Abstract
Background:
Whether hypothyroidism is related to non-alcoholic fatty liver disease (NAFLD) is controversial. Our aim was to investigate the relationship between NAFLD and hypothyroidism that may predict the NAFLD potential of these lesions and new prevention strategies in hypothyroidism patients.
Methods:
Totally 51,407 hypothyroidism patients with average 28.23% NAFLD were analyzed by Revman 5.3 and Stata 15.1 softwares in the present study. The PubMed and Embase databases were systematically searched for works published through May 9, 2020.
Results:
The blow variables were associated with an increased risk of NAFLD in hypothyroidism patients as following: increased of thyroid stimulating hormone (TSH) levels (odds ratio [OR] = 1.23, 1.07–1.39, P = .0001); old age (mean difference [MD] = 3.18, 1.57–4.78, P = .0001); increased of body mass index (BMI) (MD = 3.39, 2.79–3.99, P < .000001); decreased of free thyroxine 4 (FT4) levels (MD = –0.28, –0.53 to –0.03, P = .03). In addition, FT3 (MD = 0.11, –0.09–0.3, P = .29) had no association with the risk of NAFLD in hypothyroidism patients.
Conclusion:
Our systematic review identified results are as following: hypothyroidism was positively associated with the risk of NAFLD. The increased concentration of TSH levels maybe a risk factor that increased incidence of NAFLD. The BMI of NAFLD patients was significantly higher than that of non-NAFLD patients. Old age was significantly associated with the incidence of NAFLD. FT4 was significantly associated with the risk of NAFLD due to its negatively effect while FT3 was not significantly related to the risk of NAFLD. Taken together, the present meta-analysis provides strong evidence that hypothyroidism may play a vital role in the progression and the development of NAFLD.
Keywords: hypothyroidism, meta-analysis, non-alcoholic fatty liver disease, relationship
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is common worldwide chronic liver disease which increased substantially decade years in both developed and developing countries.[1] NAFLD is divided into 2 histological categories clinically including nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH) which cause of liver disease with prevalence estimates ranging from 25% to 45%.[2] A range of progressive liver diseases are included in NAFLD, ranging from simple steatosis to NASH, with fibrosis that usually developing into cirrhosis and hepatocellular carcinoma (HCC).[3]
From a pathogenic point of view, NAFLD is closely related to the characteristics of insulin resistance (IR) and metabolic syndrome (MetS).[4] MetS is a recognized as the risk factors of cardiovascular metabolic which including visceral overweight/obesity, atherosclerotic dyslipidemia, glucose intolerance or type 2 diabetes mellitus (T2D), and hypertension.[5] Moreover, it was reported that most of these cardiovascular metabolic risk factors come from systemic and liver IR.[6] This metabolic abnormality activates multiple molecular pathways, leading to metabolic disorders and a pro-inflammatory environment, which may cause an accumulated in the same individual and the development of MetS.[7] In addition, NAFLD is not only related to morbidity and mortality of liver, but also with the increased risk of other diseases such as chronic kidney disease and colorectal carcinoma.[8]
Hypothyroidism includes subclinical hypothyroidism and overt hypothyroidism in clinical practice. Subclinical hypothyroidism is a disease in which the level of thyroid stimulating hormone (TSH) is higher than the normal range, the level of serum free thyroxine 4 (FT4) is normal, and there is no apparently clinical symptoms.[9] Overt hypothyroidism is a disease with increased TSH levels, decreased FT4 levels, and commonly accompanied by obvious clinical manifestation.[10] Previous research has been demonstrated that hypothyroidism associated with metabolic syndrome, cardiovascular mortality, and disturbance in lipid metabolism.[11]
The relationship between NAFLD and hypothyroidism was studied more and more in recent years. Thyroid hormone (TH) is secreted by thyroid gland which plays an important role in regulating energy homeostasis, hepatic lipid metabolism, body weight, and IR.[12] It was demonstrated that decreased the concentration of TH in serum may promote hyperlipidemia and obesity, thus contributing to NAFLD.[13] In addition, it was reported that the prevalence of NAFLD is negatively correlated with FT4 levels, and the reduction of FT4 levels contributes to the occurrence of NAFLD.[14] Furthermore, previous studies have revealed that thyroid hormone receptor (THR) agonist reduced hepatic steatosis.[15] However, the relationship between NAFLD and hypothyroidism remain controversial. It has been demonstrated that hypothyroidism was correlated with NAFLD while conducted statistical analysis on the thyroid function and other data of 4648 physical examination patients.[16] Furthermore, it has been demonstrated that subclinical hypothyroidism and low-normal thyroid function were associated with NAFLD.[17] However, it was also reported that there was no correlation between hypothyroidism and NAFLD with a statistical analysis on the data of 832 volunteers.[18] Moreover, it was also found that NAFLD was not associated with thyroid hormone levels and hypothyroidism.[19] In addition, clinical data supporting connection is incomplete, and the pathophysiology underlying the association remains unclear. Therefore, a systematic review and meta-analysis is needed to determine and better characterize the proposed relationship between NAFLD and hypothyroidism.
2. Methods
2.1. Search strategy
The relevant published articles including PubMed and Embase databases were used to identify until May 9, 2020. The following keywords were used in searching: “hypothyroidism OR thyroid dysfunction OR thyroid stimulating OR TSH” AND “nonalcoholic fatty liver disease OR NAFLD OR nonalcoholic fatty liver OR NAFL OR nonalcoholic steatohepatitis OR NASH OR fatty liver.” Relevant articles were used to broaden the search scope, and all retrieved studies, reviews, and conference abstracts were retrieved by the computer. If multiple published studies describe the same population, we extract only the most complete or recent one. Two authors (XZ and BL) independently completed the selection process and resolved the differences through discussion.
2.2. Selection criteria
The selection strategy used the following criteria: English language studies; prospective or retrospective original studies or cohort that explored the relationship between hypothyroidism and NAFLD; to diagnose of NAFLD was based on standardized ultrasound examination or pathologic examination; biochemical test was needed in diagnosis of hypothyroidism.
The exclusion criteria were adapted to exclude studies from meta-analysis as following: non-English language studies; reviews, case reports, editorials, letters to editors, congress abstracts, commentaries, practice guidelines, meetings or conference records; insufficient data (e.g., <30 patients in the research); the diagnosis of NAFLD is based only on studies of serum liver enzyme levels or other NAFLD surrogate markers (e.g., using fatty liver index); the diagnosis of hypothyroidism is wrong or non-standardized collection; studying period beyond 10 years.
2.3. Data extraction
Two authors (BL and YZ) abstracted the following data from the included articles: first author, country, publication years, study design, case number, diagnosis method, covariate adjustment, definition of hypothyroidism, and Newcastle-Ottawa quality assessment scale (NOS). Any disagreements were resolved by a third investigator (XZ). The NOS was used to assess the quality of the study.
2.4. Ethical statement
The meta-analysis was strictly followed the ethical approval that referenced publication articles which related to human care, handling, sampling, and administration procedures should be approved by ethics committee.
3. Statistical analysis
All statistical analysis were performed using Ravman Manager version 5.3 (Cochrane Collaboration, Oxford, UK) and Stata Statistical Software version 15.1 (StataCorp LP, College Station, TX). The magnitude of the effect of each study was calculated by odds ratio (OR) and the mean difference (MD) of the 95% confidence interval (CI) briefly. This meta-analysis used a combined OR and the MD of 95% CI method to assess the strength of the association between NAFLD and the risk of hypothyroidism. A P value of <.05 was considered statistically significant unless otherwise specified. In addition, the heterogeneity was quantified using the Q-test and the I2 statistic. When P > .1 and I2 < 50%, a fixed-effect model was applied; otherwise, a random-effects model was used. The possibility of publication bias was evaluated using the funnel plot.
4. Results
After searching, a total of 806 studies were initially considered for inclusion in the meta-analysis. Forty three records were excluded by language and duplicate; 79 studies were excluded in the form of reviews, case reports, editorials, letters to the editor, and summaries of conference or meeting proceedings; 371 records were excluded by the screening of title or abstract; 296 records were excluded by studying period beyond 10 years or insufficient data. After a full review, a total of 17 studies which met our selection criteria were finally included in meta-analysis. The selection flowchart of research is presented in Fig. 1. The basic characteristics of the studies were included in Table 1. There is no significant asymmetry of Begg funnel plot exist in all the risk factor analyses.
Figure 1.
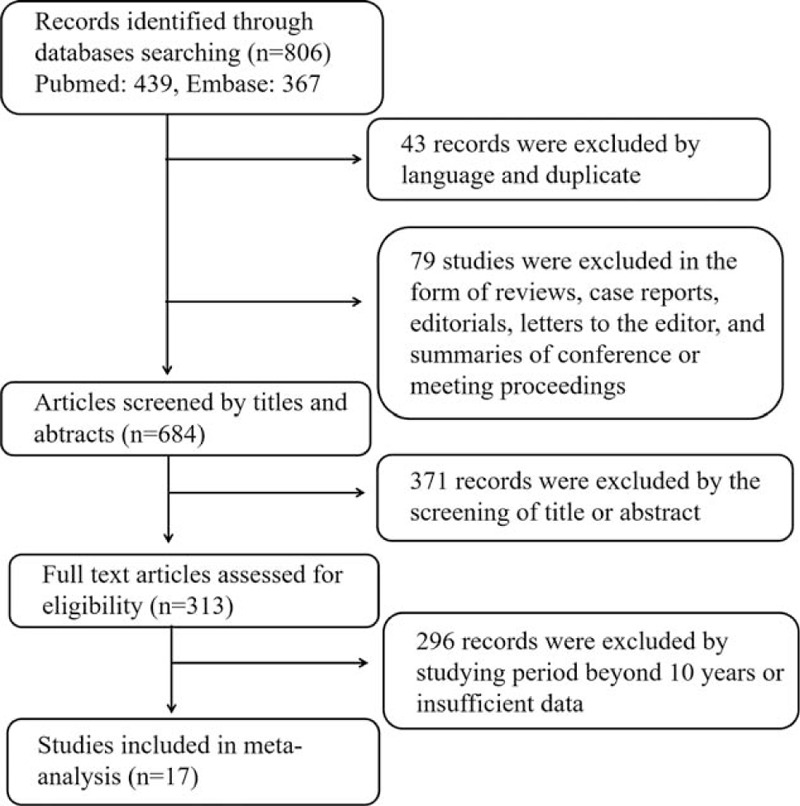
Flow chart of the study selection process.
Table 1.
Basic characteristics of included studies on the association between hypothyroidism and NAFLD.
| First author | Country | Publication years | Study design | Case number | Diagnosis method | Covariate adjustment | Definition of hypothyroidism | NOS |
| Assem[20] | Egypt | 2018 | Cross-sectional | 90 | Ultrasound | Age, sex, TPO-Ab, insulin resistance. | Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. | 6 |
| Bano[21] | Netherlands | 2016 | Retrospective study | 9419 | Ultrasound | Age, sex, follow-up time, use of hypolipidemic drugs, cardiovascular. | Subclinical hypothyroidism (TSH > 4.0 mIU/L and FT4 levels within the reference range), Overt hypothyroidism (TSH > 4.0 mIU/L and FT4 levels < 0.85 ng/dL). | 9 |
| Chung[16] | Korea | 2012 | Cross-sectional study | 2324 | Ultrasound | Gender, Age, BMI, Waist circumference, Triglyceride, SBP, DBP, diabetes mellitus, hypertension | Subclinical hypothyroidism (TSH > 4.1 mIU/L; normal FT4 concentration); Overt hypothyroidism: FT4 level < 0.7 ng/dL. | 7 |
| Ding[22] | China | 2015 | Retrospective study | 1815 | Liver-biopsy | Age, gender, HBeAg, serum thyroid function test. | Overt hypothyroidism (FT4 < 7.86 pmol/L and TSH > 5.30 mIU/L). Subclinical hypothyroidism (higher TSH level with normal range of FT4). | 8 |
| Escudé[23] | Spain | 2020 | Cross-sectional, retrospective study | 10116 | Ultrasound | Age, sex, height, weight, waist circumference, BMI, hypertension, dyslipidemia, obesity. | Subclinical hypothyroidism (TSH >4.94 IU/mL and normal FT4 levels); clinical hypothyroidism (high TSH and FT4 level <0.7 IU/mL). | 8 |
| Eshraghian[18] | Iran | 2013 | Cross-sectional study | 832 | Ultrasound | Age, sex, weight, height, BMI, waist circumference, hip circumference, waist-to-hip ratio. | Subclinical hypothyroidism (TSH >5.2 mIU/L, normal FT4 concentration); overt hypothyroidism FT4 <11.5 pmol/L and TSH >5.2 mIU/L). | 9 |
| Gökmen[24] | Turkey | 2015 | Retrospective study | 115 | Ultrasound | Insulin resistance, enlarged waist circumference, elevated body mass index, higher FT3/FT4 ratio, hypertriglyceridemia. | TSH >4.1 mIU/L | 7 |
| Kaltenbach[25] | Germany | 2016 | Prospective cross-sectional study | 332 | Ultrasound | Age, gender, BMI-SDS. | Subclinical hypothyroidism (TSH > 4 μU/mL, normal thyroxine). | 7 |
| Kassem[26] | Egypt | 2017 | Prospective cross-sectional study | 60 | Ultrasound | Age, gender. | Reference value (TSH between 0.27 and 4.2 IU/mL, FT3 between 2.57 and 4.43 pg/mL, FT4 between 0.93 and 1.71 ng/mL). | 6 |
| Kim[27] | Korea | 2017 | Retrospective study | 425 | Ultrasonography | Age, sex, BMI, waist circumference, diabetes, hypertension, smoking. | Subclinical hypothyroidism (TSH >4.5 mIU/L, with a normal thyroid hormone T4 level between 0.7 and 1.8 ng/dL). | 9 |
| Lee[28] | Korea | 2015 | Retrospective study | 18544 | Ultrasound | Sex, age, BMI, TGs, HDL | Subclinical hypothyroidism (TSH > 4.2 mIU/L, normal FT4); overt hypothyroidism (TSH > 4.2 mIU/L, FT4 < 10.97 ng/dL). | 8 |
| Ludwig[29] | Germany | 2015 | Retrospective study | 2445 | Ultrasound | Gender, BMI, WHR, metabolic syndrome, diabetes mellitus. | TSH >3.4 IU/mL, TT4 between 12.8 and 20.4 pmol/L, TT3 between 3.9 and 6.7 pmol/L. | 7 |
| Pacifico[30] | Italy | 2017 | Retrospective study | 402 | Ultrasound | Age, gender, pubertal status, BMI-SDS. | Subclinical hypothyroidism (TSH > 4.1 mIU/L with normal FT4); overt hypothyroidism (TSH ≥ 4.1 mIU/L with FT4 < 0.7 ng/dL) | 9 |
| Pagadala[31] | American | 2011 | Retrospective case-control study | 676 | Histological | Gender, BMI, ethnicity, diabetes, HTN, hyperlipidemia. | Hypothyroidism (TSH > 4.8 IU/mL) | 8 |
| Parikh[32] | India | 2015 | Retrospective study | 800 | Ultrasound | Gender, BMI. | Subclinical hypothyroidism (TSH > 5.5 IU/mL but <10 IU/mL); overt hypothyroidism (TSH > 10 IU/mL). | 8 |
| Tahara[33] | Japan | 2019 | Cross-sectional study | 2134 | Ultrasonography | BMI, triglyceride, high-density lipoprotein-cholesterol, hypertension, diabetes. | Subclinical hypothyroidism (TSH > 4.00 μU/L with an FT4 level between 0.90 and 1.80 ng/dL). | 8 |
| Xu[34] | China | 2011 | Case-control study | 878 | Ultrasound | Age, gender, metabolic syndrome status, smoking status, BMI, WC, SBP, DBP, TC,TG, HDL-C. | Subcinical hypothyroidism (TSH > 5.5 IU/mL or <0.5 IU/mL FT4 > 14.41 pmol/L or <7.85 pmol/L). | 8 |
4.1. Prevalence of NAFLD and variables in hypothyroidism
The prevalence of NAFLD was clinicopathological variable in different study, ranging from 21.67% to 66.67%. Overall, NAFLD was confirmed among 14,514 patients of totally 51,407 hypothyroidism patients in this systematic review and meta-analysis. In addition, a fix-effects model was applied in the analysis involving NAFLD and hypothyroidism (I2 = 37.5%). It was indicated that hypothyroidism was significantly related to NAFLD in patients (OR = 1.25, 95% CI = 1.04–1.46, P = .156) (Fig. 2).
Figure 2.
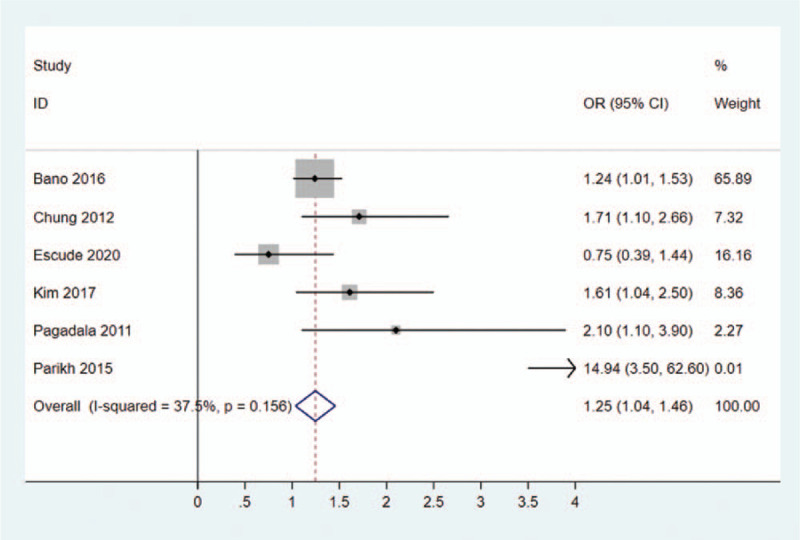
Forest plots of the relationship between hypothyroidism and NAFLD. NAFLD = non-alcoholic fatty liver disease.
4.2. Risk factors for NAFLD in hypothyroidism patients
4.2.1. TSH
A random-effects model and input continuous data were selected using inverse variance method to calculated (I2 = 75.8%). The results indicated that a significant association was existed between TSH (the concentration of TSH levels increased) and NAFLD in hypothyroidism patients (OR = 1.23, 95% CI = 1.07–1.39, P = .0001) (Table 2, Fig. 3).
Table 2.
Risk factors for NAFLD in hypothyroidism patients.
| Risk factor | OR/MD | 95% CI | P value |
| TSH | OR = 1.23 | 1.07–1.39 | .0001 |
| Age | MD = 3.18 | 1.57–4.78 | .0001 |
| BMI | MD = 3.39 | 2.79–3.99 | <.000001 |
| FT3 | MD = 0.11 | -0.09–0.31 | .29 |
| FT4 | MD = -0.28 | -0.53–-0.03 | .03 |
Figure 3.
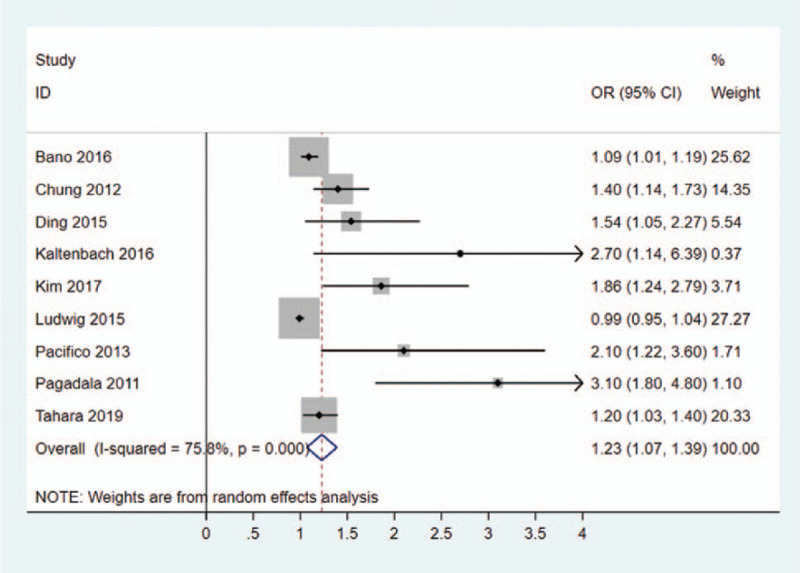
Forest plots of the association between TSH and NAFLD in hypothyroidism patients. NAFLD = non-alcoholic fatty liver disease, TSH = thyroid stimulating hormone.
4.2.2. Age
A random-effects model and input continuous data were selected using inverse variance method to calculated (P < .00001, I2 = 97%). Eight included studies were investigated for the relationship between age and NAFLD in hypothyroidism patients. It was revealed that age (old age) exhibited a high ratio of NAFLD in hypothyroidism patients (OR = 3.18, 95% CI = 1.57–4.78, P = .0001) (Fig. 4).
Figure 4.
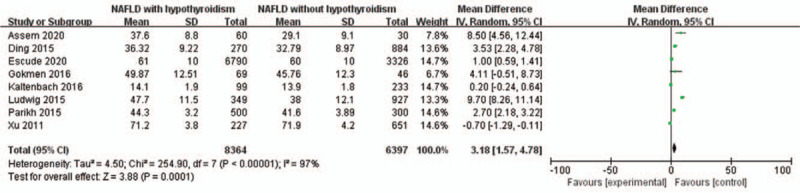
Forest plots of the association between age and NAFLD in hypothyroidism patients. NAFLD = non-alcoholic fatty liver disease.
4.2.3. BMI
A random-effects model was utilized to analyze the data (I2 = 98%). It was demonstrated that the increased of BMI was significantly related to NAFLD in hypothyroidism patients (MD = 3.39, 95% CI = 2.79–3.99, P < .000001) (Fig. 5).
Figure 5.
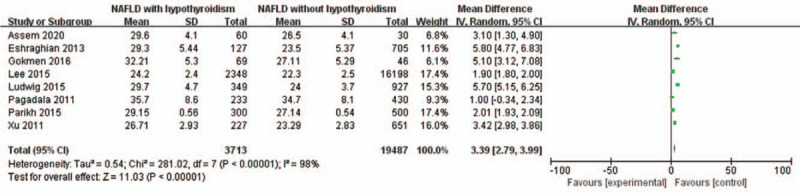
Forest plots of the association between BMI and NAFLD in hypothyroidism patients. BMI = body mass index, NAFLD = non-alcoholic fatty liver disease.
4.2.4. FT3
A random-effects model was utilized to analyze the data (I2 = 82%). Six included studies were evaluated for the relationship between free triiodothyronine (FT3) and NAFLD in hypothyroidism patients. It was revealed that FT3 was not associated with NAFLD in hypothyroidism patients (MD = 0.29, 95% CI = 0.11–0.31, P = .28) (Fig. 6).
Figure 6.
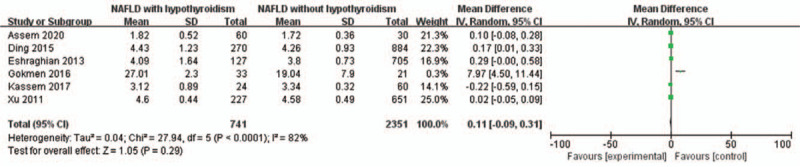
Forest plots of the association between FT3 and NAFLD in hypothyroidism patients. FT3 = free triiodothyronine, NAFLD = non-alcoholic fatty liver disease.
4.2.5. FT4
Seven studies were analyzed for the correlation between FT4 and NAFLD in hypothyroidism patients. A random-effects model was used to analyze the data (I2 = 96%). It was demonstrated that NAFLD was significantly negatively related to a low concentration of FT4 levels in hypothyroidism patients (MD = –0.28, 95% CI = –0.53 to –0.03, P = .03) (Fig. 7).
Figure 7.
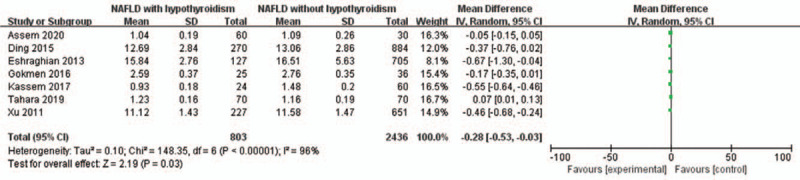
Forest plots of the association between FT4 and NAFLD in hypothyroidism patients. FT4 = free thyroxine 4, NAFLD = non-alcoholic fatty liver disease.
5. Discussion
Hypothyroidism is a common disease of the endocrine system that affects lifelong health due to insufficiency of thyroid secretion caused thyroid dysfunction.[35] NAFLD represents fatty infiltration of the liver without excessive alcohol consumption.[36] Previous studies has demonstrated that hypothyroidism (including subclinical hypothyroidism and overt hypothyroidism) is a independent significant risk factor and may leading to MetS for NAFLD.[37] It is revealed that hypothyroidism may directly result in NAFLD irrespective of other metabolic risk factors.[24,38] In addition, it was also showed that there was no significant correlation between NAFLD and subclinical, overt, or overall hypothyroidism, and there was no significant difference in TH level between participants with and without NAFLD.[19] Base on different research have contrary conclusion, whether hypothyroidism is related to NAFLD is necessary to study deeply. Therefore, the purpose of the present meta-analysis was providing the most comprehensive and updated assessment of the relationship between NAFLD with hypothyroidism. It was showed that the association between the pathogenesis of NAFLD and hypothyroidism was significant involving 17 articles with a total of 14,514 patients. These finding suggested that an association between hypothyroidism and NAFLD.
TSH changes in hypothyroidism include increased secretion, slowed degradation, and diminished or absent TSH circadian rhythms which has proved that an important factor for hypothyroidism.[39] It was reported that hypothyroidism was related to NAFLD in a dose-dependent manner as in the range of upper TSH levels.[26,40] Furthermore, previous research has been demonstrated that serum TSH levels was markedly affected in NAFLD patients in the general population, even those within the reference range. According to our analysis data, hypothyroidism patients with relatively high TSH levels was in risk of developing NAFLD (OR = 1.23, P = .0001). Our finding was consistent with previous report.
Age is considered to be a major prognostic factor for risk of NAFLD in patients with hypothyroidism. Previous studies demonstrated that old age was related to the increased risk of in NAFLD patients.[41] In addition, it was reported that the relationship between hypothyroidism and the lipid profile, which is substantially influenced by age, especially in patients with mild thyroid impairment (TSH < 10 mIU/L).[42] However, lipid profile is closely related to NAFLD that occur in patients related to age. Therefore, it's necessary to found out the relationship between age and hypothyroidism in NAFLD patients. In the present meta-analysis, it was revealed that the patients with old age for hypothyroidism may have the increased risk of NAFLD in clinical practice (MD = 3.18, P = .0001).
Body mass index (BMI) is considered to be the most useful predictive factor for the onset of NAFLD which means a poorer prognosis ultimately.[43] It was also demonstrated that BMI is associated with dysfunction of thyroid function especially hypothyroidism.[44] Furthermore, positive relationship have been reported between FT3/FT4 ratio and BMI in patients with obesity among NAFLD patients.[45] Based on our analysis data, the risk of NAFLD was related to BMI in hypothyroidism patients (MD = 3.39, P < .000001) which is analogous with previous research.
Six studies were analyzed in the meta-analysis for the correlation between FT3 and NAFLD in hypothyroidism patients. It was observed that there is no significant relationship of free triiodothyronine (FT3) concentrations with NAFLD.[46] On the other hand, the decreased concentration of FT3 symbolized hypothyroidism symptom for patients in clinical.[47] In our meta-analysis, it was found that FT3 has no association with NAFLD in hypothyroidism patients (MD = 0.11, P = .29).
Among the clinical and pathological features that can be evaluated, FT4 is an important factor for NAFLD in hypothyroidism patients. It was demonstrated that the prevalence of NAFLD was negatively correlated with the level of FT4, and the decrease of FT4 was helpful to the occurrence of NAFLD.[48] Furthermore, the decreased in FT4 concentration also marks the symptoms of hypothyroidism in newly diagnosed patients.[49] Our finding indicated that decreased the concentration of FT4 was relatively higher risk of developing NAFLD in hypothyroidism patients (MD = –0.28, P = .03). Besides, the FT3/FT4 ratio can be used as an indicator of peripheral deiodinase activity which related to hypothyroidism. It was reported that the increased conversion of FT4 to FT3 is a compensatory mechanism for fat accumulation to increase energy expenditure association with increased deiodinase activity which was related to NAFLD.[50]
The latest meta-analysis which only including 3 studies showed that hypothyroidism was not independently associated with the risk of NAFLD in the median 5 years.[51] This finding may be caused by the small number of included studies. A total of 17 studies were included to discuss the relationship between hypothyroidism and NAFLD. The number of included studies was more than before, and according to the NOS evaluation criteria, the scores of the included studies were 6 to 9 with high quality. Therefore, the results were highly reliable. In addition, because the time relationship between liver and thyroid disease is not clear, the causal relationship between hypothyroidism and NAFLD is not yet fully established.
There still exist some limitations in the present meta-analysis. Firstly, the heterogeneity is significant in some studies. We attempted to explore the heterogeneity between studies through a random effects model to give a more conservative effect estimate. Secondly, criteria of diagnosis cannot reach uniformity. Due to liver biopsy is not feasible in the general population, some non-standardization methods such as ultrasound, computed tomography, magnetic resonance imaging, and spectroscopy are commonly used to diagnose NAFLD. Thirdly, only 17 studies and recent 10 years studies were included for investigating the relationship between NAFLD and hypothyroidism. In future studies, large-scale and long-term randomized controlled trials should be conducted in different populations to provide more important evidence. Fourthly, although some confounding factors were considered and excluded, some lifestyle differences may also affected the final results. Fifthly, the meta-analysis only used a combination of OR and MD with 95% CI to assess the strength of the association between NAFLD and the risk of hypothyroidism. However, in statistical analysis method, RR (risk ratio) and HR (risk ratio) were still be used to evaluate the correlation. A variety of statistical methods can be used in the future research. Sixthly, only Revman 5.3 and Stata 15.1 softwares were used in the meta-analysis, more software such as Meta analyst, MIX, Meta-DiSc, Metawin, and R should be used for different types of meta-analysis in the future.
6. Conclusion
It was revealed that the major findings as following: hypothyroidism was positively associated with the risk of NAFLD. The elevated TSH levels maybe a risk factor which independently associated with NAFLD. The BMI of NAFLD patients was significantly higher than that of non-NAFLD patients. Old age was significantly associated with the incidence of NAFLD. FT4 was significantly associated with the risk of NAFLD while FT3 was not significantly related to the risk of NAFLD. Taken together, the present meta-analysis provides strong evidence that hypothyroidism may play an important role in the progression and the development of NAFLD.
Acknowledgments
The authors want to thank Jingxin Mao (ORCID: 0000-0002-2813-1702, Southwest University, China) for revision of the manuscript.
Author contributions
Conceptualization: Yang Zou.
Data curation: Bin Li.
Formal analysis: Bin Li, Yang Zou.
Funding acquisition: Yang Zou.
Methodology: Xiaoxu Zeng.
Project administration: Yang Zou.
Resources: Yang Zou.
Software: Bin Li.
Visualization: Bin Li.
Writing – original draft: Xiaoxu Zeng.
Writing – review & editing: Xiaoxu Zeng.
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, FT3 = free triiodothyronine, FT4 = free thyroxine 4, HCC = hepatocellular carcinoma, IR = insulin resistance, MD = mean difference, MetS = metabolic syndrome, NAFL = nonalcoholic fatty liver, NAFLD = non-alcoholic fatty liver disease, NASH = nonalcoholic steatohepatitis, NOS = Newcastle-Ottawa quality assessment scale, OR = odds ratio, T2D = type 2 diabetes mellitus, THR = thyroid hormone receptor, TSH = thyroid stimulating hormone.
How to cite this article: Zeng X, Li B, Zou Y. The relationship between non-alcoholic fatty liver disease and hypothyroidism: a systematic review and meta-analysis. Medicine. 2021;100:17(e25738).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
BMI = body mass index, FT3 = free triiodothyronine, FT4 = free thyroxine 4, MD = mean difference, NAFLD = non-alcoholic fatty liver disease, NOS = Newcastle-Ottawa quality assessment scale, TSH = thyroid stimulating hormone.
BMI = body mass index, CI = confidence interval, FT3 = free triiodothyronine, FT4 = free thyroxine 4, MD = mean difference, NAFLD = non-alcoholic fatty liver disease, OR = odds ratio.
References
- [1].Sugimoto K, Takei Y. Clinicopathological features of non-alcoholic fatty liver disease. Hepatol Res 2011;41:911–20. [DOI] [PubMed] [Google Scholar]
- [2].Gunn NT, Shiffman ML. The use of liver biopsy in nonalcoholic fatty liver disease: when to biopsy and in whom. Clin Liver Dis 2018;22:109–19. [DOI] [PubMed] [Google Scholar]
- [3].Su Q, Kumar V, Sud N, et al. MicroRNAs in the pathogenesis and treatment of progressive liver injury in NAFLD and liver fibrosis. Adv Drug Deliv Rev 2018;129:54–63. [DOI] [PubMed] [Google Scholar]
- [4].Hogden A, Greenfield D, Nugus P, et al. Engaging in patient decision-making in multidisciplinary care for amyotrophic lateral sclerosis: the views of health professionals. Patient Prefer Adherence 2012;6:691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Koopman AD, Rauh SP, van Riet E, et al. The association between social jetlag, the metabolic syndrome, and type 2 diabetes mellitus in the general population: the new Hoorn study. J Biol Rhythms 2017;32:359–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Huang RC, Beilin LJ, Ayonrinde O, et al. Importance of cardiometabolic risk factors in the association between nonalcoholic fatty liver disease and arterial stiffness in adolescents. Hepatology 2013;58:1306–14. [DOI] [PubMed] [Google Scholar]
- [7].Than A, Cheng Y, Foh L-C, et al. Apelin inhibits adipogenesis and lipolysis through distinct molecular pathways. Mol Cell Endocrinol 2012;362:227–41. [DOI] [PubMed] [Google Scholar]
- [8].Ionică FE, Negreş S, Bejenaru L, et al. Pharmacological approaches for nonalcoholic fatty liver disease. Rom J Diabetes Nutr Metab Dis 2016;23:313–8. [Google Scholar]
- [9].Gao C, Yang B, Guo Q, et al. High thyroid-stimulating hormone level is associated with the risk of developing atherosclerosis in subclinical hypothyroidism. Horm Metab Res 2015;47:220–4. [DOI] [PubMed] [Google Scholar]
- [10].Saini V, Yadav A, Arora MK, et al. Correlation of creatinine with TSH levels in overt hypothyroidism-A requirement for monitoring of renal function in hypothyroid patients? Clin Biochem 2012;45:212–4. [DOI] [PubMed] [Google Scholar]
- [11].Delitala AP, Fanciulli G, Maioli M, et al. Subclinical hypothyroidism, lipid metabolism and cardiovascular disease. Eur J Intern Med 2017;38:17–24. [DOI] [PubMed] [Google Scholar]
- [12].Coppola M, Glinni D, Moreno M, et al. Thyroid hormone analogues and derivatives: actions in fatty liver. World J Hepatol 2014;6:114–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pacifico L, Anania C, Ferraro F, et al. Thyroid function in childhood obesity and metabolic comorbidity. Clin Chim Acta 2012;413:396–405. [DOI] [PubMed] [Google Scholar]
- [14].Liu Y, Wang W, Yu X, et al. Thyroid function and risk of non-alcoholic fatty liver disease in Euthyroid subjects. Ann Hepatol 2018;17:779–88. [DOI] [PubMed] [Google Scholar]
- [15].Cable EE, Finn PD, Stebbins JW, et al. Reduction of hepatic steatosis in rats and mice after treatment with a liver-targeted thyroid hormone receptor agonist. Hepatology 2009;49:407–17. [DOI] [PubMed] [Google Scholar]
- [16].Chung GE, Kim D, Kim W, et al. Non-alcoholic fatty liver disease across the spectrum of hypothyroidism. J Hepatol 2012;57:150–6. [DOI] [PubMed] [Google Scholar]
- [17].Kim D, Kim W, Joo SK, et al. Subclinical hypothyroidism and low-normal thyroid function are associated with nonalcoholic steatohepatitis and fibrosis. Clin Gastroenterol Hepatol 2018;16:123.e1–31.e1. [DOI] [PubMed] [Google Scholar]
- [18].Eshraghian A, Dabbaghmanesh MH, Eshraghian H, et al. Nonalcoholic fatty liver disease in a cluster of Iranian population: thyroid status and metabolic risk factors. Arch Iran Med 2013;16:584–9. [PubMed] [Google Scholar]
- [19].Jaruvongvanich V, Sanguankeo A, Upala S. Nonalcoholic fatty liver disease is not associated with thyroid hormone levels and hypothyroidism: a systematic review and meta-analysis. Eur Thyroid J 2017;6:208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Assem M, Fawzi M, Ibrahim A, et al. Thyroid dysfunction and insulin resistance in patients with nonalcoholic fatty liver disease. Egypt J Inter Med 2018;30:97–101. [Google Scholar]
- [21].Bano A, Chaker L, Plompen EP, et al. Thyroid function and the risk of nonalcoholic fatty liver disease: the Rotterdam Study. J Clin Endocrinol Metab 2016;101:3204–11. [DOI] [PubMed] [Google Scholar]
- [22].Ding WJ, Wang MM, Wang GS, et al. Thyroid function is associated with non-alcoholic fatty liver disease in chronic hepatitis B-infected subjects. J Gastroenterol Hepatol 2015;30:1753–8. [DOI] [PubMed] [Google Scholar]
- [23].Escudé AM, Pera G, Arteaga I, et al. Relationship between hypothyroidism and non-alcoholic fatty liver disease in the Spanish population. Med Clin (Barc) 2020;154:01–6. [DOI] [PubMed] [Google Scholar]
- [24].Gökmen FY, Ahbab S, Ataoğlu HE, et al. FT3/FT4 ratio predicts non-alcoholic fatty liver disease independent of metabolic parameters in patients with euthyroidism and hypothyroidism. Clinics (Sao Paulo) 2016;71:221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kaltenbach TM, Graeter T, Oeztuerk S, et al. Thyroid dysfunction and hepatic steatosis in overweight children and adolescents. Pediatr Obes 2017;12:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kassem A, Khalil F, Ramadan MR, et al. Association and impact of non-alcoholic fatty liver disease on thyroid function. Int J Curr Res Med Sci 2017;3:87–100. [Google Scholar]
- [27].Kim D, Kim W, Joo SK, et al. Subclinical hypothyroidism and low-normal thyroid function are associated with nonalcoholic steatohepatitis and fibrosis. Clin Gastroenterol Hepatol 2018;16:123–31. [DOI] [PubMed] [Google Scholar]
- [28].Lee KW, Bang KB, Rhee EJ, et al. Impact of hypothyroidism on the development of non-alcoholic fatty liver disease: a 4-year retrospective cohort study. Clin Mol Hepatol 2015;21:372–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ludwig U, Holzner D, Denzer C, et al. Subclinical and clinical hypothyroidism and non-alcoholic fatty liver disease: a cross-sectional study of a random population sample aged 18 to 65 years. BMC Endocr Disord 2015;15:41–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pacifico L, Bonci E, Ferraro F, et al. Hepatic steatosis and thyroid function tests in overweight and obese children. Int J Endocrinol 2013;2013:01–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pagadala MR, Zein CO, Dasarathy S, et al. Prevalence of hypothyroidism in nonalcoholic fatty liver disease. Dig Dis Sci 2012;57:528–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Parikh P, Phadke A, Sawant P. Prevalence of hypothyroidism in nonalcoholic fatty liver disease in patients attending a tertiary hospital in western India. Indian J Gastroenterol 2015;34:169–73. [DOI] [PubMed] [Google Scholar]
- [33].Tahara K, Akahane T, Namisaki T, et al. Thyroid-stimulating hormone is an independent risk factor of non-alcoholic fatty liver disease. JGH Open 2020;4:400–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Xu C, Xu L, Yu C, et al. Association between thyroid function and nonalcoholic fatty liver disease in euthyroid elderly Chinese. Clin Endocrinol 2011;75:240–6. [DOI] [PubMed] [Google Scholar]
- [35].Dimitri P. The role of GLIS3 in thyroid disease as part of a multisystem disorder. Best Pract Res Clin Endocrinol Metab 2017;31:175–82. [DOI] [PubMed] [Google Scholar]
- [36].Zheng J, Zhou Y, Zhang K, et al. Association between nonalcoholic fatty liver disease and subclinical atherosclerosis: a cross-sectional study on population over 40 years old. BMC Cardiovasc Disord 2018;18:147–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Filipovic B, Forbes A, Tepes B, et al. Nonalcoholic fatty liver disease. Can J Gastroenterol Hepatol 2018;2018:01–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lim JS, Mietus-Snyder M, Valente A, et al. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol 2010;7:251–6. [DOI] [PubMed] [Google Scholar]
- [39].Eisenberg MC, Santini F, Marsili A, et al. TSH regulation dynamics in central and extreme primary hypothyroidism. Thyroid 2010;20:1215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Tao Y, Gu H, Wu J, et al. Thyroid function is associated with non-alcoholic fatty liver disease in euthyroid subjects. Endocr Res 2015;40:74–8. [DOI] [PubMed] [Google Scholar]
- [41].Tognini S, Polini A, Pasqualetti G, et al. Age and gender substantially influence the relationship between thyroid status and the lipoprotein profile: results from a large cross-sectional study. Thyroid 2012;22:1096–103. [DOI] [PubMed] [Google Scholar]
- [42].McPherson S, Hardy T, Dufour J-F, et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol 2017;112:740–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Miyake T, Kumagi T, Hirooka M, et al. Body mass index is the most useful predictive factor for the onset of nonalcoholic fatty liver disease: a community-based retrospective longitudinal cohort study. J Gastroenterol 2013;48:413–22. [DOI] [PubMed] [Google Scholar]
- [44].Han C, Li C, Mao J, et al. High body mass index is an indicator of maternal hypothyroidism, hypothyroxinemia, and thyroid-peroxidase antibody positivity during early pregnancy. BioMed Res Int 2015;2015:01–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Biondi B. Thyroid and Obesity: An Intriguing Relationship. 2010;Oxford, UK: Oxford University Press, 3614–3617. [DOI] [PubMed] [Google Scholar]
- [46].Ittermann T, Haring R, Wallaschofski H, et al. Inverse association between serum free thyroxine levels and hepatic steatosis: results from the study of health in pomerania. Thyroid 2012;22:568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Mahajan AS, Lal R, Dhanwal DK, et al. Evaluation of autonomic functions in subclinical hypothyroid and hypothyroid patients. Indian J Endocrinol Metab 2013;17:460–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Rahman F, Hossain S, Biswas SK, et al. Evaluation of thyroid function status in sonographically suggested fatty liver subjects. Bangl J Nucl Med 2015;18:125–30. [Google Scholar]
- [49].van den Berg EH, van Tienhoven-Wind LJ, Amini M, et al. Higher free triiodothyronine is associated with non-alcoholic fatty liver disease in euthyroid subjects: the lifelines cohort study. Metabolism 2017;67:62–71. [DOI] [PubMed] [Google Scholar]
- [50].Doin FC, Rosa-Borges M, Martins MR, et al. Diagnosis of subclinical central hypothyroidism in patients with hypothalamic-pituitary disease by Doppler echocardiography. Eur J Endocrinol 2012;166:631–5. [DOI] [PubMed] [Google Scholar]
- [51].Mantovani A, Nascimbeni F, Lonardo A, et al. Association between primary hypothyroidism and nonalcoholic fatty liver disease: a systematic review and meta-analysis. Thyroid 2018;28:1270–84. [DOI] [PubMed] [Google Scholar]


