Abstract
The SARS-CoV-2 infection has been predominately associated with lung disease. However, emerging evidence has associated the COVID-19 infection with a hypercoagulable state. This hypercoagulable state can occur despite the use of anticoagulants and antiplatelets. In fact, it may even be the presenting symptom of COVID–19 in some patients. Thromboembolism associated with COVID–19 carries a worse prognosis and should be identified as early as possible. Therefore, we report 2 patients with arterial thrombosis in the form of limb ischemia in the setting of COVID-19.
Keywords: COVID–19, Severe acute respiratory syndrome-coronavirus-2, SARS-CoV-2, Thrombosis, Arterial, Acute limb ischemia
Introduction
The first case of severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) was described in Wuhan, China in December 2019 and the World Health Organization declared the disease (Coronavirus disease 2019, COVID-19) a pandemic in March 2020 [1]. Disease manifestation can vary from an asymptomatic form to severe disease—characterized by hypoxemic respiratory failure, septic shock, and multiorgan dysfunction (MOD) that may require invasive mechanical ventilation and supportive treatment in critical care units [2]. It can manifest with a wide range of complications (gastrointestinal, neurological, thromboembolic and cardiovascular). It has been reported that in severe forms of COVID-19, a hypercoagulable state, characterized by micro-and macro–vascular thrombotic angiopathy occurs [3]. We report two patients with COVID-19 and arterial thrombosis.
Case 1
A 73-year-old male with no past medical history (PMH) was brought by family to the Emergency Department (ED) for shortness of breath (SOB), confusion and right leg pain of 6 days duration. He denied any recent falls, trauma, syncope, nausea, vomiting, chest pain. Vital signs on presentation were temperature 36.4°C, heart rate 99 beats per minute, respiratory rate 16 breaths per minute, blood pressure 165/83 mmHg, and oxygen saturation 89% on room air. On examination, his right foot was dark bluish in color, cold to touch, and the dorsalis pedis pulse was not palpable. Rest of the physical examination was unremarkable.
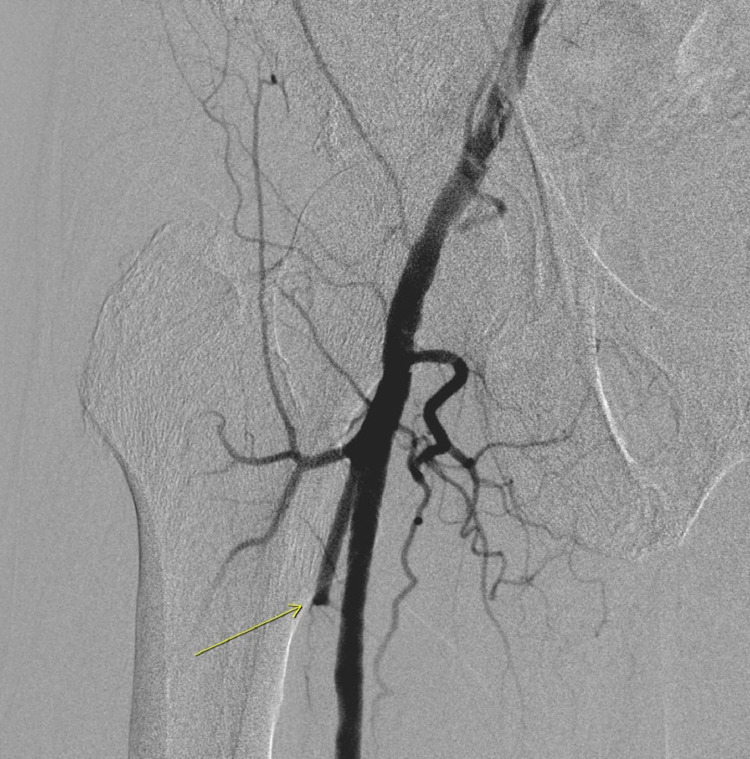
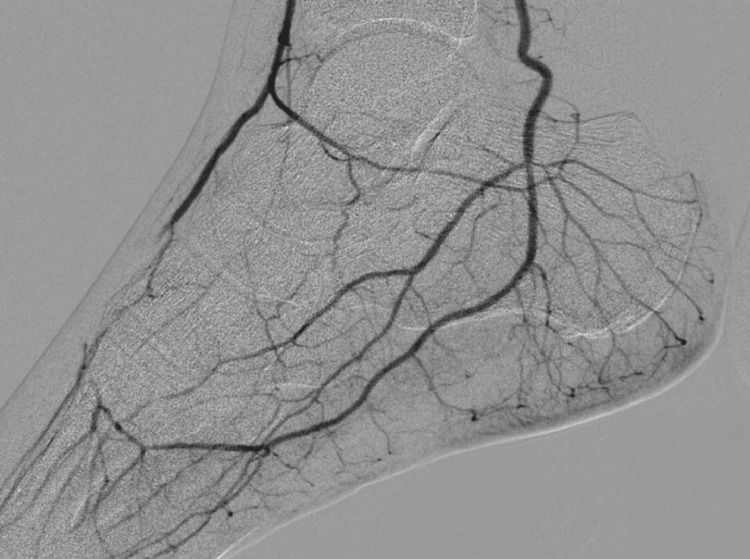
Labs were as follows: 11.9 K/mm3 (reference: 4.5-11 K/mm3), Hemoglobin: 15.3 g/dl (reference: 12-16 g/dl), platelets: 451 K/mm3 (reference: 140-440 K/mm3); sodium: 135 meq/L (reference: 135-145 meq/L); potassium 4.1 meq/L (reference: 3.5-5 meq/L), creatine 1.08 mg/dl (reference: 0.6-1.30 mg/dl), blood urea nitrogen (BUN) 29 mg/dl (reference: 7-23 mg/dl), ferritin: 760 ng/mL [16.4-294.0]; D-Dimer 2.08 mcg/mL (reference: less than 0.5); prothrombin time (PT) 13.5 seconds (reference: 12.2-14.9 seconds); international normalized ratio (INR) 1.2 (reference: less than 1), partial thromboplastin time (PTT) 26. (reference: 21.3-35.1 seconds), fibrinogen 572 (523-572); lactate dehydrogenase (LDH) 381 U/L (reference: 140-271 U/L), C-reactive protein (CRP) 22.3 mg/L (reference: less than 10 mg/L), Erythrocyte sedimentation rate (ESR) 71 mg/L (reference: less than 10 mg/L). Chest X-ray showed interstitial infiltrates throughout the right lung and to a lesser degree left base (Fig. 1). Electrocardiogram (EKG) showed no evidence of acute ischemia or arrhythmias. Computed tomography (CT) scan head was unremarkable. Arterial duplex ultrasound showed extensive thrombosis of the right popliteal artery, tibioperoneal trunk, the anterior tibial, posterior tibial artery, peroneal artery and dorsalis pedis artery. Reverse transcription polymerase chain reaction (RT-PCR) nasal swab was negative; however, given clinical presentation (SOB, hypoxia), chest x–ray findings (bilateral interstitial infiltrates) and SARS-CoV-2 IgG positive there was strong suspicion for COVID-19. The patient was started on ceftriaxone, azithromycin, and therapeutic anticoagulation with heparin, ipratropium–-albuterol and dexamethasone. Vascular surgery was consulted, and he underwent an angiogram (Fig. 2-6) and thrombolysis with Tissue Plasminogen Activator (tPA) and continued on therapeutic anticoagulation with heparin drip. Patient's post–operative course was uncomplicated, and he was discharged on apixaban.
Fig. 3.
Angiogram showing thrombosis of the femoral artery that passing through adductor hiatus within adductor magnus muscle.
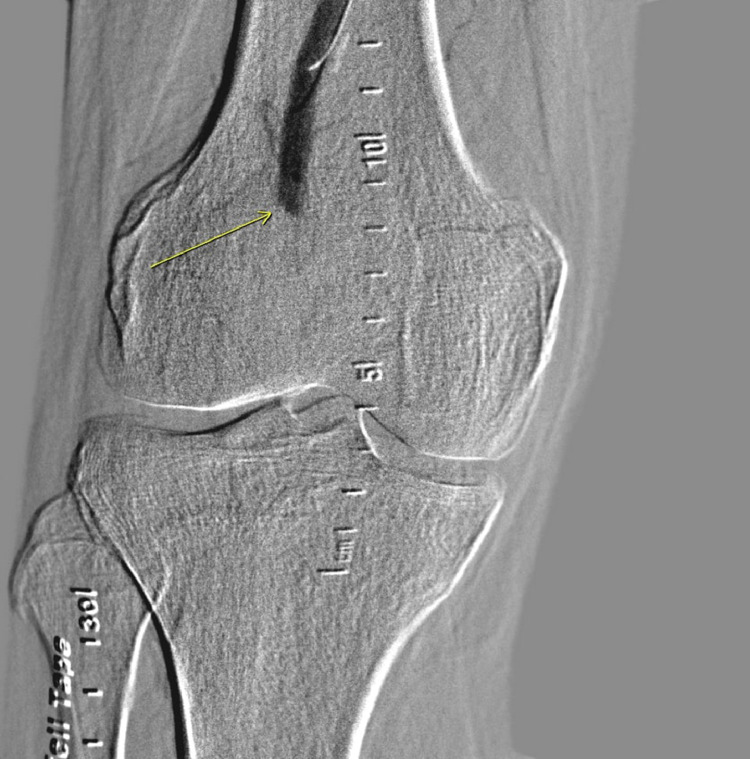
Fig. 4.
Angiogram showing thrombosis of popliteal artery.
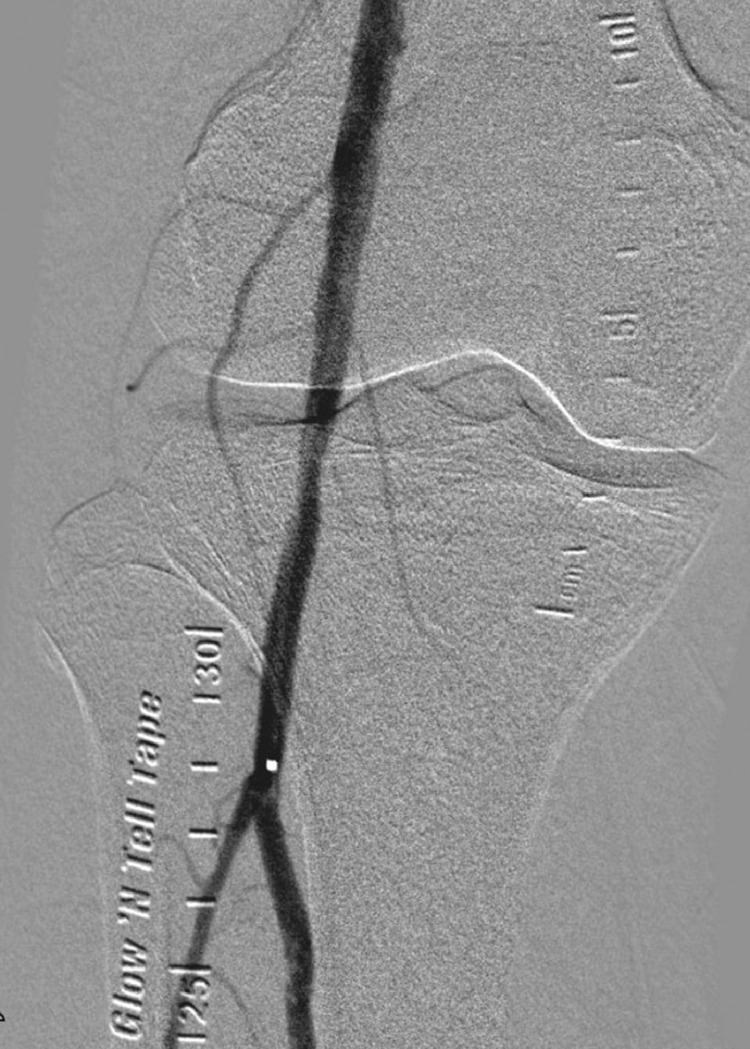
Fig. 5.
Angiogram showing restoration of blood flow to the femoral artery and popliteal artery.
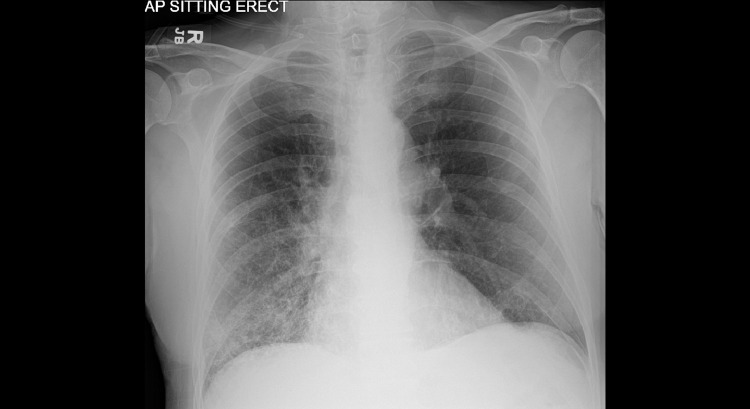
Fig. 1.
Chest X-ray showing interstitial infiltrates throughout the right lung and to a lesser degree left base.
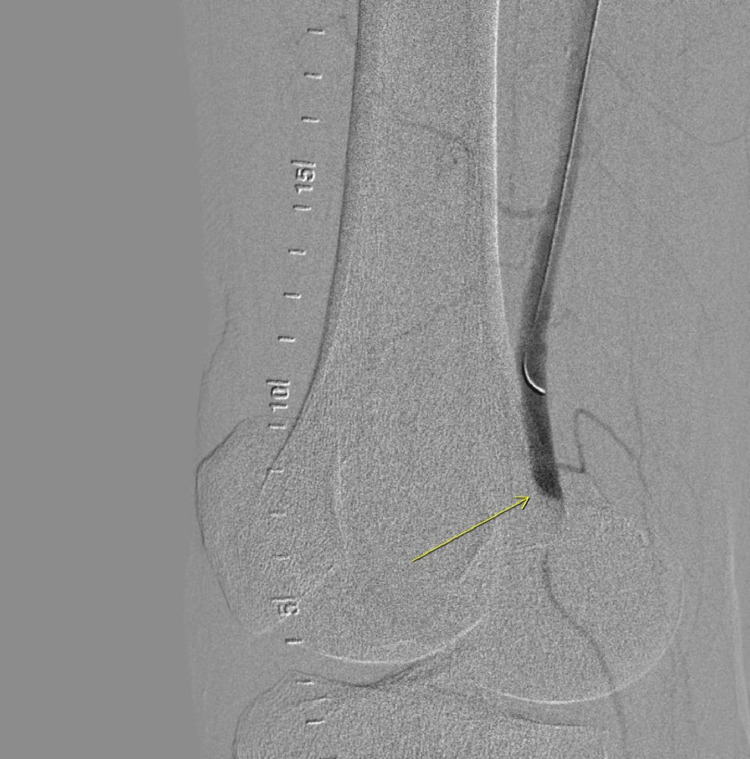
Fig. 2.
Angiogram showing thrombosis of the profunda femoris and lateral femoral circumflex artery.
Fig. 6.
Angiogram showing restoration of the blood flow in the anterior tibial, deep plantar and dorsal digital arteries after intervention.
Case 2
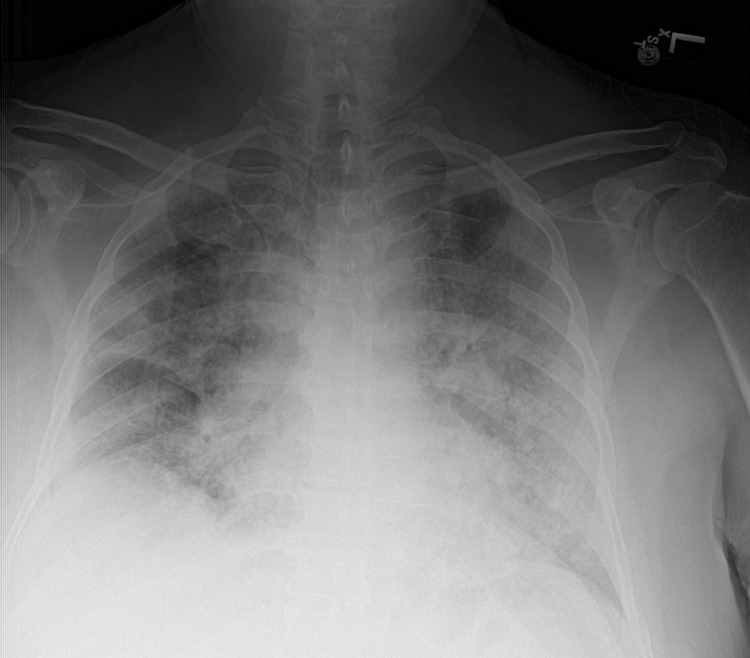
A 63-year-old male with PMH of hypertension presented to the ED with complaints of fever, progressively worsening shortness of breath, and cough for three days. He denied any known sick contacts. Of note, patient tested negative for COVID approximately 12 days prior to symptom onset. He denied chest pain, myalgias, nausea, vomiting, diarrhea or abdominal pain. In the ED, initial vital signs were significant for: temperature 36.5°C, heart rate 115 beats per minute, respiratory rate 24-26 breaths per minute, blood pressure 140/82 mmHg, oxygen saturation 80% on room air. On physical exam, the patient was in mild respiratory distress with use of accessory muscles. Chest X-ray showed mild cardiomegaly and bilateral hazy infiltrates throughout both lung fields (Fig. 7). RT-PCR nasal swab was positive for SARS-CoV-2. Labs were as follows- WBC: 6.9 K/mm3, hemoglobin:13.6 g/dl, platelets: 290 k/mm3, sodium: 138 mEq/L; potassium 3.2 mEq/L, creatinine 0.88 mg/dL, BUN 10 mg/dL, ferritin: 1212 ng/mL, CRP: 268.2 mg/L, LDH 303 unit/L, D-Dimer 2.16 mcg/mL, ESR: 108 mm/hr, Prothrombin time (PT): 14.0 seconds, the INR 1.1, partial thromboplastin time (PTT): 32.7 seconds.
Fig. 7.
Chest X-ray showing mild cardiomegaly and bilateral hazy infiltrates throughout both lung fields.
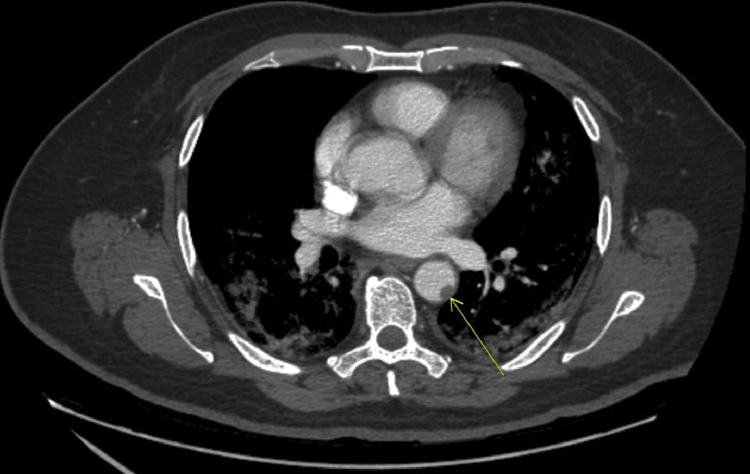
The patient was started on steroids, ceftriaxone, azithromycin, remdesivir and oxygen support. He received convalescent plasma also. He was also started on enoxaparin 40 subcutaneous for DVT prophylaxis. However, on day 5 of his hospitalization, he complained of acute severe left lower extremity pain. Left foot was cold to touch and a diminished dorsalis pedis pulse. Computed tomography angiogram (CT angiogram) of abdomen, aorta and iliofemoral showed an intraluminal clot in the descending thoracic aorta (Fig. 8) with evidence of embolic phenomenon to the left anterior tibial, peroneal artery and distal posterior tibial (Fig. 9). The patient was started on therapeutic heparin drip and vascular surgery was consulted. His clinical condition improved with anticoagulation and without invasive intervention. He was transitioned to apixaban and was discharged on hospital day 14.
Fig. 8.
Coronal view of CT-Angiogram showing clot in the descending thoracic aorta.
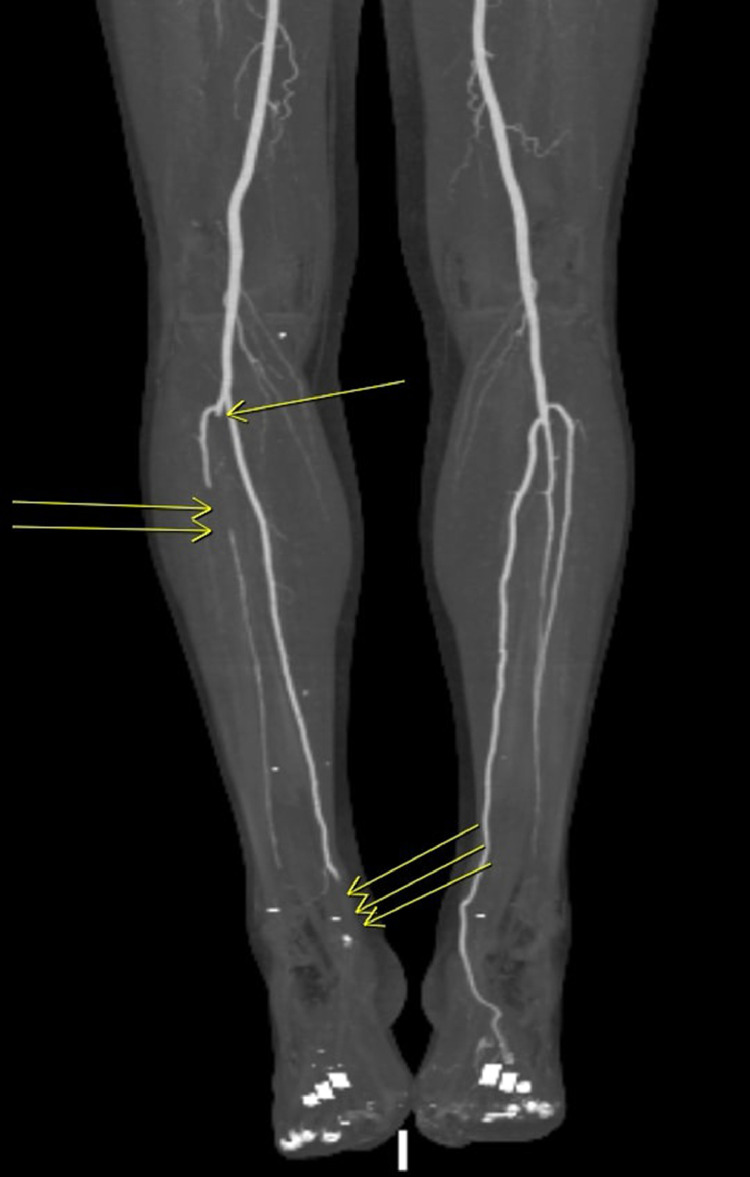
Fig. 9.
CTA showing embolic phenomenon to the left lower extremity: proximal peroneal artery (single arrow), anterior tibial (double arrow) and distal posterior tibial artery just above the ankle (triple arrow).
Discussion
In over a year, the Coronavirus pandemic caused by respiratory syndrome coronavirus-2 (SARS-CoV-2) has led to many detrimental consequences and led to significant morbidity and mortality throughout the world. COVID-19 presents with a wide spectrum of clinical manifestations, from asymptomatic to a severely extensive inflammatory response that ultimately leads to pulmonary damage and end-organ failure [4]. While severe respiratory failure is the main culprit, the association between COVID-19 and coagulation dysfunction has been recently reported [4].
While there have been ample reports of venous thromboembolism in patients with SARS-CoV-2, literature on arterial thrombosis is limited. Recent studies have shown that abnormal coagulation dysfunction is associated with poor prognosis in patients with COVID-19 [5]. In a study by Etkin et. al, the authors evaluated a total of 49 patients with arterial thromboembolism and observed a high rate of death (46%) and a high rate of limb ischemia—up to 18% of limb loss were noted in patients with COVID-19 [6]. Kashi et. al, in one article, analyzed seven patients with COVID-19 who experienced serious arterial thrombotic events despite the use of antiplatelet or anticoagulation [7].
Goldman et al, in a retrospective study, compared patients who had CT angiogram performed—16 patients with confirmed positive SARS-CoV-2 and control 32 patients who tested negative for SARS-CoV-2. It was observed that all patients (100%) who tested positive for SARS-CoV-2 had at least one thrombus while only 69% of patients who tested negative for SARS-CoV-2 had thrombi. Furthermore, they observed that death or limb amputation were more common amongst COVID-19 positive patients and thrombus burden also. Patients with COVID-19 who presented with symptoms of leg ischemia only were more likely to avoid amputation or death than patients who had pulmonary or systemic symptoms [8].
The coagulation dysfunction is most likely secondary to the extensive inflammation from COVID-19 [3]. Furthermore, the coagulation dysfunction can be described by the Virchow's triad which consists of vascular wall injury, stasis of blood, and hypercoagulability [9]. The glycocalyx layer in the endothelium secretes tPA that prevents platelets from biding and also inhibits the activation of the clotting cascade. Invasion by the COVID-19 virus of the endothelium can lead to disruption of this process and promote formation of thrombus [9]. Additionally, abnormal blood flow and hyper viscosity can lead to both thrombosis and further endothelial injury. This is also complicated by the presence of microthrombi that is linked to COVID that further leads to turbulent blood flow [9]. Finally, the low levels of tPA associated with endothelial damage, and inhibition of clot–lysing pathway, collectively promotes a hypercoagulable environment which is amplified by platelet dysfunction, and compliment activation [3]. Furthermore, extensive inflammatory response predisposed persons with COVID-19 to severe disseminated intravascular coagulation (DIC). These patients were found to have elevated levels of D-dimer, and fibrin degradation levels [3].
Conclusion
SARS-CoV-2 causes severe hypoxic respiratory failure; however, there have been ample reports of many extrapulmonary manifestation including hematological complications such as severe coagulopathy. We report a case series of two patients with COVID-19 with arterial thrombosis. Our small case series adds to the available literature. Due to the fact that thrombosis can occur in spite of use of anticoagulation and antiplatelets, one needs to be cognizant of the occurrence and the associated poor prognosis related to COVID-19 coagulopathy.
Financial Disclosures
None
Footnotes
Competing Interests: None of the authors have any conflict of interest to disclose.
References
- 1.Hu B, Guo H, Zhou P. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19(3):141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behzad S, Aghaghazvini L, Radmard AR. Extrapulmonary manifestations of COVID-19: radiologic and clinical overview. Clin Imaging. 2020;66:35–41. doi: 10.1016/j.clinimag.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang N, Li D, Wang X. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Etkin Y, Conway AM, Silpe J. Acute arterial thromboembolism in patients with COVID-19 in the new york city area. Ann Vasc Surg. 2021;70:290–294. doi: 10.1016/j.avsg.2020.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kashi M, Jacquin A, Dakhil B. Severe arterial thrombosis associated with Covid-19 infection. Thromb Res. 2020;192:75–77. doi: 10.1016/j.thromres.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldman IA, Ye K, Scheinfeld MH. Lower-extremity arterial thrombosis associated with COVID-19 is characterized by greater thrombus burden and increased rate of amputation and death. Radiology. 2020;297(2):E263–E2E9. doi: 10.1148/radiol.2020202348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed S, Zimba O, Gasparyan AY. Thrombosis in Coronavirus disease 2019 (COVID-19) through the prism of Virchow's triad. Clin Rheumatol. 2020;39(9):2529–2543. doi: 10.1007/s10067-020-05275-1. [DOI] [PMC free article] [PubMed] [Google Scholar]