Abstract
Background
Renal denervation (RDN) is as an effective treatment for heart failure (HF), but its effects on cardiac function of patients with HF are not well documented. Here, the aim was to investigate RDN’s effect on patients with chronic systolic HF, by conducting a single-center, prospective, randomized, and controlled study.
Methods
Sixty patients with chronic systolic HF were randomly assigned to the RDN or control groups, receiving percutaneous catheter-based RDN with radiofrequency ablation and drug treatment, respectively. All patients performed a 6-minute walk test, echocardiography, blood pressure measurement, and biochemical test, at both baseline and in a 6-month follow up.
Results
Over 6-month follow up, patients in RDN group showed a decrease in N-terminal pro-B-type natriuretic peptide (440.1 ± 226.5 pg/mL vs. 790.8 ± 287.0 pg/mL, p < 0.001, Cohen’s d = 1.14), an increase in left ventricular ejection fraction (39.1 ± 7.3% vs. 35.6 ± 3.3%, p = 0.017, Cohen’s d = 0.61), improved New York Heart Association class assessment (p = 0.01, Cohen’s d = 0.66), and decreased blood pressures (p < 0.001, Cohen’s d = 0.91), without reporting hypotension and syncope amaurosis. No significant between-group difference was observed for glomerular filtration rate and heart rate.
Conclusions
Renal denervation which effectively and safely improves patient’s cardiac function as well as exercise tolerance, could be considered as an effective treatment for chronic systolic HF.
Keywords: renal denervation, sympathetic nervous system, heart failure, blood pressure, cardiology
Introduction
Chronic heart failure (HF) is a common disease suffered by around 100 million people all over the world. There were 5.1 million people in United States at present a number which increases at a rate of 800,000 cases per year whosuffer from this disease. HF is believed to be a major cause of sudden cardiac death, and previous studies have shown that the death rate for patients with chronic HF is 6–9 times higher than those in the normal population. Around 300,000 deaths are caused by HF every year in United States [1]. The medical expense on HF reachedas much as $30 billion in 2012, and has kept growing in recent years [2].
Excessive activity of the sympatheic nerve system is believed as one of the major causes of the HF progression. Sympathetic activation involves efferent and afferent pathways to regulate cardiovascular functions such as blood pressure (BP) and heart rate, in response to acute stress like volume depletion or excessive vasodilatation [3]. Excessive activity of the sympathetic nervous system has a direct adverse consequence for cardiovascular diseases, particularly for HF and hypertension [4]. A proper suppression of the sympathetic nervous system activity may be able to improve the conditions of patients with HF. Particularly, catheterbased renal sympathetic denervation (RDN), which can specificly and effectively reduce the activity of sympatheic nervous system [3, 5], which is considered a proper treatment for HF.
Renal denervation was implemented by selective sympathetic denervation of the human kidney with radiofrequency energy ablation. It was firstly proposed to treat resistant hypertension [6, 7], but its treatment effect for this specific disease was not as good as had been imagined. HTN-3 study found that there was no significant difference in BP between the RDN and sham groups [8]. Even though, its ability in suppressing the activity of the sympathetic nervous system suggests it is a possible application in the treatment of HF.
To explore the treatment effect of RDN on HF, several animal models, e.g. pig and rat models, were established [9–11] and the performance of RDN on these animal models were quite promissing. However, these animal based results were insufficient to be considered as direct evidence for the application of RDN in clinical practice; more experiments on humans should be conducted to eliminate the concerns regarding its effectiveness and safety to patients with HF. A first-in-man study of chronic systolic HF has already been conducted [12], and suggests that patient exercise tolerances were improved after the intervention of RDN. Nevertheless, small sample size and its single-group non-blinded and non-randomized nature limited the validity of these research findings. Thus, the aim presently was to comprehensively investigate the effectiveness and safety of catheter-based RDN in the treatment of chronic systolic HF, by conducting a randomized controlled trial with a larger sample size.
Methods
Patients
Sixty patients with chronic systolic HF (New York Heart Association [NYHA] class II or III) were recruited in this study. All patients were not less than 18 years old. The inclusion criteria were: (1) left ventricular ejection fraction (LVEF) should be smaller than 40% at echocardiography or N-terminal pro-B-type natriuretic peptide (NT-proBNP) should be larger than 125 pg/mL; (2) glomerular filtration rate (GFR) should not be smaller than 45 mL/min/1.73 m2 and systolic BP should not be smaller than 100 mmHg. The exclusion criteria were: (1) patients with renal artery stenosis (in history or revealed by imaging), type I diabetes, severe heart valvar disease, and myocardial infarction or cerebrovascular accident 6 months prior were excluded; (2) patients who were or would have been in pregnancy during the study.
The study was approved by the ethics committee of Putuo Hospital Affiliated to Shanghai University of Traditional Chinese Medicine. All patients had been informed in advance and signed a consent form.
Experimental design
This was a single-centre, prospective, randomized and controlled study. The 60 recruited patients were equally and randomly assigned to the intervention group undergoing catheter-based RDN and the control group receiving drug treatment with the random envelope method, there were 30 patients in each group. All involved patients were followed up for 6 months.
RDN intervention
Before the RDN intervention, patients were given enteric-coated acetylsalicylic acid (300 mg) or clopidogrel (300 mg) by chewing. During the operation, patients were given heparin (6000–8000 U) by intravenous injection. Femoral artery puncture were conducted after skin preparation in right fold inguen and disinfection; then 7 F vascular sheath was imbedded. Renal arteriography was conducted in both left and right with a JR4 catheter. Spiral ablation was performed with an imbedded 6 F radiofrequency ablation catheter (Ablation instrument [39D-72X]: Johnson Medical Instrument Co. Ltd.) in temperature control mode (8–10 W, 50°C) to both left and right renal arteries. There were 4–6 ablation points in the left and right renal arteries respectively. The effective ablation time for each point was 60 s and the interval between two neighbouring points was 0.5 cm. Renal arteriography was conducted after operation.
Study assessment
Efficacy endpoint
All patients accepted NT-proBNP test, echocardiographic LVEF assessment and NYHA class assessment both before the operation and after 6-month follow up. Six-minute walk test was also conducted both preoperatively and monthly during the following period. Changes in these tests were treated as the efficacy endpoint in this study.
Safety endpoint
All patients accepted the office BP measurement both preoperatively and monthly during the follow up. Heart rate and GFR were also measured both before the operation and after 6-month follow up. Changes in these measurements were treated as safety points in this study.
Pharmacological therapies
All patients underwent pharmacotherapy for HF with a maximal tolerated dose prior to denervation, including beta-blockers, angiotensin converting enzyme (ACE) inhibitors or endothelin receptor antagonist, and spironolactone. No change of medications was permitted prior to denervation. During the following, patients were given standard HF care and the physicians could freely adjust the dosage of pharmacotherapy according to patient condition, e.g., changes in BP and heart rate.
Statistical analysis
Statistical analyses were carried out with the SPSS 21.0 statistical analysis package (SPSS Inc., New York, USA). All measurement data were presented as mean ± standard deviation (SD); while, the enumeration data were presented as a percentage number. The comparison of measurement data was achieved by the student independent t-test with a prior checking of dataset normality by Shapiro-Wilk test; and for enumeration data, they were compared by χ2 test or the Fisher exact test. Statistical difference was defined as the situation where p-value was smaller than 0.05.
Results
Baseline clinical characteristics
Sixty patients (13 females and 47 males) with a mean age of 60.2 ± 11.6 years were enrolled in this randomized controlled study between January 2014 and July 2015. Their mean body mass index was 26.7 ± 2.7 kg/m2. Among these patients, 65.0% were with hypertension, 58.3% were with coronal heart disease, 11.7% were with atrial fibrillation, and 25% were with type 2 diabetes. All patients were diagnosed as chronic HF (NYHA class II or III, NT-proBNP level is 791.2 ± 363.7 pg/mL, 6-minute walk distance is 213.8 ± 65.9 m, and the LVEF is 34.9 ± 3.2% during the echocardiogram). There was a mean GFR of 100.6 ± 33.9 mL/min/1.73 m2, mean office systolic BP was 142.6 ± 22.6 mmHg, mean diastolic BP was 80.8 ± 12.6 mmHg, and mean heart rate was 69.1 ± 7.3 bpm. In addition, 95% of patients were treated with ACE inhibitors or angiotensin II receptor blockers, 78.3% with beta-blockers, 16.7 % with aldosterone antagonists, 43.3% with calcium-channel blockers, 6.7% with digoxin, and 50% with loop diuretics.
Bilateral renal denervation was successfully performed in all 30 patients in the RDN group. No statistically significant difference was found between the two groups in age, sex, most comorbidities, reported duration spent on pharmacotherapy for HF, and assessments of effectiveness and safety endpoints (Table 1).
Table 1.
Baseline patient characteristics, demographics, background medications, efficacy and safety endpoints parameters in renal denervation and control groups.
| Renal denervation group (n = 30) | Control group (n = 30) | P | |
|---|---|---|---|
| Age [years] | 59.0 (12.1) | 61.3 (11.1) | 0.446 |
| Sex [male] | 25 (83.3%) | 22 (73%) | 0.347 |
| Body mass index [kg/m2] | 26.8 (2.6) | 26.7 (2.8) | 0.939 |
| Medical history: | |||
| Hypertension | 21 (70.0%) | 18 (60.0%) | 0.417 |
| Coronary heart disease | 17 (56.7%) | 18 (60.0%) | 0.793 |
| Atrial fibrillation | 4 (13.3%) | 3 (10.0%) | 0.688 |
| Type 2 diabetes | 8 (26.7%) | 7 (23.3%) | 0.766 |
| Patients receiving drug class: | |||
| ACE or ARB | 29 (96.7%) | 28 (93.3%) | 1.000 |
| Beta-blockers | 23 (76.7%) | 24 (80.0%) | 0.754 |
| Aldosterone antagonists | 6 (20.0%) | 4 (13.3%) | 0.488 |
| Calcium-channel blockers | 14 (46.7%) | 12 (40.0%) | 0.602 |
| Digoxin | 3 (10%) | 1 (3.3%) | 0.612 |
| Loop diuretics | 14 (46.7%) | 16 (53.3%) | 0.606 |
| Hemoglobin | 119.1 (25.9) | 112.3 (24.5) | 0.299 |
| Efficacy endpoint: | |||
| NT-proBNP [pg/mL] | 797.7 (356.1) | 784.7 (377.1) | 0.892 |
| Six minute walk test [m] | 217.5 (69.5) | 210.0 (63.0) | 0.666 |
| NYHA class: | |||
| Class II | 17 (56.7%) | 16 (53.3%) | 0.795 |
| Class III | 13 (43.3%) | 14 (46.7%) | 0.795 |
| Echocardiographic LVEF [%] | 35.0 (3.2) | 34.8 (3.2) | 0.872 |
| Safety endpoint: | |||
| GFR [mL/min/1.73 m2] | 104.8 (35.9) | 96.4 (31.8) | 0.337 |
| Symbolic BP [mmHg] | 142.0 (24.8) | 143.2 (20.7) | 0.844 |
| Diastolic BP [mmHg] | 79.8 (12.6) | 81.7 (12.9) | 0.579 |
| Heart rate [bpm] | 68.7 (7.9) | 69.4 (6.9) | 0.728 |
Data are mean (standard deviation) or number (%). ACE — angiotensin-converting enzyme inhibitor; ARB — angiotensin II receptor blocker; BP — blood pressure; GFR — glomerular filtration rate; LVEF — left ventricular ejection fraction; NT-proBNP — N-terminal pro-B-type natriuretic peptide; NYHA — New York Heart Association
Efficacy endpoint
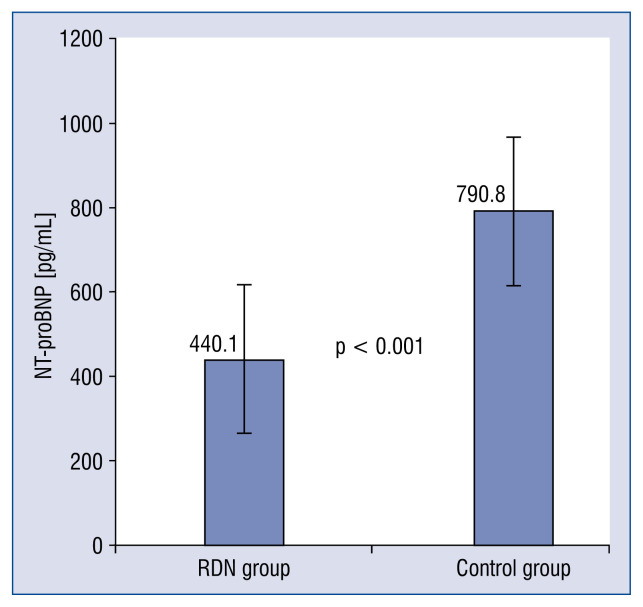
All patients in both groups were followed up for 6 months. Results of the Student independent t-test on NT-proBNP indicates that the NT-proBNP levels in RDN group (440.1 ± 226.5 pg/mL) were significantly lower (p < 0.001, Cohen’s d = 1.14) than those in the control group (790.8 ± 287.0 pg/mL) in the 6th month follow up (Fig. 1).
Figure 1.
Mean N-terminal pro B-type natriuretic peptide (NT-proBNP) level at 6 months after renal denervation (RDN) in the RDN and control groups. A significant decrease in the RDN group was observed. Error bars indicate standard errors.
The effects of RDN on cardiac functions of patients with chronic HF can be indicated by the results of independent t-tests on echocardiographic parameters between the two groups (Table 2). It is seen that LVEF significantly increased (p = 0.017, Cohen’s d = 0.61) in the RDN group (39.1 ± 7.3%) when compared with the control group (35.6 ± 3.3%) at the end of the 6-month follow up. Further, left ventricular end systolic diameters in the RDN group (46.4 ± 4.7 mm) were significantly smaller (p < 0.001, Cohen’s d = 0.87) than those in the control group (50.2 ± 3.1 mm); nevertheless, no statistically significant difference was found in left ventricular end diastolic diameter and interventricular septal thickness between the two groups (p > 0.05).
Table 2.
Changes in echocardiographic parameters before and 6 months after randomization in the renal denervation (RDN) and control groups.
| Parameter | Baseline | 6 months | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| RDN | Control | P | RDN | Control | P | |
| LVEF [%] | 35.0 ± 3.2 | 34.8 ± 3.2 | 0.872 | 39.1 ± 7.3 | 35.6 ± 3.3 | 0.017 |
| LVESD [mm] | 50.3 ± 3.3 | 51.4 ± 2.4 | 0.156 | 46.4 ± 4.7 | 50.2 ± 3.1 | < 0.001 |
| LVEDD [mm] | 63.3 ± 4.5 | 63.6 ± 4.7 | 0.082 | 60.0 ± 6.7 | 62.5 ± 4.5 | 0.106 |
| IVST [mm] | 10.3 ± 1.3 | 10.2 ± 1.3 | 0.848 | 9.5 ± 0.9 | 9.6 ± 0.9 | 0.575 |
Data are mean ± standard deviation. LVEF — left ventricular ejection fraction; LVESD — left ventricular end systolic diameter; LVEDD — left ventricular end diastolic diameter; IVST — interventricular septal thickness
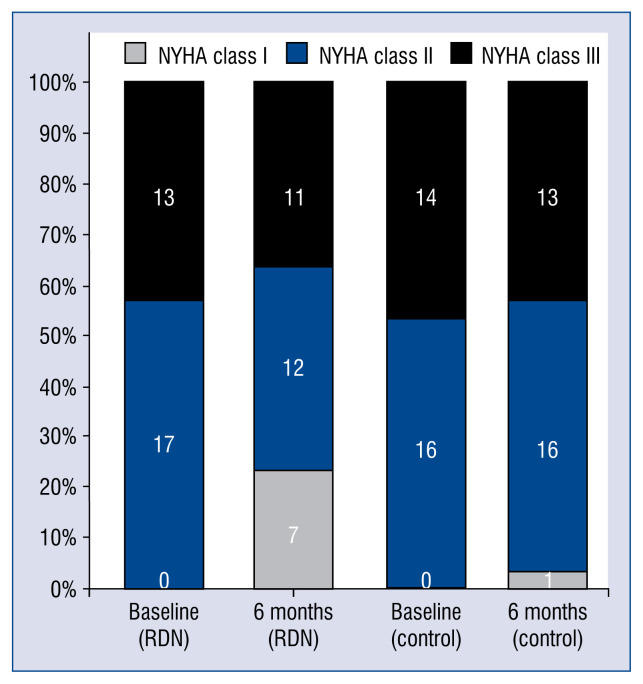
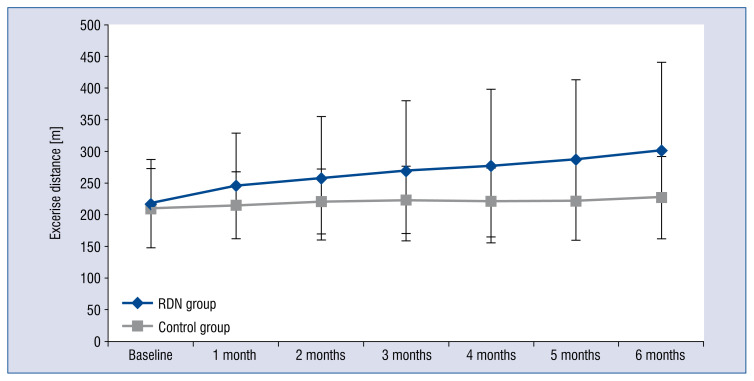
After 6-month follow up, patients in the RDN group showed an improvement of cardiac function, with 7 patients showing better results of NYHA class assessment (Fig. 2). The 6-minute walk distance in the RDN group (301.2 ± 139.5 m) was significantly increased (p = 0.01, Cohen’s d = 0.66) when compared with the control group (227.2 ± 65.0 m) in the 6th month (Fig. 3).
Figure 2.
New York Heart Association (NYHA) class assessment in the renal denervation (RDN) and control groups at baseline and at 6 months after RDN. Black bars indicate NYHA class III. Blue bars indicate NYHA class II. Gray bars indicate NYHA class I.
Figure 3.
Change in 6 minute walk test before and after renal denervation (RDN) for RDN and control groups. A 6 minute walk test was measured at baseline and over a 6 month follow-up. A significant improvement in 6 minute walking distance was observed in the RDN group over 6 months. Error bars indicate standard errors.
Safety endpoint
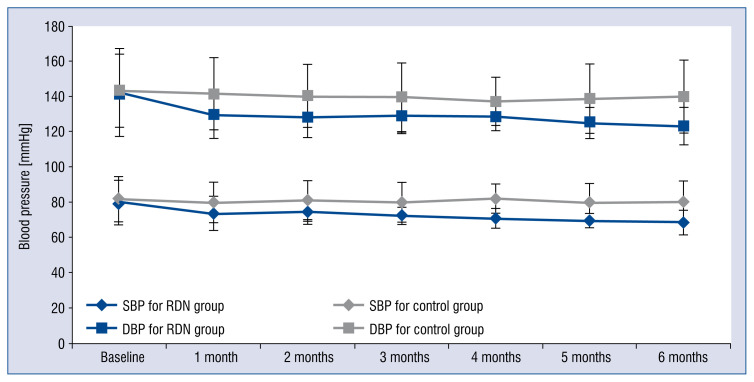
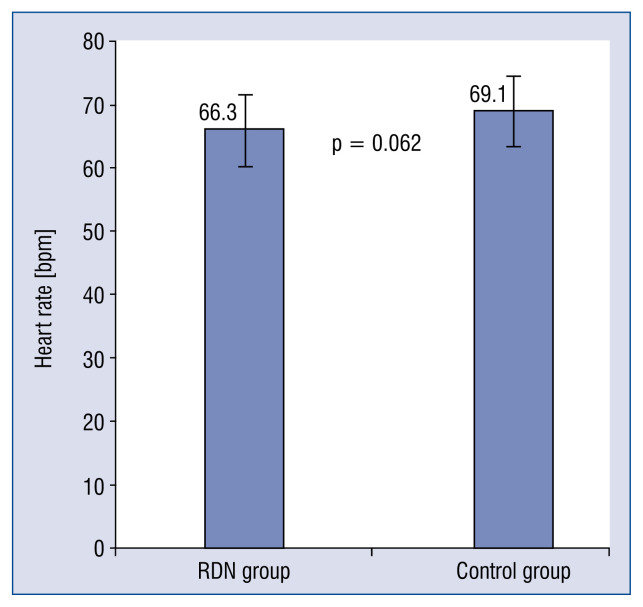
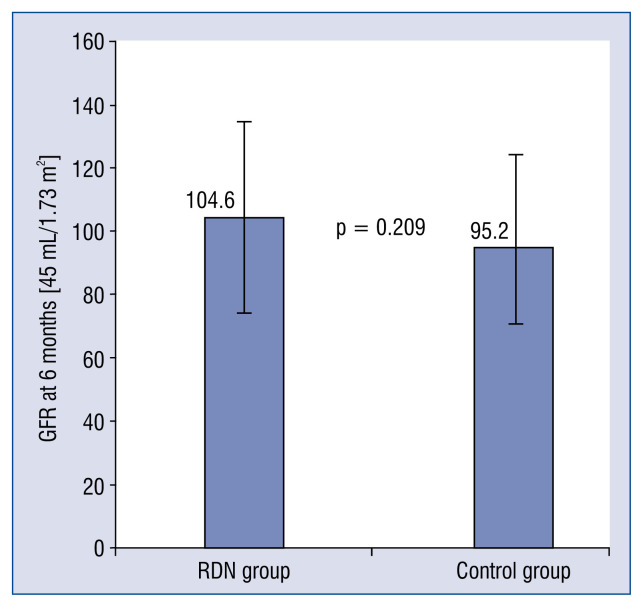
In the 6th month, the office BP of patients in the RDN group (123.3 ± 0.9 / 68.5 ± 7.0 mmHg) significantly decreased (p < 0.001, Cohen’s d = 0.91) when compared with those in the control group (139.8 ± 20.7 / 80.2 ± 11.8 mmHg), as shown in Figure 4; nevertheless, no patient reported symptoms of hypotension syncope or amaurosis during the follow up period. No significant difference was found for heart rates (p = 0.062, Cohen’s d = 0.49) and GFR (p = 0.209, Cohen’s d = 0.33) between the two groups after 6-month follow up, even though there was a slight decrease in heart rate and an increase in GFR were observed (Figs. 5, 6).
Figure 4.
Paired changes of systolic blood pressure (SBP) and diastolic blood pressure (DBP) before and after renal denervation (RDN) for RDN and control groups. Blood pressure was measured at baseline and over a 6 month follow-up. Significant reductions in blood pressure were observed in the RDN group over 6 months. Error bars indicate standard errors.
Figure 5.
Mean heart rate at 6 months after renal denervation (RDN) in the RDN and control groups. No significant between-group differences were observed. Error bars indicate standard errors.
Figure 6.
Mean glomerular filtration rate (GFR) at 6 months after renal denervation (RDN) in the RDN and control groups. No significant between-group differences were observed. Error bars indicate standard errors.
Discussion
This was a prospective randomized and controlled study to explore the effectiveness and safety of cathedral-based RDN on patients with chronic systolic HF. The improvement of exercise tolerance, decrease of NT-proBNP level, and increase of LVEF at echocardiography, suggest the feasibility and effectiveness of RDN in treatment of chronic systolic HF. In addition, no amaurosis and syncope in the RDN group, as well as no significant difference between-group of GFR further indicate the safety of RDN treatment.
Renal denervation has never been proposed as an effective way to treat resistant hypertension [6, 7], but its effect on this specific disease was inconclusive due to disappointing results acquired in the SIMPLICITY HTN-3 study, no significant difference was found in reduced BP between the RDN and sham groups [8]. Even though, recent studies proposed that RDN might be an effective way for the treatment of HF [12], due to its abilities in suppressing activities of the sympathetic nervous system and renin–angiotensin–aldosterone system (RASS) by blocking the renal sympathetic nerve. It should be noted that excessive activation of the sympathetic nerves and the over expression of RASS are believed to be major contributors to the cause and development of HF [13]. Actually, excessive activation of the renal sympathetic nerves can lead to renal vasoconstriction and increase renin secretion as well as proximal tubular sodium reabsorption [14]. This kind of chronic stimulation caused by excessive activation of the sympathetic nerves can lead to volume overloading, myocardial remodeling and cardiovascular function deterioration [15].
Accumulating animal evidence suggests that RDN is able to bring hemodynamic changes and improve cardiac functions. For example, RDN showed an effective inhibition on RASS in a porcine model of pacing-induced HF [11]. After pacing, the RDN group has shown a significantly higher LVEF at echocardiography and a significantly lower plasma concentration of renin, relative to the control group. In contrast, although a significant elevated plasma concentration of aldosterone was reported in the control group, no significant change in the aldosterone level was observed in the RDN group. Similar results have been found in a rat model of isoproterenol induced chronic HF. It was reported that RDN down-regulated the protein expression of angiotensin II in myocardial tissue of rats left atrial, and thereby improved the cardiac function, shown by an increased LVEF [10]. Moreover, RDN was reported to be able to improve the cardiac function and inhibit myocardial remodeling in rats with post-myocardial infarction [9]. Further animal study has suggested that denervation intervention can ameliorate progression of left ventricular hypertrophy in spontaneously hypertensive rats, probably due to the reduction of BP and lower expression of inflammatory factors (e.g., TLR4, NF-κB, TNF-α, and IL-6) in myocardial tissue [16].
Though the positive results have been well confirmed in animal studies, the effects of RDN on patients with HF are still not well documented, and some indirect evidence were reported in studies on treatment of resistant hypertension. For example, a study including 72 patients reported that RDN could ameliorate the cardiac function, indicated by an improvement in left ventricular mass index, left ventricular mass/body surface area ratio, left ventricular wall stress, as well as LVEF values [17]. Another study also reported that the resistant hypertension patients were also accompanied with left ventricular hypertrophy and diastolic dysfunction [18]. These results together suggest that RDN would be promising treatment for HF, especially for that accompanied with hypertension. Actually, the REACH-Pilot study has once investigated the effects of RDN on patients with HF and reported that RDN could improve patient exercise tolerances [12]. Nevertheless, this study was limited by its small sample size and its single-group non-blinded and non-randomized nature [19]. Here, more patients were recruited and a randomized controlled trial was performed for more convincing results. Obtained results verfied the values of RDN in improving the cardiac function and treating patients with HF.
The RDN group in this study had shown a reduction in BP of approximately 20 mmHg over 6-month follow up. However, such changes in BP did not accompany any symptoms of hypotension, indicating that this intervention did not lead to symphonic problems in patients with chronic systolic HF. Furthermore, patients receiving RDN showed no changes in GFR and heart rate before and after the procedures. The reduction of BP has been suggested to potentially dampen the impairment of renal function, as a decreased BP may help reduce the sympathetic outflow to the kidney [6]. In this study, the hemodynamic changes and normal renal function show no adverse effects on the kidneys of patients with chronic systolic BP, suggesting the safety of RDN.
Limitations of the study
Several limitations need to be noted in this study. First, the number of patients with systolic HF for RDN procedure was not large, thereby limiting statistical power. However, the improvement of cardiac function in the present population is in line with that of previous studies in hypertension with a large sample size. Second, non-blinded or sham-procedure group was not provided. The results, therefore, may contain bias. A randomized controlled trial with a sham group should be done to further investigate the effectiveness of RDN in HF. Third, whether RDN treatment in chronic systolic HF could improve a patients’ diuretic need or resistance warrants further investigation.
Conclusions
Catheter-based RDN with radiofrequency ablation has shown to be very promising in improving cardiac function and exercise tolerance for patients with chronic systolic HF, in the present single-center, randomized, controlled trial. No adverse effects on the kidneys of patients should also be noted. Thus, this innovative technique can be an effective and safe new therapy for chronic systolic HF, even though more long-term experimental and clinical evidence should be provided in the future studies.
Acknowledgements
This study was supported by National Natural Science of China (grant No. 81303145); Key Projects of Shanghai Municipal Health Bureau (grant No. 20134003); and Key Medical Discipline Construction Projects of Shanghai Municipal Health Bureau (grant No. ZK2015A147).
Footnotes
Conflict of interest: None declared
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.Dhakal P, Liu K, Kozman H, et al. Renal Denervation in Heart Failure: A New Therapeutic Paradigm. Clin Med Insights Cardiol. 2015;9(Suppl 1):101–104. doi: 10.4137/CMC.S18754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Böhm M, Ewen S, Kindermann I, et al. Renal denervation and heart failure. Eur J Heart Fail. 2014;16(6):608–613. doi: 10.1002/ejhf.83.. [DOI] [PubMed] [Google Scholar]
- 4.Schlaich MP, Socratous F, Hennebry S, et al. Sympathetic activation in chronic renal failure. J Am Soc Nephrol. 2009;20(5):933–939. doi: 10.1681/ASN.2008040402. [DOI] [PubMed] [Google Scholar]
- 5.Patel HC, Dhillon PS, Mahfoud F, et al. The biophysics of renal sympathetic denervation using radiofrequency energy. Clin Res Cardiol. 2014;103(5):337–344. doi: 10.1007/s00392-013-0618-6. [DOI] [PubMed] [Google Scholar]
- 6.Renal sympathetic denervation in patients with treatmentresistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376(9756):1903–1909. doi: 10.1016/s0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 7.Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multi-centre safety and proof-of-principle cohort study. Lancet. 2009;373(9671):1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 8.Bhatt DL, Kandzari DE, O’Neill WW, et al. SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370(15):1393–1401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 9.Abramovitch J, Arviddson B, Dunn K, et al. The radiation tolerance of specific optical fibers for the LHC upgrades. Physics Procedia. 2012;37:1630–1643. doi: 10.1016/j.phpro.2012.03.751.. [DOI] [Google Scholar]
- 10.Liu Q, Li Z, Zhang Qi, et al. [Effects of renal denervation on left atrial fibrosis in rats with isoproterenol induced chronic heart failure]. Zhonghua Xin Xue Guan Bing Za Zhi. 2015;43(12):1040–1045. [PubMed] [Google Scholar]
- 11.Xie Y, Liu Q, Xu Y, et al. [Effect of catheter-based renal sympathetic denervation in pigs with rapid pacing induced heart failure]. Zhonghua Xin Xue Guan Bing Za Zhi. 2014;42(1):48–52. [PubMed] [Google Scholar]
- 12.Davies JE, Manisty CH, Petraco R, et al. First-in-man safety evaluation of renal denervation for chronic systolic heart failure: primary outcome from REACH-Pilot study. Int J Cardiol. 2013;162(3):189–192. doi: 10.1016/j.ijcard.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 13.Hasking GJ, Esler MD, Jennings GL, et al. Norepinephrine spill-over to plasma in patients with congestive heart failure: evidence of increased overall and cardiorenal sympathetic nervous activity. Circulation. 1986;73(4):615–621. doi: 10.1161/01.cir.73.4.615. [DOI] [PubMed] [Google Scholar]
- 14.Florea VG, Cohn JN. The autonomic nervous system and heart failure. Circ Res. 2014;114(11):1815–1826. doi: 10.1161/CIRCRESAHA.114.302589. [DOI] [PubMed] [Google Scholar]
- 15.Kshatriya S, Kozman H, Siddiqui D, et al. The kidney in heart failure: friend or foe? Am J Med Sci. 2012;344(3):228–232. doi: 10.1097/MAJ.0b013e318242a631. [DOI] [PubMed] [Google Scholar]
- 16.Tan LH, Li XG, Guo YZ, et al. [Effect of renal sympathetic denervation on left ventricular hypertrophy and inflammatory factors in spontaneously hypertensive rats]. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2013;42(5):550–555. [PubMed] [Google Scholar]
- 17.Mahfoud F, Urban D, Teller D, et al. Effect of renal denervation on left ventricular mass and function in patients with resistant hypertension: data from a multi-centre cardiovascular magnetic resonance imaging trial. Eur Heart J. 2014;35(33):2224–2231. doi: 10.1093/eurheartj/ehu093. [DOI] [PubMed] [Google Scholar]
- 18.Brandt MC, Mahfoud F, Reda S, et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol. 2012;59(10):901–909. doi: 10.1016/j.jacc.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 19.Patel HC, Rosen SD, Hayward C, et al. Renal denervation in heart failure with preserved ejection fraction (RDT-PEF): a randomized controlled trial. Eur J Heart Fail. 2016;18(6):703–712. doi: 10.1002/ejhf.502. [DOI] [PubMed] [Google Scholar]