Abstract
Background
Although it has been realized that restrictive mitral valve annuloplasty (MVA) may result in clinically significant functional mitral stenosis (MS), it still cannot be predicted. The purpose of this study was to identify risk factors for clinically significant functional MS following restrictive MVA surgery for chronic ischemic mitral regurgitation (CIMR).
Methods
One hundred and fourteen patients who underwent restrictive MVA with coronary artery bypass grafting (CABG) for treatment of CIMR were retrospectively reviewed. Clinically significant functional MS was defined as resting transmitral peak pressure gradient (PPG) ≥ 13 mmHg.
Results
During the follow-up period (range 6–12 months), 28 (24.56%) patients developed clinically significant functional MS. The PPG at follow-up was significantly higher than that measured in the early postoperative stage (3–5 days after surgery). Moreover, there was a linear correlation between the two measurements (r = 0.398, p < 0.001). Annuloplasty size ≤ 27 mm and early postoperative PPG ≥ 7.4 mmHg could predict clinically significant functional MS at 6–12 months postoperatively.
Conclusions
Chronic ischemic mitral regurgitation patients treated with restrictive MVA and CABG have significant increases in PPG postoperatively. Annuloplasty size ≤ 27 mm and early postoperative PPG ≥ 7.4 mmHg can predict clinically significant functional MS at 6–12 months after surgery.
Keywords: chronic ischemic mitral regurgitation, restrictive mitral valve annuloplasty, coronary artery bypass grafting, functional mitral stenosis, transmitral peak pressure gradient
Introduction
Chronic ischemic mitral regurgitation (CIMR) is a frequent complication of ischemic heart disease and is associated with poor outcomes. Restrictive mitral valve annuloplasty (MVA) combined with coronary artery bypass grafting (CABG) is a traditional procedure for the surgical management of CIMR [1, 2]. Although restrictive MVA can reduce the risk of recurrence of mitral regurgitation (MR), it is intuitive that it may cause functional mitral stenosis, leading to higher transmitral peak pressure gradient (PPG) and pulmonary arterial pressure (PAP) and, indeed, several reports in literature support this concept [3–6]. Recent studies suggest that resting PPG is an independent predictor of increased systolic PAP during exercise, and that a PPG ≥ 13 mmHg is the best predictor of poor performance on the 6-minute walk test (6MWT) distance [4–7]. What is more, reduced 6MWT distance powerfully predicts morbidity and mortality in patients with heart failure [5, 8].
Since functional mitral stenosis has great negative influence on postoperative outcome, it is vital to identify factors that can predict whether or not a patient will develop clinically significant functional mitral stenosis after restrictive MVA surgery. Therefore, the objective of the present study was to identify the risk factors for the development of clinically significant functional mitral stenosis in CIMR patients undergoing restrictive MVA and CABG. In addition, this study attempts to throw light on measures that can be taken to avoid the development of clinically significant functional mitral stenosis.
Methods
Patients and study design
This study was approved by the Human Research Ethics Committee of the Fuwai Hospital and was performed in accordance with the Declaration of Helsinki and the approved guidelines. From November 2008 to October 2015, 257 consecutive CIMR patients underwent restrictive MVA and CABG in Fuwai hospital. These patients were eligible for inclusion in the study. CIMR was defined by echocardiographic and coronary angiographic findings according to accepted criteria, i.e., 1) MR occurring more than 16 days after myocardial infarction; 2) ≥ 70% stenosis of at least one coronary artery, with wall motion abnormalities of the corresponding left ventricular (LV) segment; and 3) Type I/IIIb leaflet dysfunction according to the Carpentier classification [9].
From the initial cohort of 257 patients, 143 were excluded for various reasons [9], i.e., 1) Presence of aortic valve regurgitation or stenosis, congenital valvular heart disease, infective endocarditis, or recurrent MR (i.e., postoperative MR ≥ 2+ at follow-up); or 2) Performance of other procedures, such as LV reconstruction/reshaping, partial band/pericardial annuloplasty, or procedures other than ring annuloplasty for mitral valve plasty. Thus, the final study cohort was comprised of 114 patients.
Baseline patient characteristics, echocardiography data, operative data, and surgical techniques were collected from the Division of Cardiovascular Surgery database and individual medical records. Patients were followed up through the internet or telephone interviews and outpatient department records. Oral informed consent was obtained from all patients via questionnaire. The echocardiography findings and outcomes at 6–12 months after discharge were analyzed. Median follow-up was 8 months (interquartile range 6–10 months). At the end of follow-up (May 31, 2016), the completeness of follow-up was 96.21%. All collected data were sent to a core lab (State Key Laboratory of Cardiovascular Disease, Beijing, China) for analysis.
Surgical technique
Restrictive MVA and CABG were performed by senior surgeons with special interest in mitral valve surgery. Both procedures were performed by median sternotomy. The ring size was determined by measurements of the intertrigonal distance and anterior leaflet height. A successful repair was defined as leaflet coaptation of ≥ 0.8 cm and MR ≤ 1 and mitral valve area > 1.5 cm2 at transesophageal echocardiography performed at the end of cardiopulmonary bypass. Complete revascularization was achieved in all patients after CABG. All patients received the same perioperative care. Intraoperative transesophageal echocardiography was routinely used.
Three kinds of complete symmetric rings (Carpentier-Edwards Physio Annuloplasty Ring, Carpentier-Edwards Physio II Annuloplasty Ring, and Medtronic Duran AnCore Ring) were used in the present study. The prosthesis sizes were 26 mm (n = 9), 27 mm (n = 29), 28 mm (n = 30), 29 mm (n = 14), 30 mm (n = 15), 31 mm (n = 4), 32 mm (n = 12), and 33 mm (n = 1).
Echocardiography
Echocardiographic measurements followed American Society of Echocardiography guidelines [10]. MR was classified as mild (grade 1+), moderate (grade 2+), or severe (grades 3+ and 4+). Of the 114 patients, 110 suffered from MR of grades 3+, while 4 suffered from MR of grades 4+. Two-dimensional and Doppler transthoracic echocardiography examinations were performed before operation, in the early postoperative stage (3–5 days postoperatively), and at follow-up (6–12 months postoperatively). Clinically significant functional mitral stenosis was defined as resting PPG ≥ 13 mmHg [5].
Statistical analysis
Continuous and categorical variables were reported as mean ± standard deviation and percentages, respectively. The χ2 test and the t test were used to compare the preoperative characteristics between groups. Logistic regression was used to identify the independent predictors of PPG ≥ 13 mmHg (i.e., clinically significant functional mitral stenosis) at follow-up. To reduce the chance of overfitting the models, models were fit “reduced” which contained only variables whose univariate tests of associations with outcome, based on t test or the χ2 test, were < 0.10 for outcome of PPG ≥ 13 mmHg at follow-up. This approach yielded a subset of 5 predictors (denoted with a asterisk in Tables 1 and 2) from the original set of 40. Because this data set was too small and variety of annuloplasty great, to fit the model, the study population were classified into four groups according to the size of annuloplasty: 26–27 mm group (n = 38; 33.33%), 28–29 mm group (n = 44; 38.60%), 30–31 mm group (n = 19; 16.67%), and 32–33 mm group (n = 13; 11.40%). Model-based probabilities were analyzed using receiver operating characteristic (ROC) curves. Area under the curve (AUC) summarizes information about outcome included in the “predictor” set, with a value of 1 as the maximum. All reported p values are two-sided, and values of p < 0.05 were considered to indicate statistical significance. All statistical analyses were performed by SPSS 13.0 software (SPSS, Inc., Chicago, IL) and Graph Pad Prism release 5 (Graph Pad Software Inc, La Jolla, Calif) statistical packages.
Table 1.
Preoperative demographic and clinical data of non-stenosis and stenosis patients.
| Variables | Non-stenosis (n = 86) | Stenosis (n = 28) | P |
|---|---|---|---|
| Age [years] | 58.42 ± 8.24 | 59.63 ± 8.92 | 0.554 |
| Sex (male) | 77 (89.53%) | 21 (75.00%) | 0.062* |
| Body surface area [m2] | 1.91 ± 0.16 | 1.92 ± 0.18 | 0.773 |
| EUROscore | 5.62 ± 1.21 | 5.57 ± 1.07 | 0.861 |
| Diabetes | 25 (29.07%) | 6 (21.43%) | 0.430 |
| Hypertension | 53 (61.63%) | 17 (60.71%) | 0.931 |
| Hyperlipidemia | 37 (43.02%) | 10 (35.71%) | 0.495 |
| COPD | 4 (4.65%) | 1 (3.57%) | 0.804 |
| Carotid artery stenosis | 4 (4.65%) | 3 (10.71%) | 0.273 |
| History of PCI | 5 (5.81%) | 3 (10.71%) | 0.399 |
| History of heart failure | 18 (20.93%) | 3 (10.71%) | 0.204 |
| Atrial fibrillation | 11 (12.79%) | 3 (10.71%) | 0.768 |
| Aneurysm | 7 (8.14%) | 2 (7.14%) | 0.864 |
| Grade on CCS angina scale: | |||
| Total | 0.231 | ||
| I | 51 (59.30%) | 18 (64.29%) | |
| II | 14 (16.28%) | 4 (14.29%) | |
| III | 8 (9.30%) | 5 (17.86%) | |
| IV | 13 (15.12%) | 1 (3.57%) | |
| NYHA class III or IV | 48 (55.81%) | 16 (57.14%) | 0.902 |
| Left main CAD | 23 (26.74%) | 7 (25.00%) | 0.968 |
| CABG/patient | 2.89 ± 0.70 | 2.81 ± 0.79 | 0.624 |
| Distal anastomoses: | 0.751 | ||
| 1 | 4 (4.65%) | 2 (7.14%) | |
| 2–4 | 70 (81.40%) | 21 (75.00%) | |
| > 4 | 12 (13.95%) | 5 (17.86%) | |
| Anastomoses/patient | 3.52 ± 0.95 | 3.41 ± 1.08 | 0.594 |
| LIMA | 81 (94.19%) | 23 (82.14%) | 0.062* |
| ACC time | 113.36 ± 38.05 | 105.11 ± 24.11 | 0.183 |
| CPB time | 158.84 ± 47.91 | 146.89 ± 32.99 | 0.146 |
| IABP | 2 (2.33%) | 1 (3.57%) | 0.729 |
| Prosthesis size: | 0.010* | ||
| 26–27 mm | 22 (25.58%) | 16 (57.14%) | |
| 28–29 mm | 36 (41.86%) | 8 (28.57%) | |
| 30–31 mm | 13 (15.12%) | 2 (7.14%) | |
| 32–33 mm | 15 (17.44%) | 2 (7.14%) | |
| Concomitant procedure: | |||
| Modified maze procedure | 1 (1.16%) | 1 (3.57%) | 0.434 |
| Tricuspid annuloplasty | 6 (6.98%) | 1 (3.57%) | 0.491 |
| Mechanical ventilation time [h] | 25.98 ± 16.68 | 26.38 ± 22.03 | 0.920 |
| ICU stay time [h] | 67.30 ± 50.37 | 76.54 ± 56.22 | 0.446 |
Indicates variables entered into logistic regression.
Data are shown as mean ± standard deviation or number (percentage). COPD — chronic obstructive pulmonary disease; PCI — percutaneous coronary intervention; CCS — Canadian Cardiovascular Society; NYHA — New York Heart Association functional class; CAD — coronary artery disease; CABG — coronary artery bypass grafting; LIMA — left internal mammary artery; ACC — aortic cross-clamp; CPB — cardiopulmonary bypass; IABP — intra-aortic balloon pump; ICU — intensive care unit
Table 2.
Preoperative, early postoperative, and follow-up Doppler echocardiography data for non-stenosis and stenosis patients.
| Variables | Non-stenosis (n = 86) | Stenosis (n = 28) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Preoperative | Postoperative | Follow-up | Preoperative | Postoperative | Follow-up | |
| LVEF [%] | 51.03 ± 11.24 | 52.13 ± 10.10 | 53.05 ± 9.82 | 50.71 ± 7.14 | 51.61 ± 7.14 | 54.29 ± 8.63 |
| LVEDD mid-ventricle [mm] | 57.59 ± 6.29 | 52.65 ± 6.35 | 54.44 ± 6.60 | 58.48 ± 5.17 | 53.25 ± 4.61 | 55.18 ± 5.16 |
| LVESD mid-ventricle [mm] | 38.72 ± 8.70 | 35.63 ± 7.87 | 35.94 ± 7.84 | 38.95 ± 5.24 | 35.89 ± 4.97 | 35.48 ± 6.39 |
| LAD [mm] | 41.48 ± 5.08* | 37.49 ± 4.56 | 41.21 ± 5.68 | 44.37 ± 6.18# | 39.50 ± 5.52 | 46.07 ± 5.92# |
| Grade of mitral regurgitation: | ||||||
| 3+ | 82 (95.35%) | – | – | 28 (100%) | – | – |
| 4+ | 4 (4.65%) | – | – | 0 | – | – |
| Tricuspid regurgitation: | 29 (33.72%) | – | 16 (18.60%) | 11 (39.29%) | – | 10 (35.71%) |
| 0/1+/2+/3+/4+ | 57/22/5/2/0 | 70/15/1/0/0 | 17/9/1/1/0 | 18/10/0/0/0 | ||
| Pulmonary hypertension: | 10 (11.63%) | – | 6 (6.98%) | 4 (14.29%) | – | 4 (14.29%) |
| Systolic PAP < 40 mmHg | 5 | – | 2 | 3 | – | 1 |
| Systolic PAP 40–60 mmHg | 3 | – | 3 | 1 | – | 2 |
| Systolic PAP > 60 mmHg | 2 | – | 1 | 0 | – | 1 |
| Transmitral mean gradient [mmHg] | 3.24 ± 1.00 | 3.28 ± 1.02 | 3.57 ± 1.10 | 6.93 ± 1.97# | ||
| PPG [mmHg]: | – | 6.41 ± 2.29* | 7.39 ± 2.36 | – | 9.04 ± 3.28# | 16.19 ± 3.95# |
| PPG < 7.4 mmHg | – | 61 (70.93%) | – | – | 7 (25.00%) | – |
| PPG ≥ 7.4 mm Hg, n (%) | – | 25 (29.07%) | – | – | 21 (75.00%) | – |
Data are shown as mean ± standard deviation or number (percentage).
Indicates variables entered into logistic regression.
p < 0.05 (non-stenosis group vs. stenosis group).
LVEF — left ventricular ejection fraction; LVEDD — left ventricular end-diastolic dimension; LVESD — left ventricular end-systolic dimension; LAD — left atrial dimension. PAP — pulmonary artery pressure; PPG — transmitral peak pressure gradient
Results
Comparison of early postoperative PPG vs. follow-up PPG
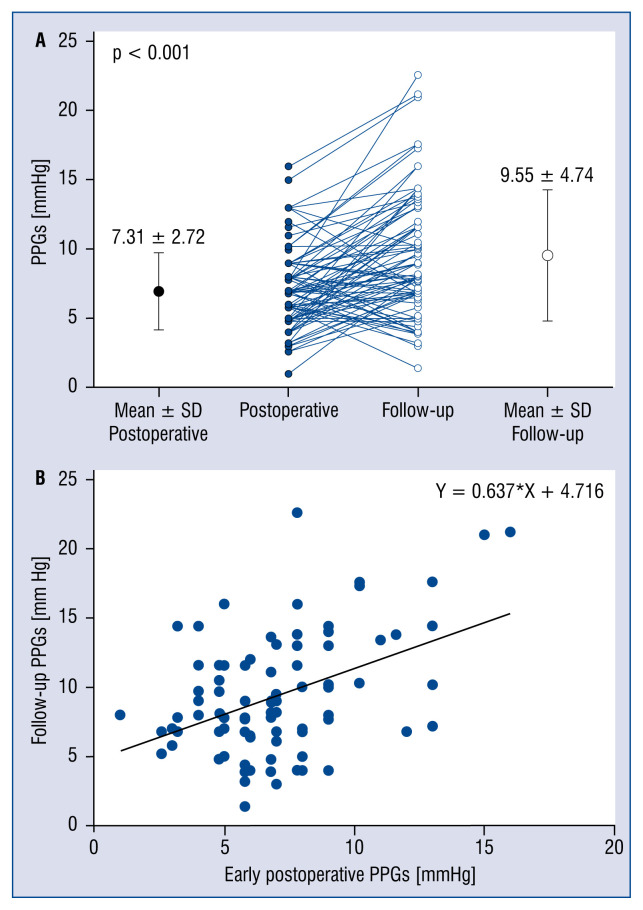
Follow-up PPG was significantly higher than early postoperative PPG (9.55 ± 4.74 mmHg vs. 7.31 ± 2.72 mmHg; p < 0.001; Fig. 1A). There was linear correlation between the two (r = 0.398; p < 0.001); the relationship between the early postoperative PPG (X) and PPG at follow-up (y) could be expressed as: Y = X × 0.637 + 4.716 (Fig. 1B).
Figure 1.
A. Comparison of early postoperative transmitral peak pressure gradient (PPG) vs. follow-up PPG for the overall population; B. Relationship between early postoperative and follow-up PPG; X — PPG at early postoperative stage; Y — PPG at follow-up; SD — standard deviation.
Comparison of demographics and clinical features between stenosis and non-stenosis patients
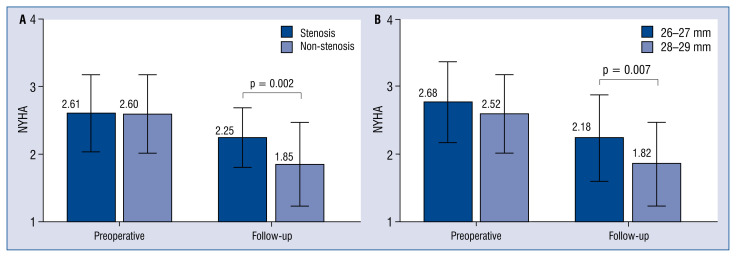
There were no deaths during the first 6 months. Between 6 and 12 months postoperatively, 3 patients died. A large proportion (28/114 patients; 24.56%) were diagnosed with clinically significant functional mitral stenosis (i.e., PPG ≥ 13 mmHg) at follow-up. Stenosis and non-stenosis patients had similar characteristics at baseline (Tables 1, 2). There was no significant difference between the two groups in grade of MR. The echocardiographic results showed that, compared with the non-stenosis group, both the PPG in early postoperative stage and at follow-up, and the follow-up transmitral mean gradient were significantly higher in the stenosis group (all p < 0.001). After adjustment for the diameter of the preoperative left atrium, the stenosis patients had significantly larger left atrial diameter than the non-stenosis patients at follow-up (p < 0.001). The stenosis patients also had significantly worse New York Heart Association (NYHA) functional class at follow-up (p = 0.002; Fig. 2A).
Figure 2.
A. Comparison of preoperative and follow-up New York Heart Association (NYHA) functional class of the stenosis patients and the non-stenosis patients. There was no significant difference in preoperative NYHA class, but the follow-up NYHA class was significantly higher in stenosis patients than in non-stenosis patients; B. Comparison of preoperative and follow-up NYHA class for patients with 26–27 mm and 28–29 mm prosthesis. There was no significant difference in preoperative NYHA class, but the follow-up NYHA class was significantly higher in patients with the 26–27 mm prosthesis than in patients with the 28–29 mm prosthesis.
Predicting occurrence of clinically significant functional mitral stenosis at follow-up
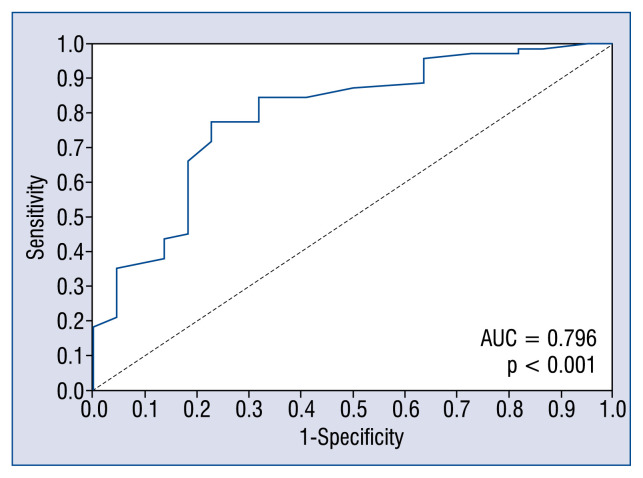
In forward multiple stepwise regression analyses, the size of annuloplasty (p = 0.021) and early postoperative PPG (p < 0.001) were independently associated with clinically significant functional mitral stenosis at follow-up (Table 3). In ROC curve analysis, both the size of annuloplasty (AUC = 0.68; p = 0.005) and early postoperative PPG (AUC = 0.75; p < 0.001) could effectively predict clinically significant functional mitral stenosis at follow-up. Annuloplasty size of ≤ 27 mm (sensitivity 74%, specificity 57%) and early postoperative PPG of ≥ 7.4 mmHg (sensitivity 71%, specificity 71%) could effectively separate patients who developed clinically significant functional mitral stenosis at follow-up from those who did not. Importantly, the combination of the two had the best performance (AUC = 0.80; p < 0.001; Fig. 3).
Table 3.
Logistic regression analysis for outcome of clinically significant functional mitral stenosis.
| Variable | Univariable | Stepwise multivariable | ||
|---|---|---|---|---|
|
|
|
|||
| Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | |
| Sex | 2.852 (0.950–8.561) | 0.062 | ||
| LIMA | 0.284 (0.076–1.066) | 0.062 | ||
| Prosthesis size | 0.708 (0.545–0.920) | 0.010 | 0.714 (0.536–0.950) | 0.021 |
| Early postoperative PPG | 1.399 (1.169–1.675) | < 0.001 | 1.407 (1.164–1.701) | < 0.001 |
| Preoperative LAD | 1.102 (1.015–1.198) | 0.021 | ||
CI — confidence interval; LIMA — left internal mammary artery; PPG — transmitral peak pressure gradient; LAD — left atrial dimension
Figure 3.
Receiver operating characteristic curve generated by the combination of the size of annuloplasty and early postoperative transmitral peak pressure gradient; AUC — area under the curve.
Comparison of demographics and clinical features between the 26–27 mm group and the 28–29 mm group
Having found that an annuloplasty size ≤ 27 mm could effectively predict the occurrence of clinically significant functional mitral stenosis at follow-up, we compared the clinical features of the 26–27 mm group and the 28–28 mm group. The two groups had similar characteristics, including body surface area (BSA), at baseline (Tables 4, 5). There was no significant difference in early postoperative PPG or transmitral mean gradient between the two groups, but both the follow-up PPG and transmitral mean gradient were significantly higher in the 26–27 mm group (both p = 0.001). The intensive care unit (ICU) stay time was markedly longer for the 26–27 mm group than the 28–29 mm group. The left atrium of the 26–27 mm group was significantly larger, both at the early postoperative stage (p = 0.003) and at follow-up (p = = 0.030). Furthermore, although the distribution of NYHA functional class in the two groups was similar preoperatively, the NYHA functional class was significantly worse in the 26–27 mm group at follow-up (p = 0.007; Fig. 2B).
Table 4.
Preoperative demographic and clinical data for the 26–27 mm group and the 28–29 mm group.
| Variables | 26–27 mm (n = 38) | 28–29 mm (n = 44) | P |
|---|---|---|---|
| Age [years] | 60.42 ± 8.19 | 57.86 ± 9.33 | 0.194 |
| Sex (male) | 31 (81.58%) | 39 (88.64%) | 0.367 |
| Body surface area [m2] | 1.91 ± 0.15 | 1.87 ± 0.12 | 0.151 |
| EUROscore | 5.58 ± 1.22 | 5.73 ± 1.17 | 0.576 |
| Diabetes | 11 (28.95%) | 12 (27.27%) | 0.866 |
| Hypertension | 23 (60.53%) | 24 (54.55%) | 0.585 |
| Hyperlipidemia | 15 (39.47%) | 19 (43.18%) | 0.734 |
| COPD | 2 (5.26%) | 2 (4.55%) | 0.881 |
| Carotid artery stenosis | 2 (5.26%) | 2 (4.55%) | 0.881 |
| History of PCI | 3 (7.89%) | 3 (6.82%) | 0.852 |
| History of heart failure | 7 (18.42%) | 7 (15.91%) | 0.763 |
| Atrial fibrillation | 8 (21.05%) | 4 (9.09%) | 0.125 |
| Aneurysm | 4 (10.53%) | 3 (6.82%) | 0.549 |
| Grade on CCS angina scale: | |||
| No angina | 13 (34.21%) | 16 (36.36%) | 0.839 |
| Class III or IV | 6 (15.79%) | 7 (15.91%) | 0.988 |
| NYHA class III or IV | 24 (63.16%) | 22 (50.00%) | 0.231 |
| Left main CAD | 12 (31.58%) | 11 (25.00%) | 0.470 |
| Distal anastomoses: | 0.186 | ||
| 1 | 2 (5.26%) | 2 (4.55%) | |
| 2–4 | 27 (71.05%) | 38 (88.64%) | |
| > 4 | 9 (23.68%) | 4 (6.82%) | |
| Anastomoses/patient | 3.58 ± 1.15 | 3.42 ± 0.79 | 0.475 |
| Grafts/patient | 2.87 ± 0.70 | 2.86 ± 0.77 | 0.962 |
| LIMA | 34 (89.47%) | 39 (88.64%) | 0.904 |
| ACC time [min] | 107.24 ± 21.94 | 104.50 ± 33.31 | 0.658 |
| CPB time [min] | 153.97 ± 32.94 | 145.91 ± 37.81 | 0.310 |
| Mechanical ventilation time [h] | 28.76 ± 23.88 | 26.56 ± 15.08 | 0.619 |
| ICU stay time [h] | 89.86 ± 71.45 | 56.27 ± 27.97 | 0.012 |
Data are shown as mean ± standard deviation or number (percentage). COPD — chronic obstructive pulmonary disease; PCI — percutaneous coronary intervention; CCS — Canadian Cardiovascular Society; NYHA — New York Heart Association functional class; CAD — coronary artery disease; CABG — coronary artery bypass graft; IABP — intra-aortic balloon pump; LIMA — left internal mammary artery; ACC — aortic cross-clamp; CPB — cardiopulmonary bypass; ICU — intensive care unit
Table 5.
Preoperative, early postoperative, and follow-up Doppler echocardiography data for the 26–27 mm group and the 28–29 mm group.
| Variables | 26–27 mm (n = 38) | 28–29 mm (n = 44) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Preoperative | Postoperative | At follow-up | Preoperative | Postoperative | At follow-up | |
| LVEF [%] | 49.84 ± 10.30 | 51.13 ± 8.51 | 51.53 ± 8.93 | 50.50 ± 9.10 | 51.75 ± 8.10 | 54.09 ± 7.65 |
| LVEDD mid-ventricle [mm] | 58.86 ± 6.02 | 53.47 ± 5.31 | 55.92 ± 5.88 | 57.30 ± 6.33 | 52.48 ± 5.29 | 53.86 ± 6.43 |
| LVESD mid-ventricle [mm] | 40.20 ± 8.05 | 36.32 ± 6.23 | 37.43 ± 7.45 | 38.74 ± 7.62 | 35.56 ± 6.05 | 34.96 ± 6.21 |
| LAD [mm] | 43.11 ± 5.99 | 40.00 ± 5.19 | 44.53 ± 5.45 | 42.00 ± 5.44 | 36.75 ± 4.43# | 41.59 ± 6.44# |
| Grade of mitral regurgitation 3+ | 38 (100%) | – | – | 44 (100%) | – | – |
| Tricuspid regurgitation | 16 (42.11%) | – | 9 (23.68%) | 14 (31.82%) | – | 12 (27.27%) |
| Pulmonary hypertension | 4 (10.53%) | – | 4 (10.53%) | 6 (13.64%) | – | 5 (11.36%) |
| Transmitral mean gradient [mmHg] | 3.45 ± 1.24 | 5.30 ± 2.36 | 3.42 ± 0.85 | 3.62 ± 1.71# | ||
| PPG [mmHg] | – | 7.44 ± 3.01 | 12.07 ± 5.43 | – | 7.05 ± 2.53 | 8.34 ± 3.79# |
p < 0.05 (26–27 mm group vs. 28–29 mm group).
LVEF — left ventricular ejection fraction; LVEDD — left ventricular end-diastolic dimension; LVESD — left ventricular end-systolic dimension; LAD — left atrial dimension; PPG — transmitral peak pressure gradient.
Discussion
According to available research, this study is the first to compare mitral gradients at different postoperative stages in CIMR patients undergoing restrictive MVA and CABG. A major finding of this study was that follow-up PPG was significantly higher than early postoperative stage PPG. Interestingly, Murashita et al. [11] showed that restrictive MVA for degenerative regurgitation resulted in small mitral valve gradients, which decreased with time after discharge. Transmitral gradients are determined by both transmitral flow and the severity of the intrinsic stenosis [4, 12]. Unless there is corresponding increase in mitral effective orifice area (EOA) [4], the improvement in LV diastolic function after CABG will result in higher mitral gradients [13].
Previous studies have demonstrated that when ischemic MR is treated by restrictive MVA, MR is often traded for mitral stenosis, which may outweigh the benefits of this procedure on the hemodynamic and clinical outcomes [5]. The occurrence of functional mitral stenosis after restrictive MVA has not been well studied despite its relatively high incidence (9–54%) [8, 12, 14]. Importantly, recent findings show that PPG can independently predict elevated systolic PAP during exercise as well as reduced 6MWT distance [4–7], which is a powerful predictor of morbidity and mortality in patients with heart failure [5, 8]. Thus, elevated resting peak mitral gradients correlate well with functional mitral stenosis and could serve as a good predictor of poor functional capacity [5]. However, several studies have shown that transmitral gradients do not correlate with functional capacity and an elevated gradient should not be considered as detrimental to functional capacity. Rubino et al. [3] divided 125 consecutive patients into two groups according to mean transmitral gradient (Δp = 5 mmHg) at predischarge echocardiography. They found that mild mitral stenosis did not affect echocardiographic, functional, or clinical outcomes [3]. In this regard, it should be noted that PPG correlated with functional capacity better than mitral mean gradient, and PPG ≥ 13 mmHg was the best predictor of reduced 6MWT distance [5]; furthermore, the induction of mild mitral stenosis did not affect functional and echocardiographic outcomes [3]. Bertrand et al. [12] concluded that an increased transmitral gradient in a patient with normal cardiac output might be acceptable and may have no impact on functional capacity. However, their study had several limitations, including a small study sample of only 23 patients, which might have led to type II statistical errors; in addition, the fact that not all patients with ischemic MR participated in the study might have introduced a selection bias [12].
Magne et al. [5] have shown that the best predictors of reduced 6MWT distance in restrictive MVA patients were PPG and mitral mean gradient, with PPG ≥ 13 mmHg being the best predictor of reduced 6MWT distance (sensitivity 92%, specificity 82%). On the basis of this important finding, the present study defined clinically significant functional mitral stenosis as PPG ≥ 13 mmHg at follow-up. In the current study, although the stenosis and the non-stenosis patients had similar characteristics at baseline, the former had larger left atrial diameter and worse NYHA functional class. Thus, the present findings confirmed Magne et al.’s [5] conclusion that PPG ≥ 13 mmHg at follow-up can contribute greatly to the diagnosis of clinically significant functional mitral stenosis.
Unlike in mitral valve replacement, the EOA of the mitral valve in patients undergoing restrictive MVA is influenced not only by the size of annuloplasty but also by the severity of subvalvular leaflet tethering [6, 15]. Therefore, we still cannot predict prosthesis–patient mismatch preoperatively by BSA and EOA. A recent study showed that use of smaller prostheses (26 mm) for degenerative MR might result in higher PPG and a larger left atrium [16]. In the present study, although the 26–27 mm group and the 28–29 mm group had similar characteristics (including BSA) at baseline, the former had longer ICU stay, higher PPG at follow-up, and worse NYHA functional class. Thus, the results of the present study show that when BSA is ≥ 1.90 m2, an annuloplasty of 26–27 mm size might result in functional mitral stenosis and poor functional capacity. Therefore, when the BSA is ≥ 1.90 m2, it is advisable to avoid annuloplasties that are ≤ 27 mm in size; in these cases, an annuloplasty of size no smaller than 28 mm or mitral valve replacement would be a better alternative. Adherence to this rule should help prevent the possibility of postoperative functional mitral stenosis. Furthermore, because the transmitral pressure gradient tends to increase postoperatively due to the improvement in LV function after CABG, a larger prosthesis should be chosen when the option is between two sizes of prosthesis.
Of note, this study showed that early postoperative PPG ≥ 7.4 mmHg can predict the occurrence of clinically significant functional mitral stenosis at follow-up. Therefore, patients with early postoperative PPG ≥ 7.4 mmHg need special attention, as they may potentially suffer prosthesis–patient mismatch in the future and require treatment.
Limitations of the study
There are still some limitations. This study reports retrospective data from a single center and is subject to all the limitations inherent to this design. In addition, the small study sample might have led to type II statistical errors. Another limitation is that echocardiographic measurements, such as cardiac output and systolic PAP, were available only for a subset of patients. However, with detailed information about NYHA functional class, evaluation still found a correlation between PPG and functional capacity. As this is a retrospective study, there was a lack of detailed data as to the degree of tethering for all patients. Of note, transmitral pressure gradient is a combination of all reasons that affect the transmitral flow including mitral valve tethering. With details of transmitral pressure gradient, functional mitral stenosis at follow-up could be predicted effectively.
Conclusions
The present results demonstrate that postoperative PPG increases significantly in CIMR patients undergoing restrictive MVA and CABG. To reduce the risk of occurrence of clinically significant functional mitral stenosis, annuloplasties of ≤ 27 mm should be avoided in patients with BSA ≥ 1.90 m2, and a larger prosthesis should be chosen when the option is between two sizes of prosthesis. Of note, patients with early postoperative PPG ≥ 7.4 mmHg are at high risk of developing clinically significant functional mitral stenosis. Further studies are needed to determine selection of annuloplasties for patients with conditions other than CIMR to avoid functional mitral stenosis.
Acknowledgements
The authors thank Shanglin Chen (MD) for the assistance provided in the statistical analysis.
Footnotes
Conflict of interest: None declared
References
- 1.Kron IL, Acker MA, Adams DH, et al. 2015 The American Association for Thoracic Surgery Consensus Guidelines: Ischemic mitral valve regurgitation. J Thorac Cardiovasc Surg. 2016;151(4):940–956. doi: 10.1016/j.jtcvs.2015.08.127. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg. 2014;148(1):e1–e132. doi: 10.1016/j.jtcvs.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Rubino AS, Onorati F, Santarpia G, et al. Impact of increased transmitral gradients after undersized annuloplasty for chronic ischemic mitral regurgitation. Int J Cardiol. 2012;158(1):71–77. doi: 10.1016/j.ijcard.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Fino C, Iacovoni A, Ferrero P, et al. Restrictive mitral valve annuloplasty versus mitral valve replacement for functional ischemic mitral regurgitation: an exercise echocardiographic study. J Thorac Cardiovasc Surg. 2014;148(2):447–53e2. doi: 10.1016/j.jtcvs.2013.05.053. [DOI] [PubMed] [Google Scholar]
- 5.Magne J, Sénéchal M, Mathieu P, et al. Restrictive annuloplasty for ischemic mitral regurgitation may induce functional mitral stenosis. J Am Coll Cardiol. 2008;51(17):1692–1701. doi: 10.1016/j.jacc.2007.11.082. [DOI] [PubMed] [Google Scholar]
- 6.Kubota K, Otsuji Y, Ueno T, et al. Functional mitral stenosis after surgical annuloplasty for ischemic mitral regurgitation: importance of subvalvular tethering in the mechanism and dynamic deterioration during exertion. J Thorac Cardiovasc Surg. 2010;140(3):617–623. doi: 10.1016/j.jtcvs.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cahalin LP, Mathier MA, Semigran MJ, et al. The six-minute walk test predicts peak oxygen uptake and survival in patients with advanced heart failure. Chest. 1996;110(2):325–332. doi: 10.1378/chest.110.2.325. [DOI] [PubMed] [Google Scholar]
- 8.Kainuma S, Taniguchi K, Daimon T, et al. Does stringent restrictive annuloplasty for functional mitral regurgitation cause functional mitral stenosis and pulmonary hypertension? Circulation. 2011;124(11 Suppl):S97–106. doi: 10.1161/CIRCULATIONAHA.110.013037. [DOI] [PubMed] [Google Scholar]
- 9.Lorusso R, Gelsomino S, Vizzardi E, et al. Mitral valve repair or replacement for ischemic mitral regurgitation? The Italian Study on the Treatment of Ischemic Mitral Regurgitation (ISTIMIR) J Thorac Cardiovasc Surg. 2013;145(1):128–139. doi: 10.1016/j.jtcvs.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 10.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Murashita T, Greason KL, Suri RM, et al. Mitral valve gradient after valve repair of degenerative regurgitation with restrictive annuloplasty. J Thorac Cardiovasc Surg. 2016;151(1):106–109. doi: 10.1016/j.jtcvs.2015.08.078. [DOI] [PubMed] [Google Scholar]
- 12.Bertrand PB, Gutermann H, Smeets CJP, et al. Functional impact of transmitral gradients at rest and during exercise after restrictive annuloplasty for ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2014;148(1):183–187. doi: 10.1016/j.jtcvs.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Lawson WE, Seifert F, Anagnostopoulos C, et al. Effect of coronary artery bypass grafting on left ventricular diastolic function. Am J Cardiol. 1988;61(4):283–287. doi: 10.1016/0002-9149(88)90931-9. [DOI] [PubMed] [Google Scholar]
- 14.Williams ML, Daneshmand MA, Jollis JG, et al. Mitral gradients and frequency of recurrence of mitral regurgitation after ring annuloplasty for ischemic mitral regurgitation. Ann Thorac Surg. 2009;88(4):1197–1201. doi: 10.1016/j.athoracsur.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 15.Bertrand PB, Verbrugge FH, Verhaert D, et al. Mitral valve area during exercise after restrictive mitral valve annuloplasty: importance of diastolic anterior leaflet tethering. J Am Coll Cardiol. 2015;65(5):452–461. doi: 10.1016/j.jacc.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawamoto N, Fujita T, Hata H, et al. Prosthesis-patient mismatch due to small ring annuloplasty in patients with degenerative mitral insufficiency. J Cardiol. 2016;68(2):141–147. doi: 10.1016/j.jjcc.2015.09.007. [DOI] [PubMed] [Google Scholar]