Abstract
An association between antipsychotic drugs and pneumonia has been demonstrated in several studies; however, the risk for pneumonia caused by specific antipsychotics has not been extensively studied. The underlying mechanism is still unknown, and several receptor mechanisms have been proposed. Therefore, using a combined pharmacovigilance-pharmacodynamic approach, we aimed to investigate safety signals of US Food and Drug Administration (FDA)-approved antipsychotics for reporting pneumonia and the potential receptor mechanisms involved. A disproportionality analysis was performed to detect a signal for reporting “infective-pneumonia” and “pneumonia-aspiration” and antipsychotics using reports submitted between 2004 and 2019 to the FDA adverse events spontaneous reporting system (FAERS) database. Disproportionality was estimated using the crude and the adjusted reporting odds ratio (aROR) and its 95% confidence interval (CI) in a multivariable logistic regression. Linear regressions investigated the relationship between aROR and receptor occupancy, which was estimated using in vitro receptor-binding profiles. Safety signals for reporting infective-pneumonia were identified for clozapine (LL = 95% 3.4, n = 546 [aROR: 4.8]) as well as olanzapine (LL = 95% 1.5, n = 250 [aROR: 2.1]) compared with haloperidol, while aRORs were associated with higher occupancies of muscarinic receptors (beta = .125, P-value = .016), yet other anti-muscarinic drugs were not included as potential confounders. No safety signals for reporting pneumonia-aspiration were detected for individual antipsychotics. Multiple antipsychotic use was associated with both reporting infective-pneumonia (LL 95%: 1.1, n = 369 [aROR:1.2]) and pneumonia-aspiration (LL 95%: 1.7, n = 194 [aROR: 2.0]). Considering the limitations of disproportionality analysis, further pharmacovigilance data and clinical causality assessment are needed to validate this safety signal.
Keywords: antipsychotics, pneumonia, disproportionality, FAERS, safety signal
Introduction
Antipsychotic drugs are widely prescribed to treat schizophrenic disorders, bipolar disorder, major depression, as well as other psychiatric conditions. They are usually prescribed chronically, especially in adult and elderly populations, and their use is not rarely off-label. Thus, their safety profile should be monitored carefully especially considering their association with higher incidences of serious physical disorders that might arise from acutely occurring side effects, such as thromboembolisms, diabetic ketoacidosis, cardiac arrhythmias, liver injuries, seizures, and pneumonia.1 In 2005, the US Food and Drug Administration (FDA) issued a warning for atypical antipsychotics, which was extended in 2008 to conventional antipsychotics as well, because of their association with an increased risk of mortality in elderly patients off-label treated for dementia-related behavioral symptoms. In the reported cases, pneumonia was among the most common causes of death.2,3 Many observational studies, registries, randomized control trials, and pharmacovigilance database analyses also suggest the association between antipsychotic use and pneumonia.4–6 The underlying mechanism of antipsychotic-associated pneumonia is still unknown, and several receptor mechanisms have been proposed, eg, dopaminergic and histaminergic as well as muscarinic receptors.7 However, most of the studies examining the safety profile lump antipsychotics or use outdated classification systems, such as typical/atypical, which do not reflect precisely the receptor-binding profile.8,9 Therefore, there are sparse data on the risk of individual antipsychotics and the potential receptor mechanisms of antipsychotic-associated pneumonia.7
Nationwide registries such as the Taiwanese10,11 and Danish12 registries have been used in case control and self-controlled studies to highlight a possible association. The registries studies have strengths and limitations. In the Taiwan studies, the data originated from nationwide claim databases and the investigators attempted to explore temporal relationships and dose-dependent associations to promote better understanding of the results while the case-control design (matched for age, sex, first psychiatric admission) accounted for some control for confounding factors. However, there were also several limitations, including generalization of finding outside the country of origin, in different age groups, indications, as well as non-hospitalized patients. The Danish nationwide study focused on eliminating possible confounding by using a self-controlled design; however, several confounding factors including patients’ lifestyle and residual confounding were still unaccounted, while small clozapine user representation might have affected the final result. The results of 2 studies using disproportionality analysis of the VigiBase, the World Health Organization’s Pharmacovigilance Database, have also been published recently.13,14 They focused on the clozapine-associated pneumonia and mortality using the standard analysis of VigiBase, the statistical shrinkage to observed-to-expected ratios, which may have desirable properties for the detecting patterns in large-scale pharmacovigilance data.15 However, they analyzed only the most frequently used second-generation antipsychotics, ie, clozapine, risperidone, olanzapine, quetiapine, in contrast to our analysis (all FDA-approved antipsychotics with available data).
In the present study, we aim to enhance the current understanding of individual antipsychotics safety by using a previously published methodology with a combined pharmacovigilance-pharmacodynamic approach.16–18 Although the disproportionality analysis of the FDA Adverse Event Reporting System (FAERS) has certain inherent limitations of spontaneous reporting system databases such as underreporting, indication bias, and missing data of reports, exploring signals using disproportionality measures remains a valuable tool for early signal detection in post-marketing drug safety surveillance. In the present study, we investigated the association between different antipsychotics and pneumonia using a single comparator to allow for an easy classification of antipsychotics regardless of class in association with their risk for reporting pneumonia. This method is an easy to comprehend approach for clinicians who need to incorporate the study results in everyday decision-making since haloperidol is a familiar compound. Additionally, there are not many studies investigating whether polypharmacy of antipsychotics is associated with an increased risk for reporting pneumonia. Thus, the aim of the present study was to explore: (1) whether a variation between individual antipsychotics and reporting of pneumonia is present by detecting safety signals for individual antipsychotics and pneumonia in the FDA pharmacovigilance database, (2) whether polypharmacy of antipsychotics is associated with an increased risk for reporting pneumonia, and (3) the association between the risk for reporting pneumonia and neurotransmitter receptor occupancy.
Methods
The FAERS is a pharmacovigilance database containing reports on suspected adverse drug reactions in individual patients originated mainly but not exclusively from the United States (supplementary e-table 4).19The OpenVigil2.1-MedDRA interface,20 a tool that operates on cleaned FDA data (verified and normalized drug names) and incorporates the MedDRA terminology,21 was used to retrieve reports submitted between the first quarter of 2004 and July 2019 (period available via OpenVigil2.1-MedDRA). We included reports with at least 1 of the 21 FDA-approved antipsychotics (ATC N05A* excluding lithium). Only reports concerning adults of at least 18 years were included. Reports with missing data concerning the age, sex, reporting year, reporting country, or drug name were excluded. Reports were originally sorted based on their unique Individual Safety Report and further screened for overlapping case ID numbers, ie, different versions of the same report resubmitted and creating duplications in the database. Reports containing only non-FDA approved antipsychotics were excluded due to possible under-reporting, while droperidol (N05AD08), prochlorperazine (N05AB04), and pimavanserin (N05AX17) were not included to avoid indication bias.
Study Design
A case-non-case design was used on the pharmacovigilance data extracted from the FAERS database. Cases were the reports with an adverse event included in the narrow scope of the standardized MedDRA query (SMQ) “infective-pneumonia.” 21 The MedDRA preferred term (PT) “pneumonia-aspiration,” a term not included in the former SMQ, was selected as a secondary case definition since aspiration is an important cause of pneumonia especially in the elderly.22 All other events were defined as non-cases. To be included in the analysis, an antipsychotic had to have at least 100 unique reports in FAERS with at least 1 pneumonia-related report. As a result, molindone (N05AE02) was excluded. The final sample consisted of reports of 20 antipsychotics (aripiprazole, asenapine, brexpiprazole, cariprazine, chlorpromazine, clozapine, fluphenazine, haloperidol, iloperidone, loxapine, lurasidone, olanzapine, paliperidone, perphenazine, pimozide, quetiapine, risperidone, thiothixene, trifluoperazine, and ziprasidone).
Definition of Exposure and Receptor Occupancy
Reports including one antipsychotic were classified as reports with single antipsychotic use, ie, patients receiving a single active substance classified under ATC N05A while reports including more than one active substance in the N05A ATC group were classified as multiple antipsychotic use. In the analysis of single antipsychotic use, we compared each individual antipsychotic with haloperidol, which is a frequently used active comparator of antipsychotic drugs. Drug-receptor interactions for 10 receptors (serotonin receptors: 5-HT1A, 5-HT2A, 5-HT2C, 5-HT7; adrenergic receptor alpha1/2 (regardless of subtype); muscarinic receptors (regardless of subtype); dopamine receptors D2, D3; and histamine receptor H1) and different individual antipsychotics were quantified using the receptor occupancy theory. Occupancy (%) was estimated using the equation , where CU (nM) is the unbound drug concentration in blood and Ki (nM) is the inhibitory constant for each drug.23 The CU was calculated using the equation , where FU is the unbound drug fraction, CT (ng/ml) the drug concentration in blood, and MW the molecular weight. To estimate the total drug concentration in blood CT, we used the upper limit of the therapeutic reference range of each antipsychotic reported24 or due to data unavailability, an observational study for thiothixene.25 The MW of antipsychotics was extracted from International Union of Basic and Clinical Pharmacology (IUPHAR) database and the unbound drug fraction (FU) from Drugbank,26 or when data were not available in the review of Lombardo et al.27 The in vitro Ki for the aforementioned human receptors was extracted from psychoactive drug screening program database,25 and when not available, from IUPHAR/British Pharmacological Society.26The median was calculated when more than one values were available for a receptor (supplementary e-table 2).
Potential Confounding Factors
We investigated the following potential confounding factors using multivariable logistic regression: age, sex, reporting year, reporting country, concomitant antibiotic drugs, and other drugs associated with pneumonia (corticosteroids, immune-suppressants, benzodiazepines and benzodiazepine-related drugs, acid-suppressive drugs, and drugs with potential extrapyramidal symptoms) (supplementary e-table 1).28–31 We also investigated the co-reporting of agranulocytosis, defined as at least an adverse event included in the SMQ “agranulocytosis,” in a post hoc subgroup analysis.
Data Analysis
Report characteristics between cases and non-cases were compared with a Mann-Whitney U test for not normally distributed continuous variables (age and reporting year) and with a chi-square test for categorical variables (sex, use of antibiotics, use of pneumonia-related drugs, and reporter country). A disproportionality analysis was performed to detect a signal for reporting “infective-pneumonia” or “pneumonia-aspiration” and antipsychotics. Disproportionality was estimated using the crude and the adjusted reporting odds ratio (aROR) and its 95% confidence intervals (CIs) in a multivariable logistic regression. The aROR was defined as the odds of pneumonia-related reports with the drug exposure (individual antipsychotics or multiple antipsychotic use) divided by the odds of the reference (haloperidol or single antipsychotic use, respectively) adjusted for predefined potential confounders.20 The primary multivariable logistic regression model was conducted for each antipsychotic drug compared with haloperidol or multiple compared with single antipsychotics as case (infective/aspiration pneumonia or not) ~ antipsychotic (investigated antipsychotic vs haloperidol or multiple vs single antipsychotic) + sex (male vs female) + age (in years) + country (United States or not United States) + reporting year (in years) + co-reporting of antibiotics drugs (yes or not) + other co-reporting drugs associated with pneumonia (yes or not). A positive disproportionality signal was identified when more than 3 reports (n) were detected and the lower confidence limit of the 95% two-sided CI of the aROR (LCL 95%) was greater that one.19
The robustness of the results for the primary case definition “infective-pneumonia” was tested with post hoc subgroup analyses for age groups (=<65 vs >65 years old), reporting country (United States or not United States), and the co-reporting of agranulocytosis. Subgroup effects were investigated with a treatment-by-group interaction test added in the multivariable logistic regression of the primary analysis.
Furthermore, we conducted linear regression models to identify a potential association between infective-pneumonia and receptor occupancy. The natural logarithm of the point estimate of aROR (individual antipsychotics vs haloperidol) was the dependent variable and the natural logarithm of the estimated occupancy for a receptor the independent variable. Antipsychotics with 3 or less cases of pneumonia were excluded from this analysis.32
All analyses were performed using the base stats package and visualization using ggplot2 version 3.2.133 in R version 3.6.1.34 Alpha was set at 2-sided .05. Since disproportionality analyses are observational and exploratory (hypothesis-generating) study designs,35 adjustment to multiple testing was not conducted.36
Results
Descriptive Characteristics of Cases/Non-cases
From the 6 932 328 FAERS reports available via OpenVigil2.1 for the period from 2014 to July 2019, 119 019 unique reports of 20 FDA-approved antipsychotics were collected forming the final sample (supplementary e-figure 1). The most frequently reported antipsychotics in single use were quetiapine (26.0%), clozapine (12.1%), aripiprazole (11.4%), olanzapine (11.5%), and risperidone (8.8%), while multiple antipsychotic use accounted for 18.9% of the final sample.
Cases, ie, “infective-pneumonia” and “pneumonia-aspiration,” were identified in 1858 (1.6%) and 694 (0.6%) reports, respectively. In comparison to non-cases, cases were older (mean age [SD] 54.1 [17.6] vs 46.4 [16.8]), more frequently males (55% vs 47% males), originated more frequently outside the United States (58% vs 43%), as well as concomitant use of pneumonia-related drugs (43% vs 32%) and antibiotics (4% vs 2%). No difference was detected in the reporting year (Table 1).
Table 1.
Population Characteristics of Cases and Non-cases
| Cases (n = 1858 “infective-pneumonia” and 694 “pneumonia-aspiration”) | Non-cases (n = 116 474) | P-value | |
|---|---|---|---|
| Age | |||
| Mean (SD) | 54.1 (17.6) | 46.4 (16.8) | <.001 |
| Median | 54 | 45 | |
| Sex | |||
| Female (%) | 1101 (45) | 62 312 (53) | <.001 |
| Reporting country | |||
| United States (%) | 1030 (42) | 66 468 (57) | <.001 |
| Reporting year | |||
| Mean (SD) | 2012.4 (4.1) | 2012.5 (3.9) | .9 |
| Median | 2012 | 2012 | |
| Concomitant drugs | |||
| Pneumonia-related drugs (%) | 1028 (42) | 37 597 (32) | <.001 |
| Antibiotics (%) | 97 (4) | 2283 (2) | <.001 |
Note: P-values from Mann-Whitney U test for continuous (age and reporting year) and chi-squared for the categorical variables (sex and reporting country).
Disproportionality Analysis
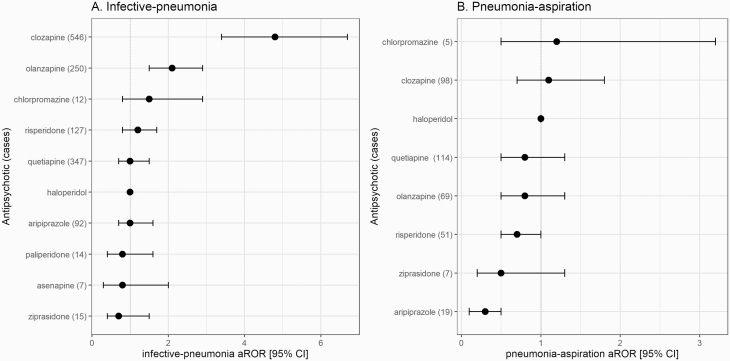
Among reports with single antipsychotic use, a signal for reporting “infective-pneumonia” was identified for clozapine (LL 95%: 3.4, n = 546, aROR: 4.8) and olanzapine (LL 95%: 1.5, n = 250, aROR: 2.1) compared with haloperidol (figure 1A). All other antipsychotics generated no signal and no signals were detected for “pneumonia-aspiration,” yet aripiprazole had a lower risk for reporting “pneumonia-apiration” than haloperidol (n = 19; aROR 0.3, 95% CI: 0.1–0.5) (figure 1B and supplementary e-table 3). Post hoc, we found no subgroup differences for “infective-pneumonia” between elderly and younger patients, US and not US reports, as well as reports with or without agranulocytosis, apart for a lower risk of aripiprazole in US reports (supplementary e-table 4). Multiple antipsychotic use was also associated with reporting “infective-pneumonia” (LL 95%: 1.1, n = 369, aROR:1.2) and also with “pneumonia-aspiration” (LL 95%: 1.7, n = 194, aROR: 2.0) compared with single antipsychotic use.
Fig. 1.
Disproportionality analysis in the US Food and Drug Administration (FDA) adverse events spontaneous reporting system (FAERS) for the association between individual antipsychotics and (A) infective-pneumonia or (B) pneumonia-aspiration. The differential risk for reporting pneumonia of antipsychotics comparison to haloperidol (adjusted reporting odds ratio [aROR] = 1, 40 cases with infective pneumonia and 25 with pneumonia aspiration) was quantified as aROR, adjusted to age, sex, reporting country, reporting year, and concomitant medication use. Some investigated antipsychotics are not displayed since there were less than 3 cases.
Relationship Between Disproportionality for Reporting Infective Pneumonia and Receptor Occupancy
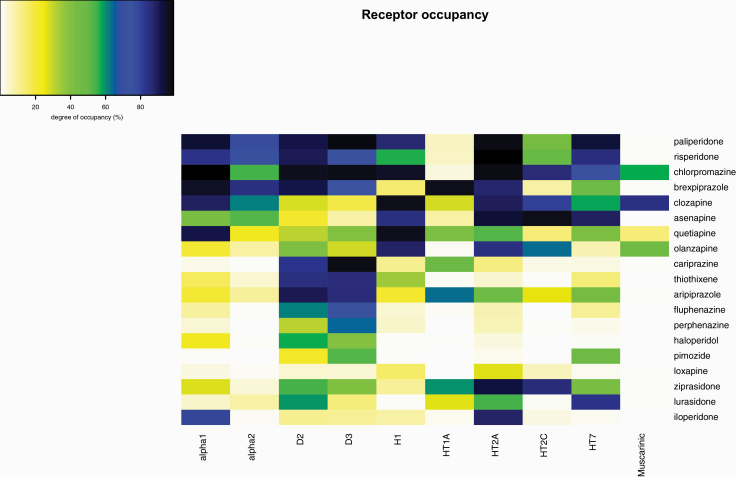
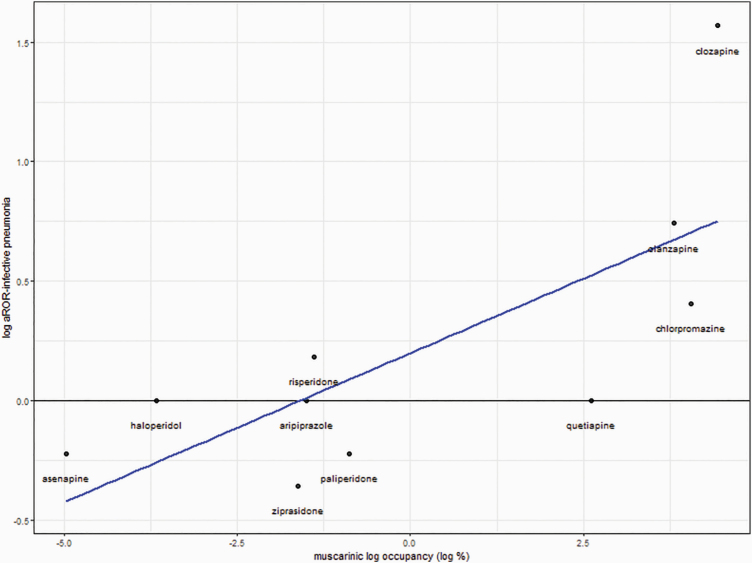
Due to limited data for trifluoperazine, receptor occupancy was calculated for 19 out of the 20 investigated antipsychotics (figure 2). Results of the linear regression models are reported in table 2 and scatter plots in figure 3 and supplementary e-figure 2. A significant relationship between muscarinic receptor occupancy and the risk for reporting “infective pneumonia” was detected (beta = .125, t-value = 3.04, P-value = .016). It seems that the results were mainly driven by olanzapine and clozapine (figure 3), and co-reporting of other antimuscarinic medication was not investigated as confounding factors. No significant relationships were found for the other receptors.
Fig. 2.
Occupancy of antipsychotics to neurotransmitter receptors. The occupancy of receptors was estimated using the receptor theory, the in vitro affinities to human receptors, and the maximum therapeutic reference range. Due to limited data, occupancies for trifluoperazine could not be estimated.
Table 2.
Linear Regression Models Between adjusted reporting odds ratio (aROR) and Receptor Occupancy
| Receptor | Beta | tValue | P-value | R 2 (%) |
|---|---|---|---|---|
| Adrenergic alpha1 | .180 | 0.631 | .546 | 4.74 |
| Adrenergic alpha2 | .085 | 0.720 | .492 | 7.09 |
| D2 | −.275 | −0.784 | .456 | 7.13 |
| D3 | −.168 | −0670 | .522 | 5.31 |
| H1 | .088 | 0.875 | .407 | 8.74 |
| 5-HT1A | −.017 | −0.164 | .874 | 0.34 |
| 5-HT2A | .084 | 0.444 | .669 | 2.40 |
| 5-HT2C | .048 | 0.538 | .605 | 3.50 |
| 5-HT7 | −.008 | −0.06 | .954 | 0.04 |
| Muscarinic | .125 | 3.04 | .016 | 53.6 |
Note: Beta represents the coefficient of the linear regression model using the natural logarithms of aROR as dependent and the natural logarithm of receptor occupancy as independent variables. The results were based on 9 data points each representing an antipsychotic drug.
Bold P-value 0.05 is significant result only for muscarinic receptor.
Fig. 3.
Linear regression model of the relationship between the natural logarithm of aROR for reporting infective-pneumonia and the natural logarithm of the occupancy on muscarinic receptors.
Discussion
The present pharmacovigilance-pharmacodynamic study investigated the risk for reporting pneumonia of individual antipsychotic drugs and its relationship with their pharmacodynamic profile. We have previously used this type of disproportionality analysis of the FAERS database to detect other safety signal of central nervous system acting drugs in combination with their pharmacodynamic profile.16Of the 19 investigated antipsychotics compared with haloperidol, safety signals were detected for clozapine and olanzapine-associated infective pneumonia, while no differences for pneumonia aspiration were identified. Additionally, multiple antipsychotic use compared with single use was associated with both reporting infective and aspiration pneumonia. A recently published umbrella review of 68 observational studies which quantified the risk of life-threatening medical events associated with exposure to antipsychotics found a strong association between antipsychotic exposure and risk of pneumonia (pooled OR: 1.84, 95% CI: 1.62–2.09).37 This association is also supported by a meta-analysis of randomized-controlled trials assessing somatic serious adverse events related to second-generation antipsychotic drugs which found that pneumonia and pneumonia aspiration were among the reasons of death with the highest absolute difference between second-generation antipsychotics and placebo and occurred mainly in older people.7
Data on individual antipsychotics are sparse and inconsistent,7 and associations have been suggested for clozapine,10–13 haloperidol,10,38 olanzapine,10,11,38 quetiapine,10,11 risperidone,10,12,39 and zotepine.10,11 The aforementioned studies implement different study designs including case-control, self-controlled, and disproportionality analysis in Vigibase and use different comparators which might explain the differences in findings. Our study aimed to investigate which antipsychotics might have a higher risk with reporting pneumonia rather to establish an association between antipsychotics and pneumonia, since haloperidol, a frequently used active comparator for antipsychotics, was used as a control. According to our results, clozapine and olanzapine had an increased risk for reporting infective-pneumonia compared with haloperidol. Therefore, additional vigilance might be required with these antipsychotics and especially with clozapine.40 Serious respiratory infections could decrease clozapine metabolism leading to increased clozapine levels and additional toxicity.40–42 This is consistent with studies on VigiBase that suggested pneumonia as a major cause of clozapine-associated mortality.13,14 Nevertheless, it should be noted that clozapine treatment compared with other antipsychotics might be associated with a lower overall mortality rate.43
Several receptor mechanisms have been proposed to explain antipsychotic-associated pneumonia. Based on our pharmacodynamic analysis, the acetylcholine muscarinic receptors were suggested as a potential receptor mechanism, yet other anti-muscarinic drugs, which are frequently used with for antipsychotic-induced extrapyramidal symptoms, were not accounted as confounding factors. Indeed, anticholinergic medications have been previously associated with an increased risk for pneumonia in elderly.44 In our analysis, the association might have been driven by clozapine and olanzapine, which seem to act as partial agonists/allosteric modulators on these receptors in comparison to other antipsychotics.45 Cholinergic effects could lead among others to sedation, dry mouth or hypersalivation (especially with clozapine), esophageal dilatation, and gastrointestinal hypomotility.7,46,47 Due to the small statistical power of linear regressions, other receptor mechanisms cannot be excluded, eg, antidopaminergic (eg, extrapyramidal symptoms), antiserotonergic, and antihistaminergic effects (eg, sedation).7
The exact pathways from receptors to pneumonia are still unclear. For example, antipsychotic-induced sedation could lead to falls and subsequently to fractures, hospitalization and pneumonia,48 or antipsychotic-induced sedation, hypersalivation, and extrapyramidal symptoms could lead to dysphagia, aspiration, and pneumonia.47 Except for a lower risk for the dopamine partial agonist aripiprazole, there were no differences among individual antipsychotics for reporting pneumonia-aspiration. Aripiprazole is usually selected for less severe and more autonomous participants, who might have a lower risk for pneumonia, yet a more benign profile of this antipsychotic cannot be excluded.49 Additionally, multiple antipsychotic use was associated with a higher risk, similarly to prior studies that used the Taiwanese claims database.11 This could suggest a multifactorial mechanism and synergistic effects, which warrant further investigation. In addition, antipsychotics might directly increase the risk for pneumonia, such as via downstream effects of thromboxane A2 receptor (TBXA2R) and platelet-activating receptor (PTAFR) on the alveolar capillary unit.9 Neurotransmitter receptors are also expressed in immune cells50 and antipsychotic drugs could alter immune responses.51 Clozapine is well known for inducing agranulocytosis, yet we did not find an effect of agranulocytosis in the risk of reporting “infective-pneumonia” (supplementary e-table 4). Nevertheless, clozapine has been recently associated with antibody deficiency, which could also lead to infections.52,53 The potential mechanism of olanzapine-associated pneumonia has not been widely investigated in the literature. A possible suggestion is that olanzapine and clozapine share a similar chemical structure and thus a similar pharmacodynamic profile.54
This study has certain limitations inherent to pharmacovigilance databases. First, we used a multivariable logistic regression to account for potential confounding factors and quantify the disproportionality signals, a method frequently used in case/non-case studies.16,17,55–57 However, there is no gold standard measure of disproportionality and other methods have been suggested, eg, the Bayesian shrinkage to observed-expected ratios.13–15 Nevertheless, it has been suggested that measures of disproportionality are comparable when 4 or more cases are available,58 which was the cutoff used to identify disproportionality signals in our study. Second, we aimed to investigate a long list of reported potential confounding factors, yet residual confounding cannot be excluded. For example, indication bias could have confounded the safety signal of clozapine for “infective-pneumonia,” the only antipsychotic indicated for treatment-resistant schizophrenia. Generally, patients treated with clozapine have more severe symptoms compared with patients taking other antipsychotics, a baseline difference that should be taken into consideration when comparing clozapine to other antipsychotic drugs. A nationwide self-controlled study using the Danish Registries tried to accommodate for this difference by using a mirror image design. Clozapine presented the greatest increase in number of pneumonia cases (0.64%) after its initiation; however, due to the limited size of the sample, the association was not statistically significant. This study also highlighted that patients treated with clozapine were more frequently receiving early retirement and leaving in solidarity.12 Accordingly, a study suggested that the association between pneumonia and antipsychotics might be confounded by physical comorbidities, poor functioning, and the severity of symptoms,59 factors that are not reported in pharmacovigilance databases. In a similar vein, aripiprazole may be used in less severe patients with a lower risk of pneumonia.49 Third, underreporting is expected in pharmacovigilance databases, yet reporting rates may be similar in drugs of the same therapeutic class.60–63 Therefore, using haloperidol as reference, a frequently used reference of antipsychotic drugs, may have reduced the effects of differential reporting rates in comparison to using all drugs in the database as reference.64 Reporting rates may also vary depending on the reporter, the drug, the adverse event, or time, thus resulting in selective reporting with adverse drug reactions of high perceived severity more likely being reported. The term notoriety effect refers to an increase in reporting a specific drug-event pair after media coverage, yet it is argued that important notoriety bias is not present in FAERS.65,66 When using retrospective data, it is not always possible to identify these events that might shift the reporting pattern.60,64 Fourth, data quality may also be an issue with missing, incorrect, or vague information; duplicate reports; and reported event being due to treated condition, another condition, or another drug being common in pharmacovigilance databases.63,67,68Furthermore, since the collected reports date as far back as Q4/2003, several parameters such as changes in reporting requirements, coding dictionaries for products and/or events, data entry and coding processes, inconsistent database structure architecture, and malicious reporting and spam must be considered.68 Fifth, a limitation regarding our pharmacodynamic approach is that in vitro affinities and the estimated occupancy do not always reflect the functional effects of the drug and also that total drug concentrations in blood were used to estimate CU, since data on unbound cerebrospinal fluid concentrations of antipsychotics are generally unavailable.69 We also cannot exclude a potential residual confounding effect of the co-treatment with anti-muscarinic medications, since the analysis was not adjusted for them. Finally, the results of our disproportionality analysis were not adjusted to multiple testing and they should be only considered as hypothesis generating, and confirmation in large cohort and case-control studies is needed.
Despite their limitations, the spontaneous reporting system and disproportionality analysis are extremely valuable tools for safety monitoring. The strength of this study is the inclusion of numerous reports with real-world antipsychotic drug use data (ie, 119 019) investigating not only hospitalized patients but also outpatients, the attempt to work with individual antipsychotics rather than with antipsychotic groups as well as the investigation of possible increased risk for reporting pneumonia when multiple antipsychotics are used. Additionally, we reviewed available pharmacodynamic sources to map the pharmacodynamic profile of the antipsychotics in order to correlate the risk for reporting pneumonia with occupancy on neurotransmitter receptors. This type of pharmacovigilance-pharmacodynamic direct combination is a relatively new proposed method to study receptor mechanisms of adverse events in safety reports databases.12
In conclusion, our results suggest a safety signal of clozapine and olanzapine for reporting infective pneumonia and the muscarinic receptors as a potential mechanism of this association. Multiple antipsychotic use was also associated with an increased risk for reporting both infective and aspiration pneumonia. Even though our study design does not allow any causality proof and an appropriate clinically performed causality assessment is needed to validate our results, it is a step toward understanding the safety profile of antipsychotic drugs and optimizing their use among individual patients.
Supplementary Material
Acknowledgments
None. SL has received honoraria as a consultant/advisor and/or for lectures from Angelini, Böhringer Ingelheim, Geodon & Richter, Janssen, Johnson & Johnson, Lundbeck, LTS Lohmann, MSD, Otsuka, Recordati, SanofiAventis, Sandoz, Sunovion, TEVA. The rest of the authors declare no conflicts of interest.
References
- 1. Correll CU, Detraux J, De Lepeleire J, De Hert M. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry. 2015;14(2):119–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Center for Drug Evaluation and Research. Postmarket drug safety information for patients and providers – Information on conventional antipsychotics. 2008. https://wayback.archive-it.org/7993/20171101120041/https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm107211.htm. Accessed December 25, 2019. [Google Scholar]
- 3. Center for Drug Evaluation and Research. Postmarket drug safety information for patients and providers – Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. 2005. https://wayback.archive-it.org/7993/20170113112252/http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm053171.htm. Accessed December 25, 2019. [Google Scholar]
- 4. Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146(11):775–786. [DOI] [PubMed] [Google Scholar]
- 5. Pratt N, Roughead EE, Salter A, Ryan P. Choice of observational study design impacts on measurement of antipsychotic risks in the elderly: a systematic review. BMC Med Res Methodol. 2012;12:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schneeweiss S, Setoguchi S, Brookhart A, Dormuth C, Wang PS. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ. 2007;176(5):627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dzahini O, Singh N, Taylor D, Haddad PM. Antipsychotic drug use and pneumonia: systematic review and meta-analysis. J Psychopharmacol. 2018;32(11):1167–1181. [DOI] [PubMed] [Google Scholar]
- 8. Siafis S, Davis JM, Leucht S. Antipsychotic drugs: from “major tranquilizers” to neuroscience-based-nomenclature. Psychol Med. 2019;84(10):2405–2414. [DOI] [PubMed] [Google Scholar]
- 9. Sultana J, Calabró M, Garcia-Serna R, et al. Biological substantiation of antipsychotic-associated pneumonia: systematic literature review and computational analyses. PLoS One. 2017;12(10):e0187034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang SY, Liao YT, Liu HC, Chen WJ, Chen CC, Kuo CJ. Antipsychotic drugs, mood stabilizers, and risk of pneumonia in bipolar disorder. J Clin Psychiatry.. 2013;74(01):e79–e86. [DOI] [PubMed] [Google Scholar]
- 11. Kuo CJJ, Yang SYY, Liao YTT, et al. Second-generation antipsychotic medications and risk of pneumonia in schizophrenia. Schizophr Bull. 2013;39(3):648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rohde C, Siskind D, de Leon J, Nielsen J.. Antipsychotic medication exposure, clozapine, and pneumonia: results from a self-controlled study. Acta Psychiatr Scand. 2019;142:78–86. [DOI] [PubMed] [Google Scholar]
- 13. De Leon J, Sanz EJ, De Las Cuevas C. Data from the World Health Organization’s pharmacovigilance database supports the prominent role of pneumonia in mortality associated with clozapine adverse drug reactions. Schizophr Bull. 2020;46(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Leon J, Sanz EJ, Norén GN, De Las Cuevas C. Pneumonia may be more frequent and have more fatal outcomes with clozapine than with other second-generation antipsychotics. World Psychiatry. 2020;19(1):120–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Norén GN, Hopstadius J, Bate A. Shrinkage observed-to-expected ratios for robust and transparent large-scale pattern discovery. Stat Methods Med Res. 2013;22(1):57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Siafis S, Papazisis G. Detecting a potential safety signal of antidepressants and type 2 diabetes: a pharmacovigilance-pharmacodynamic study. Br J Clin Pharmacol. 2018;84(10):2405–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Montastruc F, Palmaro A, Bagheri H, Schmitt L, Montastruc JL, Lapeyre-Mestre M. Role of serotonin 5-HT 2C and histamine H 1 receptors in antipsychotic-induced diabetes: a pharmacoepidemiological-pharmacodynamic study in VigiBase. Eur Neuropsychopharmacol. 2015;25(10):1556–1565. [DOI] [PubMed] [Google Scholar]
- 18. Lapeyre-Mestre M, Montastruc F. Interest of pharmacoepidemiology for pharmacodynamics and analysis of the mechanism of action of drugs. Therapie. 2019;74(2):209–214. [DOI] [PubMed] [Google Scholar]
- 19. Sakaeda T, Tamon A, Kadoyama K, Okuno Y. Data mining of the public version of the FDA adverse event reporting system. Int J Med Sci. 2013;10(7):796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Böhm R, von Hehn L, Herdegen T, et al. OpenVigil FDA – inspection of U.S. American adverse drug events pharmacovigilance data and novel clinical applications. PLoS One. 2016;11(6):e0157753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. MedDRA Maintenance and Support Services Organization. Introductory guide to MedDRA version 22.0. McLean, Virginia. 2019. http://www.meddra.org/how-to-use/support-documentation. [Google Scholar]
- 22. van der Maarel-Wierink CD, Vanobbergen JN, Bronkhorst EM, Schols JM, de Baat C. Risk factors for aspiration pneumonia in frail older people: a systematic literature review. J Am Med Dir Assoc. 2011;12(5):344–354. [DOI] [PubMed] [Google Scholar]
- 23. Kenakin T. Principles: receptor theory in pharmacology. Trends Pharmacol Sci. 2004;25(4):186–192. [DOI] [PubMed] [Google Scholar]
- 24. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9–62. [DOI] [PubMed] [Google Scholar]
- 25. Roth BL, Lopez E, Patel S, Kroeze WK. The multiplicity of serotonin receptors: uselessly diverse molecules or an embarrassment of riches? Neuroscientist. 2000;6(4):252–262. [Google Scholar]
- 26. Harding SD, Sharman JL, Faccenda E, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res.. 2018;46(D1):D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lombardo F, Berellini G, Obach RS. Trend analysis of a database of intravenous pharmacokinetic parameters in humans for 1352 drug compounds. Drug Metab Dispos. 2018;46(11):1466–1477. [DOI] [PubMed] [Google Scholar]
- 28. Ryan P, Ramsay E, Pratt N, Salter A, Roughead EE. Risk of hospitalization for hip fracture and pneumonia associated with antipsychotic prescribing in the elderly: a self-controlled case-series analysis in an Australian health care claims database. Drug Saf. 2011;34(7):567–575. [DOI] [PubMed] [Google Scholar]
- 29. Egberts ACG, Schobben AFAM, Jansen PAF, Knol W, Souverein PC, Van Marum RJ. Antipsychotic drug use and risk of pneumonia in elderly people. J Am Geriatr Soc. 2008;56(4):661–666. [DOI] [PubMed] [Google Scholar]
- 30. Sun G, Zhang L, Zhang L, Wu Z, Hu D. Benzodiazepines or related drugs and risk of pneumonia: a systematic review and meta-analysis. Int J Geriatr Psychiatry. 2019;34(4):513–521. [DOI] [PubMed] [Google Scholar]
- 31. Shin HW, Chung SJ. Drug-induced Parkinsonism. J Clin Neurol. 2012;8(1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nguyen TTH, Pariente A, Montastruc JL, et al. An original pharmacoepidemiological–pharmacodynamic method: application to antipsychotic-induced movement disorders. Br J Clin Pharmacol.. 2017;83(3):612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wickham H. Ggplot2: Elegant Graphics for Data Analysis. 2009. New York, NY: Springer. [Google Scholar]
- 34. R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. 2018. https://www.R-project.org/. [Google Scholar]
- 35. Montastruc JL, Sommet A, Bagheri H, Lapeyre-Mestre M. Benefits and strengths of the disproportionality analysis for identification of adverse drug reactions in a pharmacovigilance database. Br J Clin Pharmacol. 2011;72(6):905–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990;1(1):43–46. [PubMed] [Google Scholar]
- 37. Papola D, Ostuzzi G, Gastaldon C, et al. Antipsychotic use and risk of life-threatening medical events: umbrella review of observational studies. Acta Psychiatr Scand. 2019;140(3):227–243. [DOI] [PubMed] [Google Scholar]
- 38. Trifiro G, Gambassi G, Sen EF, Caputi AP, Bagnardi V, Brea J. Association of community-acquired pneumonia with antipsychotic drug use in elderly patients. Ann Intern Med. 2010;152(7):418–425. [DOI] [PubMed] [Google Scholar]
- 39. Gambassi G, Sultana J, Trifirò G. Antipsychotic use in elderly patients and the risk of pneumonia. Expert Opin Drug Saf. 2014;14(1):1–6. [DOI] [PubMed] [Google Scholar]
- 40. de Leon J, Ruan CJ, Schoretsanitis G, Kane JM. Dose and safety concerns of clozapine: worldwide package inserts need revisions. Schizophr Res. 2020;216:2–4. [DOI] [PubMed] [Google Scholar]
- 41. De Leon J, Diaz FJ. Serious respiratory infections can increase clozapine levels and contribute to side effects: a case report. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(6):1059–1063. [DOI] [PubMed] [Google Scholar]
- 42. De Leon J, Ruan CJ, Verdoux H, Wang C. Clozapine is strongly associated with the risk of pneumonia and inflammation. Gen Psychiatr. 2020;33(2):e100183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vermeulen JM, Van Rooijen G, Van De Kerkhof MPJ, Sutterland AL, Correll CU, De Haan L. Clozapine and long-term mortality risk in patients with schizophrenia: a systematic review and meta-analysis of studies lasting 1.1–12.5 years. Schizophr Bull. 2019;45(2):315–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chatterjee S, Carnahan RM, Chen H, Holmes HM, Johnson ML, Aparasu RR. Anticholinergic medication use and risk of pneumonia in elderly adults: a nested case-control study. J Am Geriatr Soc. 2016;64(2):394–400. [DOI] [PubMed] [Google Scholar]
- 45. Siafis S, Tzachanis D, Samara M, Papazisis G. Antipsychotic drugs: from receptor-binding profiles to metabolic side effects. Curr Neuropharmacol. 2018;16(8):1210–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Olten B, Bloch MH. Meta regression: relationship between antipsychotic receptor binding profiles and side-effects. Prog Neuropsychopharmacol Biol Psychiatry. 2018;84(Pt A):272–281. [DOI] [PubMed] [Google Scholar]
- 47. Cicala G, Barbieri MA, Spina E, de Leon J. A comprehensive review of swallowing difficulties and dysphagia associated with antipsychotics in adults. Expert Rev Clin Pharmacol. 2019;12:1–16. [DOI] [PubMed] [Google Scholar]
- 48. Schneider-Thoma J, Efthimiou O, Bighelli I, et al. Second-generation antipsychotic drugs and short-term somatic serious adverse events: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6(9):753–765. [DOI] [PubMed] [Google Scholar]
- 49. Correll CU, Cañas F, Larmo I, et al. Individualizing antipsychotic treatment selection in schizophrenia: characteristics of empirically derived patient subgroups. Eur Psychiatry.. 2011;26 ( Suppl 1):3–16. [DOI] [PubMed] [Google Scholar]
- 50. Franco R, Pacheco R, Lluis C, Ahern GP, O’Connell PJ. The emergence of neurotransmitters as immune modulators. Trends Immunol. 2007;28(9):400–407. [DOI] [PubMed] [Google Scholar]
- 51. Muller N, Schwarz MJ. The role of immune system in schizophrenia. Curr Immunol Rev. 2010;6(3):213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ponsford M, Castle D, Tahir T, et al. Clozapine is associated with secondary antibody deficiency. Br J Psychiatry. 2018;214(2):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ponsford MJ, Steven R, Bramhall K, et al. Clinical and laboratory characteristics of clozapine-treated patients with schizophrenia referred to a national immunodeficiency clinic reveals a B-cell signature resembling common variable immunodeficiency (CVID). J Clin Pathol. 2020;73(9):587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Flanagan RJ, Dunk L. Haematological toxicity of drugs used in psychiatry. Hum Psychopharmacol. 2008;23 Suppl 1:27–41. [DOI] [PubMed] [Google Scholar]
- 55. Revet A, Montastruc F, Roussin A, Raynaud JP, Lapeyre-Mestre M, Nguyen TTH. Antidepressants and movement disorders: a postmarketing study in the world pharmacovigilance database. BMC Psychiatry. 2020;20(1):308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Minnema LA, Giezen TJ, Souverein PC, Egberts TCG, Leufkens HGM, Gardarsdottir H. Exploring the association between monoclonal antibodies and depression and suicidal ideation and behavior: a VigiBase study. Drug Saf. 2019;42(7):887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chrétien B, Lelong-Boulouard V, Chantepie S, et al. Haematologic malignancies associated with clozapine v. all other antipsychotic agents: a pharmacovigilance study in VigiBase®. Psychol Med. 2020;10:1–8. doi: 10.1017/S0033291720000161. PMID: 32036793. [DOI] [PubMed] [Google Scholar]
- 58. Van Puijenbroek EP, Bate A, Leufkens HGM, Lindquist M, Orre R, Egberts ACG. A comparison of measures of disproportionality for signal detection is spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf.. 2002;11(1):3–10. [DOI] [PubMed] [Google Scholar]
- 59. Chan HY, Lai CL, Lin YC, Hsu CC. Is antipsychotic treatment associated with risk of pneumonia in people with serious mental illness? The roles of severity of psychiatric symptoms and global functioning. J Clin Psychopharmacol. 2019;39(5):434–440. [DOI] [PubMed] [Google Scholar]
- 60. Alatawi YM, Hansen RA. Empirical estimation of under-reporting in the U.S. Food and Drug Administration Adverse Event Reporting System (FAERS). Expert Opin Drug Saf. 2017;16(7):761–767. [DOI] [PubMed] [Google Scholar]
- 61. Hazell L, Shakir SAW. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29(5):385–396. [DOI] [PubMed] [Google Scholar]
- 62. Pierfitte C, Bégaud B, Lagnaoui R, Moore ND. Is reporting rate a good predictor of risks associated with drugs? Br J Clin Pharmacol. 1999;47(3):329–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bate A, Evans SJ. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. 2009;18(6):427–436. [DOI] [PubMed] [Google Scholar]
- 64. Faillie JL. Case-non-case studies: principle, methods, bias and interpretation. Therapie. 2019;74(2):225–232. [DOI] [PubMed] [Google Scholar]
- 65. de Boissieu P, Kanagaratnam L, Abou Taam M, Roux MP, Dramé M, Trenque T. Notoriety bias in a database of spontaneous reports: the example of osteonecrosis of the jaw under bisphosphonate therapy in the French national pharmacovigilance database. Pharmacoepidemiol Drug Saf. 2014;23(9):989–992. [DOI] [PubMed] [Google Scholar]
- 66. Neha R, Subeesh V, Beulah E, Gouri N, Maheswari E. Existence of notoriety bias in FDA adverse event reporting system database and its impact on signal strength. Hosp Pharm. 2019:0018578719882323. doi: 10.1177/0018578719882323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Izem R, Sanchez-Kam M, Ma H, Zink R, Zhao Y. Sources of safety data and statistical strategies for design and analysis: postmarket surveillance. Ther Innov Regul Sci. 2018;52(2):159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Duggirala HJ, Tonning JM, Smith E, et al. Use of data mining at the Food and Drug Administration. J Am Med Inform Assoc. 2016;23(2):428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Watson J, Wright S, Lucas A, et al. Receptor occupancy and brain free fraction. Drug Metab Dispos. 2009;37(4):753–760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.