Abstract
Advanced gastric cancer (AGC) patients are not tolerant to the toxicities of traditional chemotherapy and its second-line therapeutic regimens are limited. The aim of the present study is to evaluate the efficacy and safety of apatinib combined with S-1 as the second-line therapy for AGC patients.
Patients with AGC were enrolled in this study. Patients received oral apatinib (250 mg to 500 mg once daily) and S-1(40 mg/m2 twice daily) on days 1–14. Each cycle was 28 days and one course of treatment consisted of 2 cycles. Clinical efficacy and adverse events (AEs) were observed. Kaplan–Meier method was used for survival analysis.
From November 2015 to December 2017, 58 AGC patients who failed first-line chemotherapy were enrolled and assessed retrospectively. According to the Response Evaluation Criteria in Solid Tumors (RECIST) standard, all patients were evaluable for response. None achieved CR, and 10 (17.2%) achieved PR (95% CI 7.2%–27.3%). SD was observed in 58.6% (34/58) of patients (95% CI 45.6%–71.7%) and NR in 24.1% (14/58) of patients (95% CI 12.8%–35.5%). The objective response rate (ORR) and the disease control rate (DCR) were 17.2% and 75.8% respectively. The median progression-free survival (PFS) and median overall survival (OS) were 143.1 days (95% CI 121.7–164.5) and 211.6 days (95% CI 162.9–219.7) respectively. The multivariate analysis showed that the ECOG PS was the independent factor of PFS and OS for AGC patients (PFS: HR = 3.565, 95% CI: 2.25–5.65, P < .001; OS: HR = 3.676, 95% CI: 2.29–5.89, P < .001). The main AEs were fatigue (72.4%), hypertension (46.6%), and leukopenia (48.3%).
Apatinib combined with S-1 showed promising efficiency and was well tolerated as the second-line therapy for AGC patients. ECOG PS was the independent factor of PFS and OS for AGC patients. AEs were moderate and controllable, and leukopenia or hypertension was predictable factors for the PFS and OS of AGC patients.
Keywords: apatinib, gastric cancer, S-1, targeted therapy
1. Introduction
Gastric cancer (GC) is a major public health problem worldwide, and is a common digestive tract tumors.[1] GC remains second in terms of incidence among all cancers and the second leading cause of cancer-related death in China.[2,3] In China more than 80% of GC patients are not diagnosed until they have reached advanced stage,[4] and chemotherapy is commonly used as the first-line therapy for advanced GC (AGC). Currently, the most common first-line chemotherapy for AGC patients is fuoropyrimidines plus platinum compounds, with a median overall survival (OS) ranging from 9.3 to 15.3 months.[5–8] Yamada Y et al reported that oxaliplatin plus S-1 compared with cisplatin plus S-1 showed the median OS was 14.1 and 13.1 months as the first-line therapy in AGC patients.[7] In another study, docetaxel plus cisplatin and S-1 were compared with cisplatin and S-1, and the OS was 14.2 and 15.3 months respectively.[8] For the second-line therapies, irinotecan, taxanes (docetaxel, paclitaxel) or ramucirumab were recommended for AGC patients with median OS ranging from 3.6 to 10.9 months.[9–11] Sym SJ et al reported a median OS of 6.7 months and ORR of 20% in AGC patients treated with FOLFIRI in the second-line setting.[9] COUGAR-02 study showed docetaxel monotherapy provided patients with a median OS of 5.2 months as the second-line therapy.[10] However, the prognosis of AGC patients remains disappointing, with a median OS less than 1 year.[5,6] AGC patients are not tolerant to the toxicities of traditional chemotherapy and its second-line therapeutic regimens are limited. Therefore, it is imperative to explore safe and effective therapies for AGC after failure of the first-line chemotherapy.
Apatinib is an oral tyrosine kinase inhibitor that can combine with VEGFR2 and block its downstream targets such as JAK/STAT3.[12] It was approved by the China Food and Drug Administration (CFDA) as the third-line therapy for AGC in October 2014.[13,14] S-1 is a widely used chemotherapy drug and has been demonstrated a notable effect in the treatment for GC.[15] Some studies have shown that antiangiogenic drug combined with chemotherapy improves the outcome of cancer therapy, such as colorectal cancer and GC.[16,17] However, the combination apatinib and S-1 in the treatment of GC is scarce. Given the absence of approved second-line therapies for AGC, we performed a retrospective study to evaluate the efficacy and safety of apatinib combined with S-1 as a second-line therapy.
2. Materials and methods
2.1. Patients
Between November 2015 and December 2017, 58 AGC patients who failed the first-line chemotherapy were enrolled in the Affiliated People's Hospital of Jiangsu University. Before treatment, all patients underwent a physical examination, routine hematologic testing, and abdominal computed tomography (CT). Inclusion criteria contained:
-
1.
age between 20 and 78 years;
-
2.
histologically confirmed AGC;
-
3.
failure of the first-line chemotherapy;
-
4.
at least one measurable lesion;
-
5.
recurrence or metastasis was confirmed by CT;
-
6.
an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–2;
-
7.
sufficient hematopoietic, hepatic and renal function;
-
8.
a life expectancy of at least 12 weeks.
Exclusion criteria contained:
-
1.
diagnosis of any other primary tumors within the last 5 years;
-
2.
uncontrolled hypertension;
-
3.
evidence of central nervous system metastases;
-
4.
with bleeding tendency or receiving anticoagulants;
-
5.
with ulcers, intestinal obstruction or perforation.
The study was approved by the Ethics Committee of the Affiliated People's Hospital of Jiangsu University and conducted according to the Declaration of Helsinki. All patients provided written informed consent.
2.2. Treatment
Apatinib (CFDA approval no. H20140105) and S-1 (CFDA approval no. H20100135) were obtained from Jiangsu Hengrui Medicine Co., Ltd (Lianyungang, China). Patients received oral apatinib (250 mg or 500 mg once daily) and oral S-1 (40 mg/m2 twice daily) on days 1–14. Each cycle was 28 days and one course of treatment consisted of 2 cycles. The dose of apatinib was modified following recommendation by an expert consensus.[18] If obvious adverse events (AEs) occurred, dose reduction was necessary. Treatment continued until disease progression, unacceptable toxicity, or any other reason.
2.3. Efficacy and safety
The primary end point was progression-free survival (PFS), and the secondary end points were objective response rate (ORR), disease control rate (DCR), OS, and safety. PFS was calculated from the date of initial treatment to the date of disease progression or death. OS was calculated from the date of initial treatment to death or the last follow-up. The clinical efficacy was assessed according to Response Evaluation Criteria In Solid Tumors (RECIST) version 1.1 and was classified as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). The ORR represented the percentage of patients with a CR or PR, and DCR was defined as the percentage of patients with a CR, PR, or SD. Treatment response was evaluated every 2 cycles. AEs were graded by the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
2.4. Follow-up and outcome
Since starting therapy, follow-up by clinic consultation or telephone was conducted at least twice per month. The following items were recorded: the disease course, symptoms, AEs, status when follow-up was terminated (death, survival, or withdrawal), cause of death, and survival time (in days). Patients were observed until PD, death, or at the end of the study.
2.5. Statistical analysis
All statistical analyses were performed with SPSS (version 22.0; IBM Corporation, Armonk, NY, USA). The χ2 test was used for enumeration data. Measurement data were expressed as mean ± standard deviation. Survival curves were calculated with the Kaplan-Meier method and compared through the log-rank test. Cox proportional hazards analysis was used for univariate and multivariate analyses to explore the effects of clinicopathologic variables on survival. P < .05 was considered statistically significant.
3. Results
3.1. Patient characteristics
A total of 58 AGC patients were enrolled, including 40 men (69%) and 18 women (31%), with a median age of 63.7 years (range: 31–78). The clinical characteristics of the 58 patients were documented in Table 1. All patients received a first-line chemotherapy, and the chemotherapy regimens included: (1) oxaliplatin (130 mg/m2) on day 1 and S-1(40 mg/m2 twice daily) on days 1–14; (2) docetaxel (75 mg/m2) or paclitaxel (135 mg/m2) on day 1 and S-1(40 mg/m2 twice daily) on days 1–14; (3) oxaliplatin (130 mg/m2) on day 1 and capecitabine (1000 mg/m2) bid on days 1–14. Prior surgery of the primary tumor had been performed in 66% of the patients. All patients were presented with metastatic disease. The predominant metastatic sites were abdominal lymph nodes (45, 77.6%), peritoneum (31, 53.4%), liver (25, 43.1%), and lung (14, 24.1%).
Table 1.
Summary of the clinical characteristics of the 58 AGC patients.
| Characteristics | N | % |
| Gender | ||
| Male | 40 | 69 |
| Female | 18 | 31 |
| Age (years) | ||
| ≤65 | 27 | 46.6 |
| >65 | 31 | 53.4 |
| ECOG PS | ||
| 0 | 26 | 44.8 |
| 1 | 15 | 25.9 |
| 2 | 17 | 29.3 |
| Metastatic sites, n | ||
| ≤2 | 31 | 53.4 |
| >2 | 27 | 46.6 |
| Surgery of tumor | ||
| no | 17 | 29.3 |
| yes | 41 | 70.7 |
3.2. Initial dosage and adjustment
Thirty-five patients were initially administered and then maintained at a dosage of 250 mg per day. Twenty-three patients were initially administered and then maintained at a dosage of 500 mg per day, and 6 required a dosage reduction to 250 mg. The causes of dosage reduction in these patients included hypertension, leukopenia and proteinuria.
3.3. Survival analysis
The date of the last follow-up was March 2018, and the median follow-up time was 385.0 days (range: 339.6–430.5). The reasons for discontinued follow-up were death, withdrawal, or termination of follow-up. At the endpoint of the follow-up, 51 patients (87.9%) died from related cause, 5 patients (8.6%) remained alive, and 2 patients lost to follow-up. All patients were evaluable for response (Table 2). None achieved CR, and 10 (17.2%) achieved PR (95% CI 7.2%–27.3%). SD was observed in 58.6% (34/58) of patients (95% CI 45.6%–71.7%) and NR in 24.1% (14/58) of patients (95% CI 12.8%–35.5%). The ORR and DCR were 17.2% and 75.8% respectively. The median PFS was 143.1 days (95%CI 121.7–164.5), and the median OS was 211.6 days (95%CI 162.9–219.7) (Fig. 1).
Table 2.
Responses assessed per RECIST version 1.1.
| Tumor response | apatinib combined with S-1(N = 58) |
| Objective response | 17.2% |
| Disease control rate | 75.8% |
| Overall response | |
| Complete response (n, %) | 0 |
| Partial response (n, %) | 10 (17.2%) |
| Stable disease (n, %) | 34 (58.6%) |
| Progressive disease (n, %) | 14 (24.1%) |
Figure 1.
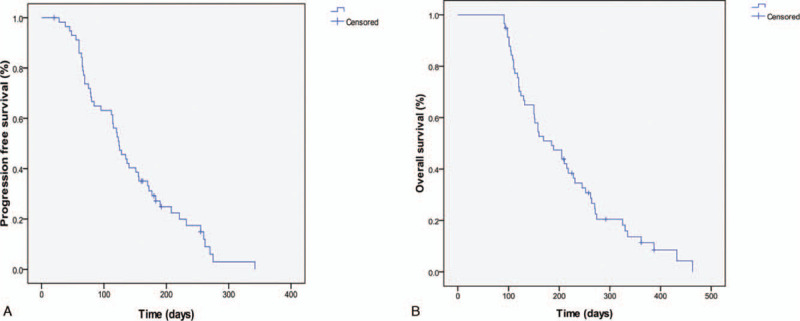
Kaplan–Meier estimates of PFS and OS. (A) PFS for the overall population and the median PFS was 143.1 ± 73.6days (95%CI 121.7–164.5). (B) OS for the overall population and the median OS was 211.6 ± 96.8 days (95%CI 162.9–219.7).
In the univariate analysis, ECOG PS was associated with longer OS and PFS (PFS: HR = 3.281, 95% CI: 2.14–5.04, P < .001; OS: HR = 3.748, 95% CI: 2.37–5.92, P < .001). The multivariate analysis showed that ECOG PS was the independent factor of PFS and OS for the AGC patients (PFS: HR = 3.565, 95% CI: 2.25–5.65, P < .001; OS: HR = 3.676, 95% CI: 2.29–5.89, P < .001). In addition, we found that age, gender and metastatic sites were not the predictors of OS and PFS.
3.4. Safety
The treatment-related AEs, which mainly manifested as fatigue (72.4%), hypertension (46.6%), hand-foot syndrome (24.1%), proteinuria (13.8%) and leukopenia (48.3%), were acceptable and manageable. No grade 4 AEs were observed in our study. The main treatment-related AEs are summarized in Table 3.
Table 3.
Incidence of AEs during the treatment.
| Adverse events | Grade 1 or 2 (n) | Grade 3 (n) | Rate (%) |
| Hematologic | |||
| Leukopenia | 26 | 2 | 48.3 |
| Anemia | 6 | 0 | 10.3 |
| Thrombocytopenia | 12 | 0 | 20.7 |
| Nonhematologic | |||
| Fatigue | 37 | 5 | 72.4 |
| Hypertension | 26 | 1 | 46.6 |
| Proteinuria | 8 | 1 | 15.5 |
| Hand-foot syndrome | 14 | 1 | 25.9 |
| Diarrhea | 3 | 0 | 5.2 |
We found that leukopenia and hypertension were associated with improved clinical outcomes. In patients with and without leukopenia, the median PFS was 187.5 vs. 101.1 days (P < .001; Fig. 2A), and OS was 267.4 vs. 158.6 days (P < .001; Fig. 2A). In patients with and without hypertension, the median PFS was 187.6 vs. 104.3 days (P < . 01; Fig. 2B), and OS was 277.1 vs. 154.0 days (P < .001; Fig. 2B). The multivariate analysis showed that leukopenia (HR = 0.369, 95% CI: 0.17–0.82, P = .014) and hypertension (HR = 0.305, 95% CI 0.14–0.69; P = .004) were independent factors of PFS. Similarly, leukopenia (HR = 0.342, 95% CI: 0.15–0.76, P = .009) and hypertension (HR = 0.280, 95% CI 0.13–0.63; P = .002) were verified as independent factors for predicting OS.
Figure 2.
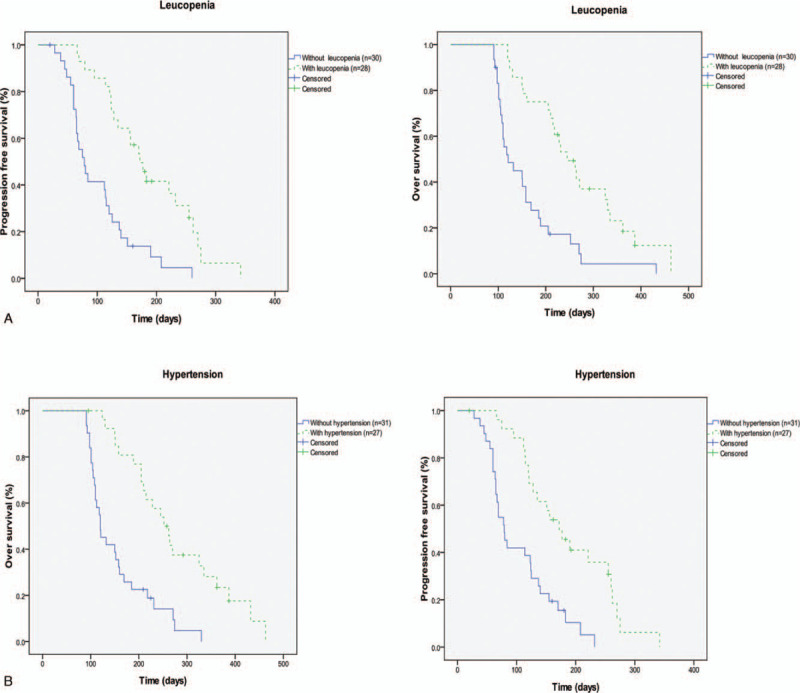
Univariate analysis of biomarkers as predictive factors for PFS and OS Kaplan–Meier curves for PFS: leucopenia (A), hypertension (B).
4. Discussion
Angiogenesis is closely related to tumor growth, proliferation, progression and metastasis, and antiangiogenesis has been demonstrated as an attractive target in cancer therapy.[12] Because its efficacy was poor when used alone, antiangiogenic therapy was usually combined with chemotherapy. But the efficacy of antiangiogenic therapy combined with chemotherapy is controversial for GC. RAINBOW trial demonstrated that ramucirumab combined with chemotherapy improved median PFS and ORR compared with the chemotherapy only,[19] but ramucirumab plus FOLFOX obtained contrasting results.[20]
Apatinib has been proven to be effective as the third-line therapy for AGC patients.[13,14] Zhang Y et al reported that apatinib monotherapy significantly improved PFS compared with placebo (4.43 months vs 3.8 months) as the second-line therapy.[21] The addition of antiangiogenic drugs to chemotherapy improved the OS of AGC patients compared with chemotherapy alone. Xiang Wang et al reported that the combination of apatinib with chemotherapy had significantly longer median PFS versus those receiving apatinib monotherapy (5.03 months vs 3.33 months, P = .003).[22] Therefore, apatinib combined with chemotherapy as the second-line therapy was associated with an increased survival benefit. However, there is insufficient evidence for apatinib combined with S-1 as the second-line therapy for AGC. So we observed the clinical efficacy and toxicity of apatinib combined with S-1 as the second-line therapy for AGC.
We observed that 75.8% of AGC patients achieved DCR. The median PFS in our study was 143.1 days and the median OS was 211.6 days. The efficacy in our study was promising as compared with that in previous studies. In Lu B et al study, the DCR was 73.68% and the PFS was 3.72 months during the combination apatinib with chemotherapy as the second-line therapy for AGC patients.[23] Yesong Guo et al reported that apatinib combined with chemotherapy showed an improved DCR (58.4% vs 41.9%, P = .041) in GC patients as compared with chemotherapy alone.[24] This also verified our conclusions. Moreover, the median PFS in our study was similar with that in RAINBOW study (median PFS was 4.4 months), which was the only successful antiangiogenic drug combined with chemotherapy in the second-line setting[19] Fangfang Lv et al reported the median PFS was 5.1 months and the median OS was 6.3 months with S-1 monotherapy as the second-line therapy.[25] Although cross-trial comparisons were difficult, we believed that the efficacy of combining apatinib with S-1 was better than that of S-1 monotherapy. Moreover, in our study, Cox regression analysis showed that ECOG PS was an independent prognostic factor of AGC, which was in accordance with the results of clinical trials on apatinib monotherapy. At the end of the follow-up, 5 patients remain alive. By analyzing the characteristics of these 5 patients, we considered that the reasons made these patients so responsive to this therapy were few metastatic sites, good ECOG score and obvious AEs.
Hypertension, proteinuria, and hand-foot syndrome are considered to be the most common AEs related to antiangiogenic agents. In our study, fatigue, leukopenia and hypertension were the most common AEs. However, treatment-related AEs were moderate and controllable, and the severity of those AEs was similar to or better than those observed in the previous studies.[13,14] No treatment-related death was recorded. No indicator for the efficacy of apatinib has been identified, but AEs attributable to VEGFR TKIs have been reported as predictive biomarkers for treatment efficacy.[26] In the analysis of AEs and efficacy, we found that patients with hypertension or leukopenia tended to have longer OS and PFS, so leukopenia or hypertension might serve as potential predictors of the clinical efficacy. Previous studies showed that the presence of AEs might serve as a potential biomarker of antitumor efficacy in GC patients,[22,26] but the mechanisms have not been fully elucidated. The reasons may be the inhibition of VEGF pathway in non-tumor cells. Blood pressure might increase with inhibition of VEGFR in vascular endothelial cells.[27] In our study, leukopenia was associated with improved clinical outcomes, and the reasons may lie in: leukopenia is a mild or moderate reduction and mainly occurs in patients with large doses (500 mg) of apatinib, and the dosage of apatinib may be related to the efficacy, but large dose of apatinib may lead to bone marrow suppression, which leads to leukopenia. As apatinib-induced AEs could partly reflect the inherent host biology that caused the difference in VEGF blockade, they could be served as prognostic biomarker of VEGF pathway inhibition efficacy. Due to the small sample size of our study, prospective studies are needed to validate these AEs as potential biomarkers.
Apatinib has consistently demonstrated manageable toxicity at daily doses of 250 mg to 850 mg. However, in some clinical trials, a lower apatinib dosage was used.[22] Furthermore, the appropriate dosage of apatinib in the second-line therapy is unknown. According to the recommendation by an expert consensus,[18] we used 250 mg or 500 mg once daily as the initial dosage of apatinib. Our clinical observations suggested that most patients were able to tolerate these dosages. Some patients underwent dose modification because of AEs and the treatment was continued in all patients after dose modification. Taking these findings into account, we suggested that the initial dosage for apatinib should be 250 mg or 500 mg.
Aptinib could increase the effects of S-1, but the synergistic effects are not fully understood. The synergistic effects may lie in:
-
1.
Apatinib normalizes tumor blood vessels, thereby increasing the local chemotherapeutic drug concentration of the tumor;[28]
-
2.
Apatinib can enhance the anti-tumor effect of chemotherapy. Peng et al found that apatinib combined with chemotherapy had a synergistic effect in the human nasopharyngeal carcinoma xenograft model.[29] Feng et al also found that apatinib enhanced the antitumor effect of docetaxel in the nude mice transplantation model;[30]
-
3.
Multidrug resistance (MDR) is the main reason for the failure of chemotherapy. Apatinib can reverse MDR by inhibiting the efflux function of multiple ABC transporters;[31]
-
4.
Apatinib may reduce inhibitory effect of Treg-mediated immunosuppression.[32]
One strength of our study is that both apatinib and S-1 are orally administered, meaning that this is a cost-effective treatment option for AGC patients, especially for outpatients. However, we acknowledged that this study had some limitations. Firstly, it was a retrospective study in a single institution; secondly, the cohort in our study is relatively small and could result in bias; thirdly, it was a single-arm study with no control group for comparison, and thus selection bias could not be ruled out. Despite the absence of control, we compared our results with previous studies, which revealed that anti-angiogenic drugs combined with chemotherapy showed positive effects on PFS and OS in GC patients. Peng W et al reported that patients receiving apatinib combined with chemotherapy had significantly longer median PFS versus those receiving apatinib monotherapy in the second-line therapy.[33] Additionally, another study showed that apatinib combined with chemotherapy significantly improved the patient's ORR and DCR compared with chemotherapy alone in GC.[34] In our study, the median PFS of apatinib combined with S-1 was similar with the results of previous studies;[33,34] however, due to the small sample size of our study, multicenter randomized controlled double-blind clinical trials are expected in the future.
5. Conclusions
Overall, the combination of apatinib and S-1 achieved relatively satisfactory results as the second-line therapy for AGC. AEs were moderate and controllable, and leukopenia or hypertension was predictable factors for the PFS and OS of AGC patients. Because of its tolerable AEs, convenient administration, and improved outcomes, apatinib combined with S-1 could be a therapeutic option for AGC patients after the first-line chemotherapy.
Author contributions
Conceptualization: Zhi-Yuan Qiu, Yan Wang.
Data curation: Rong Qin, Meifang Chen.
Formal analysis: Zhao Zhang.
Methodology: Guang-Yu Tian, Han He.
Writing – original draft: ZhiYuan Qiu, Yan Xi.
Writing – review & editing: ZhiYuan Qiu, Yan Xi, Yan Wang.
Footnotes
Abbreviations: AEs = adverse events, AGC = Advanced gastric cancer, CFDA = China Food and Drug Administration, CR = complete response, CT = computed tomography, CTCAE = Common Terminology Criteria for Adverse Events, DCR = disease control rate, ECOG PS = Eastern Cooperative Oncology Group performance status, MDR = Multidrug resistance, ORR = objective response rate, OS = overall survival, PD = progressive disease, PFS = progression-free survival, PR = partial response, RECIST = Response Evaluation Criteria In Solid Tumors, SD = stable disease.
How to cite this article: Qiu ZY, Qin R, Tian GY, Zhang Z, Chen M, He H, Xi Y, Wang Y. Apatinib combined with S-1 as second-line therapy in advanced gastric cancer. Medicine. 2021;100:17(e25630).
This research was funded by Science Foundation of the Affiliated People's Hospital of Jiangsu University (Y2019021-S), “Liu Ge Yi Gong Cheng” of Jiangsu Province (Grant No. LGY2017022), Youth Foundation of Jiangsu Province (Grant No. QNRC2016448) and Natural Science Foundation of Jiangsu Province (BK20161354) and Social Development Foundation of Zhenjiang (SH2019069).
The authors declare no conflict of interest.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
ECOG PS = Eastern Cooperative Oncology Group Performance Status.
RECIST = response evaluation criteria in solid tumors.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Chen W, Sun K, Zheng R, et al. Cancer incidence and mortality in China, 2014. Chin J Cancer Res 2018;30:01–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zheng R, Zeng H, Zhang S, et al. Estimates of cancer incidence and mortality in China, 2013. Chin J Cancer 2017;36:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zong L, Abe M, Seto Y, et al. The challenge of screening for early gastric cancer in China. Lancet 2016;388:2606. [DOI] [PubMed] [Google Scholar]
- [5].Boku N, Yamamoto S, Fukuda H, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol 2009;10:1063–9. [DOI] [PubMed] [Google Scholar]
- [6].Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687–97. [DOI] [PubMed] [Google Scholar]
- [7].Yamada Y, Higuchi K, Nishikawa K, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol 2015;26:141–8. [DOI] [PubMed] [Google Scholar]
- [8].Yamada Y, Boku N, Mizusawa J, et al. Docetaxel plus cisplatin and S-1 versus cisplatin and S-1 in patients with advanced gastric cancer (JCOG1013): an open-label, phase 3, randomised controlled trial. Lancet Gastroenterol Hepatol 2019;4:501–10. [DOI] [PubMed] [Google Scholar]
- [9].Sym SJ, Hong J, Park J, et al. A randomized phase II study of biweekly irinotecan monotherapy or a combination of irinotecan plus 5-fluorouracil/leucovorin (mFOLFIRI) in patients with metastatic gastric adenocarcinoma refractory to or progressive after first-line chemotherapy. Cancer Chemother Pharmacol 2013;71:481–8. [DOI] [PubMed] [Google Scholar]
- [10].Ford HE, Marshall A, Bridgewater JA, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol 2014;15:78–86. [DOI] [PubMed] [Google Scholar]
- [11].Shitara K, Takashima A, Fujitani K, et al. Nab-paclitaxel versus solvent-based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): an open-label, randomised, non-inferiority, phase 3 trial. Lancet Gastroenterol Hepatol 2017;2:277–87. [DOI] [PubMed] [Google Scholar]
- [12].Lee SH, Jeong D, Han YS, et al. Pivotal role of vascular endothelial growth factor pathway in tumor angiogenesis. Ann Surg Treat Res 2015;89:01–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li J, Qin S, Xu J, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo controlled, parallel-arm, phase II trial. J Clin Oncol 2013;31:3219–25. [DOI] [PubMed] [Google Scholar]
- [14].Li J, Qin S, Xu J, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol 2016;34:1448–54. [DOI] [PubMed] [Google Scholar]
- [15].Sanford M. S-1 (Teysuno®): a review of its use in advanced gastric cancer in non-Asian populations. Drugs 2013;73:845–55. [DOI] [PubMed] [Google Scholar]
- [16].Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–42. [DOI] [PubMed] [Google Scholar]
- [17].Van Cutsem E, de Haas S, Kang YK, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol 2012;30:2119–27. [DOI] [PubMed] [Google Scholar]
- [18].Expert Committee on Safety Management of Anti-Tumor Drugs of Chinese Society of Clinical Oncology. Expert consensus on the clinical application of apatinib in gastric cancer. Chin Clin Oncol 2015;20:841–7. [Google Scholar]
- [19].Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a doubleblind, randomised phase 3 trial. Lancet Oncol 2014;15:1224–35. [DOI] [PubMed] [Google Scholar]
- [20].Yoon HH, Bendell JC, Braiteh FS, et al. Ramucirumab combined with FOLFOX as front-line therapy for advanced esophageal, gastroesophageal junction, or gastric adenocarcinoma: a randomized, double-blind, multicenter Phase II trial. Ann Oncol 2016;27:2196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang Y, Gou M, Han C, et al. Efficacy and safety of apatinib as second-line therapy for advanced gastric cancer: a single-center observational study. Antican Drugs 2018;29:184–9. [DOI] [PubMed] [Google Scholar]
- [22].Xiang Wang, Ruixing Zhang, Nan Du, et al. An open label, multicenter, noninterventional study of apatinib in advanced gastric cancer patients (AHEAD-G202). Ther Adv Med Oncol 2020;12:01–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lu B, Lu C, Sun Z, et al. Combination of apatinib mesylate and second-line chemotherapy for treating gastroesophageal junction adenocarcinoma. J Int Med Res 2019;47:2207–14. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [24].Yesong Guo, Jinhai Tang, Xinen Huang, et al. Efficacy and toxicity of apatinib combined with or without chemotherapy for patients with advanced or metastatic chemotherapy-refractory gastric adenocarcinoma: A prospective clinical study. Medicine 2019;98:e13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fangfang Lv, Xin Liu, Biyun Wang, et al. S-1 monotherapy as second line chemotherapy in advanced gastric cancer patients previously treated with cisplatin/infusional fluorouracil. Int J Clin Exp Pathol 2014;7:3293–8. [PMC free article] [PubMed] [Google Scholar]
- [26].Xinyang Liu, Shukui Qin, Zhichao Wang, et al. Early presence of anti-angiogenesis-related adverse events as a potential biomarker of antitumor effcacy in metastatic gastric cancer patients treated with apatinib: a cohort study. J Hematol Oncol 2017;10:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tang JR, Markham NE, Lin YJ, et al. Inhaled nitric oxide attenuates pulmonary hypertension and improves lung growth in infant rats after neonatal treatment with a VEGF receptor inhibitor. Am J Physiol Lung Cell Mol Physiol 2004;287:L344–351. [DOI] [PubMed] [Google Scholar]
- [28].Sui Peng, Yanyan Zhang, Hong Peng, et al. Intracellular autocrine VEGF signaling promotes EBDC cell proliferation, which can be inhibited by apatinib. Cancer Lett 2016;373:193–202. [DOI] [PubMed] [Google Scholar]
- [29].Qiuxia Peng, Yunwei Han, Yanling Zhang, et al. Apatinib inhibits VEGFR-2 and angiogenesis in an in vivo murine model of nasopharyngeal carcinoma. Oncotarget 2017;8:52813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Feng X, Zhang Y, Li T, et al. Sequentially administrated of pemetrexed with icotinib/erlotinib in lung adenocarcinoma cell lines in vitro. Oncotarget 2017;8:114292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mi YJ, Liang YJ, Huang HB, et al. Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res 2010;70:7981–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Huiqin Luo, Yifu He, Wenju Chen, et al. Effect of apatinib on serum CD4+CD25+ T cells, NK Cells, and T Cells subgroup in malignant tumor. Technol Cancer Res Treat 2019;18:01–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Peng W, Zhang F, Wang Z, et al. Large Scale, multicenter, prospective study of apatinib in advanced gastric cancer: a real-world study from China. Cancer Manag Res 2020;12:6977–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Honggang Cheng, Aixia Sun, Qingbo Guo, et al. Efficacy and safety of apatinib combined with chemotherapy for the treatment of advanced gastric cancer in the Chinese population: a systematic review and meta-analysis. Drug Des Devel Ther 2018;12:2173–83. [DOI] [PMC free article] [PubMed] [Google Scholar]


