Abstract
Programmed death protein 1 (PD-1) pathway is one of the most critical mechanisms in tumor biology of hepatocellular carcinoma (HCC). The study aimed to assess the prognostic influence of pretransplant serum soluble PD-1 (sPD-1) in patients undergoing liver transplantation for treatment of HCC.
Data from 229 patients with HCC who underwent living donor liver transplantation between January 2010 and December 2015 were retrospectively evaluated. Stored serum samples were used to measure sPD-1 concentrations.
Overall survival (OS) and disease-free survival (DFS) rates were 94.3% and 74.5% at 1 year; 78.2% and 59.2% at 3 years; and 75.4% and 55.5% at 5 years, respectively. Prognostic analysis using pretransplant serum sPD-1 with a cut-off of 93.6 μg/mL (median value of the study cohort) did not have significant prognostic influence on OS (P = .69) and DFS (P = .26). Prognostic analysis using sPD-1 with a cut-off of 300 μg/mL showed similar OS (P = .46) and marginally lower DFS (P = .070). Combination of Milan criteria and sPD-1 with a cutoff of 300 μg/mL showed similar outcomes of OS and DFS in patients within and beyond Milan criteria. Multivariate analysis revealed that only Milan criteria was an independent prognostic for OS and DFS, but pretransplant sPD1 with a cut-off of 300 μg/mL did not become a prognostic factor.
The results of this study demonstrate that pretransplant serum sPD-1 did not show significant influences on post-transplant outcomes in patients with HCC. Further large-scale, multicenter studies are necessary to clarify the role of serum sPD-1 in liver transplantation recipients.
Keywords: hepatocellular carcinoma, immune checkpoint, prognosis, recurrence, tumor biology
1. Introduction
Hepatocellular carcinoma (HCC) is an established indication for liver transplantation (LT) and is a major indication for living donor liver transplantation (LDLT). However, post-transplant HCC recurrence is associated with poor outcomes and LT candidates are therefore carefully selected to minimize the risk of tumor recurrence. Various selection criteria have been proposed,[1–6] but most have yes-or-no dual concepts and additional prognostic biomarkers are required to accurately predict post-transplant tumor recurrence.
Increasing evidence suggests that cancer immune suppression and immune escape plays an essential role in tumor progression. Among these processes, activation of the programmed death protein 1 (PD-1) pathway is one of the most critical mechanisms of tumor evasion, inhibiting T-cell proliferation, inducing T-cell exhaustion, and enhancing the activity of regulatory T cells.[7] In addition to the studies of membrane-bound PD-1, soluble PD-1 (sPD-1) has been detected in the peripheral blood of patients with various malignancies, including HCC. Some clinical studies have evaluated the prognostic value of sPD-1 in patients undergoing hepatic resection for HCC, but the influence of sPD-1 concentration remains debatable.[8–10] Furthermore, the influence of pretransplant sPD-1 on the post-transplant prognosis of patients with HCC has not yet been investigated.
In this study, the prognostic influence of pretransplant serum sPD-1 was assessed in patients who had undergone LDLT for treatment of HCC in a high-volume LT center.
2. Patients and methods
2.1. Study design and patient selection
This was a retrospective, single-center study of post-transplant outcomes in patients with HCC. The aim was to estimate the prognostic value of pretransplant serum sPD-1 expression on patient overall survival (OS) and HCC-specific disease-free survival (DFS).
The LT database of the Asan Medical Center was searched to identify adult patients (≥18 years of age) with HCC who had undergone primary LDLT during the 6-year period from January 2010 to December 2015. Patients were excluded if they had an incidental diagnosis of HCC in the explanted liver, re-transplantation, treatment-induced nonviable HCC (complete pathological response) in the explanted liver, or combined HCC-cholangiocarcinoma or early post-transplant mortality (<3 months). Matching was conducted using the list of peripheral blood storage at the Bio-Resource Center of the Asan Medical Center, and >500 patients were identified whose serum samples were stored in a fresh-frozen state at −80°C. After preliminary analysis of serum sPD-1 expression, patients were randomly selected to achieve 1:1 matching between the patients with and without post-transplant HCC recurrence.
Follow-up to determine tumor recurrence and patient survival was conducted until June 2019 or patient death through review of institutional medical records and with assistance from the National Health Insurance Service in Korea. The study protocol was approved by the institutional Review Board of the Asan Medical Center (No. 2019–0599), which waived the requirement for informed consent due to the retrospective nature of this study. This study was performed in accordance with the ethical guidelines of the World Medical Association Declaration of Helsinki 2013.
2.2. Post-transplant follow-up and treatment for HCC recurrence
All patients underwent regular follow-up examinations every month during the first year and every 3 months thereafter. Detailed follow-up protocols based on the relative risk of HCC recurrence have been previously described.[11] General principles of treatment for recurrent HCC were applied to the patients with post-transplant HCC recurrence.[11–14] Various locoregional treatments for HCC recurrence were initially performed if indicated and systemic chemotherapy, including sorafenib, was provided as the final treatment modality. No patient received immunotherapy to treat HCC recurrence.
2.3. Institutional immunosuppressive regimens
The primary immunosuppressive regimen protocols used to treat adult LT recipients at our institution included an interleukin-2 receptor inhibitor, an intraoperative steroid bolus, an intravenous or oral calcineurin inhibitor (tacrolimus is preferred), and corticosteroid recycling beginning on day 1, with adjunctive mycophenolate mofetil administered to patients showing calcineurin inhibitor-associated adverse effects or to augment immunosuppression. Because everolimus has been covered by social health insurance in Korea only since early 2016, none of our patients have been administered everolimus or other mammalian target of rapamycin (mTOR) inhibitor as the primary immunosuppressant. Since 2016, calcineurin inhibitors were replaced with everolimus when post-transplant HCC recurrence or de novo malignancy developed.[13]
2.4. Serum sPD-1 assay
The concentrations of sPD-1 were measured quantitatively in sera by using enzyme-linked immunosorbent assay kit (Boster Biological Technology, Pleasanton, CA). For sPD-1 detection, the plasma was diluted 5 times with the sample diluent provided in the kit. The testing samples, blank samples, and standard controls were used to analyze the quality of sPD-1. Then, 50 μL of standard controls or samples were added to the appropriate wells in triplicate. Subsequently, 100 μL of horseradish peroxidase–conjugated antibody was added to each well except the blank wells, covered with an adhesive strip, and incubated for 1 hour at 37°C. After washing the microtiter Plate 5 times, 50 μL of substrate A and 50 μL of substrate B were added to each well. The samples were gently mixed and incubated in the dark for 15 minutes at 37°C. Finally, 50 μL of stop solution was added to each well, and the plates were analyzed at 450 nm using a Victor X3 Plate Reader (PerkinElmer, Waltham, MA) (Bio-Rad Laboratories, Hercules, CA). A standard curve was generated by plotting the average optical density (450 nm) on the vertical axis versus the corresponding concentration on the horizontal axis.
2.5. Statistical analysis
Numerical data are presented as the mean and standard deviation or median and range. Continuous variables were compared using Student t test or analysis of variance test, and incidence variables were compared using the χ2 test. Survival curves were generated using the Kaplan–Meier method and compared using a log-rank test. Cox proportional hazard regression was used for multivariate analysis and presented as hazard ratio (HR) with 95% confidence interval (95% CI). Harrell's c-index was used for assessment of prediction accuracy. A P value <.05 was considered to indicate a statistically significant difference. Statistical analyses were performed using SPSS version 22 (IBM, New York, NY) and Stata version 15 (StataCorp, College Station, TX).
3. Results
3.1. Patient demographics
A total of 229 patients were eligible for inclusion of this study. The clinicopathological features of the study cohort are summarized in Table 1. A total of 154 (67.2%) patients received pretransplant HCC treatments: transarterial chemoembolization (n = 137); radiofrequency ablation (n = 41); external beam radiotherapy (n = 17); systemic chemotherapy (n = 3); and hepatic resection (n = 49). The extent of hepatic resection was hemihepatectomy (n = 10), central bisectionectomy (n = 2), monosectionectomy (n = 11), and monosegmentectomy and partial hepatectomy (n = 26).
Table 1.
Clinicopathological features of the study patients.
| Parameters | All cases | HCC recurrence group | No recurrence group | P |
| Case no. | 229 | 102 | 127 | |
| Age, y | 53.6 ± 9.5 | 52.7 ± 5.9 | 53.2 ± 6.6 | .52 |
| Sex, male:female (n) | 195:34 | 90:12 | 105:22 | .24 |
| Background liver disease | .44 | |||
| Hepatitis B virus infection | 200 (87.3) | 91 (89.2) | 109 (85.8) | |
| Hepatitis C virus infection | 18 (7.9) | 5 (4.9) | 13 (10.2) | |
| Alcoholic liver disease | 6 (2.6) | 3 (2.9) | 3 (2.4) | |
| Others | 5 (2.2) | 3 (2.9) | 2 (1.6) | |
| MELD score | 12.1 ± 6.5 | 11.6 ± 6.1 | 12.3 ± 6.8 | .46 |
| AFP, ng/mL | 26.4 (1.0–42,200) | 31.8 (1.0–42,200) | 22.6 (1.2–5097) | .017 |
| PIVKA-II, mAU/mL | 36 (9–20,000) | 54 (9–20,000) | 30 (9–20,000) | .102 |
| Explant pathology | ||||
| Tumor no. | 1 (1–25) | 2 (1–25) | 1 (1–12) | <.001 |
| Maximal tumor diameter, cm | 2.9 (0.4–11.0) | 3.5 (0.7–11.0) | 2.6 (0.4–11.0) | <.001 |
| Total tumor volume, mL | 28.7 (0.1–1176) | 61.1 (0.7–1176) | 16.7 (0.1–1011) | <.001 |
| Macrovascular invasion | 11 (4.8) | 7 (6.9) | 4 (3.1) | .19 |
| Microvascular invasion | 78 (34.1) | 59 (57.8) | 19 (15.0) | <.001 |
| Within Milan criteria | 122 (53.3) | 37 (36.3) | 85 (66.9) | .16 |
| Within UCSF criteria | 148 (64.6) | 55 (53.9) | 93 (73.2) | .002 |
| Within Asan Medical Center criteria | 175 (76.4) | 65 (63.7) | 110 (86.6) | <.001 |
| ABO-incompatible transplantation | 48 (21.0) | 27 (26.5) | 21 (16.5) | .066 |
| Graft-recipient weight ratio | 1.11 ± 0.22 | 1.11 ± 0.21 | 1.11 ± 0.23 | .98 |
| Graft volume to recipient SLV, % | 61.9 ± 10.2 | 62.1 ± 9.8 | 61.7 ± 10.5 | .78 |
| Graft type | .14 | |||
| Right liver graft | 211 (92.1) | 97 (95.1) | 114 (89.8) | |
| Left liver graft | 4 (1.7) | 2 (2.0) | 2 (1.6) | |
| Dual grafts | 14 (6.1) | 3 (2.9) | 11 (8.7) |
Patients satisfied the Milan criteria in 122 (53.3%), the University of California San Francisco criteria in 148 (64.6%), and the Asan Medical Center criteria in 175 (76.4%) cases.[1–3]
3.2. Overall and disease-free patient survival
During the mean follow-up period of 64.5 ± 31.1 months, HCC recurrence and all-cause patient death occurred in 102 (44.5%) and 58 (25.3%) patients, respectively. The causes of patient death were HCC recurrence-related in 51 patients and due to other causes in 51 patients. The OS and DFS rates were 94.3% and 74.5% at 1 year; 78.2% and 59.2% at 3 years; and 75.4% and 55.5% at 5 years, respectively (Fig. 1).
Figure 1.

Post-transplant patient survival curves. Overall (A) and disease-free (B) patient survival curves are shown.
3.3. Pretransplant serum sPD-1 concentration
The distribution of serum sPD-1 expression is shown in Figure 2. The mean and median concentrations of pretransplant serum sPD-1 were 136.7 ± 182.2 μg/mL and 93.6 (range: 7.8–1864.8) μg/mL, respectively. The mean and median pretransplant serum sPD-1 concentration in patients with versus without post-transplant HCC recurrence was 160.0 ± 218.8 μg/mL versus 101.4 μg/mL, and 121.3 ± 145.5 μg/mL versus 88.8 μg/mL, respectively (P = .15).
Figure 2.

Distribution of pretransplant serum soluble PD-1 concentration. PD-1 = programmed death protein 1.
Receiver-operating characteristic curve analysis of tumor recurrence showed that the area under the curve was 0.557 (Fig. 3). Because the pattern of serum sPD-1 expression showed no specific points in sensitivity and specificity, it was not possible to establish a cutoff value indicating significantly different tumor recurrence rates.
Figure 3.
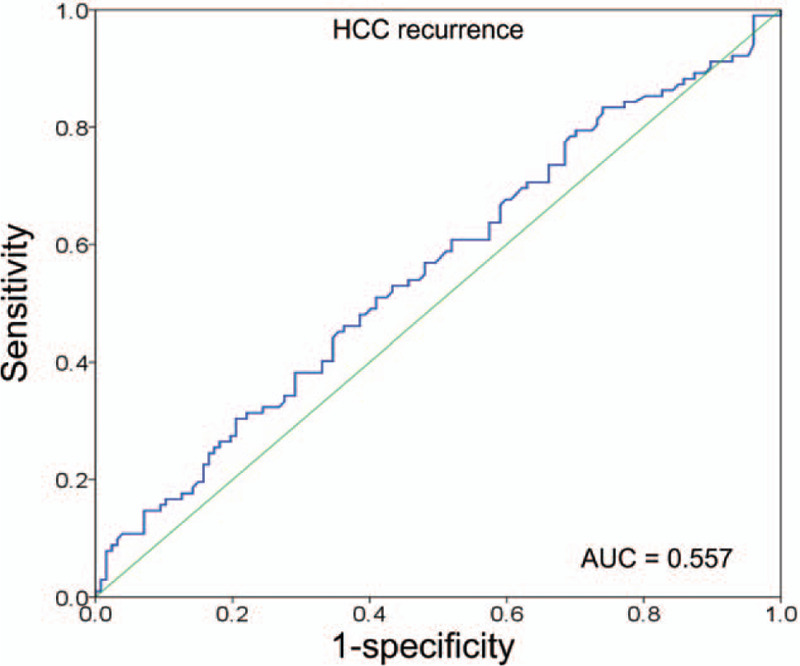
Receiver-operating characteristic curve analysis of HCC recurrence. AUC = area under the curve, HCC = hepatocellular carcinoma.
3.4. Prognosis analysis according to pretransplant serum sPD-1 concentration
Subgroup analyses on OS and DFS were performed after serum sPD-1 stratification into 18 groups, in which there was no statistical difference in OS (P = .38) and DFS (P = .17) (Fig. 4A). The correlation between serum sPD-1 expression and DFS was not high to draw out a reliable cutoff value in serum sPD-1 expression (Harrell c-index = 0.54). There was a tendency that serum sPD-1 concentration >300 μg/mL resulted in higher tumor recurrence rates (P = .070). Thus, further analyses were performed using two cut-offs in serum sPD-1 expression, each one with 93.6 μg/mL (median value of this study cohort) and 300 μg/mL.
Figure 4.

Patient survival curves according to the pretransplant serum soluble PD-1 concentration. Overall (left) and disease-free (right) patient survival curves according to the stratification of 18 groups (A), at the cut-off of 93.6 μg/mL (B), and 300 μg/mL (C). sPD-1 = soluble programmed death protein 1.
Patients with serum sPD-1 >93.6 μg/mL (n = 115 [50.2%]) showed similar OS (P = .69) and DFS (P = .26) than those with serum sPD-1 ≤93.6 μg/mL (n = 114) (Fig. 4B). Patients with serum sPD-1 >300 μg/mL (n = 13 [5.7%]) showed similar OS (P = .46) and marginally lower DFS (P = .070) than those with serum sPD-1 ≤300 μg/mL (n = 216 [94.3%]) (Fig. 4C).
3.5. Prognosis analysis according to the combinations of the Milan criteria and serum sPD-1
The Milan criteria showed a statistically significant prognostic contrast in OS and DFS (both P < .001) (Fig. 5A).
Figure 5.

Overall (left) and disease-free (right) patient survival curves according to the Milan criteria (A) and combination with the pretransplant serum soluble PD-1 concentration at the cut-off of 93.6 μg/mL (B) and 300 μg/mL (C). PD-1 = programmed death protein 1.
Through the combination of the Milan criteria and serum sPD-1 with a cut-off of 93.6 μg/mL, the OS and DFS curves were further stratified into four groups. The OS curves according to sPD-1 concentration showed very similar finding in patients within the Milan criteria (P = .96) and in patients beyond the Milan criteria (P = .69). The DFS curves according to sPD-1 concentration showed similar finding in patients within the Milan criteria (P = .78) and marginal difference in patients beyond the Milan criteria (P = .098) (Fig. 5B).
Through the combination of the Milan criteria and serum sPD-1 with a cut-off of 300 μg/mL, the OS and DFS rates were further stratified into 4 groups. The OS curves according to sPD-1 concentration showed very similar finding in patients within the Milan criteria (P = .26) and in patients beyond the Milan criteria (P = .98). The DFS curves according to sPD-1 concentration showed similar findings in patients within the Milan criteria (P = .11) and in patients beyond the Milan criteria (P = .21) (Fig. 5C).
3.6. Risk factor analysis
Univariate analyses on OS and DFS using pretransplant α-fetoprotein (AFP), maximal tumor diameter, tumor number, Milan criteria, and pretransplant serum sPD-1 with a cutoff of 300 μg/mL were performed. Pretransplant AFP, tumor number and pretransplant serum sPD-1 >300 μg/mL were not significant prognostic factors. Maximal tumor diameter >2.9 cm and the Milan criteria were significant prognostic factors on univariate analysis regarding OS and DFS (Table 2).
Table 2.
Univariate and multivariate analyses on the posttransplant outcomes.
| Parameters | Overall patient survival | Disease-free patient survival | |||||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||||||
| 5-y Recurrence rate | P | Hazard ratio | 95% CI | P | 5-y Survival rate | P | Hazard ratio | 95% CI | P | ||
| Pretransplant AFP | ≤26.4 ng/mL | 79.1% | .13 | 57.7% | .28 | ||||||
| >26.4 ng/mL | 71.8% | 53.4% | |||||||||
| Maximal tumor diameter | ≤2.9 cm | 87.5% | .001 | 1 | 70.0% | <.001 | 1 | ||||
| >2.9 cm | 65.4% | 1.76 | 0.96–3.22 | .070 | 41.5% | 1.77 | 0.97–3.24 | .065 | |||
| Viable tumor number | Single | 82.1% | .056 | 62.6% | .076 | ||||||
| Multiple | 71.6% | 52.4% | |||||||||
| Milan criteria | Within | 85.2% | <.001 | 1 | 69.4% | <.001 | 1 | ||||
| Beyond | 64.2% | 2.17 | 1.19–3.97 | .011 | 39.1% | 2.17 | 1.19–3.94 | .009 | |||
| Serum-soluble PD-1 | ≤300 μg/mL | 74.9% | .46 | 57.0% | .070 | 1 | |||||
| >300 μg/mL | 84.6% | 30.8% | 1.3 | 0.64–2.63 | .47 | ||||||
Cox proportional hazard analysis on OS and DFS was performed with the Milan criteria, maximal tumor diameter >2.9 cm and pretransplant serum sPD-1 >300 μg/mL. The Milan criteria was the only independent prognostic factor for OS (HR = 2.17 [1.19–3.97], P = .011) and DFS (HR = 2.17 [1.19–3.94], P = .009) (Table 2).
4. Discussion
Predicting the post-transplant prognosis in patients undergoing LT for HCC can be challenging as tumor burden before LT varies greatly, and tumor biology is heterogeneous and is influenced by continued immunosuppression. To reduce the risk of post-transplant HCC recurrence, many selection criteria have been proposed to date.[1–6,15] These criteria are usually based on tumor size and number, although some also include serologic tumor markers such as AFP and protein induced by vitamin K absence or antagonist-II. However, to accurately predict the risk of post-transplant HCC recurrence, it is essential to include other prognostic biomarkers that can provide additional information regarding tumor biology. In the present study, pretransplant serum sPD-1 was evaluated whether it could be a potential prognostic biomarker in LT recipients with HCC. To our knowledge, this is the first study on pretransplant serum sPD-1 expression in LT recipients.
There is an increasing body of evidence to suggest that cancer immune suppression and immune escape play an essential role in tumor progression. Activation of the PD-1 pathway is one of the most critical mechanisms in tumor evasion, inhibiting T-cell proliferation, inducing T-cell exhaustion, and enhancing the activity of regulatory T cells.[7] HCC commonly develops against a background of chronic liver disease, which promotes an immunosuppressive status in the liver, as well as T-cell exhaustion.[16,17] During HCC growth and development, the effective antitumor immune surveillance in the liver microenvironment is impaired, in which the PD-1/programmed death-ligand 1 (PD-L1) signaling pathway is involved.[18] In patients with HCC, the expression of PD-1 increases in CD8+ T cells,[19] and the high frequency of both circulating and tumor-infiltrating PD-1+ CD8+ T cells is associated with tumor progression following hepatic resection of HCC.[20] High PD-1 expression on tumor-infiltrating lymphocytes and the correlation between an exhausted phenotype and impaired effector function have been observed in HCC patients.[21,22] In a previous study, we demonstrated that high PD-1 expression in the HCC tissue is associated with aggressive biological tumor features, and there was a high correlation between PD-1 expression in the HCC tissue and peripheral blood CD8+ T cells.[22] PD-1 and PD-L1 are the main mediators of immunosuppression within the tumor microenvironment. The expression level of PD-1/PD-L1 may act as a biomarker to predict disease progression and long-term survival.[23]
Many costimulatory molecules in immunoregulation pathways are found in both membrane-bound and soluble forms. The soluble form usually arises from proteolytic cleavage of the membrane-bound form of the costimulatory protein and is the process by which the soluble forms of PD-1/PD-L1 are produced. Evidence suggests that the blood levels of sPD-1/PD-L1 are associated with the clinicopathological characteristics, treatment response, and survival outcomes in patients with lung cancer and multiple myeloma.[24,25] In patients who had undergone surgical resection for HCC, high PD-L1 expression was associated with significantly poorer OS and DFS than in those with low PD-L1 expression; however, high PD-1 expression was associated with improved outcomes.[8,9] By contrast, another study has shown no significant correlation between PD-1/PD-L1 expression and patient survival.[10]
To date, there is no consensus on the prognostic cutoff values for serum sPD-1 in patients with HCC. In the present study, prognostic analyses revealed that the sPD-1 concentration with cut off values of 93.6 μg/mL and 300 μg/mL did not have significant prognostic impact. High sPD-1 concentration showed slightly higher tumor recurrence in the patients, but such effect was not proven statistically in the present study. Based on our observation, we presumed that pretransplant serum sPD-1 concentration might not be associated with biological aggressiveness of HCC in LT recipients.
In a previous study of murine HCC, delivery of sPD-1 into the tumor site using adeno-associated virus resulted in enhanced antitumor immune effects and ultimately reduced tumor growth and prolonged long-term survival.[26] Other preclinical studies have indicated that sPD-1 is bioactive and could counteract the immunosuppressive effect of the PD-1/PD-L1 pathway, leading to restored T-cell function, a decreased number of regulatory T cells, and enhanced antitumor immunity.[25,27] These findings might explain why the elevated level of sPD-1 appears to play a role in patients undergoing hepatic resection for HCC.
The status of various checkpoint receptors is often evaluated to determine the response of their inhibitors. To date, there is one PD-1 study in LT[28] because patients with solid organ transplants have been excluded from PD-1/PD-L1 inhibitor clinical trials due to concerns regarding allograft rejection. In that study, 7 LT recipients with metastatic cancer, including 5 cases of HCC, underwent PD-1 inhibitor therapy. Rejection was observed in 2 of 7 patients. One patient achieved a complete response, 3 patients had progressive disease and 3 patients discontinued therapy before restaging assessment. In a collective review of 14 cases of LT recipients who had been treated with immune checkpoint inhibitors,[29] liver graft rejection was reported in 4 of 14 cases; in 3 cases with lethal outcome, rejection occurred within 3 weeks since the initiation of therapy. Survival outcome data were available in 12 cases and showed a median value of 1.2 months. However, in 4 patients showing a response to treatment, survival period ranged between 4 and 18 months. So far, the decision to treat LT recipients with immune checkpoint inhibitors is to be considered as ultima ratio to be weighed against the possibility of graft loss and fatal organ failure.
Exposure to high calcineurin inhibitor concentration is known to be a significant risk factor for HCC recurrence.[30] It is reported that tacrolimus upregulated the expression of PD-1 on the T-cell surface inhibiting proliferation and activation of effector T cells, favoring prevention of graft rejection and occurrence of HCC recurrence simultaneously[31]; thus, a combination therapy with low-dose tacrolimus and mycophenolate has been used for LT recipients with high risk of HCC recurrence.[32] We previously reported that mTOR inhibitor administration showed improved post-recurrence survival in LT recipients; thus, everolimus has been frequently administered in LT recipients with HCC recurrence after its coverage by the social health insurance in Korea.[13]
The present study has limitations. First, this is a retrospective, single-center study in a hepatitis B virus-endemic country; therefore, the majority of HCCs had developed in hepatitis B virus-infected livers. Second, the study patients were intentionally selected to match the numbers of patients with and without post-transplant HCC recurrence, which could have introduced a selection bias. Third, the association of serum sPD-1 and membrane-bound PD-1 in the HCC tissue was not assessed.
In conclusion, the results of this study demonstrate that pretransplant serum sPD-1 may not have significant influences on post-transplant outcomes in patients with HCC, although there were some potential prognostic influences from very high expression of serum sPD-1. Additional large-scale, multicenter studies and detailed mechanism studies are required to clarify the role of serum sPD-1 in LT recipients.
Author contributions
Conceptualization: Hwang S. Data curation: Na BG, Park GC, Ahn CS, Kim KH, Moon DB, Song GW, Ha TY, Jung DH, and Yoon YI. Formal analysis: Hwang S, Kim YK and Lee KJ. Funding acquisition: Hwang S. Methodology: Lee KJ, Kim YK, Yang HJ and Tak E. Project administration: Hwang S and Lee SG. Visualization: Hwang S, Kim YK and Lee KJ. Writing (original draft): Hwang S, Na BG, Kim YK and Kee KJ. Writing (review and editing): Hwang S, Na BG and Kim YK.
Conceptualization: Shin Hwang.
Data curation: Yun Kyu Kim, Gil-Chun Park, Chul-Soo Ahn, Ki-Hun Kim, Deok-Bog Moon, Tae-Yong Ha, Gi-Won Song, Dong-Hwan Jung, Young-In Yoon, Yo-Han Park.
Formal analysis: Shin Hwang, Kyung Jin Lee.
Investigation: Yo-Han Park.
Methodology: Yun Kyu Kim, Kyung Jin Lee, Hunji Yang, Eunyoung Tak.
Project administration: Shin Hwang.
Supervision: Shin Hwang, Sung-Gyu Lee.
Validation: Shin Hwang.
Visualization: Shin Hwang, Kyung Jin Lee.
Writing – original draft: Byeong-Gon Na, Shin Hwang, Kyung Jin Lee.
Writing – review & editing: Byeong-Gon Na, Shin Hwang, Kyung Jin Lee.
Footnotes
Abbreviations: AFP = α-fetoprotein, CI = confidence interval, DCP = des-γ-carboxyprothrombin, DFS = disease-free survival, HCC = hepatocellular carcinoma, HR = hazard ratio, LDLT = living donor liver transplantation, LT = liver transplantation, MELD = model for end-stage liver disease, mTOR = mammalian target of rapamycin, OS = Overall survival, PD-1 = Programmed death protein 1, PD-L1 = programmed death-ligand 1, SLV = standard liver volume, sPD-1 = soluble PD-1, UCSF = University of California San Francisco.
How to cite this article: Na BG, Kim YK, Hwang S, Lee KJ, Park GC, Ahn CS, Kim KH, Moon DB, Ha TY, Song GW, Jung DH, Yang H, Yoon YI, Tak E, Park YH, Lee SG. Absence of association between pretransplant serum soluble programmed death protein-1 level and prognosis following living donor liver transplantation in patients with hepatocellular carcinoma. Medicine. 2021;100:17(e25640).
BGN, YKK equally contributed to this study.
The authors report no conflicts of interest.
This study was supported by research funding from the Korean Society of Hepato-Biliary Pancreatic Surgery (grant no. 2019-01).
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Data are presented as mean ± SD or median (range) or n (%).
AFP = α-fetoprotein, HCC = hepatocellular carcinoma, MELD = model for end-stage liver disease, PIVKA-II = protein induced by vitamin K absence or antagonist-II, SLV = standard liver volume, UCSF = University of California San Francisco.
AFP = α-fetoprotein, CI = confidence interval, PD-1 = programmed death protein 1.
References
- [1].Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. New Engl J Med 1996;334:693–9. [DOI] [PubMed] [Google Scholar]
- [2].Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: Expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394–403. [DOI] [PubMed] [Google Scholar]
- [3].Lee SG, Hwang S, Moon DB, et al. Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl 2008;14:935–45. [DOI] [PubMed] [Google Scholar]
- [4].Toso C, Asthana S, Bigam DL, et al. Reassessing selection criteria prior to liver transplantation for hepatocellular carcinoma utilizing the Scientific Registry of Transplant Recipients database. Hepatology 2009;49:832–8. [DOI] [PubMed] [Google Scholar]
- [5].Fujiki M, Takada Y, Ogura Y, et al. Significance of des-gamma-carboxy prothrombin in selection criteria for living donor liver transplantation for hepatocellular carcinoma. Am J Transplant 2009;9:2362–71. [DOI] [PubMed] [Google Scholar]
- [6].Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 2009;10:35–43. [DOI] [PubMed] [Google Scholar]
- [7].Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med 2016;8:328rv4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chang B, Huang T, Wei H, et al. The correlation and prognostic value of serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death-ligand 1 (sPD-L1) in patients with hepatocellular carcinoma. Cancer Immunol Immunother 2019;68:353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gao Q, Wang XY, Qiu SJ, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 2009;15:971–9. [DOI] [PubMed] [Google Scholar]
- [10].Chang B, Shen L, Wang K, et al. High number of PD-1 positive intratumoural lymphocytes predicts survival benefit of cytokine-induced killer cells for hepatocellular carcinoma patients. Liver Int 2018;38:1449–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hwang S, Moon DB, Ahn CS, et al. Risk-based long-term screening for hepatocellular carcinoma recurrence after living donor liver transplantation. Transplant Proc 2013;45:3076–84. [DOI] [PubMed] [Google Scholar]
- [12].The Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines: management of chronic hepatitis B. Clin Mol Hepatol 2016;22:18–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jung DH, Tak E, Hwang S, et al. Antitumor effect of sorafenib and mammalian target of rapamycin inhibitor in liver transplantation recipients with hepatocellular carcinoma recurrence. Liver Transpl 2018;24:932–45. [DOI] [PubMed] [Google Scholar]
- [14].Hwang S, Kim YH, Kim DK, et al. Resection of pulmonary metastases from hepatocellular carcinoma following liver transplantation. World J Surg 2012;36:1592–602. [DOI] [PubMed] [Google Scholar]
- [15].Mazzaferro V, Sposito C, Zhou J, et al. Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterology 2018;154:128–39. [DOI] [PubMed] [Google Scholar]
- [16].Ringelhan M, Pfister D, O’Connor T, et al. The immunology of hepatocellular carcinoma. Nat Immunol 2018;19:222–32. [DOI] [PubMed] [Google Scholar]
- [17].Elsegood CL, Tirnitz-Parker JE, Olynyk JK, et al. Immune checkpoint inhibition: Prospects for prevention and therapy of hepatocellular carcinoma. Clin Transl Immunol 2017;6:e161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shrestha R, Prithviraj P, Anaka M, et al. Monitoring immune checkpoint regulators as predictive biomarkers in hepatocellular carcinoma. Front Oncol 2018;8:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang BJ, Bao JJ, Wang JZ, et al. Immunostaining of PD-1/PD-Ls in liver tissues of patients with hepatitis and hepatocellular carcinoma. World J Gastroenterol WJG 2011;17:3322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shi F, Shi M, Zeng Z, et al. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer 2011;128:887–96. [DOI] [PubMed] [Google Scholar]
- [21].Zhou G, Sprengers D, Boor PPC, et al. Antibodies against immune checkpoint molecules restore functions of tumor-infiltrating t cells in hepatocellular carcinomas. Gastroenterology 2017;153:1107–19. [DOI] [PubMed] [Google Scholar]
- [22].Kim HD, Song GW, Park S, et al. Association between expression level of PD1 by tumor-infiltrating CD8+ T cells and features of hepatocellular carcinoma. Gastroenterology 2018;155:1936–50. [DOI] [PubMed] [Google Scholar]
- [23].Moris D, Rahnemai-Azar AA, Zhang X, et al. Program death-1 immune checkpoint and tumor microenvironment in malignant liver tumors. Surg Oncol 2017;26:423–30. [DOI] [PubMed] [Google Scholar]
- [24].Sorensen SF, Demuth C, Weber B, et al. Increase in soluble PD-1 is associated with prolonged survival in patients with advanced EGFR-mutated non-small cell lung cancer treated with erlotinib. Lung Cancer 2016;100:77–84. [DOI] [PubMed] [Google Scholar]
- [25].Wang L, Wang H, Chen H, et al. Serum levels of soluble programmed death ligand 1 predict treatment response and progression free survival in multiple myeloma. Oncotarget 2015;6:41228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shin SP, Seo HH, Shin JH, et al. Adenovirus expressing both thymidine kinase and soluble PD1 enhances antitumor immunity by strengthening CD8 T-cell response. Mol Ther 2013;21:688–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Geng H, Zhang GM, Xiao H, et al. HSP70 vaccine in combination with gene therapy with plasmid DNA encoding sPD-1 overcomes immune resistance and suppresses the progression of pulmonary metastatic melanoma. Int J Cancer 2006;118:2657–64. [DOI] [PubMed] [Google Scholar]
- [28].DeLeon TT, Salomao MA, Aqel BA, et al. Pilot evaluation of PD-1 inhibition in metastatic cancer patients with a history of liver transplantation: the Mayo Clinic experience. J Gastrointest Oncol 2018;9:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Munker S, De Toni EN. Use of checkpoint inhibitors in liver transplant recipients. United European Gastroenterol J 2018;6:970–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Vivarelli M, Cucchetti A, Piscaglia F, et al. Analysis of risk factors for tumor recurrence after liver transplantation for hepatocellular carcinoma: key role of immunosuppression. Liver Transpl 2005;11:497–503. [DOI] [PubMed] [Google Scholar]
- [31].Li J, Bai Y, Wang L, et al. Regulatory effect of FK506 on CD152 and PD-1 in the liver allorecipients. Transplant Proc 2008;40:1495–7. [DOI] [PubMed] [Google Scholar]
- [32].Kang SH, Hwang S, Ha TY, et al. Tailored long-term immunosuppressive regimen for adult liver transplant recipients with hepatocellular carcinoma. Korean J Hepatobiliary Pancreat Surg 2014;18:48–51. [DOI] [PMC free article] [PubMed] [Google Scholar]


