Abstract
Background
Acinetobacter species have been a leading cause of nosocomial infections, causing significant morbidity and mortality over the entire world including Ethiopia. The most important features of A. baumannii are its ability to persist in the hospital environment and rapidly develop resistance to a wide variety of antibiotics. This study aimed to determine trend of antimicrobial resistance in Acinetobacter species over a five years period.
Method
A retrospective data regarding occurrence and antimicrobial resistance of Acinetobacter species recovered from clinical specimens referred to the national reference laboratory was extracted from microbiology laboratory data source covering a time range from 2014 to 2018. Socio-demographic characteristics and laboratory record data was analyzed using SPSS 20.
Results
A total of 102 strains of Acinetobacter species were analyzed from various clinical specimens. Majority of them were from pus (33.3%) followed by blood (23.5%), urine (15.6%) and body fluid (11.7%). Significant ascending trends of antimicrobial resistance was shown for meropenem (12.5% to 60.7%), ceftazidime (82.1% to 100%), ciprofloxacin (59.4% to 74.4%), ceftriaxone (87.1% to 98.6%), cefepime (80.0% to 93.3%) and pipracillin- tazobactam (67.8% to 96.3%). However, there was descending trend of antimicrobial resistance for tobramycin (56.5% to 42.8%), amikacin (42.1% to 31.4%) and trimethoprim-sulfamethoxazole (79.0 to 68.2%). The overall rate of carbapenem non-susceptible and multidrug resistance rates in Acinetobacter species were 56.7% and 71.6%.respectively.
Conclusion
A five year antimicrobial resistance trend analysis of Acinetobacter species showed increasing MDR and resistance to high potent antimicrobial agents posing therapeutic challenge in our Hospitals and health care settings. Continuous surveillance and appropriate infection prevention and control strategies need to be strengthened to circumvent the spread of multidrug resistant pathogens in health care facilities.
Introduction
Acinetobacter species are aerobic gram-negative bacilli that can cause healthcare-associated infections and can survive for prolonged periods in the environment and on the hands of healthcare workers [1]. Acinetobacter was first described in 1911 as Micrococcus calcoaceticus by Beijerinck, a Dutch microbiologist who isolated the organism from soil. Since then, it has had several names, nowadays known as Acinetobacter since the 1950s [2,3]. The genus Acinetobacter consists of more than 30 species, of which A. baumannii, and to a lesser extent genomic species 3 and 13TU, are mostly associated with clinical environment and nosocomial infections [3].
According to most recent scientific literature, Acinetobacter species are the second most common non-fermenting gram negative pathogens isolated from clinical specimens after Pseudomonas aeruginosa [2]. Acinetobacter baumannii has become increasingly responsible for causing hospital acquired infections (HAI), particularly in intensive care units (ICUs) [4]. It has been isolated from blood, sputum, skin, pleural fluid, and urine, usually in device associated infections [5]. The species are excellent biofilm producing bacteria, which facilitate their survival in hospital environments and are frequently found on the skin and in the respiratory and urinary tracts of hospitalized patients [6].
Acinetobacter baumannii is one of the most challenging pathogens among ESKAPE pathogens, standing for Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, A.baumannii, P.aeruginosa, and Enterobacteriaceae, capable of “escaping” from common antibacterial treatments due to its particular antibiotic resistance [7]. The bacteria produce naturally occurring AmpC β-lactamases, as well as naturally occurring oxacillinases (OXAs) with carbapenemase activity [8].
Multi-drug resistance (MDR) in Acinetobacter species is defined as non-susceptible to at least 1 agent in ≥3 antimicrobial categories [9]. The species are becoming increasingly resistant to nearly all routinely prescribed antimicrobial agents, including aminoglycosides, fluoroquinolones, and broad-spectrum β-lactams. The majority of strains are resistant to cephalosporin class of antimicrobials and resistance to carbapenems is increasingly reported [10]. Carbapenems which were once the mainstay therapy are no longer effective in controlling the infections caused by this organism. The foremost implication of infection with carbapenem resistant A. baumannii is the need to use "last-line" antibiotics such as colistin, polymyxin B, or tigecycline [11]. Sulbactam, a β-lactamase inhibitor, has good in vitro activity against Acinetobacter species and has been used successfully for treating carbapenem-resistant strains [12].
Risk factors for multi-drug resistant Acinetobacter colonization and infection include prolonged length of hospital stay, exposure to central venous catheterization, urinary catheterization, prior exposure to strong antimicrobials, greater severity of illness, surgery and receipt of invasive procedures [13].
People who have weakened immune systems, chronic lung disease, or diabetes, hospitalized patients, especially very ill patients on a ventilator, those with a prolonged hospital stay, those who have open wounds, or any person with invasive devices like urinary catheters are at greater risk for Acinetobacter infection. The infection can spread to susceptible persons by person-to-person contact or contact with contaminated surfaces [14].
Globally, MDR strains of Acinetobacter species are causing lethal hospital outbreaks often characterized by high morbidity and mortality. Due to the emergence of colistin resistance MDR-A. baumannii in clinical setting, WHO labeled the organism as critical pathogen [15]. A Center for Disease Control and Prevention (CDC) report, in the United States, in 2013, highlighted MDR Acinetobacter as a serious threat that causes ≈7,000 infections and ≈500 deaths each year. Nearly half of the strains isolated from persons with healthcare-associated infections reported to the CDC National Healthcare Safety Network in 2014 were carbapenem-nonsusceptible. Infections with carbapenem-resistant A. baumannii have been associated with death rates as high as 52% [16]. In Ethiopia, even though many studies had been conducted on antimicrobial resistance, there is still limited data showing MDR Acinetobacter infections associated morbidity and mortality at national level. Therefore, this study would provide information on the pattern of multidrug resistant Acinetobacter species isolated from different clinical specimens.
Materials and methods
Study design
A retrospective study design was followed to determine the prevalence of multidrug resistance Acinetobacter species and a trend analysis of antimicrobial susceptibility pattern among clinical specimens referred to the national reference laboratory of the Ethiopian Public Health Institute.
Study period and area
The laboratory recorded data from 2014 to 2018 was analyzed from January 2019 to June 2019 in Addis Ababa. National clinical bacteriology and mycology reference laboratory under Ethiopian Public Health Institute is designed to receive microbiological specimens from St. Paulus Hospital, Aabet Hospital, Ras Desta Damtew Hospital, St. Peter Hospital, Yekatit Hospital, Minilik II Hospital, Federal Police Hospital, Alert Hospital and other health facilities of Addis Ababa city administration.
Inclusion criteria
The study included all Acinetobacter species isolated from all ages and microbiological specimens referred to national reference laboratory during the study period. However, incomplete clinical information on patients and antimicrobial susceptibility testing report that did not comply Clinical and Laboratory Standards Institute guideline was excluded.
Data extraction method
A laboratory recorded routine data in which all samples types analyzed was incorporated in the analysis. A standardized questionnaire was used to collect socio-demographic characteristics, clinical history, hospitalization and antibiotic treatment. All records of Acinetobacter species and antimicrobial susceptibility test was collected in a questionnaire and entered to SPSS version 20.
Statistical analysis
Statistical analysis was performed using the SPSS version 20. Chi-square test and descriptive statistics (cross tab, frequency and proportion) were used to compare the trend of antimicrobial resistance rate, prevalence of MDR Acinetobacter species and resistance rates in empirically treated and untreated patients. P-value less than 0.05 were considered statistically significant.
Ethical consideration
The study was conducted after ethical clearance was obtained from Ethiopian Public Health Institute (EPHI) scientific and ethical review committee (SERC). The IRB of EPHI has given us a waiver of consent to conduct the study.
Data quality assurance
Retrospective check on quality record of media preparation per manufacturer instruction and laboratory Standard Operating Procedures (SOP) followed was conducted. We verified whether media, regents and antimicrobial agents have met expiration date and quality control parameters per clinical laboratory standard institute (CLSI). In addition, performance of quality control strains ATCC and sample storage system in laboratory was assessed from past records.
Results
Specimen source and demographical characteristics
A total of 102 Acinetobacter strains were isolated from various clinical specimens. Sixty percent of them were from males and 40% of them were from females with a mean age of 30.79 (SD±19.18). Pus/wound was the major source of the isolates (33.3%), followed by blood (23.5%), urine (15.6%), body fluid (11.7%), ear (4.9%), cerebrospinal fluid (3.9%), tracheal aspirate (1.9%), sputum (0.9%) and throat (0.9%).
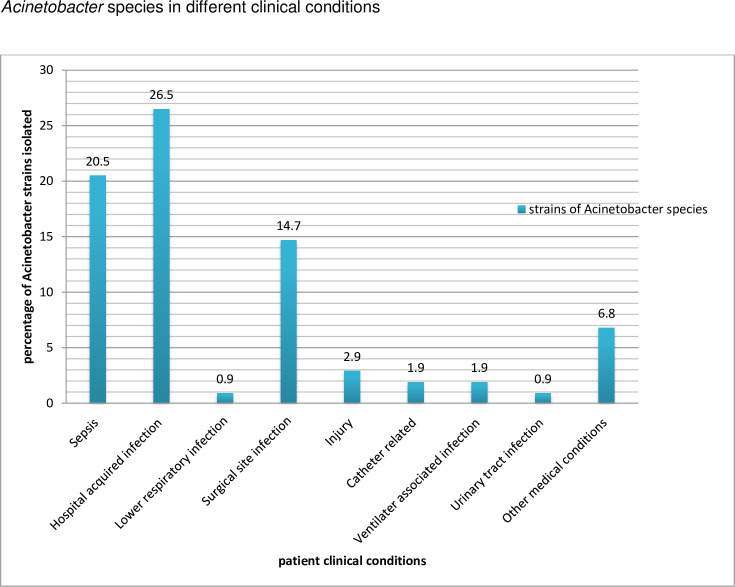
Regarding the specimen and isolate sources, the majority were from St. Paulus Hospital (30.4%) followed by Aabet Hospital (23.5%), Ras Desta Damtew Hospital (16.7%), St. Peter Hospital (9.8%), Yekatit 12 Hospital (4.9%), Minilik II Hospital (3.9%), Federal Police Hospital (2.9%), Alert Hospital (0.09%) and others from health facilities (6.8%). Acinetobacter species were mostly recovered from hospital acquired infection (HAI) (26.5%) followed by sepsis (20.5%) and surgical site infection (14.7%). The distribution of Acinetobacter species according to different clinical diagnosis of patients is indicated in Fig 1.
Fig 1. Distribution of Acinetobacter species in relation to clinical conditions.
Empirical treatment practice
Among the total of 102 patients, 26.5% of them provided specimen without initiation of antimicrobial therapy while 73.5% of them have taken antibiotic treatment empirically. Ceftriaxone was the most frequently prescribed (15.7%) antibiotics followed by ciprofloxacin and meropenem (7.8%). In addition, 84.3% of the patients have taken combined drugs (two or more antibiotics) before providing biological specimen. Vancomycin and ceftazidime were the most combined drugs prescribed that accounted for 9.8% (10/102) as shown in Table 1.
Table 1. Empirical treatment status of patients practice in health care settings from 2014–2018, Ethiopia.
| Trends of antibiotics prescription (n = 102) | ||
|---|---|---|
| Prescription of antibiotics | Frequency | Percent |
| No antibiotics | 27 | 26.5 |
| Gentamycin | 4 | 3.9 |
| gentamycin+ceftriaxone+Vancomycin | 1 | 1.0 |
| gentamycin+cotrimoxzole+Augmentinn | 1 | 1.0 |
| Cefepime | 2 | 2.0 |
| Meropenem | 8 | 7.8 |
| Meropenem +Vancomycin | 3 | 2.9 |
| Ceftriaxone | 16 | 15.7 |
| cefepime +ceftriaxone | 1 | 1.0 |
| ceftriaxone+ciprofloxacin+Vancomycin | 4 | 3.9 |
| ceftraixone+ciprofloxacin +cotrimoxazole+cefepime | 1 | 1.0 |
| ceftriaxone +Vancomycin | 3 | 2.9 |
| ceftraiaxone+Vancomycin +ceftazidime | 1 | 1.0 |
| Ciprofloxacin | 8 | 7.8 |
| Vancomycin | 5 | 4.9 |
| Cftazidime +Vancomycin | 10 | 9.8 |
| Augmentin | 2 | 2.0 |
| OTHERS | 5 | 4.9 |
| Total | 102 | 100.0 |
Trends of Antimicrobial resistance (AMR)
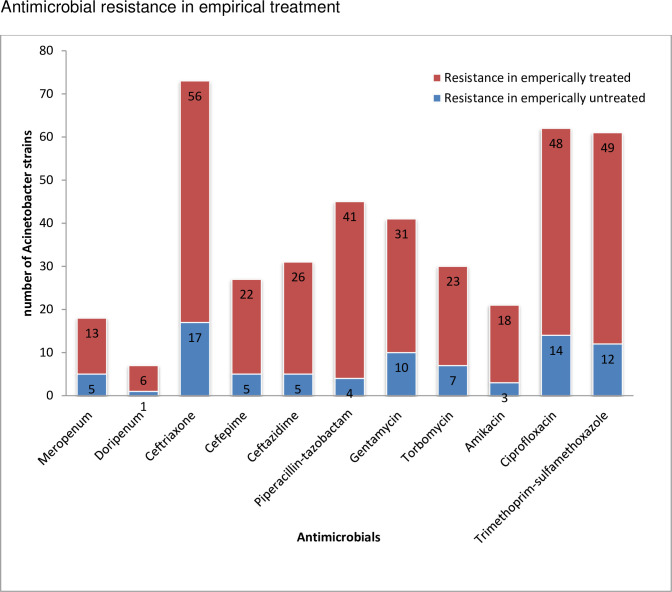
All the isolates were tested for antimicrobials recommended for non sacrolytic bacteria according to CLSI guidelines. The antimicrobial resistance among isolates from empirically treated patients was proportionally high compared to empirically untreated patients [Fig 2].
Fig 2. Antimicrobial resistance among strains isolated from empirically treated and untreated patients referred from health facilities to Ethiopian public health institute, 2014–2018.
The trends of antimicrobial resistance in the last two years (2017 to 2018) were as follows.
Group A antimicrobial agents
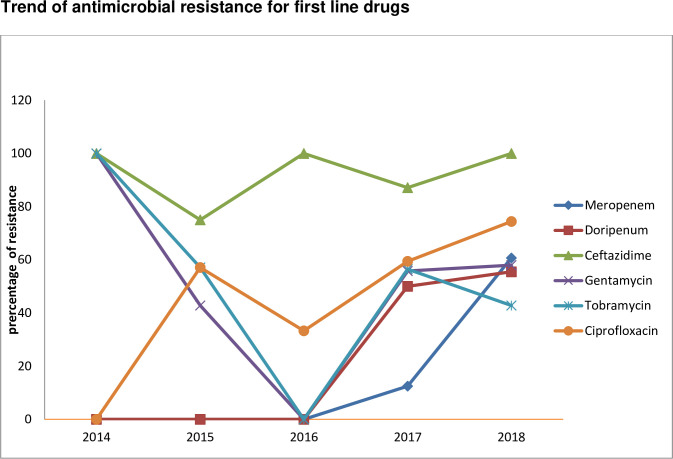
An increasing trend of antimicrobial resistance among antibiotics appropriate for inclusion in a routine, primary testing panel as well as routine reporting of the result for Acinetobacter species was observed as follows: meropenem (12.5% to 60.7%), doripenem (50.0% to 55.5%), ceftazidime (82.1% to 100%), ciprofloxacin (59.4% to 74.4%), gentamycin (55.8% to 58.0%); however, a decreasing trend of resistance was observed for tobramycin (56.5% to 42.8%) [Fig 3]. In addition, the prevalence of carbapenem non susceptible Acinetobacter species in all tested specimen types was 56.7% (21/37).
Fig 3. Trends of antimicrobial resistance for first line drugs among the Acinetobacter species from 2014 to 2018.
Group B antimicrobial agents
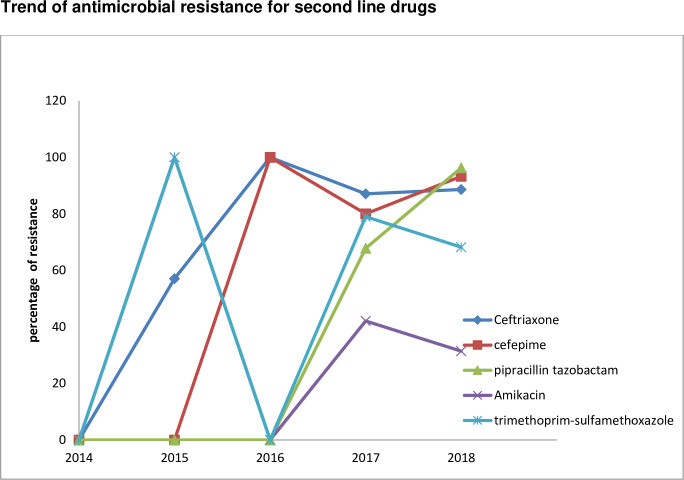
Antimicrobial resistance for second option of antibiotics have also shown ascending trend: ceftriaxone (87.1% to 88.6%), cefepime (80.0% to 93.3%), and pipracillin tazobactam (67.8% to 96.3%), Amikacin (42.1% to 31.4%), trimethoprim-sulfamethoxazole (79.0 to 68.2%) [Fig 4].
Fig 4. Trends of antimicrobial resistance for second option of drugs among the Acinetobacter species from 2014 to 2018.
In vitro activity of meropenem and doirpenem against Acinetobacter species were effective from 2014–2016; however, the resistance rate increased from 2017 to 2018.
The overall trends of antimicrobial classes over period of time are shown in Table 2.
Table 2. Trend of antimicrobial resistance in Acinetobacter species from 2014 to 2018.
| Antimicrobial classes | Antimicrobials | Disk content | % Resistance per year | ||||
|---|---|---|---|---|---|---|---|
| 2014 | 2015 | 2016 | 2017 | 2018 | |||
| Carbapenem | Meropenum | 10ug | 0 | 0 | 0 | 12.5 | 60.7 |
| Doripenum | 10ug | 0 | 0 | 0 | 50.0 | 55.5 | |
| Cephalosporin | Ceftriaxone | 30ug | 0 | 57.1 | 100 | 87.1 | 98.6 |
| Cefepime | 30ug | 0 | 0 | 100 | 80 | 93.3 | |
| Ceftazidime | 30ug | 100 | 75 | 100 | 82.1 | 100 | |
| Beta lactams | Piperacillin-tazobactam | 100/10ug | 0 | 0 | 0 | 67.8 | 96.3 |
| Aminoglycosides | Gentamycin | 10ug | 100 | 42.8 | 0 | 55.8 | 58.0 |
| Tobramycin | 10ug | 100 | 57.1 | 0 | 56.5 | 42.8 | |
| Amikacin | 30ug | 0 | 100 | 0 | 42.1 | 31.4 | |
| Fluoroquinoles | Ciprofloxacin | 5ug | 0 | 57.1 | 33.3 | 59.4 | 74.4 |
| Folate pathway inhibitors | Trimethoprim-sulfamethoxazole | 1.25/23.75ug | 0 | 100 | 0 | 79.0 | 68.2% |
Discussions
Infection due to Acinetobacter species is a major challenge within the health care facilities and the community in general due to their high drug resistance even to the high potent drugs such as carbapenems. In our study 102 Acinetobacter species strains were analyzed to investigate the trends of antimicrobial resistance. In the analysis we found that more than 70% of patients had taken antibiotics empirically before getting confirmed culture and antimicrobial susceptibility testing (AST) result showing Acinetobacter infection. This problem is reported in many published works elsewhere and this could contribute to the rise of exposure to multidrug resistance infection [17,18].
The observed increase in the prevalence of MDR Acinetobacter was statically significant over a period of time (chi- square, = 15.8, p value = 0.003). This might be due to the fact that the laboratory capacity to detect and conduct antimicrobial resistance testing has increased over the past years.
The overall prevalence of MDR among the isolates was 71.6% which is comparable with a report from Saudi Arabia (74%) [19] and Bosnia and Herzegovina (78.4%) [20]. However, the current finding was higher than the study conducted in Jimma which isolated the MDR Acinetobacter species from wound specimen at a rate of 57.2% [21], and in Iran at a rate of 56.7% [22]. The antimicrobial resistance profile and prevalence of hospital associated pathogens varies from place to place.
The prevalence of MDR Acinetobacter from the current study is found to be lower than a previous study done in Selected Referral Hospitals in Ethiopia involving surgical site of infection (95.7%) [23] This could be due the increasing trend of early awareness of clinicians on the treatment of Acinetobacter infection with appropriate drugs based on the microbiology laboratory identification and antimicrobials susceptibility testing results. Currently, carbapenem resistance Acinetobacter is included as target pathogen in the national AMR surveillance system in Ethiopia and this might also have contributed the decrease in the MDR status of Acinetobacter species identified in the clinical setting.
The trend analysis in antimicrobial resistance showed that in the year 2018 there was a high resistance rate for ceftriaxone (98.6%), cefepime (93.3%), ceftazidime (100%),and ciprofloxacin (74.4%) which is comparable with similar study in neighboring country Sudan which reported resistance rates as follows: ceftriaxone (95%), cefepime (92%), ceftazidime (96%) and ciprofloxacin (91%) [24].
Compared to the previous years, carbapenem resistance in Acinetobacter has increased by more than 50% by 2018 and this finding is comparable with previous studies conducted in East Africa [25] and in south East Asia [26].
The resistance to aminoglycoside from the current study was found to vary among the different agents under this antimicrobial class. While resistance to gentamycin was shown to increase from 48% to 58% from 2015 to 2018, resistance to tobramycin and amikacin was below 50%. The resistance rate reported here for tobramycin is in line with a study report from Morocco [27].
Conclusion
In conclusion, there has been an increasing trend of antimicrobials resistance in Acinetobacter species isolated from different sample source. Acinetobacter infection would remain a therapeutic challenge in our Hospitals and health care settings due to the increasing rate of Acinetobacter species with traits of MDR and resistance to high potent antimicrobial agents. Continuous surveillance and appropriate infection prevention and control program needs to be strengthened to circumvent the spread of these pathogens in the health care facilities.
Limitation of the study
Although Colistin (polymyxin E) is an antibiotic used as a last-resort for multidrug-resistant gram negative infections’ including Acinetobacter, this agent was not tested in the present study. Further molecular characterization of Acinetobacter exploring the genes responsible for MDR was not performed. All strains were not tested against carbapenem due to lack of supplies for first three years of the study.
Supporting information
(ZIP)
Acknowledgments
We express our grateful appreciation to national clinical bacteriology and mycology reference laboratory staffs Amete mihret, Negga Asamene, Rajaha Abubeker, Surafel Fentaw,Abebe Assefa, Degefu Beyene, Dejene Shiferaw, Tesfa Addis, Yonas Mekonen,Yohanise Yitagesu,Abera Abdeta, Meseret Assefa and Etsehiwot Adamu for their technical support.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Sunenshine RH, Wright MO, Maragakis LL, Harris AD, Song X, Hebden J, et al. Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalization. Emerging infectious diseases, 2007; 13(1):97. 10.3201/eid1301.060716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osman EA. Nagwa El Mustafa El Amin. High prevalence of multidrug resistant Acinetobacter species in Khartoum Intensive Care Units (ICUs). American Journal of Research Communication, 2015:3(2):35–42. [Google Scholar]
- 3.Turton JF, Shah J, Ozongwu C, Pike R. Incidence of Acinetobacter species other than A. baumannii among clinical isolates of Acinetobacter: evidence for emerging species. Journal of clinical microbiology, 2010; 48(4):1445–9. 10.1128/JCM.02467-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anitha M, Monisha DM, Mohamed A Sulthan, Pandurangan S. Emergence and Prevalence of Acinetobacter baumannii in Tertiary Care Hospital Settings. Sch. Acad. J. Biosci., 2016; 4(4A):335–341. [Google Scholar]
- 5.Mayasari E, Siregar C. Prevalence of Acinetobacter baumannii isolated from clinical specimens in adam malik hospital. Majalah Kedokteran Andalas, 2015; 37(1):1–7. [Google Scholar]
- 6.Poorzargar P, Javadpour S, Karmostaji A. Distribution and antibiogram pattern of Acinetobacter infections in Shahid Mohammadi Hospital, Bandar Abbas, Iran. Bimonthly Journal of Hormozgan University of Medical Sciences, 2017; 20(6):396–403. [Google Scholar]
- 7.Xie R, Zhang XD, Zhao Q, Peng B, Zheng J. Analysis of global prevalence of antibiotic resistance in Acinetobacter baumannii infections disclosed a faster increase in OECD countries. Emerging microbes & infections, 2018:7(1):31. 10.1038/s41426-018-0038-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kock MM, Bellomo AN, Storm N, Ehlers MM. Prevalence of carbapenem resistance genes in Acinetobacter baumannii isolated from clinical specimens obtained from an academic hospital in South Africa. Southern African Journal of Epidemiology and Infection, 2013; 28(1):28–32. [Google Scholar]
- 9.Magiorakos AP, Srinivasan A, Carey RT, Carmeli Y, Falagas MT, Giske CT, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clinical microbiology and infection, 2012; 18(3):268–81. 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 10.Sohail M, Rashid A, Aslam B, Waseem M, Shahid M, Akram M, et al. Antimicrobial susceptibility of Acinetobacter clinical isolates and emerging antibiogram trends for nosocomial infection management. Revista da Sociedade Brasileira de Medicina Tropical, 2016; 49(3):300–4. 10.1590/0037-8682-0111-2016 [DOI] [PubMed] [Google Scholar]
- 11.Nath H, Barkataki D. Prevalence of ESBL and MBL producing Acinetobacter Isolates in Clinical Specimens in Tertiary Care Hospital, Assam, India. Int. J. Curr. Microbiol. App. Sci., 2016; 5(11):515–22. [Google Scholar]
- 12.Oliveira MS, Prado GV, Costa SF, Grinbaum RS, Levin AS. Ampicillin/sulbactam compared with polymyxins for the treatment of infections caused by carbapenem-resistant Acinetobacter species. Journal of antimicrobial chemotherapy, 2008; 61(6):1369–75. [DOI] [PubMed] [Google Scholar]
- 13.Aedh A. Prevalence of Acinetobacter infections among Intensive Care Unit’s patients in Najran. Int. J. Curr. Res. Med. Sci., 2017; 3(5):122–8. [Google Scholar]
- 14.Falagas ME, Karveli E. The changing global epidemiology of Acinetobacter baumannii infections: a development with major public health implications. Clin Microbiol Infect, 2007; 13: 117–119. 10.1111/j.1469-0691.2006.01596.x [DOI] [PubMed] [Google Scholar]
- 15.Abadi AT, Rizvanov AA, Haertlé T, Blatt NL. World Health Organization report: current crisis of antibiotic resistance. BioNanoScience, 2019; 9(4):778–88. [Google Scholar]
- 16.Bulens SN, Sarah HY, Walters MS, Jacob JT, Bower C, Reno J, et al. Carbapenem-Nonsusceptible Acinetobacter baumannii, 8 US Metropolitan Areas, 2012–2015. Emerging infectious diseases. 2018; 24(4):727. 10.3201/eid2404.171461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Dorzi HM, Asiri AM, Shimemri A, Tamim HM, Al Johani SM, Al Dabbagh T, et al. Impact of empirical antimicrobial therapy on the outcome of critically ill patients with Acinetobacter bacteremia. Annals of thoracic medicine, 2015; 10(4):256. 10.4103/1817-1737.164302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prata-Rocha ML, Gontijo-Filho PP, de Melo GB. Factors influencing survival in patients with multidrugresistant Acinetobacter baumannii infection. The Brazilian Journal of Infectious Diseases, 2012; 16(3):237–41. [PubMed] [Google Scholar]
- 19.Almaghrabi MK, Joseph MR, Assiry MM, Hamid ME. Multidrug-resistant Acinetobacter baumannii: an emerging health threat in Aseer Region, Kingdom of Saudi Arabia. Canadian Journal of Infectious Diseases and Medical Microbiology, 2018; 2018. 10.1155/2018/9182747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rebic V, Masic N, Teskeredzic S, Aljicevic M, Abduzaimovic A, Rebic D. The importance of Acinetobacter species in the hospital environment. Medical Archives, 2018; 72(5):325. 10.5455/medarh.2018.72.330-334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Godebo G, Kibru G, Tassew H. Multidrug-resistant bacterial isolates in infected wounds at Jimma University Specialized Hospital, Ethiopia. Annals of clinical microbiology and antimicrobials, 2013; 12(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.KHALTABADI FR, Moniri R, Dastehgoli K. Multi-drug resistant Acinetobacter-derived cephalosporinase and OXAsetC genes in clinical specimens of Acinetobacter species isolated from teaching hospital. Iran. Jundishapur Journal of Microbiology (Jjm), 2013, 6 (2); 181–185. [Google Scholar]
- 23.Dessie W, Mulugeta G, Fentaw S, Mihret A, Hassen M, Abebe E. Pattern of bacterial pathogens and their susceptibility isolated from surgical site infections at selected referral hospitals, Addis Ababa, Ethiopia. International journal of microbiology, 2016; 2016. 10.1155/2016/2418902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omer MI, Gumaa SA, Hassan AA, Idris KH, Ali OA, Osman MM, et al. Prevalence and resistance profile of Acinetobacter baumannii clinical isolates from a private hospital in Khartoum, Sudan. Am J Microbiol Res., 2015; 3(2):76–9. [Google Scholar]
- 25.Ssekatawa K, Byarugaba DK, Wampande E, Ejobi F. A systematic review: the current status of carbapenem resistance in East Africa. BMC research notes, 2018; 11(1):629. 10.1186/s13104-018-3738-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu LY, Apisarnthanarak A, Khan E, Suwantarat N, Ghafur A, Tambyah PA. Carbapenem-resistant Acinetobacter baumannii and Enterobacteriaceae in south and Southeast Asia. Clinical microbiology reviews, 2017; 30(1):1–22. 10.1128/CMR.00042-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uwingabiye J, Frikh M, Lemnouer A, Bssaibis F, Belefquih B, Maleb A, et al. Acinetobacter infections prevalence and frequency of the antibiotics resistance: comparative study of intensive care units versus other hospital units. Pan African Medical Journal, 2016; 23(1). 10.11604/pamj.2016.23.191.7915 [DOI] [PMC free article] [PubMed] [Google Scholar]