Abstract
Background
Recent clinical studies have shown that treatment with LCZ696, a complex containing the angiotensin receptor blocker valsartan and neprilysin inhibitor sacubitril, improves the prognosis of heart failure patients with a reduced ejection fraction. This study evaluated whether LCZ696 affects left ventricular hypertrophy, fibrosis, and hemodynamics in isoproterenol (ISO)-treated rats compared with valsartan alone.
Methods
Male Wistar rats received subcutaneous saline (n = 10), subcutaneous ISO (2.4 mg/kg/day; n = 10), subcutaneous ISO + oral LCZ696 (60 mg/kg/day; n = 20) (ISO-LCZ), or subcutaneous ISO + oral valsartan (30 mg/kg/day; n = 20) (ISO-VAL) for 7 days.
Results
LCZ696 and valsartan did not significantly reduce the increased heart weight/body weight ratio in rats treated with ISO. Echocardiography showed that the deceleration time shortened by ISO was restored by LCZ696 but not valsartan alone (p = 0.01 vs. the ISO group). Histological analysis showed that cardiac interstitial fibrosis increased by ISO was decreased significantly by LCZ696 but not valsartan alone (control: 0.10 ± 0.14%; ISO: 0.41 ± 0.32%; ISO-LCZ: 0.19 ± 0.23% [p < 0.01 vs. the ISO group]; ISO-VAL: 0.34 ± 0.23% [p = 0.34 vs. the ISO group]). Quantitative polymerase chain reaction showed that mRNA expression of Tgfb1, Col1a1, Ccl2, and Anp increased by ISO was significantly attenuated by LCZ696 but not valsartan alone (p < 0.05 vs. the ISO group).
Conclusions
LCZ696 improves cardiac fibrosis, but not hypertrophy, caused by continuous exposure to ISO in rats.
Keywords: cardiac hypertrophy, neprilysin, angiotensin receptor blocker, fibrosis
Introduction
Left ventricular (LV) hypertrophy (LVH) is reported to be involved in heart failure with a preserved ejection fraction (HFpEF) [1]. HFpEF is associated with considerable morbidity and mortality, and the risk for adverse outcomes increases with the severity of diastolic dysfunction [2]. Treatment with anti-hypertensive drugs may be effective to prevent HFpEF. However, randomized controlled trials of antihypertensive drugs, including angiotensin-converting enzyme inhibitors and angiotensin receptor blockers (ARBs), have been generally disappointing with no convincing demonstration of mortality or morbidity reductions [3–5]. Accordingly, therapies for HFpEF are of great clinical interest.
Inhibition of neprilysin is an attractive strategy to increase natriuretic peptide levels. Natriuretic peptides stimulate diuresis and vasodilation, improve myocardial relaxation, and reduce LVH [6]. Recently, a first-in class dual angiotensin receptor and neprilysin inhibitor (ARNI), which contains equimolar amounts of valsartan and sacubitril, has been developed [7]. In the PARAMOUNT study (prospective comparison of ARNI with ARBs for management of HFpEF), LCZ696 reduced the serum level of N-terminal pro-B-type natriuretic peptide (NT-proBNP) more than valsartan at 12 weeks and was well tolerated when used for HFpEF [8]. In the PARADIGM-HF study (prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure), LCZ696 was also effective in heart failure patients with a reduced ejection fraction (HFrEF) [9]. However, the mechanism underlying the effectiveness of LCZ696 is poorly understood and little is known about its effects in HFpEF patients with LVH.
It was previously reported that continuous infusion of isoproterenol (ISO) induces cardiac hypertrophy and diastolic dysfunction in rats [10, 11]. The current study investigated whether LCZ696 affects LVH, fibrosis, and hemodynamics in ISO-treated rats.
Methods
Ethical approval
All procedures were performed in accordance with the institutional guidelines for animal research and approved by the Animal Care and Use Committee of Okayama University (OKU-2012297).
Experimental animals and drugs
Male Wistar rats weighing 187–211 g were purchased from Japan SLC (Shizuoka, Japan). Rats were housed at an animal facility in a 12-h lightdark cycle and were provided with standard rat chow and water ad libitum. LCZ696 and valsartan were kindly supplied by Novartis Pharma AG (Basel, Switzerland). LCZ696 includes molecular moieties of valsartan and sacubitril at a 1:1 ratio. L-ISO was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Study protocol
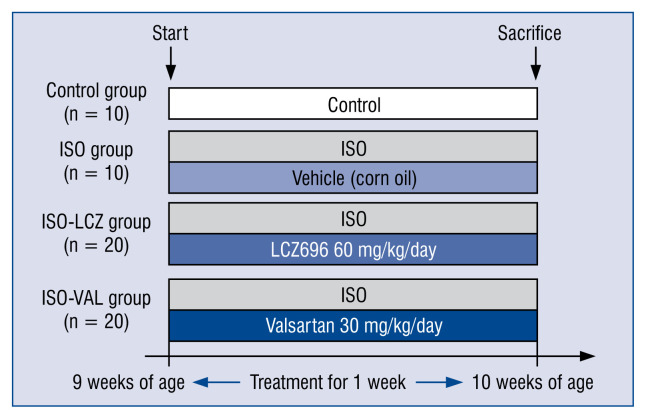
The detailed protocol is shown in Figure 1 and a previous study [11]. Delivery of ISO or saline was performed by subcutaneously implanting an osmotic minipump (Alzet, model 2001; 1.0 μL/h) into the neck under 2% isoflurane anesthesia. LCZ696 and valsartan were dissolved in corn oil and administered by gastric gavage. Rats were divided into four groups and treated for 7 days: control group (subcutaneous HCl [pH 4] in saline, n = 10); ISO group (subcutaneous ISO [2.4 mg/kg/day] and oral corn oil, n = 10); ISO-LCZ group (subcutaneous ISO [2.4 mg/kg/day] and oral LCZ696 [60 mg/kg/day], n = 20); and ISO-VAL group (subcutaneous ISO [2.4 mg/kg/day] and oral valsartan [30 mg/kg/day], n = 20). Systolic blood pressure (SBP) was measured in rats using a tail cuff attached to a MK-2000ST Blood Pressure Monitor for Rats (Muromachi, Tokyo, Japan).
Figure 1.
Schematic of the treatments; ISO — subcutaneous isoproterenol (2.4 mg/kg/day); LCZ — LCZ2696; VAL — valsartan.
Hemodynamic measurements
Seven days after infusion, rats were anesthetized with 2% isoflurane, and a micro-tip pressure transducer (Millar Instruments Inc., Houston, TX, USA) was inserted into the right carotid artery. The catheter was advanced into the LV cavity. After a 5 min period of stabilization, heart rate, LV systolic pressure (LVSP), LV end-diastolic pressure (LVEDP), and developed LV pressure (dLVP = = LVSP − LVEDP) were measured. For indices of contractility and relaxation, the maximal rates of increase and decrease in LVP dp/dt maximum and dp/dt minimum were determined. Tau was calculated according to a previous report [12].
Echocardiography
Seven days after infusion, transthoracic echocardiography was performed using a 10-MHz phased array transducer (Aplio ver. 6.0; Toshiba, Tokyo, Japan) under 2% isoflurane, as described previously [11]. M-mode echocardiography was performed using the parasternal short-axis view at the level of papillary muscles. The LV posterior wall thickness (PWT) and interventricular septal diastolic wall thickness (IVST) were measured during diastole (d) and systole (s), as were the LV internal diameter at end-diastole (LVDd) and LV internal diameter at end-systole (LVDs). Fractional shortening (FS) was then calculated according to the formula: FS = [(LVDd − LVDs)/LVDd] × 100. The apical 4-chamber view was used to assess early and late transmitral peak diastolic flow velocities (E and A waves, respectively).
Histology
The LV was fixed with 4% paraformaldehyde in phosphate buffered saline, embedded in paraffin, and cut into 5 μm-thick sections. The sections were stained with Masson’s trichrome to detect fibrosis and examined by light microscopy. In sections stained with Masson’s trichrome, interstitial fibrosis was measured using computer-assisted image analysis, and the percentage of fibrosis was calculated [13, 14]. The widths of 30 individual cardiomyocytes were measured in each sample. The percentage of the fibrotic area in the LV was analyzed using Image J software (ver. 1.47).
Quantitative PCR
Total RNA was extracted from heart tissue using Trizol (Invitrogen, Carlsbad, CA, USA). Total RNA (2 μg) was reverse transcribed using ReverTra Ace (Toyobo, Osaka, Japan). cDNAs were diluted 5-fold before conventional reverse transcription-polymerase chain reaction (RT-PCR) amplification or 50-fold before quantitative PCR analysis. Real-time PCR assays were performed to assess gene expression of rat Tgfbeta1, Col1a1, Ccl2, Anp, and Gapdh using the corresponding primer pairs (Rn00572010_m1, Rn01523309_m, 1Rn00664637_g1, Rn00580555_m1, and Rn01775763_g1, respectively; Applied Biosystems) using the StepOnePlus Real-Time PCR System (Applied Biosystems). Glyceraldehyde-3-posphate dehydrogenase (GAPDH) served as an internal control. The ΔΔCt method was used to analyze expression level of each gene.
Measurements of NT-proBNP
Serum NT-proBNP was measured using an enzyme-linked immunosorbent assay (MBS704791, MyBioSource, San Diego, CA, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation. For comparisons of each parameter among groups, statistical analysis was performed by analysis of variance with Bonferroni tests. A p-value of less than 0.05 was considered as statistically significant. Data were analyzed using SPSS 17.0 for Windows (ver. 24; SPSS Inc., Chicago, IL, USA).
Results
Heart weights and SBP
Body weight, heart weight, and the heart-to-body weight ratio are shown in Table 1. No difference was observed in body weights among the three groups at day 7 post-treatment with ISO, ISO-LCZ, and ISO-VAL. The increases in heart weight and heart-to-body weight ratio observed in the ISO group were not significantly suppressed in ISO-LCZ and ISO-VAL groups (p = 0.33). There was no difference in heart-to-body weight ratios between ISO-LCZ and ISO-VAL groups. Table 2 shows the changes in SBP derived from the tail cuff. SBP at day 7 in ISO-LCZ and ISO-VAL groups was significantly suppressed compared to that in control and ISO groups. However, there was no difference in SBP at day 7 between ISO-LCZ and ISO-VAL groups (p = 0.11).
Table 1.
Effects of LCZ696 on heart weight and systolic blood pressure.
| Control | ISO | ISO-LCZ | ISO-VAL | |
|---|---|---|---|---|
| Number of rats | 10 | 10 | 20 | 20 |
| Body weight [g] | 242 ± 15 | 238 ± 8 | 236 ± 7 | 232 ± 11 |
| Heart weight [g] | 0.81 ± 0.08 | 1.05 ± 0.11* | 0.99 ± 0.10* | 0.99 ± 0.08* |
| Heart weight/body weight × 103 | 3.35 ± 0.27 | 4.47 ± 0.39* | 4.14 ± 0.30* | 4.30 ± 0.30* |
p < 0.05 vs. control.
Values represent the mean ± standard deviation; ISO — isoproterenol; LCZ — LCZ696; VAL — valsartan
Table 2.
Change in systolic blood pressure.
| Control | ISO | ISO-LCZ | ISO-VAL | |
|---|---|---|---|---|
| Baseline | 119 ± 6 | 117 ± 8 | 120 ± 6 | 118 ± 5 |
| Day 7 | 116 ± 3 | 119 ± 5 | 112 ± 4*† | 110 ± 4*† |
p < 0.05 vs. control;
p < 0.05 vs. ISO.
Values represent the mean ± standard deviation; ISO — isoproterenol; LCZ — LCZ696; VAL — valsartan
LV hemodynamics
Cardiac catheterization and echocardiography were performed at day 7 (Table 3). During cardiac catheterization, increases in the heart rate, LVSP, LVEDP, and dP/dt maximum and a decrease in minimum dp/dt were observed in the ISO group compared with the control group. LCZ696 or valsartan alone did not suppress the increased heart rate and LVSP. However, LCZ696 and valsartan alone suppressed the increases in LVEDP and dP/dt maximum significantly in ISO-LCZ and ISO-VAL groups compared with the control group. However, there were no differences in the cardiac catheterization parameters between ISO-LCZ and ISO-VAL groups. During echocardiography, increases in heart rate, IVST, and PWT and a decrease in the deceleration time were observed in the ISO group compared with the control group. LCZ696 or valsartan alone did not suppress the IVST or PWT. The deceleration time shortened by ISO was restored by LCZ696 but not valsartan alone. There was significant difference in the deceleration times between ISO-LCZ and ISO-VAL groups (p = 0.01).
Table 3.
Effects of LCZ696 on left ventricular hemodynamics and echocardiographic data.
| Control (n = 10) | ISO (n = 10) | ISO-LCZ (n = 20) | ISO-VAL (n = 20) | |
|---|---|---|---|---|
| Cardiac catheterization | ||||
| Heart rate [bpm] | 408 ± 30 | 498 ± 36* | 477 ± 54* | 459 ± 30*# |
| LVSP [mmHg[ | 101 ± 16 | 115 ± 10* | 113 ± 9* | 110 ± 14* |
| LVEDP [mmHg] | 2.3 ± 1.3 | 3.5 ± 1.1* | 2.5 ± 1.6 | 2.4 ± 1.5 |
| dP/dtmax [mmHg/s] | 8104 ± 1705 | 12798 ± 4227* | 8450 ± 1122† | 7838 ± 1246† |
| dP/dtmin [mmHg/s] | −7107 ± 1077 | −8598 ± 1837* | −6941 ± 969† | −6468 ± 982† |
| Tau [ms] | 10 ± 1 | 10 ± 2 | 9 ± 2 | 10 ± 3 |
| Echocardiography | ||||
| LVDd [mm] | 6.1 ± 0.5 | 6.1 ± 0.4 | 6.2 ± 0.4 | 6.1 ± 0.5 |
| LVDs [mm] | 3.5 ± 0.3 | 3.6 ± 0.2 | 3.6 ± 0.3 | 3.4 ± 0.3 |
| FS [%] | 44 ± 5 | 43 ± 7 | 41 ± 3 | 42 ± 2 |
| IVST [mm] | 1.0 ± 0.1 | 1.3 ± 0.1* | 1.3 ± 0.1* | 1.3 ± 0.1* |
| PWT [mm] | 1.1 ± 0.1 | 1.4 ± 0.2* | 1.4 ± 0.1* | 1.3 ± 0.1* |
| E/A | 1.5 ± 0.1 | 1.4 ± 0.2 | 1.3 ± 0.1 | 1.4 ± 0.2 |
| Deceleration time [ms] | 36.3 ± 4.2 | 34.2 ± 6.7* | 38.5 ± 4.7†‡ | 33.8 ± 5.5* |
p < 0.05 vs. control,
p < 0.05 vs. ISO and
p < 0.05 vs. ISO-VAL.
Values represent the mean ± standard deviation; ISO — isoproterenol; LCZ — LCZ696; VAL — valsartan; LVSP — left ventricular systolic pressure; LVEDP — left ventricular end-diastolic pressure; LVDd — left ventricular end-diastolic diameter; LVDs — left ventricular end-systolic diameter; FS — fractional shortening; IVST — interventricular septal thickness; PWT — posterior wall thickness; E — early transmitral peak diastolic flow velocity; A — late transmitral peak diastolic flow velocity
Histology
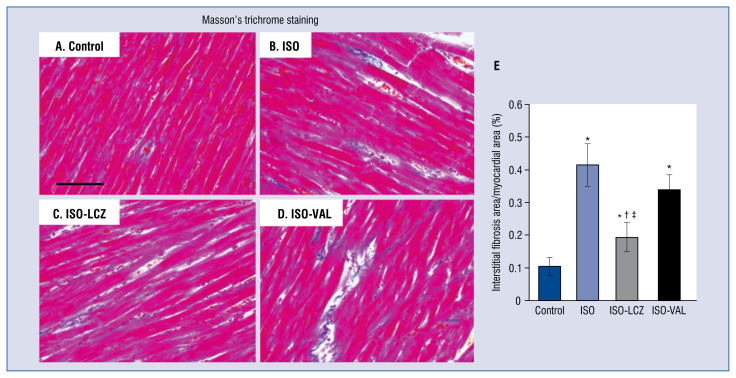
Masson’s trichrome staining revealed that areas of cardiac fibrosis were significantly smaller in ISO-LCZ group, but not in ISO-VAL group, compared with ISO group (area of fibrosis: control, 0.10 ± 0.14%; ISO, 0.41 ± 0.32%; ISO-LCZ, 0.19 ± 0.23%; ISO-VAL, 0.34 ± 0.23) (Fig. 2). The reduction of cardiac fibrosis by LCZ696 was significantly greater than that by valsartan alone (p = 0.01).
Figure 2.
Masson’s trichrome staining of hearts. Myocardial fibrosis is evident as blue staining. A. Heart sections from a control rat; B. Isoproterenol (ISO)-treated rat; C. ISO-treated rat administered LCZ696 (ISO-LCZ); D. ISO-treated rat administered valsartan (ISO-VAL). Scale bar = 100 μm. E. Bar graph shows the area of fibrosis (%). Values represent the mean ± standard deviation; n = 10 in control and ISO groups; n = 20 in ISO and ISO-VAL groups; *p < 0.05 vs. control, †p < 0.05 vs. ISO; ‡p < 0.05 vs. ISO-VAL.
Cardiac gene expression
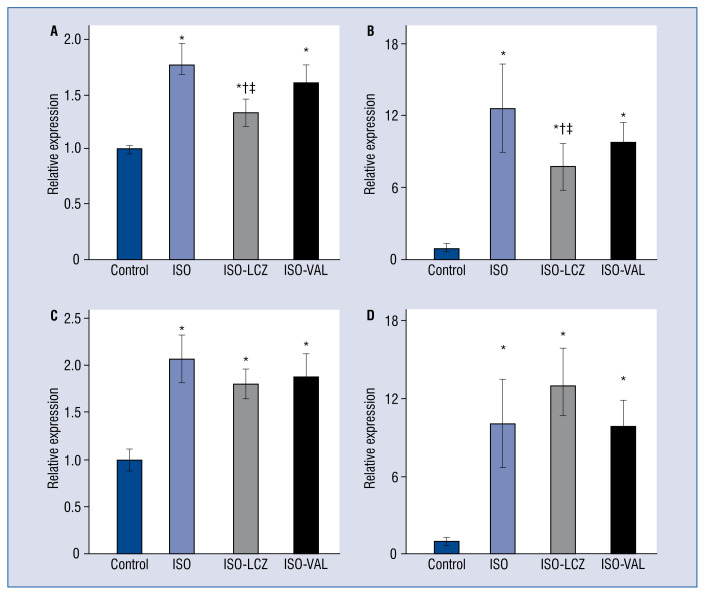
Figure 3 shows mRNA expression levels in the heart after treatments. The gene expression of Tgfb1 and Clo1a1, which are involved in cardiac fibrosis, was significantly increased in the control group. LCZ696, but not valsartan, significantly reduced Tgfb1 and Col1a1 mRNAs (Fig. 3A, B). The decreases in Tgfb1 and Col1a1 expression in the ISO-LCZ group were significantly greater than those in the ISO-VAL group (p = 0.02 and p = 0.03, respectively). The increased expression of Ccl2, which is an inflammatory marker, was not significantly reduced by LCZ696 or valsartan (Fig. 3B). In addition, gene expression of Anp, which is a marker of a failing heart, was not decreased by LCZ696 or valsartan (Fig. 3C). There were no differences in Ccl2 or Anp expression between ISO-LCZ and ISO-VAL groups.
Figure 3.
Cardiac gene expression in hearts. Total RNA was extracted from cardiac tissues and analyzed for mRNA expression of Tgfb1 (A), Col1a1 (B), Ccl2 (C), and Anp (D) by real-time RT-PCR analysis. ISO — isoproterenol-treated rats; ISO-LCZ — ISO-treated rats administered LCZ696; ISO-VAL — ISO-treated rats administered valsartan. Values represent the mean ± standard deviation; n = 10 in control and ISO groups; n = 20 in ISO and ISO-VAL groups; *p < 0.05 vs. control, †p < 0.05 vs. ISO; ‡p < 0.05 vs. ISO-VAL.
Measurements of NT-proBNP
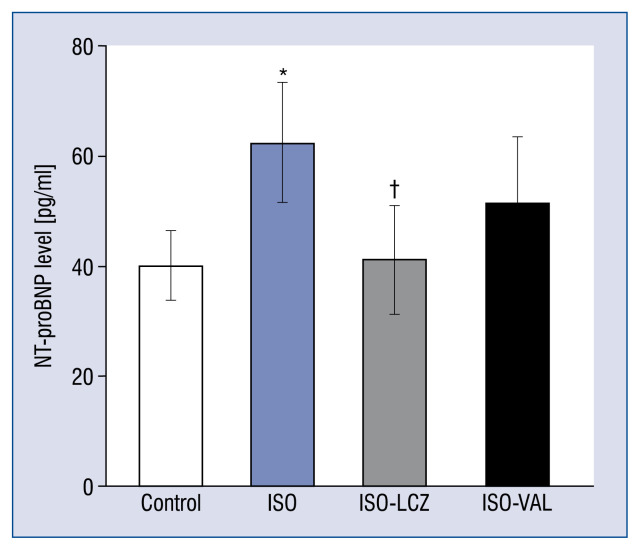
Figure 4 shows the serum NT-proBNP level after 7 days of treatments. The increase in the NT-proBNP level in the ISO group was significantly suppressed in the ISO-LCZ group but not the ISO-VAL group (control, 40.1 ± 6.2 pg/mL; ISO, 62.4 ± 10.9 pg/mL; ISO-LCZ, 41.2 ± 9.8 pg/mL, p = 0.04 vs. ISO; ISO-VAL, 54.2 ± 9.5 pg/mL; p = 0.48 vs. ISO).
Figure 4.
Serum N-terminal pro-B-type natriuretic peptide (NT-proBNP) was measured for 7 days after treatments. ISO — isoproterenol-treated rats; ISO-LCZ — ISO-treated rats administered LCZ696; ISO-VAL — ISO-treated rats administered valsartan. Values represent the mean ± standard deviation; n = 10 in control and ISO groups; n = 20 in ISO and ISO-VAL groups; *p < 0.05 vs. control; †p < 0.05 vs. ISO.
Discussion
In this study, it was found that short term treatment with LCZ696 significantly prevented LV fibrosis, but not LVH, caused by continuous infusion of ISO compared with valsartan alone. Additionally, significantly greater reductions in Tgfb1 and Col1a1 mRNA expression were induced by LCZ696 than valsartan alone. Echocardiography showed that LCZ696 significantly improved the decreased deceleration time, a parameter of LV diastolic functions, compared with valsartan alone. These results suggest more favorable effects of LCZ696 on LV fibrosis and hemodynamics in ISO-treated rats compared with valsartan alone.
von Lueder et al. [15] reported that LCZ696 reduces cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy in rats. Suematsu et al. [16] reported that LCZ696 improves HFrEF in mice with streptozotocin-induced diabetes. These studies used a model of HFrEF, which differs from the current study involving evaluation of a model without a reduced ejection fraction. As a model without a reduced ejection fraction, other studies evaluated LVH in rats with spontaneous hypertension, which was suppressed by valsartan [17, 18]. A model of chronic beta-adrenergic stimulation in rats has also been reported. ISO treatment for 2 weeks induced LVH and fibrosis that were prevented by an angiotensin II type I receptor blocker such as losartan and valsartan [19, 20]. However, a hemodynamic study was not performed in these models of LVH. According to available research, this is the first animal study performed to investigate the hemodynamic effect of LCZ696 on LV functions in the rat hypertrophied heart with a preserved ejection fraction. Improvement in cardiac fibrosis by LCZ696 was consistently observed in this study as well as previous studies. However, in contrast to the results obtained using rats with spontaneous hypertension [17], the current study did not reveal a protective effect on LVH by LCZ696 or valsartan alone. An explanation is the difference in mechanisms of cardiac hypertrophy and fibrosis induction between ISO-treated rats and spontaneously hypertensive rats. ISO directly affects cardiac hypertrophy and fibrosis in addition to elevated SBP. Another possibility is that the duration of administration in the present study was relatively short compared with that in previous studies. Therefore, a lower dose of ISO and long-term treatment may address these issues.
In this study, 60 mg/kg LCZ and 30 mg/kg valsartan was selected because several experimental studies using rat models employed these doses of valsartan and LCZ696 [16, 17, 21, 22]. The previous studies showed greater decreases in SBP induced by LCZ696 than that by valsartan alone. In contrast, the present study showed that SBP at day 7 did not statistically differ between the two groups. This finding suggests that a greater suppression of LV fibrosis by LCZ696 compared with that by valsartan alone is independent of blood pressure. To confirm this effect independently of blood pressure, future experiments should employ other models such as spontaneous hypertension rats in which blood pressure is matched between LCZ696 and valsartan groups by adjusting their doses.
There are several potential mechanisms underlying the protective effect of LCZ696 in preventing ISO-induced cardiac fibrosis. In experimental HFrEF in streptozotocin-induced diabetic mice, the mRNA level of transforming growth factor beta (TGF-β) in the LV was reported to be suppressed in LCZ696 group compared with that of control and valsartan groups [16]. These results were in line with the present study showing that LCZ696 significantly limited the increase in TFG-β upon ISO treatment. One mechanism underlying the suppression of TGF-α caused by specific inhibition of neprilysin may be the involvement of natriuretic peptides. First, the inhibition of neprilysin by sacubitril prevents breakdown of endogenous natriuretic peptides, which inhibits cardiac fibrosis [6]. Second, it has been reported that circulating angiotensin II levels increase after treatment with LCZ696 in Wistar Kyoto rats [23]. After blockade of angiotensin type I receptor, stimulation of angiotensin type II receptor via circulating angiotensin II would reduce cardiac fibrosis. Thus, the combination of angiotensin type I receptor and neprilysin inhibitors may augment their favorable effects through the angiotensin type II pathway. Finally, another mechanism may involve bradykinin. Bradykinin, which is a substrate of neprilysin, has been reported to suppress myocardial fibrosis [24], and its effect may be enhanced by angiotensin receptor-blocking therapy [25]. It is therefore possible that neprilysin inhibitor treatment contributes to cardioprotection by inhibiting bradykinin metabolism. Seki et al. [26] reported that inhibition of neprilysin with LCZ696 does not appear to have an additional benefit over valsartan alone for the endothelial functions of arteries in spontaneously hypertensive rats. Their study suggests that vasoactive peptides, such as C-type natriuretic peptide, bradykinin, and substance P, which can accumulate because of neprilysin inhibition, may not play a crucial role in the improvement of endothelial functions. The difference in target organs between their study and this one may have contributed to the favorable effects of LCZ696 compared with valsartan alone in the present study. Seki et al. [26] focused on endothelial functions, whereas here the study focused on cardiac tissue including cardiomyocytes and fibroblasts. Another explanation is the difference in animal model. This rat model was simulated with ISO, and the elevation of blood pressure was less than that in a spontaneously hypertensive rat. Further basic and clinical studies are warranted to clarify the underlying mechanism of these differences.
In the current study, LCZ696 significantly improved cardiac hemodynamics derived from catheterization in the ISO group. LVEDP in ISO-LCZ and ISO-VAL groups did not increase compared with the control group without a significant change in SBP. Notably, the echocardiographic findings suggested a significantly greater improvement in the deceleration time, a parameter of LV diastolic functions, by LCZ696 compared with valsartan alone. It was previously reported that cardiomyocyte stiffness, which is associated with diastolic heart failure, increases in ISO-induced hypertrophied hearts [10]. Although cardiomyocyte stiffness was not evaluated directly in this study, suppression of cardiac fibrosis may contribute to improvement of diastolic dysfunction in the hypertrophied heart.
The current study demonstrated that increased NT-proBNP levels in ISO group were significantly reduced by LCZ696 but not valsartan alone. Because BNP is degraded by neprilysin, LCZ696 may affect the BNP level. NT-proBNP, which is cleaved from proBNP along with BNP, is not a substrate of neprilysin degradation. Thus, a change in the NT-proBNP level reflects LV wall stress even under the use of LCZ696. The PARAMOUNT study showed that LCZ696 reduced the NT-proBNP level more than valsartan alone at 12 weeks of therapy in patients with HFpEF [8]. Considering both these findings and those of the current study, LCZ696 might have a favorable effect on the prognosis of HFpEF patients, and a further large scale study evaluating cardiovascular events is thus warranted.
Limitations of the study
There are several limitations in this study. First, different doses of LCZ696 and valsartan were not tested. Therefore, dose dependencies of LCZ696 and valsartan in the present model are unknown. Second, Gu et al. [7] reported that plasma concentration-time profiles of valsartan are similar between administration of a single oral dose of 400 mg LCZ696 and 320 mg valsartan in healthy human participants. 60 mg/kg LCZ696 and 30 mg/kg valsartan was chosen, which are similar to those in previous studies of valsartan and LCZ696 in rat models [16, 17, 21, 22], because pharmacodynamic data for LCZ696 could not be found in the ISO-treated rat model. Therefore, it cannot be denied that the pharmacodynamics of these drugs may have influencedthe present results. Third, the duration of LCZ696 or valsartan treatments in the ISO-treated model was short. Long-term treatment may facilitate understanding the effect of LCZ696 on LVH, fibrosis, and hemodynamics in ISO-treated rats.
Conclusions
LCZ696 improved LV fibrosis caused by continuous exposure to ISO in this population of rats. The results suggest the possibility of using LCZ696 to therapeutically target LVH and diastolic LV dysfunction.
Footnotes
Conflict of interest: Toru Miyoshi and Hiroshi Ito have received the research funding for this study through the concentration with Novartis Pharma K.K. The other authors have no conflicts of interest in relation to the materials presented in this article.
References
- 1.Lazzeroni D, Rimoldi O, Camici PG. From left ventricular hypertrophy to dysfunction and failure. Circ J. 2016;80(3):555–564. doi: 10.1253/circj.CJ-16-0062. [DOI] [PubMed] [Google Scholar]
- 2.Bursi F, Weston SA, Redfield MM, et al. Systolic and diastolic heart failure in the community. JAMA. 2006;296(18):2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 3.Cleland J, Tendera M, Adamus J, et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27(19):2338–2345. doi: 10.1093/eurheartj/ehl250.. [DOI] [PubMed] [Google Scholar]
- 4.Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362(9386):777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 5.Massie BM, Carson PE, McMurray JJ, et al. I-PRESERVE Investigators. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359(23):2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 6.Zois NE, Bartels ED, Hunter I, et al. Natriuretic peptides in cardiometabolic regulation and disease. Nat Rev Cardiol. 2014;11(7):403–412. doi: 10.1038/nrcardio.2014.64. [DOI] [PubMed] [Google Scholar]
- 7.Gu J, Noe A, Chandra P, et al. Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi) J Clin Pharmacol. 2010;50(4):401–414. doi: 10.1177/0091270009343932. [DOI] [PubMed] [Google Scholar]
- 8.Solomon SD, Zile M, Pieske B, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380(9851):1387–1395. doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
- 9.McMurray J, Packer M, Desai A, et al. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. N Engl J Med. 2014;371(11):993–1004. doi: 10.1056/nejmoa1409077.. [DOI] [PubMed] [Google Scholar]
- 10.Sumita Yoshikawa W, Nakamura K, Miura D, et al. Increased Passive Stiffness of Cardiomyocytes in the Transverse Direction and Residual Actin and Myosin Cross-Bridge Formation in Hypertrophied Rat Hearts Induced by Chronic Beta-Adrenergic Stimulation. Circulation Journal. 2013;77(3):741–748. doi: 10.1253/circj.cj-12-0779.. [DOI] [PubMed] [Google Scholar]
- 11.Miyoshi T, Nakamura K, Yoshida M, et al. Effect of vildagliptin, a dipeptidyl peptidase 4 inhibitor, on cardiac hypertrophy induced by chronic beta-adrenergic stimulation in rats. Cardiovasc Diabetol. 2014;13:43. doi: 10.1186/1475-2840-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen C, Rodriguez L, Levine RA, et al. Noninvasive measurement of the time constant of left ventricular relaxation using the continuous-wave Doppler velocity profile of mitral regurgitation. Circulation. 1992;86(1):272–278. doi: 10.1161/01.cir.86.1.272. [DOI] [PubMed] [Google Scholar]
- 13.Gallego B, Arévalo MA, Flores O, et al. Renal fibrosis in diabetic and aortic-constricted hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2001;280(6):R1823–R1829. doi: 10.1152/ajpregu.2001.280.6.R1823. [DOI] [PubMed] [Google Scholar]
- 14.Takemoto M, Egashira K, Tomita H, et al. Chronic Angiotensin-Converting Enzyme Inhibition and Angiotensin II Type 1 Receptor Blockade : Effects on Cardiovascular Remodeling in Rats Induced by the Long-term Blockade of Nitric Oxide Synthesis. Hypertension. 1997;30(6):1621–1627. doi: 10.1161/01.hyp.30.6.1621.. [DOI] [PubMed] [Google Scholar]
- 15.von Lueder TG, Wang BH, Kompa AR, et al. Angiotensin receptor neprilysin inhibitor LCZ696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy. Circ Heart Fail. 2015;8(1):71–78. doi: 10.1161/CIRCHEARTFAILURE.114.001785. [DOI] [PubMed] [Google Scholar]
- 16.Suematsu Y, Miura SI, Goto M, et al. LCZ696, an angiotensin receptor-neprilysin inhibitor, improves cardiac function with the attenuation of fibrosis in heart failure with reduced ejection fraction in streptozotocin-induced diabetic mice. Eur J Heart Fail. 2016;18(4):386–393. doi: 10.1002/ejhf.474. [DOI] [PubMed] [Google Scholar]
- 17.Kusaka H, Sueta D, Koibuchi N, et al. LCZ696, Angiotensin II Receptor-Neprilysin Inhibitor, Ameliorates High-Salt-Induced Hypertension and Cardiovascular Injury More Than Valsartan Alone. Am J Hypertens. 2015;28(12):1409–1417. doi: 10.1093/ajh/hpv015. [DOI] [PubMed] [Google Scholar]
- 18.Biala A, Finckenberg P, Korpi A, et al. Cardiovascular effects of the combination of levosimendan and valsartan in hypertensive Dahl/Rapp rats. J Physiol Pharmacol. 2011;62(3):275–285. [PubMed] [Google Scholar]
- 19.Grimm D, Holmer SR, Riegger GA, et al. Effects of beta-receptor blockade and angiotensin II type I receptor antagonism in isoproterenol-induced heart failure in the rat. Cardiovasc Pathol. 1999;8(6):315–323. doi: 10.1016/s1054-8807(99)00021-6. [DOI] [PubMed] [Google Scholar]
- 20.Al-Mazroua HA, Al-Rasheed NM, Korashy HM. Downregulation of the cardiotrophin-1 gene expression by valsartan and spironolactone in hypertrophied heart rats in vivo and rat cardiomyocyte H9c2 cell line in vitro: a novel mechanism of cardioprotection. J Cardiovasc Pharmacol. 2013;61(4):337–344. doi: 10.1097/FJC.0b013e318283a565. [DOI] [PubMed] [Google Scholar]
- 21.Suematsu Y, Jing W, Nunes A, et al. LCZ696 (Sacubitril/Valsartan), an Angiotensin-Receptor Neprilysin Inhibitor, Attenuates Cardiac Hypertrophy, Fibrosis, and Vasculopathy in a Rat Model of Chronic Kidney Disease. J Card Fail. 2018;24(4):266–275. doi: 10.1016/j.cardfail.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Jing W, Vaziri ND, Nunes A, et al. LCZ696 (Sacubitril/valsartan) ameliorates oxidative stress, inflammation, fibrosis and improves renal function beyond angiotensin receptor blockade in CKD. Am J Transl Res. 2017;9(12):5473–5484. [PMC free article] [PubMed] [Google Scholar]
- 23.Polhemus DJ, Trivedi RK, Gao J, et al. Renal Sympathetic Denervation Protects the Failing Heart Via Inhibition of Neprilysin Activity in the Kidney. J Am Coll Cardiol. 2017;70(17):2139–2153. doi: 10.1016/j.jacc.2017.08.056. [DOI] [PubMed] [Google Scholar]
- 24.Fujii M, Wada A, Ohnishi M, et al. Endogenous bradykinin suppresses myocardial fibrosis through the cardiac-generated endothelin system under chronic angiotensin-converting enzyme inhibition in heart failure. J Cardiovasc Pharmacol. 2004;44(Suppl 1):S346–S349. doi: 10.1097/01.fjc.0000166265.09550.7c. [DOI] [PubMed] [Google Scholar]
- 25.Sato M, Engelman RM, Otani H, et al. Myocardial protection by preconditioning of heart with losartan, an angiotensin II type 1-receptor blocker: implication of bradykinin-dependent and bradykinin-independent mechanisms. Circulation. 2000;102(19 Suppl 3):III346–III351. doi: 10.1161/01.cir.102.suppl_3.iii-346. [DOI] [PubMed] [Google Scholar]
- 26.Seki T, Goto K, Kansui Y, et al. Angiotensin II receptor-neprilysin inhibitor sacubitril/valsartan improves endothelial dysfunction in spontaneously hypertensive rats. J Am Heart Assoc. 2017;6(10) doi: 10.1161/JAHA.117.006617. [DOI] [PMC free article] [PubMed] [Google Scholar]