Abstract
Background and aim
Coronary artery anatomy frequently affects location of atherosclerotic plaques and subsequent culprit lesions. We sought to clarify whether presence or absence of Ramus Intermedius coronary artery (RI) would affect location of culprit lesions in acute left circumflex (LCX) coronary artery occlusion.
Methods
The study included 180 patients, 100 with a diagnosis of non-ST elevation myocardial infarction (NSTEMI) and 80 with ST elevation myocardial infarction (STEMI). All culprit lesions were located in the LCX coronary artery. RI group included 45 patients and the No RI group included 135 patients.
Results
Culprit LCX lesions were similarly located at a comparable distance from LCX ostium in both groups and the presence of RI was not associated with significantly more proximally located culprit LCX lesions (34.7 ± 15.2 mm compared to 30.8 ± 17.9 mm respectively, p > 0.05). The frequency distribution of culprit lesions’ distance from LCX ostium showed no significant difference between both groups in any of the segments studied (10 mm each). There was no significant difference between both groups regarding markers of myocardial necrosis size as cardiac biomarkers (peak cardiac troponin-T 1077.4 ± 361.2 pg/dl vs 926 ± 462.2 pg/dl respectively, p = 0.13), (peak creatine kinase-MB 232.2 ± 81 ng/dl vs 194.7 ± 99.2 ng/dl respectively, p = 0.07) or left ventricular ejection fraction (EF 46.3 ± 6.3% vs 48.3 ± 8.3% respectively, p = 0.76).
Conclusion
Presence of RI coronary artery, as an additional flow divider, may not be associated with more proximal culprit lesions, compared to its absence, in cases of acute LCX coronary artery occlusion. Possible underlying pathophysiologic mechanisms remain to be clarified.
Keywords: Left circumflex coronary artery, Ramus intermedius artery, Culprit lesion, STEMI, NSTEMI
1. Introduction
Coronary atherosclerosis and its complications as acute myocardial infarction (AMI) and stroke continue to be a major cause of morbidity and mortality worldwide [1]. It is well-studied that culprit coronary atherosclerotic lesions tend to cluster in the proximal coronary arteries [1,2]. Culprit lesions in AMI are also known to locate close to coronary bifurcations (flow dividers) and major curvatures that prompt atherosclerotic plaques to progress and finally rupture [1,2].
Acute myocardial infarctions secondary to acute left circumflex coronary artery occlusion frequently impose a clinical problem regarding diagnosis and accomplishing reperfusion in a timely fashion. This is mainly because of difficulties in diagnosis on initial electrocardiogram (ECG). This could explain the under-representation of acute left circumflex (LCX) occlusion in acute coronary syndrome cases requiring emergent coronary reperfusion [3–5]. However, it has also been suggested that the underrepresentation of the LCX as a culprit artery could be actually because of the lower probability of LCX plaques to rupture. It was noticed that proximal LCX segments were even less prone to develop vulnerable plaques that subsequently rupture [6].
In their work, Galbraith et al., found that the presence of Ramus Intermedius (RI) was associated with more proximal left anterior descending coronary artery (LAD) lesions and hence larger anterior infarctions. They suggested that anatomy-induced flow disturbances (by RI as an additional flow divider) have important clinical implications [7].We sought to study whether same findings of the previous study [7] could be replicated with the LCX as the culprit artery, i.e. whether presence or absence of RI coronary artery would affect location of culprit lesions in acute left circumflex (LCX) coronary artery occlusion.
2. Methods
This prospective cross-sectional study was conducted on patients with acute myocardial infarction (AMI) who were admitted to the coronary care unit of cardiology department, Zagazig University Hospitals, Egypt in the period from January 2017 to February 2020. Included patients had coronary angiography during admission for either primary percutaneous coronary intervention (PPCI) in cases of ST elevation myocardial infarction (STEMI) or non-primary PCI in cases of non-ST elevation myocardial infarction (NSTEMI), with the culprit lesions located in the LCX artery. AMI was diagnosed as per current universal definition for acute myocardial infarction [8].
The study included 180 patients, 100 patients with a diagnosis of NSTEMI and 80 patients with STEMI. Inclusion criteria (1) coronary angiography done during the index hospitalization for AMI. (2) Culprit lesions located in the LCX coronary artery.
Exclusion criteria were (1) previous coronary artery bypass grafting (2) acute LCX occlusion with undelaying etiology other than coronary atherosclerotic plaque with superimposed thrombosis (as dissection or embolism) (3) no clearly identifiable culprit lesion (4) previous angioplasty at the site of the culprit lesion.
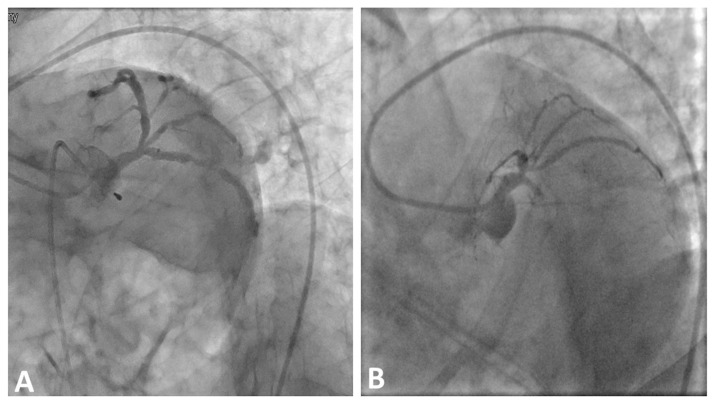
Study patients were divided into two groups according to the presence or absence of RI coronary artery. Ramus Intermedius coronary artery was said to be present in left main coronary artery trifurcations with the intermediate artery ostial diameter ≥ 1 mm[7]. All RI arteries were identified in the left anterior oblique (LAO) caudal view (spider view) (Fig. 1). RI was present in 45 patients (25%) and absent in 135 (75%) patients.
Fig. 1.
Two examples of LCX culprit lesions with RI coronary artery present (A) and absent RI (B) shown in LAO caudal (spider) view.
Written informed consent was obtained from all patients and the study was approved by the local ethical committee and the institutional review board.
Acute LCX occlusion was diagnosed by the absence of anterograde flow i.e. Thrombolysis in myocardial infarction (TIMI) 0/1, and/or presence of LCX thrombus. Cases with TIMI flow grade >1 were also included, provided that there was ST elevation on the ECG and/or presence of LCX thrombus on the atherosclerotic lesion (9 patients had TIMI = 2 flow).
Quantitative coronary angiography (QCA) analysis of LCX culprit lesion was performed using a standard software package (Q angio XA, Medis, Leiden, the Netherlands). For vessel classification, Coronary Artery Surgery Study (CASS) coronary map was used [9] and to avoid and minimize vessel foreshortening, right anterior oblique view with caudal angulation was used for measurement of distance from LCX ostium to the culprit LCX lesion [9].
Distance from the ostium of the LCX coronary artery to LCX culprit lesion was assessed (in millimeters) and defined as the distance from the ostium to the part of lesion with highest degree of percent stenosis (on QCA) [6].
Coronary angiograms were reported by two independent reviewers who were blinded to the clinical and ECG data.
Markers of myocardial necrosis were measured and compared between both studied groups, which included peak troponin-T, peak creatine kinase-MB and echocardiography-assessed left ventricular ejection fraction (LVEF).
2.1. Statistical analysis
Statistical analyses were performed using SPSS software (version 20.0, SPSS, Chicago, IL). Quantitative variables were assessed as mean ± SD, while qualitative ones as numbers and percentages.
Chi-square test was used to assess qualitative variables and T test for quantitative variables (study data showed normal distribution). A two tailed P-value <0.05 was considered significant.
3. Results
There was no significant difference between RI and No-RI groups regarding any of the demographic criteria, total ischemic times, long term medications, medications used during course of hospital admission, use of anti-platelets or 1-month clinical events as shown in Table 1.
Table 1.
Demographic and clinical characteristics of study patients.
| Character | RI (n = 45) | No RI (n = 135) | p value |
|---|---|---|---|
| Age (years) | 54.4 ± 11.2 | 57.1 ± 13.7 | 0.41 |
| Sex (male) (n, %) | 32 (73.3%) | 105 (77.7%) | 0.15 |
| Hypertension (n, %) | 27 (60) | 74 (54.8) | 0.55 |
| Diabetes Mellitus (n, %) | 11 (24.4) | 27 (20) | 0.32 |
| Smoking (n, %) | 23 (51.1) | 61 (45.1) | 0.14 |
| Dyslipidemia (n, %) | 21 (46.6) | 54 (40) | 0.86 |
| Family history of CAD (n, %) | 12 (26.6) | 28 (20.7) | 0.45 |
| Previous MI | 11 (24.4) | 30 (22.2) | 0.82 |
| Heart rate at presentation (b/m) | 87.4 ± 12.6 | 92.8 ± 18.4 | 0.64 |
| Total ischemic times (symptoms-to-balloon) (min) | 289.5 ± 114.2 | 276.6 ± 141.8 | 0.14 |
| MVD (n, %) | 29 (64.4%) | 92 (68.1) | 0.85 |
| Clinical events (1-month): | |||
| Heart failure (1-month) (n, %) | 9 (20) | 30 (22.2) | 0.76 |
| All-cause mortality (1-month) (n, %) | 4 (8.9) | 11 (8.1) | 0.86 |
| Cardiac death (1-month) (n, %) | 4 (8.9) | 11 (8.1) | 0.86 |
| Long-term medications: | |||
| Beta Blockers (n, %) | 15 (33.3) | 53 (39.2) | 0.62 |
| ACE/ARB (n, %) | 12 (26.7) | 30 (22.2) | 0.78 |
| Statins (n, %) | 8 (17.8) | 18 (13.3) | 0.65 |
| Aldosterone Receptor Blockers (n, %) | 7 (15.6) | 18 (13.3) | 0.69 |
| Aspirin (n, %) | 18 (40) | 61 (45.1) | 0.31 |
| Medications during hospital admission: | |||
| Beta Blockers (n, %) | 37 (82.2) | 106 (78.5) | 0.76 |
| ACE/ARB (n, %) | 23 (51.1) | 75 (56.1) | 0.62 |
| Aldosterone Receptor Blockers (n, %) | 27 (60) | 73 (54.1) | 0.52 |
| Dual anti-platelets (n, %) | 45 (100) | 135 (100) | 1 |
| Statins (n, %) | 36 (80) | 117 (86.7) | 0.48 |
| Dual anti-platelets after discharge | 45 (100) | 133 (98.5) | 0.92 |
ACE/I: Angiotensin converting enzyme inhibitors. ARB: Angiotensin receptor blockers. MVD (multi-vessel disease, other non-culprit significant plaques, >70% stenosis present in same or other vessels).
There was also no significant difference between both groups regarding markers of myocardial necrosis as cardiac biomarkers (cardiac troponin and CK-MB) and LVEF as shown in Table 2. There was also no significant difference between both groups regarding the presence of multivessel disease with significant luminal stenosis >70% in the culprit or other vessels (Table 2).
Table 2.
Ejection fraction, troponin-T and cardiac enzymes in both groups.
| Variable | RI (n = 45) | No RI (n = 135) | P value |
|---|---|---|---|
| LVEF (%) | 46.3 ± 6.3 | 48.3 ± 8.3 | 0.67 |
| Peak Troponin-T (pg/dl) | 1077.4 ± 361.2 | 926 ± 462.2 | 0.13 |
| CK_MB (ng/dl) | 232.2 ± 81 | 194.7 ± 99.2 | 0.07 |
LVEF: left ventricular ejection fraction.
Table 3 shows that culprit LCX lesions were similarly located at a comparable distance from the LCX ostium in both groups and that the presence of RI was not associated with more proximally located culprit LCX lesions.
Table 3.
Distance from LCX ostium to culprit LCX lesion in both groups.
| Anatomical parameter | RI | No RI | p value |
|---|---|---|---|
| Distance from LCX ostium (mm) | 34.7 ± 15.2 | 30.8 ± 17.9 | 0.32 |
The frequency distribution of culprit lesions’ distance from the LCX ostium showed no significant difference between both groups in any of the studied segments (10 mm each). Culprit lesions in both groups were mainly located 20–40 mm from the LCX ostium (75.5% in the RI group compared to 76.9% in the No RI group) as shown in Table 4.
Table 4.
The frequency distribution of distance of culprit lesions from LCX ostium.
| Distance from culprit to LCX ostium (mm) | RI | No RI | P value |
|---|---|---|---|
| 0–10 | 1 (2.22%) | 4 (3%) | 0.81 |
| 10–20 | 5 (11.1%) | 20 (14.8%) | 0.72 |
| 20–30 | 20 (44.4%) | 65 (48.1%) | 0.61 |
| 30–40 | 14 (31.1%) | 39 (28.8%) | 0.63 |
| 40–50 | 4 (8.9%) | 7 (5.1%) | 0.57 |
| >50 | 1 (2.22%) | 0 | – |
4. Discussion
Plaque rupture is related to intrinsic composition of the individual plaques (i.e. vulnerability) as well as extrinsic forces exerted on plaques (rupture triggers) [10,11]. Intrinsic forces predispose plaques to rupture, whereas extrinsic forces may precipitate disruption if vulnerable plaques are present [12,13]. Pathology studies have shown that >50% of vulnerable plaques were proximally located in coronary tree, one third in midportion, and the rest were in the distal portion of these arteries [14]. Plaque rupture was most frequently encountered in the proximal LAD (66%) followed by RCA (18%) and least found in the LCX (14%) [14].
Many observational studies have validated low shear stress hypothesis of plaque initiation and progression which involved mainly outer side of bifurcations and regions of major curvatures in the arterial tree, where shear stress is lowest [15–19]. On the other hand, plaque rupture and its relation to local shear stress seems to be more complex. In general, plaque rupture is most frequently encountered at area of cap shoulders where shear stress is relatively higher enough to cause endothelial damage followed by rupture of the already thin cap fibroatheroma [20].
We found no significant difference regarding distribution of culprit lesions in LCX coronary artery in presence or absence of RI coronary artery. It was rather expected that culprit LCX lesions would be more proximally located in cases of RI presence, as was the case in LAD culprit lesions in STEMI in the presence of RI [7]. This difference might be peculiar to proximal LCX segments, that have been possibly attributed to LCX coronary artery having lowest longitudinal strain compared to other epicardial coronary segments [6]. Insignificant difference regarding location of LCX culprit lesions in both groups also explains the almost similar extent of myocardial necrosis markers as reflected by nonsignificant differences in peak cardiac biomarkers and left ventricular ejection fraction. This was also reflected on non-significant difference in 1-month hard events as heart failure and mortality. Many confounders that could affect plaques composition, location and vulnerability (as co-morbidities like hypertension, diabetes mellitus, dyslipidemia, etc.) were present in both groups albeit with no significant difference.
Epicardial coronaries are cyclically subjected to both circumferential and longitudinal deformations that might contribute to destabilization and eventual rupture of vulnerable plaques [21]. In their work to study LCX as the culprit artery in STEMI and to define the distribution of such culprit lesions in relation to other two major epicardial coronaries [6], D. Ghanem et al. concluded that LCX plaques seemed less prone to rupture and that its proximal segment was even less so. They also studied LCX culprit lesions in NSTEMI and stable angina and found that culprit and total disease distribution were similar in NSTEMI and chronic stable angina. They suggested that lower LCX longitudinal strain (and subsequently relatively lower shear stress) might have contributed to reduced plaque rupture in STEMI [6]. Using a coronary computed tomographic angiographic (CCTA) database, they created a dynamic 3-dimensional coronary artery anatomic model [6] with retrospective ECG gating. They found that in the studied cases, the mean LAD systolic longitudinal strain was 9.5% ± 2.9% and for RCA was 10.1% ± 3.9%, compared with 1.5% ± 2.4% for the LCX (P < 0.001). They suggested that significant difference in shortening (referred to as squeezing theory) has contributed substantially to the lower prevalence of the proximal LCX culprit lesions in STEMI [6].
We found that majority of culprit LCX lesions in both groups were located 20–40 mm from the LCX ostium. This was similar to the findings of Katritsis et al. who found that culprit LCX lesions were located 29.73 ± 14.39 mm from the LCX ostium [22].
Presence of coronary segments where plaques are relatively less liable to rupture is very inviting for both clinical and basic research studies to explore possible mechanisms. Differential shear stress and longitudinal strain along specific segments of coronary tree might be reflected on abundance of local mediators with subsequent effects on plaque progression and vulnerability. This could have potential therapeutic implications in both atherosclerotic plaque progression and acute coronary syndromes management.
5. Limitations of the study
This work has some limitations. First, this was a single center study with small number of patients. Second, the presence of many confounders that could affect plaque location, morphology and vulnerability (age, hypertension, diabetes mellitus, dyslipidemia, etc.). Clustering of many risk factors of CAD in the same patient could have affected plaques’ progression and characteristics. Third, infarct size should have been assessed by more accurate imaging modalities as cardiac magnetic resonance imaging (CMR).
6. Conclusion
Presence of RI coronary artery, as an additional flow divider, may not be associated with more proximal culprit lesions, compared to its absence, in cases of acute LCX coronary artery occlusion.
Abbreviations
- RI
Ramus Intermedius coronary artery
- NSTEMI
Non-ST elevation myocardial infarction
- STEMI
ST elevation myocardial infarction
- LCX
Leftcircumflex
- LAD
Left anterior descending coronary artery
- AMI
Acute myocardial infarction
- PPCI
Primary percutaneous coronary intervention
- LVEF
Left ventricular ejection fraction
- CMR
Magnetic resonance imaging
Footnotes
Author’s contribution
Conception and design of Study: Ahmed El Zayat, Mohey Eldeeb. Literature review: Ahmed El Zayat, Mohey Eldeeb, Marwa Gad. Acquisition of data: Mohey Eldeeb, Marwa Gad, Ismail M Ibrahim. Analysis and interpretation of data: Mohey Eldeeb, Marwa Gad, Ismail M Ibrahim. Research investigation and analysis: Ahmed El Zayat, Mohey Eldeeb, Ismail M Ibrahim. Data collection: Mohey Eldeeb, Marwa Gad. Drafting of manuscript: Marwa Gad, Ismail M Ibrahim. Revising and editing the manuscript critically for important intellectual contents: Ahmed El Zayat, Mohey Eldeeb, Marwa Gad. Data preparation and presentation: Mohey Eldeeb, Marwa Gad, Ismail M Ibrahim. Supervision of the research: Ahmed El Zayat, Mohey Eldeeb, Marwa Gad, Ismail M Ibrahim. Research coordination and management: Ahmed El Zayat, Mohey Eldeeb, Marwa Gad.
Conflicts of interest
None to declare.
Funding
None.
References
- 1.Wang JC, Normand SL, Mauri L, et al. Coronary artery spatial distribution of acute myocardial infarction occlusions. Circulation. 2004;110:278–84. doi: 10.1161/01.CIR.0000135468.67850.F4. [DOI] [PubMed] [Google Scholar]
- 2.Gibson CM, Kirtane AJ, Murphy SA, et al. Distance from the coronary ostium to the culprit lesion in acute ST-elevation myocardial infarction and its implications regarding the potential prevention of proximal plaque rupture. J Thromb Thrombolysis. 2003;15:189–96. doi: 10.1023/B:THRO.0000011374.60110.bc. [DOI] [PubMed] [Google Scholar]
- 3.Huey BL, Beller GA, Kaiser DL, Gibson RS. A comprehensive analysis of myocardial infarction due to left circumflex artery occlusion: comparison with infarction due to right coronary artery and left anterior descending artery occlusion. J Am Coll Cardiol. 1988;12:1156–66. doi: 10.1016/0735-1097(88)92594-6. [DOI] [PubMed] [Google Scholar]
- 4.Wellens HJJ, Conover MB. The ECG in Emergency Decision Making. St Louis: Saunders Elsevier; 2006. p. 304. [Google Scholar]
- 5.O’Gara PT, Kushner FG, Ascheim DD, et al. ACCF/AHA guideline for the management of ST elevation myocardial infarction. J Am Coll Cardiol. 2013;61:e78–140. doi: 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Ghanim D, Kusniec F, Kinany W, Qarawani D, Meerkin D, Taha K, Amir O, Carasso S. Left circumflex coronary artery as the culprit vessel in ST-segment-elevation myocardial infarction, Texas Hear. Install J. 2017;44:320–5. doi: 10.14503/THIJ-16-5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galbraith EM, McDaniel MC, Jeroudi AM, Kashlan OR, Suo J, Giddens D, Samady H. Comparison of location of “culprit lesions” in left anterior descending coronary artery among patients with anterior wall ST-segment elevation myocardial infarction having ramus intermedius coronary arteries versus patients not having such arteries. Am J Cardiol. 2010;106:162–6. doi: 10.1016/j.amjcard.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 8.Kristian Thygesen, Alpert Joseph S, Jaffe Allan S, Chaitman Bernard R, Bax Jeroen J, Morrow David A, White Harvey D. Fourth universal definition of myocardial infarction (2018): ESC Scientific Document Group. Eur Heart J. 2019;40(3):237–69. doi: 10.1093/eurheartj/ehy462. [DOI] [PubMed] [Google Scholar]
- 9.Alderman E, Stadius M. The angiographic definitions of the bypass angioplasty revascularization investigation. Coron Artery Dis. 1992;3:1189–207. Corpus ID: 78859841. [Google Scholar]
- 10.Mann J, Davies MJ. Mechanisms of progression in native coronary artery disease: role of healed plaque disruption. Heart. 1999;82:265–8. doi: 10.1136/hrt.82.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alderman EL, Corley SD, Fisher LD, Chaitman BR, Faxon DP, Foster ED, et al. Five-year angiographic follow-up of factors associated with progression of coronary artery disease in the coronary artery surgery study (CASS). CASS participating investigators and staff. J Am Coll Cardiol. 1993;22:1141–54. doi: 10.1016/0735-1097(93)90429-5. [DOI] [PubMed] [Google Scholar]
- 12.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–71. doi: 10.1161/01.CIR.92.3.657. [DOI] [PubMed] [Google Scholar]
- 13.Lee RT, Kamm RD. Vascular mechanics for the cardiologist. J Am Coll Cardiol. 1994;23:1289–95. doi: 10.1016/0735-1097(94)90369-7. [DOI] [PubMed] [Google Scholar]
- 14.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–8. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 15.Asakura T, Karino T. Flow patterns and spatial distribution of atherosclerotic lesions in human coronary arteries. Circ Res. 1990;66:1045–66. doi: 10.1161/01.res.66.4.1045. [DOI] [PubMed] [Google Scholar]
- 16.Bharadvaj BK, Mabon RF, Giddens DP. Steady flow in a model of the human carotid bifurcation. Part I – flow visualization. J Biomech. 1982;15:349–62. doi: 10.1016/0021-9290(82)90057-4. [DOI] [PubMed] [Google Scholar]
- 17.Motomiya M, Karino T. Flow patterns in the human carotid artery bifurcation. Stroke. 1984;15:50–6. doi: 10.1161/01.str.15.1.50. [DOI] [PubMed] [Google Scholar]
- 18.Zarins CK, Giddens DP, Bharadvaj BK, Sottiurai VS, Mabon RF, Glagov S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res. 1983;53:502–14. doi: 10.1161/01.res.53.4.502. [DOI] [PubMed] [Google Scholar]
- 19.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–60. doi: 10.1152/physrev.1995.75.3.519. . Kat 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattsson EJ, Kohler TR, Vergel SM, Clowes AW. Increased blood flow induces regression of intimal hyperplasia. Arterioscler Thromb Vasc Biol. 1997;17:2245–9. doi: 10.1161/01.atv.17.10.2245. [DOI] [PubMed] [Google Scholar]
- 21.Corban MT, Eshtehardi P, Suo J, McDaniel MC, Timmins LH, Rassoul-Arzrumly E, et al. Combination of plaque burden, wall shear stress, and plaque phenotype has incremental value for prediction of coronary atherosclerotic plaque progression and vulnerability. Atherosclerosis. 2014;232(2):271–6. doi: 10.1016/j.atherosclerosis.2013.11.049. [DOI] [PubMed] [Google Scholar]
- 22.Katritsis DG, Efstathopoulos EP, Pantos J, Korovesis S, Kourlaba G, Kazantzidis S, Marmarelis V, Voridis E. Anatomic characteristics of culprit sites in acute coronary syndromes. J Intervent Cardiol. 2008;21:140–50. doi: 10.1111/j.1540-8183.2007.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]