to the left of the vertical line indicate a lower incidence of adverse events for the SML group and values to the right indicate a lower incidence of adverse events for the PDT group.
4. Discussion
The cCSC in particular may be a serious therapeutic problem, often leading to significant visual impairment. Recently, a growing number of clinical trials have used SML and PDT to treat cCSC; however, the results were inconsistent. In our present study, we enrolled 9 studies on cCSC.
To our knowledge, this is the first separate meta-analysis that assesses efficacy and safety of SML versus PDT. In terms of the effect on BCVA, both treatments demonstrated good stabilization effect after treatment. SML seemed to be more effective in increasing BCVA than PDT at all-time points up to 7 to 8 months after treatment (Fig. 2, Fig. 3). With regard to CMT, it showed the same trend but no statistical significance all follow-up time points (Fig. 4). However, PDT showed more effective than SML in reducing SFCT, and the superiority of PDT was statistically significant (Fig. 5).
PDT has proven effective in causing choroidal vascular remodeling and the reduction of choroidal exudation,[27] and this study also found that PDT significantly reduced subfoveal choroidal thickness compared with SML treatment. Considering the theories of pathophysiology of CSC largely incriminating choroidal disorder with increased thickness, choriocapillary hyperpermeability, vascular congestion, and venous dilatation with exudation of serous fluid via weakened RPE to eventually cause SRF and visual loss,[5,28] the goal of treatment should be to interrupt these mechanism and cause the resorption of SRF. The PDT groups got a high percentage of complete resolution of SRF,[29,30] indicating that the choriocapillary hyperpermeability plays a more important role in the occurrence and development of cCSC. At the same time, both treatments showed high treatment response and percentage of complete resolution of SRF, and there was no statistical difference between the 2 treatments (Fig. 6, Fig. 7).
Subthreshold micropulse laser (SML) treatment has been successfully used in cCSC with morphological and functional success achieved in the majority of cases.[31–34] Energy of SML is delivered to the tissue in a series of very short impulses, between which there are intervals that enable the tissue to cool down, preventing heat accumulation to a level that is lethal to the RPE.[35,36] Although it is believed that in CSC pathological abnormalities occur in choroid, rather than in the RPE, it is the RPE that transfers SRF to choroidal vessels. The laser energy might be the stimulation of the RPE, which leads to repair of the inner blood retinal barrier,[37] the restoration of the RPE blood retinal barrier, and increased retinal cell adhesion.[38] By normalizing RPE function, SML treatment improves the transretinal pump to eliminate the SRF. Direct effects at the points of leakage identified in fluorescein angiography are obtained on the RPE and only a minor thermal energy is released to the choroid and neurosensory retina, and thus avoiding to damage to those structures.[26] No detectable damage founded at choroid and neurosensory retina in previous studies.[39–41] This phenomenon was also indirectly confirmed in the current meta-analysis, showing a superior advantage over PDT in improving BCVA and reducing CMT. Meanwhile, SML is comfortable for the patients and not especially expensive compared with PDT.
Although both treatments showed less adverse events than conventional lasers treatment, in current study, the present study showed a lower incidence of adverse treatment effects of the SML for both morphology and visual function in comparison to PDT (Fig. 8), and no serious complications occurred after SML treatment, including RPE atrophy and CNV. Only 1 study had a serious adverse reaction unrelated to the treatment itself after SML treatment.[9] Multiple studies showed serious adverse events associated with PDT, including choroidal non-perfusion, RPE atrophy and CNV.[23,24,42] In current review, 2 patients developed the CNV after treatment of half-dose PDT and a moderate allergic reaction happened to 1 eye.[10,12]
On the other hand, there are still several limitations in our study. First, more than half of the included studies were observational studies, which are susceptible to have selection bias. Second, in meta-analysis of included trials, outcomes were measured at different follow-up times and this may induce heterogeneity. Third, fewer than 45% of studies included more than 100 patients, which may lead to bias due to small study effects. Fourth, the mean symptom duration before treatment varied widely between included studies, which may lead to heterogeneity.
In conclusion, based on a limited number of studies available at the present time, SML seems to be superior over PDT in improving BCVA. SML is a cost-effective, less destructive with less potential adverse effects like allergic reaction and neovascularization. Therefore, it may be considered as a competitive alternative to PDT, and as one of the first-line treatments for cCSC. Further randomized prospective studies are needed with a larger sample size and longer follow-up time to determine its role and superiority in cCSC.
Author contributions
Conceptualization: Zhizhong Wu, Huixing Wang, Junsheng An.
Data curation: Zhizhong Wu.
Investigation: Zhizhong Wu.
Methodology: Zhizhong Wu, Huixing Wang.
Software: Zhizhong Wu.
Validation: Zhizhong Wu.
Writing – original draft: Zhizhong Wu.
Writing – review & editing: Zhizhong Wu, Huixing Wang, Junsheng An.
Footnotes
Abbreviations: BCVA = best-corrected visual acuity, CI = confidence intervals, cCSC = chronic central serous chorioretinopathy, CMT = central macular thickness, CNV = choroidal neovascularisation, logMAR = logarithm of the minimum angle of resolution, PDT = photodynamic therapy, RCT = randomized clinical trials, RPE = retinal pigment epithelium, SRF = subretinal fluid, SML = subthreshold micropulse laser, SFCT = subfoveal central thickness, WMD = weighted mean difference.
How to cite this article: Wu Z, Wang H, An J. Comparison of the efficacy and safety of subthreshold micropulse laser with photodynamic therapy for the treatment of chronic central serous chorioretinopathy: a meta-analysis. Medicine. 2021;100:17(e25722).
ZW and HW contributed equally to this work.
The authors have no funding and conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Data are presented as mean ± standard deviation where applicable. M = month, PDT = photodynamic therapy, RCT = randomized clinical trial, SML = subthreshold micropulse laser, Y = year.
References
- [1].Nicholson B, Noble J, Forooghian F, et al. Central serous chorioretinopathy: update on pathophysiology and treatment. Surv Ophthalmol 2013;58:103–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Spaide RF, Campeas L, Haas A, et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology 1996;103:2070–80. [DOI] [PubMed] [Google Scholar]
- [3].Gemenetzi M, De Salvo G, Lotery AJ. Central serous chorioretinopathy: an update on pathogenesis and treatment. Eye 2010;24:1743–56. [DOI] [PubMed] [Google Scholar]
- [4].Ross A, Ross AH, Mohamed Q. Review and update of central serous chorioretinopathy. Curr Opin Ophthalmol 2011;22:166–73. [DOI] [PubMed] [Google Scholar]
- [5].PRüNTE C, Flammer J. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol 1996;121:26–34. [DOI] [PubMed] [Google Scholar]
- [6].van Rijssen TJ, Singh SR, van Dijk EHC, et al. Prospective evaluation of changes in choroidal vascularity index after half-dose photodynamic therapy versus micropulse laser treatment in chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol 2020;258:1191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ho M, Lai FHP, Ng DSC, et al. Analysis of choriocapillaris perfusion and choroidal layer changes in patients with chronic central serous chorioretinopathy randomised to micropulse laser or photodynamic therapy. Br J Ophthalmol 2020;105:555–60. [DOI] [PubMed] [Google Scholar]
- [8].van Rijssen TJ, van Dijk EHC, Scholz P, et al. Focal and diffuse chronic central serous chorioretinopathy treated with half-dose photodynamic therapy or subthreshold micropulse laser: PLACE trial report No. 3. Am J Ophthalmol 2019;205:01–10. [DOI] [PubMed] [Google Scholar]
- [9].van Dijk EHC, Fauser S, Breukink MB, et al. Half-dose photodynamic therapy versus high-density subthreshold micropulse laser treatment in patients with chronic central serous chorioretinopathy: the PLACE trial. Ophthalmology 2018;125:1547–55. [DOI] [PubMed] [Google Scholar]
- [10].Roca JA, Wu L, Fromow-Guerra J, et al. Yellow (577 nm) micropulse laser versus half-dose verteporfin photodynamic therapy in eyes with chronic central serous chorioretinopathy: results of the Pan-American Collaborative Retina Study (PACORES) Group. Br J Ophthalmol 2018;102:1696–700. [DOI] [PubMed] [Google Scholar]
- [11].Ntomoka CG, Rajesh B, Muriithi GM, et al. Comparison of photodynamic therapy and navigated microsecond laser for chronic central serous chorioretinopathy. Eye 2018;32:1079–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Scholz P, Altay L, Fauser S. Comparison of subthreshold micropulse laser (577 nm) treatment and half-dose photodynamic therapy in patients with chronic central serous chorioretinopathy. Eye (Lond) 2016;30:1371–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ozmert E, Demirel S, Yanik O, et al. Low-fluence photodynamic therapy versus subthreshold micropulse yellow wavelength laser in the treatment of chronic central serous chorioretinopathy. J Ophthalmol 2016;2016:3513794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kretz FT, Beger I, Koch F, et al. Randomized clinical trial to compare micropulse photocoagulation versus half-dose verteporfin photodynamic therapy in the treatment of central serous chorioretinopathy. Ophthalmic Surg Lasers Imaging Retina 2015;46:837–43. [DOI] [PubMed] [Google Scholar]
- [15].Rabiolo A, Bandello F. Eplerenone is not superior to placebo for chronic central serous chorioretinopathy. Lancet 2020;395:252–3. [DOI] [PubMed] [Google Scholar]
- [16].Lotery A, Sivaprasad S, O’Connell A, et al. Eplerenone for chronic central serous chorioretinopathy in patients with active, previously untreated disease for more than 4 months (VICI): a randomised, double-blind, placebo-controlled trial. Lancet 2020;395:294–303. [DOI] [PubMed] [Google Scholar]
- [17].Wang SK, Sun P, Tandias RM, et al. Mineralocorticoid receptor antagonists in central serous chorioretinopathy: a meta-analysis of randomized controlled trials. Ophthalmol Retina 2019;3:154–60. [DOI] [PubMed] [Google Scholar]
- [18].Kumari E, Baidya K, Khan R. Efficacy of drug eplerenone in the management of chronic central serous chorioretinopathy. J Evidence Based Med Healthcare 2019;6:3280–4. [Google Scholar]
- [19].Schwartz R, Habot-Wilner Z, Martinez MR, et al. Eplerenone for chronic central serous chorioretinopathy-a randomized controlled prospective study. Acta Ophthalmol 2017;95:e610–8. [DOI] [PubMed] [Google Scholar]
- [20].Rahimy E, Pitcher JD, Hsu J, et al. A randomized double-blind placebo-control pilot study of eplerenone for the treatment of central serous chorioretinopathy (ecselsior). Retina 2018;38:962–9. [DOI] [PubMed] [Google Scholar]
- [21].Lim JI, Glassman AR, Aiello LP, et al. Collaborative retrospective macula society study of photodynamic therapy for chronic central serous chorioretinopathy. Ophthalmology 2014;121:1073–8. [DOI] [PubMed] [Google Scholar]
- [22].Chan W-M. Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: a novel treatment at the primary disease level. Br J Ophthalmol 2003;87:1453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fujita K, Imamura Y, Shinoda K, et al. One-year outcomes with half-dose verteporfin photodynamic therapy for chronic central serous chorioretinopathy. Ophthalmology 2015;122:555–61. [DOI] [PubMed] [Google Scholar]
- [24].Hanumunthadu D, Tan AS, Singh SR, et al. Management of chronic central serous chorioretinopathy. Indian J Ophthalmol 2018;66:1704–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yu AK, Merrill KD, Truong SN, et al. The comparative histologic effects of subthreshold 532- and 810-nm diode micropulse laser on the retina. Invest Ophthalmol Vis Sci 2013;54:2216–24. [DOI] [PubMed] [Google Scholar]
- [26].Chen SN, Hwang JF, Tseng LF, et al. Subthreshold diode micropulse photocoagulation for the treatment of chronic central serous chorioretinopathy with juxtafoveal leakage. Ophthalmology 2008;115:2229–34. [DOI] [PubMed] [Google Scholar]
- [27].Ruiz-Moreno JM, Lugo FL, Armadá F, et al. Photodynamic therapy for chronic central serous chorioretinopathy. Acta Ophthalmologica 2010;88:371–6. [DOI] [PubMed] [Google Scholar]
- [28].Donald J, Gass M. Pathogenesis of disciform detachment of the neuroepithelium. Am J Ophthalmol 1967;63:661/689–87/115. [PubMed] [Google Scholar]
- [29].Tang PH, Shields R, Silva RA. Optical coherence tomography angiography findings in chronic central serous chorioretinopathy after photodynamic therapy. Ophthalmic Surg Lasers Imaging Retina 2019;50:25–32. [DOI] [PubMed] [Google Scholar]
- [30].Vladimir S, Konstantine P, Johann R. Half-time photodynamic therapy in treatment of chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol 2018;256:2027–34. [DOI] [PubMed] [Google Scholar]
- [31].Yadav NK, Jayadev C, Mohan A, et al. Subthreshold micropulse yellow laser (577 nm) in chronic central serous chorioretinopathy: safety profile and treatment outcome. Eye 2015;29:258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kim JY, Park HS, Kim SY. Short-term efficacy of subthreshold micropulse yellow laser (577-nm) photocoagulation for chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol 2015;253:2129–35. [DOI] [PubMed] [Google Scholar]
- [33].Khurram J, Malik RB, Kapil M, et al. Low-intensity/high-density subthreshold microPulse diode laser for chronic central serous chorioretinopathy. Retina (Philadelphia, Pa) 2015;35:532–6. [DOI] [PubMed] [Google Scholar]
- [34].Arsan A, Kanar HS, Sonmez A. Visual outcomes and anatomic changes after sub-threshold micropulse yellow laser (577-nm) treatment for chronic central serous chorioretinopathy: long-term follow-up. Eye 2018;32:726–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Vujosevic S, Martini F, Longhin E, et al. Subthreshold micropulse yellow laser versus subthreshold micropulse infrared laser in center-involving diabetic macular edema: morphologic and functional safety. Retina 2015;35:1594–603. [DOI] [PubMed] [Google Scholar]
- [36].Inagaki K, Ohkoshi K, Ohde S. Spectral-domain optical coherence tomography imaging of retinal changes after conventional multicolor laser, subthreshold micropulse diode laser, or pattern scanning laser therapy in Japanese with macular edema. Retina 2012;32:1592–600. [DOI] [PubMed] [Google Scholar]
- [37].Bresnick G. Diabetic maculopathy. A critical review highlighting diffuse macular edema. Ophthalmology 1983;90:1301–17. [DOI] [PubMed] [Google Scholar]
- [38].Breukink MB, Downes SM, Querques G, et al. Comparing half-dose photodynamic therapy with high-density subthreshold micropulse laser treatment in patients with chronic central serous chorioretinopathy (the PLACE trial): study protocol for a randomized controlled trial. Trials 2015;16: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Moore SM, Chao DL. Application of subthreshold laser therapy in retinal diseases: a review. Expert Rev Ophthalmol 2018;13:311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Scholz P, Altay L, Fauser S. A review of subthreshold micropulse laser for treatment of macular disorders. Adv Ther 2017;34:1528–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gawęcki M. Micropulse laser treatment of retinal diseases. J Clin Med 2019;8: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Liu Y, Wang X, Zhu M, et al. Choroidal neovascularization emerged right from the focal choroidal excavation in eyes with central serous chorioretinopathy post half-dose photodynamic therapy: a case report. BMC Ophthalmol 2019;19: [DOI] [PMC free article] [PubMed] [Google Scholar]


