Abstract
Background:
Diffusion-weighted imaging (DWI) has shown promise to screen for breast cancer without a contrast injection, but image distortion and low spatial resolution limit standard single-shot DWI. Multishot DWI methods address these limitations but introduce shot-to-shot phase variations requiring correction during reconstruction.
Purpose:
To investigate the performance of two multishot DWI reconstruction methods, multiplexed sensitivity encoding (MUSE) and shot locally low-rank (shot-LLR), compared to single-shot DWI in the breast.
Study Type:
Prospective.
Population:
A total of 45 women who consented to have multishot DWI added to a clinically indicated breast MRI.
Field Strength/Sequences:
Single-shot DWI reconstructed by parallel imaging, multishot DWI with four or eight shots reconstructed by MUSE and shot-LLR, 3D T2-weighted imaging, and contrast-enhanced MRI at 3T.
Assessment:
Three blinded observers scored images for 1) general image quality (perceived signal-to-noise ratio [SNR], ghosting, distortion), 2) lesion features (discernment and morphology), and 3) perceived resolution. Apparent diffusion coefficient (ADC) of the lesion was also measured and compared between methods.
Statistical Tests:
Image quality features and perceived resolution were assessed with a mixed-effects logistic regression. Agreement among observers was estimated with a Krippendorf’s alpha using linear weighting. Lesion feature ratings were visualized using histograms, and correlation coefficients of lesion ADC between different methods were calculated.
Results:
MUSE and shot-LLR images were rated to have significantly better perceived resolution (P < 0.001), higher SNR (P < 0.005), and a lower level of distortion (P < 0.05) with respect to single-shot DWI. Shot-LLR showed reduced ghosting artifacts with respect to both MUSE (P < 0.001) and single-shot DWI (P < 0.001). Eight-shot DWI had improved perceived SNR and perceived resolution with respect to four-shot DWI (P < 0.005).
Data Conclusion:
Multishot DWI enables increased resolution and improved image quality with respect to single-shot DWI in the breast. Shot-LLR reconstructs multishot DWI with minimal ghosting artifacts. The improvement of multishot DWI in image quality increases with an increased number of shots.
CONTRAST-ENHANCED MAGNETIC RESONANCE IMAGING (CE-MRI) of the breast is a highly sensitive method, providing a detailed depiction of lesion morphology, but is only approved for breast cancer screening in women with a greater than a 20% lifetime risk of developing breast cancer.1,2 However, recent studies strongly support the use of CE-MRI for screening a much wider population of women due to its sensitivity to prognostically significant cancers,3,4 and its increased sensitivity with respect to mammography in women with dense breast tissue.5 One primary obstacle to the wider adoption of MRI for breast cancer screening is the incompatibility of conventional CE-MRI protocols for wider population-based screening. While shortening times for CE-MRI studies by using abbreviated protocols is one step toward a more screening-amenable test, removing the need for intravenous contrast injections may have a substantial impact on increasing MRI accessibility.6–8 Additionally, concerns about receiving gadolinium contrast injections year after year may currently be affecting compliance in the high-risk population.9, 10
Diffusion-weighted imaging (DWI), which has been widely investigated for a range of breast cancer imaging roles, has the potential to serve as the primary imaging technique in a noncontrast-enhanced breast cancer screening MRI protocol.11,12 However, the single-shot echo-planar imaging (EPI) trajectory utilized in conventional DWI for motion-robustness has a large echo spacing and a long readout window, which causes degradation of image quality, including off-resonance-induced distortion and T2-decay-induced blurring. These image quality and resolution limitations, particularly in comparison to CE-MRI, are a major obstacle to clinical adoption of DWI for breast cancer detection and characterization.13
Many advanced DWI methods14–17 have been developed with the aim of improving overall image quality and resolution. One promising direction to reduce the artifacts in single-shot DWI (ss-DWI) is to acquire k-space in multiple segments with shortened readout and echo spacing while also enabling higher resolution. The trade-off of breaking the EPI trajectory into multiple segments is that motion will result in phase variations between the acquired segments of k-space. The phase variation between the segments must then be corrected during reconstruction. In readout-segmented methods, the EPI trajectory is divided into segments that are shortened in the readout direction. Readout-segmented DWI (rs-DWI) of the breast has been investigated with promising results.18–21
An alternative method for segmenting the trajectory is interleaved DWI (which we refer to as multishot DWI in this article) in which the EPI trajectory is broken into multiple segments but along the phase-encoding direction. Compared to rs-DWI, the polarity of the readout gradient in multishot DWI does not need to switch as frequently, meaning potentially better distortion performance.22 However, the shot-to-shot phase variations result in severe ghosting artifacts as opposed to blurring in rs-DWI. The improvement in image quality and available resolution should become greater as the number of shots is increased; however, this also increases the complexity of resolving phase variations.
Multiplexed sensitivity-encoding (MUSE) is a multishot DWI reconstruction method in which low-resolution phase from each shot is determined with a sensitivity encoding (SENSE) reconstruction without using extra navigator data.16 A recent study of MUSE DWI with two shots and a parallel imaging factor of 2 in the breast in 30 patients reported consistent tumor apparent diffusion coefficients (ADCs) and improved signal-to-noise ratio (SNR) with respect to single-shot DWI.23 The recently developed shot locally low-rank (shot-LLR) is an alternative multishot reconstruction method in which underlying assumptions about the relationships between different shots are used to bypass the phase-estimation step,24 enabling a higher number of shots. Shot-LLR can handle more complicated phase variations and has shown promising results for high-resolution DWI, but its performance has not been widely investigated in the breast.
The aim of this study was to characterize the performance of these two multishot DWI methods, shot-LLR and MUSE, including the effect of increasing the number of shots, in comparison to single-shot DWI in patients undergoing clinically-indicated breast MRI.
Materials & Methods
Patients
From October 2018 through January 2019, patients undergoing a clinically-indicated breast MRI at a single site were recruited to have a multishot DWI acquisition added to their exam. All recruitment followed Institutional Review Board (IRB) policies and patients who agreed to participate signed written informed consent. A breast radiologist with 28 years of breast MRI expertise (B.D.) analyzed each case to identify lesions and determine the lesion type based on prior imaging findings or pathology from surgical excision. We recorded examination indications and lesion size was measured based on the longest diameter of the lesion in the axial plane on the peak MRI series by a breast radiologist with 6 years of breast MRI experience (S.P.).
Image Acquisition and Reconstruction
For each consented patient, a multishot DWI acquisition was added to our institution’s standard-of-care scan protocol, which included a single-shot DWI acquisition. Both DWI sequences were acquired prior to the injection of contrast for the CE-MRI acquisition. Single-shot DWI utilized a parallel imaging factor of four with eight averages. For the first 15 patients, the multishot acquisition used four shots, while in the next 30 patients it used eight shots. All multishot acquisitions utilized a single repetition (average); thus, the number of shots per slice for eight-shot DWI matched that of the single-shot DWI acquisition. The imaging parameters of the multishot acquisition were matched to those of the single-shot DWI acquisition except for in-plane resolution, which was 2.1 × 2.1 mm2 for single-shot DWI and 1 × 1 mm2 for the multishot DWI acquisitions (Table 1). All scans were acquired with two b-values (0 and 600 s/mm2) and readout direction anterior–posterior, utilizing a dual-volume shim and performed with a 3T GE MR750 scanner (General Electric Healthcare, Waukesha, WI) with a 16-channel Sentinelle breast coil (In Vivo, Orlando, FL) and water-only excitation. The standard protocol also included a 3D T2-weighted acquisition (0.9 × 0.9 × 2 mm3) and a CE-MRI acquisition (0.7 × 0.7 × 1.2 mm3). All sequences were acquired bilaterally in the axial plane.
TABLE 1.
Scan Parameters of the Single-Shot and Multishot DWI Sequences
| Sequences | FOV (cm) | In-plane resolution (mm) | Slice thickness (mm) | b-value (s/mm)2 | Diffusion-encoding direction | Number of shots | Repetitions | Parallel imaging factor | Effective echo spacing (us) | Scan time |
|---|---|---|---|---|---|---|---|---|---|---|
| Single-shot DWI | 34 | 2,13 | 5 | 0,600 | 3-in-1 | 1 | 8 | 4 | 165 | 2 min 18 sec |
| Four-shot DWI | 34 | 1 | 5 | 0,600 | 3-in-1 | 4 | 1 | 1 | 258 | 1 min 16 sec |
| Eight-shot DWI | 34 | 1 | 5 | 0,600 | 3-in-1 | 8 | 1 | 1 | 131 | 2 min 22 sec |
“3-in-1” means diffusion gradients were applied simultaneously along the three orthogonal (x,y,z) axes.
Single-shot data were reconstructed using the product parallel imaging algorithm (array spatial sensitivity encoding technique, ASSET) (General Electric Healthcare). MUSE and shot-LLR reconstructions were applied to the multishot data. The online product MUSE reconstruction (General Electric Healthcare)16 was used in this study. Shot-LLR was implemented in Python based on the Berkeley Advanced Reconstruction Toolbox25 with a regularization parameter of 0.0008.24 We utilized the algorithm provided by GE’s Orchestra (software development kit v. 1.7–1) for Nyquist artifact correction and ramp sampling correction before the shot-LLR reconstruction.
Observer Study
We conducted a blinded observer study to assess the performance of the three DWI methods, with three areas of focus: 1) image quality (perceived SNR, ghosting, distortion); 2) lesion appearance (discernment and morphology); and 3) perceived resolution. Three different breast radiologists (D.I., S. P., D.S.) with 28, 6, and 3 years, respectively, of breast MRI experience participated in the study. Each case in the study included three types of diffusion-weighted images (ss-DWI, MUSE, shot-LLR). In each of the three parts of the study, images from all cases and methods were presented in a randomized order, and observers, who were blinded to the case number and method, assessed each image independently. Observers did not have access to images from previous examinations or any other information about the patient or diagnosis.
IMAGE QUALITY.
The first part of the reader study focused on overall image quality. All 45 acquired cases were included in this part of the analysis. Readers reviewed a single axial bilateral image central in the breast and rated 1) perceived SNR, 2) level of ghosting artifacts, and 3) level of distortion. All three categories utilized a 5-point rating scale with 1 worst and 5 best (Fig. 1, part 1).
FIGURE 1:

Illustration of the three parts (image quality, lesion analysis, and perceived resolution) in the observer study. A 5-point rating scale was used to assess three features of image quality (perceived SNR, level of ghosting, and distortion) (Part 1), and two features of lesion appearance (conspicuity and depiction of morphology) (Part 2). Perceived resolution was determined by radiologists who compared the b = 0 s/mm2 image from the DWI scan with the patient’s high-resolution T2-weighted scan resampled to 16 different resolutions and chose the resampled T2 image in which depiction of features best matched that of the b = 0 s/mm2 image.
LESION ANALYSIS.
The second part of the observer study focused on lesion features of DWI in comparison to CE-MRI as well as lesion ADC. Only cases in which a benign or malignant lesion had been identified were included in this part of the study. The reader was shown each diffusion-weighted image side-by-side with the respective high-resolution peak contrast image from the CE-MRI acquisition. Images were chosen from a central slice through the lesion and best matched between the DWI and contrast-enhanced images. Each contrast-enhanced image had an arrow pointing to the lesion of interest, but the diffusion-weighted image did not. The reader rated lesion conspicuity and depiction of lesion morphology on the diffusion-weighted images with respect to these two lesion characteristics on the contrast-enhanced images. As with the image quality ratings, a 5-point rating scale was utilized for lesion feature assessment with a rating of 1 indicating the lowest level of performance and a rating of 5 indicating the highest level of performance. Specific rating descriptions are shown in Fig. 1, part 2. For lesion morphology, the readers gave a single rating for an overall depiction of morphology vs. focusing on a specific morphologic feature.
Independent from the observer study, we calculated ADC in a region of interest (ROI) within the lesion on the designated central slice for each DWI method. ROIs were drawn based on consensus of two radiologists (S.C., S.O.) with breast MRI expertise on the qualitative diffusion-weighted image in the region with the highest signal, avoiding regions exhibiting necrosis or hemorrhage or signal voids due to biopsy markers.12 We calculated the ADC values for each voxel and compared the mean ADC values across the drawn ROI.
PERCEIVED RESOLUTION.
The final component of the reader study included all patients and focused on the perceived resolution. In this assessment, a single nondiffusion-weighted image (b = 0 s/mm2) from the DWI scan was compared to an aligned T2-weighted image downsampled at different resolutions (Fig. 1, part 3). For each case, the high-resolution T2-weighted images from the 3D T2-weighted scan (0.9 × 0.9 × 2 mm3) were first downsampled along the slice-encoding direction to match the slice thickness of the diffusion-weighted images (5 mm). Then the resultant images were further downsampled in-plane to 15 different resolutions from 1 × 1 mm2 to 4.5 × 4.5 mm2 with 0.25 mm spacing using bilinear interpolation. The reader was shown the nondiffusion-weighted image from the EPI scan along with the 16 T2-weighted images (the original image without in-plane downsampling and 15 images with an increasing level of downsampling). The reader was asked to assess the depiction of structures in the nondiffusion-weighted image from the EPI scan against their depiction in each of the series of downsampled T2 images, and to choose the single downsampled T2 image in which the depiction of structures across the entire field of view (FOV) best matched that of the nondiffusion-weighted image. The resolution of the matched image (perceived resolution) was used as the basis for statistical analysis.
Statistical Analysis
For image features and perceived resolution assessment, a mixed-effects logistic regression was used with fixed effects of acquisition/reconstruction and shot numbers and random effects of observer and subjects. For image feature analysis, ratings were dichotomized into ratings of 4 or 5 vs. ratings of 1, 2, and 3. P < 0.05 was considered statistically significant. Agreement among observers was estimated for the three image quality features and perceived resolution with a Krippendorf’s alpha using linear weighting. Mixed effects logistic regression and Krippendorf’s alpha were calculated using Stata 16.1 (StataCorp, College Station, TX). For lesion features, histograms were utilized to identify trends in the rankings and benign and malignant lesions were analyzed independently. Pearson correlations of mean lesion ADC between different methods were calculated and tested against the null hypothesis of zero correlation.
Results
Patients and Lesions
In total, 45 patients (age range 28–73 years, median age 51 years) provided written informed consent and had the multishot DWI acquisition added to their exam. None of the cases were excluded from the study except one for the lesion analysis described below. The clinical examination indications for the 45 cases were as follows: 28 high-risk screening, six evaluation of extent of known tumor, five evaluation of a breast abnormality, four screening for reasons other than high risk, and two evaluation of response to neoadjuvant chemotherapy. Fifteen lesions (six malignant, nine benign) from 15 cases were identified on the peak CE-MRI data. Pathology, confirmed through surgical excision or comparison to prior imaging studies, was as follows: one ductal carcinoma in situ (DCIS), two invasive ductal cancer (IDC), one triple negative breast cancer (TNBC), one recurrent phyllodes tumor, four benign lymph nodes, one benign mass, one radial scar, one benign fat necrosis, one fibroadenomatoid change, and one mixed inflammatory infiltrates. One malignant lesion, an extensive nonmass enhancement, was difficult to discern on both the CE and diffusion-weighted images and was not included in lesion analysis. Lesion size ranged from 0.2 to 3.4 cm with a mean value of 1.2 cm.
Observer Study
IMAGE QUALITY.
Representative cases demonstrating the overall image quality of the three methods with respective image quality ratings are shown in Fig. 2. For perceived SNR, both MUSE (P = 0.002) and shot-LLR (P = 0.001) were rated significantly higher than single-shot, but were not different from each other (P = 0.73) and eight-shot cases were rated as significantly better than four-shot cases (P = 0.004) (Fig. 3a). Shot-LLR was rated as having significantly fewer ghosting artifacts than both single-shot DWI (P < 0.001) and MUSE (P < 0.001), and MUSE was not significantly better than single-shot DWI (P = 0.23) (Fig. 3b). Across all three observers for both four-shot and eight-shot cases, shot-LLR received a rating of 5, indicating no ghosting artifacts, for 42 cases compared with six for MUSE and 15 for single-shot imaging (of 45 total cases). For level of distortion, both shot-LLR (P < 0.001) and MUSE (P = 0.015) were significantly better than single-shot DWI, but were not significantly different from each other (P = 0.19). Eight-shot was nominally better than four-shot, but not significantly (P = 0.069) (Fig. 3c). For all three image feature ratings, interobserver agreement was highest for ghosting (0.5) but low overall for SNR (0.22) and distortion (0.23).
FIGURE 2:

Representative examples of the single-shot images (column 1), MUSE images (column 2), and shot-LLR images (column 3). Each row is from a different patient (row 1: four-shot, rows 2–4: eight-shot). Averaged ratings for each case and method are included (S: SNR, G: ghosting artifact level, D: distortion artifact level). In all cases the improved tissue structure detail achieved with the higher-resolution multishot methods (columns 2 and 3) in comparison to the single-shot methods was evident. For the cases in rows 1 and 2, both multishot methods successfully reconstructed the images without severe ghosting artifacts, while there might be artifacts due to incomplete fat suppression (yellow arrows in row 2, MUSE and shot-LLR). The eight-shot cases in rows 3 and 4 were instances in which shot-LLR more effectively eliminated ghosting artifacts due to phase errors between the shots in comparison to MUSE (yellow arrows).
FIGURE 3:
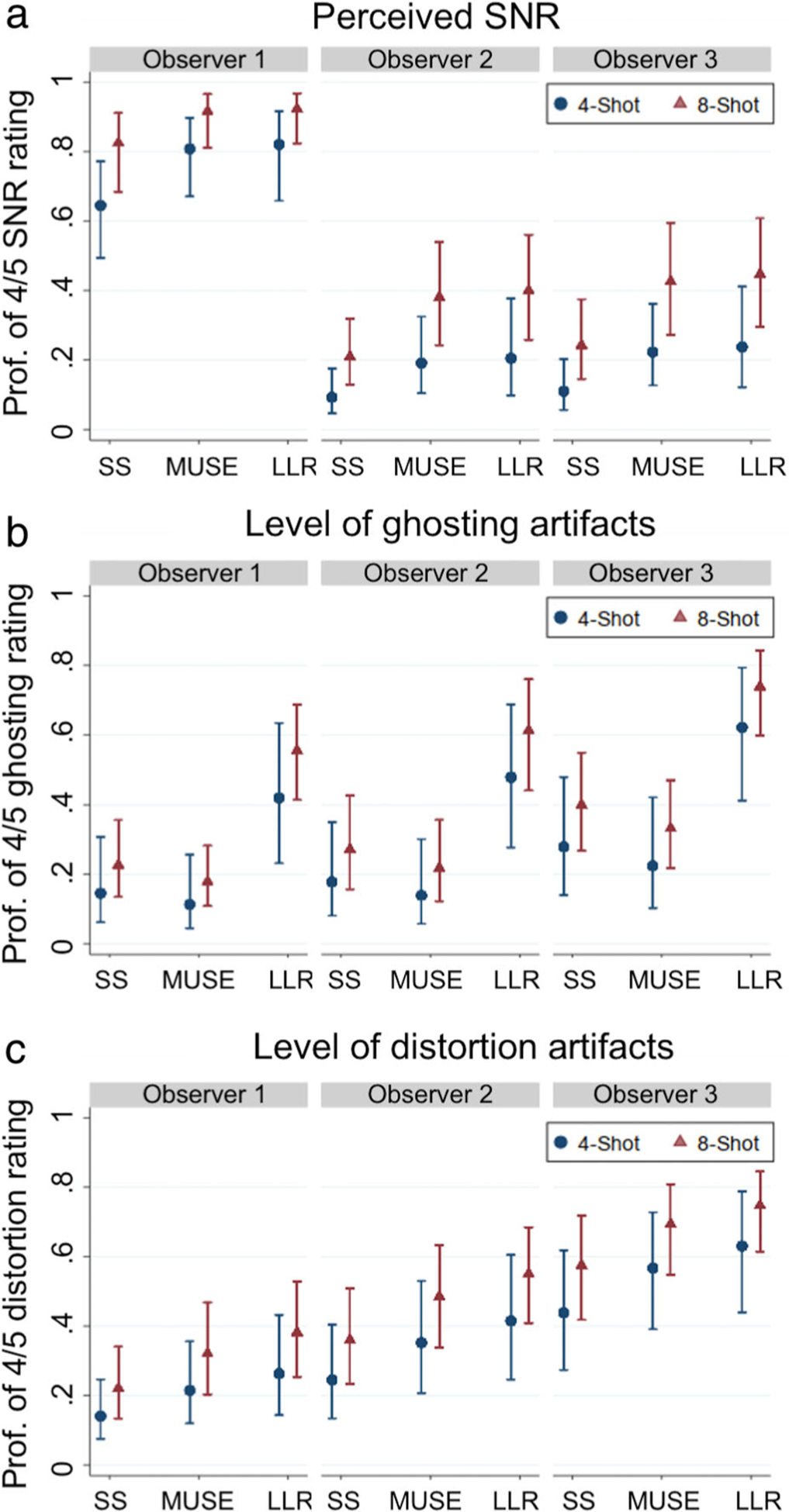
Plots of the probability of a 4 or 5 (two highest performance ratings) for each observer and shot/method combination (SS: single-shot, LLR: shot-LLR). For single-shot DWI, the four-shot/eight-shot differentiation indicates which respective multishot acquisition was acquired for that patient. For perceived SNR (a) both MUSE and shot-LLR were more likely to have a 4 or 5 rating in comparison to single-shot DWI with significance for acquisitions with both four-shot (blue) and eight-shot (red). Observer one differed from the other two observers in that the median ratings for all cases were higher, although the improvement with multishot vs. single-shot and eight-shot vs. four-shot was still indicated. The probability of achieving a rating of 4 or 5 indicating little or no ghosting artifact was higher with significance for shot-LLR vs. both MUSE and single-shot DWI (b). However, the performance of MUSE for the presence of ghosting artifact was not distinguished from that of single-shot DWI. For distortion rating, shot-LLR and MUSE both significantly outperformed single-shot DWI (c).
LESION ANALYSIS.
Representative cases showing three of the malignant lesions included in the study, with the respective lesion discernment and morphology ratings, are shown in Fig. 4. Considering the aggregate of the ratings for the depiction of malignant lesion morphology (Fig. 5b), almost all (29/30) ratings for the multishot methods were 4 or 5 (depiction of lesion morphology equivalent or better than that of the CE image), while for single-shot DWI a majority (10/15) were rated as 3 (visible but less well-defined than that of the CE image) or lower. With regard to the discernment of the malignant lesions (Fig. 5a), a majority of the single-shot (13/15) and multishot (28/30) cases were rated as 4 or 5. There was no clear distinction of depiction of lesion morphology and lesion discernment between methods for the benign lesions (Fig. 5c,d).
FIGURE 4:

Single-shot (column 1), multishot MUSE (column 2), multishot shot-LLR (column 3), and contrast-enhanced MRI (column 4) for a triple negative breast cancer (a), recurrent phyllodes tumor with hematoma/sarcoma (b), and invasive ductal carcinoma (c). The numbers represent the averaged ratings for each case and method (D: discernment, M: morphology).
FIGURE 5:

Histogram of the scores for malignant (a,b) and benign (c,d) lesion ratings of discernment and morphology. Ratings of 1 or 2 represented “invisible” or “barely perceptible” with respect to the contrast-enhanced MRI, respectively. A rating of 3 represented “visible but less well-defined than contrast-enhanced MRI.” Most multishot cases had a rating of 4 or 5, representing “equivalent or superior depiction of lesion morphology with respect to CE-MRI,” respectively.
ADC values from multishot images reconstructed by both MUSE and shot-LLR showed good correlation with those from single-shot imaging, with correlation coefficients of 0.90 (P < 0.001) and 0.90 (P < 0.001), respectively (Fig. 6). In addition, the ADC values from these two different multishot reconstruction techniques were highly correlated, with an R2 value of 0.97 (P < 0.001).
FIGURE 6:

Scatterplot of the comparisons of ADC values from the DWI methods applied. ADC values of both MUSE and shot-LLR showed a good correlation with those of single-shot DWI, with correlation coefficients of 0.90 (P < 0.001) and 0.90 (P < 0.001), respectively.
PERCEIVED RESOLUTION.
For all combinations of methods and number of shots, the mean perceived resolution was lower than the prescribed resolution, which was 2.1 mm for single-shot imaging, and 1 mm for multishot imaging (Fig. 7). The averaged perceived resolutions among all three observers were 2.7 mm for single-shot imaging, 2.3 mm for four-shot MUSE, 2.5 mm for four-shot shot-LLR, 1.6 mm for eight-shot MUSE, and 1.7 mm for eight-shot shot-LLR. Perceived resolution was significantly better for shot-LLR (P < 0.001) and MUSE (P < 0.001) vs. single-shot imaging, while shot-LLR had a slightly lower perceived resolution than MUSE (P = 0.005). Eight-shot had a significantly better resolution than four-shot (P < 0.001). Interobserver agreement on perceived resolution was 0.22.
FIGURE 7:

Perceived resolution was worse than the prescribed resolution (2.1 mm for single-shot, 1 mm for MUSE and shot-LLR) for all methods. For single-shot DWI, the four-shot/eight-shot differentiation indicates which respective multishot acquisition was acquired for that patient. MUSE and shot-LLR both had significantly higher perceived resolution than single-shot (P < 0.001); while MUSE, although the difference was small, had significantly higher perceived resolution in comparison to shot-LLR (P = 0.005). For both multishot methods, eight-shot imaging (red) had significantly higher perceived resolution than four-shot imaging (blue) (P < 0.001).
Discussion
Achieving DWI with resolution and image quality comparable to that of CE-MRI would be a major step toward the realization of noncontrast-enhanced screening MRI for breast cancer. In this study, multishot DWI, reconstructed by both shot-LLR and MUSE, achieved lower distortion, higher perceived SNR, and higher perceived resolution for both four-shot and eight-shot acquisitions in comparison to single-shot DWI. Shot-LLR outperformed MUSE for the appearance of ghosting artifacts, while MUSE showed a slightly higher perceived resolution compared to shot-LLR. Eight-shot DWI had improved SNR and perceived resolution with respect to four-shot DWI for both methods.
Shot-LLR and MUSE for both four-shot and eight-shot acquisitions resulted in higher perceived SNR in comparison to the single-shot acquisition. This higher perceived SNR in the multishot methods was achieved despite the higher prescribed resolution (1 × 1 mm2) without a compensatory increase in scan time (four-shot 1 minute 16 seconds, eight-shot 2 minutes 22 seconds) in comparison to the single-shot DWI (2.1 × 2.1 mm2, 2 minutes 18 seconds). These results may be in part due to the fact that the reconstruction of each average in single-shot DWI using parallel imaging can be inherently SNR-limited,16 while in multishot methods all k-space lines are acquired and jointly reconstructed, which enables high SNR and resolution.24
For multishot methods, the rating of level of ghosting artifacts reflects the methods’ ability to resolve phase variations between shots. Although each NEX (number of excitations) is reconstructed independently in single-shot imaging, ghosting artifacts might still exist due to the failure of parallel imaging. Notably in our study, shot-LLR showed significantly reduced ghosting artifacts compared with MUSE for the four-shot and eight-shot acquisitions. Shot-LLR also outperformed single-shot DWI with parallel imaging in terms of ghosting artifacts, while MUSE did not. This difference may be because the phase estimation for each shot with parallel imaging in MUSE may fail when the number of shots exceeds an appropriate parallel imaging factor for the receive coil. Since shot-LLR jointly reconstructs data from all shots, the improvements of shot-LLR over MUSE and single-shot DWI may be more pronounced with a limited number of coil elements. In the previous study of MUSE in the breast with two shots and a parallel imaging factor of 2, ghosting artifact was not specifically assessed and improvement of overall artifact of MUSE vs. single-shot DWI was limited, with only a 5% improvement from one of two readers. Therefore, the results of our study build on this previous work by 1) separating the analysis of these two prominent artifacts of ghosting and distortion, 2) using a number of shots as high as eight (reduced distortion artifacts), and 3) including the shot-LLR reconstruction (reduced ghosting artifacts).
Distortion in DWI depends on the velocity of k-space coverage in the phase-encode direction. Although single-shot DWI with a parallel imaging factor of four and a four-shot acquisition both segment k-space by a factor of four, the four-shot acquisition in this study would be expected to have greater distortion than the single-shot acquisition because the increased readout-direction resolution extends the effective echo spacing by 50%. However, the level of distortion of shot-LLR and MUSE for both four-shot and eight-shot acquisitions were rated to be better than single-shot imaging. This study assessed the perceived distortion without reference to an undistorted acquisition and the level of distortion can vary from case to case based on breast size, shape, and shimming. Quantitative, reference-based distortion assessments utilized in previous studies have confirmed the improvement in distortion with multisegment vs. single-shot methods and provide an option for more explicit distortion assessment in future studies.19,20
For both the four-shot and eight-shot acquisitions, the multishot methods allowed for a higher prescribed in-plane resolution of 1 × 1 mm2. This in-plane resolution was chosen to match the 1 × 1 mm2 in-plane resolution required of clinical CE-MRI acquisitions.26 The improvement of the depiction of lesion morphology achieved with both multishot methods vs. single-shot DWI with parallel imaging was evident in the image examples and ratings of the malignant lesions. Although only a small number of lesions were presented in this study, the results emphasized the potential for multishot methods to achieve a depiction of lesion morphology similar to that of CE-MRI. It was notable that across all observers, for malignant lesions, that all but one of the multishot ratings for depiction of lesion morphology were four or five, which indicated depiction of lesion morphology equivalent or better than that of the lesion on the CE image. Depiction of morphology on the single-shot diffusion-weighted images was most frequently rated as having depiction of lesion morphology that was visible but less well-defined than on CE-MRI.
The improved depiction of lesion morphology was achieved while maintaining the degree of lesion discernment between the methods, indicating that the multishot methods did not impact diffusion contrast. This improved depiction of lesion morphology with the multishot methods aligns with findings of previous work.20,27 A study in the breast in which a lower in-plane resolution of 1.9 × 1.4 mm2 was utilized for both single and multisegment DWI also reported an improvement of depiction of morphological detail of segmented DWI vs. ss-DWI, demonstrating the improvement due to reduced blurring and distortion.20 A smaller study of 36 lesions, in which both rs-EPI and ss-EPI were acquired with 1 × 1 mm2 in-plane resolution with unilateral acquisitions, also reported an improvement in depiction of anatomical detail with the multisegment method.27 Improvement of depiction morphology was not as pronounced for benign lesions, which could be due to the lower frequency of fine morphologic details in some of these lesions. However, the ADC values for both benign and malignant lesions were highly correlated between all three methods, also aligning with the findings of a recent study of MUSE vs. single-shot DWI in the breast.23 While no standard exists for ADC values, the ADC values we calculated aligned well with reported ADC ranges for the specific tumor types previously reported in the literature.28
The goal of the perceived resolution assessment was to gain an understanding of the resolution of images from the EPI scan as perceived by the radiologist, rather than relying on the prescribed resolution (based on the acquisition parameters). The perceived resolution was chosen by each observer by comparing nondiffusion-weighted images with the high-resolution T2 images from the CUBE scan downsampled at different levels. Perceived resolution for both single-shot and multishot methods was lower than the prescribed acquisition resolution. As all acquisitions utilize an EPI trajectory, some degree of signal decay, blurring, and localized distortion are always present whether or not the readout is shortened; therefore, these differences are not unexpected. The perceived resolution was significantly higher with both shot-LLR and MUSE in comparison to the single-shot method. MUSE achieved a small but significant increase in perceived resolution in comparison to shot-LLR. This might be the result of regularization of the shot-LLR reconstruction, which was the same for all subjects and slices and might cause some smoothing. The significant increase in perceived resolution between eight-shot and four-shot cases demonstrates that, although the perceived resolution will not exactly match the prescribed resolution in DWI scans, the ability to continue to shorten readout length by increasing the number of shots further reduces distortion and blurring and, consequently, further improves the depiction of tissue morphology.
The interobserver agreement was lower for imaging features and perceived resolution, with higher agreement achieved for the rating of ghosting artifacts. In particular, for the rating of perceived SNR, one observer gave a much higher percentage of ratings of 4 or 5 than the other two observers. While in this study observers were simply asked to use their best judgment in aligning a rating with a strength of image feature, future studies could incorporate training or sample cases to coordinate the perception of the radiologists, although gaining insight on variable perception is also informative. Despite these differences in actual rankings chosen, statistically significant differences between the methods were achieved. In this work we chose to focus on observer-based metrics instead of quantitative metrics. While quantitative metrics are also imperative for characterizing method performance, observed-based metrics may allow for insight into the diagnostic impact of a new method. For example, perceived resolution indicates that the depiction of morphology may align more closely with a lower resolution due to the remaining blurring and localized distortion of DWI despite the prescribed in-plane resolution matching that of CE-MRI.
The desire to develop an MRI screening exam that does not require contrast injection motivates the use of DWI in the breast8; however, DWI has also been widely investigated for a number of other applications in breast MRI.28–30 Chief among these has been the investigation of DWI as an adjunct to CE-MRI to improve specificity31,32 with a recent multi-institution study reporting improved diagnosis and reduced biopsies with DWI.29 Further, the role of DWI as an adjunct to CE-MRI has included the assessment of the potential role of DWI in an abbreviated CE-MRI protocol, with one study reporting that it may provide diagnostic information complementary to that conventionally garnered from the contrast washout phase.33 Finally, DWI has also been reported to be effective at predicting and tracking response to treatment.30,34 Thus, while the ability to improve overall image quality and resolution of breast DWI is of interest for a noncontrast screening paradigm, advanced DWI acquisition and reconstruction methods have the potential to impact these other applications of breast MRI, as well.
Limitations
A b-value of 600 s/mm2 is below the b-value 800 s/mm2 that is generally recommended as the optimal b-value at which to perform DWI in the breast.12 At our institution, clinical DWI is acquired with a b-value of 600 s/mm2, so we chose to acquire the multishot acquisitions at a matching b-value. While this limits the immediate generalizability of these results, the demonstrated higher perceived SNR of the multishot acquisitions achieved with only one signal average indicates that increasing the b-value to at minimum 800 s/mm2 with the multishot methods will be feasible. Shot-LLR enables robust reconstruction of multishot DWI with an expense of increased reconstruction time, which is about 1 minute for each slice. Parallel computing or some neural-network-based reconstruction methods may be helpful in accelerating this process. Another concern about multishot imaging is the increased scan time due to the increased number of shots. Multislice imaging, especially recently developed multiband multislice imaging, could help reduce the scan time of multishot imaging.35–37 Navigator data that provides phase information for each shot may improve the performance of MUSE and shot-LLR for a high number of shots. Further, the multishot acquisition is compatible with deep-learning methods that can be used to greatly decrease the reconstruction time and/or to improve through-plane resolution.38–40 Future studies should include a larger number of lesions with a higher b-value and focus on a screening-specific population.
Conclusion
The results of this study demonstrate that two multishot DWI methods, MUSE and shot-LLR, provide improved performance in comparison to single-shot DWI in the breast in terms of general image quality and depiction of lesions. The advantages of increasing the number of shots with multishot DWI in the breast are also delineated with perceived SNR and perceived resolution that improves with eight vs. four shots. In particular, the superior performance of shot-LLR compared to MUSE for resolving shot-to-shot ghosting artifacts may be advantageous for wide clinical implementation of the method. Finally, these results support that the continued refinement of advanced DWI methods may facilitate implementation of a noncontrast-enhanced screening breast MRI protocol.
Acknowledgment
We thank Lloyd Estkowski and Ann Shimakawa for technical support and helpful discussions.
Contract grant sponsor: National Institutes of Health; Contract grant numbers: R01-EB009055, P41-EB015891; Contract grant sponsor: GE Healthcare.
References
- 1.Monticciolo DL, Newell MS, Moy L, Niell B, Monsees B, Sickles EA. Breast cancer screening in women at higher-than-average risk: Recommendations from the ACR. J Am Coll Radiol 2018;15:408–414. [DOI] [PubMed] [Google Scholar]
- 2.Saslow D, Boetes C, Burke W, et al. American cancer society guidelines for breast screening with MRI as an adjunct to mammography. Obstet Gynecol Surv 2007;62:458–460. [DOI] [PubMed] [Google Scholar]
- 3.Kuhl CK, Strobel K, Schild HH. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology 2017;283:361–370. [DOI] [PubMed] [Google Scholar]
- 4.Sung JS, Stamler S, Brooks J, et al. Breast cancers detected at screening MR imaging and mammography in patients at high risk: Method of detection reflects tumor histopathologic results. Radiology 2016;280: 716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakker MF, De Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med 2019;381:2091–2102. [DOI] [PubMed] [Google Scholar]
- 6.Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): First post-contrast subtracted images and maximum-intensity projection ☒A novel approach to breast cancer screening with MRI. J Clin Oncol 2014;32: 2304–2310. [DOI] [PubMed] [Google Scholar]
- 7.Mango VL, Morris EA, David Dershaw D, et al. Abbreviated protocol for breast MRI: Are multiple sequences needed for cancer detection? Eur J Radiol 2015;84:65–70. [DOI] [PubMed] [Google Scholar]
- 8.Amornsiripanitch N, Bickelhaupt S, Shin HJ, et al. Diffusion-weighted MRI for unenhanced breast cancer screening. Radiology 2019;293: 504–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gulani V, Calamante F, Shellock FG, Kanal E, Reeder SB. Gadolinium deposition in the brain: Summary of evidence and recommendations. Lancet Neurol 2017;16:564–570. [DOI] [PubMed] [Google Scholar]
- 10.Pullicino R, Radon M, Biswas S, Bhojak M, Das K. A review of the current evidence on gadolinium deposition in the brain. Clin Neuroradiol 2018;28:159–169. [DOI] [PubMed] [Google Scholar]
- 11.Partridge SC, Nissan N, Rahbar H, Kitsch AE, Sigmund EE. Diffusion-weighted breast MRI: Clinical applications and emerging techniques. J Magn Reson Imaging 2017;45:337–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baltzer P, Mann RM, Iima M, et al. Diffusion-weighted imaging of the breast—A consensus and mission statement from the EUSOBI international breast diffusion-weighted imaging working group. Eur Radiol 2020;30:1436–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinker K, Moy L, Sutton EJ, et al. Diffusion-weighted imaging with apparent diffusion coefficient mapping for breast cancer detection as a stand-alone parameter: Comparison with dynamic contrast-enhanced and multiparametric magnetic resonance imaging. Invest Radiol 2018; 53:587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holdsworth SJ, Skare S, Newbould RD, Bammer R. Robust GRAPPA-accelerated diffusion-weighted readout-segmented ( RS ) -EPI. Magn Reson Med 2009;1640:1629–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porter DA, Heidemann RM. High resolution diffusion-weighted imaging using readout-segmented echo-planar imaging, parallel imaging and a two-dimensional navigator-based reacquisition. Magn Reson Med 2009;475:468–475. [DOI] [PubMed] [Google Scholar]
- 16.Chen NK, Guidon A, Chang HC, Song AW. A robust multi-shot scan strategy for high-resolution diffusion weighted MRI enabled by multiplexed sensitivity-encoding (MUSE). Neuroimage 2013;72:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solomon E, Nissan N, Furman-haran E, et al. Overcoming limitations in diffusion-weighted mri of breast by spatio-temporal encoding. Magn Reson Med 2015;73:2163–2173. [DOI] [PubMed] [Google Scholar]
- 18.Baltzer PAT, Bickel H, Spick C, et al. Potential of noncontrast magnetic resonance imaging with diffusion-weighted imaging in characterization of breast lesions: Intraindividual comparison with dynamic contrast-enhanced magnetic resonance imaging. Invest Radiol 2018;53: 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bogner W, Weber M, Helbich TH, Trattnig S. Readout-segmented echo-planar imaging improves the diagnostic performance of diffusion-weighted MR breast examinations at 3.0 T. Radiology 2012;263:64–76. [DOI] [PubMed] [Google Scholar]
- 20.Kim YJ, Kim SH, Kang BJ, et al. Readout-segmented echo-planar imaging in diffusion-weighted MR imaging in breast cancer: Comparison with single-shot echo-planar imaging in image quality. Korean J Radiol 2014;15:403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park JY, Shin HJ, Shin KC, et al. Comparison of readout segmented echo planar imaging (EPI) and EPI with reduced field-of-vIew diffusion-weighted imaging at 3t in patients with breast cancer. J Magn Reson Imaging 2015;42:1679–1688. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Ma X, Zhang Z, et al. A comparison of readout segmented EPI and interleaved EPI in high-resolution diffusion weighted imaging. Magn Reson Imaging 2018;47(November 2017):39–47. [DOI] [PubMed] [Google Scholar]
- 23.Daimiel Naranjo I, Lo Gullo R, Morris EA, et al. High-spatial-resolution multishot multiplexed sensitivity-encoding diffusion-weighted imaging for improved quality of breast images and differentiation of breast lesions: A feasibility study. Radiol Imaging Cancer 2020;2:e190076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu Y, Levine EG, Tian Q, et al. Motion-robust reconstruction of multi-shot diffusion-weighted images without phase estimation through locally low-rank regularization. Magn Reson Med 2019;81:1181–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uecker M, Ong F, Bahri D, Tamir JI, Virtue P, Cheng JY, Lustig M, Zhang T: Berkeley advanced reconstruction toolbox. In: Proc 23rd Annual Meeting ISMRM, Toronto; 2015, 2486. [Google Scholar]
- 26.Kuhl C. The current status of breast MR imaging. Part I. Choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice. Radiology 2007;244:356–378. [DOI] [PubMed] [Google Scholar]
- 27.Kanao S, Kataoka M, Iima M, et al. High-resolution diffusion-weighted MRI of the breast using readout-segmented EPI and single-shot EPI. Imaging Med 2017;9:185–190. [Google Scholar]
- 28.Bickel H, Pinker-Domenig K, Bogner W, et al. Quantitative apparent diffusion coefficient as a noninvasive imaging biomarker for the differentiation of invasive breast cancer and ductal carcinoma in situ. Invest Radiol 2015;50:95–100. [DOI] [PubMed] [Google Scholar]
- 29.Rahbar H, Zhang Z, Chenevert TL, et al. Utility of diffusion-weighted imaging to decrease unnecessary biopsies prompted by breast MRI: A trial of the ECOG-ACRIN cancer research group (A6702). Clin Cancer Res 2019;25:1756–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu S, Ren R, Chen Z, et al. Diffusion-weighted imaging in assessing pathological response of tumor in breast cancer subtype to neoadjuvant chemotherapy. J Magn Reson Imaging 2015;42:779–787. [DOI] [PubMed] [Google Scholar]
- 31.Chen X, Li WL, Zhang YL, Wu Q, Guo YM, Bai ZL. Meta-analysis of quantitative diffusion-weighted MR imaging in the differential diagnosis of breast lesions. BMC Cancer 2010;10.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, Tang M, Min Z, Lu J, Lei X, Zhang X. Accuracy of combined dynamic contrast-enhanced magnetic resonance imaging and diffusion-weighted imaging for breast cancer detection: A meta-analysis. Acta Radiol 2016;57:651–660. [DOI] [PubMed] [Google Scholar]
- 33.Dietzel M, Ellmann S, Schulz-Wendtland R, et al. Breast MRI in the era of diffusion weighted imaging: Do we still need signal-intensity time curves? Eur Radiol 2020;30:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Partridge SC, Zhang Z, Newitt DC, et al. Diffusion-weighted MRI findings predict pathologic response in neoadjuvant treatment of breast cancer: The ACRIN 6698 multicenter trial. Radiology 2018;289: 618–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Filli L, Ghafoor S, Kenkel D, et al. Simultaneous multi-slice readout-segmented echo planar imaging for accelerated diffusion-weighted imaging of the breast. Eur J Radiol 2016;85:274–278. [DOI] [PubMed] [Google Scholar]
- 36.Ohlmeyer S, Laun FB, Palm T, et al. Simultaneous multislice echo planar imaging for accelerated diffusion-weighted imaging of malignant and benign breast lesions. Invest Radiol 2019;54:524–530. [DOI] [PubMed] [Google Scholar]
- 37.Liao C, Stockmann J, Tian Q, et al. High-fidelity, high-isotropic-resolution diffusion imaging through gSlider acquisition with B1+ and T1 corrections and integrated ΔB0/Rx shim array. Magn Reson Med 2019;83 :56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delbany M, Bustin A, Poujol J, et al. One-millimeter isotropic breast diffusion-weighted imaging: Evaluation of a superresolution strategy in terms of signal-to-noise ratio, sharpness and apparent diffusion coefficient. Magn Reson Med 2019;81:2588–2599. [DOI] [PubMed] [Google Scholar]
- 39.Hu Y, Xu Y, Tian Q, et al. RUN-UP: Accelerated multishot diffusion-weighted MRI reconstruction using an unrolled network with U-Net as priors. Magn Reson Med 2020;1–12. https://onlinelibrary.wiley.com/doi/full/10.1002/mrm.28446?casa_token=FByCNycRHIUAAAAA%3AadrDSS7NZtV7rVQdhhMezbsT8U7f1nVpn_FMdT4Q0M1h-zkQsqmEzrB2zIJMhUK3Oi3T3WYot8O27A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tian Q, Bilgic B, Fan Q, et al. Improving in vivo human cerebral cortical surface reconstruction using data-driven super-resolution. Cereb Cortex 2020;1–20. https://academic.oup.com/cercor/advance-article-abstract/doi/10.1093/cercor/bhaa237/5901562?redirectedFrom=fulltext#207171494. [DOI] [PMC free article] [PubMed] [Google Scholar]


