Abstract
There is emerging evidence of high mortality rates after the first diagnosis of psychotic disorder. The objective of this study was to estimate the standardized mortality ratio (SMR) in a population-based cohort of individuals with a first diagnosis of schizophrenia-spectrum psychotic disorder (SSD). The cohort included a population-based sample of individuals with a first diagnosis of SSD based on the first diagnosis occurring during hospitalization or in an outpatient setting between 2007 and 2010 in Ontario, Canada. All patients were followed for 5 years after the first diagnosis. The primary outcome was SMR, including all-cause, suicide-related, accidental, and other causes. Between 2007 and 2010, there were 2382 patients in the hospitalization cohort and 11 003 patients in the outpatient cohort. Over the 5-year observation period, 97 (4.1%) of the hospitalization cohort and 292 (2.7%) of the outpatient cohort died, resulting in an SMR of 13.6 and 9.1, respectively. In both cohorts, suicide was the most common cause of death. Approximately 1 in 25 patients with a first diagnosis of SSD during hospitalization, and 1 in 40 patients with a first diagnosis of SSD in an outpatient setting, died within 5 years of first diagnosis in Ontario, Canada. This mortality rate is between 9 and 13 times higher than would be expected in the age-matched general population. Based on these data, timely access to services should be a public health priority to reduce mortality following a first diagnosis of an SSD.
Keywords: schizophrenia, first episode, mortality
Introduction
Individuals with psychotic disorders, such as schizophrenia and schizoaffective disorders (ie, a schizophrenia- spectrum psychotic disorder [SSD]), have excess mortality reducing their lifespan by up to 15 years.1,2 The main causes of this excess mortality are cardiovascular disease and suicide.1,2 However, the causes and risk of mortality are not equally distributed throughout the life span of an individual with SSD. In younger people, after a first diagnosis, the main causes of the elevated risk of mortality appears to be suicide and suicide-related injury,3 not cardiovascular disease. These findings are corroborated by a recent study suggesting that individuals with schizophrenia are more likely to die by suicide at a younger age than individuals who have died by suicide without a diagnosis of schizophrenia.4 The early mortality observed in individuals following a first diagnosis of SSD is striking for two reasons. First, the causes of death in this period appear to be related more to suicide than early manifestations of chronic diseases, such as cardiovascular disease, which are more likely to appear later in the lifespan.5 Second, there is evidence that early mortality may be preventable with access to adequate resources.6 Thus, there is early evidence that suggests that mortality early after the onset of schizophrenia is both more common than once thought and potentially preventable if individuals have access to high-quality care.
The objective of the present study was to estimate the risk of mortality among a population-based cohort of individuals with a first diagnosis of SSD and to estimate standardized mortality rates by comparing to mortality rates, including cause-specific mortality rates, in an age-matched general population comparison group. Secondary objectives included determining access to health services 30 days and 1 year following the first SSD diagnosis and, among those who died, 30 days and 1 year preceding their death.
Methods
Study Design and Data Sources
We used a retrospective cohort design to identify all residents in Ontario, Canada with a first diagnosis of SSD between January 1, 2007, and December 31, 2010, between 16 and 45 years of age. In Ontario, health care is publicly funded,7 and the province’s population is nearly 15 million people. Individuals were followed for 5 years following their cohort enrollment index date. The following datasets were used: the Ontario Health Insurance Program (OHIP) database for information related to physician visits, including diagnoses; the Ontario Mental Health Reporting System (OMHRS) for information about hospitalizations occurring in designated psychiatric beds; the Canadian Institute for Health Information Discharge Abstract Database (CIHI-DAD) for information about hospitalizations occurring in nonpsychiatric beds; the National Ambulatory Care Reporting System (NACRS) for information related to Emergency Department (ED) visits; the Registered Persons Database (RPDB) for information related to patient demographics; and the Ontario Registrar General Vital Statistics–Death (ORGD) database for information on deaths, including the cause of death. We also used Census data for information on neighborhood-level income. These datasets were linked using unique encoded identifiers and analyzed at ICES. The use of data in this project using anonymized data was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a Research Ethics Board.
Participants
Individuals were assigned to two separate cohorts depending on the site of first diagnosis. The validated diagnostic algorithm to detect individuals with a psychotic disorder requires either one SSD diagnosis (DSM-IV codes 295.x; ICD9 code 295) from a hospitalization or three physician-based diagnoses of SSD over a period of 36 months.8 We created two separate cohorts, one in which individuals were enrolled based on the SSD diagnosis during hospitalizations and one in which individuals were enrolled based on the three outpatient diagnoses over 36 months. We need to create two cohorts because calculating survival time between the two cohorts was different. The outpatient cohort has a survival bias based on the requirement of three diagnoses (eg, up to an extra 36 months of survival required to enter the cohort), whereas hospitalized cohort patients enter the cohort immediately, making the survival time estimates between the two cohorts noncomparable. There is also some diagnostic uncertainty at the time of first diagnosis of schizophrenia.9,10 Accordingly, we conducted a sensitivity analysis using a separate cohort that included individuals with a diagnosis of psychotic disorder not otherwise specified (DSM-IV and ICD9 code 298), in addition to the individuals with schizophrenia and schizoaffective disorder.
Individuals were excluded if they were younger than 16 or older than 45 years of age to capture individuals with a first diagnosis of an SSD. They were also excluded if they had a diagnosis of schizophrenia in the 12 months prior to cohort enrollment to ensure we were enrolling first episode (incident) cases of SSD. Patients were also excluded if they were non-Ontario residents at the time of diagnosis, or ineligible for OHIP in the year prior to diagnosis. These individuals were excluded because we would not know whether the diagnosis was a “first diagnosis”; it is possible that these individuals could have had a prior diagnosis in another province, for example. For the cohort that was enrolled via diagnosis during hospitalization, patients were excluded if their hospitalization length of stay was less than 72 h (these “short stay” hospitalizations may not have accurate diagnoses).
Outcomes
The primary outcome was all-cause mortality within 5 years of incident diagnosis. We also described the number of different causes of death; due to the relatively small number of deaths, we were only able to report on three categories of mortality: accidental, suicide-related, and “other” causes of death. The “other” category included all causes of mortality that were not accidental or suicide-related causes, the most common of which were related to cardiovascular disease.
We measured health service utilization 30 days and 1 year following incident diagnosis. We measured physician visits, including psychiatrist, primary care, and other physician visits. We measured ED visits, categorized as mental health-related and nonmental health-related. We also measured ED visits related to suicide attempts, using a validated diagnostic algorithm.11 We further measured hospitalizations, categorized as mental health- and nonmental health-related. Among the patients who died within 5 years of their incident schizophrenia diagnosis, we measured the above health service utilization outcomes 30 days and 1 year prior to death date.
Data Analysis
We calculated standardized mortality ratios (SMRs), which is the ratio between the observed number of deaths in the SSD population compared with the expected number of deaths based on the age- and sex-standardized Ontario population, indirectly standardizing for both age and sex.12 We also calculated SMRs stratified by sex, by age category, and by cause of death. For all SMR calculations, we used Ontario’s population as of 2011 as the standard population (per Canadian Institute for Health Information [CIHI] data and guidelines) to generate the expected number of deaths and SMRs.13 SMR calculations were performed separately for each cohort entry modality (hospital or outpatient setting).
We also conducted three sensitivity analyses producing mortality and standardized mortality rates in three different cohorts. The first sensitivity analysis not only included a cohort similar to the primary study cohort but also included psychotic disorder not otherwise specified, a less specific diagnosis than schizophrenia or schizoaffective disorder. In the second and third sensitivity analysis, we recreated our study cohort by revising the algorithm for outpatient diagnoses to be less stringent. We created two separate cohorts by changing the entry criteria to require only two diagnoses of SSD within 24 months and one diagnosis of SSD within 24 months.
All analyses were completed using SAS Enterprise Guide v9.4, with an ICES-created macro used for SMR calculations.
Results
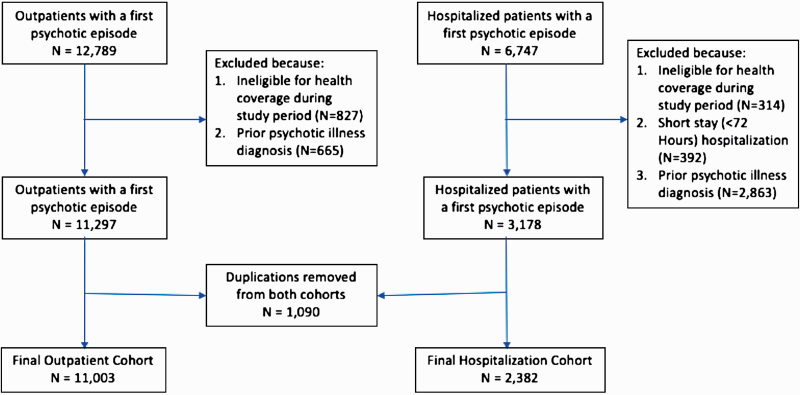
Between January 1, 2007, and December 31, 2010, 12 789 patients between the ages of 16 and 45 were enrolled in the cohort via outpatient encounters (Appendix figure 1). After exclusions due to ineligibility of health care coverage (N = 827) and those with a diagnosis of a psychotic disorder 12 months prior to cohort entry (N = 665), there were 11 297 outpatients with a diagnosis of an SSD disorder. For the cohort with patients enrolled via hospitalization, there were 6747 with a diagnosis of SSD between the ages of 16 and 45 years. A total of 314 patients were excluded due to health care coverage ineligibility, and 392 patients were excluded who had a short stay hospitalization. Finally, 2863 patients were excluded because they had a diagnosis of SSD in the 12 months prior to cohort entry, resulting in a final hospitalization cohort of 3178 patients. After accounting for 1090 patients who were in both the hospitalization and outpatient cohorts, there were 2382 patients in the first episode SSD hospitalization cohort and 11 003 patients with a first episode SSD diagnosis via outpatient enrollment. Over the 5-year follow-up period, altogether 610 patients (93 in the hospital-diagnosed sample and 517 in the outpatient-diagnosed sample) were censored due to leaving the province.
The demographic and health care utilization characteristics in the 1 year prior to cohort entry for both cohorts are described in table 1. The hospital-diagnosed cohort was similar to the outpatient-diagnosed cohort with respect to sex, age, and neighborhood income distribution. A greater proportion of the hospital-diagnosed vs outpatient-diagnosed cohort (16.1% vs 9.8%) had no physician visits in the year prior to cohort entry. The hospital-diagnosed cohort was also more likely to have prior mental health-related hospitalizations (21.0% vs 16.6%; table 1).
Table 1.
Baseline demographic characteristics and health care utilization 1 y prior to cohort entry
| Hospital cohort N (%) | Outpatient cohort N (%) | Standardized difference | |
|---|---|---|---|
| Total | 2382 | 11 003 | |
| Demographic characteristics | |||
| Sex | |||
| Male | 1547 (64.9%) | 6785 (61.7%) | 0.07 |
| Female | 835 (35.1%) | 4218 (38.3%) | 0.07 |
| Age (numeric, y) | |||
| Mean (SD) | 30.15 ± 8.61 | 29.46 ± 8.61 | 0.08 |
| Median (IQR) | 29 (22–38) | 28 (22–37) | 0.08 |
| Age categories (y) | |||
| 16–20 | 379 (15.9%) | 2054 (18.7%) | 0.07 |
| 21–25 | 497 (20.9%) | 2409 (21.9%) | 0.03 |
| 26–30 | 398 (16.7%) | 1753 (15.9%) | 0.02 |
| 31–35 | 358 (15.0%) | 1584 (14.4%) | 0.02 |
| 36–40 | 355 (14.9%) | 1564 (14.2%) | 0.02 |
| 41–45 | 395 (16.6%) | 1639 (14.9%) | 0.05 |
| Neighborhood income quintile | |||
| 1 (low) | 763 (32.0%) | 3381 (30.7%) | 0.03 |
| 2 (medium–low) | 492 (20.7%) | 2323 (21.1%) | 0.01 |
| 3 (medium) | 423 (17.8%) | 1906 (17.3%) | 0.01 |
| 4 (medium–high) | 373 (15.7%) | 1803 (16.4%) | 0.02 |
| 5 (high) | 297 (12.5%) | 1512 (13.7%) | 0.04 |
| Rural residence | |||
| No | 2148 (90.2%) | 10 108 (91.9%) | 0.06 |
| Prior healthcare utilization* | |||
| Physician visits | |||
| No visits | 384 (16.1%) | 1083 (9.8%) | 0.19 |
| PCP visit(s) | 1758 (73.8%) | 9205 (83.7%) | 0.24 |
| Psychiatrist visit(s) | 1049 (44.0%) | 4866 (44.2%) | 0.00 |
| PCP and psychiatrist visit(s) | 856 (35.9%) | 4282 (38.9%) | 0.06 |
| ED visit | |||
| Mental health-related | 585 (24.6%) | 2428 (22.1%) | 0.06 |
| Self-harm | 48 (2.0%) | 206 (1.9%) | 0.01 |
| Nonmental health-related | 982 (41.2%) | 4068 (37.0%) | 0.09 |
| Hospitalization | |||
| Mental health-related | 500 (21.0%) | 1822 (16.6%) | 0.11 |
| Nonmental health-related | 137 (5.8%) | 508 (4.6%) | 0.05 |
*Health care utilization was measured 1 y prior to the hospitalization date for patients in the hospitalization cohort or 1 y prior to the first outpatient diagnosis for the outpatient cohort.
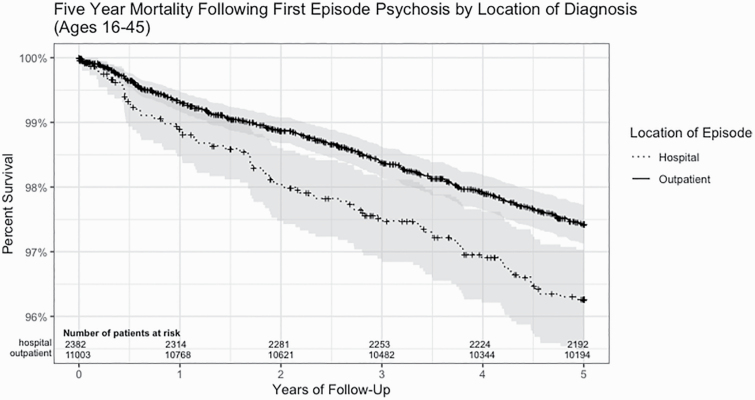
Among the hospital-diagnosed cohort, 97 (4.1%) of the patients died within 5 years of cohort entry. Among the outpatient-diagnosed cohort, 292 (2.7%) died within 5 years of cohort entry. The sex, age, and causes of death are described in table 2. A greater proportion of females died in the hospital-diagnosed cohort (33.0% vs 27.7%). The age at death was slightly younger in the outpatient-diagnosed cohort. A greater proportion of the outpatient-diagnosed cohort patients had an “accidental” cause of death compared with hospital-diagnosed cohort patients (28.8% vs 18.6%). The SMRs are described in table 3. The overall age- and sex-standardized SMR for the hospitalized cohort was 13.62 (95% CI: 11.05–16.47) and the overall SMR for the outpatient cohort was 9.11 (95% CI: 8.10–10.19). The SMR varied when stratified by sex, age, and cause of death for both the hospital and outpatient cohorts (table 3). A Kaplan–Meier 5-year survival curve is shown in figure 1.
Table 2.
Characteristics of patients who died within the hospital-diagnosed and outpatient-diagnosed cohorts
| Hospital cohort N (%) | Outpatient cohort N (%) | Standardized difference | |
|---|---|---|---|
| Total deaths | 97 | 292 | 0.08 |
| Sex | |||
| Male | 65 (67.0%) | 211 (72.3%) | 0.11 |
| Female | 32 (33.0%) | 81 (27.7%) | 0.11 |
| Age (numeric, y) | |||
| Mean (SD) | 34.13 (8.46) | 32.25 (8.81) | 0.22 |
| Median (IQR) | 34 (28–42) | 33 (25–41) | 0.21 |
| Age category (y) | |||
| 16–20 | 8 (8.2%) | 33 (11.3%) | 0.10 |
| 21–25 | 10 (10.3%) | 51 (17.5%) | 0.21 |
| 26–30 | 14 (14.4%) | 43 (14.7%) | 0.01 |
| 31–35 | 19 (19.6%) | 51 (17.5%) | 0.05 |
| 36–40 | 18 (18.6%) | 37 (12.7%) | 0.16 |
| 41–45 | 28 (28.9%) | 77 (26.4%) | 0.06 |
| Cause of death | |||
| Accidental | 18 (18.6%) | 84 (28.8%) | 0.24 |
| Suicide | 27 (27.8%) | 88 (30.1%) | 0.05 |
| Other | 52 (53.6%) | 120 (41.1%) | 0.21 |
Table 3.
Mortality and SMRs
| Hospital-diagnosed cohort (N = 2382) | Total person-time of follow-up (y) | Observed deaths | Crude mortality rate/1000 persons/y (95% CI) | Expected death count | SMR (95% CI) | SMR |
|---|---|---|---|---|---|---|
| All-cause mortality | 11 613 | 97 | 8.35 (6.77–10.19) | 7.12 | 13.62 (11.05–16.47) | 13.62 |
| Sex | ||||||
| Male | 7543 | 65 | 8.62 (6.65–10.98) | 4.37 | 14.89 (11.48–18.71) | 14.89 |
| Female | 4070 | 32 | 7.86 (5.38–11.10) | 2.76 | 11.60 (7.92–15.96) | 11.60 |
| Age category at cohort entry (y) | ||||||
| 16–24 | 3895 | 17 | 4.36 (2.54–6.99) | 1.41 | 12.08 (7.01–18.47) | 12.08 |
| 25–29 | 2046 | 11 | 5.38 (2.68–9.62) | 0.83 | 13.22 (6.58–22.24) | 13.22 |
| 30–34 | 1770 | 23 | 12.99 (8.24–19.50) | 1.11 | 20.72 (13.12–30.05) | 20.72 |
| 35–39 | 1691 | 11 | 6.51 (3.25–11.64) | 1.20 | 9.14 (4.55–15.38) | 9.14 |
| 40–45 | 2211 | 35 | 15.83 (11.03–22.02) | 2.54 | 13.80 (9.59–18.72) | 13.80 |
| Cause of death | ||||||
| Accidental | 11 613 | 18 | 1.55 (0.92–2.45) | 2.15 | 8.39 (4.95–12.69) | 8.39 |
| Suicide | 11 613 | 27 | 2.32 (1.53–3.38) | 1.52 | 17.81 (11.69–25.10) | 17.81 |
| Other* | 11 613 | 52 | 4.48 (3.34–5.87) | 1.89 | 27.45 (20.54–35.50) | 27.45 |
| Outpatient-diagnosed cohort N = 11 003 | ||||||
| All-cause mortality | 54 223 | 292 | 5.39 (4.79–6.04) | 32.04 | 9.11 (8.10–10.19) | 9.11 |
| Sex | ||||||
| Male | 33 360 | 211 | 6.32 (5.509–7.24) | 18.50 | 11.40 (9.92–13.00) | 11.40 |
| Female | 20 863 | 81 | 3.88 (3.08–4.83) | 13.54 | 5.98 (4.75–7.36) | 5.98 |
| Age category at cohort entry (y) | ||||||
| 16–24 | 20 044 | 72 | 3.59 (2.81–4.52) | 7.20 | 10.00 (7.82–12.44) | 10.00 |
| 25–29 | 9074 | 50 | 5.51 (4.09–7.26) | 3.69 | 13.56 (10.05–17.57) | 13.56 |
| 30–34 | 7829 | 42 | 5.36 (3.87–7.25) | 4.76 | 8.82 (6.36–11.69) | 8.82 |
| 35–39 | 7696 | 43 | 5.59 (4.04–7.53) | 5.48 | 7.85(5.68–10.37) | 7.85 |
| 40–45 | 9580 | 85 | 8.87 (7.09–10.97) | 10.77 | 7.89(6.30–9.66) | 7.89 |
| Cause of death | ||||||
| Accidental | 54 223 | 84 | 1.55 (1.24–1.92) | 9.89 | 8.50(6.77–10.41) | 8.50 |
| Suicide | 54 223 | 88 | 1.62 (1.30–2.00) | 6.95 | 12.66(10.15–15.45) | 12.66 |
| Other* | 54 223 | 120 | 2.21 (1.82–2.65) | 8.45 | 14.20 (11.77–16.86) | 14.20 |
*“Other” causes of death is an aggregate measure of causes that are neither accidental nor due to suicide. This category was collapsed due to small cell sizes.
Fig. 1.
Five-year survival after cohort entry.
The health care utilization 30 days and 1 year following diagnosis is described in table 4. Notably, 37% of the hospital-diagnosed cohort patients and 38% of the outpatient-diagnosed cohort patients did not see any physician within 30 days of cohort enrollment. By 1 year, only 8% of the hospital-diagnosed cohort patients and 3% of the outpatient-diagnosed cohort patients still had not seen a physician for mental health-related reasons. Psychiatric hospitalizations were common following cohort entry. Among the hospital-diagnosed cohort patients, 13% experienced a psychiatric rehospitalization within 30 days of index hospitalization discharge. Among the outpatient-diagnosed cohort patients, 28% of patients experienced a psychiatric hospitalization within 30 days of cohort enrollment. Table 4 also shows the health care utilization 30 days and 1 year prior to death among the patients who died in each of the two cohorts. For patients who died in the two cohorts, approximately 50% had not seen a physician within 30 days of death. Within 1 year of death, 18% of the hospital-diagnosed cohort and 11% of the outpatient-diagnosed cohort had not seen a physician, but approximately 35% of the patients in both cohorts had a psychiatric hospitalization in the year prior to death.
Table 4.
Health service utilization 30 d and 1 y following cohort entry and prior to death
| Health service utilization after cohort entry | Hospital cohort N (%) | Outpatient cohort N (%) | Standardized difference |
|---|---|---|---|
| Total | 2382 | 11 003 | |
| 30 d following diagnosis | |||
| No visits | 877 (36.8) | 4157 (37.8) | 0.02 |
| PCP visit(s) | 818 (34.3) | 4151 (37.7) | 0.07 |
| Psychiatrist visit(s) | 982 (41.2) | 3855 (35.0) | 0.13 |
| PCP and psychiatrist visit(s) | 344 (14.4) | 1422 (12.9) | 0.04 |
| ED visit | |||
| Mental health related | 156 (6.5) | 447 (4.1) | 0.11 |
| Self-harm | 8 (0.3) | 34 (0.3) | 0.00 |
| Nonmental health related | 213 (8.9) | 684 (6.2) | 0.10 |
| Hospitalization | |||
| Mental health related | 301 (12.6) | 3094 (28.1) | 0.39 |
| Nonmental health related | 35 (1.5) | 79 (0.7) | 0.07 |
| 1 y following diagnosis | |||
| No visits | 199 (8.4) | 328 (3.0) | 0.23 |
| PCP visit(s) | 1778 (74.6) | 9502 (86.4) | 0.30 |
| Psychiatrist visit(s) | 1700 (71.4) | 8003 (72.7) | 0.03 |
| PCP and psychiatrist visit(s) | 1333 (56.0) | 6876 (62.5) | 0.13 |
| ED visit | |||
| Mental health related | 523 (22.0) | 1914 (17.4) | 0.11 |
| Self-harm | 50 (2.1) | 191 (1.7) | 0.03 |
| Nonmental health related | 857 (36.0) | 3817 (34.7) | 0.03 |
| Hospitalization | |||
| Mental health related | 952 (40.0) | 4598 (41.8) | 0.04 |
| Nonmental health related | 132 (5.5) | 522 (4.7) | 0.04 |
| Health service utilization prior to death | |||
| Total deaths | 97 | 292 | 0.08 |
| 30 d before death | |||
| Physician visits | |||
| No visits | 46 (47.4%) | 143 (49.0%) | 0.03 |
| PCP visit(s) | 29 (29.9%) | 99 (33.9%) | 0.09 |
| Psychiatrist visit(s) | 21 (21.6%) | 68 (23.3%) | 0.04 |
| PCP and psychiatrist visit(s) | 6 (6.2%) | 27 (9.2%) | 0.11 |
| ED visit | |||
| Mental health-related | 7 (7.2%) | 25 (8.6%) | 0.05 |
| Self-harm | ≤5 | ≤5 | 0.21 |
| Nonmental health-related | 11 (11.3%) | 49 (16.8%) | 0.16 |
| Hospitalization | |||
| Mental health-related | 6 (6.2%) | 23 (7.9%) | 0.07 |
| Nonmental health-related | ≤5 | 12 (4.1%) | 0.05 |
| 1 y before death | |||
| Physician visits | |||
| No visits | 17 (17.5%) | 32 (11.0%) | 0.19 |
| PCP visit(s) | 63 (64.9%) | 213 (72.9%) | 0.17 |
| Psychiatrist visit(s) | 52 (53.6%) | 185 (63.4%) | 0.20 |
| PCP and psychiatrist visit(s) | 38 (39.2%) | 140 (47.9%) | 0.18 |
| ED visit | |||
| Mental health-related | 29 (29.9%) | 99 (33.9%) | 0.09 |
| Self-harm | ≤5 | 18 (6.2%) | 0.04 |
| Nonmental health-related | 55 (56.7%) | 162 (55.5%) | 0.02 |
| Hospitalization | |||
| Mental health-related | 36 (37.1%) | 104 (35.6%) | 0.03 |
| Nonmental health-related | 18 (18.6%) | 48 (16.4%) | 0.06 |
In the first sensitivity analysis that also included psychotic disorder not otherwise specified, the cohort was larger, reflective of the inclusion of more subjects, but the SMR was similar (Appendix table 1). In the second and third sensitivity analyses requiring only two diagnoses of SSD within 24 months and one diagnosis of SSD within 24 months, the cohorts shifted patients from the hospital-diagnosed cohort to the outpatient-diagnosed cohort and increased the cohort size slightly, but generated very similar SMR results as in the primary study cohort (Appendix table 2).
Discussion
Among Ontario residents with a first SSD diagnosis, 1 in 25 (4%) who had a first diagnosis during hospitalization and 1 in 40 (2.5%) who had a first diagnosis during outpatient contact died within 5 years. Overall, patients who received a first diagnosis during hospitalization were over 13 times more likely to die within 5 years compared with age-matched, population-based controls, and patients who were diagnosed in outpatient settings were 9 times more likely to die. Additionally, more than one-third of patients did not have physician follow-up within 30 days of first diagnosis, and 50% of those who died saw no physician in the 30 days prior to the date of death. Furthermore, as many as 17.5% of the hospital-diagnosed and 11.0% of the outpatient-diagnosed cohorts, had no physician contact even one year prior to death.
The findings of this study are consistent with previous studies of mortality in first episode psychosis.3,14,15 There have been recent, population-based analyses of the mortality gap in all adults with schizophrenia, and these studies reveal that cardiovascular diseases, as well as suicide, are significant contributors to the persistent mortality gap.1,2,16 This finding has led to a focus on addressing cardiovascular risk factors in individuals with schizophrenia and other psychotic disorders.1 However, in the period of time following first diagnosis, suicide, and unnatural deaths, as was shown in our study, have emerged as the main causes of death.4,5,14,17 This finding, along with early evidence that access to services focused on individuals with first episode psychosis substantially reduces mortality,6 suggests that addressing mortality associated with schizophrenia likely requires aggressive efforts to engage these individuals in treatment, and intensive suicide risk assessment and management in the years following first diagnosis. “Light touch” efforts have also been shown to reduce suicidal attempts following ED visits as well, and are effective low-cost interventions that can reduce mortality.18,19 System-level solutions are required, given recent data from our group that suggest that 60% of patients are not seeing a psychiatrist within 30 days of first diagnosis.20 Furthermore, in the present cohort study, nearly 30% of patients did not see a psychiatrist within a year of first diagnosis.
There are many strengths to this study. First, we used population-based data to develop a cohort that is followed for 5 years. The use of a validated algorithm meant that we had a highly representative sample that included patients who only engaged the system on an outpatient basis at cohort entry. Second, we were able to follow all patients for 5 years with minimal (5%) loss to follow-up. Third, the outcome, mortality, is routinely measured within the population-based data. Several limitations are worth mentioning, however. First, our validated algorithm is, like most health administrative data algorithms, highly specific with relatively low sensitivity. We conducted several sensitivity analyses to assess the impact of this, all of which revealed consistent results to our primary analysis. However, we have likely missed cases of schizophrenia for a number of reasons; requiring health care utilization for cohort entry means that individuals who have a psychotic disorder but never access the system will be missed. Second, our health care utilization measures include physician-based visits only. There are nonphysician-based health care services for individuals with early psychosis that are not represented in our data. We may have thus underestimated the true health care utilization of the two cohorts. Nevertheless, access to physicians is considered critical in the care of early psychosis,21 as maintenance antipsychotic treatment for SSD is considered crucial.22 Third, data were unavailable to determine reasons for the lack of physician contact, that is, whether this was related to patient nonadherence due to lack of illness insight or illness worsening, fragmentation of care, or other reasons that could guide interventions to mitigate the suboptimal engagement in people with early phase SDD. Nevertheless, it is clear that early, integrated care models for people with early-phase SSD improve outcomes over usual care23 indicating that such models should be favored whenever possible. Finally, we did not capture risk factors for mortality, such as comorbid substance use14 and nonadherence to antipsychotic treatment.24–27 Finally, Ontario has some catchmented, dedicated early psychosis services, which could mean that our mortality data are an underestimate (given prior work showing that engagement is higher in early psychosis services23 and that service users have reduced mortality compared with nonusers6). The availability of such services stands in contrast to most other jurisdictions around the world, other than countries such as Australia, and Ireland, for instance.28 In the US, the “EPI-NET” initiative and On-Track sites are closing the gap through the creation of dedicated service networks and communities of practice.29
In a population-based cohort of patients who were diagnosed with SSD in Ontario during hospitalization or outpatient contact, 5-year mortality was 13-fold and 9-fold elevated, respectively, compared with the general population. These findings provide a public health imperative to ensure timely and sustained access to care for this population, and to invest in the development of relatively low-cost interventions that can enable and support such access.
Acknowledgments
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). Parts of this material are based on data and/or information compiled and provided by Ontario’s Ministry of Health and Long-term Care and CIHI. The analyses, conclusions, opinions, and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred. PK acknowledges funding from the Canadian Institutes of Health Research (CIHR) and the Ministry of Health and Long-term Care. AV acknowledges funding from the National Institute of Mental Health, CIHR, National Sciences and Engineering Research Council, Canada Foundation for Innovation, CAMH Foundation, and the University of Toronto.
Appendix Table 1: Mortality and Standardized Mortality Ratios - Cohort including Psychotic Disorder Not Otherwise Specified.
| Hospital-diagnosed Cohort N = 2,382 | Total person time of follow-up (years) | Observed Deaths | Crude Mortality rate per 1,000 persons/year | Expected Death Count | SMR (95% CI) |
|---|---|---|---|---|---|
| All-Cause Mortality | 15019 | 130 | 8.66 (7.23–10.28) | 9.04 | 14.38 (12.01–16.96) |
| Sex | |||||
| Male | 8921 | 80 | 8.97 (7.11–11.16) | 5.03 | 15.92 (12.61–19.58) |
| Female | 6098 | 50 | 8.20 (6.09–10.81) | 4.02 | 12.45 (9.23–16.12) |
| Age Category (years) | |||||
| 16–24 | 5419 | 29 | 5.35 (3.58–7.69) | 1.96 | 14.77 (9.90–20.67) |
| 25–29 | 2397 | 17 | 7.09 (4.13–11.36) | 0.98 | 17.38 (10.08–26.57) |
| 30–34 | 2337 | 26 | 11.13 (7.27–16.30) | 1.46 | 17.87 (11.62–25.31) |
| 35–39 | 2188 | 19 | 8.68 (5.23–13.56) | 1.57 | 12.12 (7.27–18.16) |
| 40–45 | 2678 | 39 | 14.56 (10.36–19.91) | 3.07 | 12.71 (9.03–17.00) |
| Cause of Death | |||||
| Accidental | 15019 | 28 | 1.86 (1.24–2.69) | 2.75 | 10.18 (6.76–14.30) |
| Suicide | 15019 | 41 | 2.73 (1.96–3.70) | 1.94 | 21.11(15.16–28.10) |
| Other | 15019 | 61 | 4.06 (3.11–5.22) | 2.39 | 25.51 (19.52–32.33) |
| Outpatient-diagnosed Cohort N = 11,003 | |||||
| All-Cause Mortality | 68293 | 403 | 5.90 (5.34–6.51) | 40.53 | 9.94 (9.00–10.94) |
| Sex | |||||
| Male | 41084 | 280 | 6.82 (6.04–7.66) | 23.12 | 12.11 (10.73–13.57) |
| Female | 27209 | 123 | 4.52 (3.76–5.39) | 17.40 | 7.07 (5.87–8.37) |
| Age Category (years) | |||||
| 16–24 | 25482 | 88 | 3.45 (2.77–4.25) | 9.16 | 9.61 (7.70–11.72) |
| 25–29 | 11374 | 67 | 5.89 (4.57–7.48) | 4.63 | 14.48 (11.21–18.14) |
| 30–34 | 9509 | 50 | 5.26 (3.90–6.93) | 5.79 | 8.63 (6.41–11.20) |
| 35–39 | 9554 | 63 | 6.59 (5.07–8.44) | 6.82 | 9.24 (7.10–11.66) |
| 40–45 | 12374 | 135 | 10.91 (9.15–12.91) | 14.05 | 9.61 (8.06–11.30) |
| Cause of Death | |||||
| Accidental | 68293 | 108 | 1.58 (1.30–1.91) | 12.44 | 8.68 (7.12–10.40) |
| Suicide | 68293 | 109 | 1.60 (1.31–1.93) | 8.75 | 12.46 (10.23–14.91) |
| Other | 68293 | 186 | 2.72 (2.35–3.14) | 10.71 | 17.36 (14.96–19.95) |
Appendix Table 2: Mortality and Standardized Mortality Ratios – Cohorts requiring 2 psychotic disorder diagnoses within 24 months and 1 psychotic disorder diagnosis within 12 months.
| Hospital-diagnosed Cohort - Outpatient Diagnosis Requires 2 Billings in 24 Months. | |||||
|---|---|---|---|---|---|
| N = 1,943 | Total person time of follow-up | Observed Deaths | Crude Mortality rate per 1,000 persons/year | Expected Death Count | SMR (95% CI) |
| All-Cause Mortality | 9,504 | 71 | 7.47 (5.83–9.42) | 5.45 | 13.02 (10.17–16.23) |
| Sex | |||||
| Female | 3,337 | 27 | 8.09 (5.33–11.77) | 2.16 | 12.5 (8.23–17.66) |
| Male | 6,167 | 44 | 7.13 (5.18–9.58) | 3.29 | 13.36 (9.71–17.62) |
| Age Category (years) | |||||
| 16–24 | 3,949 | 16 | 4.05 (2.32–6.58) | 1.42 | 11.23 (6.42–17.47) |
| 25–29 | 1,593 | 7 | 4.39 (1.77–9.05) | 0.65 | 10.84 (4.27–20.22) |
| 30–34 | 1,282 | 13 | 10.14 (5.40–17.34) | 0.8 | 16.35 (8.62–26.28) |
| 35–39 | 1,169 | 8 | 6.84 (2.95-13,48) | 0.83 | 9.6 (4.12–17.47) |
| 40+ | 1,511 | 27 | 17.87 (11.78–26.00) | 1.74 | 15.54 (10.22–21.92) |
| Cause of Death | |||||
| Accidental | 9,504 | 13 | 1.37 (0.73–2.34) | 1.74 | 7.48 (3.96–12.08) |
| Suicide | 9,504 | 23 | 2.42 (1.53–3.63) | 1.21 | 18.97 (12.03–27.57) |
| Other | 9,504 | 35 | 3.68 (2.57–5.12) | 0.98 | 35.54 (24.86–48.53) |
| Outpatient-diagnosed Cohort - Outpatient Diagnosis Requires 2 Billings in 24 Months | |||||
| N = 15,317 | Total person time of follow-up | Observed Deaths | Crude Mortality rate per 1,000 persons/year | Expected Death Count | SMR (95% CI) |
| All-Cause Mortality | 75,615 | 360 | 4.76 (4.28–5.28) | 44.85 | 8.03 (7.22–8.88) |
| Sex | |||||
| Female | 30,594 | 102 | 3.33 (2.72–4.05) | 19.59 | 5.21 (4.25–6.27) |
| Male | 45,021 | 258 | 5.73 (5.05–6.47) | 25.26 | 10.21 (9.01–11.50) |
| Age Category (years) | |||||
| 16–24 | 27,769 | 99 | 3.57 (2.90–4.34) | 9.98 | 9.92 (8.06–11.97) |
| 25–29 | 12,336 | 64 | 5.19 (4.00–6.63) | 5.01 | 12.77 (9.84–16.10) |
| 30–34 | 10,766 | 56 | 5.20 (3.93–6.75) | 6.55 | 8.55 (6.46–10.94) |
| 35–39 | 10,897 | 48 | 4.40 (3.25–5.84) | 7.72 | 6.22 (4.58–8.10) |
| 40+ | 13,847 | 93 | 6.72 (5.42–8.23) | 15.49 | 6 (4.85–7.29) |
| Cause of Death | |||||
| Accidental | 75,615 | 111 | 1.47 (1.21–1.77) | 13.71 | 8.09 (6.66–9.67) |
| Suicide | 75,615 | 112 | 1.48 (1.22–1.78) | 9.66 | 11.6 (9.55–13.84) |
| Other | 75,615 | 137 | 1.81 (1.52–2.14) | 8.1 | 16.92 (14.20–19.86) |
| Hospital-diagnosed Cohort - Outpatient Diagnosis Requires 1 Billing in 12 Months | |||||
| N = 1,359 | Total person time of follow-up | Observed Deaths | Crude Mortality rate per 1,000 persons/year | Expected Death Count | SMR (95% CI) |
| All-Cause Mortality | 6,664 | 47 | 7.05 (5.18–9.38) | 3.83 | 12.26 (9.01–16.03) |
| Sex | |||||
| Female | 2,409 | 20 | 8.3 (5.07–12.82) | 1.55 | 12.9 (7.87–19.18) |
| Male | 4,255 | 27 | 6.35 (4.18–9.23) | 2.28 | 11.82 (7.80–16.73) |
| Age Category (years) | |||||
| 16–24 | 2,695 | 12 | 4.45 (2.30–7.78) | 0.97 | 12.33 (6.36–20.36) |
| 25–29 | 1,158 | <=5 | 4.32 (1.40–10.08) | 0.47 | 10.64 (3.36–22.01) |
| 30–34 | 904 | 7 | 7.74 (3.11–15.95) | 0.56 | 12.61 (4.95–23.48) |
| 35–39 | 830 | <=5 | 6.02 (1.96–14.06) | 0.59 | 8.5 (2.67–17.53) |
| 40+ | 1,077 | 18 | 16.71 (9.91–26.41) | 1.23 | 14.61 (8.65–22.18) |
| Cause of Death | |||||
| Accidental | 6,664 | 7 | 1.05 (0.42–2.16) | 1.22 | 5.74 (2.27–10.78) |
| Suicide | 6,664 | 17 | 2.55 (1.49–4.08) | 0.85 | 19.99 (11.62–30.64) |
| Other | 6,664 | 23 | 3.45 (2.19–5.18) | 0.69 | 33.18 (21.10–48.35) |
| Outpatient-diagnosed Cohort - Outpatient Diagnosis Requires 1 Billing in 12 Months | |||||
| N = 28,057 | Total person time of follow-up | Observed Deaths | Crude Mortality rate per 1,000 persons/year | Expected Death Count | SMR (95% CI) |
| All-Cause Mortality | 138,596 | 616 | 4.44 (4.10–4.81) | 84.21 | 7.31 (6.75–7.90) |
| Sex | |||||
| Female | 61,921 | 195 | 3.15 (2.72–3.62) | 39.57 | 4.93 (4.26–5.64) |
| Male | 76,675 | 421 | 5.49 (4.98–6.04) | 44.64 | 9.43 (8.55–10.35) |
| Age Category (years) | |||||
| 16–24 | 47,084 | 131 | 2.78 (2.33–3.30) | 16.89 | 7.76 (6.48–9.14) |
| 25–29 | 22,876 | 97 | 4.24 (3.44–5.17) | 9.26 | 10.48 (8.49–12.66) |
| 30–34 | 20,445 | 105 | 5.14 (4.20–6.22) | 12.44 | 8.44 (6.90–10.13) |
| 35–39 | 20,669 | 97 | 4.69 (3.81–5.73) | 14.65 | 6.62 (5.37–8.00) |
| 40+ | 27,522 | 186 | 6.76 (5.82–7.80) | 30.82 | 6.04 (5.20–6.93) |
| Cause of Death | |||||
| Accidental | 138,596 | 181 | 1.31 (1.12–1.51) | 17.8 | 9.83 (8.74–11.70) |
| Suicide | 138,596 | 175 | 1.26 (1.08–1.46) | 25.15 | 7.2 (5.97–8.03) |
| Other | 138,596 | 260 | 1.88 (1.65–2.12) | 15.21 | 17.1 (15.08–19.24) |
Appendix Figure 1: Cohort Flow Chart.
Conflict of Interest statement
Dr. Correll has been a consultant and/or advisor to or has received honoraria from Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, Gerson Lehrman Group, IntraCellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, Medscape, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Sumitomo Dainippon, Sunovion, Supernus, Takeda, and Teva. He has provided expert testimony for Janssen and Otsuka. He served on a Data Safety Monitoring Board for Lundbeck, Rovi, Supernus, and Teva. He received grant support from the Berlin Institute of Health (BIH), Janssen, the National Institute of Mental Health (NIMH), Patient Centered Outcomes Research Institute (PCORI), Takeda, and the Thrasher Foundation. He received royalties from UpToDate and is also a stock option holder of LB Pharma.
References
- 1. Olfson M, Gerhard T, Huang C, Crystal S, Stroup TS. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72(12):1172–1181. [DOI] [PubMed] [Google Scholar]
- 2. Gatov E, Rosella L, Chiu M, Kurdyak PA. Trends in standardized mortality among individuals with schizophrenia, 1993–2012: a population-based, repeated cross-sectional study. CMAJ. 2017;189(37):E1177–E1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Simon GE, Stewart C, Yarborough BJ, et al. Mortality rates after the first diagnosis of psychotic disorder in adolescents and young adults. JAMA Psychiatry. 2018;75(3):254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zaheer J, Jacob B, de Oliveira C, Rudoler D, Juda A, Kurdyak P. Service utilization and suicide among people with schizophrenia spectrum disorders. Schizophr Res. 2018;202:347–353. [DOI] [PubMed] [Google Scholar]
- 5. Björkenstam C, Björkenstam E, Hjern A, Bodén R, Reutfors J. Suicide in first episode psychosis: a nationwide cohort study. Schizophr Res. 2014;157(1–3):1–7. [DOI] [PubMed] [Google Scholar]
- 6. Anderson KK, Norman R, MacDougall A, et al. Effectiveness of early psychosis intervention: comparison of service users and nonusers in population-based health administrative data. Am J Psychiatry. 2018;175(5):443–452. [DOI] [PubMed] [Google Scholar]
- 7. Ontario Ministry of Health and Long-term Care. Health Insurance Act , R.S.O. 1990, c. H.6. Toronto, ON: Ontario Ministry of Health and Long-term Care; 2020. [Google Scholar]
- 8. Kurdyak P, Lin E, Green D, Vigod S. Validation of a population-based algorithm to detect chronic psychotic illness. Can J Psychiatry. 2015;60(8):362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Amin S, Singh SP, Brewin J, Jones PB, Medley I, Harrison G. Diagnostic stability of first-episode psychosis. Comparison of ICD-10 and DSM-III-R systems. Br J Psychiatry. 1999;175:537–543. [DOI] [PubMed] [Google Scholar]
- 10. Addington J, Chaves A, Addington D. Diagnostic stability over one year in first-episode psychosis. Schizophr Res. 2006;86(1–3):71–75. [DOI] [PubMed] [Google Scholar]
- 11. Bethell J, Rhodes AE. Identifying deliberate self-harm in emergency department data. Health Rep. 2009;20(2):35–42. [PubMed] [Google Scholar]
- 12. Ahmad OB, Boschi-Pinto C, Lopez AD, Murray CJ, Lozano R, Inoue M.. Age Standardization of Rates: A New WHO Standard. Geneva: World Health Organization; 2001. [Google Scholar]
- 13. Canadian Institute for Health Information. Making Sense of Health Indicators: Statistical Considerations. Ottawa, ON: CIHI; 2010. [Google Scholar]
- 14. Reininghaus U, Dutta R, Dazzan P, et al. Mortality in schizophrenia and other psychoses: a 10-year follow-up of the ӔSOP first-episode cohort. Schizophr Bull. 2015;41(3):664–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Doyle R, O’Keeffe D, Hannigan A, et al. The iHOPE-20 study: mortality in first episode psychosis-a 20-year follow-up of the Dublin first episode cohort. Soc Psychiatry Psychiatr Epidemiol. 2019;54(11):1337–1342. [DOI] [PubMed] [Google Scholar]
- 16. Correll CU, Solmi M, Veronese N, et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry. 2017;16(2):163–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nordentoft M, Madsen T, Fedyszyn I. Suicidal behavior and mortality in first-episode psychosis. J Nerv Ment Dis. 2015;203(5):387–392. [DOI] [PubMed] [Google Scholar]
- 18. Inagaki M, Kawashima Y, Kawanishi C, et al. Interventions to prevent repeat suicidal behavior in patients admitted to an emergency department for a suicide attempt: a meta-analysis. J Affect Disord. 2015;175:66–78. [DOI] [PubMed] [Google Scholar]
- 19. Miller IW, Camargo CA Jr, Arias SA, et al. Suicide prevention in an emergency department population: the ED-SAFE study. JAMA Psychiatry. 2017;74(6):563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anderson KK, Kurdyak P. Factors associated with timely physician follow-up after a first diagnosis of psychotic disorder. Can J Psychiatry. 2017;63(4):268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Addington D, Abidi S, Garcia-Ortega I, Honer WG, Ismail Z. Canadian guidelines for the assessment and diagnosis of patients with schizophrenia spectrum and other psychotic disorders. Can J Psychiatry. 2017;62(9):594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Correll CU, Rubio JM, Kane JM. What is the risk-benefit ratio of long-term antipsychotic treatment in people with schizophrenia? World Psychiatry. 2018;17(2):149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Correll CU, Galling B, Pawar A, et al. Comparison of early intervention services vs treatment as usual for early-phase psychosis: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2018;75(6):555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Strålin P, Hetta J. Medication, hospitalizations and mortality in 5 years after first-episode psychosis in a Swedish nation-wide cohort. Early Interv Psychiatry. 2019;13(4):902–907. [DOI] [PubMed] [Google Scholar]
- 25. Vermeulen JM, van Rooijen G, van de Kerkhof MPJ, Sutterland AL, Correll CU, de Haan L. Clozapine and long-term mortality risk in patients with schizophrenia: a systematic review and meta-analysis of studies lasting 1.1-12.5 years. Schizophr Bull. 2019;45(2):315–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274–280. [DOI] [PubMed] [Google Scholar]
- 27. Taipale H, Tanskanen A, Mehtälä J, Vattulainen P, Correll CU, Tiihonen J. 20-year follow-up study of physical morbidity and mortality in relationship to antipsychotic treatment in a nationwide cohort of 62,250 patients with schizophrenia (FIN20). World Psychiatry. 2020;19(1):61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Malla A, Iyer S, McGorry P, et al. From early intervention in psychosis to youth mental health reform: a review of the evolution and transformation of mental health services for young people. Soc Psychiatry Psychiatr Epidemiol. 2016;51(3):319–326. [DOI] [PubMed] [Google Scholar]
- 29. Dixon LB, Goldman HH, Srihari VH, Kane JM. Transforming the treatment of schizophrenia in the United States: the RAISE initiative. Annu Rev Clin Psychol. 2018;14:237–258. [DOI] [PMC free article] [PubMed] [Google Scholar]