Abstract
Alpha fetoprotein (AFP) level is the gold standard diagnostic tool for detection and monitoring hepatocellular carcinoma (HCC) but with low sensitivity. Thus, the identification of alternative or combined serum markers of HCC is highly needed. Therefore, the aim of this work was to verify the value of serum midkine (MDK), Dickkopf-related protein 1 (DKK1), and alpha-L-fucosidase (AFU) in detection of HCC.
We recruited 244 subjects to the present study; 89 with liver cirrhosis, 86 cirrhotic hepatitis C virus (HCV) induced HCC, and 69 apparently healthy volunteers as controls. Serum AFP, MDK, DKK1, and AFU were measured by ELISA.
Patients with HCC showed significantly higher serum MDK, DKK1, and AFU levels compared with those patients with liver cirrhosis and healthy controls (X2 = 179.56, 153.94, and 90.07 respectively) (P < .001 in all). In HCC cases, neither of MDK, DKK1, or AFU was correlated with tumor number. On the other hand, only serum DKK1 was significantly higher in lesions >5 cm, those with portal vein thrombosis and advanced HCC stage. Receiver operator characteristic (ROC) curve analysis showed that serum MDK levels discriminated between cirrhosis and HCC at a sensitivity of 100%, a specificity of 90% at cut-off value of >5.1 ng/mL.
Although our results showed that serum MDK, DKK-1, and AFU are increased in HCC cases only MDK may be considered as the most promising serological marker for the prediction of the development of HCC in cirrhotic HCV patients.
Keywords: alpha-L-fucosidase, biological markers of hepatocellular carcinoma, chronic hepatitis C, Dickkopf-related protein 1, hepatocellular carcinoma, liver cirrhosis, midkine, serum midkine
Key Points
No single biomarker was found to have optimal performance for diagnosis of HCC.
Serum MDK, DKK-1, and AFU are increased in HCC cases and they could complement each other and improve the early diagnostic rate.
MDK alone may be considered as the most promising serological marker for the prediction of the development of HCC in cirrhotic HCV patients.
1. Introduction
Hepatocellular carcinoma (HCC) is the fourth most common cancer and third leading cause of cancer death worldwide.[1] It has been recognized that liver cirrhosis is the most common risk factor for the development of HCC as about 80% of HCC develop in cirrhotic liver.[2]
Egypt has high prevalence of hepatitis C virus (HCV) antibody of about 10.0%, and active viraemia HCV RNA of 7.0% according to the last Egyptian Demographic Health Survey.[3] Hence the remarkable high incidence of HCV related HCC in Egypt as demonstrated in several center studies.[4,5]
The diagnosis of HCC patients is difficult particularly in the early disease development hence alphafetoprotein (AFP) which is widely used as a diagnostic biomarker has a suboptimal performance.[6]
Midkine (MDK) is a heparin-binding secreted growth factor protein of a highly regulated gene family which has a pivotal role in cell growth and angiogenesis.[7] Over expression of MDK was observed in various human malignancies including HCC at both mRNA and protein level.[8]
Wingless related integration site (WNT/B catenin pathway) plays an important role in cellular homeostasis[9] and interruption of which may result in developmental abnormalities and diseases particularly cancers.[10] Secreted WNT antagonists play a role in regulation of WNT/ B catenin, of these antagonists are the Dickkopf (DKK) proteins’ family.[11]
DKK-1 block the interaction between the secreted WNT ligands and the low-density lipoprotein receptor related protein 5/6 resulting in B catenin degradation.[12] The oncogenic role of DKK-1 as well as its prognostic value in cancer outcome was highlighted by several studies.[13]
Serum alpha-L-fucosidase (AFU) is one of the liposomal enzymes that is widely present in tissues and body fluid. AFU is engaged in the breakdown of fucose containing glycoconjugates.[14] Several studies have revealed increases serum levels of AFU in patients with HCC.[15]
Therefore, the combined use of these promising biomarkers would aid early accurate diagnosis. Our study aimed to verify the value of serum MDK, DKK1, and AFU in detection of HCC development in cirrhotic HCV patients.
2. Patients and methods
This observational cross-sectional study was performed in 175 patients; 89 patients were cirrhotic HCV patients without HCC (group I) and 86 cirrhotic HCV patients with HCC (group II). Besides, 69 apparently healthy volunteers were included as a control group (group III), who were presenting to the outpatient clinic or the inpatient ward of the main University hospital between March 2016 and February 2017. Figure 1.
Figure 1.
Flow chart of patients.
Inclusion criteria: Inclusion of patients with these criteria; HCV positive proven by PCR, age: 18 to 65 years, any sex, possibility of continuous communication during the study period. Ultrasound (US) (for all patients) and Triphasic CT examination was performed for those with US proven hepatic focal lesion to define HCC patients.
Exclusion criteria: Patients with these conditions were excluded from the study: Chronic diseases, for example, chronic hepatitis B virus (HBV) infection, diabetes, renal failure, and patients with other malignancies. Patients who fail to sign the informed consent were excluded.
All patients and controls were subjected to proper and detailed history taking and thorough clinical examination. After overnight fasting, blood sampling of 10 mL and the sample was derived into 2 halves; 5 mL for routine laboratory investigation[16]; CBC, liver function tests (AST, ALT, total bilirubin, total protein and albumin, prothrombin time and activity, and AFP) and renal function tests, viral markers for hepatitis B (HBs Ag) and hepatitis C (anti-HCV) by ELISA, and PCR for HCV-RNA.[17]
The other 5 mL were centrifuged, and separate aliquots of serum were frozen at −80 °C for the time of analysis of serum Midkine by a commercially available enzyme-linked immunosorbent assay kit (Human Midkine PicoKine ELISA Kit [Boster Biological Technology, Pleasanton CA, Catalog # EK1235]), serum DKK1 by using a commercially available enzyme-linked immunosorbent assay kit (Human DKK-1 PicoKine ELISA Kit (Boster Biological Technology, Pleasanton CA, Catalog # EK0867), and serum AFU by a commercially available enzyme-linked immunosorbent assay kit (Human Tissue alpha-L-fucosidase, ELISA Kit [EIAab www.eiaab.com, Catalog No: E0807h]).
2.1. Ethical approval
All patients and healthy controls who participated in the present study signed an informed consent form. The study protocol was approved by the Ethics Committee of the Faculty of Medicine and is in accordance with the Helsinki Declaration of 1975.
2.2. Statistical methods
Differences between groups were analyzed with the unpaired t test or the Mann–Whitney U test, where appropriate. Results were expressed as median and interquartile range (IQR)and were analyzed by using the Independent-samples Kruskal–Wallis test. To assess the accuracy of the diagnostic tests, the matched data sets (chronic liver diseases patients and HCC patients) regarding AFP, MDK, DKK1, and AFU were analyzed by using receiver operator characteristic (ROC) curve analysis. All statistical procedures were performed using SPSS software, version 20 for Windows (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.).
3. Results
The clinical and demographic data of the 3 studied groups are shown in Table 1. The mean age of HCC patients was significantly higher than those with liver cirrhosis and controls (P = .00 and .01 respectively). In addition, patients with HCC had statistically significant lower platelet count than those with liver cirrhosis and controls (P < .001 in both).
Table 1.
The clinical and demographic data of the 3 studied groups.
| Variable | Group I liver cirrhosis (n = 89) | Group II HCC (n = 86) | Group III healthy (n = 69) | P∗ | Significance between groups |
| Age, y | 52 (47–60) | 59 (55–62) | 50 (46–55) | <.001 | I–II/II–III |
| ALT, U/L | 39 (27–54.5 | 42.5 (25–74.5) | 26 (24–45) | .002 | I–III/II–III |
| AST, U/L | 59 (42–83) | 68.5 (45.75–92) | 24 (19–54) | .003 | I–III/II–III |
| Albumin, g/dL | 2.7 (2.1–3.2) | 2.7 (2.1–3.3) | 3.8 (3.5–5) | <.001 | I–III/II–III |
| Total bilirubin, mg/dL | 1.7 (1.1–2.4) | 1.5 (1–3.4) | 1 (0.5–1.2) | .035 | I–III/II–III |
| Platelet count (×109 L−1) | 111.5 (71–143) | 76 (55–124) | 180 (152–250) | <.001 | I–II/I–III/II–III |
| Child class (A/B/C) | 21/41/27 | 26/32/28 | |||
| BCLC classification (A/B/C/D) | 7/34/12/33 |
3.1. Serum AFP, MDK, DKK1, and AFU
Serum AFP was significantly higher in group II than in groups I and III (Z = –6.41 and –8.81 respectively) (P = .00 and .00 respectively). Also, serum AFP was significantly higher in group I than in group III (Z = –5.77) (P = .00) Table 2.
Table 2.
Serum AFP, MDK, DKK1, and AFU in the 3 studied groups.
| Variable | Group I liver cirrhosis (n = 89) | Group II HCC (n = 86) | Group III healthy (n = 69) | X2 | P∗ | Significance between groups |
| AFP, ng/mL Median (IQR) | 8.8 (2.9–30) | 108.3 (11.75–516.67) | 2.6 (0.8–5.3) | 98.5 | .00∗ | All |
| MDK, ng/mL Median (IQR) | 1.96 (1.08–2.8) | 12.23 (9.38–21.44) | 1.1 (1.09–2.77) | 153.94 | .00∗ | All |
| DKK1, ng/mL | 1.82 (1.46–3.42) | 7.58 (3.42–20.68) | 0.72 (0.48–0.88) | 179.56 | .00∗ | All |
| AFU, U/L Median (IQR) | 0.38 (0.25–1.35) | 0.67 (0.37–1.87) | 0.23 (0.21–0.26) | 90.07 | .00∗ | All |
Serum MDK was significantly higher in group II than in groups I and III (Z = 10.38, 10.58) (P = .00) and in group I than in group III (Z = 3.12 and P = .00).
Also, DKK-1 was significantly higher in HCC cases compared with cirrhotic and controls (Z = 9.66, 10.55) (P = .00). Moreover, it was significantly higher in cirrhotic patients than controls (Z = 9.20) (P = .00) Table 2.
As regards AFU, it was significantly higher in group II than in groups I and III (Z = 2.75, 8.99) (P = .00) and in group I than in group III (Z = 7.15 and P = .00) Table 2.
In group II (HCC patients), multiple lesions were found in 53 patients and 33 patients presented with single focal lesion. Early HCC cases were 41 (BCLC A+B) while late HCC cases were 45 (BCLC C+D). Portal vein thrombosis was present in 12 patients and absent in 74 patients. Lesions >5 cm were observed in 36 patients while 50 patients had lesions <5 cm.
Serum MDK was higher in patients with multiple focal lesions, lesions >5 cm and those with portal vein thrombosis (median = 14.43, 12.88, and 16.44 respectively) than in single focal lesion, lesions <5 cm and those without portal vein thrombosis (median = 10.41, 12.18, and 12.1 respectively) but was statistically insignificant (Z = 1.00, 0.71, and 1.89) (P = .31, .47, and .06 respectively).
Furthermore, lesions >5 cm have significantly higher levels of DKK-1 than those <5 cm (Z = 3.88 and P = .00). Also, those with portal vein thrombosis have higher levels of DKK-1 than those without it (Z = 4.99 and P = .00). DKK-1 levels were significantly higher in advanced HCC stage than in early ones (Z = 2.64 and P = .00).
Although serum DKK-1 levels were higher in those with multiple focal lesions than in patients with single lesion but that was statistically insignificant (median 9.37 and 5.98 respectively) (Z = 1.86, P = .06).
Regarding AFU, it was observed that serum AFU doesn’t have any association with tumor number, size, severity, or portal vein thrombosis.
ROC curve analysis reveals that serum MDK and DKK1 at cut off 5.1 and 2.3 ng/mL respectively showed better performance than AFP at a cut off ≥10 and AFU at a cut off 0.37 μ/L in differentiation between HCC patients and those with liver cirrhosis. (Table 3, Fig. 2).
Table 3.
Sensitivity, specificity, PPV, NPV, and accuracy of the studied parameters for prediction of HCC.
| AUC | CI 95% | Cutoff | Sensitivity | Specificity | PPV | NPV | Accuracy | |
| AFP, ng/mL | 0.83 | 0.73–0.89 | 10 | 78% | 45% | 61.5% | 71.2% | 65.14% |
| Midkine, ng/mL | 0.95 | 0.95–0.99 | 5.1 | 100% | 90% | 89% | 100% | 94% |
| DKK-1, ng/mL | 0.92 | 0.93–0.97 | 2.3 | 89% | 80% | 80.2% | 88,6% | 84% |
| AFU, U/L | 0.62 | 0.69–0.81 | 0.37 | 74% | 50% | 58.33% | 65.67% | 61.1% |
Figure 2.
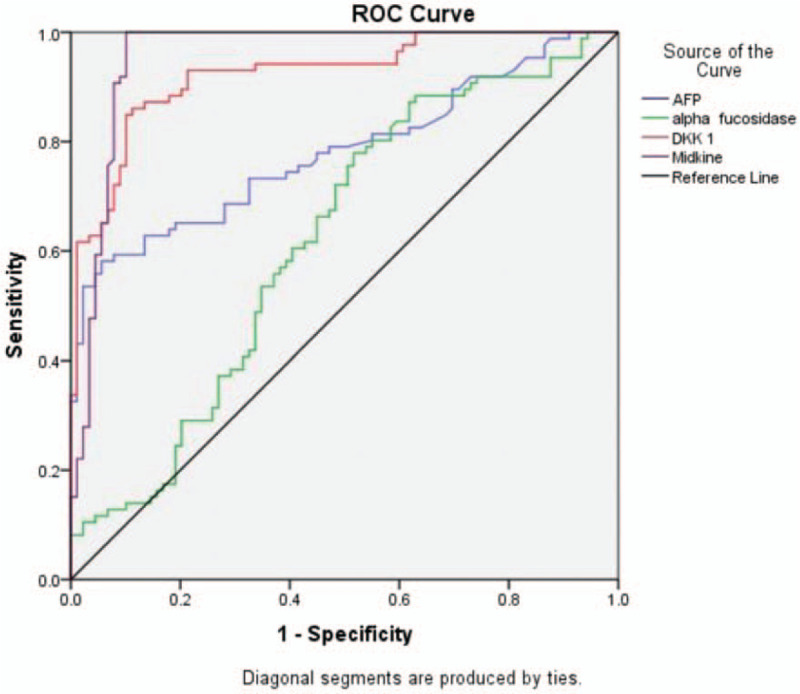
Receiver-operator characteristics (ROC) curve analyses showing the diagnostic potential of serum AFP, AFU, DKK1, and MDK to detect HCC occurrence. AFP = alpha fetoprotein, AFU = alpha-L-fucosidase, DKK1 = Dickkopf-related protein 1, HCC = hepatocellular carcinoma, MDK = midkine.
Using 10 ng/mL as a diagnostic cut-off value for AFP, it was positive in 78% of (67/86) HCC patients and negative in 22% (19/86) of them. While in those HCC patients with negative AFP, serum AFU was positive in 15/19 (79%), serum DKK1 was positive in 16/19 (84.2%), and serum MDK was positive in 19/19 (100%).
A logistic regression analysis was performed to ascertain the effects of serum MDK, DKK1, and AFU on the like hood that cirrhotic HCV patients would have HCC. The logistic regression model was statistically significant X2 = 137.45 and P = .000. Among the 3 variables tested, only serum MDK and DKK1 significantly predicted HCC development in cirrhotic HCV patients (P = .00 and .017 respectively).
4. Discussion
Serum biomarkers are reliable potential tools for screening and diagnosis of HCC. Meanwhile, no single biomarker was found to have optimal performance for diagnosis of HCC. Therefore, combination of several biomarkers have been shown to complement each other and improve the early diagnostic rate.[18]
Evidence that MDK has a significant role in cancer process such as proliferation, anti-apoptosis, and angiogenesis has been documented in many solid tumors, including HCCs.[19]
In this study, serum MDK was significantly elevated in patients with HCC compared with those with cirrhosis (P < .001).
In addition, ROC curves showed a higher classification power of MDK (93.54% of HCC patients) to detect HCC patients at a cutoff value of 5.1 ng/mL with respect to AFP at a cutoff value of 10 ng/mL (36% of HCC patients). This indicates that serum MDK could be a useful marker with a lower false-positive rate in diagnosing and differentiating HCC from liver cirrhosis.
Our results were in agreement with Zhu et al,[20] who showed that serum MDK may serve as a novel diagnostic tumor marker for the detection of HCC, particularly for those with negative AFP and/or at an early stage. However, most of the HCC patients in their study are HBV-related. Similar findings were reported by Hung et al[21] and Shaheen et al.[22]
MDK was significantly elevated in HCC tissue as well as serum in Zhu et al[20] study with AUC of 0.91% and 95% confidence interval (CI) 0.89–0.93 similar to ours 0.95% and 95% CI of 0.92–0.98. In Zhu study,[20] 13.2% of patients with liver cirrhosis had elevated serum MDK (false positive) versus 10.11% in our study.
In Shaheen et al,[22] serum MDK was higher in HCC patients than in those with liver cirrhosis and controls. Also, out of 40 HCC patients 15 have AFP <20, in those 15 patients MDK was higher than cut off 0.387 ng/mL in 14/15. Furthermore, MDK was positive (above the cut off value of in 19/19 cases of HCC with negative AFP) (100%).
A large-scale multicenter study was performed with patients affected by liver diseases including HCC, chronic hepatitis B virus (HBV), or liver cirrhosis to assess whether DKK1 could serve as an alternative biomarker to alpha-fetoprotein (AFP) for HCC diagnosis.[23]
Our study revealed significant higher serum DKK-1 levels in HCC patients compared with healthy controls. This was in accordance with previous studies done by Kim et al[24] and Bakr et al.[25] In addition, several studies reported high expression of DKK-1 in hepatocellular carcinoma.[26]
We found a significant elevation of serum DKK-1 levels in cases with lesions >5 cm compared with smaller one (<5 cm). This finding was in accordance with Bakr et al[25] and Dala et al[27] this also was similar in late stage disease and in those with portal vein thrombosis.
Dala et al[27] showed that DKK-1 at a cut off value of 1.122 ng/mL had a sensitivity of 80%, specificity of 77.1% with an area under curve of 0.810 and P value of <.001 while our cut off was 2.3 ng/mL that showed sensitivity of 89%, specificity of 80% with an area under curve of 0.92 and P value of <.001.
However, patients with multiple HCC lesions showed no significant difference of DKK-1 levels compared with single lesions. These results were in agreement with previous studies that showed positive correlation between serum DKK-1 and tumor size ≥5 cm.[23,28]
We have noticed significant increased serum DKK-1 levels in patients with BCLC (C–D) than BCLC (A–B) stages. Similar results were reported by Kim et al,[24] and Bakr et al.[25]
Moreover, in 31 cases of HCC who have normal AFP, DKK-1 was higher above the cut off value in 28 cases. This also in accordance with Bakr et al[25] who reported elevated DKK-1 in all cases with normal AFP. This finding was similar to results of Tao et al[29] and Gomceli et al.[30]
Also, Mohamed et al,[31] in Egyptian patients with HCC found that 17/40 have normal AFP but DKK-1 was above the cut off value. Also, in their study at cut off value of 400 ng/mL AFP (sensitivity 28% and specificity 100%) while ours 100% specificity was associated with 30% sensitivity.
Alpha-L-fucosidase enzyme has been proposed as a marker of HCC.[32–35] The results of our study confirm the work of the previously mentioned studies of an increased serum activity of alpha-L-fucosidase enzyme in patients with HCC. The reason for the increase of alpha-L-fucosidase is still unknown. One possible explanation is an increased synthesis of proteins by tumor with a consequent increase in fucose turnover.[36]
Our results revealed that AFU was also higher in those with liver cirrhosis than healthy controls which is consistent with the studies that suggested that elevated AFU was not directly derived from tumors.[37] El-Houseini et al[37] reported that AFU could be used as a diagnostic biomarker of HCC 6 months before detection by ultrasound.
Our study included 60/86 (Child B+C) of HCC cases, high levels of cytokines in advanced cirrhotic disease process could upregulate AFU levels.[38]
In AFP negative HCC patients, serum AFU was positive in 15 patients (79%). Similar finding was reported by Wang et al,[39] who showed that 17 patients with HCC with negative AFP have higher AFU but the difference between our study and Wang is their cut off value for AFP is >400 ng/mL while ours was 10 ng/mL.
Our study revealed that AFU sensitivity and specificity were 60.5% and 57.3% for HCC diagnosis which is lower than AFP (64% and 82%). These findings were in accordance with Bukofzer et al,[32] who reported less sensitivity and specificity of AFU than AFP. While Zhang et al[40] showed the sensitivity of AFU was 72.4% and the specificity was 63.8%.
Alpha-L-fucosidase enzyme showed no relation with the size of the tumor. This finding came in agreement with Takahashi et al[41] who found no relationship between alpha-L-fucosidase enzyme activity and tumor size.
This study had its potential limitations. First, the small number of patients, and this was owing to the pilot nature of the study to discover the efficacy of these markers together. Also, our patient cohort did not include hepatocellular carcinoma secondary to other etiologies than HCV. So, larger multicenter studies investigating these markers would help to define the cut off levels and recommend their use as an alternative biomarker for HCC. On the other hand, this was the first study to combine these 3 promising biomarkers.
5. Conclusions
Although our results showed that serum MDK, DKK-1, and AFU are increased in HCC cases only MDK may be considered as the most promising serological marker for the prediction of the development of HCC in cirrhotic HCV patients.
Acknowledgments
The authors would thank all the nursing staff and laboratory technicians who helped in this work.
Author contributions
Conceptualization: Ayman El-Shayeb.
Data curation: Mariam Salah Zaghloul.
Formal analysis: Mariam Salah Zaghloul.
Funding acquisition: Nihal El-Habachi.
Investigation: Amal Mansour.
Methodology: Amal Mansour.
Project administration: Ayman El-Shayeb, Nihal El-Habachi.
Software: Mariam Salah Zaghloul.
Supervision: Ayman El-Shayeb.
Validation: Mariam Salah Zaghloul.
Visualization: Mariam Salah Zaghloul.
Writing – original draft: Mariam Salah Zaghloul.
Writing – review & editing: Ayman El-Shayeb, Nihal El-Habachi, Amal Mansour.
Footnotes
Abbreviations: AFP = alpha fetoprotein, AFU = alpha-L-fucosidase, DKK1 = Dickkopf-related protein 1, HCC = hepatocellular carcinoma, IQR = interquartile range, MDK = midkine, ROC = receiver operator characteristic, WNT/B = wingless related integration site.
How to cite this article: El-Shayeb AF, El-Habachi NM, Mansour AR, Zaghloul MS. Serum midkine is a more sensitive predictor for hepatocellular carcinoma than Dickkopf-1 and alpha-L-fucosidase in cirrhotic HCV patients. Medicine. 2021;100:17(e25112).
Ethics approval and consent to participate: All patients and healthy controls who participated in the present study signed an informed consent form. The study protocol was approved by the Ethics Committee of the Faculty of Medicine, Alexandria University, Egypt and is in accordance with the Helsinki Declaration of 1975.
Availability of data and materials: The data that support the findings of this study are available from [Alexandria clinical research center (ACRC)] but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of [ACRC].
This study was supported by a research grant from the Science and Technology Development Fund (STDF), Egypt (Grant No. 5215) to Professor Dr. Nihal ELHabachi.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Data are given as median (IQR). Reference intervals are given in parentheses. HCC = hepatocellular carcinoma.
Significant at P ≤ .05.
Data are given as median (IQR). Reference intervals are given in parentheses. AFP = alpha fetoprotein, AFU = alpha-L-fucosidase, DKK1 = Dickkopf-related protein 1, HCC = hepatocellular carcinoma, MDK = midkine
Significant at P ≤ .05.
AFP = alpha fetoprotein, AFU = alpha-L-fucosidase, AUC = area under the curve, CI = confidence interval, DKK1 = Dickkopf-related protein 1, HCC = hepatocellular carcinoma, NPV = negative predictive value, PPV = positive predictive value.
References
- [1].Herszenyi L, Tulassay Z. Epidemiology of gastrointestinal and liver tumors. Eur Rev Med Pharmacol Sci 2010;14:249–58. [PubMed] [Google Scholar]
- [2].Shaker MK, Abdella HM, Khalifa MO, et al. Epidemiological characteristics of hepatocellular carcinoma in Egypt: a retrospective analysis of 1313 cases. Liver Int 2013;33:1601–6. [DOI] [PubMed] [Google Scholar]
- [3].Gomaa A, Allam N, Elsharkway A, et al. Hepatitis C infection in Egypt: prevalence, impact and management strategies. Hepat Med 2017;9:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Abd-Elsalam S, Elwan N, Soliman H, et al. Epidemiology of liver cancer in Nile delta over a decade: a single-center study. South Asian J Cancer 2018;7:24–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ziada DH, El Sadany S, Soliman H, et al. Prevalence of hepatocellular carcinoma in chronic hepatitis C patients in Mid Delta, Egypt: a single center study. J Egypt Natl Canc Inst 2016;28:257–62. [DOI] [PubMed] [Google Scholar]
- [6].Farinati F, Marino D, De Giorgio M, et al. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol 2006;101:524–32. [DOI] [PubMed] [Google Scholar]
- [7].Muramatsu T. Midkine and pleiotrophin: two related proteins involved in development, survival, inflammation and tumorigenesis. J Biochem 2002;132:359–71. [DOI] [PubMed] [Google Scholar]
- [8].Ruan M, Ji T, Wu Z, et al. Evaluation of expression of midkine in oral squamous cell carcinoma and its correlation with tumour angiogenesis. Int J Oral Maxillofac Surg 2007;36:159–64. [DOI] [PubMed] [Google Scholar]
- [9].Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer 2013;13:11–26. [DOI] [PubMed] [Google Scholar]
- [10].Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell 2012;149:1192–205. [DOI] [PubMed] [Google Scholar]
- [11].Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 2006;25:7469–81. [DOI] [PubMed] [Google Scholar]
- [12].Zorn AM. Wnt signalling: antagonistic Dickkopfs. Curr Biol 2001;11:R592–5. [DOI] [PubMed] [Google Scholar]
- [13].Hall CL, Kang S, MacDougald OA, et al. Role of Wnts in prostate cancer bone metastases. J Cell Biochem 2006;97:661–72. [DOI] [PubMed] [Google Scholar]
- [14].Johnson SW, Alhadeff JA. Mammalian alpha-L-fucosidases. Comp Biochem Physiol B 1991;99:479–88. [DOI] [PubMed] [Google Scholar]
- [15].Zhao YJ, Ju Q, Li GC. Tumor markers for hepatocellular carcinoma. Mol Clin Oncol 2013;1:593–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Burtis CA, Ashwood ER. Tietz Fundamentals of Clinical Chemistry. WB Saunders: Philadelphia, PA; 1996. [Google Scholar]
- [17].Eddleston ALWF. Viral hepatitis, scientific basis and clinical management. Gut 1994;35:1679. [Google Scholar]
- [18].Tsuchiya N, Sawada Y, Endo I, et al. Biomarkers for the early diagnosis of hepatocellular carcinoma. World J Gastroenterol 2015;21:10573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Choudhuri R, Zhang H-T, Donnini S, et al. An angiogenic role for the neurokines midkine and pleiotrophin in tumorigenesis. Cancer Res 1997;57:1814–9. [PubMed] [Google Scholar]
- [20].Zhu W-W, Guo J-J, Guo L, et al. Evaluation of midkine as a diagnostic serum biomarker in hepatocellular carcinoma. Clin Cancer Res 2013;19:3944–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hung Y-J, Lin ZH, Cheng T-I, et al. Serum midkine as a prognostic biomarker for patients with hepatocellular carcinoma. Am J Clin Pathol 2011;136:594–603. [DOI] [PubMed] [Google Scholar]
- [22].Shaheen KY, Abdel-Mageed AI, Safwat E, et al. The value of serum midkine level in diagnosis of hepatocellular carcinoma. Int J Hepatol 2015;2015:146389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Shen Q, Fan J, Yang X-R, et al. Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large-scale, multicentre study. Lancet Oncol 2012;13:817–26. [DOI] [PubMed] [Google Scholar]
- [24].Kim SU, Park JH, Kim H-S, et al. Serum Dickkopf-1 as a biomarker for the diagnosis of hepatocellular carcinoma. Yonsei Med J 2015;56:1296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bakr HG, Elfarargy OM, Elnaggar AM. Clinical significance of serum Dikkopf-1 (Dkk-1) as a diagnostic marker for hepatocellular carcinoma. Int J Sci Res 2016;5: [Google Scholar]
- [26].Yu B, Yang X, Xu Y, et al. Elevated expression of DKK1 is associated with cytoplasmic/nuclear β-catenin accumulation and poor prognosis in hepatocellular carcinomas. J Hepatol 2009;50:948–57. [DOI] [PubMed] [Google Scholar]
- [27].Dala A, Badr M, Habib M, et al. Diagnostic value of serum Dickkopf-1 as a predictor of hepatocellular carcinoma in patients with liver cirrhosis. Menoufia Med J 2019;32:359–62. [Google Scholar]
- [28].Tung EKK, Mak CKM, Fatima S, et al. Clinicopathological and prognostic significance of serum and tissue Dickkopf-1 levels in human hepatocellular carcinoma. Liver Int 2011;31:1494–504. [DOI] [PubMed] [Google Scholar]
- [29].Tao Y-M, Liu Z, Liu H-L. Dickkopf-1 (DKK1) promotes invasion and metastasis of hepatocellular carcinoma. Dig Liver Dis 2013;45:251–7. [DOI] [PubMed] [Google Scholar]
- [30].Gomceli I, Bostanci EB, Ozer I, et al. A novel screening biomarker in gastric cancer: serum Dickkopf-1. Hepatogastroenterology 2012;59:1661–4. [DOI] [PubMed] [Google Scholar]
- [31].Mohamed FZ, Barakat LAA, Radwan NH, Khedr MMM. Comparative study between DKK-1 and AFP for diagnosing of hepatocellular carcinoma among egyptian patients. Eur J Pharma Med Res 2016;3:08. [Google Scholar]
- [32].Bukofzer S, Stass P, Kew M, et al. Alpha-l-fucosidase as a serum marker of hepatocellular carcinoma in southern african blacks. Br J Cancer 1989;59:417–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Giardina MG, Matarazzo M, Morante R, et al. Serum α-L-fucosidase activity and early detection of hepatocellular carcinoma. Cancer 1998;83:2468–74. [PubMed] [Google Scholar]
- [34].Ishizuka H, Nakayama T, Matsuoka S, et al. Prediction of the development of hepato-cellular-carcinoma in patients with liver cirrhosis by the serial determinations of serum alpha-L-fucosidase activity. Intern Med 1999;38:927–31. [DOI] [PubMed] [Google Scholar]
- [35].Tangkijvanich P, Tosukhowong P, Bunyongyod P, et al. Alpha-L-fucosidase as a serum marker of hepatocellular carcinoma in Thailand. Southeast Asian J Trop Med Public Health 1999;30:110–4. [PubMed] [Google Scholar]
- [36].Deugnier Y, David V, Brissot P, et al. Serum α-L-fucosidase: a new marker for the diagnosis of primary hepatic carcinoma? Hepatology 1984;4:889–92. [DOI] [PubMed] [Google Scholar]
- [37].El-Houseini ME, El-Sherbiny M, Awad M, et al. Serum alpha-L-fucosidase enzyme activity as a marker for hepatocellular carcinoma: comparison with AFP using ROC analysis. J Egypt Natl Cancer Inst 2001;13:227–83. [Google Scholar]
- [38].Leonardi GC, Candido S, Cervello M, et al. The tumor microenvironment in hepatocellular carcinoma. Int J Oncol 2012;40:1733–47. [DOI] [PubMed] [Google Scholar]
- [39].Wang C-C, Iyer SG, Low JK, et al. Perioperative factors affecting long-term outcomes of 473 consecutive patients undergoing hepatectomy for hepatocellular carcinoma. Ann Surg Oncol 2009;16:1832–42. [DOI] [PubMed] [Google Scholar]
- [40].Zhang S-Y, Lin B-D, Li B-R. Evaluation of the diagnostic value of alpha-l-fucosidase, alpha-fetoprotein and thymidine kinase 1 with ROC and logistic regression for hepatocellular carcinoma. FEBS Open Bio 2015;5:240–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Takahashi H, Saibara T, Iwamura S, et al. Serum α-L-fucosidase activity and tumor size in hepatocellular carcinoma. Hepatology 1994;19:1414–7. [PubMed] [Google Scholar]


