Abstract
Background:
Malnutrition is commonly observed after stroke and is closely associated with poor clinical outcomes. So, early nutrition support is particularly crucial for severe stroke patients. However, a significant number of critically ill patients are intolerant to enteral nutrition (EN). Probiotics have been widely used in malnutrition by various diseases and have a low incidence of enteral intolerance. So, we aim to elucidate the efficacy of probiotics in EN in improving the nutritional status and clinical prognosis of severe stroke patients with nasal feeding.
Method:
Embase, PubMed, Sinomed, Web of Science, Cochrane Library, China National Knowledge Infrastructure, Wanfang database, and Vip Journal Integration Platform were searched from inception to March 31, 2021. Randomized controlled trials that applied probiotics in patients with severe stroke were included. The data were extracted and the risk of bias was assessed independently by 2 evaluators.
Results:
Twenty-four studies comprising 2003 participants of randomized controlled trials were included. The result of pooled analyses showed that probiotics in EN were associated with better outcomes than EN alone on Glasgow Coma Scale score (mean difference [MD] = 1.03, 95% confidence intervals [CI]: 0.78–1.27; P < .00001), infection events (odds ratio [OR] = 0.25, 95% CI: 0.15–0.43; P < .00001), rate of intestinal flora dysbiosis (OR = 0.24, 95% CI: 0.12–0.48; P < .0001), gastrointestinal complications (OR = 0.25, 95% CI: 0.16–0.37, P < .00001), time to reach target nutrition (MD = −1.80, 95% CI: −2.42 to 1.18, P < .00001), prealbumin content (MD = 25.83, 95% CI: 13.68–37.99, P < .0001).
Conclusion:
Our results demonstrated that probiotics supplementation might be an effective intervention for improving the clinical prognosis in severe stroke patients with nasal feeding, but no significant effect on increasing muscle circumference.
Keywords: enteral nutrition, nutrition statue, probiotics, severe stroke
1. Introduction
Malnutrition is commonly observed after stroke and is strongly related to poor clinical outcomes.[1] It is mostly attributed to dysphagia, disorders of consciousness, mobility impairments, and gastrointestinal dysfunction, among which dysphagia is the main risk factor for malnutrition in stroke patients.[2] The prevalence of malnutrition after stroke ranges from 6.1% to 62%.[3,4] The evidence showed that early nutritional status after stroke was independently related to long-term prognosis.[2,5] Malnutrition is often accompanied by low cholesterol levels which weaken immunity by suppressing the innate and acquired immune response, and deteriorate the inflammation response by increasing the expression of proinflammatory factors, which are associated with high incidence of cognitive impairment, disability and mortality after stroke.[6–8] So, early nutrition support is particularly crucial for severe stroke patients.
Enteral nutrition (EN) is safer than parenteral nutrition and is regarded as a more effective treatment of malnutrition. However, a considerable number of critically ill patients are intolerant to EN.[9] Probiotics have been widely used for malnutrition caused by various diseases without safety issues. Robertson et al[10] confirmed that probiotics intervention could significantly decrease the incidence of necrotising enterocolitis and late-onset sepsis. In neurological disorders, probiotics therapy has shown beneficial clinical effects. A clinical trial found that probiotics in EN therapy were superior to EN alone in reducing gastrointestinal dysfunction and length of intensive care unit stay in patients of severe traumatic brain injury.[11]
However, the effectiveness of probiotics in severe stroke patients with nasal feeding has not been systematically evaluated. So the present study is necessary to fill this gap. The aim of this review is to investigate the effect of the probiotics on nutrition status and clinical efficacy in severe stroke patients with nasal feeding.
2. Methods
This study was undertaken according the PRISMA guidelines, and had registered this review on PROSPERO: CRD42020173643. https://www.crd.york.ac.uk/prospero/.
2.1. Search strategy
We searched Embase (from 1974 to March 31, 2021), PubMed (before March 31, 2021), Sinomed (before March 31, 2021), Web of Science (from 1950 to March 31, 2021), Cochrane Library (before March 31, 2021), China National Knowledge Infrastructure (from 1999 to March 31, 2021), VIP Journal Integration Platform (from 1989 to March 31, 2021), and Wanfang database (before March 31, 2021) for randomized controlled trials that assessed the effect of probiotics in EN on severe stroke patients published. The search was conducted using the following terms: (stroke OR apoplexy OR cerebral hemorrhage OR cerebrovascular accident OR cerebral infarction OR brain infarction OR cerebral haemorrhage OR cerebral apoplexy OR cerebrovascular disease OR brain vascular accident) AND (probiotics OR prebiotics OR symbiotic OR lactobacillus OR synbiotics OR lactobacterium OR bifidobacterium OR lactobacilli OR lactic acid bacteria).
2.2. Study selection
2.2.1. Inclusion criteria
-
1.
The study design was randomized controlled trials.
-
2.
All subjects were under a clinical diagnosis of severe stroke or Glasgow Coma Scale score ≤9.
-
3.
Treatment course ≥14 days.
2.2.2. Exclusion standards
-
1.
Patients with heart, liver, kidney, and other severe organ failure, or malignancy, metabolic diseases.
-
2.
Patients who were allergic to EN or probiotics, and those with mental illness.
-
3.
The study lacking crucial outcome indexes was excluded.
2.3. Data extraction and outcome measures
The data were extracted independently by 2 evaluators using prespecified extraction forms. Any discrepancies were resolved through negotiation by the third evaluator. The following data were extracted from studies: first author, publication year; number of patients (male/female), age, intervention, course of treatment; Glasgow Coma Scale score, infection rate, rate of intestinal flora dysbiosis, gastrointestinal complication, time to reach target nutrition, mid arm muscle circumference, and prealbumin content.
2.4. Risk of bias assessment
The quality of included studies was assessed using the Cochrane Collaboration “Risk of bias” assessment tool,[12] which included aspects as follows: sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other biases. Any disagreement was resolved by consulting and discussing among all authors. The scores of modified Jadad Scale vary from 0 to 7 points. An article with Jadad score of 1 to 3 was low-quality and a score of 4 to 7 was high-quality.
2.5. Statistical analysis
Review Manager Software (Version 5.3; Oxford, England) was used for statistical analysis. Categorical data were assessed employing odds ratio (OR) with 95% confidence interval (CI) and continuous data were analyzed using mean difference (MD) with 95% CI. Heterogeneity among studies was assessed by the I-squared (I2) test. I2 ≤ 50% was considered low heterogeneity, and the fixed-effects model was adopted; otherwise, it was deemed to be significant heterogeneity, and the random-effects model was adopted. To avoid biases caused by methodological differences among studies, we used the sensitivity analyses to find the source of heterogeneity and inconsistency. Full-text was evaluated to find the research of the origins of heterogeneity, and investigated its influence on meta-analysis. Publication bias was evaluated by funnel plots when the number of literatures was more than 10. When P < .05, the difference was considered statistically significant.
2.6. Ethical approval
Ethical approval and informed consent of patients were not needed because we only collected data from previous studies that had been published and did not recruit patients.
3. Results
3.1. Study selection
The study selection process was shown in Figure 1. A total of 1225 potentially eligible articles were found through databases. Among them, 1162 publications were excluded for duplication or inconsistent with inclusion criteria by reading the title and abstract. Sixty-three full-texts of articles were reviewed, and eventually 24 articles were included in the final selection.
Figure 1.
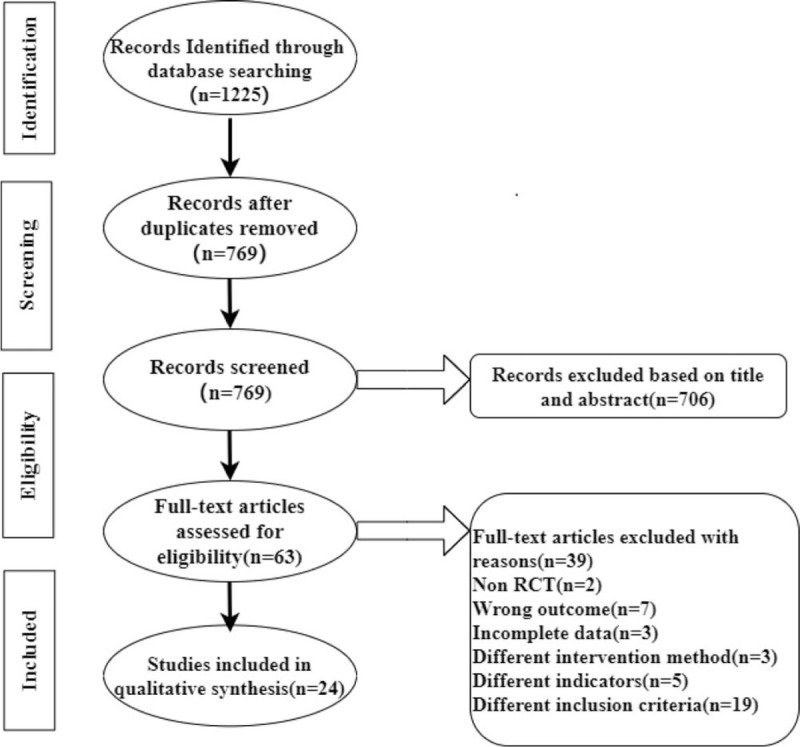
Flowchart of study selection process.
3.2. Study characteristics
The main characteristics of the included studies were summarized in Table 1. The 24 studies were published between 2013 and 2021. A total of 2003 participants were included in this review, of which 57% were males. A total of 1007 patients were allocated to probiotics supplement with EN. The sample size of each study ranged from 56 to 124, and the participants mostly were 60 to 80 years’ old. The treatment course ranged from 14 to 60 days.
Table 1.
Trials characteristics.
| Sample size (M/F) | Age, y (¯X± S) | Intervention | |||||||
| First author (year) | Treatment | Control | Treatment | Control | Duration of illness | Treatment | Control | Duration of treatment | Result |
| Wang et al (2021)[13] | 51 (28/23) | 51 (26/25) | 68.23 ± 8.72 | 67.62 ± 8.21 | ≤48 h | Probiotics + EN + basic treatment | Basic treatment + EN | 14 days | F G |
| Dong (2020)[14] | 36 (−/−) | 36 (−/−) | 61.1 ± 1.9 | 61.1 ± 1.9 | — | Probiotics + EN + basic treatment | Basic treatment + EN | 14 days | B C |
| Liang et al (2020)[15] | 30 (−/−) | 30 (−/−) | 60.19 ± 18.65 | 62.13 ± 13.97 | — | Probiotics + EN + basic treatment | Basic treatment + EN | 60 days | C |
| Ma (2020)[16] | 47 (25/22) | 46 (26/20) | 52 ± 6 | 52 ± 6 | ≤72 h | Probiotics + EN + basic treatment | Basic treatment + EN | 20 days | A F |
| Wang (2020)[17] | 30 (20/10) | 30 (18/12) | 66.18 ± 5.42 | 65.07 ± 2.46 | ≤24 h | Probiotics + EN + basic treatment | Basic treatment + EN | 16 days | G |
| Xie (2020)[18] | 30 (15/15) | 30 (16/14) | 61.9 ± 4.5 | 61.8 ± 4.7 | ≤72 h | Probiotics + EN + basic treatment | Basic treatment + EN | 14 days | C F |
| Chen (2019)[19] | 35 (20/15) | 35 (19/16) | 75.47 ± 4.59) | 75.23 ± 4.52 | — | Probiotics + EN + basic treatment | Basic treatment + EN | 14 days | B D G |
| Chen (2019)[20] | 34 (18/16) | 34 (19/15) | 72.06 ± 6.43 | 72.06 ± 6.43 | — | Probiotics + EN + basic treatment | Basic treatment + EN | 14 days | C |
| Jin et al (2019)[21] | 28 (13/15) | 28 (17/11) | 62.18 ± 11.12 | 62.07 ± 10.94 | <48 h | Probiotics + EN + basic treatment | Basic treatment + EN | 14 days | A D F |
| Li et al (2019)[22] | 43 (24/19) | 43 (27/16) | 60.9 ± 8.6 | 61.66 ± 10.64 | — | Probiotics + EN + basic treatment | Basic treatment + EN | 14 days | B C G |
| Li and Li (2019)[23] | 40 (24/16) | 40 (25/15) | 70.21 ± 0.62 | 69.74 ± 0.44 | ≤72 h | Probiotics + EN + basic treatment | Basic treatment + EN | 14 days | C E |
| Dong (2018)[24] | 41 (24/17) | 41 (23/18) | 62.4 ± 4.1 | 63.4 ± 4.3 | — | Probiotics + EN + basic treatment | Basic treatment + EN | 28 days | A C G |
| Gao (2018)[25] | 40 (21/19) | 40 (15/25) | 58.2 ± 2.1 | 51.1 ± 2.3 | — | Probiotics + EN + basic treatment | Basic treatment + EN | 14 days | C G |
| Sun et al (2018)[26] | 50 (26/24) | 50 (27/23) | 71.52 ± 7.08 | 72.17 ± 7.22 | — | Probiotics + EN + basic treatment | Basic treatment + EN | 14 days | C E |
| Wu (2018)[27] | 31 (18/13) | 32 (16/16) | 58.48 ± 8.09 | 58.59 ± 9.97 | — | Probiotics + EN + basic treatment | Basic treatment + EN | 21 days | A D F |
| Zhou (2018)[28] | 45 (28/17) | 45 (27/18) | 70.17 ± 5.54 | 69.78 ± 4.97 | — | Probiotics + EN + basic treatment | Basic treatment + EN | 14 days | C |
| Zhang et al (2017)[29] | 44 (24/20) | 44 (23/21) | _ | _ | <6 h | Probiotics + EN + basic treatment | Basic treatment + EN | 14 days | F |
| Chen and Chen (2016)[30] | 45 (25/20) | 45 (23/22) | 69.9 ± 7.2 | 70.3 ± 6.7 | <3 days | Probiotics + EN + basic treatment | Basic treatment + EN | 14 days | A |
| Hou (2016)[31] | 38 (20/18) | 38 (22/16) | 72.15 ± 7.56 | 71.89 ± 7.42 | ≤3 days | Probiotics + EN + basic treatment | Basic treatment + EN | 14 days | A C E |
| Hou et al (2016)[32] | 38 (20/18) | 38 (22/16) | 72.2 ± 7.6 | 71.9 ± 7.4 | ≤3 days | Probiotics + EN + basic treatment | Basic treatment + EN | 14 days | A |
| Wu et al (2016)[33] | 62 (33/29) | 62 (34/28) | 54.22 ± 4.29 | 54.13 ± 4.56 | — | Probiotics + EN + basic treatment | Basic treatment + EN | 14 days | C F |
| Bai (2014)[34] | 61 (44/17) | 59 (47/12) | 64.55 ± 10.45 | 64.37 ± 10.63 | ≤72 h | Probiotics + EN + basic treatment | Basic treatment + EN | 14 days | F |
| Wu et al (2014)[35] | 47 (25/22) | 40 (21/19) | 72.5 ± 8.4 | 72.2 ± 9.1 | ≤72 ho | Probiotics + EN + basic treatment | Basic treatment + EN | 21 days | A B |
| Bai et al (2013)[36] | 61 (−/−) | 59 (−/−) | — | — | ≤72 h | Probiotics + EN + basic treatment | Basic treatment + EN | 28 days | D |
3.3. Quality assessment
Among these 24 studies, 23 studies[13–22,24–35] described detailed random grouping methods. The rest of the researches[23] did not mention specific grouping methods. In incomplete outcome data, selective reporting, and other bias domain, all studies were considered to have a low risk of bias. Also, the included studies in our meta-analysis had unclear risk in terms of the allocation concealment and blinding for outcome assessment. The quality of these articles was presented in Supplementary Table 2.
4. Meta-analysis results
4.1. Glasgow Coma Scale score
Eight trials[16,21,24,27,30–32,35] reported the effect of probiotics on Glasgow Coma Scale score, and the results revealed that probiotics in EN were associated with a significant improvement on Glasgow Coma Scale score (MD = 1.03, 95% CI: 0.78–1.27; P < .00001) without significant heterogeneity (I2 = 46%) (Fig. 2).
Figure 2.
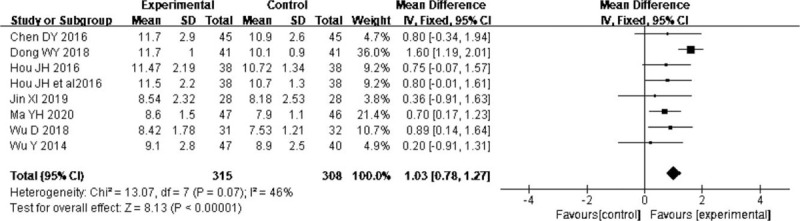
Forest plots of effect of probiotics on the Glasgow Coma Scale.
4.2. Infection rate
Six trials[13,17,19,22,24,25] showed the effect of probiotics on infection rate; the result suggested that probiotics in EN were associated with lower infection events (OR = 0.25, 95% CI: 0.15–0.43; P < .00001). There was no significant heterogeneity (I2 = 40%) (Fig. 3)
Figure 3.
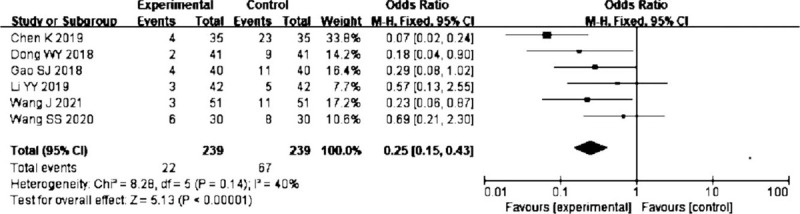
Forest plots of effect of probiotics on the infection rate.
4.3. Rate of intestinal flora dysbiosis
Four trials[14,19,22,35] reported the effect of probiotics on intestinal flora dysbiosis. The pooled result showed a lower incidence of intestinal flora dysbiosis in probiotics group (OR = 0.24, 95% CI: 0.12–0.48; P < .0001) without significant heterogeneity (I2 = 0%) (Fig. 4).
Figure 4.
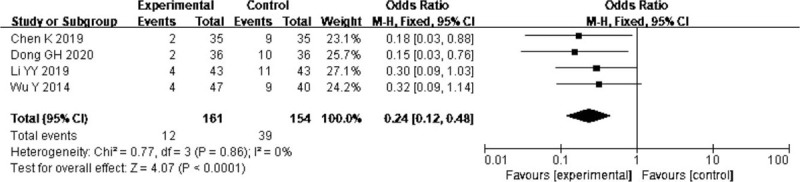
Forest plots of effect of probiotics on the rate of intestinal flora dysbiosis.
4.4. Gastrointestinal complication
Twelve trials[14,15,18,20,22,23–26,28,31,33] reported the effect of probiotics on gastrointestinal complications, and the pooled analysis revealed that the probiotics in EN decreased the gastrointestinal complications (OR = 0.25, 95% CI: 0.16–0.37; P < .00001) without significant heterogeneity (I2 = 0%) (Fig. 5).
Figure 5.
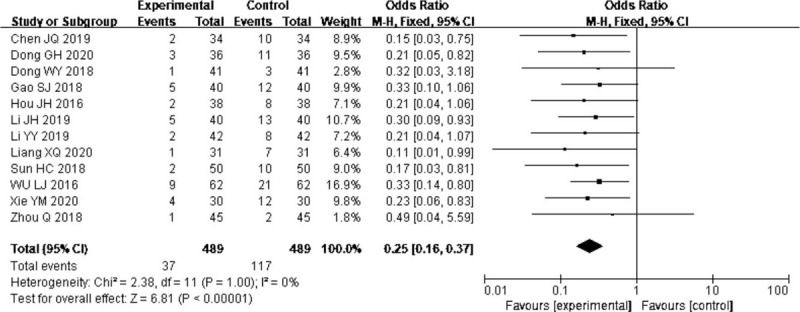
Forest plots of effect of probiotics on the gastrointestinal complications.
4.5. Time to reach target nutrition
Four trials[19,21,27,36] reported the effect of probiotics on time to reach target nutrition. The pooled result showed that compared to EN alone, probiotics in EN were associated with shorter time to reach target nutrition (MD = −1.80, 95% CI: −2.42 to 1.18 P < .00001). There was significant heterogeneity (I2 = 95%) (Fig. 6), but the main outcomes were not affected by excluding any particular study, so the random-effect model was selected.
Figure 6.
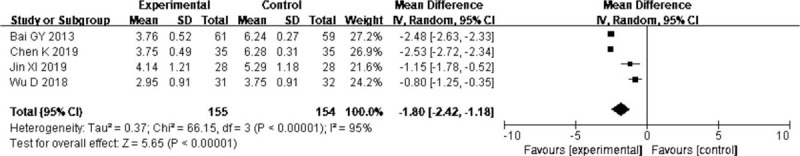
Forest plots of effect of probiotics on the time to reach target nutrition.
4.6. Mid arm muscle circumference
Three trials[23,26,31] reported the effect of probiotics on mid arm muscle circumference, and result revealed that probiotics in EN exerted no positive effect on mid arm muscle circumference (MD = 1.76, 95% CI: 0.37–3.89; P = .1). There was significant heterogeneity (I2 = 91%) (Fig. 7).
Figure 7.
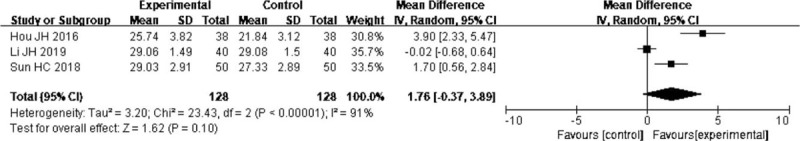
Forest plots of effect of probiotics on the mid arm muscle circumference.
4.7. Prealbumin content
Eight trials[13,16,18,21,27,29,33,34] reported the effect of probiotics on prealbumin content, and the pooled result showed that probiotics were associated with improvement on prealbumin content (MD = 25.83, 95% CI: 13.68–37.99, P < .0001). There was significant heterogeneity (I2 = 93%) (Fig. 8), but the main outcomes were not affected by excluding any particular study, so the random-effect model was selected.
Figure 8.
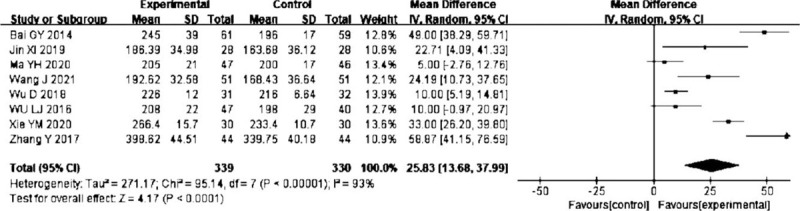
Forest plots of effect of probiotics on the prealbumin content.
4.8. Publication bias
The funnel plot was used to evaluate the publication bias of the 12 randomized controlled trials. Figure 9 showed the publication bias of the whole study was small.
Figure 9.
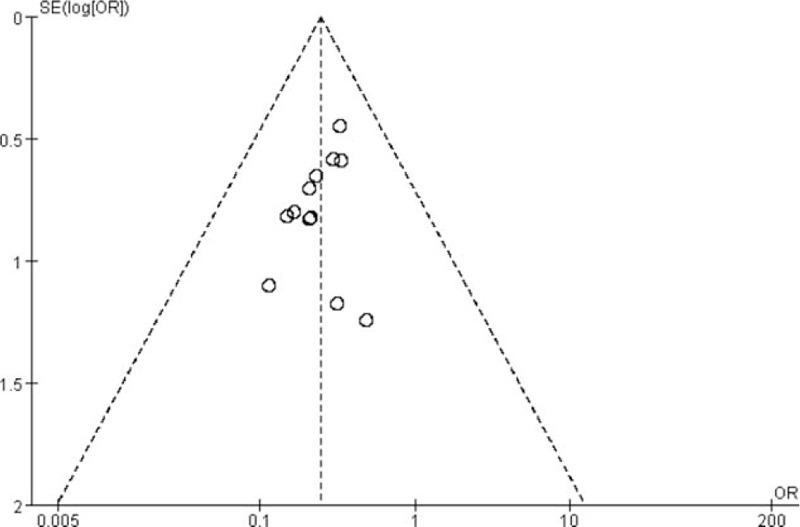
Funnel plot of publication bias.
5. Discussion
Our analysis suggested that probiotics had a positive impact on balancing the intestinal flora. The application of probiotics ameliorated gastrointestinal function, nutritional status, and immunity, and what's more improved Glasgow Coma Scale score, which indicated that probiotics were beneficial in improving the state of consciousness.
The results showed that the time to reach target nutrition was decreased, and prealbumin content was improved in the group of probiotics in EN. This meant that compared to EN alone, probiotics in EN could improve nutrition status. The studies included in this meta-analysis suggested that probiotics in EN reduced the incidence of gut microbiome dysbiosis, protected intestinal mucosal integrity, and alleviated gastrointestinal complications.[14,15,18–20,22–26,28,31,33,35] The mechanism of improving the nutritional status of probiotics was that probiotics could stabilize intestinal homeostasis, accelerate gastrointestinal peristalsis, and inhibit disuse atrophy of intestinal mucosal.[37,38] With the restoration of gastrointestinal function, probiotics ultimately facilitated the absorption of nutrients.[39] However, the effect on muscle circumference needs to be further validated.
Present researches confirmed that probiotics had positive effect on reducing the incidence of infection and state of consciousness in patients with severe stroke. Infection after stroke, accompanied by inflammatory responses, facilitated the aggregation of proinflammatory cytokines into the region of brain injury and aggravated neurotoxicity.[40–43] Research had found that probiotics therapy may be an efficient strategy to induce persistent immune regulation of the central nervous system and reduce neuroinflammation.[44] The researches performed by Jin et al[45] and Wan et al[46] found that probiotics could improve the immunity of critically ill patients, which were consistent with the results of our study. It was important that the reduction of infection rate strongly correlates with improvement of nutritional status.[47–49] Besides, our result indicated the improvement in Glasgow Coma Scale score. However, it was not enough to prove the recovery of neurological function. Therefore, further studies are needed to confirm whether probiotics could improve neurological function in patients with severe stroke.
Based on our finding, we found that probiotics supplementation was carried out in the early stage or within 72 hours after stroke. Probiotic formula was also analyzed in this meta-analysis, and it showed that eight studies used Bifidobacterium triple viable, ten studies used Bifidobacterium tetravaccine, and 1 study added xylooligosaccharides (XOS) to probiotics. Researches showed that XOS could increase Bifidobacteria and fortify the integrity of intestinal epithelium barrier and gastrointestinal absorption function.[50,51] Based on comprehensive analysis, it is recommended to set the course of treatment to ≥14 days. Marzorati M et al[52] found 14-day probiotic intervention had a positive impact on the gut microbiome dysbiosis, as evidenced by a rapid decline in Enterobacteriaceae bacteria and increase of SCFA-producing gut flora. Wu et al[53] also discovered a positive effect of 14-day probiotic intervention on antibiotic-induced intestinal flora dysbiosis. Therefore, we expect our findings to inform the treatment of stroke recovery.
There are several limitations in this meta-analysis. First, all the studies were performed in China. The reason is that probiotics and intestinal flora are a hot topic of research in China. In summary, it was found that the number of articles published on probiotics and intestinal flora from 2015 to 2021 is twice the number published from 2010 to 2015. Secondly, there was significant heterogeneity in the analysis of the 4 indexes, and the heterogeneity might be explained by the difference of the trial design, type of probiotic strains, characteristics of patients, and course of treatment, and the heterogeneity may be a barrier to be accepted widely of our findings. Thirdly, the Glasgow Coma Scale score outcome needs to be interpreted carefully for the scores of the 2 studies not improve,[21,32,35] although the difference was statistically significant after combined analysis. In the future, high-quality randomized controlled trials will be required to determine the efficacy of various probiotic formulas with different treatment duration. Also, high-quality trials based on large human cohorts should be designed to clarify the underlying mechanisms of various probiotic formulas in ameliorating nutrition status of severe stroke.
6. Conclusion
In conclusion, our results show that probiotics in EN are useful for improving nutritional status, contributing to the clinical prognosis of severe stroke patients with nasal feeding, and providing clinical evidence for probiotics in the treatment of severe stroke patients. Considering the limitations of our meta-analysis, further research is needed to ensure a high level of evidence demonstrating the beneficial effects of probiotics in enternal nutrition.
Acknowledgments
The authors thank Weijia Lin, Yingying Li, and Man Yuan for their suggestions in article design and writing.
Author contributions
Xiaomin Liu drafted the manuscript. Jiahao Chu and Junzi Long searched related literatures, extracted data and assessed the quality of literature. Jie Zheng, Xue Cheng, and Xinmin Li revised and reviewed the article manuscript. Yasu Zhang had primary responsibility for final content. All authors approved the final manuscript.
Data curation: Jiahao Chu, Junzi Long.
Methodology: Jiahao Chu, Junzi Long.
Software: Jie Zheng, Xue Cheng.
Supervision: Yasu Zhang.
Visualization: Xue Cheng.
Writing – original draft: Xiaomin Liu.
Writing – review & editing: Xinmin Li.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, MD = mean difference, OR = odds ratio.
How to cite this article: Liu X, Zhang Y, Chu J, Zheng J, Cheng X, Li X, Long J. Effect of probiotics on the nutritional status of severe stroke patients with nasal feeding that receive enteral nutrition: a protocol for systematic review and meta-analysis of randomized controlled trials. Medicine. 2021;100:17(e25657).
The authors report no conflicts of interest.
Funding Sources: The study supported by National Natural Science Foundation of China (ID: 81704135), Foundation of He’nan Educational Committee (ID: 18A360012), and the key scientific and technological project of Henan Province (ID: 192102310424).
The datasets generated during and/or analyzed during the current study are publicly available.
Supplemental digital content is available for this article.
¯X = mean value, A = Glasgow Coma Scale score, B = rate of intestinal flora dysbiosis, C = gastrointestinal complication, D = time to reach target nutrition. E: mid arm muscle circumference, EN = enteral nutrition, EN = enteral nutrition, F = female, F = prealbumin content, G = infection rate, M = male, S = standard deviation.
References
- [1].FOOD Trial Collaboration. Poor nutritional status on admission predicts poor outcomes after stroke: observational data from the FOOD trial. Stroke 2003;34:1450–6. [DOI] [PubMed] [Google Scholar]
- [2].Gomes F, Emery PW, Weekes CE. Risk of malnutrition is an independent predictor of mortality, length of hospital stay, and hospitalization costs in stroke patients. J Stroke Cerebrovasc Dis 2016;25:799–806. [DOI] [PubMed] [Google Scholar]
- [3].Foley NC, Salter KL, Robertson J, et al. Which reported estimate of the prevalence of malnutrition after stroke is valid? Stroke 2009;40:e66–74. [DOI] [PubMed] [Google Scholar]
- [4].Mosselman MJ, Kruitwagen CL, Schuurmans MJ, et al. Malnutrition and risk of malnutrition in patients with stroke: prevalence during hospital stay. J Neurosci Nurs 2013;45:194–204. [DOI] [PubMed] [Google Scholar]
- [5].Wierzbicki K, Horyniecki M, Mamak D, et al. Does the nutritional status of acute stroke patients affect the neurological status in the early post-stroke period? Neurol Res 2020;4:01–7. [DOI] [PubMed] [Google Scholar]
- [6].Pinto A, Tuttolomondo A, Di Raimondo D, et al. Risk factors profile and clinical outcome of ischemic stroke patients admitted in a Department of Internal Medicine and classified by TOAST classification. Int Angiol 2006;5:261–7. [PubMed] [Google Scholar]
- [7].Tuttolomondo A, Pedone C, Pinto A, et al. Gruppo Italiano di Farmacoepidemiologia dell’Anziano (GIFA) researchers. Predictors of outcome in acute ischemic cerebrovascular syndromes: The GIFA study. Int J Cardiol 2008;125:391–6. [DOI] [PubMed] [Google Scholar]
- [8].Tuttolomondo A, Di Sciacca R, Di Raimondo D, et al. Effects of clinical and laboratory variables and of pretreatment with cardiovascular drugs in acute ischaemic stroke: a retrospective chart review from the GIFA study. Int J Cardiol 2011;151:318–22. [DOI] [PubMed] [Google Scholar]
- [9].Gungabissoon U, Hacquoil K, Bains C, et al. Prevalence, risk factors, clinical consequences, and treatment of enteral feed intolerance during critical illness. JPEN J Parenter Enteral Nutr 2015;39:441–8. [DOI] [PubMed] [Google Scholar]
- [10].Robertson C, Savva GM, Clapuci R, et al. Incidence of necrotising enterocolitis before and after introducing routine prophylactic Lactobacillus and Bifidobacterium probiotics. Arch Dis Child Fetal Neonatal Ed 2020;105:380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pavelescu D, Mirea L, Grintescu I. Could selected probiotics have beneficial effects on clinical outcome of severe traumatic brain injury patients? Crit Care 2014;18:472.25128022 [Google Scholar]
- [12].Higgins JP, Altman DG, Gotzsche PC. The Cochrane collaboration's tool for assessing risk of bias in randomized trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang J, Ru R, Sun J, et al. Effects of enteral nutrition combined with probiotics on nutritional status in patients with acute ischemic stroke after neural intervention therapy. Journal of Chengdu Medical College 2021;16:29–32. [Google Scholar]
- [14].Dong GH. Clinical effect of probiotics combined with enteral nutrition on patients with severe stroke. Chinese Remedies & Clinics 2020;20:1687–8. [Google Scholar]
- [15].Liang XQ, Mou D, Lei WJ, et al. Effect of probiotics-combined early enteral nutrition treatment in treatment of patients with severe cerebral hemorrhage. Modern Hospitals 2020;20:1227–9. [Google Scholar]
- [16].Ma YH. Effect of Bifidobacterium tetani combined with enteral nutrition on gastrointestinal and neurological functions in patients with severe cerebral hemorrhage. Chinese Remedies & Clinics 2020;20:1474–6. [Google Scholar]
- [17].Wang SS. Early enteral nutrition combined with symbiogenic preparations on the improvement of cellular immune function in elderly hypertensive patients with cerebral hemorrhage. J Mod Med Health 2020;20:1474–6. [Google Scholar]
- [18].Xie YM. Effects of early enteral nutrition combined with probiotics on nutritional status and gastrointestinal function in nasogastric stroke patients. Journal of North Pharmacy 2020;17: 44-45, 52. [Google Scholar]
- [19].Chen K. Analysis of the effect of probiotics combined with early indwelling gastric tube nasal feeding enteral nutrition support in elderly patients with severe stroke. Yiyao Qianyan 2019;9:155–6. [Google Scholar]
- [20].Chen JQ. Effect of Bifidobacterium tetravaccine tablets combined with enteral nutrition on gastrointestinal complications and intestinal mucosal barrier function in patients with severe stroke. Med Forum 2019;23:2397–8. [Google Scholar]
- [21].Ji X, Shi Y, Yuan B, et al. The effect of early enteral nutrition supplemented with probiotics on ventilator-associated pneumonia in patients with severe stroke. Chin J Microecol 2019;31:174–8. [Google Scholar]
- [22].Li YY, Yan RY, Li DC, et al. Effect of probiotic enteral nutrition intervention on intestinal flora and inflammatory state in patients with severe cerebral hemorrhage after operation. Hebei Med 2019;25:572–7. [Google Scholar]
- [23].Li JH, Li JY. Effect of Bifidobacterium tetravaccine tablets combined with enteral nutrition on gastrointestinal complications and intestinal mucosal barrier function in patients with severe stroke. Journal of China Prescription Drug 2019;17:125–6. [Google Scholar]
- [24].Dong WY, Chen H, Zhu FL. Effects of Bifidobacterium tetravaccine tablets on neurological function and nutritional status in patients with severe ischemic stroke. Chinese Journal of Rural Medicine and Pharmacy 2018;25:06–7. [Google Scholar]
- [25].Gao SJ. Effect of early enteral nutrition combined with probiotics in patients with acute severe stroke. China Medical Engineering 2018;26:34–7. [Google Scholar]
- [26].Sun HC, Zhang XG, Liu Z, et al. Effect of bifidobacterium tetravaccine Tablets on intestinal function in patients with severe stroke. Chin J Microecol 2018;30:1292–6. [Google Scholar]
- [27].Wu D. Effect of Early Enteral Nutrition With Bifidobacterium on Nutritional Status in Patients With Severe Cerebral Hemorrhage. Hebei: Hebei Medical University; 2018. [Google Scholar]
- [28].Zhou Q. Effect of bifid triple viable capsules combined with early enteral nutrition on nutritional status and complications in patients with severe stroke. Diet Health 2018;5:66–7. [Google Scholar]
- [29].Zhang Y, Wang J, Zhu HL, et al. Effects of Bifidobacterium-containing enteral nutrition intervention on the nutritional status and intestinal flora disturbance in patients with the severe cerebral infarction. Journal of Hainan Medical University 2017;23:1723–5. [Google Scholar]
- [30].Chen DY, Chen J. Influence of bifid triple viable capsules combined with early enteral nutrition on nutriture and complication of patients with severe stroke. Journal of Brain and Nervous Diseases 2016. 79–81. [Google Scholar]
- [31].Hou JH. Influence of Bifidobacterium tetravaccine tablets combined with enteral nutrition on complications of digestive canal and intestinal mucous membrane barrier function of patients with severe stroke. Chinese Journal of Pharmaco epidemiology 2016;25: 11-13, 42. [Google Scholar]
- [32].Hou JH, Zhuo JM, Zhong WG, et al. Effects of Bifidobacterium tetravaccine tablets on nutritional status and immune indexes in patients with severe stroke. Chinese Journal of Rural Medicine and Pharmacy 2016;23:06–7. [Google Scholar]
- [33].Wu LJ, Li JP, Guo BW. Additional Bifidobacteria in severe stroke patients receiving enteral nutrition. Parenteral & Enteral Nutrition 2016;23:220–2. [Google Scholar]
- [34].Bai GY. Clinical Research of Probiotics Enteral Nutrition for the Treatment of Critically Ill Patients With Cerebral Apoplexy. Xinjiang: Xinjiang Medical University; 2014. [Google Scholar]
- [35].Wu Y, Yu Y, Liu JF, et al. Preliminary study on application of early enteral nutrition combined with Probiotics for acute severe stroke Patients. Clinical Medicine of China 2014;30:01–4. [Google Scholar]
- [36].Bai GY, Fan M, Tao YL, et al. Probiotics with enteral nutrition therapy clinical observation of patients with severe stroke. Guangdong Trace Elements Science 2013;20:10–4. [Google Scholar]
- [37].d’Ettorre G, Rossi G, Scagnolari C, et al. Probiotic supplementation promotes a reduction in T-cell activation, an increase in Th17 frequencies, and a recovery of intestinal epithelium integrity and mitochondrial morphology in ART-treated HIV-1-positive patients. Immun Inflamm Dis 2017;5:244–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wen Y, Li J, Long Q, et al. The efficacy and safety of probiotics for patients with constipation-predominant irritable bowel syndrome: a systematic review and meta-analysis based on seventeen randomized controlled trials. Int J Surg 2020;79:111–9. [DOI] [PubMed] [Google Scholar]
- [39].Yu XY, Yin HH, Zhu JC. Increased gut absorptive capacity in rats with severe head injury after feeding with probiotics. Nutrition 2011;27:100–7. [DOI] [PubMed] [Google Scholar]
- [40].Tuttolomondo A, Maida C, Pinto A. Diabetic foot syndrome: immune- inflammatory features as possible cardiovascular markers in diabetes. World J Orthop 2015;6:62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Petta S, Valenti L, Tuttolomondo A, et al. Interferon lambda 4 rs368234815 TT > (G variant is associated with liver damage in patients with nonalcoholic fatty liver disease. Hepatology 2017;66:1885–93. [DOI] [PubMed] [Google Scholar]
- [42].Di Raimondo D, Tuttolomondo A, Buttà C, et al. Metabolic and anti-inflammatory effects of a home-based programme of aerobic physical exercise. Int J Clin Pract 2013;67:1247–53. [DOI] [PubMed] [Google Scholar]
- [43].Tuttolomondo A, Maida C, Pinto A. Diabetic foot syndrome as a possible cardiovascular marker in diabetic patients. J Diabetes Res 2015;2015:268390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Frank MG, Fonken LK, Watkins LR, et al. Could probiotics be used to mitigate neuroinflammation? ACS Chem Neurosci 2019;10:13–5. [DOI] [PubMed] [Google Scholar]
- [45].Jin Y, Xu H, Chen Y, et al. Therapeutic effect of Bifidobacterium combined with early enteral nutrition in the treatment of severe acute pancreatitis: a pilot study. Eur Rev Med Pharmacol Sci 2018;22:4018–24. [DOI] [PubMed] [Google Scholar]
- [46].Wan G, Wang L, Zhang G, et al. Effects of probiotics combined with early enteral nutrition on endothelin-1 and C-reactive protein levels and prognosis in patients with severe traumatic brain injury. J Int Med Res 2019;48:300060519888112. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [47].Kokura Y, Wakabayashi H, Nishioka S, et al. Nutritional intake is associated with activities of daily living and complications in older inpatients with stroke. Geriatr Gerontol Int 2018;18:1334–9. [DOI] [PubMed] [Google Scholar]
- [48].Nii M, Maeda K, Wakabayashi H, et al. Nutritional improvement and energy intake are associated with functional recovery in patients after cerebrovascular disorders. J Stroke Cerebrovasc Dis 2016;25:57–62. [DOI] [PubMed] [Google Scholar]
- [49].Alwarawrah Y, Kiernan K, MacIver NJ. Changes in nutritional status impact immune cell metabolism and function. Front Immunol 2018;9:1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sheng K, He S, Sun M, et al. Synbiotic supplementation containing Bifidobacterium infantis and xylooligosaccharides alleviates dextran sulfate sodium-induced ulcerative colitis. Food Funct 2020;11:3964–74. [DOI] [PubMed] [Google Scholar]
- [51].Finegold SM, Li Z, Summanen PH, et al. Xylooligosaccharide increases bifidobacteria but not lactobacilli in human gut microbiota. Food Funct 2014;5:436–45. [DOI] [PubMed] [Google Scholar]
- [52].Marzorati M, Abbeele PVD, Bubeck SS, et al. Bacillus subtilis HU58 and Bacillus coagulans SC208 probiotics reduced the effects of antibiotic-induced gut microbiome dysbiosis in an M-SHIME® model. Microorganisms 2020;8:1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wu J, Gan T, Zhang Y, et al. The prophylactic effects of BIFICO on the antibiotic-induced gut dysbiosis and gut microbiota. Gut Pathog 2020;12:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


