Abstract
Background
Current knowledge on Rickettsia felis infection in humans is based on sporadic case reports. Here we conducted a retrospective seroepidemiological survey of R. felis infection among febrile patients visiting a medical center in Taipei.
Methodology/Principal findings
A total of 122 patients with suspected rickettsioses presenting with fever of unknown origin (FUO) but tested negative for scrub typhus, murine typhus, or Q fever were retrospectively identified during 2009 to 2010. The archived serum samples were examined for the presence of antibodies against R. felis, Rickettsia japonica, and Rickettsia typhi using microimmunofluorescence (MIF) assay. Serological evidence of Rickettsia exposure was found in 23 (19%, 23/122) patients. Eight patients had antibodies reactive to R. felis, including four with current infection (a ≥4-fold increase in IgG titer between acute and convalescent sera). The clinical presentations of these four patients included fever, skin rash, lymphadenopathy, as well as more severe conditions such as pancytopenia, hepatomegaly, elevated liver enzymes/bilirubin, and life-threatening acute respiratory distress syndrome. One of the patients died after doxycycline was stopped after being tested negative for scrub typhus, Q fever, and murine typhus.
Conclusions
Rickettsia felis is a neglected flea-borne pathogen in Taiwan, and its infection can be life-threatening. Further prospective studies of the prevalence of R. felis among patients with FUO and compatible clinical manifestations are warranted.
Author summary
Based on sporadic case reports, Rickettsia felis is believed to cause mild infection in humans. However, the true threat of the infection might be underestimated since the laboratory diagnoses of the pathogen were not routinely performed. We conducted a retrospective seroepidemiological survey among patients with clinical symptoms compatible with rickettsioses but negative for scrub typhus, Q fever, and murine typhus at a medical center in Taipei. Immunofluorescence assay was carried out to detect antibodies against R. felis, Rickettsia japonica, and Rickettsia typhi. We found that 23 out of 122 (19%, 23/122) such patients were positive for at least one species of rickettsiae, including 4 patients with active R. felis infection. One of these four patients died after stopping the antibiotic treatment after tested negative for scrub typhus, Q fever, and murine typhus. Our results showed that R. felis infection is a neglected disease in Taiwan and could be potentially life-threatening.
Introduction
Rickettsia felis is an obligate intracellular bacterium, formerly belonging to the transitional group of Rickettsia species [1]. The organism was found sustained in cat flea (Ctenocephalides felis), which is believed to be its primary vector and reservoir, through vertical transmission including transovarial and transstadial transmission [2–5]. Human case of R. felis infection was described in 1994, and since then, the disease has become an emerging zoonosis reported worldwide [6–14].
The transmission biology of R. felis is more complicated than previously thought, as more field surveys have detected the bacterial DNA in various arthropods, including fleas, ticks, mites, and mosquitoes [15]. Cat flea is presumed to be the most common vector of R. felis and transmits the pathogen to vertebrate hosts through infectious bites [16]. Co-migration of humans and domestic animals harboring cat fleas probably contributes to the widespread distribution of R. felis [17, 18]. Nevertheless, the prevalence of R. felis within cat fleas fluctuated according to generations and colonies. For example, studies have observed infection rates of 35% to 96% from a single colony over the course of one year and 0 to 100% from F1 progeny for different cat flea colonies [5, 19]. Vertebrate blood source was also proposed to affect the sustainability of R. felis in cat fleas [3]. Therefore, horizontal amplification or mammalian reservoir hosts are potentially crucial for the maintenance of R. felis within vector populations. Indeed, horizontal transmission has been demonstrated by cofeeding experiments [16, 20]. A recent report also showed domestic dogs (Canis familiaris) were able to sustain prolong periods of rickettsemia [21]. Due to cat flea’s nature of lacking host specificity, several mammals carrying cat fleas, such as opossums, raccoons, and rats have been implicated in the transmission cycle of R. felis, too [6, 22–26].
Rickettsia felis infection causes flea-borne spotted fever (cat flea typhus). The typical clinical presentations included fever, myalgias, arthralgia, nausea, vomiting, hepatitis, photophobia, hearing loss, and other non-specific symptoms [8, 9, 27, 28]. Studies also detected R. felis in afebrile subjects as well as skin lesions and intact skin [29–32]. Rickettsia felis infection has traditionally been characterized as a mild illness compared to other rickettsial diseases. Most patients became afebrile after 3 days of therapy with doxycycline or a fluoroquinolone, and more than 80% of patients had the infection resolved within 7 days [33]. However, neurological symptoms have been reported in Mexico and Sweden, and deaths had been attributed to R. felis infection based on the evidence of pathogen DNA in the cerebrospinal fluid of two patients presented with meningoencephalitis in Indonesia [8, 14, 34]. Coinfections with other pathogens, such as Plasmodium, increased the difficulty of diagnosis [35]. Interestingly, fewer than 15% of patients recalled any exposure to risk animals [2].
In Taiwan, R. felis infection has been demonstrated to be endemic in cat fleas [36–38]. Sporadic cases of human infection were also reported [13, 39]. However, given that Taiwan Centers for Disease Control (Taiwan CDC) only tested scrub typhus, Q fever, epidemic typhus, and murine typhus for notified suspected rickettsioses, it is likely that cases of R. felis (and other emerging rickettsioses) had been overlooked or unrecognized [40–45]. Herein, we conducted a retrospective seroepidemiological survey of R. felis infection among patients with clinically suspected rickettsioses. Microimmunofluorescence (MIF) assay was carried out with archived serum samples to detect antibodies against the pathogen, and medical records of the patients were reviewed.
Methods
Ethical statement
The study procedure has been reviewed and approved by the NTUH Research Ethic Committee (REC #201106109RB). The committee waived the need for signed informed consent.
Study setting and human subjects
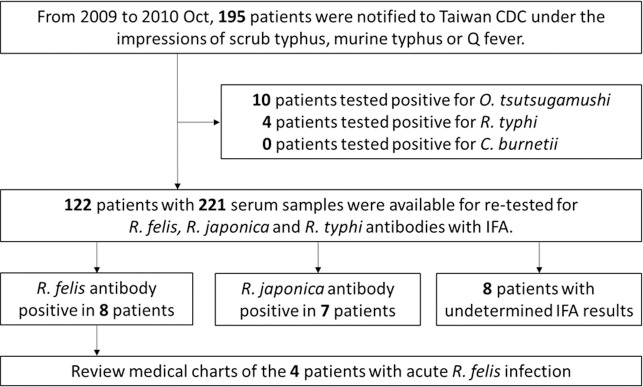
National Taiwan University Hospital (NTUH), a 2,800-bed university hospital, provides both primary and tertiary referral care in Taipei, Taiwan. During January 1, 2009 to October 31, 2010, 195 patients presented to NTUH with febrile illness or symptoms resembling those of notifiable rickettsioses, such as persistent high fever, headache, back pain, chills, swollen lymph nodes, rash, eschar, etc. Their blood samples were sent to the Taiwan CDC for laboratory diagnoses. Ten and four patients were diagnosed with scrub typhus and murine typhus, respectively. Of the 181 patients tested negative for scrub typhus, murine typhus, and Q fever, 122 patients gave sufficient amount of blood samples and were included in this retrospective study. Fig 1 illustrated the study design.
Fig 1. Study design flowchart.
The flow diagram showed the experimental design of the study.
Microimmunofluorescence assay
MIF assay was performed to detect antibodies against Rickettsia spp. Besides R. felis, a combination of antigens was used to help to determine the etiological agent, i.e. Rickettsia typhi for typhus group (TG) rickettsiae and Rickettsia japonica for spotted fever group (SFG) rickettsiae [46].
Briefly, inactivated whole-cell antigens were dotted on chamber slides and fixed and permeabilized in ice-cold methanol for 10 minutes. The slides were dried and preserved at -80°C until usage. The reactivity of antigens was first verified by banked animal antisera such as cats infested by fleas harboring R. felis and rodents positive for antibodies against SFG or TG rickettsiae [47]. Then a pool of human serum samples previously tested negative for R. felis, SFG rickettsiae, and TG rickettsiae antibodies was used as negative control. Sera from 10 patients or individuals with R. felis and TG rickettsiae infections or SFG rickettsiae antibodies were pooled and served as positive control. The pooled positive and negative control sera were included for each test batch. After reacting with serum sample diluted at 1:64 at 37°C for 30 minutes, slides were washed 3 times in PBS-Tween 20 for 5 min and rinsed with distilled water for a few seconds. Serum antibodies were then labeled with goat anti-human IgG+A+M (H+L)-FITC (Invitrogen, Inc., Waltham, MA, USA) at room temperature in dark for 30 minutes. Unreactive antibodies were washed off by immersing the slides 3 times in PBS-Tween 20 for 5 min. Coverslips were mount onto the slides by mounting media (phosphate buffered saline:glycerol = 3:7) before the slides were examined with a fluorescence microscope (Leica Microsystem, Singapore) by two researchers independently. Images were documented with a SPOT digital camera (RT color, Diagnostic Instruments Inc., Sterling Heights, MI, USA) [48].
For positive samples, further assays were carried out to detect IgM and IgG using specific secondary antibodies. Prior to detection of IgM, sera were treated by rheumatoid factor absorbent (Serion Immundiagnostica GmbH, Wurzburg, Germany). Serum samples were serially diluted to 1:4,096 to determine the titers of antibodies. Patients were considered exposed to the pathogen if (1) IgG titer was ≥1:64 or IgM titer was ≥1:32 for single blood sampling; or (2) seroconversion occurred between paired samples, i.e. a ≥4-fold rise of specific IgG. When cross-reactions occurred, a rickettsial antigen was considered to represent the etiological agent when the antibody titers against this antigen were ≥2-fold higher than the titers of antibodies against other antigens. Otherwise, it would be classified as undetermined infection [49].
Clinical features
Current/recent infection was defined by exhibiting a ≥4-fold rise in antibody titers between paired serum samples. The medical records of patients who currently/recently infected by R. felis were retrieved and reviewed. Information about the initial symptoms and signs, clinical course, complications, treatment, and outcomes was analyzed.
Results
Exposure to R. felis, R. typhi, and R. japonica
A total of 221 archived serum samples, including 99 paired sera and 23 samples collected either during acute or convalenscent phase, from 122 patients with fever of unknown origin (FUO) were tested by MIF assay. Evidence of rickettsiae exposure was discovered in 23 (19%, 23/122) patients (Table 1). All these patients had antibodies cross-reactive to at least two groups of rickettsial antigens. Eight of the 23 patients were suspected to be infected by R. felis (patient 1–8), i.e. having antibodies titers against R. felis ≥2-fold higher than those against R. typhi or R. japonica. Four of these patients (patient 1–4) exhibited seroconversion, indicating active R. felis infection, while the other 4 patients might be infected by R. felis currently or exposed to the pathogen in the past, which could not be determined from single blood samples. On the other hand, high titer antibodies against R. japonica were observed in patient 9–15, and the seroconversion in 5 of them (patient 9–13) implied the patients were suffering from infection of SFG rickettsiae. The causative rickettsial agents of the remaining 8 patients (patient 16–23) were undetermined due to the equal antibody titers against two or more groups of rickettsial antigens. In addition, none of these 122 patients showed a higher titer of antibodies against R. typhi than the other two rickettsial antigens, thus recent R. typhi infection was not suggested.
Table 1. Antibodies against Rickettsia spp. in patients with suspected rickettsioses but tested negative for scrub typhus, Q fever, or murine typhus.
| Types of rickettsial infection | Patient | 1st sampling (days after onset) | 2nd sampling (days after onset) | R. felis | R. typhi | R. japonica | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| acute phase | convalescent phase | acute phase | convalescent phase | acute phase | convalescent phase | ||||||||||
| IgG | IgM | IgG | IgM | IgG | IgM | IgG | IgM | IgG | IgM | IgG | IgM | ||||
| R. felis | 1 | 9 | 19 | — | — | 1,024 | — | — | — | 64 | — | — | — | 512 | — |
| 2 | 7 | 61 | — | — | 512 | — | — | — | 64 | — | — | — | 128 | — | |
| 3 | 17 | 28 | 512 | — | 2,048 | — | — | — | — | — | 512 | — | 512 | — | |
| 4 | 8 | 18 | 256 | — | 1,024 | — | — | — | — | — | 64 | — | 128 | — | |
| 5 | 13 | NA | NA | NA | 1,024 | — | NA | NA | 64 | — | NA | NA | 512 | — | |
| 6 | 4 | NA | 1,024 | — | NA | NA | 512 | — | NA | NA | 512 | — | NA | NA | |
| 7 | 32 | NA | NA | NA | 1,024 | — | NA | NA | 128 | — | NA | NA | 512 | — | |
| 8 | 33 | NA | NA | NA | 512 | — | NA | NA | — | — | NA | NA | 128 | — | |
| Spotted fever group (SFG) rickettsiae | 9 | 2 | 16 | — | — | 128 | — | — | — | 64 | — | — | — | 1,024 | — |
| 10 | 6 | 13 | — | — | 1,024 | — | — | — | 256 | — | — | — | 2,048 | — | |
| 11 | 11 | 25 | — | — | 1,024 | 512 | — | — | — | 512 | — | — | 2,048 | 512 | |
| 12 | 5 | 25 | 1,024 | — | 1,024 | — | 64 | — | 512 | — | 64 | — | 4,096 | — | |
| 13 | 4 | 18 | — | — | 256 | — | — | — | — | — | — | — | 1,024 | — | |
| 14 | 34 | NA | NA | NA | 512 | — | NA | NA | 128 | — | NA | NA | 1,024 | — | |
| 15 | 3 | NA | 512 | — | NA | NA | 256 | — | NA | NA | 1024 | — | NA | NA | |
| Undetermined rickettsial infection | 16 | 8 | 43 | 512 | 512 | 2,048 | 256 | 128 | 1,024 | 64 | 1,024 | 512 | 1,024 | 512 | 1,024 |
| 17 | 4 | 18 | — | — | 2,048 | — | — | — | — | — | — | — | 2,048 | — | |
| 18 | 8 | 17 | — | — | 512 | 512 | — | — | — | 512 | — | — | 256 | 512 | |
| 19 | 7 | 21 | — | — | 1,024 | — | — | — | 1,024 | — | — | — | 1,024 | — | |
| 20 | 98 | NA | NA | NA | 1,024 | — | NA | NA | 256 | — | NA | NA | 1,024 | — | |
| 21 | 4 | NA | 1,024 | 1,024 | NA | NA | 256 | 256 | NA | NA | 128 | 1,024 | NA | NA | |
| 22 | 3 | NA | 1,024 | — | NA | NA | 512 | — | NA | NA | 1,024 | — | NA | NA | |
| 23 | 4 | NA | 128 | 128 | NA | NA | 512 | 1,024 | NA | NA | 2,048 | 512 | NA | NA | |
NA: sample not available
—: negative result
The eight patients potentially exposed to R. felis were 14 to 77 years of age (median age of 40 years), including 5 males and 3 females (Table 2). Most of them came from northern Taiwan except Patient 1 had a recent travel history to Guangzhou, China. The seasons of onset were mainly winter and spring. None of the patients reported having contact with animals or experiencing flea bites.
Table 2. Demographic information of the eight patients with potential Rickettsia felis infection.
| Blood sample | Patient | Age/Sex | Race | Onset date (yr/mo) | Risk factors | symptoms/signs and lab findings | |||
|---|---|---|---|---|---|---|---|---|---|
| Travel history or location | Occupation | Animal contact | Cluster of similar cases | ||||||
| Paired | 1 | 49/M | Asian | 2009/12 | Guangzhou, China | businessman | denied | none | fever, muscle ache, skin rashes |
| 2 | 47/F | Asian | 2010/1 | Taipei, Taiwan | hotel housekeeper | denied | none | fever, skin rashes, pancytopenia, abnormal liver function, hepatomegaly | |
| 3 | 77/M | Asian | 2010/3 | Hsinchu, Taiwan | retired | denied | none | abnormal liver function | |
| 4 | 26/F | Asian | 2010/4 | Taipei, Taiwan | nurse | denied | none | fever, skin rashes, neck lymphadenopathy, abdomen pain | |
| Single blood samples* | 5 | 14/M | Asian | 2009/6 | Taipei, Taiwan | student | denied | none | fever, skin rashes, consciousness change |
| 6 | 29/M | Asian | 2009/12 | New Taipei City, Taiwan | dining business | denied | none | fever, headache, abnormal liver function | |
| 7 | 33/F | Asian | 2009/11 | Taoyuan, Taiwan | business | denied | none | fever | |
| 8 | 76/M | Asian | 2010/3 | New Taipei City, Taiwan | retired | denied | none | fever | |
*The illness episode for which the serum sample was taken may not be caused by active R. felis infection.
Clinical features of the R. felis-infected patients
Patient 1
A 49-year-old male returning from Guangzhou, China presented to the physician with fever and myalgias. The patient was admitted after the initial diagnosis of influenza was excluded by the rapid test. The patient developed acute respiratory distress syndrome (ARDS) on the following days and was intubated. Levofloxacin and doxycycline were empirically given for 7 days, and his ARDS gradually improved. Bacterial culture and serology for scrub typhus, Q fever, typhoid fever, and leptospirosis were all negative. Skin rashes developed over axillaries, inguinal area and then progressed to whole lower extremities. Doxycycline was discontinued and methylprednisolone 40 mg per day was started on day 8. Under steroid therapy, however, the patient’s condition deteriorated. Despite intensive supportive care, the patient died from ARDS on day 30. Evidence of R. felis infection was identified in the study. Antibody titer was increased to 1:1,024 during convalescent phase (Table 1).
Patient 2
This 47-year-old woman was admitted due to fever and skin rashes lasting for one week. The patient was a hotel housekeeper and denied other specific travel history or insect bite. Laboratory tests carried out in another hospital found pancytopenia and abnormal liver functions. Abdominal computed tomography showed hepatomegaly without structural lesions. Ceftriaxone and doxycycline were started empirically under tentative diagnosis of scrub typhus or Q fever. Fever subsided on the next days. Complete blood cell count and liver functions gradually returned to normal range. The patient was discharged on day 7 although the serological tests were negative for both scrub typhus and Q fever. MIF assay using paired sera revealed a ≥4-fold increase in IgG titer against R. felis, suggesting the patient was suffering from R. felis infection (Table 1).
Patient 3
A 77-year-old male, with diabetes mellitus and chronic kidney disease, was admitted for general malaise without fever. Escherichia coli bacteremia with septic shock was diagnosed after admission. However, despite intravenous therapy using ceftriaxone actively against E. coli, the patient’s liver function continued to deteriorate, with elevated aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin (total and direct), alkaline phosphatase (ALP), and gamma-glutamyl transferase (GGT) up to 362 IU/L, 253 IU/L, 7.47 mg/dL, 5.4 mg/dL, 769 IU/L and 386 IU/L, respectively. Abdominal sonography did not reveal structural lesions in liver or gall bladder. Scrub typhus was suspected, but the serological tests gave negative results. A 7-day course of levofloxacin was given. The patient’s liver functions recovered, and the patient was discharged. A ≥4-fold increase in IgG titers against R. felis was identified between paired sera in the retrospective study (Table 1).
Patient 4
A 26-year-old female was admitted due to intermittent fever for one week although one week’s doxycycline had been prescribed as an outpatient. Other accompanied symptoms and signs included skin rashes, abdominal dull pain, and palpable neck lymph nodes. The patient denied any history of travel, occupation, cluster and animal contact. Blood tests revealed elevated AST (205 U/L) and ALT (325 U/L). Viral hepatitis tests and blood culture gave negative results. Doxycycline and flomoxef were given for five days, and her fever subsided on the third day of hospitalization. The patient was then discharged with oral levofloxacin. Scrub typhus was suspected, but the results of both polymerase chain reaction and serological tests were negative. Evidence of R. felis infection was provided with a ≥4-fold increase in IgG titers between paired sera in the study (Table 1).
Discussion
We conducted a seroepidemiological survey to retrospectively look for unrecognized R. felis infection among patients with suspected rickettsioses. We found evidence of R. felis exposure in 8 patients of whom 4 had been actively infected. The clinical presentations of these 4 patients included not only mild symptoms such as fever, skin rash, or lymphadenopathy in accordance with previous reports but also severe and potential life-threatening conditions including pancytopenia, hepatomegaly, elevated liver enzyme/bilirubin, and acute respiratory distress syndrome. One of the patients (patient 1) died after he was incorrectly treated with intravenous corticosteroid for presumed immunological conditions, after being tested negative for scrub typhus, Q fever, murine typhus, and epidemic typhus. In contrast, patients diagnosed with scrub typhus-associated acute respiratory distress syndrome have been successfully treated with intravenous minocycline [50, 51]. First reported in 2008, sporadic human cases had been documented both in northern and southern Taiwan [13, 39]. In the study, R. felis infection accounted for up to 21.6% (8/37) of the rickettsioses in 195 patients, suggesting a clinically significant prevalence of this disease in Taiwan. Although most of these patients presented to the hospital in winter and spring a report studying R. felis in cat fleas parasitizing stray animals in Taipei found a stable infection rate over the course of one year [37]. Our findings highlighted the importance of a correct laboratory diagnosis of rickettsial illnesses. R. felis infection should be considered as a differential diagnosis for patients present with FUO in the future.
Besides 14 patients diagnosed with scrub typhus and murine typhus by the Taiwan CDC, an additional 23 patients were identified with potential rickettsiae exposure in the study. In another retrospective research study conducted in southern Taiwan involving patients with clinically suspected Q fever, scrub typhus, murine typhus, leptospirosis, and dengue fever, serological evidence of SFG rickettsiae exposure was observed in 18 of 413 patients [39]. However, no indigenous human SFG rickettsioses have been confirmed in Taiwan to date. On the other hand, a variety of SFG rickettsiae has been documented in arthropods and small mammals. Novel species including Rickettsia sp. TwKM01, Rickettsia sp. IG-1, Rickettsia sp. RR01were isolated from Rhipicephalus haemaphysalodies, Ixodes granulatus, and Rhipicephalus sanguineus ticks, and Rickettsia sp. TwKM02 was isolated from Leptotrombidium deliense mites [38, 44, 45]. Rickettsia conorii, R. japonica, Rickettsia rickettsii, Rickettsia australis, Rickettsia helvetica, Rickettsia monacensis have been detected in fleas and ticks collected from small mammals and birds [43, 47, 52, 53]. Infections of R. conorii, R. japonica, R. rickettsii, Rickettsia sp. TwKM01, Rickettsia sp. IG-1, Rickettsia sp. TwKM02, or Rickettsia raoultii were also demonstrated in rodents by molecular analysis or serology [47, 53, 54]. Based on the concept of “One Health”, the diverse and prevalent SFG rickettsiae in the field would increase the potential risk for human SFG rickettsioses. Moreover, MIF assay yielded positive results against at least 2 species of Rickettsia with equal titers in 8 patients, thus the etiological agents were undetermined in the study. The patients could be infected either by more than one species of Rickettsia simultaneously/sequentially or by unknown agents which cross-reacted with two groups of antigens. Surveillance of Rickettsia spp. in the field and febrile patients should be taken continuously to detect possible threats in order to control the infections.
In the study, the serological results did not suggest recent R. typhi infection in any of the 122 patients presenting to NTUH in Taipei. This might be because murine typhus occurs sporadically in Taiwan, and most cases are located in the southwest and central-west districts, including Kaohsiung-Pingtung region, Tainan City, Changhua County, and Taichung City [55]. Although extensive cross-reaction of antibodies happened between R. typhi and Rickettsia prowazekii [56], the latter has not been reported in Taiwan since World War II.
There were certain limitations in our study. Although molecular methods offer better sensitivity and specificity for diagnosis of acute rickettsial infections, DNA samples from acute phase PBMC or whole blood before administration of antibiotics and autopsy specimens were unavailable in the retrospective study. We were unable to isolate the pathogens or provide direct molecular evidence of infections. Nevertheless, periods of rickettsemia may quickly subside. The serological reference method for rickettsioses is immunofluorescence analysis. Best efforts were made to retrieve paired samples (acute and convalescent) drawn approximately 2 weeks apart from the study subjects, but in some cases, samples were not collected in the recommended time frame, or only single serum samples were available (Table 1). Since some patients were referred to NTUH, the first sampling times have past 7 days after onset, however, their symptoms were not resolved while administration. Significant changes in antibody titers against R. felis and R. japonica were observed in 4 patients (patient 1–4) and 5 patients (patient 9–13), respectively, suggesting recent infections. On the other hand, for patients with single samples, a single titer result does not differentiate between past and current exposure. They might presented to the hospital due to other acute infections. MIF assay allowed the detection of antibodies against different rickettsial antigens within the same drop of diluted serum at the same time. Difference in antibody titers was used to distinguish cross-reactions between groups of rickettssial antigens, but cross-reactions between closely related species of Rickettsia could not be ruled out without the use of further serological assays, such as cross-adsorption technique and western blot. Therefore, from a critical point of view, our findings of anitbodies against R. felis in patients may be in fact caused by infection of organisms closely related to R. felis.
In conclusion, R. felis is a neglected zoonotic pathogen in Taiwan, and without properly treatment, the infection can be lethal. Considering the accumulating reports of detected SFG rickettsiae in animals and arthropods in the field, human rickettsioses are likely to happen more frequently than previously thought. This work highlighted the importance of a correct and prompt laboratory diagnosis. Inclusion of R. felis infection in routine differential diagnosis would help to guide appropriate treatment. Further prospective studies of the emerging rickettsiosis among FUO patients presented with compatible clinical manifestations are warranted.
Acknowledgments
We thank the Center for Infection Control at National Taiwan University Hospital (NTUH) for the kind help in providing notification information. This work was part of the Master thesis of the first author, Wan-Hsiu Yang, at National Taiwan University (NTU) (2014).
Data Availability
All relevant data are within the manuscript.
Funding Statement
The authors acknowledged the financial support provided by the Ministry of Health and Welfare, National Taiwan University Infectious Diseases Research and Education Center, Taipei, Taiwan, and the Ministry of Science and Technology Council (103-2314-B-002-036-MY2 to K.H.T.). The funders had no role in study design, data collection and analysis, or preparation of the manuscript.
Reference
- 1.Legendre KP, Macaluso KR. Rickettsia felis: A review of transmission mechanisms of an emerging pathogen. Trop Med Infect Dis. 2017;2(4). Epub 2018/10/03. 10.3390/tropicalmed2040064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azad AF, Sacci JB Jr., Nelson WM, Dasch GA, Schmidtmann ET, Carl M. Genetic characterization and transovarial transmission of a typhus-like Rickettsia found in cat fleas. Proc Natl Acad Sci U S A. 1992;89(1): 43–46. 10.1073/pnas.89.1.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wedincamp J Jr., Foil LD. Vertical transmission of Rickettsia felis in the cat flea (Ctenocephalides felis Bouche). J Vector Ecol. 2002;27(1): 96–101. [PubMed] [Google Scholar]
- 4.Perez-Osorio CE, Zavala-Velazquez JE, Arias Leon JJ, Zavala-Castro JE. Rickettsia felis as emergent global threat for humans. Emerg Infect Dis. 2008;14(7): 1019–1023. 10.3201/eid1407.071656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reif KE, Macaluso KR. Ecology of Rickettsia felis: a review. J Med Entomol. 2009;46(4): 723–736. 10.1603/033.046.0402 [DOI] [PubMed] [Google Scholar]
- 6.Richter J, Fournier PE, Petridou J, Haussinger D, Raoult D. Rickettsia felis infection acquired in Europe and documented by polymerase chain reaction. Emerg Infect Dis. 2002;8(2): 207–208. 10.3201/eid0802.010293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schriefer ME, Sacci JB Jr., Dumler JS, Bullen MG, Azad AF. Identification of a novel rickettsial infection in a patient diagnosed with murine typhus. J Clin Microbiol. 1994;32(4): 949–954. 10.1128/JCM.32.4.949-954.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zavala-Velazquez JE, Ruiz-Sosa JA, Sanchez-Elias RA, Becerra-Carmona G, Walker DH. Rickettsia felis rickettsiosis in Yucatan. Lancet. 2000;356(9235): 1079–1080. 10.1016/S0140-6736(00)02735-5 [DOI] [PubMed] [Google Scholar]
- 9.Raoult D, La Scola B, Enea M, Fournier PE, Roux V, Fenollar F, et al. A flea-associated Rickettsia pathogenic for humans. Emerg Infect Dis. 2001;7(1): 73–81. 10.3201/eid0701.010112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi YJ, Jang WJ, Ryu JS, Lee SH, Park KH, Paik HS, et al. Spotted fever group and typhus group rickettsioses in humans, South Korea. Emerg Infect Dis. 2005;11(2): 237–244. 10.3201/eid1102.040603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Znazen A, Rolain JM, Hammami A, Jemaa MB, Raoult D. Rickettsia felis infection, Tunisia. Emerg Infect Dis. 2006;12(1): 138–140. 10.3201/eid1201.050876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker TM, Murray CK, Richards AL, Samir A, Ismail T, Fadeel MA, et al. Concurrent infections in acute febrile illness patients in Egypt. Am J Trop Med Hyg. 2007;77(2): 390–392. [PubMed] [Google Scholar]
- 13.Tsai KH, Lu HY, Tsai JJ, Yu SK, Huang JH, Shu PY. Human case of Rickettsia felis infection, Taiwan. Emerg Infect Dis. 2008;14(12): 1970–1972. 10.3201/eid1412.080515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindblom A, Severinson K, Nilsson K. Rickettsia felis infection in Sweden: report of two cases with subacute meningitis and review of the literature. Scand J Infect Dis. 2010;42(11–12): 906–909. 10.3109/00365548.2010.508466 [DOI] [PubMed] [Google Scholar]
- 15.Parola P. Rickettsia felis: from a rare disease in the USA to a common cause of fever in sub-Saharan Africa. Clin Microbiol Infect. 2011;17(7): 996–1000. 10.1111/j.1469-0691.2011.03516.x [DOI] [PubMed] [Google Scholar]
- 16.Reif KE, Kearney MT, Foil LD, Macaluso KR. Acquisition of Rickettsia felis by cat fleas during feeding. Vector Borne Zoonotic Dis. 2011;11(7): 963–968. 10.1089/vbz.2010.0137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawrence AL, Webb CE, Clark NJ, Halajian A, Mihalca AD, Miret J, et al. Out-of-Africa, human-mediated dispersal of the common cat flea, Ctenocephalides felis: The hitchhiker’s guide to world domination. Int J Parasitol. 2019; 49(5): 321–336. 10.1016/j.ijpara.2019.01.001 [DOI] [PubMed] [Google Scholar]
- 18.Abdad MY, Stenos J, Graves S. Rickettsia felis, an emerging flea-transmitted human pathogen. Emerg Health Threats J. 2011;4: 7168. 10.3402/ehtj.v4i0.7168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reif KE, Stout RW, Henry GC, Foil LD, Macaluso KR. Prevalence and infection load dynamics of Rickettsia felis in actively feeding cat fleas. PLoS One. 2008;3(7): e2805. 10.1371/journal.pone.0002805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirunkanokpun S, Thepparit C, Foil LD, Macaluso KR. Horizontal transmission of Rickettsia felis between cat fleas, Ctenocephalides felis. Mol Ecol. 2011;20(21): 4577–4586. 10.1111/j.1365-294X.2011.05289.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ng-Nguyen D, Hii SF, Hoang MT, Nguyen VT, Rees R, Stenos J, et al. Domestic dogs are mammalian reservoirs for the emerging zoonosis flea-borne spotted fever, caused by Rickettsia felis. Sci Rep. 2020;10(1): 4151. 10.1038/s41598-020-61122-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oteo JA, Portillo A, Santibanez S, Blanco JR, Perez-Martinez L, Ibarra V. Cluster of cases of human Rickettsia felis infection from Southern Europe (Spain) diagnosed by PCR. J Clin Microbiol. 2006;44(7): 2669–2671. 10.1128/JCM.00366-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JA, Radulovic S, Schriefer ME, Azad AF. Rickettsia felis: a new species of pathogenic rickettsia isolated from cat fleas. J Clin Microbiol. 1996;34(3): 671–674. 10.1128/JCM.34.3.671-674.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boostrom A, Beier MS, Macaluso JA, Macaluso KR, Sprenger D, Hayes J, et al. Geographic association of Rickettsia felis-infected opossums with human murine typhus, Texas. Emerg Infect Dis. 2002;8(6): 549–554. 10.3201/eid0806.010350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Case JB, Chomel B, Nicholson W, Foley JE. Serological survey of vector-borne zoonotic pathogens in pet cats and cats from animal shelters and feral colonies. J Feline Med Surg. 2006;8(2): 111–117. 10.1016/j.jfms.2005.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bayliss DB, Morris AK, Horta MC, Labruna MB, Radecki SV, Hawley JR, et al. Prevalence of Rickettsia species antibodies and Rickettsia species DNA in the blood of cats with and without fever. J Feline Med Surg. 2009;11(4): 266–270. 10.1016/j.jfms.2008.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galvao MA, Zavala-Velazquez JE, Zavala-Castro JE, Mafra CL, Calic SB, Walker DH. Rickettsia felis in the Americas. Ann N Y Acad Sci. 2006;1078: 156–158. 10.1196/annals.1374.027 [DOI] [PubMed] [Google Scholar]
- 28.Galvao MA, Mafra C, Chamone CB, Calic SB, Zavala-Velazquez JE, Walker DH. Clinical and laboratorial evidence of Rickettsia felis infections in Latin America. Rev Soc Bras Med Trop. 2004;37(3): 238–240. 10.1590/s0037-86822004000300009 [DOI] [PubMed] [Google Scholar]
- 29.Maina AN, Knobel DL, Jiang J, Halliday J, Feikin DR, Cleaveland S, et al. Rickettsia felis infection in febrile patients, western Kenya, 2007–2010. Emerg Infect Dis. 2012;18(2): 328–331. 10.3201/eid1802.111372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mourembou G, Lekana-Douki JB, Mediannikov O, Nzondo SM, Kouna LC, Essone JC, et al. Possible role of Rickettsia felis in acute febrile illness among children in Gabon. Emerg Infect Dis. 2015;21(10): 1808–1015. 10.3201/eid2110.141825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mediannikov O, Fenollar F, Bassene H, Tall A, Sokhna C, Trape JF, et al. Description of "yaaf", the vesicular fever caused by acute Rickettsia felis infection in Senegal. J Infect. 2013;66(6): 536–540. 10.1016/j.jinf.2012.10.005 [DOI] [PubMed] [Google Scholar]
- 32.Mediannikov O, Socolovschi C, Million M, Sokhna C, Bassene H, Diatta G, et al. Molecular identification of pathogenic bacteria in eschars from acute febrile patients, Senegal. Am J Trop Med Hyg. 2014;91(5): 1015–1019. 10.4269/ajtmh.13-0629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noden BH, Davidson S, Smith JL, Williams F. First detection of Rickettsia typhi and Rickettsia felis in fleas collected from client-owned companion animals in the southern Great Plains. J Med Entomol. 2017;54(4): 1093–1097. 10.1093/jme/tjx069 [DOI] [PubMed] [Google Scholar]
- 34.Mawuntu AHP, Johar E, Anggraeni R, Feliana F, Bernadus JBB, Safari D et al. Rickettsia felis identified in two fatal cases of acute meningoencephalitis. PLoS Negl Trop Dis. 2020;14(2):e0007893. 10.1371/journal.pntd.0007893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mediannikov O, Socolovschi C, Edouard S, Fenollar F, Mouffok N, Bassene H, et al. Common epidemiology of Rickettsia felis infection and malaria, Africa. Emerg Infect Dis. 2013;19(11):1775–1783. 10.3201/eid1911.130361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsai KH, Lu HY, Huang JH, Wang PJ, Wang HC, Huang CG, et al. Rickettsia felis in cat fleas in Taiwan. Vector Borne Zoonotic Dis. 2009;9(5): 561–563. 10.1089/vbz.2008.0076 [DOI] [PubMed] [Google Scholar]
- 37.Tsai KH, Huang CG, Fang CT, Shu PY, Huang JH, Wu WJ. Prevalence of Rickettsia felis and the first identification of Bartonella henselae Fizz/CAL-1 in cat fleas (Siphonaptera: Pulicidae) from Taiwan. J Med Entomol. 2011;48(2): 445–452. 10.1603/me10022 [DOI] [PubMed] [Google Scholar]
- 38.Hsu YM, Lin CC, Chomel BB, Tsai KH, Wu WJ, Huang CG, et al. Identification of Rickettsia felis in fleas but not ticks on stray cats and dogs and the evidence of Rickettsia rhipicephali only in adult stage of Rhipicephalus sanguineus and Rhipicephalus haemaphysaloides. Comp Immunol Microbiol Infect Dis. 2011;34(6): 513–518. 10.1016/j.cimid.2011.09.005 [DOI] [PubMed] [Google Scholar]
- 39.Lai CH, Chang LL, Lin JN, Tsai KH, Hung YC, Kuo LL, et al. Human Spotted fever group rickettsioses are underappreciated in southern Taiwan, particularly for the species closely-related to Rickettsia felis. PLoS One. 2014. April 22;9(4): e95810. 10.1371/journal.pone.0095810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsai KH, Chung LH, Chien CH, Tung YJ, Wei HY, Yen TY, et al. Human granulocytic anaplasmosis in Kinmen, an offshore island of Taiwan. PLoS Negl Trop Dis. 2019;13(9): e0007728. 10.1371/journal.pntd.0007728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng SH, Yang SL, Ho YN, Chen HF, Shu PY. Human Case of Ehrlichia chaffeensis Infection, Taiwan. Emerg Infect Dis. 2019;25(11): 2141–2143. 10.3201/eid2511.190665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yen TY, Tung YJ, Wang HC, Tsai KH. Detection of Ehrlichia chaffeensis in a febrile patient in Kinmen, an offshore island of Taiwan. J Formos Med Assoc. 2020;119(8): 1329–1330. 10.1016/j.jfma.2019.11.019 [DOI] [PubMed] [Google Scholar]
- 43.Kuo CC, Lin YF, Yao CT, Shih HC, Chung LH, Liao HC, et al. Tick-borne pathogens in ticks collected from birds in Taiwan. Parasit Vectors. 2017;10(1): 587. 10.1186/s13071-017-2535-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsui PY, Tsai KH, Weng MH, Hung YW, Liu YT, Hu KY, et al. Molecular detection and characterization of spotted fever group rickettsiae in Taiwan. Am J Trop Med Hyg. 2007;77(5): 883–890. [PubMed] [Google Scholar]
- 45.Tsai KH, Wang HC, Chen CH, Huang JH, Lu HY, Su CL, et al. Isolation and identification of a novel spotted fever group rickettsia, strain IG-1, from Ixodes granulatus ticks collected on Orchid Island (Lanyu), Taiwan. Am J Trop Med Hyg. 2008;79(2): 256–261. [PubMed] [Google Scholar]
- 46.Uchiyama T, Uchida T, Walker DH. Species-specific monoclonal antibodies to Rickettsia japonica, a newly identified spotted fever group Rickettsia. J Clin Microbiol. 1990;28(6): 1177–1180. 10.1128/JCM.28.6.1177-1180.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuo CC, Shu PY, Mu JJ, Wang HC. High prevalence of Rickettsia spp. infections in small mammals in Taiwan. Vector Borne Zoonotic Dis. 2015;15: 13–20. 10.1089/vbz.2014.1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teysseire N, Raoult D. Comparison of Western immunoblotting and microimmunofluorescence for diagnosis of Mediterranean spotted fever. J Clin Microbiol. 1992;30(2): 455–460. 10.1128/JCM.30.2.455-460.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perez-Arellano JL, Fenollar F, Angel-Moreno A, Bolanos M, Hernandez M, Santana E, et al. Human Rickettsia felis infection, Canary Islands, Spain. Emerg Infect Dis. 2005;11(12): 1961–1964. 10.3201/eid1112.050711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bouchaib H, Eldin C, Laroche M, Raoult D, Parola P. Tick- and flea-borne rickettsioses in Tizi-Ouzou, Algeria: Implications for travel medicine. Travel Med Infect Dis. 2018;26: 51–57. 10.1016/j.tmaid.2018.11.005 [DOI] [PubMed] [Google Scholar]
- 51.Fang CT, Ferng WF, Hwang JJ, Yu CJ, Chen YC, Wang MH, et al. Life-threatening scrub typhus with meningoencephalitis and acute respiratory distress syndrome. J Formos Med Assoc. 1997;96(3): 213–216. [PubMed] [Google Scholar]
- 52.Kuo CC, Huang JL, Lin TE, Wang HC. Detection of Rickettsia spp. and host and habitat associations of fleas (Siphonaptera) in eastern Taiwan. Med Vet Entomol. 2012;26(3): 341–350. 10.1111/j.1365-2915.2012.01009.x [DOI] [PubMed] [Google Scholar]
- 53.Kuo CC, Shu PY, Mu JJ, Lee PL, Wu YW, Chung CK, et al. Widespread Rickettsia spp. infections in ticks (Acari: Ixodoidea) in Taiwan. J. Med. Entomol. 2015;52: 1096–1102. 10.1093/jme/tjv083 [DOI] [PubMed] [Google Scholar]
- 54.Kuo CC, Huang CL, Wang HC. Identification of potential hosts and vectors of scrub typhus and tick-borne spotted fever group rickettsiae in eastern Taiwan. Med. Vet. Entomol. 2011;25: 169–177. 10.1111/j.1365-2915.2010.00941.x [DOI] [PubMed] [Google Scholar]
- 55.Minahan NT, Chao CC, Tsai KH. The re-emergence and emergence of vector-borne rickettsioses in Taiwan. Trop Med Infect Dis. 2017;3(1): 1. 10.3390/tropicalmed3010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.La Scola B, Rydkina L, Ndihokubwayo JB, Vene S, Raoult D. Serological differentiation of murine typhus and epidemic typhus using cross-adsorption and Western blotting. Clin Diagn Lab Immunol. 2000;7(4): 612–616. 10.1128/cdli.7.4.612-616.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]