Abstract
Background:
Robot-assisted and laparoscopic surgery are the most minimally invasive surgical approaches for the removal of liver lesions. Minor hepatectomy is a common surgical procedure. In this study, we evaluated the advantages and disadvantages of robot-assisted vs laparoscopic minor hepatectomy (LMH).
Methods:
A systematic literature search was performed in PubMed, Embase, and the Cochrane Library to identify comparative studies on robot-assisted vs. laparoscopicminor hepatectomy up to February, 2020. The odds ratios (OR) and mean differences with 95% confidence intervals were calculated using the fixed-effects model or random-effects model.
Results:
A total of 12 studies involving 751 patients were included in the meta-analysis. Among them, 297 patients were in the robot-assisted minor hepatectomy (RMH) group and 454 patients were in the LMH group. There were no significant differences in intraoperative blood loss (P = .43), transfusion rates (P = .14), length of hospital stay (P > .64), conversion rate (P = .62), R0 resection rate (P = .56), complications (P = .92), or mortaliy (P = .37) between the 2 groups. However, the RMH group was associated with a longer operative time (P = .0003), and higher cost (P < .00001) compared to the LMH group. No significant differences in overall survival or disease free survival between the 2 groups were observed. In the subgroup analysis of left lateral sectionectomies, RMH was still associated with a longer operative time, but no other differences in clinical outcomes were observed.
Conclusions:
Although RMH is associated with longer operation times and higher costs, it exhibits the same safety and effectiveness as LMH. Prospective randomized controlled clinical trials should now be considered to obtain better evidence for clinical consensus.
Keywords: laparoscopic surgery, liver lesions, meta-analysis, minor hepatectomy, robot-assisted
1. Introduction
With the improvement of technology and instruments, laparoscopic hepatectomy has emerged as the leading minimally invasive option for patients with benign and malignant liver lesions in the past decade.[1,2] Laparoscopic hepatectomy is performed from minor peripheral segmentectomy to major hepatectomy and posterosuperior segments.[3,4] All liver segments can be reached through this minimally invasive procedure.[5] Compared to open surgery, laparoscopic hepatectomy is associated with less intraoperative blood loss, fewer postoperative complications, lower postoperative pain, improved cosmetics, a shorter hospital day, similar operative costs, and comparable oncologic outcomes.[6–12] However, laparoscopy has some inherent shortcomings, including an unstable video view that drifts from the protruding structures, a rigidity of the straight laparoscopic instrument that limits accessibility to difficult liver surface areas, and poor ergonomics that force surgeons into uncomfortable positions during long procedures.[13]
The introduction of robot-assisted hepatectomy can overcome the intrinsic limitations of conventional laparoscopy. The robotic system allows for a 3-dimensional, enlarged view of the surgical field, 7 degrees of freedom for operation, tremor filtration for accurate suture, and improved precision regarding surgical anatomy.[14,15] The procedure also allows the surgeon to remain in a seated posture, reducing fatigue-related complications. Hepatobiliary surgeons have attempted to expand the indications of robot-assisted hepatectomy. The indications for robot-assisted hepatectomy are similar to laparoscopic hepatectomy, although robot-assisted hepatectomy offers theoretical advantages. However, its practical benefits regarding clinical outcomes remain controversial.
Based on most published articles, minor hepatectomy (MH) is defined as a resection of < 3 adjacent segments and remains the most common procedure performed with robotic assistance or laparoscopy.[15] MH includes anatomic or non-anatomic segmentectomy, left lateral sectionectomy, and bisegmenmentectomies. Comparisons of the clinical outcomes between robot-assisted minor hepatectomy (RMH) and laparoscopic minor hepatectomy (LMH) have been described in several studies. Due to the relatively small patient numbers of single center studies, the level of evidence of international consensus remiains low.[16] In this study, a pool analysis of published data comparing the perioperative outcomes of robot-assisted MH with laparoscopic MH was therefore performed.
2. Methods
The study protocol was agreed by all authors and followed the Preferred Reporting Item for Systematic Reviews and Meta-Analyses statement (PRISM).[17] Patient consent was not required due to the restrospective nature of the study.
2.1. Literature selection
We performed a systematic search of PubMed, Embase, and the Cochrane Library Central for relevant articles published from inception until February 29, 2020. The search was performed by Jiming Wang and Jiangfa Li. The search terms utilized were “robotic,” “robot-assisted,” “laparoscopy,” “laparoscopic,” “liver resection,” “liver srgery,” “hepatic resection,” “hepatectomy,” “sectionnectomy,” combined with the boolean operators AND/OR. The search strategy consisted of a different combination of Medical Subject Headings (MeSH) and search terms. Only studies involving human trails that published in English were included. Reference lists of the selected studies were also searched for other relevant articles.
2.2. Inclusion and exclusion criteria
Inclusion criteria were as follows:
-
(1)
Performed a comparison of perioperative outcomes between robot-assisted MH and laparoscopic MH, including randomized clinical trials and observational clinical studies;
-
(2)
Had sufficient data to calculate statistics;
-
(3)
Included only high-quality studies if more than one originated from the same institute or authors.
Exclusion criteria were as follows:
-
(1)
Letters, editorials, case reports, experts opinions, and review articles without original data;
-
(2)
Studies without available full-texts.
2.3. Data extraction and methodological quality assessment
Datas were independently extracted and evaluated for qualification by 2 reviewers. Data were collected as follows: first author name, country, year of publication, number of patients, characteristics of the population, and outcomes of interest. Titles and abstracts were selected to exclude duplicate and irrelevant studies. Differences between the two reviewers regarding article inclusion were resolved by the consensus of other authors. The Newcastle-Ottawa scale (NOS) was used for methodological quality assessment in all studies.[18] A scoring system of NOS (range from 0 to 9) has been developed for evaluation. Only studies with scores ≥6 were considered eligible.
2.4. Outcomes of interest and definitions
Fromall included studies, the following data were extracted: operative time (minutes), intraoperative blood loss (milliliters), intraoperative transfusion rate, R0 resection rate, conversion rate, complications, hospital stay (days), 90-days mortality, cost, and other comparable data. Complications were classified according to the Clavien–Dindo grade (minor complication grade < III, major complications grade ≥ III).[19] R0 resection was defined as the absence of microscopic tumor cells within 1 mm of the margin of resection.
2.5. Statistical analysis
Data analysis was performed using RevMan 5.3 software (The Cochrane Collaboration, Oxford, UK). When the data was reported as the median and range, the mean and standard deviation were calculated according to the methods described by Hozo etal.[20] Mean differences (MD) or odd ratios (OR) were used to analyze continuous and dichotomous data, respectively. The Mantel-Haenszel method was used to calculate dichotomous variables and the inverse variance method was used to calculate continuous variables. The fixed-effects model was used to calculate all the results. If heterogeneity was obvious, the random-effects model was applied. Study heterogeneity was evaluated using χ 2 tests and I2 statistics (I2 > 50% was considered obvious heterogeneity). The level of significance was set at a P-value ≤ .05. Causes of statistical heterogeneity were investigated using sensitivity analysis or subgroup analysis. A visual inspection of the funnel plots was used to assess the risk of publication bias. Only studies that included 28 or more patients in each group were subjected to sensitivity analyses.
3. Results
The flow diagram of the meta-analysis is shown in Figure 1. A total of 650 potentially relevant articles were retrieved from the primary literature search. Following the analysis of titles and abstracts, 172 studies were excluded due to duplication, and 460 articles were deemed irrelevant. The remaining 18 full-text studies were considered eligible. Of the 18 studies, 6 were excluded, 3 of which were published by the same institution and had overlapping patient populations,[21–23] with the other 3 studies performed major hepatectomy assessments.[24–26] Finally, 12 studies (non-randomized clinical trials) published from 2010 to 2018 met the inclusion criteria and were included in the qualitative synthesis.[27–38] In total,741 patients met the inclusion criteria, including 297 robot-assisted and 444 laparoscopic MH. Study characteristics and NOS quality assessment scores are shown in Table 1. The majority of articles reported that no significant differences in patient demographics and preoperative data between the 2 groups.
Figure 1.
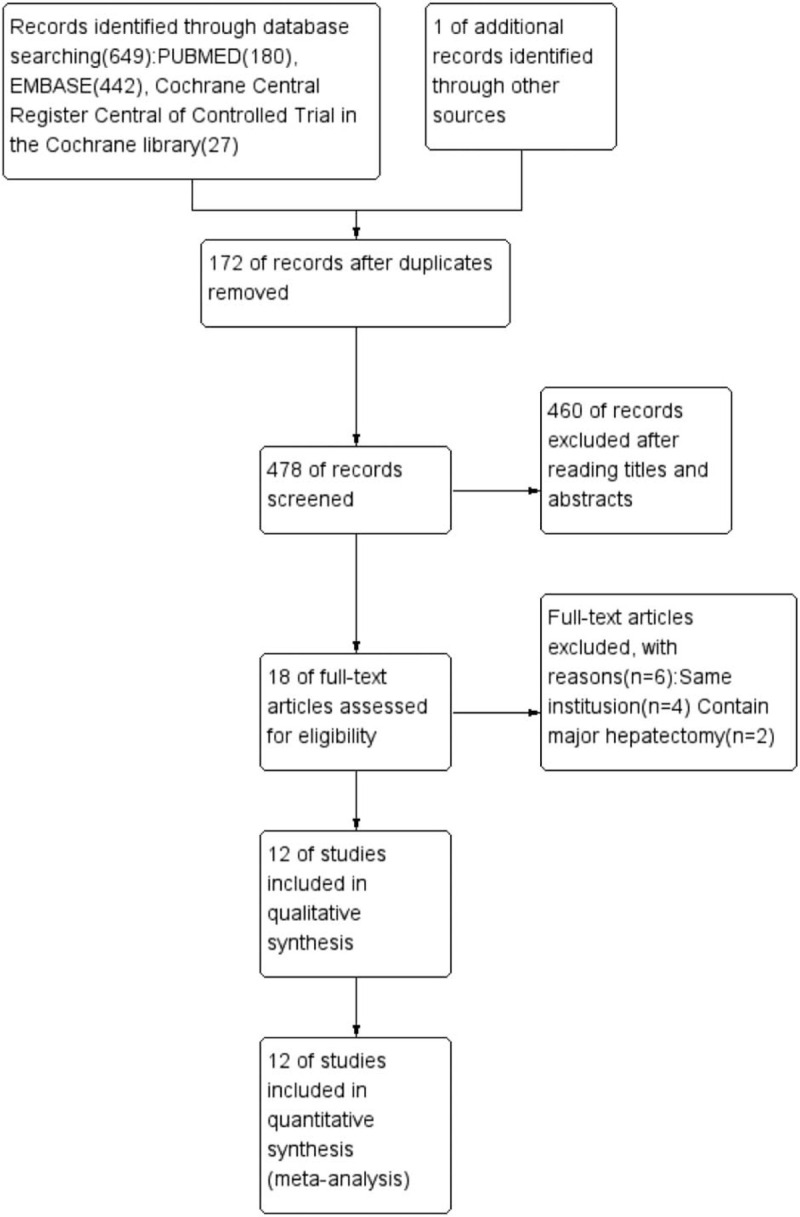
Flow chart of the literature selection.
Table 1.
Characteristics of the included studies.
| First Author | Year | Country | Study type | Approach | Cases | Age (yr) | Male | BMI (kg/m2) | Malignant | Tumor size (mm) | ASA (1/2/3/4) | NOS |
| Tranchart | 2014 | France | Retro/ | RH | 28 | 66.5 (42–84) | 13 | 26.1 (16.7–36) | 15 | 35 (6–115) | 5/14/9 | 7 |
| Comparative | LH | 28 | 66 (41–78) | 13 | 23.2 (16–33) | 17 | 40 (6–130) | 5/19/4 | ||||
| Salloum | 2016 | France | Retro/ | RLLS | 16 | 56 ± 15 | 8 | 27 ± 5 | 9 | 56 < 2,24 > 2 | 7 | |
| Case-control | LLLS | 80 | 58 ± 16 | 38 | 26 ± 5 | 48 | 10 < 2,6 > 2 | |||||
| Montalti | 2015 | Italy | Retro/ | RPS | 36 | 62 ± 13 | 21 | NA | 26 | 44.4 ± 30.6 | 3/19/13 | 7 |
| Comparative | LPS | 72 | 56.8 ± 15 | 39 | NA | 50 | 49.5 ± 35 | 10/42/20 | ||||
| Berber | 2010 | USA | Retro/ | RH | 9 | 66.6 ± 6.4 | 7 | NA | 7 | 32 ± 13 | NA | 9 |
| Comparative | LH | 23 | 66.7 ± 9.6 | 12 | NA | 21 | 29 ± 13 | NA | ||||
| Minggen HU | 2019 | China | Retro/ | RLLS | 58 | 52.2 ± 13.8 | 33 | 24.7 ± 4.0 | 31 | 47 ± 26 | 2/54/2 | 8 |
| Case-control | LLLS | 54 | 48.9 ± 13.1 | 26 | 23.8 ± 3.2 | 26 | 47 ± 28 | 5/48/1 | ||||
| Kim | 2016 | Korea | Retro/ | RLLS | 12 | 54.1 ± 12.2 | 6 | NA | 7 | 23 (20–36) | NA | 8 |
| Case-contro | LLLS | 31 | 56.4 ± 11.6 | 18 | NA | 24 | 24 (17–30) | NA | ||||
| O’Connor | 2017 | USA | Retro/ | RH | 39 | NA | NA | NA | NA | 40 (17–110) | NA | 7 |
| Comparative | LH | 50 | NA | NA | NA | NA | 4 (10–95) | NA | ||||
| Lai | 2013 | China | Retro/ | RH | 33 | NA | NA | NA | NA | NA | NA | 7 |
| Comparativel | LH | 33 | NA | NA | NA | NA | NA | NA | ||||
| Lee | 2015 | China | Retro/ | RLLS | 38 | 58.5 (20–81) | 26 | NA | 27 | 30 (11–120) | 4/31/3 | 8 |
| Case-control | LLLS | 29 | 60 (34–80) | 15 | NA | 23 | 30 (15–70) | 6/26/3 | ||||
| Croner | 2016 | Germany | Retro/ | RH | 10 | 64 (45–76) | 8 | 28 (28.3) | 10 | NA | 1 4/4 | 8 |
| Case-control | LH | 19 | 59 (32–85) | 13 | 26 (26.6) | 15 | NA | 2/7/9/1 | ||||
| Ruoyu | 2018 | China | Retro/ | RLLS | 7 | NA | NA | NA | NA | NA | NA | 7 |
| Comparativel | LLLS | 17 | NA | NA | NA | NA | NA | NA | ||||
| Packiam | 2012 | USA | Retro/ | RLLS | 11 | 57 ± 16 | 3 | 31 ± 7 | 6 | 55 (24–65) | NA | 7 |
| Comparative | LLLS | 18 | 52 ± 17 | 4 | 29 ± 7 | 8 | 44 (26–71) | NA |
3.1. Operative time
Information on operative times was provided in all studies. According to Figure 2, the operation time of the RMH group was longer than that of LMH groups (MD = 28.65, 95% confidence intervals (CI):13.12 to 44.17; P = .0003). Heterogeneity amongst the studies was obvious (I2 = 59%, P = .005).
Figure 2.
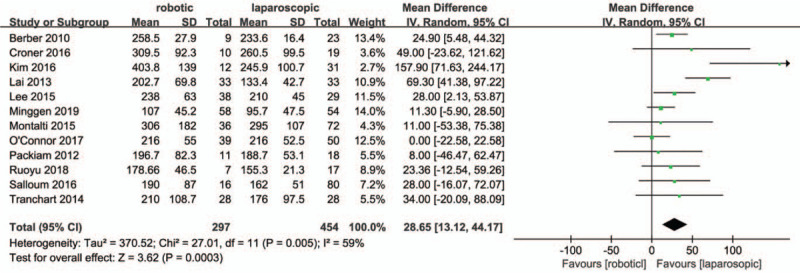
Forest plot of meta-analysis concerning operative time.
3.2. Intraoperative blood loss, transfusion rate
Ten studies[27,29,30,32–38] reported intraoperative estimated blood loss. The data in the meta-analysis showed that the operative blood loss in the RMH group was similar to that of the LMH group (MD = -12.9, 95% CI:-44.75 to 18.94; P = .43; I2 = 0%, Fig. 3). In total, eight studies[28,30,32,33,35–38] reported intraoperative transfusion rates that were similar between the RMH group and LMH group (OR = 1.87, 95% CI:0.82 to 4.26; P = .14; I2 = 0%, Fig. 4).
Figure 3.
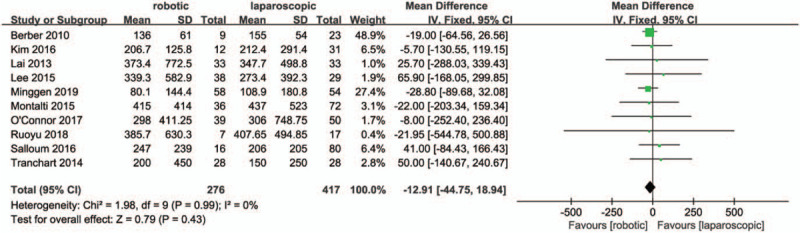
Forest plot of meta-analysis concerning blood loss.
Figure 4.
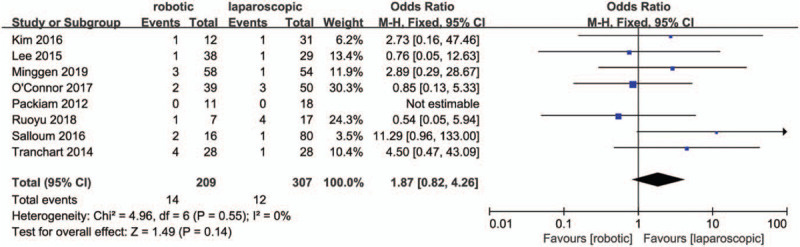
Forest plot of meta-analysis concerning transfusion rate.
3.3. Conversion rate
Ten studies[27,28,30,32–38] included in the meta-analysis reported the conversion to open rates. No significant differences between the RMH group and the LMH group regarding conversion rate were observed (OR = .86, 95% CI:0.46 to 1.58; P = .62; I2 = 33%, Fig. 5).
Figure 5.
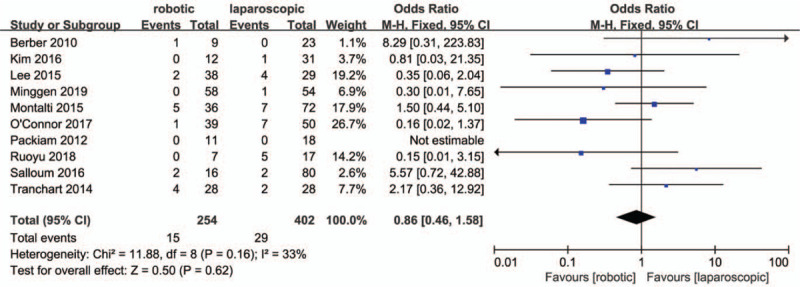
Forest plot of meta-analysis concerning conversion rate.
3.4. R0 resection rate
Seven studies[27,29,31,33,35,36,38] reported R0 resection rates, for which no heterogeneity was observed (P = .9; I2 = 0%, Fig. 6). There were no significant differences between the RMH group and LMH group regarding R0 resection rates (OR = 1.36, 95% CI:0.48 to 3.83; P = .56).
Figure 6.
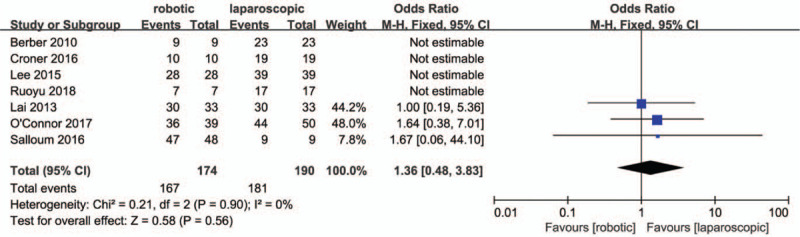
Forest plot of meta-analysis concerning R0 resection rate.
3.5. Complications
All studies reported an overall incidence of complications, for which a very low heterogenetity was observed (P = .42; I2 = 2%). The results in Figure 7. showed there were no significant differences in total complications between the 2 groups (OR = .98, 95% CI:0.63 to 1.53; P = .92). Eight studies[28,30–32,34–36,38] classified the complications. Low heterogeneity was observed for major complication rates (P = .21; I2 = 27%), whilst no heterogeneity was observed for minor compication rates (P = .95; I2 = 0%). The results in Figsure 8 and 9. showed that there were no significant differences in minor (OR = 1.01, 95% CI:0.59 to 1.74; P = .97) and major compication rates (OR = 1.33, 95% CI:0.62 to 2.85; P = .46) between the 2 groups.
Figure 7.
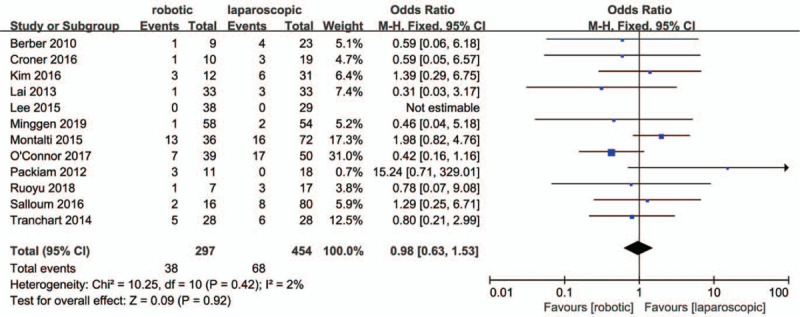
Forest plot of meta-analysis concerning overall complication rate.
Figure 8.
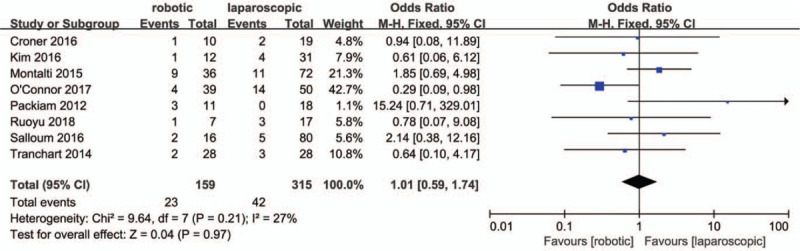
Forest plot of meta-analysis concerning minor complication rate.
Figure 9.
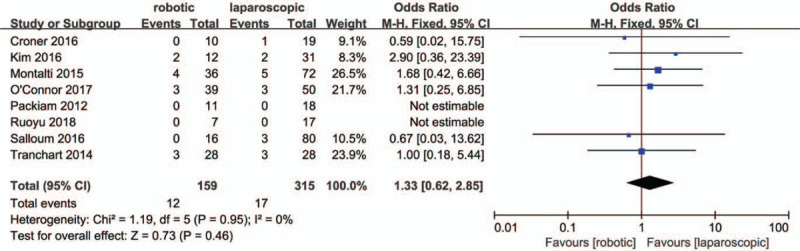
Forest plot of meta-analysis concerning major complication rate.
3.6. Hospital stay
Ten studies[28,30–38] reported the length of hospital stay. According to Fig. 10, no significant differences were observed between RMH and LMH groups regarding hospital stay (MD = -0.15, 95% CI:-0.47 to 0.77; P = .64;). Heterogeneity among the studies was obvious (P = .02, I2 = 55%).
Figure 10.
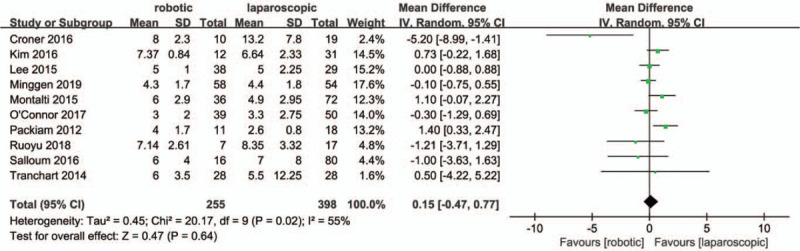
Forest plot of meta-analysis concerning hospital stay.
3.7. Postoperative mortality
Ten studies[27–31,33,34,36–38] reported post-operative mortalities. Six studies[27–29,33,37,38] reported no deaths. The meta-analysis revealed no significant differences in postoperative mortality between the 2 groups (MD = 1.86, 95% CI:0.48 to 7.14; P = .37; Fig. 11.). Heterogeneity among the studies was low (P = .29, I2 = 20%).
Figure 11.
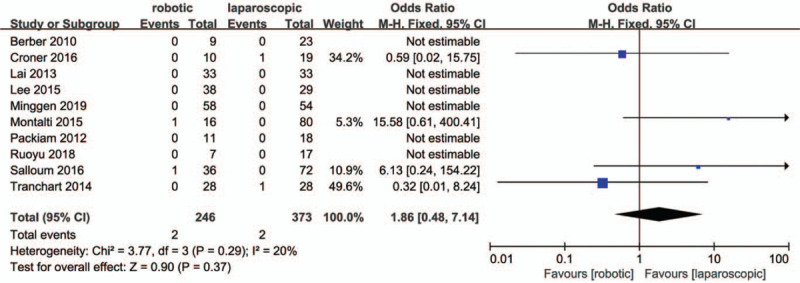
Meta-analysis Forest plot of postoperative mortality.
3.8. Perioperative costs
Five studies[28,31,32,36,37] reported data regarding perioperative costs. Two studies[32,37] reported the perioperative costs as the mean ± standard deviation. The meta-analysis demonstrated that the RMH group had higher costs than the LMH group (MD = 4206, 95% CI:2404.55 to 6007.45; p < .00001;). The importance of heterogeneity among the studies was obvious (I2 = 58%). Croner et al,[31] and Packiam et al[28] reported that the perioperative costs were higher in the RMH group compared to the LMH group (8765 Euro vs 3437 Euro;$6,553 versus $4,408; P < .05). Only Salloum et al[36] reported that no significant differences occurred in terms of costs between the 2 groups.
3.9. Overall survival (OS), disease free survival (DFS)
Montalti etal[34] reported that the OS in patients with colorectal liver metastases was similar (92.3, 64.6, and 40.4% versus 96.4, 70.8, and 62.9%, P = .24) at 1, 3, and 5 years between RMH vs. LMH groups, respectively. Berber et al[27] found that the OS and DFS werecomparable between groups when patients were followed up for an average of 14 months. Kim et al[32] reported that no significant differences were found in DFS (P = .463) or OS (P = .484) between the robotic and laparoscopic patients.
3.10. Comparison of MH in the diffierent locations
MH for lesions located in the anterolateral segments (II, III, IVb, V, and VI) represents a lower technicl challenge than lesions located in the posterosuperior (I, IVa, VII, and VIII) liver segments. In the subgroup analysis, left lateral sectionectomies were the most common MH.
A single study[34] reported that the posterosuperior hepatectomy could not be performed through the meta-analysis. There were no differences between the RMH and LMH groups regarding operative time, intraoperative blood loss, hospital stay, R0 negative margin rates and compliacations.
As for left lateral sectionectomy, 6 studies[28,32,33,36–38] were included in this meta-analysis. Only one study reported perioperative deaths. The reported outcomes of the meta-analysis are listed in Table 2 and showed a similar results outcome to the the original meta-analysis.
Table 2.
The outcomes of the meta-anlysis for robot-assisted vs laparoscopic left lateral sectionectomy.
| No. of studies | |||||||
| Outcomes of interest | No. of studies | RLLS | LLLS | OR/MD | 95%CI | P | I2 (%) |
| Operation time | 6 | 142 | 229 | 24.47 | -5.26–49.68 | .02 | 57 |
| Blood loss | 5 | 131 | 211 | -10.5 | -59.33–38.32 | .67 | 0 |
| Transfusion rate | 6 | 142 | 229 | 1.93 | 0.67–5.54 | .22 | 0 |
| conversion | 5 | 131 | 211 | -0.61 | 0.22–1.67 | .34 | 32 |
| RO resection | 3 | 82 | 65 | 1.67 | 0.06–44.1 | .76 | 0 |
| Total complications | 6 | 142 | 229 | 1.47 | 0.64–3.40 | .36 | 0 |
| Minor complication | 4 | 46 | 146 | 1.84 | 0.68–4.97 | .23 | 6 |
| Major complication | 4 | 46 | 146 | 1.80 | 0.32–10.02 | .43 | 0 |
| Hospital stay | 3 | 98 | 179 | 1.03 | 0.31–3.45 | .96 | 0 |
3.11. Sensitivity analysis and publication bias
Sensitivity analysis results that excluded studies with fewer than 28 patients in each group are shown in Table 3. Six studies[29,30,33–35,37] were in included. The results of sensitivity analyses were consistent with the original results, and heterogeneity was reduced.
Table 3.
Sensitivity analysis exclude series with less than 28 patients in each group.
| No. of studies | |||||||
| Outcomes of interest | No. of studies | RMH | LMH | OR/MD | 95%CI | P | I2 (%) |
| Operation time | 6 | 232 | 266 | 25.28 | 3.38–47.17 | .02 | 70 |
| Blood loss | 6 | 232 | 266 | -15.37 | -67.16–36.41 | .56 | 0 |
| Transfusion rate | 4 | 163 | 161 | 1.77 | 0.62–5.04 | .28 | 0 |
| conversion | 5 | 199 | 233 | -0.72 | -0.35–1.49 | .38 | 29 |
| RO resection | 4 | 148 | 122 | 1.36 | 0.48–3.83 | .56 | 0 |
| Total complications | 6 | 232 | 266 | 0.85 | 0.49–1.45 | .54 | 37 |
| Minor complication | 3 | 103 | 150 | 0.79 | 0.40–1.55 | .49 | 63 |
| Major complication | 3 | 103 | 150 | 1.35 | 0.55–3.31 | .52 | 0 |
| Hospital stay | 3 | 98 | 179 | 1.03 | 0.31–3.45 | .96 | 0 |
| Mortality | 2 | 64 | 100 | 1.36 | 0.20–9.14 | .75 | 37 |
As was shown in Figure 12, funnel plots for studies reporting the overall incidence of complication showed a basic symmetry. No significant publication bias was observed.
Figure 12.
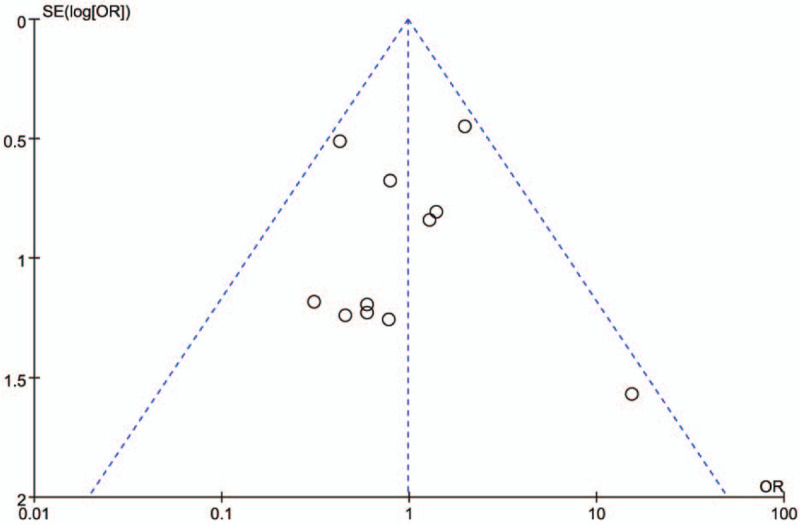
Funnel plot of the overall complication rates included in the studies.
4. Discussion
Minimally invasive hepatectomy includes hand-assisted laparoscopic, full laparoscopic and full robotic approaches. The number of laparoscopic hepatectomy is increasing exponenially worldwide. Initially, the most favorable indication for minimally invasive hepatectomy was a solitary lesion with a tumor size less than 5 cm, located in the left lateral and anterior liver segments.[39] Currently, left lateral sectionnectomy is recommended as a standard operation.[40,41] Based on the articles, the most common type of minimally invasive hepatectomy was MH. Robotic hepatectomy is still evolving and despite its advantages over laparoscopic hepatectomy, high costs, missing the haptic feedback, and long operation times limit its use. There was little evidence that RMH provided better results than LMH.[16] Although, several systematic reviews and meta-analyses have been published on the comparison between robot-assisted hepatectomy vs. laparoscopic hepatectomy for liver lesions,[42–45] to our knowledge, no detailed meta-analysis has compared the benefits of RMH vs. LMH. This meta-analysis identified twelve articles assessing RMH and LMH as 2 alternative surgical techniques, for which the clinical outcomes were measured.
The results of the meta-analysis revealed that RMH was associated with a longer operative time compared to LMH. This difference can be partially explained by the additional time to dock and undock the robot, and the time taken to exchange instruments. Another reason may be that the series includes periods corresponding to the learning curve of robot-assisted hepatectomy. With the increase in cases of robot-assisted surgery, the operation time can be significantly shortened.[46–48] The learning curve for robot-assisted hepatectomy (about 30 patients) was shorter in comparison with laparoscopic hepatectomy (about 60 patients).[47,49]
Blood loss is an important index affecting the postoperative recovery of patients with hepatectomy. Due to the high flexibility and accuracy of the robotic surgical system, it gives the possibility to perform more precise dissection and suture of vascular, especially aberrant hepatic arteries, permitting an easier bleeding control.[50,51] However, the results of this meta-analysis revealed that blood loss and transfusion rates did not significantly differ between robot-assisted and laparoscopic MH. MH includes left lateral sectionectomy, weidge resection, right anterior sectionectomy, and right posterior sectionectomy, which clearly differ in terms of complexity. Resection of the right anterior and posterior segments should be considered as equivalent to major hepatectomy. The major types of minor hepatectomy reported in these articles were left lateral sectionectomy and peripheral wedge resection, both of which are of low complexity.[52] Only as single article described a comparison of robotic assisted vs. laparoscopic parenchymal hepatectomy for lesions located in the posterosuperior segments.[34] Therefore, no significant differences in operative outcomes including blood loss, transfusion rates, conversion rates and hospital stay between the two groups were observed. However, Zhang et al found a longer length of hospital stay in robot-assisted MH group performing their meta-analysis.[45] This difference can be explained by different policies on postoperative management in different research institutions.
No statistical differences were observed regarding total complications (minor or major), mortality, or R0 resection rates, which demonstrated that the 2 approaches were comparable in terms of safety and oncologic outcome. Perioperative mortality rates were low in both groups, and most articles reported that no deaths occurred. The major cause of death was multi-organ failure, and cardiopulmonary complications. Only a small number of articles reported long-term oncologic outcomes for which the 5-year OS and DFS for patients with malignancy did not differ between RMH and LMH groups. With the increasing experience of robotic hepatectomy, intraoperative blood loss and the incidence of postoperative complications were improved. O’Connor et al[35] reported that RMH shows an improved perioperative outcome than LMH following an analysis of 25 cases.
The major drawback of advanced robotic surgery is its associated high cost. This remains the major constraint preventing its widespread use. The costs of surgery were significantly higher in the RMH group. Only Salloum et al[36] reported the total costs were similar between the 2 groups in a single-center study. In general, the cost of robotic equipment and staplers for liver resection per patient were significantly higher than that of laparoscopic equipment.[34,36] The cost of buying and maintaining robots represented an additional financial burden, which could be reduced by increasing the number of operations per-robot per-year. Robotic hepatectomy requires fewer assistants than laparoscopic hepatectomy, therefore reducing labor costs.
The results from this meta-analysis should be interpreted with caution due to several limitations. First, the included studies were retrospective and non-randomized controlled trials (NRCTs), which may exaggerate the effects of the approaches, either by unmeasured confounding factors or through patients selection bias. However, evidences exists that shows that how well-designed NRCTs data may be reliable.[53] In this meta-analysis, the included studies were high quality NRCTs in the standard of NOS. Given the limited number of comparative studies previously performed and the existence of no singe RCT, the present meta-analysis provide the best available evidence comparing the RMH with LMH. Secondly, heterogeneity among the included studies regarding operative times must be considered. Significant heterogeneity remained in the sensitivity analysis, although the results were consistent. The surgical technique of each institution, postoperative management, and the learning curve of the surgeons considerably varied among the reviewed articles. These factors may result in heterogeneity and can impact the results. Thirdly, RMH and LMH were applied to a variety of liver lesions. The outcomes may have been influenced by specific clinicopathology. Further studies should overcome the above-mentioned limitations.
In summary, we show that compared MH (low complexity), the preferred method for laparoscopy, robotic assistance fails to improve all clinical outcome, but involves significantly higher costs and longer operative times. For complex cases (tumors near the major blood vessels, obesity, aberrant hepatic arteries, etc.), RMH is a preferred.[37] For an initial learning curve, MH (low complexity) may also represent a good indication for the transition to more complex hepatectomy for liver surgeons who wish to iniiate minimally invasive hepatectomy using robotic systems. Further prospective, multicenter, and large sample RCTs are now required to further reveal the effectiveness and safety of RMH compared to LMH.
Author contributions
Conceptualization: Jiangfa Li, Songqing He.
Data curation: Jiming Wang, Jiangfa Li.
Formal analysis: Jiming Wang, Jiangfa Li, Guandou Yuan.
Funding acquisition: Songqing He.
Investigation: Jiming Wang, Jiangfa Li.
Methodology: Jiming Wang, Jiangfa Li.
Project administration: Guandou Yuan, Song-Qing He.
Resources: Song-Qing He.
Software: Jiming Wang, Jiangfa Li.
Supervision: Guandou Yuan, Songqing He.
Validation: Guandou Yuan, Songqing He.
Visualization: Jiming Wang.
Writing – original draft: Jiming Wang.
Writing – review & editing: Jiming Wang, Jiangfa Li.
Footnotes
Abbreviations: CI = confidence intervals, DFS = disease free survival, LMH = laparoscopic minor hepatectomy, MD = mean differences, MH = minor hepatectomy, NOS = Newcastle-Ottawa scale, OR = odd ratios, OS = overall survival, RMH = robot-assisted minor hepatectomy, SD = standard deviation.
How to cite this article: Wang JM, Li JF, Yuan GD, He SQ. Robot-assisted versus laparoscopic minor hepatectomy: a systematic review and meta-analysis. Medicine. 2021;100:17(e25648).
This study was supported in part by the National Natural Science Foundation of China (81771674); the 111 Project (D17011), Guangxi Key Reasearch and Development Plan (2018AD03001), the Project to Improve the Scientific Research Ability of Middle-aged and Young Teachers (2018glmcy044), Hubei Chen Xiaoping Science and Technology Development Foundation (CXPJJH118000017-02-09).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
LH = laparoscopic hepatectomy, LLLS = laparoscopic left lateral sectionectomy, LPS = laparoscopic posterosuperior hepatectomy, RH = robot-assisted hepatectomy, RLLS = robot-assisted left lateral sectionectomy, RPS = robot-assisted posterosuperior hepatectomy.
Statistically significant results are shown in bold.
CI = confidence interval, LLLS = laparoscopic left lateral sectionectomy, MD = mean difference, OR = odds ratio, RLLS = robot-assisted left lateral sectionectomy.
Statistically significant results are shown in bold.
CI = confidence interval, LMH = laparoscopic minor hepatectomy, MD = mean difference, OR = odds ratio, RMH = robot-assisted minor hepatectomy.
References
- [1].Ciria R, Cherqui D, Geller DA, et al. Comparative short-term benefits of laparoscopic liver resection. Ann Surg 2015;263:01. [DOI] [PubMed] [Google Scholar]
- [2].Cai X. Laparoscopic liver resection: the current status and the future. Hepatobiliary Surgery & Nutrition 2018;7:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kasai M, Cipriani F, Gayet B, et al. Laparoscopic versus open major hepatectomy: a systematic review and meta-analysis of individual patient data. Surgery.985-95. [DOI] [PubMed] [Google Scholar]
- [4].Araki K, Shirabe K, Yamanaka T, et al. Validation of our surgical strategy of laparoscopic liver resection for postero-superior segments. J Hepatobiliary Pancreat Sci 2017;24:A114. [Google Scholar]
- [5].Ishizawa T, Gumbs AA, Kokudo N, et al. Laparoscopic segmentectomy of the liver from segment I to VIII. Ann Surg 2012;256:959–64. [DOI] [PubMed] [Google Scholar]
- [6].Chong JU, Han DH, Choi GH, et al. Short and long-term outcomes after laparoscopic or robotic liver resection in patients with HCC located in posterosuperior segments: comparision with open liver resection. HPB 2019;21:S397. [Google Scholar]
- [7].Tozzi F, Berardi G, Vierstraete M, et al. Laparoscopic versus open approach for formal right and left hepatectomy: a propensity score matching analysis. World J Surg 2018;42:2627–34. [DOI] [PubMed] [Google Scholar]
- [8].Cho HD, Kim KH, Hwang S, et al. Comparison of pure laparoscopic versus open left hemihepatectomy by multivariate analysis: a retrospective cohort study. Surg Endosc 2018;32:643–50. [DOI] [PubMed] [Google Scholar]
- [9].Wong-Lun-Hing EM, van Dam RM, van Breukelen GJ, et al. Randomized clinical trial of open versus laparoscopic left lateral hepatic sectionectomy within an enhanced recovery after surgery programme (ORANGE II study). Br J Surg 2017;104:525–35. [DOI] [PubMed] [Google Scholar]
- [10].Komorowski AL, Mituś JW, Wysocki WM, et al. Laparoscopic and open liver resection - A literature review with meta-analysis. Arch Med Sci 2017;13:525–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kawaguchi Y, Otsuka Y, Kaneko H, et al. Comparisons of financial and short-term outcomes between laparoscopic and open hepatectomy: Benefits for patients and hospitals. Surgery Today 2016;46:535–42. [DOI] [PubMed] [Google Scholar]
- [12].T.T, G.W, T.B, et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with propensity score matching: a multi-institutional Japanese study. J Hepatobiliary Pancreat Sci 2015;22:721–7. [DOI] [PubMed] [Google Scholar]
- [13].Ballantyne GH. Robotic surgery, telerobotic surgery, telepresence, and telementoring. Review of early clinical results[J]. Surg Endosc 2002;16:1389–402. [DOI] [PubMed] [Google Scholar]
- [14].Fruscione M, Pickens R, Baker EH, et al. Robotic-assisted versus laparoscopic major liver resection: analysis of outcomes from a single center. HPB 2019;21:906–11. [DOI] [PubMed] [Google Scholar]
- [15].Giulianotti PC, Bianco FM, Daskalaki D, Gonzalez-Ciccarelli LF, Kim J, Benedetti E. Robotic liver surgery: technical aspects and review of the literature. Hepatobiliary Surgery & Nutrition. 5:311–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Liu R, Wakabayashi G, Kim HJ, et al. International consensus statement on robotic hepatectomy surgery in 2018. World J Gastroenterol 2019;25:1432–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009;151:W65–94. [DOI] [PubMed] [Google Scholar]
- [18].Wells G, Shea B, O’Connell J. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Symposium on Systematic Reviews: Beyond the Basics 2014;7: [Google Scholar]
- [19].Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lai EC, Tang CN, Yang GP, et al. Multimodality laparoscopic liver resection for hepatic malignancy--from conventional total laparoscopic approach to robot-assisted laparoscopic approach. Int J Surg (London, England) 2011;9:324–8. [DOI] [PubMed] [Google Scholar]
- [22].Tsung A, Geller DA, Sukato DC, et al. Robotic versus laparoscopic hepatectomy: a matched comparison. Ann Surg 2014;259:549–55. [DOI] [PubMed] [Google Scholar]
- [23].Tranchart H, Ceribelli C, Patiriti A, et al. Classical versus robot-assisted laparoscopic liver resection: a matched-pair comparative study. HPB 2014;16:289. [DOI] [PubMed] [Google Scholar]
- [24].Yu YD, Kim KH, Jung DH, et al. Robotic versus laparoscopic liver resection: a comparative study from a single center. Langenbecks Arch Surg 2014;399:1039–45. [DOI] [PubMed] [Google Scholar]
- [25].Magistri P, Tarantino G, Guidetti C, et al. Laparoscopic versus robotic surgery for hepatocellular carcinoma: the first 46 consecutive cases. J Surg Res 2017;217:92–9. [DOI] [PubMed] [Google Scholar]
- [26].Lai ECH, Tang CN. Long-term survival analysis of robotic versus conventional laparoscopic hepatectomy for hepatocellular Carcinoma: a comparative study. Surgical Laparoscopy, Endoscopy and Percutaneous Techniques 2016;26:162–6. [DOI] [PubMed] [Google Scholar]
- [27].Berber E, Akyildiz HY, Aucejo F, et al. Robotic versus laparoscopic resection of liver tumours. HPB 2010;12:583–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Packiam V, Bartlett DL, Tohme S, et al. Minimally invasive liver resection: robotic versus laparoscopic left lateral sectionectomy. J Gastrointest Surg 2012;16:2233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lai ECH, Yang GPC, Tang CN. Robot-assisted laparoscopic liver resection for hepatocellular carcinoma: Short-term outcome. Am J Surg 2013;205:697–702. [DOI] [PubMed] [Google Scholar]
- [30].Tranchart H, Ceribelli C, Ferretti S, et al. Traditional versus robot-assisted full laparoscopic liver resection: a matched-pair comparative study. World J Surg 2014;38:2904–9. [DOI] [PubMed] [Google Scholar]
- [31].Croner RS, Perrakis A, Hohenberger W, et al. Robotic liver surgery for minor hepatic resections: a comparison with laparoscopic and open standard procedures. Langenbecks Arch Surg 2016;401:707–14. [DOI] [PubMed] [Google Scholar]
- [32].Kim JK, Park JS, Han DH, et al. Robotic versus laparoscopic left lateral sectionectomy of liver. Surg Endosc 2016;30:4756–64. [DOI] [PubMed] [Google Scholar]
- [33].Lee KF, Cheung YS, Chong CC, et al. Laparoscopic and robotic hepatectomy: experience from a single centre. ANZ J Surg 2016;86:122–6. [DOI] [PubMed] [Google Scholar]
- [34].Montalti R, Scuderi V, Patriti A, et al. Robotic versus laparoscopic resections of posterosuperior segments of the liver: a propensity score-matched comparison. Surg Endosc 2016;30:1004–13. [DOI] [PubMed] [Google Scholar]
- [35].O’Connor V, Vuong B, Yang ST, et al. Robotic minor hepatectomy offers a favorable learning curve and may result in superior perioperative outcomes compared with laparoscopic approach. Am Surg 2017;83:1085–8. [PubMed] [Google Scholar]
- [36].Salloum C, Lim C, Lahat E, et al. Robotic-assisted versus laparoscopic left lateral sectionectomy: analysis of surgical outcomes and costs by a propensity score matched cohort study. World J Surg 2017;41:516–24. [DOI] [PubMed] [Google Scholar]
- [37].Hu M, Liu Y, Li C, et al. Robotic versus laparoscopic liver resection in complex cases of left lateral sectionectomy. Int J Surg 2019;67:54–60. [DOI] [PubMed] [Google Scholar]
- [38].Ruoyu, Guan, Kui, et al. Comparison between robotic liver resection and laparoscopic liver resection. J Surg Concepts Pract 2018;23:346–51. [Google Scholar]
- [39].Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: the Louisville Statement, 2008. Ann Surg 2009;250:825–30. [DOI] [PubMed] [Google Scholar]
- [40].Liu Z, Ding H, Xiong X, et al. Laparoscopic left lateral hepatic sectionectomy was expected to be the standard for the treatment of left hepatic lobe lesions. Medicine (United States) 2018;97(7.): [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dokmak S, Raut V, Aussilhou B, et al. Laparoscopic left lateral resection is the gold standard for benign liver lesions: a case-control study. HPB 2014;16:183–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hu L, Yao L, Li X, et al. Effectiveness and safety of robotic-assisted versus laparoscopic hepatectomy for liver neoplasms: a meta-analysis of retrospective studies. Asian J Surg 2018;41:401–16. [DOI] [PubMed] [Google Scholar]
- [43].Qiu J, Chen S, Chengyou D. A systematic review of robotic-assisted liver resection and meta-analysis of robotic versus laparoscopic hepatectomy for hepatic neoplasms. Surg Endosc 2016;30:862–75. [DOI] [PubMed] [Google Scholar]
- [44].Guan R, Chen Y, Yang K, et al. Clinical efficacy of robot-assisted versus laparoscopic liver resection: a meta analysis[J]. Asian J Surg 2019;42:19–31. [DOI] [PubMed] [Google Scholar]
- [45].Zhang L, Yuan Q, Xu Y, et al. Comparative clinical outcomes of robot-assisted liver resection versus laparoscopic liver resection: a meta-analysis. PloS One 2020;15:e0240593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chen P-D, Wu C-Y, Hu R-H, et al. Robotic major hepatectomy: Is?there a learning curve? Surgery 2017;161:642–9. [DOI] [PubMed] [Google Scholar]
- [47].Zhu P, Liao W, Ding ZY, et al. Learning curve in robot-assisted laparoscopic liver resection[J]. J Gastrointest Surg 2019;23:1778–87. [DOI] [PubMed] [Google Scholar]
- [48].Gravetz A, Sucandy I, Wilfong C, et al. Single-institution early experience and learning curve with robotic liver resections. Am Surg 2019;85:115–9. [PubMed] [Google Scholar]
- [49].Efanov M, Alikhanov R, Tsvirkun V, et al. Comparative analysis of learning curve in complex robot-assisted and laparoscopic liver resection. HPB 2017;19:818–24. [DOI] [PubMed] [Google Scholar]
- [50].Cirocchi R, D’Andrea V, Amato B, et al. Aberrant left hepatic arteries arising from left gastric arteries and their clinical importance. Surgeon 2020;18:100–12. [DOI] [PubMed] [Google Scholar]
- [51].Cirocchi R, D’Andrea V, Lauro A, et al. The absence of the common hepatic artery and its implications for surgical practice: results of a systematic review and meta-analysis. Surgeon 2019;17:172–85. [DOI] [PubMed] [Google Scholar]
- [52].Lee MK, Gao F, Strasberg SM. Perceived Complexity of Various Liver Resections: Results of a Survey of Experts with Development of a Complexity Score and Classification. Journal of the American College of Surgeons.220:64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Abraham NS, Byrne CJ, Young JM, et al. Meta-analysis of well-designed nonrandomized comparative studies of surgical procedures is as good as randomized controlled trials. J Clin Epidemiol 2010;63:238–45. [DOI] [PubMed] [Google Scholar]


