Abstract
Background
Although the safety and efficacy of left atrial (LA) appendage (LAA) closure (LAAC) in nonvalvular atrial fibrillation (NVAF) patients have been well documented in randomized controlled trials and real-world experience, there are limited data in the literature about the impact of LAAC on cardiac remodeling. The aim of the study was to examine the impact of LAAC on cardiac functional and structural remodeling in NVAF patients.
Methods
Between March 2014 and November 2016, 47 NVAF patients who underwent LAAC were included in this study (LAAC group). A control group (non-LAAC group) was formed from 141 NVAF patients without LAAC using propensity score matching. The difference-in-difference analysis was used to evaluate the difference in cardiac remodeling between the two groups at baseline and follow-up evaluations.
Results
The LAAC group had a larger increase in LA dimension, volume and volume index than the non-LAAC group (+3.9 mm, p = 0.001; +9.7 mL, p = 0.006 and +5.9 mL/m2, p = 0.011, respectively). Besides, a significant increase in E and E/e’ ratio was also observed in the LAAC group (+14.6 cm/s, p = 0.002 and +2.3, p = 0.028, respectively). Compared with the non-LAAC group, left ventricular (LV) ejection fraction and fractional shortening decreased in LAAC patients, but were statistically insignificant (−3.5%, p = 0.109 and −2.0%, p = 0.167, respectively).
Conclusions
There were significant increases in LA size and LV filling pressure among NVAF patients after LAAC. These impacts of LAAC on cardiac functional and structural remodeling may have some clinical implications that need to be addressed in future studies.
Keywords: left atrial appendage closure, atrial fibrillation, stroke prevention, diastolic function, systolic function, cardiac remodeling, difference-in-difference analysis
Introduction
Thrombus formed inside the left atrial (LA) appendage (LAA) is the most common cause of ischemic stroke in nonvalvular atrial fibrillation (NVAF) patients [1–3]. Although oral anticoagulant (OAC) has been shown to be effective in reducing the incidence of ischemic stroke, it also increases the risk of hemorrhage complications in these patients [4–6]. Percutaneous LAA closure (LAAC) is considered to be effective both in decreasing the risk of stroke and lowering the bleeding complication of OAC in NVAF patients with concomitant high bleeding risk [7–9].
Left atrial appendage has several important mechanical and endocrine functions regarding unique anatomical and physiological properties [10, 11]. When LA volume or pressure overload happens, LAA becomes a significant reservoir chamber due to its distensible ability [12]. LAA removal resulted in increased LA size, LA pressure and decreased cardiac output in animals [13]. The removal may be particularly harmful with existing heart failure because it would further reduce the cardiac output and promote heart failure. Besides, LAA is also known as the source of atrial natriuretic peptide (ANP) in the human heart. The concentration of ANP is much higher in LAA walls than in the rest of the atrial free wall and in the ventricles [11]. Patients with LAA removal were found to have a significantly lower ANP secretion and concomitant increase in salt and water retention [14, 15]. Interestingly, percutaneous LAAC with devices for ischemic stroke prevention has been shown to be associated with a significant reduction in plasma ANP [16, 17]. Therefore, both LAA mechanical and endocrine functions will be altered after LAAC. These changes can further affect the cardiac function and structure.
Although the safety and efficacy of LAAC in NVAF patients are well documented, there are limited data in the literature about the impact of percutaneous LAAC on cardiac functional and structural remodeling.
Methods
Patients
The data of NVAF patients, who underwent LAAC between March 2014 and November 2016 (LAAC group), were retrospectively collected before LAAC (baseline) and 12 months after the procedure (follow-up). All of these patients had permanent atrial fibrillation (AF,) high risk of ischemic stroke (CHA2DS2-VASc score ≥ 2), and contraindication to OAC or high risk of bleeding (HAS-BLED score ≥ 3). A control group (non-LAAC group) was formed by including NVAF patients without LAAC that were being followed-up at the documented institute. For each case in the LAAC group, three control subjects were matched (1:3 style) based on sex, age and ejection fraction (EF) using propensity score matching. Patients with incomplete data or with the following conditions were excluded from the study: device embolization, significant residual peri-device leak (≥ 5 mm) detected on follow-up transesophageal echocardiography (TEE), mitral valve stenosis, proximal AF or AF that was converted to sinus rhythm during follow-up, prosthetic valve, atrial septal defect, dilated cardiomyopathy, and moderate to severe mitral valve regurgitation.
Devices
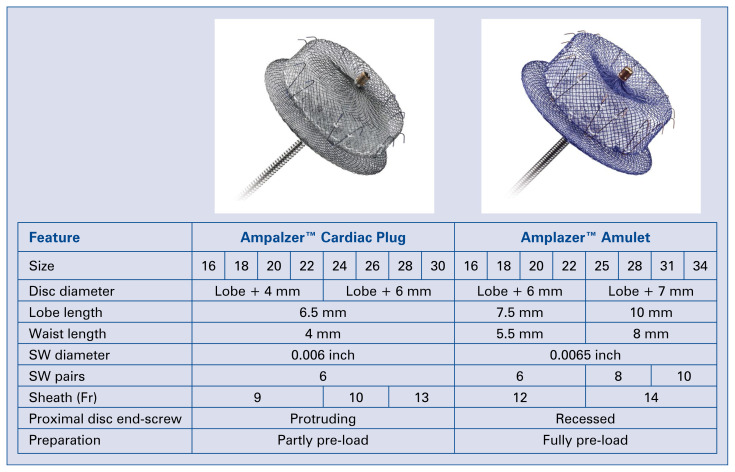
The devices used for LAAC were either Cardiac Plug or Amulet (St. Jude Medical, St. Paul, Minnesota, USA). Cardiac Plug is a self-expanding device that is made from nitinol wire mesh with a lobe and a disc connected via a waist. The design is aimed at sealing the body and ostium of the LAA. The lobe is usually implanted 10 mm inside the LAA body, and the anchoring mechanism is aided by stabilizing wires. The Amulet (or Cardiac Plug 2) is the second generation of the Cardiac Plug, which retains the basic structure of Cardiac Plug with some modifications for better performance and sealing (Fig. 1).
Figure 1.
Ampalzer™ Cardiac plug and Amplazer™ Amulet; SW — stabilising wire.
Procedure
Before the procedure, TEE was performed to assess LAA size, morphology and to confirm the absence of thrombus in LA and LAA. After trans-septal puncture, heparin was administered to achieve an active clotting time of 250–350 s [18]. At least 2 standard projections (RAO cranial and RAO caudal) were performed to obtain good visualization of the LAA. Fluoroscopy and TEE (or intra-cardiac echocardiography) imaging were used to re-evaluate the LAA and to select an appropriate device for each patient. The device size was chosen to be at least 20% larger than the measured diameter at the landing zone [18]. TEE (or intra-cardiac echocardiography) was used to check for the compression, positioning, stability of the device, peri-device leak and the relationship between device and adjacent structures. Transthoracic echocardiography, electrocardigraphy and chest X-ray were performed within 24 h after the procedure to rule out complications. All patients were discharged with dual antiplatelet therapy. The follow-up echocardiography was performed at 1 and 6 months, and then every 12 months after the procedure.
Echocardiography
Echocardiography was performed using the Philips EPIQ 7 ultrasound system for cardiology (Philips Corporation, MA, USA) with an S8-3 sector array transducer. All acquired data were stored in digital files for offline interpretation and were then evaluated by a single experienced expert in cardiovascular imaging. The measurement and quantification followed the recommendations from the American Society of Echocardiography [19, 20]. The parameters were obtained by averaging five consecutive cardiac cycles. In this study, baseline and follow-up echocardiography data were collected in both LAAC and non-LAAC groups.
In the M-mode, the following parameters were measured at parasternal long axis view: left ventricular internal dimension at end diastole (LVDd), left ventricular internal dimension at end systole (LVDs), interventricular septum thickness at end diastole (IVSd), interventricular septal thickness at end systole (IVSs), left ventricular posterior wall thickness at end diastole (LVPWd), left ventricular posterior wall thickness at end systole (LVPWs), left atrial dimension (LAD). The Teichholz method was used to calculate EF (the M-mode left ventricular ejection fraction). Cube formula was used to estimate left ventricular mass (LVM) and left ventricular mass indexed to body surface area (LVMI). Tricuspid annular plane systolic excursion (TAPSE) was measured on apical four-chamber views.
Pulse-wave Doppler was used to measure E (peak velocity of early diastolic trans-mitral flow) and deceleration time of early diastolic trans-mitral flow (DT). Tissue Doppler was used to measure e’ (peak velocity of early diastolic mitral annular motion). The E/e’ ratio was then calculated.
In two-dimensional (2D) mode, biplane left ventricular long-axis length at end diastole (LVLd) and biplane left ventricular long-axis length at end systole (LVLs) were calculated by averaging measurements from apical two-chamber and apical four-chamber views. The biplane technique of disk summation (modified Simpson’s rule) was used to calculate left ventricular end diastolic volume (EDV), left ventricular end systolic volume (ESV), left ventricular stroke volume (SV) and left ventricular ejection fraction (EF) in the 2D mode [20]. Right atrial dimension (RA), right ventricular basal and mid cavity dimensions (RVb and RVm, respectively) were measured on apical four-chamber view that was slightly focused on the right heart chambers. left atrial volume (LAV) and right atrial volume (RAV) were calculated using the area length method [20, 21]. The change of LAV index (LAVI) was defined as the difference between follow-up and baseline LAVI (ΔLAVI = follow-up LAVI – baseline LAVI). Similarly, the change of the E/e’ ratio was the difference between follow-up and baseline E/e’ ratio (ΔE/e’ = follow-up E/e’ – baseline E/e’).
Statistical analysis
To reduce the time-modified confounding effect, the difference-in-difference (DID) estimator was adopted to evaluate the impact of percutaneous LAAC on cardiac remodeling among NVAF patients [22, 23]. The analysis focused on comparing cardiac functional and structural changes that occurred between baseline and follow-up in both intervention (LAAC group) and control (non-LAAC group) groups. The model that was used for this DID regression is written as: Yit = β0+ β1Gi+ β2Tt+β3GiTt+ɛit, where, Yit is the parameter Y in participant i measured at time t (baseline or follow-up); β0 is a constant; Gi is a dummy that indicates whether this participant was in the LAAC group (Gi = 1) or in the non-LAAC group (Gi = 0); Tt is a dummy that indicates whether the parameter was measured at the follow-up (Tt = 1) or at the baseline (Tt = 0); ɛit represents the controlled variables. The main parameter of interest was β3 (the DID estimator), which indicated whether LAAC patients had more cardiac functional and structural changes over time than the NVAF patients without LAAC. The DID estimator revealed the real effect of LAAC on cardiac functional and structural remodeling after removing the effect of factors related to the time trend such as aging, disease progression, social life change, new medication, and other factors.
Statistical analyses were performed using SPSS Statistics 23.0 (IBM Corp., Armonk, NY, USA). Continuous variables were presented as the mean ± standard deviation. Categorical variables were presented as frequencies and percentages. The χ2 test was used to compare categorical variables, and for independent samples T-test was used to compare continuous variables. Multiple regression with the stepwise method was used to identify independent predictors of cardiac remodeling in the LAAC group, which had a significant association recognized using univariate analysis. A two-sided p value < 0.05 was considered statistically significant.
Results
Out of 188 NVAF patients who were included in this study, 47 were in the LAAC group, and 141 were in the non-LAAC group. The baseline characteristics of the participants are summarized in Table 1. There were no statistically significant differences in age, sex, body mass index, EF and cardiothoracic ratio between the two groups. The HAS-BLED score, the CHA2DS2-VASc score and the rate of stroke history were higher in the LAAC group than that in the non-LAAC group. However, these differences were also statistically insignificant. The mean procedure time was 94.2 ± 42.9 min, and Cardiac Plug was used in 30 (66.0%) patients.
Table 1.
The baseline characteristics of participants.
| Variable | LAAC group (n = 47) | Non-LAAC group (n = 141) | P |
|---|---|---|---|
| Age [year] | 75.1 ± 9.9 | 74.7 ± 9.7 | 0.799 |
| Male gender | 20 (42.6%) | 60 (42.6%) | 1.000 |
| BMI [kg/m2] | 24.4 ± 3.8 | 24.3 ± 3.7 | 0.953 |
| HAS-BLED score | 2.55 ± 1.41 | 2.23 ± 1.34 | 0.156 |
| CHA2DS2-VASc score | 3.83 ± 1.98 | 3.32 ± 1.65 | 0.082 |
| Labile INR | 6 (12.8%) | 20 (14.2%) | 0.807 |
| Bleeding | 8 (17.0%) | 23 (16.3%) | 0.910 |
| Stroke or TIA | 21 (44.7%) | 49 (34.8%) | 0.223 |
| Hypertension | 32 (68.1%) | 92 (65.2%) | 0.722 |
| Diabetes mellitus | 6 (12.8%) | 21 (14.9%) | 0.719 |
| Congestive heart failure | 14 (29.8%) | 35 (24.8%) | 0.502 |
| Chronic kidney disease | 5 (10.6%) | 13 (9.2%) | 0.775 |
| Vascular diseases | 7 (14.9%) | 14 (9.9%) | 0.349 |
| Heart rate [bpm] | 78.0 ± 16.9 | 80.4 ± 18.1 | 0.429 |
| Cardiothoracic ratio | 0.58 ± 0.06 | 0.59 ± 0.07 | 0.538 |
| Left ventricular ejection fraction | 56.7 ± 10.8 | 55.4 ± 11.7 | 0.425 |
LAAC — left atrial appendage closure; BMI — body mass index; INR — international normalized ratio; TIA — transient ischemic attack
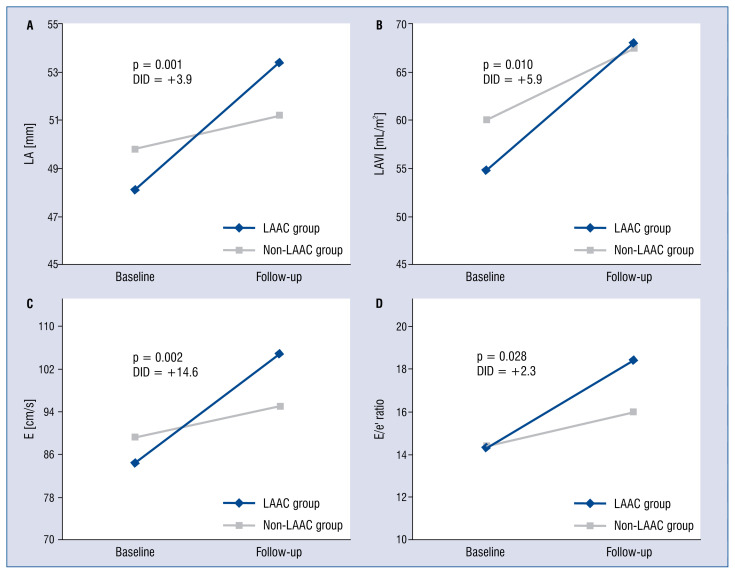
The impact of LAAC on cardiac structural remodeling is demonstrated in Table 2. There was no significant difference in structural change of left ventricle, right ventricle and right atrium between the two groups. However, the LAAC group had a higher relative increase in LAD, LAV and LAVI than the non-LAAC group with positive DID estimators (+3.9 mm, p = 0.001; +9.7 mL, p = 0.006 and +5.9 mL/m2, p = 0.011, respectively).
Table 2.
Changes on cardiac configuration evaluated by difference-in-difference (DID) analysis.
| Parameters | LAAC group (n = 47) | Non-LAAC group (n = 141) | DID estimator | 95% CI | P | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Baseline | Follow-up | Baseline | Follow-up | ||||
| M-mode | |||||||
| LAD [mm] | 48.2 ± 8.7 | 53.6 ± 9.0 | 49.9 ± 8.5 | 51.3 ± 8.6 | +3.9 | 1.9; 5.8 | 0.001 |
| LVDd [mm] | 51.1 ± 5.5 | 50.8 ± 5.7 | 51.1 ± 6.3 | 50.7 ± 6.5 | +0.7 | −1.1; 1.7 | 0.685 |
| LVDs [mm] | 34.6 ± 6.2 | 35.7 ± 6.7 | 34.1 ± 7.0 | 33.5 ± 7.5 | +1.7 | −0.1; 3.5 | 0.064 |
| IVSd [mm] | 9.5 ± 1.3 | 9.2 ± 1.0 | 10.0 ± 1.6 | 9.7 ± 1.6 | −0.2 | −0.6; 0.3 | 0.447 |
| IVSs [mm] | 13.5 ± 2.1 | 13.0 ± 1.5 | 14.2 ± 2.5 | 14.1 ± 2.6 | −0.4 | −1.2; 0.4 | 0.298 |
| LVPWd [mm] | 9.3 ± 1.2 | 8.9 ± 0.9 | 9.3 ± 1.2 | 9.5 ± 3.2 | −0.4 | −1.4; 0.6 | 0.398 |
| LVPWs [mm] | 14.1 ± 2.5 | 13.4 ± 2.1 | 14.5 ± 2.4 | 14.4 ± 2.4 | −0.6 | −1.5; 0.3 | 0.209 |
| 2D-mode | |||||||
| LVLd [mm] | 73.9 ± 8.1 | 72.4 ± 6.2 | 74.5 ± 9.2 | 74.2 ± 9.1 | −0.9 | −2.9; 1.1 | 0.383 |
| LVLs [mm] | 64.6 ± 7.8 | 62.2 ± 6.2 | 65.1 ± 8.3 | 64.3 ± 8.2 | −1.6 | −3.9; 0.6 | 0.154 |
| RVb [mm] | 33.3 ± 8.3 | 34.5 ± 4.6 | 34.0 ± 5.0 | 35.0 ± 6 | 9 +0.2 | −2.4; 2.8 | 0.887 |
| RVm [mm] | 22.3 ± 3.4 | 22.7 ± 3.0 | 22.2 ± 2.7 | 23.5 ± 4.9 | −0.9 | −2.6; 0.7 | 0.262 |
| RAD [mm] | 40.8 ± 8.8 | 40.7 ± 5.7 | 43.3 ± 8.4 | 44.0 ± 9.1 | −0.9 | −3.9; 2.4 | 0.589 |
| Volume and mass | |||||||
| EDV [mL] | 99.4 ± 29.4 | 101.3 ± 29.2 | 104.6 ± 37.3 | 107.6 ± 46.4 | −1.6 | −10.5; 7.3 | 0.722 |
| ESV [mL] | 39.1 ± 19.9 | 41.0 ± 29.1 | 42.3 ± 23.0 | 43.5 ± 29.5 | +0.3 | −5.8; 6.4 | 0.926 |
| SV [mL] | 47.0 ± 16.0 | 46.8 ± 12.2 | 48.4 ± 18.3 | 49.8 ± 18.5 | −1.5 | −6.5; 3.4 | 0.540 |
| LAV [mL] | 90.3 ± 36.7 | 108.4 ± 37.7 | 95.9 ± 35.9 | 104.2 ± 44.9 | +9.7 | 3.6; 15.9 | 0.006 |
| LAVI [mL/m2] | 54.7 ± 23.0 | 68.1 ± 23.5 | 60.0 ± 24.4 | 67.5 ± 31.4 | +5.9 | 1.7; 9.8 | 0.011 |
| RAV [mL] | 67.1 ± 39.1 | 62.3 ± 17.8 | 78.4 ± 40.6 | 76.1 ± 50.8 | −1.8 | −13.8; 10.2 | 0.764 |
| LVM [g] | 173.6 ± 46.3 | 162.1 ± 33.8 | 181.5 ± 50.1 | 176.4 ± 49.5 | −6.4 | −17.3; 4.5 | 0.246 |
| LVMI [g/m2] | 107.2 ± 26.3 | 100.2 ± 21.6 | 110.4 ± 31.0 | 107.9 ± 29.2 | −4.6 | −10.8; 3.1 | 0.280 |
LAAC — left atrial appendage closure; CI — confidence interval; LAD — left atrial dimension; LVDd/LVDs — left ventricular internal dimension at end diastole/systole; IVSd/IVSs — interventricular septum thickness at end diastole/systole; LVPWd/LVPWs — left ventricular posterior wall thickness at end diastole/systole; LVLd/LVLs — biplane left ventricular diastolic/systolic length; RVb/RBm — right ventricular base/middle dimension; RAD — right atrial dimension; EDV/ESV — biplane left ventricular volume at end diastole/systole; SV — biplane left ventricular stroke volume; LAV — left atrial volume; LAVI — left atrial volume indexed to body surface area; RAV — right atrial volume; LVM — left ventricular mass; LVMI — left ventricular mass index
The impact of LAAC on cardiac functional remodeling is presented in Table 3. In comparison with the non-LAAC group, EF and fractional shortening had more relative decrease among LAAC patients with a negative DID estimator, but these changes were statistically insignificant (−3.5%, p = 0.109 and −2.0%, p = 0.167, respectively). Additionally, there was a significant relative increase in E and E/e’ ratio observed in patients with LAAC (+14.6 cm/s, p = 0.002 and +2.3, p = 0.028, respectively).
Table 3.
Changes on cardiac function and pulmonary arterial pressure evaluated by difference-in-difference (DID) analysis.
| Parameters | LAAC group (n = 47) | Non-LAAC group (n = 141) | DID estimator | 95% CI | P | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Baseline | End-line | Baseline | End-line | ||||
| Systolic function | |||||||
| FS [%] | 32.3 ± 7.7 | 31.2 ± 7.1 | 33.4 ± 8.2 | 34.3 ± 8.3 | −2.0 | −4.9; 0.9 | 0.167 |
| EF [%] | 63.1 ± 12.1 | 60.1 ± 11.2 | 64.3 ± 12.3 | 64.8 ± 12.2 | −3.5 | −7.7; 0.8 | 0.109 |
| EF2C [%] | 58.9 ± 10.9 | 56.6 ± 9.5 | 55.3 ± 12.4 | 55.8 ± 11.1 | −2.9 | −6.6; 0.7 | 0.111 |
| EF4C [%] | 56.5 ± 12.4 | 55.2 ± 9.9 | 55.2 ± 11.0 | 55.6 ± 10.2 | −1.4 | −4.8; 1.9 | 0.404 |
| EFBP [%] | 57.7 ± 10.8 | 55.8 ± 9.2 | 55.4 ± 11.7 | 55.7 ± 10.1 | −2.2 | −5.3; 0.9 | 0.164 |
| TAPSE [mm] | 11.7 ± 2.2 | 12.0 ± 1.7 | 11.9 ± 2.3 | 11.9 ± 2.4 | +0.4 | −0.7; 1.4 | 0.476 |
| Diastolic function | |||||||
| DT [ms] | 183.5 ± 73.1 | 181.0 ± 73.1 | 182.8 ± 75.3 | 182.7 ± 71.8 | −3.79 | −36.8; 29.3 | 0.819 |
| E [cm/s] | 85.6 ± 28.4 | 106.1 ± 27.3 | 89.5 ± 26.9 | 95.3 ± 28.8 | +14.6 | 6.18; 22.9 | 0.002 |
| E/e’ ratio | 14.3 ± 6.9 | 18.4 ± 7.8 | 14.4 ± 7.3 | 16.2 ± 7.3 | +2.3 | 0.3; 4.3 | 0.028 |
| Pulmonary arterial pressure | |||||||
| PAPs | 39.1 ± 11.0 | 40.1 ± 9.5 | 39.2 ± 10.9 | 40.0 ± 12.8 | +0.24 | −3.7; 4.2 | 0.903 |
LAAC — left atrial appendage closure; CI — confidence interval; FS — left ventricular fractional shortening; EF — left ventricular ejection fraction on M-mode; EF2C — left ventricular ejection fraction on two chamber view; EF4C — left ventricular ejection fraction on four chamber view; EFBP — biplane left ventricular ejection fraction measured; TAPSE — tricuspid annular plane systolic excursion; DT — deceleration time of early diastolic trans-mitral flow; E — peak velocity of early diastolic trans-mitral flow; e’ — peak velocity of early diastolic mitral annular motion; PAPs — pulmonary arterial systolic pressure
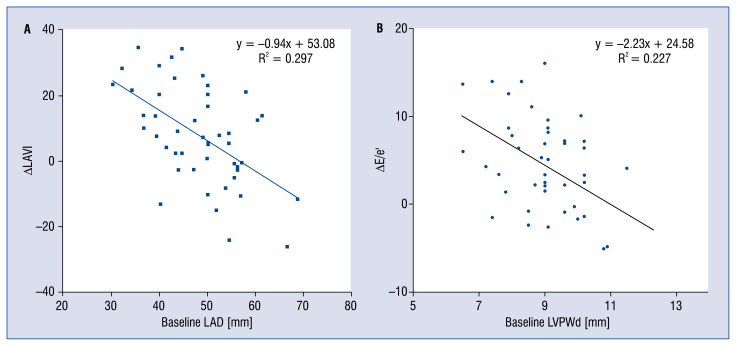
Univariate analysis showed that the relative LAVI change in the LAAC group was associated with baseline LAD (β = −0. 56, p < < 0.001), IVSd (β = −0.33, p < 0.05); RVm (β = −0.35, p < 0.05), E (β = −0.38, p < 0.05) and pulmonary arterial systolic pressure (β = −0.29, p < 0.05). Besides, the relative change of the E/e’ ratio was correlated with IVSd (β = −0.34, p < 0.05), LVPWd (β = −0.49, p < 0.001), LVM (β = −0.29, p < 0.05) and RVm (β = −0.30, p < 0.05). In multiple linear regression with the step-wise method, the predictor of the relative LAVI change was baseline LAD (β = −0.94, p < 0.001, R = 0.55), and the predictor of the relative E/e’ ratio change was baseline LVPWd (β = −2.23, p < 0.01, R = 0.477).
Discussion
The present study showed that there were several impacts of LAAC on cardiac functional and structural remodeling in NVAF patients. Significant increases were identified in LA size and LV filling pressure in this group of patients 12 months after the procedure.
Percutaneous LAAC is sometimes the only option for ischemic stroke prevention, especially in NVAF patients with contraindication for long-term OAC or high risk of bleeding. Although the safety and efficacy of this treatment have been demonstrated in randomized controlled trials and real-world experience [5–7, 24, 25], the data about the impacts of LAAC on cardiac remodeling are not well established. To evaluate the impact of this therapy on cardiac functional and structural remodeling, changes were measured and compared of echocardiographic parameters between LAAC and non-LAAC groups before and 12 months subsequent to the procedure.
A remarkable finding in this study was noted, a significantly higher increase in LAD (+3.9 mm), which may predictably lead to a higher increase in LAV (+9.7 mL) and the LAVI (+5.9 mL/m2) among LAAC patients after the procedure in comparison with non-LAAC patients (Fig. 2A, B). This LA remodeling may be explained by the alteration of LAA function after LAAC. Previous studies have shown that there was a decrease in ANP concentration after percutaneous LAA device closure for ischemic stroke prevention [15, 17]. Even though the mechanism of this phenomenon has not been fully understood, the insufficiency of this endocrine hormone may let the LA suffer more from pressure and volume overload. Besides, LAA is more compliant than the LA main chamber and plays an important role in the presence of LA pressure and/or volume overload [26, 27]. The separation between LAA and LA after complete endothelialization of the device results in the disappearance of this LAA reservoir function [14]. An animal study has shown that LA compliance decreased after removal of the LAA, and the change in compliance was associated with decreased atrial reservoir function, which was manifested by smaller reservoir volume alterations [13]. Furthermore, LAA clamping during coronary artery bypass grafting or mitral valve surgery indicated a significant increase in LA maximal dimension [14]. A recent study that evaluated the impact of LAAC on LA mechanical function also found that there was an increase in maximum LAV, LAVI and expansion index among NVAF patients who received this treatment [28]. However, these studies presented the alteration of LAD and LAV in a short time period, ranging from hours to 60 days. Another study used 3D-echocardiographic measurements in patients 6 months after LAAC with Watchman device and showed that LAV increased significantly after interventional LAAC. However, the LA enlargement did not correlate with clinical progression of heart failure [29]. In the present study, a similar consequence of LAA isolation, related to the increase in LA size, could be observed 12 months after LAAC procedure. Furthermore, multiple linear regression with the stepwise method showed that the predictor of LAVI change was baseline LAD (β = −0.94, p < 0.001, R = 0.545). Interestingly, the negative correlation suggested that if LA had had a more normal appearance at the baseline (smaller LA size), then it would be more impacted after the LAAC (Fig. 3A). Thus, clinicians should be more careful in selecting patients with a less remodeled LA for LAAC. In addition, it may be necessary to pay more attention to medical therapy and follow-up for this subgroup of patients after the procedure to reduce LA adverse remodeling.
Figure 2.
A–C. Changes in cardiac function and structure between baseline and follow-up; LA — left atrial; LAVI — left atrial volume indexed to body surface area; E — peak velocity of early diastolic trans-mitral flow; e’ — peak velocity of early diastolic mitral annular motion; DID — difference-in-difference analysis; LAAC — left atrial appendage closure.
Figure 3.
A, B. Correlation between cardiac function and configuration changes with corresponding independent predictors; LAVI — left atrial volume indexed body surface area; e — peak velocity of early diastolic transmitral flow; e’ — peak velocity of early diastolic mitral annular motion; LAD — left atrial dimension; LVPWd — left ventricular posterior wall thickness at end diastole; ΔLAVI = follow-up LAVI – baseline LAVI; E/e’ = follow-up E/e’ – baseline E/e’.
Another important finding in the present study was a significant increase in the E/e’ ratio, which indicated an increase in LV filling pressure among patients with LAAC (Fig. 2D). In AF, atrial contraction is lost, Doppler assessment of LV filling pressure is limited by the variability in cycle length and the absence of organized atrial activity [19, 30]. However, echocardiographic parameters that are independent of atrial influence can be utilized to assess LV filling pressure in AF patients. Among these parameters, the E/e’ ratio was documented to have a most reliable correlation with invasive assessment of LV filling pressure [19, 31, 32]. Thus, the increase of the E/e’ ratio in the present study was a reliable manifestation of LV filling pressures elevation among AF patients after LAAC. This may be a consequence of inadequate volume and pressure regulation in LA because of LAA function loss following LAAC procedure. Previous studies confirmed that clamping or surgical excision of the appendage resulted in an immediate increase in mean atrial pressure [13, 27]. Besides, the elevated LV filling pressure in AF patients, which was estimated by E/e’, was also found to be independently associated with atrial remodeling [33]. Therefore, the elevation of LV filling pressure was a good explanation for the increase in LAD and LAV after LAAC in this study [34–36]. Further analysis with multivariable regression showed that the change of LV filling pressure in LAAC group, represented by the E/e’ ratio, was independently associated with LVPWd (β= −2.23, p < 0.01, R = 0.477) (Fig. 3B). Besides, the correlation of the E/e’ ratio with IVSd, LVPWd, LVM in univariate analysis suggested the important role of baseline LV wall thickness in the alteration of LV filling pressure after LAAC.
The result of this study also showed no significant change in LV and RV systolic function. However, all of the negative DID estimators of EF and fractional shortening change may elicit the decrease in systolic function among NVAF patients after LAAC. Along with increase in LA size and LV filling pressure, these changes may have some long-term physiological and clinical implications that need to be addressed.
Limitations of the study
This was a retrospective study with a limited number of patients in the LAAC group. Observed changes may be caused by chance in this small study cohort. Furthermore, because only some relevant indicators related to cardiac remodeling were collected, the alteration of other parameters as well as clinical presentations may have happened and were not observed in this study. Similarly, changes in medication that may affect cardiac remodeling was not evaluated. Besides, patients were not matched for LA size at baseline, which may differently affect cardiac remodeling. Because the study population had a normal EF and elevated LV filling pressure at baseline, the results may only be specific to this population.
Conclusions
The present study showed that there was a significant increase in LA size and LV filling pressure among NVAF patients after LAAC. These impacts of LAAC on cardiac functional and structural re-modeling observed on echocardiography may have clinical implications that need to be addressed in future studies.
Acknowledgements
It is acknowledged that all authors listed meet the authorship criteria according to the latest guidelines of the International Committee of Medical Journal Editors, and all authors are in agreement with the manuscript.
Footnotes
Conflict of interest: None declared
References
- 1.Di Minno MN, Ambrosino P, Dello Russo A, et al. Prevalence of left atrial thrombus in patients with non-valvular atrial fibrillation. A systematic review and meta-analysis of the literature. Thromb Haemost. 2016;115(3):663–677. doi: 10.1160/TH15-07-0532. [DOI] [PubMed] [Google Scholar]
- 2.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 3.Yaghi S, Song C, Gray WA, et al. Left atrial appendage function and stroke risk. Stroke. 2015;46(12):3554–3559. doi: 10.1161/STROKEAHA.115.011273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherwood M, Nessel C, Hellkamp A, et al. Gastrointestinal Bleeding in Patients With Atrial Fibrillation Treated With Rivaroxaban or Warfarin. J Am Coll Cardiol. 2015;66(21):2271–2281. doi: 10.1016/j.jacc.2015.09.024.. [DOI] [PubMed] [Google Scholar]
- 5.Reddy VY, Doshi SK, Sievert H, et al. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3-Year Follow-up of the PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation) Trial. Circulation. 2013;127(6):720–729. doi: 10.1161/CIRCULATIONAHA.112.114389. [DOI] [PubMed] [Google Scholar]
- 6.Maura G, Blotière PO, Bouillon K, et al. Comparison of the short-term risk of bleeding and arterial thromboembolic events in nonvalvular atrial fibrillation patients newly treated with dabigatran or rivaroxaban versus vitamin K antagonists: a French nationwide propensity-matched cohort study. Circulation. 2015;132(13):1252–1260. doi: 10.1161/CIRCULATIONAHA.115.015710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belgaid DR, Khan Z, Zaidi M, et al. Prospective randomized evaluation of the watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: The PREVAIL trial. Int J Cardiol. 2016;219:177–179. doi: 10.1016/j.ijcard.2016.06.041. [DOI] [PubMed] [Google Scholar]
- 8.Reddy VY, Möbius-Winkler S, Miller MA, et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology) J Am Coll Cardiol. 2013;61(25):2551–2556. doi: 10.1016/j.jacc.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 9.Holmes D, Doshi S, Kar S, et al. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2015;65(24):2614–2623. doi: 10.1016/j.jacc.2015.04.025.. [DOI] [PubMed] [Google Scholar]
- 10.DeSimone CV, Gaba P, Tri J, et al. A review of the relevant embryology, pathohistology, and anatomy of the left atrial appendage for the invasive cardiac electrophysiologist. J Atr Fibrillation. 2015;8(2):1129–1187. doi: 10.4022/jafib.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hara H, Virmani R, Holmes DR, et al. Is the left atrial appendage more than a simple appendage? Catheter Cardiovasc Interv. 2009;74(2):234–242. doi: 10.1002/ccd.21983. [DOI] [PubMed] [Google Scholar]
- 12.Fastner C, Behnes M, Sartorius B, et al. Left atrial appendage morphology, echocardiographic characterization, procedural data and in-hospital outcome of patients receiving left atrial appendage occlusion device implantation: a prospective observational study. BMC Cardiovasc Disord. 2016;16:25. doi: 10.1186/s12872-016-0200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoit BD, Shao Y, Tsai LM, et al. Altered left atrial compliance after atrial appendectomy. Influence on left atrial and ventricular filling. Circ Res. 1993;72(1):167–175. doi: 10.1161/01.res.72.1.167. [DOI] [PubMed] [Google Scholar]
- 14.Beigel R, Wunderlich NC, Ho SY, et al. The left atrial appendage: anatomy, function, and noninvasive evaluation. JACC Cardiovasc Imaging. 2014;7(12):1251–1265. doi: 10.1016/j.jcmg.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Al-Saady NM, Obel OA, Camm AJ. Left atrial appendage: structure, function, and role in thromboembolism. Heart. 1999;82(5):547–554. doi: 10.1136/hrt.82.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majunke N, Sandri M, Adams V, et al. Atrial and brain natriuretic peptide secretion after percutaneous closure of the left atrial appendage with the watchman device. J Invasive Cardiol. 2015;27(10):448–452. [PubMed] [Google Scholar]
- 17.Luani B, Rauwolf T, Groscheck T, et al. Serial assessment of natriuretic peptides in patients undergoing interventional closure of the left atrial appendage. Heart Lung Circ. 2017 doi: 10.1016/j.hlc.2017.07.001. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Meier B, Blaauw Y, Khattab AA, et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion. EuroIntervention. 2015;10(9):1109–1125. doi: 10.4244/EIJY14M08_18. [DOI] [PubMed] [Google Scholar]
- 19.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Jiamsripong P, Honda T, Reuss CS, et al. Three methods for evaluation of left atrial volume. Eur J Echocardiogr. 2008;9(3):351–355. doi: 10.1016/j.euje.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Dimick JB, Ryan AM. Methods for evaluating changes in health care policy: the difference-in-differences approach. JAMA. 2014;312(22):2401–2402. doi: 10.1001/jama.2014.16153. [DOI] [PubMed] [Google Scholar]
- 23.Platt RW, Schisterman EF, Cole SR. Time-modified confounding. Am J Epidemiol. 2009;170(6):687–694. doi: 10.1093/aje/kwp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panikker S, Lord J, Jarman JWE, et al. Outcomes and costs of left atrial appendage closure from randomized controlled trial and real-world experience relative to oral anticoagulation. Eur Heart J. 2016;37(46):3470–3482. doi: 10.1093/eurheartj/ehw048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy VY, Gibson DN, Kar S, et al. Post-Approval U.S. Experience With Left Atrial Appendage Closure for Stroke Prevention in Atrial Fibrillation. J Am Coll Cardiol. 2017;69(3):253–261. doi: 10.1016/j.jacc.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 26.Spencer RJ, DeJong P, Fahmy P, et al. Changes in left atrial appendage dimensions following volume loading during percutaneous left atrial appendage closure. JACC Cardiovasc Interv. 2015;8(15):1935–1941. doi: 10.1016/j.jcin.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 27.Tabata T, Oki T, Yamada H, et al. Role of left atrial appendage in left atrial reservoir function as evaluated by left atrial appendage clamping during cardiac surgery. Am J Cardiol. 1998;81(3):327–332. doi: 10.1016/s0002-9149(97)00903-x. [DOI] [PubMed] [Google Scholar]
- 28.Coisne A, Pilato R, Brigadeau F, et al. Percutaneous left atrial appendage closure improves left atrial mechanical function through Frank-Starling mechanism. Heart Rhythm. 2017;14(5):710–716. doi: 10.1016/j.hrthm.2017.01.042. [DOI] [PubMed] [Google Scholar]
- 29.Luani B, Groscheck T, Genz C, et al. Left atrial enlargement and clinical considerations in patients with or without a residual interatrial shunt after closure of the left atrial appendage with the WATCHMAN™-device. BMC Cardiovasc Disord. 2017;17(1):294. doi: 10.1186/s12872-017-0728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagarakanti R, Ezekowitz M. Diastolic dysfunction and atrial fibrillation. J Interv Card Electrophysiol. 2008;22(2):111–118. doi: 10.1007/s10840-008-9203-8. [DOI] [PubMed] [Google Scholar]
- 31.Traversi E, Cobelli F, Pozzoli M. Doppler echocardiography reliably predicts pulmonary artery wedge pressure in patients with chronic heart failure even when atrial fibrillation is present. Eur J Heart Fail. 2001;3(2):173–181. doi: 10.1016/s1388-9842(00)00140-9. [DOI] [PubMed] [Google Scholar]
- 32.Kusunose K, Yamada H, Nishio S, et al. Clinical utility of single-beat E/e’ obtained by simultaneous recording of flow and tissue Doppler velocities in atrial fibrillation with preserved systolic function. JACC Cardiovasc Imaging. 2009;2(10):1147–1156. doi: 10.1016/j.jcmg.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 33.Kim TH, Shim C, Park J, et al. Left ventricular diastolic dysfunction is associated with atrial remodeling and risk or presence of stroke in patients with paroxysmal atrial fibrillation. J Cardiol. 2016;68(2):104–109. doi: 10.1016/j.jjcc.2015.10.008.. [DOI] [PubMed] [Google Scholar]
- 34.El Aouar LM, Meyerfreud D, Magalhães P, et al. Relationship between left atrial volume and diastolic dysfunction in 500 Brazilian patients. Arq Bras Cardiol. 2013;101(1):52–58. doi: 10.5935/abc.20130109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pritchett A, Mahoney D, Jacobsen S, et al. Diastolic dysfunction and left atrial volume. J Am Coll Cardiol. 2005;45(1):87–92. doi: 10.1016/j.jacc.2004.09.054.. [DOI] [PubMed] [Google Scholar]
- 36.Hammoudi N, Achkar M, Laveau F, et al. Left atrial volume predicts abnormal exercise left ventricular filling pressure. Eur J Heart Fail. 2014;16(10):1089–1095. doi: 10.1002/ejhf.131. [DOI] [PubMed] [Google Scholar]