Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for the novel coronavirus disease 2019 (COVID-19), has caused a global pandemic on a scale not seen for over a century. Increasing evidence suggests that respiratory droplets and aerosols are likely the most common route of transmission for SARS-CoV-2. Since the virus can be spread by presymptomatic and asymptomatic individuals, universal face masking has been recommended as a straightforward and low-cost strategy to mitigate virus transmission. Numerous governments and public health agencies around the world have advocated for or mandated the wearing of masks in public settings, especially in situations where social distancing is not possible. However, the efficacy of wearing a mask remains controversial. This interdisciplinary review summarizes the current, state-of-the-art understanding of mask usage against COVID-19. It covers three main aspects of mask usage amid the pandemic: quality standards for various face masks and their fundamental filtration mechanisms, empirical methods for quantitatively determining mask integrity and particle filtration efficiency, and decontamination methods that allow for the reuse of traditionally disposable N95 and surgical masks. The focus is given to the fundamental physicochemical and engineering sciences behind each aspect covered in this review, providing novel insights into the current understanding of mask usage to curb COVID-19 spread.
Keywords: COVID-19, SARS-CoV-2, Mask, Particle, Filtration, Decontamination
Abbreviations: BFE, bacterial filtration efficiency; CDC, Centers for Disease Control and Prevention; COVID-19, coronavirus disease 2019; ECDC, European Centre for Disease Prevention and Control; EtO, ethylene oxide; FDA, Food and Drug Administration; FFR, filtering facepiece respirator; FFP, filtering facepiece; MGB, Mass General Brigham; MPPS, most penetrating particle size; NIOSH, National Institute for Occupational Safety and Health; NNU, National Nurses United; PFE, particle filtration efficiency; PM, particulate matter; PP, polypropylene; PPE, personal protective equipment; QNFT, quantitative fit test; R0, basic reproduction number; Rt, effective reproduction number; RH, relative humidity; RPE, respiratory protective equipment; SARS-CoV-2, acute respiratory syndrome coronavirus 2; TCID50, fifty-percent tissue culture infectious dose; TIL, total inward leakage; TPI, threads per inch; UN, United Nations; UV, ultraviolet; UVC, the UV spectrum with wavelength from 200 to 280 nm; UVGI, ultraviolet germicidal irradiation; VFE, viral filtration efficiency; VHP, vaporized hydrogen peroxide; WHO, World Health Organization; ΔP, differential pressure across a mask or filter material
Graphic abstract
1. Introduction
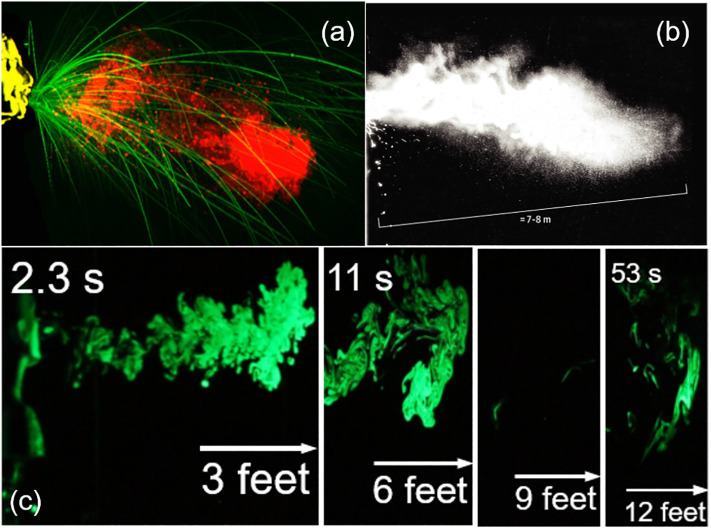
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for novel coronavirus disease 2019 (COVID-19), has caused a global pandemic, infecting more than 146 million people worldwide and causing over three million deaths since its initial outbreak in December 2019 [1]. Among all plausible routes, increasing evidence suggests that airborne transmission of SARS-CoV-2 via respiratory droplets and aerosols is most likely responsible for the rapid spread of COVID-19 [[2], [3], [4], [5], [6], [7], [8]]. Respiratory droplets, which have a relatively large size of 5–10 μm, are emitted when an infected individual coughs or sneezes (Fig. 1a) [9]. In comparison, aerosols are nuclei of respiratory droplets that form after evaporation, and are usually less than 5 μm in size [10]. Viral particles have been found in respiratory droplets and exhaled aerosols of infected individuals [11,12], and are bound to inhalable aerosols in the atmosphere, e.g., PM2.5 [7,13,14]. Accumulating evidence suggests that respiratory droplets and aerosols expelled during a sneeze or cough can travel up to 12 to 26 f. (Fig. 1b and c) [15,16], significantly farther than the 6-ft social distancing guideline recommended by the Centers for Disease Control and Prevention (CDC) of the United States. Due to their small size and hence negligible influence by gravity, aerosols generally remain afloat in the air for prolonged periods of time, causing an additional threat of airborne transmission, especially in indoor environments with poor ventilation.
Fig. 1.
Respiratory droplets and aerosols expelled during a sneeze or cough. (a) Droplet formation by a sneeze. The large millimeter-sized, semi-ballistic droplets (green) settle with gravity, while the multiphase cloud/puff comprised of small aerosols (red) propagates over a longer distance. Adapted with permission from [9]. Copyright 2020 Elsevier. (b) A picture of multiphase turbulent gas clouds formed by a human sneeze, obtained with a high-speed camera. It shows that clusters of droplets/aerosols travelled 7–8 m, i.e., up to 26 ft. Adapted with permission from ref. [15]. Copyright 2020 JAMA. (c) Time-dependent path of an emulated, uncovered, heavy cough jet. The jet is composed of vaporized droplets and aerosols, visualized with a green laser. It can be seen that the jet travels up to 12 ft within 53 s. Adapted with permission from [16]. Copyright 2020 American Institute of Physics.
Given the evidence that COVID-19 can be spread by presymptomatic and asymptomatic individuals, universal face masking has been recommended as a low-cost and efficient means of mitigating virus transmission. However, the efficacy of wearing a mask remains controversial and compliance is low in some regions. Policy makers from different governments and organizations have put forth conflicting regulations and guidelines on mask usage. On March 11, 2020, the World Health Organization (WHO) designated the COVID-19 outbreak as a pandemic. In April 2020, the WHO claimed that there was not enough medical evidence to support universal masking of healthy people and advised the public not to wear face masks unless coughing, sneezing, or caring for a patient with suspected COVID-19 symptoms. This was recommended as part of a global effort to alleviate personal protective equipment (PPE) shortages among healthcare workers [17,18]. On June 5, 2020, the WHO released new interim guidance concerning the use of masks amid the COVID-19 pandemic, separating its advice for healthcare workers and for the general public. Although there is a lack of published work evaluating the efficacy of universal masking by healthcare workers to prevent spread of SARS-CoV-2, the continuous use of masks by healthcare workers in clinical settings is widely supported. For the general public, the use of cloth masks in public settings, such as grocery stores and places of worship, especially in regions with known or suspected widespread transmission, or in situations where physical distancing is not possible, has been encouraged by the WHO and many policy makers [19].
As of July 2020, the CDC recommended that all Americans wear masks in public settings [20]. This recommendation was made, at least in part, due to a report from a hair salon in Missouri that demonstrated the efficacy of wearing masks [21]. In May 2020, two hairstylists in Springfield, Missouri received positive test results for SARS-CoV-2 and were exposed to 139 clients in total since the onset of their symptoms [21]. Both stylists, as well as all 139 clients, wore some kind of facial covering while in the salon, with the stylists wearing either a double-layered cotton face covering or a surgical mask. Despite their proximity to the infected stylists, for appointments ranging from 15 to 45 min in duration, it was found that none of the 139 clients developed COVID-19 symptoms within the two-week quarantine period. Furthermore, of the 67 clients tested, all results were negative. Interestingly, the type of face mask worn by the 139 clients varied, with only two clients wearing N95 masks, 46% wearing surgical masks and 47% wearing cloth masks [21]. Although anecdotal, this incident suggests that consistent and proper usage of facial coverings can help minimize symptomatic transmission of SARS-CoV-2 during close contact, as at a hair salon. In fact, it appears that COVID-19 transmission rates are generally lower in countries and regions where citizens are accustomed or required to adopt universal masking, such as many parts of Asia [22,23]. Simulations and mathematical models have also predicted that the adoption of universal masking would substantially curtail the spread of COVID-19 [24].
Here we review the current understanding of face masks and filtering facepiece respirators (FFRs), and their efficacy in mitigating the spread of COVID-19. First, we briefly introduce available epidemiological evidence supporting mask usage in the community to curb the spread of COVID-19. We then introduce definitions and filtration mechanisms of various face masks and FFRs. Next, we focus on the available testing methods capable of quantitatively evaluating the physical integrity and filtration efficiency of various masks, and finally, we discuss available decontamination methods to allow for the reusing of masks and FFRs. This review examines the current controversy surrounding universal mask-wearing and its efficacy in curbing COVID-19 transmission, which may help to define clearer regulations and guidelines on mask usage for both healthcare providers and the general public.
2. Epidemiological evidence of using face masks against COVID-19
Several systematic reviews of randomized controlled trials have evaluated the effect of face masks and other physical interventions in preventing the spread of influenza and other respiratory viruses, all of which were performed prior to the COVID-19 pandemic [[25], [26], [27], [28], [29], [30], [31], [32], [33]]. Although SARS-CoV-2 is also a respiratory virus, it is unknown to what degree the conclusions of these studies apply to COVID-19. Due to ethical and logistical concerns, randomized controlled trials involving population health measures are rare, and thus a wider evidence base must be examined [19].
Smaller-scale observational studies have presented real-world evidence that suggests mask-wearing helps mitigate community transmission of COVID-19. For example, one study examined the rate of secondary transmission of SARS-CoV-2 in 124 Beijing households with at least one laboratory-confirmed COVID-19 patient [34]. This retrospective cohort study found that face mask usage by the primary case and the family before the onset of symptoms in the primary case led to a 79% reduction in transmission, although it is important to note that the study did not examine the effect of different types of masks. Another study examined in-flight transmission rates of SARS-CoV-2 [35]. It was found that three flights on which masking was not required and rarely practiced resulted in mass transmission events. In contrast, a flight that enforced rigid masking, although it included 25 individuals who tested positive for COVID-19 upon arrival, only resulted in two likely transmissions, one of which involved a passenger who was seated in close proximity to five index cases [35]. In five 8-h flights containing a total of 58 COVID-19-positive passengers, but with enforced, rigid masking policies, no secondary cases were reported after two weeks among the 1500–2000 passengers on those flights [35]. In Thailand, a retrospective case-control study examined 1050 asymptomatic contacts of COVID-19 positive individuals; 211 of the contacts later tested positive, and 839 of them never tested positive [36]. It was found that compliance with mask-wearing during contact with the COVID-19 positive individuals was strongly associated with a > 70% reduced risk of infection for those who always wore a mask, compared to those who never wore one [36].
Several studies have examined the effect of universal masking at the community level. One study of Mass General Brigham (MGB), the largest healthcare system in Massachusetts, assessed the effect of hospital-wide masking policies on SARS-CoV-2 infection among healthcare workers [37]. It was found that implementation of universal masking policies at MGB was, at least in part, associated with a significant decrease in the rate of SARS-CoV-2 infection among healthcare workers [37]. In another study, the synthetic control method was used to study the effect of municipal district mask mandates, and the point in time at which they were implemented, on COVID-19 transmission in Jena, Germany [38]. It was found that, varying from region to region, face mask mandates reduced the number of newly reported SARS-CoV-2 infections between 15 and 75% within the first 20 days of their introduction. It was concluded that the community-wide usage of face masks in all public settings led to a reduction in the daily growth rate of infections by 47% [38]. A similar study performed in the United States examined the effects of state mandates for the use of face masks in public areas on the daily growth rate, as well as the effects of mask usage in certain work settings in comparison to community-wide mandates [39]. The study focused on 15 states and Washington D.C., in which all able individuals were mandated to wear a face mask in public settings. Similarly, it was found that face mask mandates were associated with a lower COVID-19 daily growth rate, and that this effect increased over time after the mandates were put in place [39].
A study of face masks performed by Goldman Sachs in June 2020 presents interesting data on self-reported mask use in different countries, finding that the percentage of people who reported wearing face masks in public is nearly 90% in East Asia, just below 70% in the United States and Germany, and less than 10% in Scandinavia [40]. Within the United States, it was found that mask usage was generally lowest in the South and highest in the Northeast, with 80% of Massachusetts residents reporting “always” wearing a face mask in public, compared to just 40% of respondents in Arizona. This study estimated that a nation-wide mask mandate could increase the percentage of the population who wear masks in public by 15%, leading to a decrease in the daily growth rate of confirmed cases by 0.6–1% [40].
A nation-wide study of Canada investigated the impact of mask mandates and other nonpharmaceutical interventions on SARS-CoV-2 growth across 34 public health regions in Ontario, as well as the effect on Canada as a whole at the country level [41]. The study of Ontario utilized variation in the timing of the adoption of mask mandates in each sub-region, and was conducted over a relatively small geographic area. The study found that within the first few weeks after the mask mandate came into effect, the average weekly number of newly diagnosed SARS-CoV-2 infections decreased by 25–31% in Ontario. On the national scale, corroborating evidence was found, with mask mandates accounting for an estimated 36–46% reduction in weekly case numbers. The study further examined self-reported mask usage, and found that mask mandates increased mask usage by 30%, significantly affecting public behavior towards masking [41].
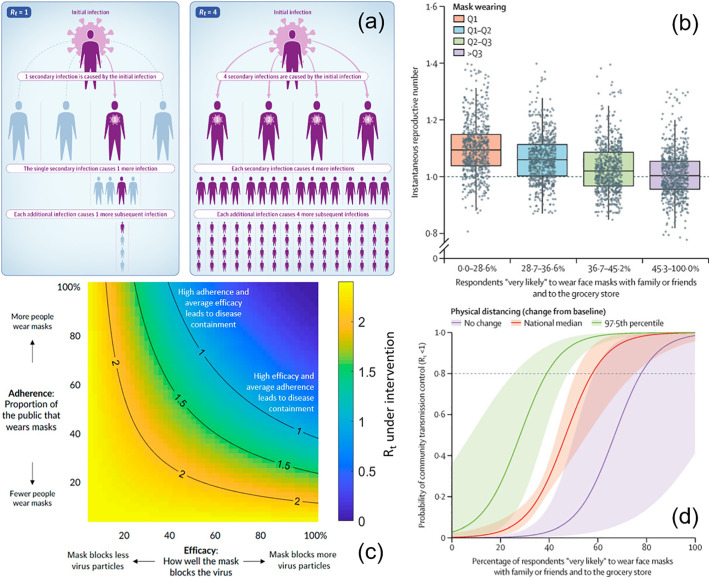
While the methods and results presented here do vary, all available epidemiologic evidence suggests that community-wide mask-wearing results in reduced rates of COVID-19 infections. In epidemiologic studies, the speed and intensity of viral spread are measured by the effective reproduction number (R t). It is defined as the average number of secondary infectious cases caused by a primary infectious case, with the implementation of any policy, regulation, mandate, and other interventions (Fig. 2a) [42,43]. The basic reproduction number (R 0) for COVID-19, without any intervention, was estimated to be in the range of 2.4 to 3.9 [19,44]. The goal of any intervention is to achieve a reduction of R t to below 1. It is predicted that community-wide mask-wearing is able to reduce R t to below 1 (Fig. 2b) [45], however the effect of mask wearing on R t depends on both adherence to policy and the efficacy of individual masks in preventing viral particles from entering the airways (Fig. 2c) [19]. It is expected that the best results in curbing COVID-19 transmission can be achieved through a combination of mask wearing and social distancing (Fig. 2d) [19].
Fig. 2.
Epidemiological impact of mask-wearing on COVID-19 transmission rate, measured by the effective reproduction number (Rt). (a) Illustration of Rt for two representative cases, Rt = 1 and Rt = 4, respectively. Adapted with permission from ref. [43]. Copyright 2020 JAMA. (b) Rt decreases with reported mask use. The plot is stratified by quartiles of the percentage of individuals who reported that they were “very likely” to wear a mask with family or friends and to the grocery store. Adapted with permission from ref. [45]. Copyright 2021 Lancet. (c) Effect of public mask wearing on Rt from an initial basic reproduction number R0 = 2.4. The blue area is what is needed to slow the spread of COVID-19. Each black line represents a specific disease transmission level with Rt indicated. Adapted with permission from ref. [19]. Copyright 2021 PNAS. (d) Combined social distancing and mask wearing has the highest impact on reducing Rt. The horizontal dashed line was placed at Rt = 0.8 for community transmission control. Adapted with permission from ref. [45]. Copyright 2021 Lancet.
3. Standards, testing methods, and efficacy of face masks
3.1. Standards and definitions
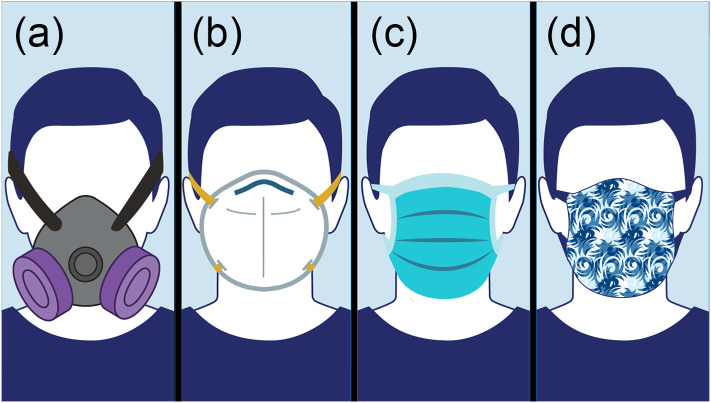
As shown in Fig. 3 , there are four general categories of face masks. Elastomeric respirators are designed to be reusable, N95 and surgical masks are designed to be disposable, while cloth masks are generally reusable, although they are not standardized or strictly regulated [46]. Elastomeric respirators are scarcely used by the general public or healthcare workers as they require maintenance and a supply of replaceable components. Hence, this review will only discuss N95, surgical, and cloth masks. A detailed comparison of these three mask categories can be found in Table 1 .
Fig. 3.
Schematics of various face masks. (a) An elastomeric respirator, equipped with a replaceable cartridge or filter, which is designed to be reusable. (b) A particle filtering respirator, commonly known as an N95 mask, which is designed to be disposable. (c) A surgical mask, also known as a medical, procedure, or dental mask, which is designed to be disposable. (d) A cloth mask, or a cloth face covering, which is not standardized or regulated. Adapted with permission from ref. [46]. Copyright 2020 United States Food and Drug Administration (FDA).
Table 1.
Summary of three main mask categories.
| Masks | Characteristics | Advantages | Disadvantages |
|---|---|---|---|
| Respirators (e.g., N95 masks) |
|
|
|
| Surgical masks |
|
|
|
| Cloth masks |
|
|
|
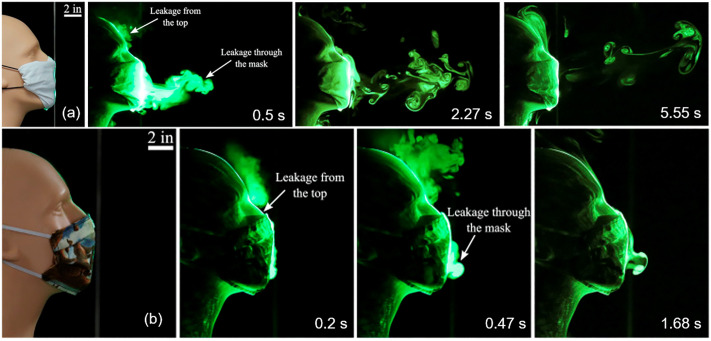
In general, any kind of face mask can impede or slow down the propagation of respiratory droplets and aerosols to a certain extent (Fig. 4 ). Different from face masks, however, the primary purpose of using face shields and goggles is to prevent particle or aerosol invasion to the eyes [47]. Research has shown that although face shields are able to block the initial forward motion of respiratory droplets, small aerosols can flow around the bottom and sides of the shield, thus becoming suspended in air and spreading to a distance of at least three feet [48]. It was found that using face shields and masks together does not significantly enhance the protection offered by wearing masks alone [47]. Therefore, the CDC does not recommend using face shields or goggles as a substitute for face masks [49].
Fig. 4.
Effect of face masks on the propagation of respiratory droplets and aerosols. (a) Time-dependent path of an emulated cough jet, through a folded cotton handkerchief mask constructed following the recommendation by the United States Surgeon General. (b) Time-dependent path of an emulated cough jet, through a homemade cloth mask stitched with two-layers of cotton quilting fabric of 70 threads per inch. In comparison to Fig. 1, it can be seen that both face masks tested significantly impeded the propagation of respiratory droplets and aerosols expelled from the emulated cough. The cloth mask (b) was able to limit the forward motion of the cough jet to within 3 in. from the mouth. Leakage of droplets and aerosols from the cloth mask occurred mostly from the gap between the nose and the mask along the top edge, indicating the importance of mask fit. Adapted with permission from ref. [16]. Copyright 2020 American Institute of Physics.
Both N95 and surgical masks are commercially available, medical-grade respiratory protective equipment (RPE) that are standardized and strictly regulated. Many countries have their own standards and naming conventions for masks and respirators. In general, the efficacy of RPE depends on their particle filtration efficiency and their quality of fit. Particle filtration efficiency (PFE) is a measure of how well the mask or respirator is able to capture particles in a specific size range. It is defined as the percentage of downstream particles over upstream particles. The fit of RPE is determined by the amount of leakage around the perimeter of the RPE.
N95 masks belong to the general category of disposable particle filtering respirators whose purpose is to protect the wearer against particulate matter (PM) in the environment [50]. In Europe, non-powered air-purifying respirators are commonly known as filtering half masks or filtering facepieces (FFPs), which can be further categorized based on their PFE into FFP1, FFP2, and FFP3, with 80%, 94%, and 99% PFE, respectively [51]. In the United States, the National Institute for Occupational Safety and Health (NIOSH) uses the letters N, R, and P to represent non-powered, air-purifying particulate respirators that are nonresistant, somewhat resistant, and strongly resistant to oil aerosols, respectively [52,53]. The number associated with a specific respirator is indicative of its efficiency level, with N95, R95, and P95 filters indicating a minimum of 95% PFE. Similarly, 99-level respirators must demonstrate at least 99% PFE, and 100-level respirators must demonstrate no less than 99.97% PFE [52]. Due to the overwhelming popularity of N95 respirators over those at other efficiency levels, we will use the term “N95 mask” to represent the broader category of particulate respirators. It should be noted that the N95 masks discussed in this review exclude those with an exhalation valve. The exhalation valve acts as a one-way check valve that allows exhaled airflow out, but restricts inhaled airflow. Consequently, an infected individual can still spread the virus via respiratory droplets that pass through the exhalation valve when wearing these masks [54]. Therefore, the CDC has recommended against the use of N95 masks with an exhalation valve for the purpose of controlling COVID-19 spread [49].
Surgical masks, also known as medical, procedure, or dental masks, serve to protect the wearer from large droplets, splashes, and sprays of fluids by establishing a physical barrier between the wearer's respiratory system (mouth and nose) and the immediate environment. In addition, surgical masks protect others from the wearer's expelled respiratory droplets by slowing down and dispersing the small droplets produced by breathing, speaking, coughing and sneezing, thereby preventing them from spreading over a large region [50]. Surgical masks are standardized by the Food and Drug Administration (FDA) in the United States, with categories 1, 2, and 3, based on their fluid resistance efficiency, with level 1 providing the least fluid resistance. ASTM International, formerly known as the American Society for Testing and Materials, evaluates the performance of surgical masks based on PFE, bacterial filtration efficiency (BFE), differential pressure (ΔP), resistance to penetration by synthetic blood, and flammability [55]. In Europe, surgical mask requirements and testing methods must comply with the EN 14683:2019 European Standard [51]. Table 2 summarizes the standard tests required for evaluating the efficacy of face masks.
Table 2.
Summary of standard tests for evaluating mask effectiveness.
| Tests | Details | Organization/Regulation |
|---|---|---|
| Particle filtration efficiency (PFE) |
|
|
| Bacterial/Viral filtration efficiency (BFE/VFE) |
|
|
| Fluid resistance |
|
|
| Pressure differential (ΔP) |
|
The fit of a face mask can be determined with the quantitative fit test (QNFT) method, e.g., using the PortaCount Respirator Fit Tester (TSI, Shoreview, MN, USA). The maximum fit factor score is set at 200, while fit factors >100 are considered adequate protection and pass the QNFT. Unlike N95s and other respirators, surgical and cloth masks are not designed to be tight-fitting. Using the QNFT, it was found that 100% of surgical masks tested failed under normal breathing conditions [56]. While an N95 respirator produced a fit factor of 100, surgical masks had a fit factor in the range of only 2.5–9.6, depending on the brand of the mask and individual face shape [56]. Up to 20–30% of droplets and aerosols could leak out, mostly from the loose-fitting sides of the surgical masks [16]. Leakage could also pose a major issue to individuals wearing N95s if the mask is not properly sealed [56]. It was found that a few 3-mm holes on N95 masks led to a drastically decreased PFE, comparable to that of a surgical mask [57].
3.2. Filtration mechanisms
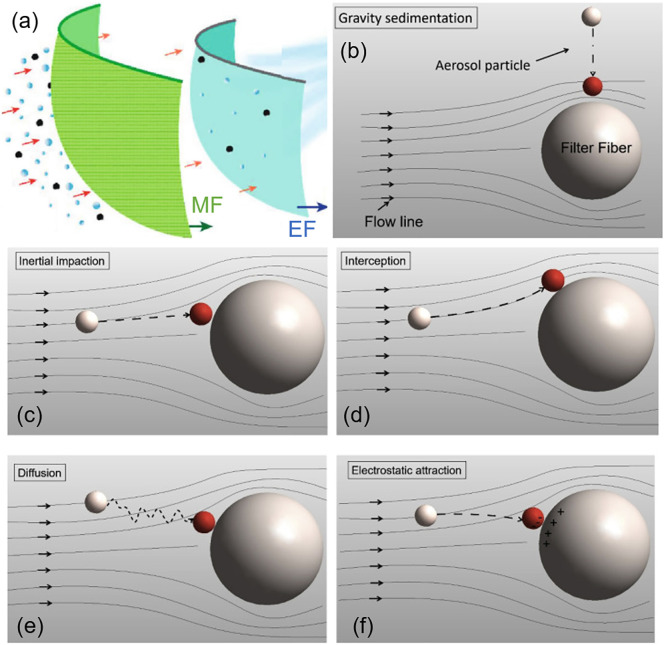
The vast majority of masks use the mechanism of filtration to function. It is important to note that a mask filter does not function as a simple strainer. Hence, the pore size of the mask alone does not determine its PFE. In general, the filtration mechanisms of masks involve both mechanical and electrostatic filtration (Fig. 5a) [58,59].
Fig. 5.
Filtration mechanisms of masks. (a) The filtration mechanisms of masks generally involve mechanical (MF) and electrostatic filtrations (EF). Adapted with permission from ref. [60]. Copyright 2020 American Chemical Society. (b) Gravity sedimentation, whereby a large particle falls and adheres to a fiber cross-section. The grey particle (top) falls onto the filter and adheres via van der Waals attraction, indicating successful filtration, shown in red. (c) Inertial impaction, whereby a large particle with large inertia travels linearly, eventually coming into contact with a fiber. The grey particle (left) does not follow the streamline around the fiber and is unable to avoid it, adhering and becoming filtered. (d) Interception, whereby a small particle is led to adhere to a fiber by motion along the streamline. (e) Diffusion, whereby Brownian motion of a small particle results in contact and adhesion to a fiber. (f) Electrostatic attraction, whereby a charged particle is trapped on a fiber of opposite charge through electrostatic attraction. Particles shown in b-f were not drawn to scale. Adapted with permission from ref. [59]. Copyright 2021 KeAi Communications Co.
Mechanical filtration utilizes non-charged fibers that trap particles via gravitational sedimentation, inertial impaction, interception, and Brownian diffusion [[59], [60], [61]]. For large particles in the size range of 1 to 10 μm, mechanical filtration in the form of gravitational sedimentation (Fig. 5b) and inertial impaction (Fig. 5c) is the most effective mechanism in capturing particles. Larger particles have greater inertia, and hence they move more linearly and are unable to flow around mask fibers. Consequently, they become stuck to fibers and cannot pass through the filter. On the other hand, smaller particles closely follow streamlines and can avoid contact with fibers via impaction and sedimentation. For small particles in the size range of 0.1 to 1 μm, interception and Brownian motion are the most prevailing mechanical mechanisms of filtration. Small particles up to 0.6 μm move along streamlines, leading them to be intercepted by filter fibers when they move closely to the fibers (Fig. 5d) [59]. For even smaller particles, especially those <0.2 μm, filtration is dominated by Brownian motion, as the random movement of these particles leads to their contact with fibers by diffusion (Fig. 5e). Once in contact, the particles adhere to the fiber via van der Waals attraction and remain stuck [59,62,63].
Electrostatic filtration uses charged fibers, called electrets, which have a quasi-permanent electric field to electrostatically attract and filter particles [64]. Charged particles, even those smaller than the pore sizes of a filter, can be attracted by electrets with the opposite charge (Fig. 5f). In theory, electrostatic attraction is independent of particle size, as Coulomb's law is independent of mass. However, the momentum of larger particles is greater, and hence the path of large particles is mostly straight. In comparison, the path of smaller particles is much less linear and predicable, and thus they have a higher tendency to be captured by electrostatic attraction [56,60]. It should be noted that the charges on fibers do not last forever, and if the filter is sufficiently loaded with particles, its electrostatic forces will be reduced over time, eventually behaving as a regular mechanical filter [65,66].
The application of electrostatic forces can significantly improve filtration efficiency of a fibrous filter, particularly for particles in the size range of 0.15–0.5 μm [67]. The addition of electrostatically charged fibers helps improve filtration efficiency at a low fiber-packing density, and therefore allows for reduced differential pressure and enhanced breathability. The efficiency of electrostatic filtration depends on the chemical composition of the particles and fibers, the charge on the particles, and the surface charge density of the fibers. Another important consideration is the accumulation of particles in the filter, also known as the particle loading. The effect of particle loading is different for solid particles and liquid droplets and aerosols [67]. Deposited liquid droplets typically form a thin film on the fiber surface, whereas solid particles have the tendency to take the shape of irregular chains in the absence of electrostatic forces [67]. In general, the efficiency of an electret filter decreases with particle loading in the early stages of filtration. However, different fiber materials exhibit complex time-dependent behavior [67]. Further study is required in this area to fully understand the effects of particle loading on filtration efficiency.
Overall, the PFE of a mask is determined by multiple factors, including those related to the mask itself, such as its pore size, fiber charge, and fit, and those related to external conditions, such as the particle size, shape, charge, and load, the airflow rate and flow conditions, the relative humidity (RH), and environmental temperature [59,68]. Among all external conditions, it has been found that the airflow rate has the most significant effect on the PFE of a mask. Interestingly, the effect of the airflow rate on the PFE can be opposite for large and small particles since different filtration mechanisms are responsible for those particles. For large particles, increasing the airflow rate would increase the PFE as a result of the larger centrifugal and inertial forces, which favor the capture of large particles by sedimentation and impaction. For small particles, decreasing the airflow rate would increase the PFE because there is more time for electrostatic attraction and Brownian diffusion to capture the particles [59,69]. Hinds and Kraske found that the effect of airflow rate on large particles (>1 μm) was insignificant, whereas the PFE for smaller particles was indeed reduced at higher flowrates [70]. As such, for the vast majority of masks, including N95s, the PFE appears to decrease at higher flow rates.
3.3. Mask testing methods
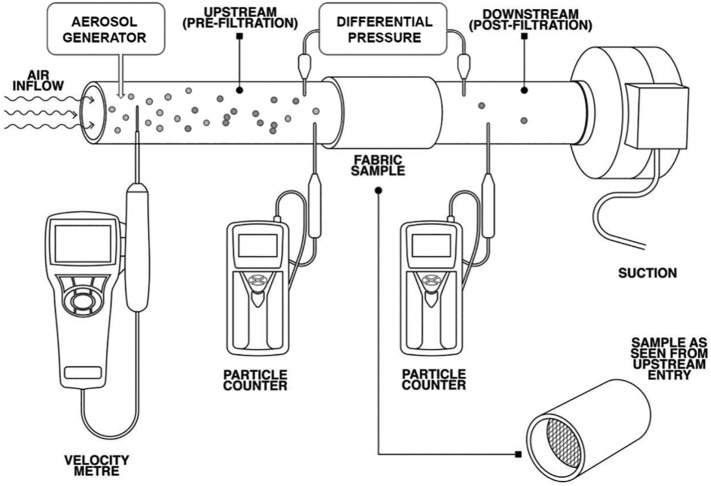
Most laboratory tests measure the PFE of face masks using a generalized conceptual setup shown in Fig. 6 [60,71,72]. A mask or its fabric sample is fixed in the cross-section of an airtight test tube with diameters ranging from 2.5 cm to 60 cm. Airflow is generated by downstream suction and the flowrate is measured with a velocity meter, such as the TSI thermal anemometer (model #AVM440). The airflow rate is controlled in the range of 1.2 CFM (~35 L/min) and 3.2 CFM (~90 L/min), which mimics human respiration rates under light and heavy workloads, respectively [65,73]. Aerosols are generated upstream using an aerosol generator, e.g., TSI particle generator (model #8026), which produces multidispersed NaCl aerosols with sizes ranging from tens of nanometers to approximately 10 μm. Aerosol concentrations before and after passing through the mask or the fabric sample are measured with a particle counter, such as the TSI optical particle sizer (model #3330), which counts particles between 0.3 and 10 μm in diameter. Breathability is determined by measuring the pressure difference (ΔP) across the mask or fabric sample using a differential pressure meter, e.g., TSI digital manometer (model #AXD620). An ideal mask should have a high PFE and a low ΔP.
Fig. 6.
General design of an experimental setup for quantitatively determining the particle filtration efficiency (PFE) of a mask or fabric material. The mask is fixed in the cross-section of an airtight test tube. Airflow is generated by downstream suction and the flowrate is measured with a velocity meter. Aerosols are generated upstream with an aerosol generator, and the aerosol concentrations before and after passing through the mask or the fabric sample are measured using a particle counter. Breathability is determined by measuring the pressure difference across the mask using a differential pressure meter. Modified with permission from ref. [72]. Copyright 2020 BMJ Publishing Group Ltd.
Surgical masks are regulated in the United States by the FDA and must undergo quality tests standardized by ASTM [68]. The ASTM PFE test uses un-neutralized latex sphere aerosols, ~0.1 μm in size, at a flowrate of 28.3 L/min and 30–50% RH [68]. The BFE test uses aerosolized bacteria, normally Staphylococcus aureus, around 3.0 μm in size, at a flowrate of 28.3 L/min [58,68]. The fluid resistance test uses synthetic blood which is squirted on the mask at varying pressures. In addition, there are flame resistance and differential pressure (ΔP) tests. A ΔP ≥0.5 cmH2O (49 Pa) is considered uncomfortable due to low breathability [74]. The viral filtration efficiency (VFE) is sometimes tested, although it is not required by the FDA nor is it standardized by ASTM. This test usually follows the same protocol as the BFE test, typically using the bacteriophage ΦX174 instead of S. aureus [58]. It has been found that the VFE of a mask is usually very close to its BFE [68].
The NIOSH 42 CFR Part 84 outlines the test necessary for a respirator to be approved as an N95. The test uses NaCl aerosols, about 0.3 μm in size, at an airflow rate of 85 L/min, corresponding to pulmonary ventilation during heavy exercise. The aerosols must be charge-neutralized, and the mask must be preconditioned at 38 °C and 85% RH for 24 h before the test. The NIOSH test is considered the strictest and most conservative test for evaluating the PFE of a mask [68]. A mask with a PFE >95% determined by the FDA's criteria may have an efficiency of <70% under the NIOSH N95 criteria. A surgical N95 mask must pass both the FDA and NIOSH requirements.
In addition to these standard methods, several low-cost testing methods have been developed for laboratory and even household tests. For example, Fischer et al. have developed a $200 setup capable of qualitatively evaluating mask efficiency during speech [75]. When an individual wearing a mask spoke inside a dark box, the propagated droplets were visualized using a laser and recorded using a cell phone camera. Neupane et al. have developed an optical microscopy setup, using a $20 sapphire ball lens, a smartphone camera, a machine shop aluminum plate, and a white LED [76,77]. This device was capable of rapidly screening face mask fabrics to determine the pore size of cloth masks, where a smaller pore size implied a higher PFE. Thus, it is implicitly assumed that the masks tested do not use electrostatic filtration .
3.4. Material and structure of N95 and surgical masks
The filters of N95 and surgical masks are mostly made of non-woven fibrous materials. The process of manufacturing non-woven fabrics is relatively easy and inexpensive, making it especially useful for mass-production of disposable masks. Due to their high electrical resistance and stability, polymeric materials such as polypropylene (PP) and polyacrylonitrile are most commonly used in masks [56]. These filter materials are capable of capturing relatively large particles (> 0.3 μm) by sedimentation and impaction, and relatively small particles (< 0.2 μm) by diffusion and electrostatic attraction [56]. The process of manufacturing non-woven micro- and nanofibers usually involves one of two techniques, meltblowing and spunbonding, in which extremely fine molten polymeric fibers are mechanically, chemically, or thermally joined together, creating a fine mesh of web-like fabric that is irregular in pore size and distribution, unlike traditional woven materials [58].
In terms of their structures, both N95 and surgical masks are composed of multilayers of non-woven fibrous materials. Surgical masks generally consist of three layers, and N95 masks have one additional middle layer to provide extra support and fit. The outer layer facing the air is typically made of hydrophobic, spunbonded, non-woven PP that repels moisture and prevents aqueous droplets from penetrating and damaging the filter. In general, this spunbonded PP layer has a fiber diameter of 20 μm and a pore size of up to 100 μm (Fig. 7c) [78]. The spunbonded PP features a weak electric charge, with a dipole charge density less than 0.5 μC/m2 [79]. Consequently, this layer has a rather poor PFE of only 6–10% [78,79]. The innermost layer is designed to absorb moisture released during exhalation and keep the mask dry, with the aim of blocking pathogen growth. The middle layer is typically made of meltblown non-woven fabric, which is the primary layer responsible for particle filtration. The meltblown PP used in N95 and surgical masks do not differ significantly in terms of their pore sizes, basis weights, and bulk densities [78]. In general, the meltblown PP used in N95 and surgical masks have a similar fiber diameter in the range of 1–10 μm, and a pore size of around 20 μm (Fig. 7a and b) [78]. However, the meltblown PP used in N95 masks usually has a much greater charge than that used in surgical masks, with a dipole charge density of around 17.3–27.2 μC/m2 [79]. Consequently, the PFE of meltblown PP used in N95 masks is generally greater than 95%, while the PFE of meltblown PP used in surgical masks usually ranges from 19 to 33% [78].
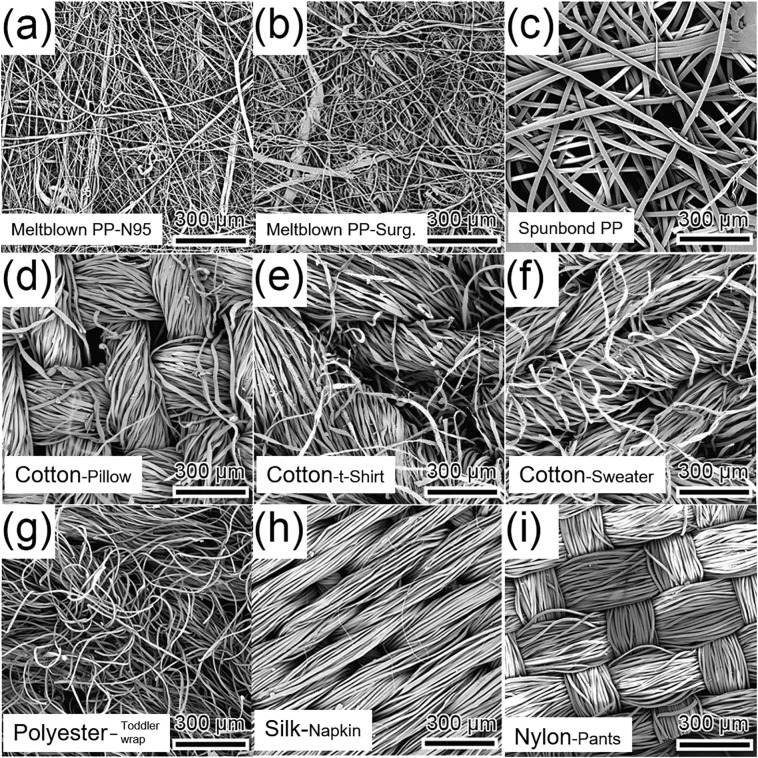
Fig. 7.
Scanning electron microscopy (SEM) images of various mask filter materials. (a) Meltblown (non-woven) polypropylene (PP) from a N95 mask. (b) Meltblown (non-woven) PP from a surgical mask. (c) Spunbonded (non-woven) PP. (d) Cotton (woven) from a pillow cover. (e) Cotton (knit) from a t-shirt. (f) Cotton (knit) from a sweater. (g) Polyester (knit) from a toddler wrap. (h) Silk (woven) from a napkin. (i) Nylon (woven) from exercise pants. Adapted with permission from ref. [78]. Copyright 2020 American Chemical Society.
3.5. Efficacy and design of cloth masks
Cloth masks, unlike N95 and surgical masks, are not standardized nor regulated by any government agency. As such, the design of cloth masks is very diverse, varying in the material, threads per inch (TPI), propensity for electrostatic charge, etc. Comparing the efficacy of different cloth masks is not a trivial task because of vast variations in their fabric, thread count, number of layers, fit, and design. In general, the PFE of cloth masks is expected to be less than that of N95 and surgical masks (Fig. 8a) [63].
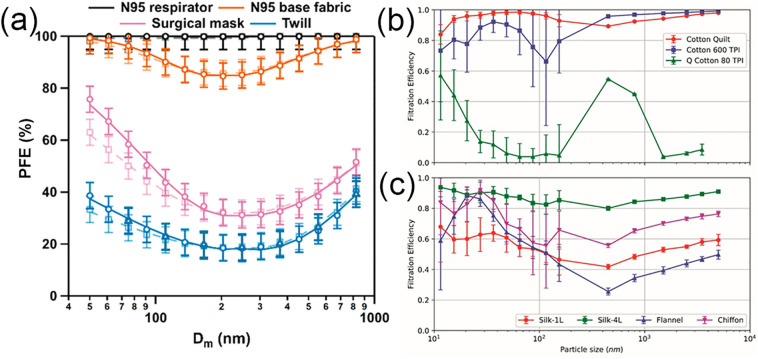
Fig. 8.
Comparison of the PFE of N95, surgical masks, and several cloth mask materials. (a) The PFEs of an N95 mask (black), the N95 base fabric (orange), a surgical mask (pink), and a cotton/polyester (65%/35%) twill blend (blue), as a function of the particle mobility diameter (Dm). This comparison suggests that the PFE ranks in the order of N95 mask > N95 base fabric > > Surgical mask > Cloth mask. It also shows that the most penetrating particle size (MPPS) of the surgical mask and cloth mask is in the range of 200–300 nm. Adapted with permission from ref. [63]. Copyright 2020 American Chemical Society. (b) The PFEs of cotton fabrics with different thread counts, including a cotton quilt consisting of two 120 TPI cotton sheets enclosing a ~ 0.5 cm thick cotton batting (red), 600 TPI cotton (blue), and 80 TPI quilted cotton (green). (c) The PFEs of different fabric materials, including one layer of natural silk (red), four layers of natural silk (green), one layer of flannel (cotton/polyester, blue), and one layer of chiffon (polyester/Spandex, purple). Adapted with permission from ref. [60]. Copyright 2020 American Chemical Society.
Using modified NIOSH N95 tests, it was found that, in general, materials with a higher thread count, i.e., smaller pore size, result in higher filtration efficiencies [64]. For example, Konda et al. found that the mean PFE of 600 TPI and 80 TPI cotton was 98% and 14%, respectively, for particles larger than 300 nm, and 79% and 9%, respectively, for particles smaller than 300 nm (Fig. 8b) [60]. Likewise, Zhao et al. measured the PFE of cotton from a sweater, t-shirt, and pillow cover, listed in descending order of their basis weights (g/m2) [78]. Their corresponding PFEs were found to be 26%, 22%, and 5%, respectively. The correlation between the PFE and thread counts of the cloth masks may be explained by pore size, as the pore size of the pillow cover is much larger than that of the sweater (Fig. 7d and f). Nevertheless, it should be noted that a higher thread count also results in a greater ΔP, i.e., the fabric is less breathable. For example, the ΔP of 600 TPI cotton is 2.5 Pa, whereas for 80 TPI cotton it is 2.2 Pa [60]. The ΔP of cotton from the sweater, t-shirt, and pillow cover is 14.5, 17.0, and 4.5 Pa, respectively [78].
Among common materials, the most effective fabric is cotton, while silk performs the worst. Using the NIOSH N95 tests, Konda et al. found the mean PFE of 600 TPI cotton, chiffon, natural silk, and flannel to be 98%, 73%, 56%, and 44%, respectively, for particles larger than 300 nm, and 79%, 67%, 54%, and 57%, respectively, for particles smaller than 300 nm (Fig. 8c) [60]. Similarly, Zhao et al. found the mean PFE of t-shirt cotton, nylon, polyester, and silk to be 22%, 23%, 18%, and 5%, respectively [78]. A BFE test with B. atrophaeus and a VFE test with the bacteriophage MS2 also showed similar results. For cotton, linen, and silk, the measured BFEs were 69%, 60%, and 58%, respectively, and the VFEs were 51%, 62%, and 54%, respectively [58]. It should be noted that even for cloth masks made of the same material, the individual PFE of the masks can be very different. This is because fabrics made of the same material, such as cotton, may have vastly different structures, weave patterns, and pore sizes (Fig. 7d-f).
The filtration efficiency of a cloth mask is also significantly affected by its number of fabric layers. Bahl et al. showed that a double-layer cloth mask made of t-shirt material was able to significantly reduce the spread of droplets generated by a cough and sneeze, in comparison to its single-layer counterpart [80]. Research shows that the PFE of cloth masks can be significantly improved by using multiple layers of the same or different materials (Fig. 8c). Taking advantage of the mechanical integrity of fabrics such as cotton, and the electrostatic attraction of fabrics such as silk, it was found that the PFEs of cloth masks made of cotton/silk, cotton/chiffon, and cotton/flannel were all higher than 90% [58]. However, these masks also had the greatest ΔP, at 3.0 Pa, in comparison to 2.5–2.7 Pa for these materials in single layers [60]. Another way to design multilayered cloth masks is to use both hydrophobic and hydrophilic layers, with the former acting to prevent water and aerosol buildup on the mask, and the latter aiding in attracting and adhering droplets to the filter [78,81]. Based on available evidence, the CDC recommends that the general public wear cloth masks with two or more layers to limit the spread of COVID-19 [49].
3.6. Comparison of N95, surgical, and cloth masks
The WHO and the Public Health Agency of Canada recommend that healthcare workers use surgical masks in the presence of COVID-19 patients, and N95 masks when performing aerosol generating procedures (AGP), such as tracheal intubation, tracheotomy, and bronchoscopy, among others [82,83]. The CDC and the European Centre for Disease Prevention and Control (ECDC) recommend the use of N95 masks for healthcare workers performing non-AGP as well [82]. Many studies have shown that surgical masks are just as effective as N95 masks in regard to protection from viral pathogens, concluding that the usage of N95 masks did not result in a significantly lower risk of respiratory infection compared to surgical masks [50,58,82,[84], [85], [86]]. A randomized controlled trial of 2862 healthcare personnel reported no significant differences in laboratory-confirmed influenza infection among users wearing N95 or surgical masks, with incidence rates of 8.2% and 7.2%, respectively [30]. Likewise, Smith et al. and Long et al. found no significant differences in laboratory-confirmed illnesses in healthcare workers and users who wore N95 vs. surgical masks [84,86]. Offeddu et al. also found no significant differences for SARS and influenza (H1N1) infections, although wearing an N95 or surgical mask reduced the risk of SARS transmission by approximately 80%, compared to not wearing a mask [87]. Thus, we conclude that there is a lack of substantial evidence from meta-analyses to support the claim that N95 masks are significantly superior to surgical masks in protecting healthcare workers caring for COVID-19 patients during non-AGP [82,84].
Laboratory testing, however, is fairly conclusive and consistent in establishing the superior performance of N95 over surgical masks in terms of their different PFEs (Fig. 8a). Using a VFE test with bacteriophage MS2, Balazy et al. demonstrated that N95 masks routinely outperformed surgical masks [65]. When being evaluated with the NIOSH NaCl test, Rengasamy et al. found that the lowest PFE for selected N95s was 98.15%, whereas the surgical masks had a mean PFE ranging from 54.72% to 88.40% [68]. Another NIOSH NaCl test found that the PFEs of different surgical masks were remarkably inconsistent, varying from 1.58% to 88.1%, while the PFEs of all N95 masks tested were consistently higher than 99.14% [57]. It should be noted that the PFE of a mask also depends on the size of particles used in the test. It was found that the most penetrating particle size (MPPS) ranges from 30 to 100 nm for N95s [65,88,89], and 200 to 500 nm for surgical masks [90]. Therefore, one should consider the PFE data reported in literature with caution, given the many different experimental conditions used by researchers and vast product-to-product variations, even for masks in the same category, as demonstrated by the surgical masks.
The difference in PFE between N95 and surgical masks becomes much less pronounced if there is a lack of a proper seal/fit and leakage occurs. At the airflow rate of 8 L/min, intact N95 and surgical masks had very different total inward leakage (TIL) of 0.31% and 3.58%, respectively [57]. The TIL refers to all aerosols that pass through the mask, including those from leaks due to poor fit. Nevertheless, after introducing six 3-mm holes on both masks, the differences in TIL between the N95 and surgical masks became statistically insignificant (38.72 vs. 35.67%) [57]. This leaking effect may explain why there seemed to be no significant differences between N95 and surgical masks in healthcare workers, as strict compliance with PPE all the time is very unlikely [87].
As for the comparison to cloth masks, although there have been studies that showed their usefulness in preventing the spread of COVID-19 for the public [56,91], it is important to bear in mind that cloth masks should not be used as a replacement for surgical masks or N95 masks and are not recommended for healthcare workers [58]. The PFEs of cloth masks are generally much lower than those of N95s (Fig. 8a). A cluster randomized trial of 1607 healthcare workers found that the attack rate of viruses was much higher with cloth masks than with surgical masks [92]. Furthermore, the attack rate of influenza-like viruses is significantly higher with cloth masks. The risk of being infected by influenza is 12 times greater when wearing cloth masks compared to surgical masks [92]. In addition, as shown in Fig. 8b and c, the PFE of cloth masks varies significantly with respect to their materials, thread counts, and layers. Hence, the filtration efficiency of cloth masks reported from literature may not be directly comparable.
4. Decontamination and reuse of masks
Since the start of the COVID-19 pandemic, there has been a noticeable global shortage of PPE [93]. A survey conducted by the National Nurses United (NNU) reported that 87% of nurses nationwide had to reuse disposable masks, and 27% of them had to provide care to COVID-19 patients without proper PPE between April 15 and May 10, 2020. This PPE shortage is mainly due to four factors: hospital budgets, demand shock, government failures, and fragile supply chain. Firstly, PPE in hospital was usually operated on a cost-passing model, meaning it is billed to the patient/insurer. As a result, there was no incentive to stockpile PPE, which has a limited lifespan [94]. Secondly, the sudden increase in demand for PPE due to the wide spread of COVID-19 led to a rapid and sharp decrease in PPE inventories. Many non-medical users, including public consumers, bought up a significant portion of available PPE, which reduced the already-limited inventory available for use by healthcare workers. Furthermore, many governments were slow to implement policies to raise PPE production and also provided poor guidance to private sector PPE distributors [94]. The uncertain supply chain has also impacted the shortage. For example, the United States has historically relied heavily on other countries to supply PPE, especially China, the world's leading exporter of PPE [94,95]. In 2018 and 2019, the US imported 31.7 and 33.8% of its supply of face masks from other countries, respectively [94]. The US-China trade war and PPE export restrictions from many countries have further exacerbated the shortages [96,97]. This shortage has also led to a drastic increase in the prices of existing PPE. In March, the WHO noted that the prices of N95 and surgical masks had increased by twofold and sixfold, respectively [96]. While the situation has improved recently, there are still PPE shortages worldwide. To address this, China has ramped production of surgical masks from 20 million per day to >110 million per day [96,97]. However, PPE shortages remain a serious problem amid this pandemic, especially in less wealthy countries.
The global shortage of PPE necessitates the development of methods to decontaminate and reuse N95 and/or surgical masks, even though they are not designed to be reused [58,98]. These methods need to meet a few requirements. Firstly, they must be able to successfully decontaminate the mask, inactivating all pathogens. This is routinely tested using an inoculating pathogen, such as H1N1, by detecting its presence pre- and post-treatment of the mask. Secondly, the decontamination method must not deteriorate the mask in such a way that its integrity, quantified by the QNFT and NIOSH NaCl test, is significantly compromised. Microscopy is useful for determining any fiber or structural variations after decontamination. Thirdly, there must not be any chemical residue or by-products left on the mask that may have an adverse health effect on the user. In addition, the cost and availability of the necessary resources and equipment, the ease and safety of the treatment, and the scalability of the method should all be taken into consideration.
Here we summarize a few methods for mask decontamination, including ultraviolet germicidal irradiation (UVGI), dry and moist heat, vaporized hydrogen peroxide (VHP), ethylene oxide (EtO), ethanol treatment, and emerging nano-enabled, self-cleaning, and reusable masks. The main characteristics of the most commonly used decontamination methods, including UVGI, dry and moist heat treatments, and VHP, are summarized in Table 3 . It should be noted that these decontamination methods are mostly developed for reusing N95 masks, with different regulations in different countries [99]. Cloth masks, on the other hand, are not strictly regulated. MacIntyre et al. showed that dirty cloth masks used by healthcare workers can be a source of contamination [100]. The CDC recommends washing cloth masks whenever they get dirty, or at least daily, using regular laundry detergent or soap [49]. After repeated wash-dry cycles, cloth masks should be disposed of, since their PFE decreases due to surface stretching, which enlarges pore sizes [76,77].
Table 3.
Summary of mask decontamination methods.
| Decontamination method | Equipment needed | Effect on viral and bacterial inactivation | Impact on particle filtration efficiency (PFE) | Impact on physical properties of masks | Safety risk and other considerations |
|---|---|---|---|---|---|
| Ultraviolet germicidal irradiation (UVGI) |
|
|
|||
| Dry heat treatment |
|
|
|
|
|
| Moist heat treatment |
|
|
|
|
|
| Vaporized hydrogen peroxide (VHP) |
|
|
|
4.1. Ultraviolet germicidal irradiation
Ultraviolet germicidal irradiation (UVGI) uses high-energy ultraviolet electromagnetic waves to inactivate viruses and microbes on a mask. The UV spectrum consists of four wavelength fragments, one of which, UVC, in the wavelength range of 200 to 280 nm, covers the most readily absorbed wavelengths by nucleic acids (i.e., 260 nm) and proteins (i.e., 280 nm) [101,102]. During the UV treatment, photons are absorbed by viral or microbial nucleic acids, thus damaging DNA/RNA and preventing replication [101,103,104]. UVGI also damages the protein capsid, albeit to a lesser extent [101]. UVGI is particularly effective against small microbes or viruses [103]. The larger the pathogen, the less effective UVGI is against it. The process of UVGI is very fast, taking only 60–70 s to effectively inactivate coronaviruses [103]. Its effectiveness is largely dependent on the intensity of radiation, measured in J/cm2, and the length of exposure.
The highest estimated contamination level or pathogen load on a mask would require a 3-Log reduction, corresponding to 99.9% reduction, in order to be considered fully disinfected [105]. Torres et al. demonstrated that a UVGI dose of 1 J/cm2 delivered over 60–70 s resulted in a 3-Log reduction on the fifty-percent tissue culture infectious dose (TCID50) of inoculated H1N1 on an N95 mask [103]. At low UV doses (<10 J/cm2), the PFE, ΔP, and physical appearance of the mask were largely unaffected [[106], [107], [108], [109]]. The PFE remained >99% for both the 3 M ™ 1860s and 1870s N95 masks while achieving a 4-Log reduction (>99.99%) on the TCID50 of H1N1 [106,110].
Nevertheless, excess exposure to UV, whether it is the intensity, duration, or the number of cycles, can greatly deteriorate masks [106,111]. Lindsley et al. tested various N95 masks with UV doses from 120 to 950 J/ cm2, on one side at 27 °C and 25% RH [104]. Using the NIOSH NaCl test at a flowrate of 5 L/min, the PFE was found to be significantly reduced by UVGI treatment. The elastic straps of the N95 masks were found to be the most vulnerable to UV degradation, with their mean breaking strength decreased by 10–21% at 590 J/cm2 [104].
It should be noted that N95 masks include a variety of types that differ in shape (e.g., cup, flat-fold, or pouch) and design features (e.g., pleats, ridges, and flaps) [105,106]. The latter may create shadows and areas of the mask where the UVGI is limited or unable to reach, thus causing non-homogeneous disinfection [106]. Another disadvantage of UVGI is that it is not readily available on a widespread scale. Most UVGI systems use low-pressure mercury lamps, which carry the risk of mercury pollution, and have led to a 2013 UN proposal to ban their use [103]. In addition, these lamps produce ozone which has toxic effects [103]. Alternatively, UVC-LED sources can be used instead [112]. However, UVC-LED has lower irradiance levels and thus more irradiation must be used, which can greatly increase cost [103].
4.2. Heat treatment
Heat treatment, including dry heat and moist heat, decontaminates a mask by irreversible coagulation and denaturation of microbial or viral proteins, usually at a temperature above 70 °C [106,113] .It has been predicted that exposure to 70 °C for only 3 min can inactivate SARS-CoV-2 by a 3-Log (99.9%) reduction [114]. Because the PP fibers used in N95 and surgical masks have a thermal degradation point of 130 °C, heat treatments should not exceed this temperature [115]. In practice, mask decontamination with heat treatments is usually operated in the temperature range between 70 and 80 °C.
Being a simple and easily scalable method, dry heat treatment uses high-temperature dry air to decontaminate a mask [115,116]. It does not require any specialized equipment other than common laboratory ovens, or even household rice cookers [106,115,117]. Microwave ovens are not recommended because microwave irradiation may lead to fiber melting [107]. Autoclaves are also discouraged as their high operation temperature and pressure lead to mask deterioration. Grinshpun et al. found severe damage to the nose clip seal and loss of strap elasticity of N95s, enough to render the masks unusable, after an autoclave treatment at 120 °C and 15 psi for 30 min [98]. One study showed that a 5-min exposure to a 70 °C environment led to a sixfold reduction in TCID50 of SARS-CoV-2 [118]. Another study showed that a 60-min exposure in a 70 °C cabinet successfully reduced SARS-CoV-2 on N95 masks to an undetectable level [115,119]. The mean fiber diameter of the mask only changed slightly from 3.88 μm to 4.21 μm after 10 cycles of dry heat treatment [115]. All heat-treated masks passed the QNFT and had a PFE >95% [115]. The BFE also exceeded 99.9% after 70 °C dry heat treatment in a laboratory incubator for 5–90 min, after 3 treatments [116]. However, higher temperatures cause a greater risk of material damage. A laboratory oven at 80 °C showed minor warping, shrinkage, and edge cracking on N95 masks, whereas lower temperatures did not [120].
The moist heat treatment uses heated high-pressure steam to decontaminate masks [106]. It is suggested that an RH of 50–85% is the most effective in inactivating microbes [118]. Microwave-generated steam took only 2 min to produce viral inactivation [103]. It was shown that moist heat treatment at 70 °C and 50% RH for 60 min successfully inactivated SARS-CoV-2 on N95 masks [115]. In another study, moist heat treatment via a common household rice cooker for 8–10 min, followed by 5 min of steaming, led to a 5-Log reduction in MS2 and S. aureus on N95 masks [121]. In comparison, dry heat treatment in an oven at 100 °C for 15 min only led to a 3-Log reduction for the same microbes [121]. The mean fiber diameter increased from 3.88 μm to 3.92 μm after 10 cycles of moist heat treatment at 50% RH, having less of an effect on the fiber diameter than dry heat treatment. It was also found that the moist heat treatment had little effect on the PFE, BFE, QNFT, and ΔP [106,115].
4.3. Vaporized hydrogen peroxide sterilization
Vaporized hydrogen peroxide (VHP) sterilization is the only method currently approved by the FDA for mask decontamination and is commercially available as the Battelle Critical Care Decontamination System (CCDS)™ [122]. Hydrogen peroxide is an oxidizing agent, producing reactive hydroxyl radicals that attack nucleic acids and proteins in order to inactivate viruses[123,124]. The vaporized form of hydrogen peroxide is more effective due to its ability to penetrate, spread out, and cover large surface areas, thus allowing for uniform contact with the mask [103]. In addition, VHP decomposes into oxygen and water vapor over time, leaving no hazardous chemical residues on the decontaminated mask [107].
Saini et al. used 11–12% VHP stabilized with silver nitrate to treat N95 masks contaminated with B. stearothermophilus spores at 23–38 °C [123]. B. stearothermophilus was used as it survived in all conditions known to inactivate SARS-CoV-2. After the VHP treatment, there was no revival of any spores and the mask was successfully disinfected for all temperatures tested. There were also no significant changes in the droplet permeability, physical appearance, and ultrastructure of the masks post-VHP treatment, even after 15 decontamination cycles [123]. Another study confirmed that a 55-min VHP treatment did not significantly change the PFE, ΔP, fit, and physical appearance of N95 masks [107]. Similar conclusions were drawn by Steinberg et al., based on a systemic literatue review [118].
Nevertheless, VHP treatment has a number of limitations. Firstly, it is a fairly expensive technology that requires specialized devices [125]. Secondly, it was found that the VHP treatment may tarnish the metal nose bands of N95 masks [107] and cause degradation of the elastic bands of the masks, especially after repeated treatment cycles [58,103]. Thirdly, prior to VHP treatment, the clearance of dirt and other impurities from the masks is necessary, since hydrogen peroxide can be deactivated by organic debris [103]. For the same reason, VHP treatment cannot be applied to masks and respirators that contain cellulose-based materials or exhalation valves [115]. In addition, masks and respirators after VHP treatment cannot be used immediately due to the presence of hydrogen peroxide residues on the inner surface. It was found that the level of hydrogen peroxide decreased to 0.6 ppm, i.e., below the safety limit of 1 ppm, 2 h after the treatment, and became undetectable after 3 h [111].
4.4. Ethylene oxide treatment
Ethylene oxide (EtO) gas has long been used in the healthcare industry as a disinfectant for medical devices. This disinfection process is fairly similar to that of VHP. Viscusi et al. implemented a decontamination process for masks using 100% EtO gas for 1 h, followed by 4-h aeration at 55 °C [107]. It showed no effect on the PFE, ΔP, or appearance of the masks [107]. Other experiments also found similar results, with the PFE >95% [106,125]. However, there is a general concern about this method, as EtO is a known carcinogen and mutagen that can cause severe adverse health effects [106]. Residual EtO was generally not found to be a risk, as it was either undetected on the masks or found in a small amount (500 ppb), which is only half the permissible exposure limit set by AccuStandard [107,125]. However, Salter et al. found a trace amount of 2-hydroxyethyl acetate, a possible carcinogen and likely a byproduct of EtO treatment, presented on masks post-treatment [125]. The potential hazard of EtO treatment necessitates further research before it can be adopted as a general method for mask decontamination.
4.5. Ethanol treatment
Ethanol is a widely used disinfectant for viral and bacterial pathogens. In particular, ethanol has been found to reduce coronavirus infectivity by approximately 4-Log or greater [123]. However, masks disinfected by soaking in 70% ethanol for 2 h, although having a negligible effect on ΔP, showed a marked decrease in the PFE [98]. It was found that the PFE of N95 masks dropped to <90% at a flowrate of 30 L/min, and < 70% at a flowrate of 85 L/min, respectively, for the MPPS using the NIOSH NaCl test [98]. Many other studies also found a decrease in PFE after ethanol treatment [106]. Ethanol treatment also appeared to affect the filter efficiency distribution across particle sizes, with the MPPS shifting from 0.2–0.3 μm to 0.5 μm pre- and post-treatment [98]. The significant reduction in the PFE of masks is most likely due to the fact that ethanol compromises electrostatic filtration by removing electric charges from the fibers [[126], [127], [128]]. It was shown that isopropanol treatment reduced the dipole charge density of N95 masks from 17.28–27.29 μC/m2 to near-zero [79]. A similar effect on the masks by ethanol treatment is not unexpected. In addition, the hydrophobic layer of surgical masks could be damaged by concentrated alcohol [120], although ethanol treatment has no visible effects on the masks [98].
4.6. Nano-enabled reusable masks
Nanotechnology has opened new horizons for developing novel reusable, self-cleaning, antimicrobial, and antiviral masks [[129], [130], [131], [132]]. To date, the application of nanotechnology in face masks is mostly centered around the use of antimicrobial/antiviral nanoparticles, nanofibers and nanoparticle coatings to entail superhydrophobicity, and the synergistic effects of water repelling and other self-cleaning functionalities such as nanoparticle-induced photothermal and photocatalytic sterilization.
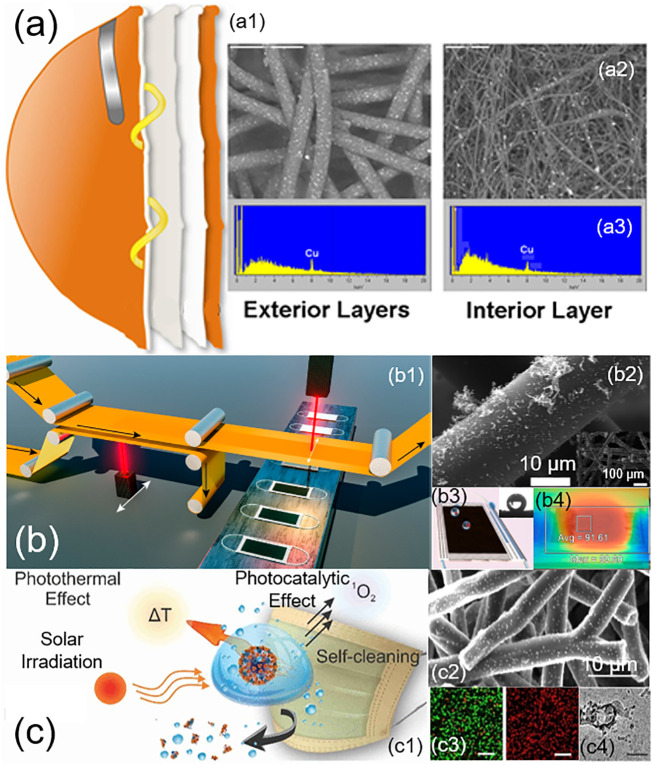
Certain metal nanoparticles, such as silver and copper, can induce potent antimicrobial and antiviral effects by releasing free ions that generate reactive oxygen species, which are capable of damaging DNA, RNA, and proteins [133,134]. Nano-enabled masks with these metal nanoparticles may effectively inactivate SARS-CoV-2 by damaging viral proteins and lipids, most notably the envelope proteins of the virus [130]. By integrating copper oxide nanoparticles into N95 masks (Fig. 9a), Borkow et al. found that these nano-enabled masks were able to inactivate the human influenza A virus (H1N1) and the avian influenza virus (H9N2) to less than 0.88- and 0.97-Log TCID50 respectively, after 30 min [135]. Meanwhile, these nano-enabled masks successfully passed all NIOSH N95 test standards. Similarly, Joe et al. showed that nano‑silver could be loaded onto various textiles and mask filters to inactivate aerosolized bacteriophage MS2 [136]. Although further research is still needed to determine the effectiveness of these nano-enabled masks on SARS-CoV-2, the use of antimicrobial and antiviral nanoparticles appears to hold great promise in developing reusable masks [137].
Fig. 9.
Nano-enabled self-cleaning, antimicrobial, antiviral, and reusable masks. (a) N95 mask impregnated with copper oxide nanoparticles. This mask consists of four layers (a1). The two external spunbond polypropylene layers contain 2.2% copper oxide nanoparticles, and one internal meltblown polypropylene (PP) layer contains 2% copper oxide nanoparticles. Scanning electron microscopy (SEM) micrographs show the ultrastructures of nanoparticle-containing exterior and interior layers (a2), along with X-ray photoelectron spectroscopy (XPS) analysis (a3). Adapted with permission from ref. [135]. Copyright 2010 PLoS ONE. (b) Laser-induced graphene surgical mask. (b1) Illustration of laser-induced forward transfer (LIFT) for roll-to-roll production of graphene-coated masks. (b2) SEM images of the graphene-coated nonwoven fiber. (b3) Illustration of the self-cleaning capacity of the mask, with the demonstrated high water contact angle on the mask. (b4) Photothermal sterilization of the mask. Surface temperature, measured with an infrared camera, increased to an average of 91.61 °C after exposure to sunlight for 5 min. Adapted with permission from ref. [141]. Copyright 2020 American Chemical Society. (c) Photoactive antiviral mask (PAM). (c1) Illustration of the self-cleaning and the antiviral mechanisms of the mask through photothermal and photocatalytic effects in response to solar irradiation. (c2) SEM images of hybrid shellac‑copper nanoparticles coated on nonwoven polypropylene fibers of a surgical mask. (c3) Confocal microscopy images that showed inactivation (represented by red color) of E. coli after 5 min exposure to solar irradiation. (c4) Disruption of virus-like particles by the PAM. Adapted with permission from ref. [143]. Copyright 2020 American Chemical Society.
Another direction taken in the development of nano-enabled masks is to utilize the fine pore sizes generated by nanofibers. El-Atab et al. have developed a reusable nanoporous membrane fabricated with a silicon-based template that is then transferred onto an ultrathin and hydrophobic polymeric film [138]. When attached to an N95 mask, this nanoporous membrane, with a pore size as small as 5 nm, is capable of effectively filtering most particles and pathogens, while having no significant impact on breathability [138]. It was found that electrospun, polymer-based nanofibers, such as polyvinylidene difluoride, offered a number of advantages over the meltblown filters used in N95 masks [139]. These advantages include uncompromised filtration efficiency, higher resistance to ethanol treatment, and sustained hydrophobicity against moisture, thus preventing bacterial growth inside the mask and allowing for multiple reuse cycles. Recently Leung and Sun have developed a charged polyvinylidene fluoride nanofiber mat using corona discharge, which can retain its electrostatic charges for up to three months with only a 1% reduction in filtration efficiency, constituting a promising breakthrough in mask technology [140]. Compared to current microfiber technology typically used in the filtration media of face masks, multimode electrostatically charged nanofiber filters have shown a superior filtration efficiency [140]. Current issues with this technology involve a balance between the nanofiber diameter and the resultant pressure drop. While the use of smaller diameter nanofibers results in enhanced capture of small aerosols less than 0.1 μm, it can also induce much higher flow resistance and thus pressure drop, decreasing breathability [140].
The latest development of nano-enabled masks has taken advantage of the combined benefits of superhydrophobicity and photothermal capacity offered by nanomaterials. Laser-induced forward transfer has been used to additively deposit graphene onto commercial surgical masks (Fig. 9b) [141]. These modified, nano-enabled surgical masks showed excellent superhydrophobicity, which effectively prevents mask contamination by repelling water droplets. Meanwhile, being an excellent broadband absorber, the integrated graphene provided these nano-enabled masks with a photothermal capacity. The surface temperature of these nano-enabled masks can be quickly increased to over 80 °C after 100 s of sunlight illumination [141]. It was found that with the combined superhydrophobic and photothermal effects, these nano-enabled masks inactivated 99.998% aerosolized bacteria after only 10 min of sunlight exposure [142]. Similar synergistic effects can be produced with hybrid shellac/copper nanoparticles. By coating commercial surgical masks with these hybrid nanoparticles, Kumar et al. developed novel photoactive antiviral masks (Fig. 9c) [143]. It was found that these nano-enabled masks can effectively inactivate bacteria, such as E. coil, to a 4-Log reduction, and can also disrupt the membrane of nanosized (~100 nm) virus-like particles made of lipid vesicles.
4.7. Comparison of decontamination methods
There are several meta-analyses that compare the performance of various mask decontamination methods [106,118,[144], [145], [146], [147]]. Although a certain degree of inconsistency exists, it appears that most meta-analyses have drawn the conclusion that VHP is the most reliable and safest method to decontaminate N95 masks for multiple cycles.
A few studies have focused on the impact of different decontamination methods on mask integrity. For example, Liao et al. studied the effects of UVGI, dry heat, moist heat, and ethanol treatments on the PFE of meltblown fabrics used in N95 masks [148]. They found that among all tested methods, moist heat at 85 °C and 30% RH had the least impact on the PFE of the filter fabrics. They were able to perform 50 cycles of the moist heat treatment without significant changes in the PFE. In comparison, UVGI induced small degradation after 20 treatment cycles. Treatments with 75% ethanol significantly decreased the PFE, and thus were not recommended for mask decontamination [148]. Ou et al. studied the effects of UVGI, 77 °C dry and moist heat, and 75% isopropanol treatments on N95 masks [149]. They found that UVGI was able to maintain the PFE of masks up to 10 treatment cycles. Between the two heat treatment methods tested, dry heat showed better compatibility with electret material by sustaining both the PFE and fit. However, the isopropanol treatment significantly decreased the PFE of electret filters by removing their electrostatic charges, and was therefore not recommended for mask decontamination [149].
Most recent studies have compared the performance of different decontamination methods by jointly evaluating their impact on mask integrity and their effectiveness in inactivating SARS-CoV-2. Kumar et al. studied the effect of UVGI, moist heat (70 and 75 °C), VHP, and EtO trestment on N95 masks following experimental contamination with SARS-CoV-2 [150]. They found that all tested methods except for UVGI were effective in eliminating the virus. This was likely due to the low UV doses used in their study, i.e., a total of 1.12 J/cm2, with 0.56 J/cm2 on each side of the mask. All these methods were able to maintain mask integrity after one treatment, while UVGI, moist heat, and VHP were able to maintain mask integrity after 10 decontamination cycles [150].
Smith et al. compared the performance and impact of 70% ethanol, UVGI (18 J/cm2), and VHP, on N95 masks contaminated with pooled clinical SARS-CoV-2 samples [151]. It was found that both ethanol and UVGI caused functional degradation of mask integrity after only one decontamination cycle, as indicated by the decreased fit factor score from 200 to less than 100, while VHP treatment showed no significant effect on the fit factor after two decontamination cycles. It was also found that out of the three decontamination methods, only ethanol treatment eliminated detectable SARS-CoV-2 RNA on all tested masks. The VHP treatment, although it did not completely eliminate infectious SARS-CoV-2 RNA on all masks tested, did result in a 5-Log reduction in SARS-CoV-2 RNA on two healthcare grade N95 masks [151].
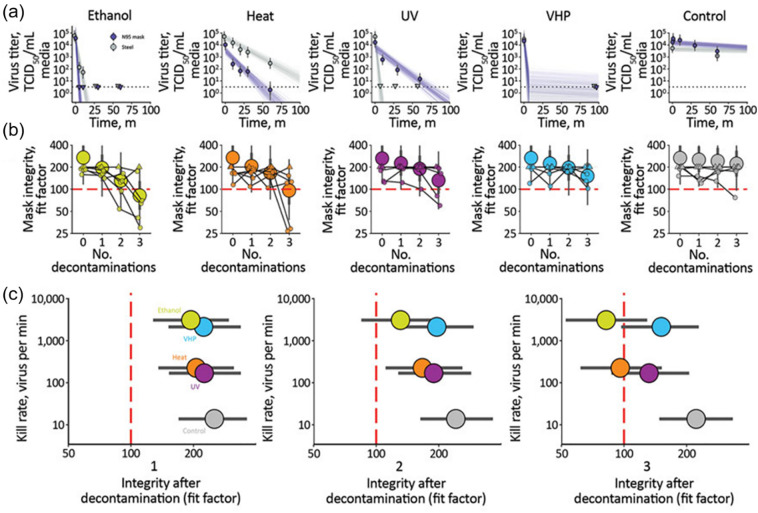
Fischer et al. compared the performance and impact of 70% ethanol, 70 °C dry heat, UVGI (UVC), and VHP on N95 masks, after each decontamination round and two hours of wear, for three consecutive decontamination and wear cycles [152]. These results are shown in Fig. 10 [152]. It was found that both VHP and ethanol yielded extremely rapid inactivation on N95 masks, in comparison to UVGI and heat treatment (Fig. 10a). All four decontamination methods were found to have negligible effects on the mask integrity after a single decontamination cycle (Fig. 10b). However, subsequent rounds of decontamination caused sharp degradation of the ethanol-treated masks, and, to a slightly lesser degree, the heat-treated masks. After the third decontamination round, only VHP- and UVGI-treated masks maintained a fit factor score higher than 100, indicating an acceptable performance. With an integrated consideration of the virus inactivation rates and impacts on the mask integrity (Fig. 10c), it was found that VHP was the most efficient method in inactivating SARS-CoV-2, while having the least adverse effect on the integrity of N95 masks for all three decontamination cycles. UVGI killed SARS-CoV-2 less rapidly than VHP, but more effectively preserved mask integrity. Dry heat at 70 °C killed the virus with a similar speed to UVGI, but failed to maintain mask integrity after two decontamination cycles. Consistent with other studies [148,151], ethanol treatment significantly impaired the physical integrity of N95 masks, and hence was not recommended [152].
Fig. 10.
Comparison of the performance and the impact of four decontamination methods, i.e., 70% ethanol, 70 °C dry heat, UVGI (UVC), and vaporized hydrogen peroxide (VHP), on N95 masks, after each decontamination round and two hours of wear, for three consecutive decontamination and wear sessions. (a) The inactivation rate of SARS-CoV-2 on N95 filter fabric, quantified with the TCID50, in comparison to the TCID50 of stainless steel. Both VHP and ethanol yielded extremely rapid inactivation on N95 masks, in comparison to UV and heat treatments. (b) Fit factors of N95 masks, quantified with QNFT, after each decontamination round. After the third decontamination round, only VHP- and UV-treated masks maintained an acceptable level of performance. (c) The overall performance of decontamination methods against SARS-CoV-2, after 1, 2, and 3 decontamination rounds, was determined by the virus kill rate, post-treatment mask integrity, and the fit factor. The ethanol treatment is shown in green, heat in orange, UV in purple, VHP in blue, and non-treatment control in grey. VHP treatment displayed the best combination of rapid inactivation of SARS-CoV-2 and preservation of N95 respirator integrity after all three decontamination cycles. Adapted with permission from ref. [152]. Copyright 2021 United States Centers for Disease Control and Prevention (CDC).
5. Concluding remarks
The COVID-19 pandemic has lasted for more than a year and caused a monumental shift in our everyday lives. Respiratory droplets and aerosols have been confirmed as the main transmission routes for SARS-CoV-2. The effectiveness of masks in population-wide scenarios has been proven to be significant, and as a result, universal face-masking has been encouraged by many national and international agencies. Mass vaccinations have been initiated in many countries from the beginning of 2021. However, there are still a number of uncertainties related to the use of vaccines, including the time needed to achieve herd immunity, their efficacy in protecting individuals from SARS-CoV-2 variants, and the probability of vaccinated individuals developing an asymptomatic infection and spreading SARS-CoV-2 to others. Before these questions can be resolved, and before a cure for COVID-19 becomes fully realized, non-pharmaceutical interventions, such as social distancing, face-masking, handwashing, and contact tracing, will likely remain the mainstay of preventative measures against COVID-19, even in the post-vaccination era.
Not all masks are created equal. While laboratory tests generally suggest that N95 masks are superior in performance to surgical masks, population studies in healthcare workers have not documented significant differences. This discrepancy may be due to the lack of proper fit when using N95s. Conversely, cloth masks generally perform poorly compared to N95 and surgical masks in laboratory tests. However, in part because of the global PPE shortage, cloth masks have become the most commonly used PPE by the general public. Despite their shortcomings, community-based research has demonstrated the efficacy of cloth masks in slowing down the spread of COVID-19. Due to a lack of standards and regulations, research is needed to identify the optimal combinations of fabric materials, number of layers, thread counts, and other properties, in order to properly engineer more effective cloth masks. Decontamination methods that allow for the reuse of N95 and surgical masks have been widely studied amid the pandemic. Nevertheless, it should be noted that all N95 and surgical masks are designed for single-use, and all decontamination methods compromise mask integrity and filtration efficiency to a certain degree. There is no one-size-fits-all decontamination method for any type of mask. The creation of standardized procedures for different types, brands, and models of masks should be considered by manufacturers and federal regulators to ensure consistency and reliability in both the production and decontamination processes. We hope this review has provided some insight into the role of face masks in curbing COVID-19 infection, and that the emerging evidence will prompt and crystalize a clear and consistent set of regulations on mask usage for both healthcare providers and the general public.
Declaration of Competing Interest
The authors declare no competing financial interests.
Acknowledgements
We thank Dr. David Easa for valuable discussion about the paper. This research was supported by the National Science Foundation (NSF) grant numbers CBET-1604119 and CBET-2011317 to Y.Y.Z.
References
- 1.World Health Organization, Coronavirus Disease (COVID-19) Pandemic https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed 2021-4-26)
- 2.Buonanno G., Stabile L., Morawska L. Estimation of airborne viral emission: quanta emission rate of SARS-CoV-2 for infection risk assessment. Environ Int. 2020;141:105794. doi: 10.1016/j.envint.2020.105794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morawska L., Cao J. Airborne transmission of SARS-CoV-2: the world should face the reality. Environ Int. 2020;139:105730. doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morawska L., Milton D.K. It is time to address airborne transmission of coronavirus disease 2019 (COVID-19) Clin Infect Dis. 2020;71:2311–2313. doi: 10.1093/cid/ciaa939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang R., Li Y., Zhang A.L., Wang Y., Molina M.J. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc Natl Acad Sci. 2020;117:14857–14863. doi: 10.1073/pnas.2009637117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang S., Mao Y., Jones R.M., Tan Q., Ji J.S., Li N., et al. Aerosol transmission of SARS-CoV-2? Evidence, prevention and control. Environ Int. 2020;144:106039. doi: 10.1016/j.envint.2020.106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zuo Y.Y., Uspal W.E., Wei T. Airborne transmission of COVID-19: aerosol dispersion, lung deposition, and virus-receptor interactions. ACS Nano. 2020;14:16502–16524. doi: 10.1021/acsnano.0c08484. [DOI] [PubMed] [Google Scholar]
- 8.Veldhuizen R.A.W., Zuo Y.Y., Petersen N.O., Lewis J.F., Possmayer F. The COVID-19 pandemic: a target for surfactant therapy? Expert Rev Respir Med. 2020:1–12. doi: 10.1080/17476348.2021.1865809. [DOI] [PubMed] [Google Scholar]
- 9.Balachandar S., Zaleski S., Soldati A., Ahmadi G., Bourouiba L. Host-to-host airborne transmission as a multiphase flow problem for science-based social distance guidelines. Int J Multiphase Flow. 2020;132:103439. [Google Scholar]
- 10.Fennelly K.P. Particle sizes of infectious aerosols: implications for infection control. Lancet Respir Med. 2020;8:914–924. doi: 10.1016/S2213-2600(20)30323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheuch G. Breathing is enough: for the spread of influenza virus and SARS-CoV-2 by breathing only. J Aerosol Med Pulm Drug Deliv. 2020;33:230–234. doi: 10.1089/jamp.2020.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma J., Qi X., Chen H., Li X., Zhang Z., Wang H., et al. Coronavirus disease 2019 patients in earlier stages exhaled millions of severe acute respiratory syndrome coronavirus 2 per hour. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1283. ciaa1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu X., Nethery R.C., Sabath M.B., Braun D., Dominici F. Air pollution and COVID-19 mortality in the United States: Strengths and limitations of an ecological regression analysis. Sci Adv. 2020;6 doi: 10.1126/sciadv.abd4049. eabd4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang D., Shi L., Zhao J., Liu P., Sarnat J.A., Gao S., et al. Urban air pollution may enhance COVID-19 case-fatality and mortality rates in the United States. The Innovation. 2020;1:100047. doi: 10.1016/j.xinn.2020.100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. JAMA. 2020;323:1837–1838. doi: 10.1001/jama.2020.4756. [DOI] [PubMed] [Google Scholar]
- 16.Verma S., Dhanak M., Frankenfield J. Visualizing the effectiveness of face masks in obstructing respiratory jets. Phys Fluids. 2020;32 doi: 10.1063/5.0016018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Majumder M.S., Mandl K.D. Early in the epidemic: impact of preprints on global discourse about COVID-19 transmissibility. Lancet Glob Health. 2020;8 doi: 10.1016/S2214-109X(20)30113-3. e627-e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization Advice on the Use of Masks in the Context of COVID-19: Interim Guidance, 5 June 2020. https://apps.who.int/iris/handle/10665/332293 (accessed 2021-2-15)
- 19.Howard J., Huang A., Li Z., Tufekci Z., Zdimal V., van der Westhuizen H.-M., et al. An evidence review of face masks against COVID-19. Proc Natl Acad Sci. 2021;118 doi: 10.1073/pnas.2014564118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brooks J.T., Butler J.C., Redfield R.R. Universal masking to prevent SARS-CoV-2 transmission-the time is now. JAMA. 2020;324:635–637. doi: 10.1001/jama.2020.13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hendrix M., Walde C., Findley K., Trotman R. Absence of apparent transmission of SARS-CoV-2 from two stylists after exposure at a hair salon with a universal face covering policy — Springfield, Missouri, may 2020. MMWR Morb Mortal Wkly Rep. 2020;69:930–932. doi: 10.15585/mmwr.mm6928e2. [DOI] [PubMed] [Google Scholar]
- 22.Galvin C.J., Li Y.J., Malwade S., Syed-Abdul S. COVID-19 preventive measures showing an unintended decline in infectious diseases in Taiwan. Int J Infect Dis. 2020;98:18–20. doi: 10.1016/j.ijid.2020.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang C.J., Ng C.Y., Brook R.H. Response to COVID-19 in Taiwan: big data analytics, new technology, and proactive testing. JAMA. 2020;323:1341–1342. doi: 10.1001/jama.2020.3151. [DOI] [PubMed] [Google Scholar]
- 24.Eikenberry S.E., Mancuso M., Iboi E., Phan T., Eikenberry K., Kuang Y., et al. To mask or not to mask: modeling the potential for face mask use by the general public to curtail the COVID-19 pandemic. Infect Dis Model. 2020;5:293–308. doi: 10.1016/j.idm.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Canini L., Andréoletti L., Ferrari P., D’Angelo R., Blanchon T., Lemaitre M., et al. Surgical mask to prevent influenza transmission in households: a cluster randomized trial. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cowling B.J., Zhou Y., Ip D.K., Leung G.M., Aiello A.E. Face masks to prevent transmission of influenza virus: a systematic review. Epidemiol Infect. 2010;138:449–456. doi: 10.1017/S0950268809991658. [DOI] [PubMed] [Google Scholar]
- 27.Brienen N.C., Timen A., Wallinga J., van Steenbergen J.E., Teunis P.F. The effect of mask use on the spread of influenza during a pandemic. Risk Anal. 2010;30:1210–1218. doi: 10.1111/j.1539-6924.2010.01428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simmerman J.M., Suntarattiwong P., Levy J., Jarman R.G., Kaewchana S., Gibbons R.V., et al. Findings from a household randomized controlled trial of hand washing and face masks to reduce influenza transmission in Bangkok. Thailand Influenza Other Respir Viruses. 2011;5:256–267. doi: 10.1111/j.1750-2659.2011.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bin-Reza F., Lopez Chavarrias V., Nicoll A., Chamberland M.E. The use of masks and respirators to prevent transmission of influenza: a systematic review of the scientific evidence. Influenza Other Respir Viruses. 2012;6:257–267. doi: 10.1111/j.1750-2659.2011.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radonovich L.J., Jr., Simberkoff M.S., Bessesen M.T., Brown A.C., Cummings D.A.T., Gaydos C.A., et al. N95 respirators vs medical masks for preventing influenza among health care personnel: a randomized clinical trial. JAMA. 2019;322:824–833. doi: 10.1001/jama.2019.11645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saunders-Hastings P., Crispo J.A.G., Sikora L., Krewski D. Effectiveness of personal protective measures in reducing pandemic influenza transmission: a systematic review and meta-analysis. Epidemics. 2017;20:1–20. doi: 10.1016/j.epidem.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Brainard J., Jones N.R., Lake I.R., Hooper L., Hunter P.R. Community use of face masks and similar barriers to prevent respiratory illness such as COVID-19: a rapid scoping review. Euro surveillance : bulletin Europeen Sur les maladies transmissibles = European Communic Disease Bulletin. 2020;25:2000725. doi: 10.2807/1560-7917.ES.2020.25.49.2000725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao J., Shiu E.Y.C., Gao H., Wong J.Y., Fong M.W., Ryu S., et al. Nonpharmaceutical measures for pandemic influenza in nonhealthcare settings-personal protective and environmental measures. Emerg Infect Dis. 2020;26:967–975. doi: 10.3201/eid2605.190994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y., Tian H., Zhang L., Zhang M., Guo D., Wu W., et al. Reduction of secondary transmission of SARS-CoV-2 in households by face mask use, disinfection and social distancing: a cohort study in Beijing. China BMJ Glob Health. 2020;5 doi: 10.1136/bmjgh-2020-002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freedman D.O., Wilder-Smith A. In-flight transmission of SARS-CoV-2: a review of the attack rates and available data on the efficacy of face masks. J Travel Med. 2020;27 doi: 10.1093/jtm/taaa178. taaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doung-Ngern P., Suphanchaimat R., Panjangampatthana A., Janekrongtham C., Ruampoom D., Daochaeng N., et al. Case-control study of use of personal protective measures and risk for SARS-CoV 2 infection, Thailand. Emerg Infect Dis. 2020;26:2607–2616. doi: 10.3201/eid2611.203003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X., Ferro E.G., Zhou G., Hashimoto D., Bhatt D.L. Association between universal masking in a health care system and SARS-CoV-2 positivity among health care workers. JAMA. 2020;324:703–704. doi: 10.1001/jama.2020.12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitze T., Kosfeld R., Rode J., Wälde K. Face masks considerably reduce COVID-19 cases in Germany. Proc Natl Acad Sci. 2020;117:32293–32301. doi: 10.1073/pnas.2015954117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lyu W., Wehby G.L. Community use of face masks and COVID-19: evidence from a natural experiment of state mandates in the US. Health Aff. 2020;39:1419–1425. doi: 10.1377/hlthaff.2020.00818. [DOI] [PubMed] [Google Scholar]
- 40.Hatzius J., Struyven D., Rosenberg I. Face masks and GDP. https://www.goldmansachs.com/insights/pages/face-masks-and-gdp.html
- 41.Karaivanov A., Lu S.E., Shigeoka H., Chen C., Pamplona S. Face masks, public policies and slowing the spread of COVID-19: Evidence from Canada. National Bureau of Economic Research Working Paper Series. 2020;27891 doi: 10.3386/w27891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ridenhour B., Kowalik J.M., Shay D.K. Unraveling R0: considerations for public health applications. Am J Public Health. 2014;104:e32–e41. doi: 10.2105/AJPH.2013.301704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inglesby T.V. Public health measures and the reproduction number of SARS-CoV-2. JAMA. 2020;323:2186–2187. doi: 10.1001/jama.2020.7878. [DOI] [PubMed] [Google Scholar]
- 44.He W., Yi G.Y., Zhu Y. Estimation of the basic reproduction number, average incubation time, asymptomatic infection rate, and case fatality rate for COVID-19: meta-analysis and sensitivity analysis. J Med Virol. 2020;92:2543–2550. doi: 10.1002/jmv.26041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rader B., White L.F., Burns M.R., Chen J., Brilliant J., Cohen J., et al. Mask-wearing and control of SARS-CoV-2 transmission in the USA: a cross-sectional study. The Lancet Digital Health. 2021;3(3):E148–E157. doi: 10.1016/S2589-7500(20)30293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.US FDA Use of Respirators, Facemasks, and Cloth Face Coverings in the Food and Agriculture Sector During Coronavirus Disease (COVID-19) Pandemic. https://www.fda.gov/food/food-safety-during-emergencies/use-respirators-facemasks-and-cloth-face-coverings-food-and-agriculture-sector-during-coronavirus (accessed 2021-2-15)
- 47.Salimnia H., Meyer M.P., Mitchell R., Fairfax M.R., Gundel A., Guru N., et al. A laboratory model demonstrating the protective effects of surgical masks, face shields, and a combination of both in a speaking simulation. Am J Infect Control. 2021;49:409–415. doi: 10.1016/j.ajic.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verma S., Dhanak M., Frankenfield J. Visualizing droplet dispersal for face shields and masks with exhalation valves. Phys Fluids. 1994;2020(32) doi: 10.1063/5.0022968. 091701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.CDC Use Masks to Slow the Spread of COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/diy-cloth-face-coverings.html (accessed 2021-2-15)
- 50.Johnson D.F., Druce J.D., Birch C., Grayson M.L. A quantitative assessment of the efficacy of surgical and N95 masks to filter influenza virus in patients with acute influenza infection. Clin Infect Dis. 2009;49:275–277. doi: 10.1086/600041. [DOI] [PubMed] [Google Scholar]
- 51.EN 14683:2019 . European Committee for Standardization; 2019. Medical Face Masks - Requirements and Test Methods. [Google Scholar]
- 52.Department of Health and Human Services Part 84 — Approval of Respiratory Protective Devices. https://www.govinfo.gov/content/pkg/CFR-2004-title42-vol1/xml/CFR-2004-title42-vol1-part84.xml (accessed 2021-2-15)
- 53.Sousan S., Garcia N., White A., Balanay J.A. Filtration efficiency of surgical sterilization fabric for respiratory protection during COVID-19 pandemic. Am J Infect Control. 2021;49:1–7. doi: 10.1016/j.ajic.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Staymates M. Flow visualization of an N95 respirator with and without an exhalation valve using Schlieren imaging and light scattering. Phys Fluids. 2020;32:111703. doi: 10.1063/5.0031996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.ASTM . West Conshohocken, PA; ASTM International: 2019. F2100-19e1, standard specification for performance of materials used in medical face masks. [Google Scholar]
- 56.O’Dowd K., Nair K.M., Forouzandeh P., Mathew S., Grant J., Moran R., et al. Face masks and respirators in the fight against the COVID-19 pandemic: a review of current materials. Adva Future Perspect Mater. 2020;13 doi: 10.3390/ma13153363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rengasamy S., Eimer B.C., Szalajda J. A quantitative assessment of the total inward leakage of NaCl aerosol representing submicron-size bioaerosol through N95 filtering facepiece respirators and surgical masks. J Occup Environ Hyg. 2014;11:388–396. doi: 10.1080/15459624.2013.866715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chua M.H., Cheng W., Goh S.S., Kong J., Li B., Lim J.Y.C., et al. Face masks in the new COVID-19 normal: materials, testing, and perspectives. Research (Washington, DC) 2020;2020:7286735. doi: 10.34133/2020/7286735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tcharkhtchi A., Abbasnezhad N., Zarbini Seydani M., Zirak N., Farzaneh S., Shirinbayan M. An overview of filtration efficiency through the masks: mechanisms of the aerosols penetration. Bioact Mater. 2021;6:106–122. doi: 10.1016/j.bioactmat.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Konda A., Prakash A., Moss G.A., Schmoldt M., Grant G.D., Guha S. Aerosol filtration efficiency of common fabrics used in respiratory cloth masks. ACS Nano. 2020;14:6339–6347. doi: 10.1021/acsnano.0c03252. [DOI] [PubMed] [Google Scholar]
- 61.Institute of Medicine . The National Academies Press; Washington, DC: 2006. Reusability of facemasks during an influenza pandemic: Facing the flu. [Google Scholar]
- 62.Bandi M.M. Electrocharged facepiece respirator fabrics using common materials. Proceed Royal Society A. 2020;476:20200469. doi: 10.1098/rspa.2020.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zangmeister C.D., Radney J.G., Vicenzi E.P., Weaver J.L. Filtration efficiencies of Nanoscale aerosol by cloth mask materials used to slow the spread of SARS-CoV-2. ACS Nano. 2020;14:9188–9200. doi: 10.1021/acsnano.0c05025. [DOI] [PubMed] [Google Scholar]
- 64.Hao W., Parasch A., Williams S., Li J., Ma H., Burken J., et al. Filtration performances of non-medical materials as candidates for manufacturing facemasks and respirators. Int J Hyg Environ Health. 2020;229:113582. doi: 10.1016/j.ijheh.2020.113582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bałazy A., Toivola M., Adhikari A., Sivasubramani S.K., Reponen T., Grinshpun S.A. Do N95 respirators provide 95% protection level against airborne viruses, and how adequate are surgical masks? Am J Infect Control. 2006;34:51–57. doi: 10.1016/j.ajic.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 66.Rengasamy A., Zhuang Z., Berryann R. Respiratory protection against bioaerosols: literature review and research needs. Am J Infect Control. 2004;32:345–354. doi: 10.1016/j.ajic.2004.04.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang C.-S. Electrostatic forces in fibrous filters—a review. Powder Technol. 2001;118:166–170. [Google Scholar]
- 68.Rengasamy S., Shaffer R., Williams B., Smit S. A comparison of facemask and respirator filtration test methods. J Occup Environ Hyg. 2017;14:92–103. doi: 10.1080/15459624.2016.1225157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qian Y., Willeke K., Grinshpun S.A., Donnelly J., Coffey C.C. Performance of N95 respirators: filtration efficiency for airborne microbial and inert particles. Am Ind Hyg Assoc J. 1998;59:128–132. doi: 10.1080/15428119891010389. [DOI] [PubMed] [Google Scholar]
- 70.Hinds W.C., Kraske G. Performance of dust respirators with facial seal leaks: I. Experimental Am Ind Hyg Assoc J. 1987;48:836–841. doi: 10.1080/15298668791385679. [DOI] [PubMed] [Google Scholar]
- 71.Hutten I.M. Butterworth-Heinemann; Oxford, UK: 2015. Handbook of nonwoven filter media. [Google Scholar]
- 72.O’Kelly E., Pirog S., Ward J., Clarkson P.J. Ability of fabric face mask materials to filter ultrafine particles at coughing velocity. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-039424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Silverman L., Lee G., Plotkin T., Sawyers L.A., Yancey A.R. Air flow measurements on human subjects with and without respiratory resistance at several work rates. AMA Arch Ind Hyg Occup Med. 1951;3:461–478. [PubMed] [Google Scholar]
- 74.Forouzandeh P., O’Dowd K., Pillai S.C. Face masks and respirators in the fight against the COVID-19 pandemic: an overview of the standards and testing methods. Saf Sci. 2021;133:104995. doi: 10.1016/j.ssci.2020.104995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fischer E., Fischer M., Grass D., Henrion I., Warren W., Westman E. Low-cost measurement of face mask efficacy for filtering expelled droplets during speech. Sci Adv. 2020;6 doi: 10.1126/sciadv.abd3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Neupane B.B., Mainali S., Sharma A., Giri B. Optical microscopic study of surface morphology and filtering efficiency of face masks. PeerJ. 2019;7 doi: 10.7717/peerj.7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Neupane B.B., Chaudhary R.K., Sharma A. A smartphone microscopic method for rapid screening of cloth facemask fabrics during pandemics. PeerJ. 2020;8 doi: 10.7717/peerj.9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao M., Liao L., Xiao W., Yu X., Wang H., Wang Q., et al. Household materials selection for homemade cloth face coverings and their filtration efficiency enhancement with Triboelectric charging. Nano Lett. 2020;20:5544–5552. doi: 10.1021/acs.nanolett.0c02211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yim W., Cheng D., Patel S.H., Kou R., Meng Y.S., Jokerst J.V. KN95 and N95 respirators retain filtration efficiency despite a loss of dipole charge during decontamination. ACS Appl Mater Interfaces. 2020;12:54473–54480. doi: 10.1021/acsami.0c17333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bahl P., Bhattacharjee S., de Silva C., Chughtai A.A., Doolan C., MacIntyre C.R. Face coverings and mask to minimise droplet dispersion and aerosolisation: a video case study. Thorax. 2020;75:1024–1025. doi: 10.1136/thoraxjnl-2020-215748. [DOI] [PubMed] [Google Scholar]
- 81.Lustig S.R., Biswakarma J.J.H., Rana D., Tilford S.H., Hu W., Su M., et al. Effectiveness of common fabrics to block aqueous aerosols of virus-like nanoparticles. ACS Nano. 2020;14:7651–7658. doi: 10.1021/acsnano.0c03972. [DOI] [PubMed] [Google Scholar]
- 82.Bartoszko J.J., Farooqi M.A.M., Alhazzani W., Loeb M. Medical masks vs N95 respirators for preventing COVID-19 in healthcare workers: a systematic review and meta-analysis of randomized trials. Influenza Other Respir Viruses. 2020;14:365–373. doi: 10.1111/irv.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luo Y., Yin K. Management of pregnant women infected with COVID-19. Lancet Infect Dis. 2020;20:513–514. doi: 10.1016/S1473-3099(20)30191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smith J.D., MacDougall C.C., Johnstone J., Copes R.A., Schwartz B., Garber G.E. Effectiveness of N95 respirators versus surgical masks in protecting health care workers from acute respiratory infection: a systematic review and meta-analysis. Cmaj. 2016;188:567–574. doi: 10.1503/cmaj.150835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jayaweera M., Perera H., Gunawardana B., Manatunge J. Transmission of COVID-19 virus by droplets and aerosols: a critical review on the unresolved dichotomy. Environ Res. 2020;188:109819. doi: 10.1016/j.envres.2020.109819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Long Y., Hu T., Liu L., Chen R., Guo Q., Yang L., et al. Effectiveness of N95 respirators versus surgical masks against influenza: a systematic review and meta-analysis. J Evid Based Med. 2020;13:93–101. doi: 10.1111/jebm.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Offeddu V., Yung C.F., Low M.S.F., Tam C.C. Effectiveness of masks and respirators against respiratory infections in healthcare workers: a systematic review and meta-analysis. Clin Infect Dis. 2017;65:1934–1942. doi: 10.1093/cid/cix681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.He X., Reponen T., McKay R.T., Grinshpun S.A. Effect of particle size on the performance of an N95 filtering Facepiece respirator and a surgical mask at various breathing conditions. Aerosol Sci Technol. 2013;47:1180–1187. doi: 10.1080/02786826.2013.829209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eshbaugh J.P., Gardner P.D., Richardson A.W., Hofacre K.C. N95 and p100 respirator filter efficiency under high constant and cyclic flow. J Occup Environ Hyg. 2009;6:52–61. doi: 10.1080/15459620802558196. [DOI] [PubMed] [Google Scholar]
- 90.Rengasamy S., Miller A., Eimer B.C., Shaffer R.E. Filtration performance of FDA-cleared surgical masks. J Int Soc Respir Prot. 2009;26:54–70. [PMC free article] [PubMed] [Google Scholar]
- 91.Isaacs D., Britton P., Howard-Jones A., Kesson A., Khatami A., Marais B., et al. Do facemasks protect against COVID-19? J Paediatr Child Health. 2020;56:976–977. doi: 10.1111/jpc.14936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.MacIntyre C.R., Seale H., Dung T.C., Hien N.T., Nga P.T., Chughtai A.A., et al. A cluster randomised trial of cloth masks compared with medical masks in healthcare workers. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2014-006577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jain U. Risk of COVID-19 due to shortage of personal protective equipment. Cureus. 2020;12 doi: 10.7759/cureus.8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cohen J., Rodgers Y.V.M. Contributing factors to personal protective equipment shortages during the COVID-19 pandemic. Prev Med. 2020;141:106263. doi: 10.1016/j.ypmed.2020.106263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Inaba M., Naito H., Sakata T., Nakao A. COVID-19 pandemic and shortage of personal protective equipment in Tokyo clinics. Acute Med Surg. 2020;7 doi: 10.1002/ams2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Burki T. Global shortage of personal protective equipment. Lancet Infect Dis. 2020;20:785–786. doi: 10.1016/S1473-3099(20)30501-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McMahon D.E., Peters G.A., Ivers L.C., Freeman E.E. Global resource shortages during COVID-19: bad news for low-income countries. PLoS Negl Trop Dis. 2020;14 doi: 10.1371/journal.pntd.0008412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Grinshpun S.A., Yermakov M., Khodoun M. Autoclave sterilization and ethanol treatment of re-used surgical masks and N95 respirators during COVID-19: impact on their performance and integrity. J Hosp Infect. 2020;105:608–614. doi: 10.1016/j.jhin.2020.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kobayashi L.M., Marins B.R., Costa P.C.D.S., Perazzo H., Castro R. Extended use or reuse of N95 respirators during COVID-19 pandemic: an overview of national regulatory authority recommendations. Infect Control Hosp Epidemiol. 2020;41:1364–1366. doi: 10.1017/ice.2020.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.MacIntyre C.R., Dung T.C., Chughtai A.A., Seale H., Rahman B. Contamination and washing of cloth masks and risk of infection among hospital health workers in Vietnam: a post hoc analysis of a randomised controlled trial. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-042045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gilbert R.M., Donzanti M.J., Minahan D.J., Shirazi J., Hatem C.L., Hayward-Piatkovskyi B., et al. Mask reuse in the COVID-19 pandemic: creating an inexpensive and scalable ultraviolet system for filtering Facepiece respirator decontamination. Glob Health Sci Pract. 2020;8:582–595. doi: 10.9745/GHSP-D-20-00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dai T., Vrahas M.S., Murray C.K., Hamblin M.R. Ultraviolet C irradiation: an alternative antimicrobial approach to localized infections? Expert Rev Anti-Infect Ther. 2012;10:185–195. doi: 10.1586/eri.11.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Torres A.E., Lyons A.B., Narla S., Kohli I., Parks-Miller A., Ozog D., et al. Ultraviolet-C and other methods of decontamination of filtering facepiece N-95 respirators during the COVID-19 pandemic. Photochem Photobiol Sci. 2020;19:746–751. doi: 10.1039/d0pp00131g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lindsley W.G., Martin S.B., Jr., Thewlis R.E., Sarkisian K., Nwoko J.O., Mead K.R., et al. Effects of ultraviolet germicidal irradiation (UVGI) on N95 respirator filtration performance and structural integrity. J Occup Environ Hyg. 2015;12:509–517. doi: 10.1080/15459624.2015.1018518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mills D., Harnish D.A., Lawrence C., Sandoval-Powers M., Heimbuch B.K. Ultraviolet germicidal irradiation of influenza-contaminated N95 filtering facepiece respirators. Am J Infect Control. 2018;46:e49–e55. doi: 10.1016/j.ajic.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Polkinghorne A., Branley J. Evidence for decontamination of single-use filtering facepiece respirators. J Hosp Infect. 2020;105:663–669. doi: 10.1016/j.jhin.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Viscusi D.J., Bergman M.S., Eimer B.C., Shaffer R.E. Evaluation of five decontamination methods for filtering facepiece respirators. Ann Occup Hyg. 2009;53:815–827. doi: 10.1093/annhyg/mep070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.O’Hearn K., Gertsman S., Sampson M., Webster R., Tsampalieros A., Ng R., et al. Decontaminating N95 and SN95 masks with ultraviolet germicidal irradiation does not impair mask efficacy and safety. J Hosp Infect. 2020;106:163–175. doi: 10.1016/j.jhin.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhao Z., Zhang Z., Lanzarini-Lopes M., Sinha S., Rho H., Herckes P., et al. Germicidal ultraviolet light does not damage or impede performance of N95 masks upon multiple uses. Environ Sci Technol Lett. 2020;7:600–605. doi: 10.1021/acs.estlett.0c00416. [DOI] [PubMed] [Google Scholar]
- 110.Lore M.B., Heimbuch B.K., Brown T.L., Wander J.D., Hinrichs S.H. Effectiveness of three decontamination treatments against influenza virus applied to filtering Facepiece respirators. Ann Occup Hyg. 2011;56:92–101. doi: 10.1093/annhyg/mer054. [DOI] [PubMed] [Google Scholar]
- 111.Cheng V.C.C., Wong S.C., Kwan G.S.W., Hui W.T., Yuen K.Y. Disinfection of N95 respirators by ionized hydrogen peroxide during pandemic coronavirus disease 2019 (COVID-19) due to SARS-CoV-2. J Hosp Infect. 2020;105:358–359. doi: 10.1016/j.jhin.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schnell E., Harriff M.J., Yates J.E., Karamooz E., Pfeiffer C.D., McCarthy J., et al. Homegrown ultraviolet germicidal irradiation for hospital-based N95 decontamination during the COVID-19 pandemic. medRxiv. 2020 2020.04.29.20085456 (accessed 2021-2-15) [Google Scholar]
- 113.Chin A.W.H., Chu J.T.S., Perera M.R.A., Hui K.P.Y., Yen H.-L., Chan M.C.W., et al. Stability of SARS-CoV-2 in different environmental conditions. The Lancet Microbe. 2020;1 doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yap T.F., Liu Z., Shveda R.A., Preston D.J. A predictive model of the temperature-dependent inactivation of coronaviruses. Appl Phys Lett. 2020;117 doi: 10.1063/5.0020782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Daeschler S.C., Manson N., Joachim K., Chin A.W.H., Chan K., Chen P.Z., et al. Effect of moist heat reprocessing of N95 respirators on SARS-CoV-2 inactivation and respirator function. Cmaj. 2020;192:E1189–e97. doi: 10.1503/cmaj.201203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pascoe M.J., Robertson A., Crayford A., Durand E., Steer J., Castelli A., et al. Dry heat and microwave-generated steam protocols for the rapid decontamination of respiratory personal protective equipment in response to COVID-19-related shortages. J Hosp Infect. 2020;106:10–19. doi: 10.1016/j.jhin.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Oh C., Araud E., Puthussery J.V., Bai H., Clark G.G., Wang L., et al. Dry heat as a decontamination method for N95 respirator reuse. Enviro Sci Technol Letters. 2020;7(9):677–682. doi: 10.1021/acs.estlett.0c00534. [DOI] [PubMed] [Google Scholar]
- 118.Steinberg B.E., Aoyama K., McVey M., Levin D., Siddiqui A., Munshey F., et al. Efficacy and safety of decontamination for N95 respirator reuse: a systematic literature search and narrative synthesis. Can J Anaesth. 2020;67:1814–1823. doi: 10.1007/s12630-020-01770-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xiang Y., Song Q., Gu W. Decontamination of surgical face masks and N95 respirators by dry heat pasteurization for one hour at 70°C. Am J Infect Control. 2020;48:880–882. doi: 10.1016/j.ajic.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Celina M.C., Martinez E., Omana M.A., Sanchez A., Wiemann D., Tezak M., et al. Extended use of face masks during the COVID-19 pandemic - thermal conditioning and spray-on surface disinfection. Polym Degrad Stab. 2020;179:109251. doi: 10.1016/j.polymdegradstab.2020.109251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li D.F., Cadnum J.L., Redmond S.N., Jones L.D., Donskey C.J. It’s not the heat, it’s the humidity: effectiveness of a rice cooker-steamer for decontamination of cloth and surgical face masks and N95 respirators. Am J Infect Control. 2020;48:854–855. doi: 10.1016/j.ajic.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu Y., Leachman S.A., Bar A. Proposed approach for reusing surgical masks in COVID-19 pandemic. J Am Acad Dermatol. 2020;83 doi: 10.1016/j.jaad.2020.04.099. e53-e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Saini V., Sikri K., Batra S.D., Kalra P., Gautam K. Development of a highly effective low-cost vaporized hydrogen peroxide-based method for disinfection of personal protective equipment for their selective reuse during pandemics. Gut Pathog. 2020;12:29. doi: 10.1186/s13099-020-00367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cai C., Floyd E.L. Effects of sterilization with hydrogen peroxide and chlorine dioxide on the filtration efficiency of N95, KN95, and surgical face masks. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Salter W.B., Kinney K., Wallace W.H., Lumley A.E., Heimbuch B.K., Wander J.D. Analysis of residual chemicals on filtering Facepiece respirators after decontamination. J Occup Environ Hyg. 2010;7:437–445. doi: 10.1080/15459624.2010.484794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Grillet A.M., Nemer M.B., Storch S., Sanchez A.L., Piekos E.S., Leonard J., et al. COVID-19 global pandemic planning: Performance and electret charge of N95 respirators after recommended decontamination methods. Exp Biol Med (Maywood, NJ) 2021;246:740–748. doi: 10.1177/1535370220976386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lin T.H., Chen C.C., Huang S.H., Kuo C.W., Lai C.Y., Lin W.Y. Filter quality of electret masks in filtering 14.6-594 nm aerosol particles: effects of five decontamination methods. PLoS One. 2017;12 doi: 10.1371/journal.pone.0186217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen C.C., Willeke K. Aerosol penetration through surgical masks. Am J Infect Control. 1992;20:177–184. doi: 10.1016/s0196-6553(05)80143-9. [DOI] [PubMed] [Google Scholar]
- 129.Imani S.M., Ladouceur L., Marshall T., Maclachlan R., Soleymani L., Didar T.F. Antimicrobial Nanomaterials and coatings: current mechanisms and future perspectives to control the spread of viruses including SARS-CoV-2. ACS Nano. 2020;14:12341–12369. doi: 10.1021/acsnano.0c05937. [DOI] [PubMed] [Google Scholar]
- 130.Weiss C., Carriere M., Fusco L., Capua I., Regla-Nava J.A., Pasquali M., et al. Toward nanotechnology-enabled approaches against the COVID-19 pandemic. ACS Nano. 2020;14:6383–6406. doi: 10.1021/acsnano.0c03697. [DOI] [PubMed] [Google Scholar]
- 131.Liao M., Liu H., Wang X., Hu X., Huang Y., Liu X., et al. A technical review of face mask wearing in preventing respiratory COVID-19 transmission. Curr Opin Colloid Interface Sci. 2021;101417 doi: 10.1016/j.cocis.2021.101417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Campos E.V.R., Pereira A.E.S., de Oliveira J.L., Carvalho L.B., Guilger-Casagrande M., de Lima R., et al. How can nanotechnology help to combat COVID-19? Opportunities and urgent need. J Nanobiotechnol. 2020;18:125. doi: 10.1186/s12951-020-00685-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang Z., Xia T., Liu S. Mechanisms of nanosilver-induced toxicological effects: more attention should be paid to its sublethal effects. Nanoscale. 2015;7:7470–7481. doi: 10.1039/c5nr01133g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhou J., Hu Z., Zabihi F., Chen Z., Zhu M. Progress and perspective of antiviral protective material. Adv Fiber Mater. 2020;2:123–139. doi: 10.1007/s42765-020-00047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Borkow G., Zhou S.S., Page T., Gabbay J. A novel anti-influenza copper oxide containing respiratory face mask. PLoS One. 2010;5 doi: 10.1371/journal.pone.0011295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Joe Y.H., Park D.H., Hwang J. Evaluation of Ag nanoparticle coated air filter against aerosolized virus: anti-viral efficiency with dust loading. J Hazard Mater. 2016;301:547–553. doi: 10.1016/j.jhazmat.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ruiz-Hitzky E., Darder M., Wicklein B., Ruiz-Garcia C., Martín-Sampedro R., del Real G., et al. Nanotechnology responses to COVID-19. Adv Healthc Mater. 2020;9:2000979. doi: 10.1002/adhm.202000979. [DOI] [PubMed] [Google Scholar]
- 138.El-Atab N., Qaiser N., Badghaish H., Shaikh S.F., Hussain M.M. Flexible Nanoporous template for the design and development of reusable anti-COVID-19 hydrophobic face masks. ACS Nano. 2020;14:7659–7665. doi: 10.1021/acsnano.0c03976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ullah S., Ullah A., Lee J., Jeong Y., Hashmi M., Zhu C., et al. Reusability comparison of melt-blown vs Nanofiber face mask filters for use in the coronavirus pandemic. ACS Appl Nano Mater. 2020;3:7231–7241. doi: 10.1021/acsanm.0c01562. [DOI] [PubMed] [Google Scholar]
- 140.Leung W.W., Sun Q. Charged PVDF multilayer nanofiber filter in filtering simulated airborne novel coronavirus (COVID-19) using ambient nano-aerosols. Sep Purif Technol. 2020;245:116887. doi: 10.1016/j.seppur.2020.116887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhong H., Zhu Z., Lin J., Cheung C.F., Lu V.L., Yan F., et al. Reusable and recyclable Graphene masks with outstanding Superhydrophobic and Photothermal performances. ACS Nano. 2020;14:6213–6221. doi: 10.1021/acsnano.0c02250. [DOI] [PubMed] [Google Scholar]
- 142.Huang L., Xu S., Wang Z., Xue K., Su J., Song Y., et al. Self-reporting and Photothermally enhanced rapid bacterial killing on a laser-induced Graphene mask. ACS Nano. 2020;14:12045–12053. doi: 10.1021/acsnano.0c05330. [DOI] [PubMed] [Google Scholar]
- 143.Kumar S., Karmacharya M., Joshi S.R., Gulenko O., Park J., Kim G.-H., et al. Photoactive antiviral face mask with self-sterilization and reusability. Nano Lett. 2021;21:337–343. doi: 10.1021/acs.nanolett.0c03725. [DOI] [PubMed] [Google Scholar]
- 144.Sarkis-Onofre R., Borges R.D.C., Demarco G., Dotto L., Schwendicke F., Demarco F.F. Decontamination of N95 respirators against SARS-CoV-2: a scoping review. J Dent. 2020;104:103534. doi: 10.1016/j.jdent.2020.103534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rodriguez-Martinez C.E., Sossa-Briceño M.P., Cortés J.A. Decontamination and reuse of N95 filtering facemask respirators: a systematic review of the literature. Am J Infect Control. 2020;48:1520–1532. doi: 10.1016/j.ajic.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zorko D.J., Gertsman S., O’Hearn K., Timmerman N., Ambu-Ali N., Dinh T., et al. Decontamination interventions for the reuse of surgical mask personal protective equipment: a systematic review. J Hosp Infect. 2020;106:283–294. doi: 10.1016/j.jhin.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cassorla L. Decontamination and reuse of N95 filtering Facepiece respirators: where do we stand? Anesth Analg. 2021;132 doi: 10.1213/ANE.0000000000005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liao L., Xiao W., Zhao M., Yu X., Wang H., Wang Q., et al. Can N95 respirators be reused after disinfection? How many times? ACS Nano. 2020;14:6348–6356. doi: 10.1021/acsnano.0c03597. [DOI] [PubMed] [Google Scholar]
- 149.Ou Q., Pei C., Chan Kim S., Abell E., Pui D.Y.H. Evaluation of decontamination methods for commercial and alternative respirator and mask materials - view from filtration aspect. J Aerosol Sci. 2020;150:105609. doi: 10.1016/j.jaerosci.2020.105609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kumar A., Kasloff S.B., Leung A., Cutts T., Strong J.E., Hills K., et al. Decontamination of N95 masks for re-use employing 7 widely available sterilization methods. PLoS One. 2020;15 doi: 10.1371/journal.pone.0243965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Smith J.S., Hanseler H., Welle J., Rattray R., Campbell M., Brotherton T., et al. Effect of various decontamination procedures on disposable N95 mask integrity and SARS-CoV-2 infectivity. J Clin Translat Sci. 2020:1–5. doi: 10.1017/cts.2020.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fischer R.J., Morris D.H., van Doremalen N., Sarchette S., Matson M.J., Bushmaker T., et al. Effectiveness of N95 respirator decontamination and reuse against SARS-CoV-2 virus. Emerg Infect Dis. 2020;26:2253–2255. doi: 10.3201/eid2609.201524. [DOI] [PMC free article] [PubMed] [Google Scholar]