Abstract
It has been suggested that the antipsychotic clozapine may modulate brain glutamate, and that this effect could contribute to its efficacy in treatment-resistant schizophrenia (TRS). The aim of this study was to examine the effects of clozapine on brain glutamate in TRS longitudinally. This study examined individuals with TRS before and 12 weeks after switching from a non-clozapine antipsychotic to treatment with clozapine as part of their normal clinical care. Proton magnetic resonance spectroscopy (1H-MRS) measured concentrations, corrected for voxel tissue content, of glutamate (Glucorr), and glutamate plus glutamine (Glxcorr) in the anterior cingulate cortex (ACC) and right caudate nucleus. Symptoms were monitored using the Positive and Negative Syndrome Scale (PANSS). Of 37 recruited patients (27 men, 39.30 years old, 84% clozapine naïve), 25 completed 1H-MRS at both timepoints. 12 weeks of clozapine was associated with a longitudinal reduction in Glucorr in the caudate (n = 23, F = 7.61 P = .01) but not in the ACC (n = 24, F = 0.02, P = .59). Percentage reduction in caudate Glucorr was positively correlated with percentage improvement in symptoms (total PANSS score, n = 23, r = .42, P = .04). These findings indicate that reductions in glutamate in the caudate nucleus may contribute to symptomatic improvement during the first months of clozapine treatment.
Keywords: 1H-MRS, magnetic resonance spectroscopy, antipsychotic, anterior cingulate cortex, caudate, psychosis
Introduction
Clozapine is the only antipsychotic recommended for patients with treatment-resistant schizophrenia (TRS).1 However, approximately half of patients with TRS do not respond to clozapine.2,3 The neurobiological mechanisms that mediate clozapine response are unclear, and it is currently not possible to predict in advance whether clozapine will be effective in improving symptoms.
Cross-sectional proton magnetic resonance spectroscopy (1H-MRS) studies indicate that patients with TRS taking non-clozapine antipsychotics have higher levels of glutamate in the anterior cingulate cortex (ACC) than patients with schizophrenia who have shown a good antipsychotic response4 and healthy volunteers.5,6 Similarly, elevated ACC glutamate levels have been linked to a poor response to conventional antipsychotic treatment early in illness.7,8 In contrast, studies examining participants with TRS taking clozapine at the time of scanning have reported no difference in ACC glutamate or Glx (the combined signal from glutamate and glutamine) in comparison to an antipsychotic-responsive group.9,10 One explanation for this difference could be that clozapine treatment reduces ACC glutamate levels. Interestingly, binding to the NMDA receptor intrachannel PCP/MK-801 site is reduced across the brain in clozapine-treated, but not typical-antipsychotic-treated patients with schizophrenia relative to healthy volunteers, which may also indicate that clozapine modulates glutamatergic systems.11 It is also possible that a reduction in ACC glutamate may relate to the degree of the clozapine response. In comparison to healthy volunteers, ACC Glx levels were elevated in clozapine-nonresponsive TRS (termed ultra-resistant schizophrenia, URS), but not in patients with TRS who had responded to clozapine.10
In addition to the ACC, schizophrenia is associated with glutamatergic elevation in the striatum.12 In first-episode psychosis, effective treatment with risperidone reduces glutamate in the right caudate (associative striatum) and this reduction correlates with improvement in symptoms.13 It is unknown whether effective clozapine treatment produces similar reductions in striatal glutamate. One cross-sectional study including patients with TRS taking clozapine found higher Glx in the putamen compared to both an URS and an antipsychotic-responsive group,9 although no group difference was found in a similar study examining the caudate.10 Overall, while effective treatment with non-clozapine antipsychotics may reduce glutamate or Glx in the ACC14–16 and striatum13 (and see17,18), it is possible that glutamate metabolites remain elevated in patients who are treatment-resistant. The effects of clozapine on glutamate in either the ACC or striatum have not been yet investigated longitudinally in humans.
While 1H-MRS measures the total amount of intracellular glutamate in the voxel and not that associated with neurotransmission specifically, effects of clozapine on glutamatergic neurotransmission have been described in preclinical research. In the rodent prefrontal cortex, clozapine attenuates the increases in extracellular glutamate efflux19–21 and disruptions in neuronal firing22 that are stimulated by blocking N-methyl-D-aspartate (NMDA) receptors. In rodents, clozapine administration also decreases glutamate concentrations in ex vivo frontal cortex tissue samples,23,24 and may decrease the in vivo 1H-MRS glutamate signal in the rat striatum.25
Whilst the effects of clozapine on brain glutamate in schizophrenia have not been investigated before, clinical studies have examined the effect of clozapine on plasma or serum glutamate levels. These studies have reported increases,26 decreases27 and no effect28,29 of clozapine administration overall. However, a good response has recently been associated with higher serum glutamate levels relative to poor clozapine response.30 Nonetheless, the relationship between peripheral and central glutamate levels is unclear and the role of glutamate in the clozapine response can be more directly evaluated by using 1H-MRS to measure brain glutamate levels in patients undergoing clozapine treatment.
The aim of this study was to measure glutamate levels in the ACC and striatum in patients with TRS before and 12 weeks after switching from their current antipsychotic to treatment with clozapine. Our main hypothesis was that glutamate would be reduced in these 2 areas over the 12-week observation period. In secondary analyses, we hypothesized that longitudinal reductions in glutamate concentration would be correlated with the degree of symptomatic improvement.
Methods
Participants and Clinical Measures
This study was approved by London South East NHS ethics committee (Ref:13/LO/1857). Participants were recruited from inpatient and outpatient services within the South London and Maudsley, and the Oxleas NHS Foundation Trusts.
The study included participants meeting ICD-10 criteria for schizophrenia (F20) or schizoaffective disorder (F25), as diagnosed by their treating psychiatrist, who were due to switch from their current antipsychotic to clozapine as part of their normal clinical care. Inclusion required that participants were clozapine naïve or had not taken clozapine for at least 3 months prior to the study. The presence of TRS was inferred from medical records and from their treating psychiatrist. Criteria included at least 2 previous trials of a non-clozapine antipsychotic, each within the recommended dose range for at least 6 weeks, a diagnosis of TRS provided by their psychiatrist and referral for clozapine initiation. Participants with mental capacity to consent provided written informed consent to study procedures. The study was also open to participants lacking capacity to consent if a consultee advised assent on their behalf. The consultee was defined as a person nonprofessionally involved in caring for the patient or concerned with their welfare (normally their next-of-kin family member). They were advised on the role of the consultee, provided with a consultee information sheet and invited to ask any questions about the study before advising on the patient’s behalf. If there was any indication from the patient, consultee, clinical team, or anyone else involved in the patient’s care that the patient would not wish to participate, they were not enrolled in the study. During study participation, study researchers maintained contact with the consultee and clinical team. If the patient expressed any objections to the study or wishes to withdraw, they were withdrawn from the study immediately. General exclusion criteria included drug dependency, as defined in DSM-IV, or pregnancy. Participants with contraindications to magnetic resonance imaging (MRI) at 3 Tesla were excluded from MRI but were able to participate in the larger study, including clinical assessment and blood sampling.
Clinical assessment and MRI/MRS were performed at baseline (−14 to 0 days before clozapine titration) and repeated 12 weeks after clozapine initiation. This period was selected as a 12-week treatment period has been associated with clozapine response in ~50% of patients.31,32 Clozapine plasma levels were measured in blood samples taken at 6 and 12 weeks to evaluate whether clozapine levels were above the indicated therapeutic threshold of 350 ng/ml,32 blood samples were also collected at 8 weeks if below threshold at 6 weeks.
Demographic and clinical history was collected through self-report and review of medical records. Clinical assessment instruments included the Positive and Negative Syndrome Scale (PANSS),33 Clinical Global Impression – Schizophrenia scale (CGI-S)34 Global Assessment of Functioning scale (GAF)35 and Personal and Social Performance scale (PSP).36 We assessed the percentage change in PANSS total score from baseline to 12 weeks using the formula: (12-week score – baseline score) / baseline score × 100, after subtracting minimum possible scores.37
Magnetic Resonance Imaging
MR data were acquired on a 3 Tesla MR750 scanner (General Electric) (supplementary methods). 1H-MRS spectrum were acquired in 8 cm3 (2 × 2 × 2 cm3) voxels prescribed in the bilateral ACC7,8 and in the right caudate nucleus13,38 (supplementary figure S1). Spectrum were acquired using a conventional PRESS (Point RESolved Spectroscopy) acquisition with 96 averages, TR = 3000 ms and with a TE = 30 ms in the ACC and a TE = 35 ms in the caudate.13
Data Processing
Spectrum were analyzed with LCModel version 6.3-0I (supplementary methods). Spectral quality was evaluated by visual inspection, and individual metabolite estimates were excluded if the Cramér Rao Lower Bound estimates of the standard deviations (%SD) were greater than >20%.
Metabolite values were corrected for voxel tissue content using the formula:
where M is the uncorrected metabolite, and WM, GM and CSF indicate the fraction of white and gray matter and cerebrospinal fluid content in the voxel. The formula assumes a CSF water concentration of 55 556 mol/m3.39,40 Voxel GM ratio was calculated as GM/(GM+WM).
Statistical Analysis
Analysis was performed in SPSS version 25, IBM. Data were inspected for normality of distribution and outlying values. Initial analysis identified any relationships between glutamatergic metabolite levels at baseline and the clinical and demographic characteristics of the sample using t-tests for categorical variables and Pearson’s correlations for continuous variables (threshold P = .05). Where significant, the potential influences of these variables were subsequently investigated during hypothesis testing. For the main analysis, repeated measures analysis of variance examined the effects of 12 weeks of clozapine treatment on Glucorr and Glxcorr levels in the ACC and caudate. In the main analysis, the threshold for statistical significance was corrected for the 2 voxels examined (P = 0.05/2 = .025). In exploratory analysis, Pearson’s correlations investigated relationships between glutamate metabolite levels and PANSS Total scores, and between the percentage change in glutamate metabolites and PANSS scores over 12 weeks.
Results
Demographic and Clinical Characteristics of the Sample
Seventy-seven participants with TRS consented to the larger study, of whom 37 completed at least one MR session (table 1). Common reasons for nonparticipation in MR included presence of MRI contraindication, being above the scanner weight limit, or being too unwell to leave the ward or tolerate the scanning session. Within patients in whom baseline PANSS scores were available, there was no significant difference in symptom severity between those who did or did not participate in MRI (mean ± SD PANSS total score: MRI: n = 37; 79.19 ± 15.02; No MRI: n = 24; 74.21 ± 19.44; t(59) = −0.46; P = .65). Twenty-five participants had complete data, including 1H-MRS and PANSS scores at both baseline and 12 weeks. A further 10 participants completed 1H-MRS at baseline (total baseline 1H-MRS = 35), of whom 7 subsequently left the study, and 3 completed 12-week PANSS but not 12-week 1H-MRS. An additional 2 participants declined baseline 1H-MRS but completed all PANSS ratings and 12-week MRS (total 12-week 1H-MRS = 27). This resulted in PANSS ratings in a total of 37 patients at baseline, and 30 patients at 12 weeks. Reasons for drop-out over the observation period (total n = 7), included not commencing clozapine (n = 2), discontinuing clozapine due to poor tolerability or other medical concern (n = 4) or moving outside the participating NHS Trusts (n = 1). Concomitant use of GABA-acting (eg, benzodiazepines) and/or serotonergic (eg, selective serotonin reuptake inhibitor antidepressants) was relatively common (32% and 51% of the sample, respectively, table 1), as were psychiatric comorbidities and previous substance use (table 1).
Table 1.
Demographic and Clinical Characteristics of the Total Sample
| Age | 39.30 ± 13.56 |
| Sex (M / F) | 27 / 10 |
| Age of onset | 25.51 ± 8.74 |
| Duration of illness | 15.08 ± 9.19 |
| Previous antipsychotic trials (median; range; min; max) | 3; 8; 2; 10 |
| Previous clozapine trial (Y / N) | 6 / 31 |
| Number of hospitalizations (median; range; min; max) | 3; 12; 0; 12 |
| Capacity to consent (Y/N) | 32 / 5 |
| Diagnosis (F20 / F25) | 30 / 7 |
| Comorbid psychiatric diagnosis | |
| Affective disorder | 6 |
| Anxiety disorder | 1 |
| Epilepsy | 1 |
| Personality disorder | 1 |
| Post-traumatic stress disorder | 1 |
| None | 27 |
| Antipsychotic before switching to clozapine (N): | |
| Olanzapine | 10 |
| Aripiprazole | 5 |
| Amisulpride | 4 |
| Risperidone | 5 |
| Quetiapine | 4 |
| Flupentixol | 2 |
| Asenapine | 1 |
| Haloperidol | 1 |
| Zuclopenthixol | 1 |
| Antipsychotic dose at baseline mean ± SD CPZE/mg per day | 219.41 ± 184.85 |
| Antidepressant medication: N (Y / N) | 12 / 25 |
| GABAergic medication: N (Y / N) | 18/19 |
| GABAergic medication: N (BDZ/Val/Li/Zop/PreG/Lam) | 11/7/3/1/1/1 |
| Current tobacco use N (Y / N) | 15 / 22 |
| Current alcohol current use N (Y / N) | 10 / 27 |
| Substance abuse ever N (Y / N) | 25 / 12 |
| Substance abuse ever by type (N): | |
| Cannabis | 23 |
| Cocaine | 13 |
| Amphetamines | 11 |
| Hallucinogens | 8 |
| Ketamine | 6 |
| Legal Highs | 5 |
| Inhalants | 2 |
| Heroin | 1 |
| Current Substance use N, (Y / N) | 7 / 30 |
| Current Substance use type (N) | |
| Cannabis | 6 |
| Cocaine | 4 |
| Amphetamines | 1 |
| Hallucinogens | 0 |
| Ketamine | 0 |
| Legal Highs | 0 |
| Inhalants | 0 |
| Heroin | 0 |
Note: Values are provided as mean ± SD unless otherwise stated. CGI-S, Clinical Global Impression- Schizophrenia; CPZE, Chlorpromazine equivalent dose; GAF, Global Assessment of Functioning; PANSS, Positive and Negative Syndrome Scale; PSP, Personal and Social Performance. Diagnosis, F20, Schizophrenia; F25, Schizoaffective disorder; CNS medication, 5-HT, serotonergic antidepressants (including citalopram, duloxetine, escitalopram, fluoxetine, mirtazapine, sertraline, reboxetine, venlafaxine); GABA, GABAergic medication (including BDZ, benzodiazepines; Val, valproate; Li, lithium; Zop, zopiclone; PreG, pregabalin; Lam, lamotrigine).
Symptom severity and functioning were improved after 12 weeks of clozapine compared to baseline (table 2). At 12 weeks, the mean plasma clozapine level was 0.46 ± 0.28 ng/ml (supplementary table 1). The percentage change in PANSS total score was associated with clozapine dose at 12 weeks (r = −.50, n = 30, P = .01), likely reflecting the prescription of higher doses to individuals showing less symptomatic improvement. The change in total PANSS score was not related to plasma clozapine level at 12 weeks (P > .05).
Table 2.
Symptoms and Functioning at Baseline and 12 Weeks
| Symptoms and Functioning | |||
|---|---|---|---|
| Baseline | 12 Weeks | ||
| Total sample (Baseline N = 37; 12 Weeks N = 30) | |||
| PANSS—Positive | 18.00 ± 5.87 | 13.57 ± 4.85 | |
| PANSS—Negative | 19.22 ± 7.52 | 15.37 ± 6.20 | |
| PANSS—General | 35.04 ± 7.23 | 26.80 ± 6.08 | |
| PANSS—Total | 72.19 ± 15.01 | 55.77 ± 14.26 | |
| CGI-S (median; range; min; max) | 5; 3; 4; 7 | 4; 3; 3; 6 | |
| GAF | 46.22 ± 10.45 | 59.90 ± 10.35 | |
| PSP | 52.27 ± 14.80 | 62.27 ± 10.85 | |
| Sample with PANSS at both timepoints (N = 30) | |||
| PANSS—Positive | 18.00 ± 6.17 | 13.57 ± 4.85 | T(29) = 6.68; P < .01 |
| PANSS—Negative | 18.30 ± 7.17 | 15.37 ± 6.19 | T(29) = 3.47; P < .01 |
| PANSS—General | 34.03 ± 7.09 | 26.80 ± 6.08 | T(29) = 7.06; P < .01 |
| PANSS—Total | 70.33 ± 15.51 | 55.77 ± 14.26 | T(29) = 7.71; P < .01 |
| CGI-S (median; range; min; max) | 5; 3; 4; 7 | 4; 3; 3; 6 | Z = 4.36; P < .01 |
| GAF | 47.13 ± 10.72 | 59.90 ± 10.35 | T(29) = 5.92; P < .01 |
| PSP | 52.97 ± 15.33 | 62.27 ± 10.85 | T(29) = 3.64; P < .01 |
Note: Values are provided as mean ± SD unless otherwise stated. CGI-S, Clinical Global Impression- Schizophrenia; GAF, Global Assessment of Functioning; PANSS, Positive and Negative Syndrome Scale; PSP, Personal and Social Performance Scale.
1H-MRS Spectral Quality
Of the 35 baseline 1H-MRS sessions, all ACC spectrum were included in the analyses, but Glu and Glx values were excluded in one caudate spectra due to CRLB > 20%. Of the 27 12-week 1H-MRS sessions, CRLB > 20% led to exclusion of Glu and Glx in one ACC spectra and Glx but not Glu in one caudate spectra. At 12 weeks, ACC but not caudate spectrum were acquired in 2 participants. This resulted in ACC glutamate values for 35 patients at baseline, 26 at 12 weeks, and 24 at both timepoints, and caudate glutamate values for 34 patients at baseline, 25 at 12 weeks, and 23 at both timepoints. All values for metabolites, spectral quality, and voxel tissue composition are presented in supplementary tables 2 and 3. GM ratio of the ACC voxel was significantly lower at 12 weeks compared to at baseline. There were no significant differences between timepoints in caudate voxel tissue composition (supplementary table 2).
Baseline Glutamate Metabolite Levels and Sample Characteristics
Baseline glutamate metabolite levels in the ACC and caudate were not associated with age, duration of illness, age at illness onset, sex, current smoking status, presence of GABAergic medication or voxel GM ratio (P > .05). Baseline ACC Glucorr was higher in clozapine naïve participants compared to those who had previously taken clozapine (mean ± SD: ACC Glucorr naïve: 14.80 ± 2.11, n = 29; retrial: 12.62 ± 3.12, n = 6; t(33) = 2.12; P = .04). ACC Glxcorr showed a similar effect of previous clozapine exposure (naïve: 20.73 ± 3.82, n = 29; retrial: 16.13 ± 4.48, n = 6; t(33) = 2.50; P = .02). The elevation in ACC glutamate metabolites in clozapine naïve compared to retrial participants remained significant when covarying for ACC GM ratio (Glucorr: F(32) = 4.29; P = .05; Glxcorr: F(32) = 5.85; P = .02). Caudate Glucorr did not differ between clozapine naïve and retrial participants (P > .6). Baseline ACC Glucorr was also higher in participants who reported drinking alcohol compared to those who did not (ACC Glucorr non-alcohol drinkers 13.82 ± 2.18, n = 26; alcohol drinkers: 16.19 ± 2.26, n = 9; t(33) = 2.78; P = .01), including when covarying for ACC GM ratio (F(32) = 8.44; P = .01). This effect was present at trend-level for ACC Glxcorr (P = .08). Caudate Glucorr, was higher in participants prescribed antidepressants at baseline (Caudate Glucorr no antidepressant 11.18 ± 1.98, n = 24; antidepressant: 14.23 ± 5.72, n = 10; t(32) = 2.34; P = .03), for caudate Glxcorr this effect had a significance value of P = .13.
Effect of 12-Week Clozapine on Glutamate Levels
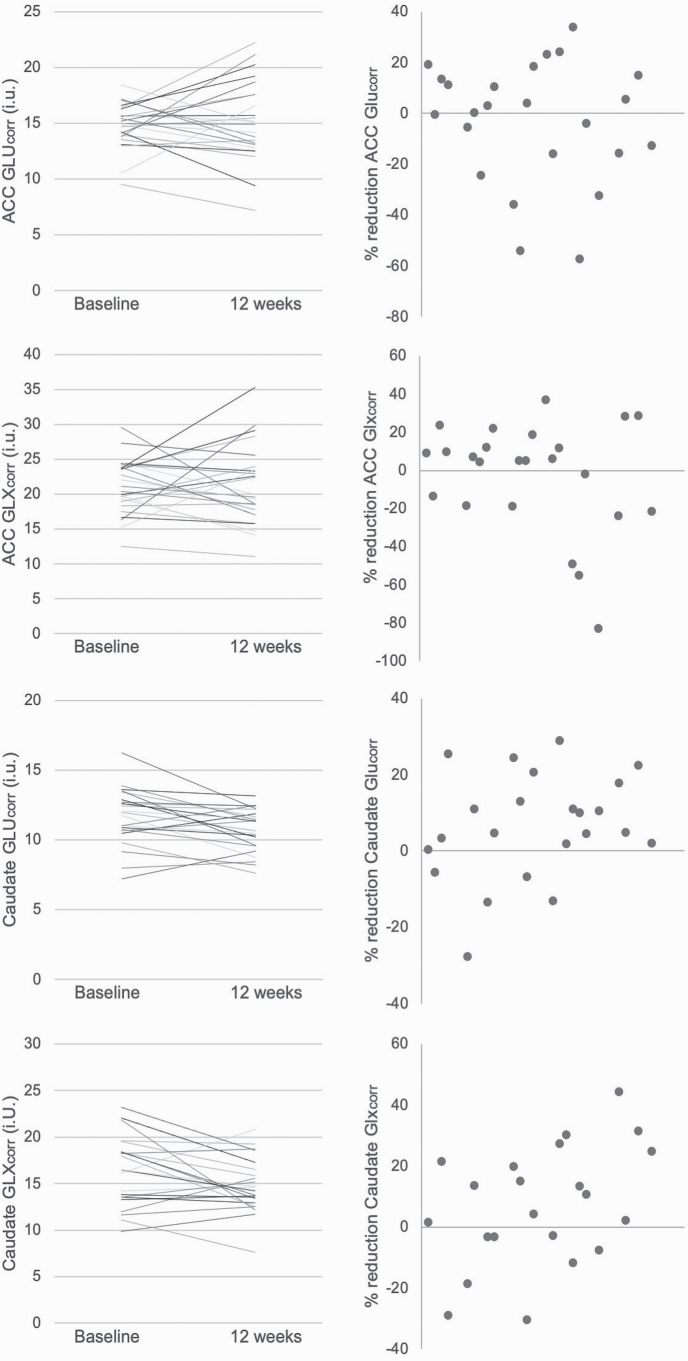
In the ACC, there was no significant difference in Glucorr or Glxcorr at baseline compared to after 12 weeks of clozapine treatment (table 3, figure 1). This was also the case when analysis was restricted to participants who were clozapine naïve at baseline, or who did not drink alcohol, or when covarying for the numerical difference in voxel GM ratio over time, clozapine dose, or plasma levels at 12 weeks (P > .05).
Table 3.
Glutamatergic Metabolites in the Anterior Cingulate Cortex and Right Caudate at Baseline and After 12 Weeks of Clozapine in Participants With Treatment-Resistant Schizophrenia
| Anterior Cingulate Cortex | |||
|---|---|---|---|
| Metabolite | Baseline | 12 Weeks | Repeated Measures ANOVA |
| Total sample | |||
| Glucorr | 14.43 ± 2.41 (35) | 15.46 ± 3.68 (26) | |
| Glxcorr | 19.94 ± 4.42 (35) | 21.40 ± 5.57 (26) | |
| Completed sample | |||
| Glucorr | 14.77 ± 1.99 (24) | 15.15 ± 3.58 (24) | F (1,23) = 0.02, P = .59 |
| Glxcorr | 21.05 ± 3.95 (24) | 21.15 ± 5.68 (24) | F (1,23) = 0.01, P = .93 |
| Right Caudate Nucleus | |||
| Metabolite | Baseline | 12 Weeks | |
| Total sample | |||
| Glucorr | 12.08 ± 3.69 (34) | 10.85 ± 1.58 (25) | |
| Glxcorr | 17.39 ± 5.56 (34) | 14.90 ± 2.81 (25) | |
| Completed sample | |||
| Glucorr | 11.66 ± 2.00 (23) | 10.73 ± 1.56 (23) | F (1,22) = 7.61, P = .01 |
| Glxcorr | 16.34 ± 3.75 (22) | 14.78 ± 2.96 (22) | F (1,21) = 4.73, P = .04 |
Note: Data is provided for the total number of subjects in which 1H-MRS was available each timepoint (Total sample) and for the sample who completed 1H-MRS at both timepoints (Completed Sample). Data are presented as mean ± SD (n). Glu, Glutamate; Glx, Glutamate + Glutamine. The reduction in caudate Glucorr was significant after multiple comparisons correction (bold).
Fig. 1.
Values in individual participants for Glutamate (Glucorr) and Glx (Glxcorr, glutamate plus glutamine) in the anterior cingulate cortex (ACC) and right caudate before and 12 weeks after switching to clozapine (left). The right column presents data as percentage reduction. In the caudate, Glucorr was significantly reduced after 12 weeks of clozapine treatment (P = .01).
In the caudate, Glucorr was significantly reduced after 12 weeks of clozapine treatment compared to at baseline (table 3, figure 1). The reduction in caudate Glucorr remained significant when covarying for numerical difference in caudate voxel GM ratio over time (P = .005), antidepressant use at baseline (P = .02), clozapine dose (P = .05), or clozapine plasma levels at 12 weeks (P = .003). Caudate Glxcorr showed a similar pattern of reduction over time, but this was not significant following multiple comparisons correction (table 3).
Relationships Between Glutamatergic Metabolites and Symptoms
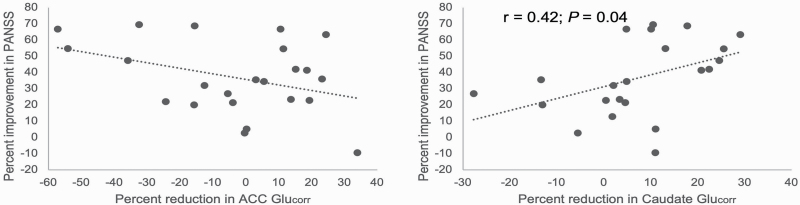
The relationships between percentage change in ACC glutamate metabolites and percentage change in PANSS total score were nonsignificant (ACC Glucorr: n = 24; r = −.36; P = .08; figure 2). In the caudate, there was a direct relationship, such that greater reductions in Glucorr, but not Glxcorr were associated with greater improvements in symptoms (n = 23, r = .42, P = .04), which was also significant when controlling for numerical difference in caudate GM ratio over time (P = .03). Relationships between baseline Glucorr and Glxcorr and total PANSS score at baseline or 12 weeks were nonsignificant.
Fig. 2.
Relationships between the percentage reduction in Glucorr in the anterior cingulate cortex (ACC) and caudate and the percentage improvement in PANSS total scores 12 weeks after switching to clozapine treatment. The percentage reduction in symptoms was not significantly correlated with the percentage reduction in Glucorr in the (ACC), (n = 24; r = −.36; P = .08). In the caudate, greater reductions in Glucorr were associated with greater improvements in symptoms (n = 23, r = .42, P = .04).
Discussion
Although modulation of brain glutamate has been suggested to contribute to the efficacy of clozapine, this has only been supported by indirect evidence.30,41–43 In this study, we examined glutamate levels in the ACC and caudate in patients with TRS before and after 12 weeks of switching to clozapine treatment. We found that reductions in glutamate levels in the caudate over this period, and that this reduction was associated with improvements in symptoms. In contrast, there was no change in glutamatergic metabolites in the ACC. Secondary findings included observations that ACC glutamate was higher in clozapine naïve than re-trial participants, and in those who had consumed alcohol in the last week, and that caudate glutamate at baseline was higher in patients who were also taking an antidepressant medication. Overall, our main findings indicate that glutamate reductions in the caudate, but not ACC, occur over the first months of clozapine treatment.
We hypothesized that clozapine would reduce ACC glutamate levels, on the basis of preclinical research,19–21 and because the elevations ACC glutamate levels which have been observed in patients who have responding poorly to antipsychotics4,5,7,8 are not apparent in patients who are taking and have responded to clozapine.9,10 Contrary to this hypothesis, however, we did not observe reductions in ACC glutamate over 12 weeks of clozapine treatment, suggesting ACC glutamate levels are relatively stable over the initial months following clozapine initiation. It should be noted that previous cross-sectional studies of patients who were taking clozapine included participants who were already stabilized on clozapine therapy for several years.9,10 Although the number of patients in our study who had taken clozapine previously was small, this subsample had lower ACC glutamate at baseline compared to those who were clozapine naïve. This could indicate that reductions in ACC glutamate metabolites might be observed after longer-term clozapine exposure. A second interpretation is that reductions in ACC glutamate are only observed in patients who have minimal symptom severity after clozapine treatment. In the study of Iwata,10 ACC Glx did not differ between healthy volunteers and clozapine-treated patients with CGI-S and positive symptom scores of mild or less. While on average symptoms improved during our study, 83% (25/30) of participants still had CGI-S scores of ≥4 (moderate severity) at 12 weeks, so were relatively more unwell.
In the caudate, glutamate reduced over 12 weeks of clozapine treatment, and this reduction correlated with the degree of symptomatic improvement. Reductions in glutamate metabolites in the caudate are also observed over 4 weeks of antipsychotic treatment in first-episode psychosis.13 The caudate nucleus modulates information flow through corticostriatal loops to control cognitive and emotional behavior.44 It is one of the major regions implicated in schizophrenia and in the action of dopamine D2 receptor antagonist antipsychotics.45,46 Clozapine could decrease caudate glutamate levels via its interactions with multiple receptor subtypes in cortical as well as subcortical regions47 as well as potentially through more direct effects on the glutamatergic system.24,48–51 Given evidence for cortical abnormalities in TRS,52 studies examining corticostriatal connectivity during clozapine treatment would be useful to investigate these mechanisms further. As the 1H-MRS signal includes all MR-visible glutamate in the voxel, future pharmacological studies in animals combining 1H-MRS with invasive techniques to examine neurotransmission may provide further mechanistic information.
A secondary and exploratory finding was that ACC glutamate was elevated in patients who reported consuming alcohol in the week prior to imaging, compared to those who did not. We are not aware of previous research examining the effects of alcohol intake on brain glutamate levels in patients with schizophrenia specifically. 1H-MRS studies in alcohol use disorder have reported increases in ACC glutamate during initial (24 hour) withdrawal but reductions during drinking or extended abstinence.53–55 We excluded participants meeting the criteria for current substance dependence, as is common within schizophrenia neuroimaging studies. Our findings indicate that a more detailed analysis of the potential effects of alcohol intake may be warranted, including intake below threshold levels for dependence. Similarly, the observation that caudate glutamate levels were higher in patients who were taking an antidepressant highlights the importance of considering potential influences of concomitant medications. Nonetheless, our main findings of no change in ACC glutamate and a reduction in caudate glutamate over clozapine treatment did not change when alcohol or antidepressant use were included as variables in the respective analyses.
Strengths of our study include the longitudinal design and our ability to recruit a relatively unwell patient group at the time of clozapine initiation. Our study had provision to include patients who lacked mental capacity to consent, in order to aid the generalizability of the research to the clinical population. However, a significant proportion of the total sample were unable or declined to participate in MRI. This may impact on the generalizability of the MRI study, including potentially biasing results towards less unwell TRS patients who may also have been more likely to respond to clozapine. As participants had significant illness and clozapine is the only recommended antipsychotic for TRS, we did not investigate the effects of clozapine on glutamate in comparison to placebo or an alternative active compound. These comparisons would be required to specifically attribute the reductions in symptoms or caudate glutamate levels to clozapine treatment rather than nonspecific aspects of study participation. A general limitation of 1H-MRS at 3 Tesla is that the glutamate signal has an estimated contamination by glutamine, albeit of <10%, and that glutamine alone cannot be reliably estimated.4,56 Findings should also be interpreted in the context that 1H-MRS measures the total amount of intracellular glutamate, and the voxels include contributions from gray matter, white matter, and CSF. High bandwidth Very Spatially Selective Outer Volume Suppression pulses were used to suppress signal from outside the voxel, which may have led to underestimation of the unsuppressed water peak and overestimation of metabolite concentrations.57
In summary, the main finding of this study is that 1H-MRS measures of glutamate metabolites in the caudate were reduced over 12 weeks of clozapine treatment and that this was associated with symptom reduction in TRS. In contrast, ACC glutamate was not significantly altered by clozapine over the observation period. Future studies could examine whether reductions in ACC glutamate occur over a longer period of clozapine treatment and the relationship with clinical outcome and could also examine corticostriatal connectivity to provide deeper mechanistic insight.
Supplementary Material
Acknowledgments
We would like to thank T. Collier for assistance with participant recruitment, R. Case for data entry. G.J.B. receives honoraria for teaching from General Electric Healthcare, who also part fund a PhD studentship, and acts as a consultant for IXICO. J.M.S. has received honoraria from Hoffman la Roche and Janssen Pharmaceuticals and is PI on a research study sponsored by Takeda Pharmaceuticals. F.B. became an employee at COMPASS Pathways plc after the completion of this work. This work is unrelated to COMPASS Pathways plc. The remaining authors report no relevant financial interests or potential conflicts of interest. This study supports data sharing, in line with MRC policy. To apply for access to the anonymized study data, please contact Alice.Egerton@kcl.ac.uk
Funding
This work was funded by the Medical Research Council, UK, Grant MR/L003988/1 to A.E. This study presents independent research funded in part by the National Institute for Health Research (NIHR), Biomedical Research Centre at South London and Maudsley National Health Service (NHS) Foundation Trust and King’s College London.
References
- 1. Kane JM, Agid O, Baldwin ML, et al. Clinical guidance on the identification and management of treatment-resistant schizophrenia. J Clin Psychiatry. 2019;80(2):18com12123. [DOI] [PubMed] [Google Scholar]
- 2. Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789–796. [DOI] [PubMed] [Google Scholar]
- 3. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–1223. [DOI] [PubMed] [Google Scholar]
- 4. Mouchlianitis E, Bloomfield MA, Law V, et al. Treatment-resistant schizophrenia patients show elevated anterior cingulate cortex glutamate compared to treatment-responsive. Schizophr Bull. 2016;42(3):744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Demjaha A, Egerton A, Murray RM, et al. Antipsychotic treatment resistance in schizophrenia associated with elevated glutamate levels but normal dopamine function. Biol Psychiatry. 2014;75(5):e11–e13. [DOI] [PubMed] [Google Scholar]
- 6. Tarumi R, Tsugawa S, Noda Y, et al. Levels of glutamatergic neurometabolites in patients with severe treatment-resistant schizophrenia: a proton magnetic resonance spectroscopy study. Neuropsychopharmacology. 2020;45(4):632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Egerton A, Broberg BV, Van Haren N, et al. Response to initial antipsychotic treatment in first episode psychosis is related to anterior cingulate glutamate levels: a multicentre 1H-MRS study (OPTiMiSE). Mol Psychiatry. 2018;23(11):2145–2155. [DOI] [PubMed] [Google Scholar]
- 8. Egerton A, Brugger S, Raffin M, et al. Anterior cingulate glutamate levels related to clinical status following treatment in first-episode schizophrenia. Neuropsychopharmacology. 2012;37(11):2515–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goldstein ME, Anderson VM, Pillai A, Kydd RR, Russell BR. Glutamatergic neurometabolites in clozapine-responsive and -resistant schizophrenia. Int J Neuropsychopharmacol. 2015;18(6):pyu117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iwata Y, Nakajima S, Plitman E, et al. Glutamatergic neurometabolite levels in patients with ultra-treatment-resistant schizophrenia: a cross-sectional 3T proton magnetic resonance spectroscopy study. Biol Psychiatry. 2019;85(7):596–605. [DOI] [PubMed] [Google Scholar]
- 11. Bressan RA, Erlandsson K, Stone JM, et al. Impact of schizophrenia and chronic antipsychotic treatment on [123I]CNS-1261 binding to N-methyl-D-aspartate receptors in vivo. Biol Psychiatry. 2005;58(1):41–46. [DOI] [PubMed] [Google Scholar]
- 12. Merritt K, Egerton A, Kempton MJ, Taylor MJ, McGuire PK. Nature of glutamate alterations in Schizophrenia: a meta-analysis of proton magnetic resonance spectroscopy studies. JAMA Psychiatry. 2016;73(7):665–674. [DOI] [PubMed] [Google Scholar]
- 13. de la Fuente-Sandoval C, Leon-Ortiz P, Azcarraga M, et al. Glutamate levels in the associative striatum before and after 4 weeks of antipsychotic treatment in first-episode psychosis: a longitudinal proton magnetic resonance spectroscopy study. JAMA psychiatry 2013;70(10):1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kegeles LS, Mao X, Stanford AD, et al. Elevated prefrontal cortex gamma-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. ArchGenPsychiatry 2012;69(5):449–459. [DOI] [PubMed] [Google Scholar]
- 15. Choe BY, Suh TS, Shinn KS, Lee CW, Lee C, Paik IH. Observation of metabolic changes in chronic schizophrenia after neuroleptic treatment by in vivo hydrogen magnetic resonance spectroscopy. Invest Radiol. 1996;31(6):345–352. [DOI] [PubMed] [Google Scholar]
- 16. Goto N, Yoshimura R, Kakeda S, et al. Six-month treatment with atypical antipsychotic drugs decreased frontal-lobe levels of glutamate plus glutamine in early-stage first-episode schizophrenia. Neuropsychiatr Dis Treat. 2012;8:119–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Egerton A, Bhachu A, Merritt K, McQueen G, Szulc A, McGuire P. Effects of antipsychotic administration on brain glutamate in schizophrenia: a systematic review of longitudinal 1H-MRS Studies. Front Psychiatry. 2017;8:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kubota M, Moriguchi S, Takahata K, Nakajima S, Horita N. Treatment effects on neurometabolite levels in schizophrenia: a systematic review and meta-analysis of proton magnetic resonance spectroscopy studies. Schizophr Res. 2020. [DOI] [PubMed] [Google Scholar]
- 19. Abekawa T, Ito K, Koyama T. Role of the simultaneous enhancement of NMDA and dopamine D1 receptor-mediated neurotransmission in the effects of clozapine on phencyclidine-induced acute increases in glutamate levels in the rat medial prefrontal cortex. Naunyn Schmiedebergs Arch Pharmacol. 2006;374(3):177–193. [DOI] [PubMed] [Google Scholar]
- 20. Amitai N, Kuczenski R, Behrens MM, Markou A. Repeated phencyclidine administration alters glutamate release and decreases GABA markers in the prefrontal cortex of rats. Neuropharmacology. 2012;62(3):1422–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. López-Gil X, Babot Z, Amargós-Bosch M, Suñol C, Artigas F, Adell A. Clozapine and haloperidol differently suppress the MK-801-increased glutamatergic and serotonergic transmission in the medial prefrontal cortex of the rat. Neuropsychopharmacology. 2007;32(10):2087–2097. [DOI] [PubMed] [Google Scholar]
- 22. Homayoun H, Moghaddam B. Fine-tuning of awake prefrontal cortex neurons by clozapine: comparison with haloperidol and N-desmethylclozapine. Biol Psychiatry. 2007;61(5):679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McLoughlin GA, Ma D, Tsang TM, et al. Analyzing the effects of psychotropic drugs on metabolite profiles in rat brain using 1H NMR spectroscopy. J Proteome Res. 2009;8(4):1943–1952. [DOI] [PubMed] [Google Scholar]
- 24. Fukuyama K, Kato R, Murata M, Shiroyama T, Okada M. Clozapine normalizes a glutamatergic transmission abnormality induced by an impaired NMDA receptor in the thalamocortical pathway via the activation of a group III metabotropic glutamate receptor. Biomolecules 2019;9(6):234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bærentzen S, Casado-Sainz A, Lange D, et al. The chemogenetic receptor ligand clozapine N-Oxide induces in vivo neuroreceptor occupancy and reduces striatal glutamate levels. Front Neurosci. 2019;13:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Evins AE, Amico ET, Shih V, Goff DC. Clozapine treatment increases serum glutamate and aspartate compared to conventional neuroleptics. J Neural Transm (Vienna). 1997;104(6-7):761–766. [DOI] [PubMed] [Google Scholar]
- 27. Hons J, Vasatova M, Cermakova E, Doubek P, Libiger J. Different serine and glycine metabolism in patients with schizophrenia receiving clozapine. J Psychiatr Res. 2012;46(6):811–818. [DOI] [PubMed] [Google Scholar]
- 28. Yamamori H, Hashimoto R, Fujita Y, et al. Changes in plasma D-serine, L-serine, and glycine levels in treatment-resistant schizophrenia before and after clozapine treatment. Neurosci Lett. 2014;582:93–98. [DOI] [PubMed] [Google Scholar]
- 29. Tortorella A, Monteleone P, Fabrazzo M, Viggiano A, De Luca L, Maj M. Plasma concentrations of amino acids in chronic schizophrenics treated with clozapine. Neuropsychobiology. 2001;44(4):167–171. [DOI] [PubMed] [Google Scholar]
- 30. Krivoy A, Hochman E, Sendt KV, et al. Association between serum levels of glutamate and neurotrophic factors and response to clozapine treatment. Schizophr Res. 2018;192:226–231. [DOI] [PubMed] [Google Scholar]
- 31. Meltzer HY, Bastani B, Kwon KY, Ramirez LF, Burnett S, Sharpe J. A prospective study of clozapine in treatment-resistant schizophrenic patients. I. Preliminary report. Psychopharmacology (Berl). 1989;99(Suppl):S68–S72. [DOI] [PubMed] [Google Scholar]
- 32. Rosenheck R, Evans D, Herz L, et al. How long to wait for a response to clozapine: a comparison of time course of response to clozapine and conventional antipsychotic medication in refractory schizophrenia. Schizophr Bull. 1999;25(4):709–719. [DOI] [PubMed] [Google Scholar]
- 33. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 34. Haro JM, Kamath SA, Ochoa S, et al. The Clinical Global Impression-Schizophrenia scale: a simple instrument to measure the diversity of symptoms present in schizophrenia. Acta psychiatrica Scandinavica Supplementum 2003; 107 ( 416):16–23. [DOI] [PubMed] [Google Scholar]
- 35. Hall RC. Global assessment of functioning. A modified scale. Psychosomatics. 1995;36(3):267–275. [DOI] [PubMed] [Google Scholar]
- 36. Nasrallah H, Morosini P, Gagnon DD. Reliability, validity and ability to detect change of the personal and social performance scale in patients with stable schizophrenia. Psychiatry Res. 2008;161(2):213–224. [DOI] [PubMed] [Google Scholar]
- 37. Leucht S, Davis JM, Engel RR, Kane JM, Wagenpfeil S. Defining ‘response’ in antipsychotic drug trials: recommendations for the use of scale-derived cutoffs. Neuropsychopharmacology. 2007;32(9):1903–1910. [DOI] [PubMed] [Google Scholar]
- 38. McQueen G, Lally J, Collier T, et al. Effects of N-acetylcysteine on brain glutamate levels and resting perfusion in schizophrenia. Psychopharmacology (Berl). 2018;235(10):3045–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kreis R, Ernst T, Ross BD. Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magn Reson Med. 1993;30(4):424–437. [DOI] [PubMed] [Google Scholar]
- 40. Gasparovic C, Song T, Devier D, et al. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn Reson Med. 2006;55(6):1219–1226. [DOI] [PubMed] [Google Scholar]
- 41. Goff DC, Henderson DC, Evins AE, Amico E. A placebo-controlled crossover trial of D-cycloserine added to clozapine in patients with schizophrenia. Biol Psychiatry. 1999;45(4):512–514. [DOI] [PubMed] [Google Scholar]
- 42. Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37(1):4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Javitt DC. Is the glycine site half saturated or half unsaturated? Effects of glutamatergic drugs in schizophrenia patients. Curr Opin Psychiatry. 2006;19(2):151–157. [DOI] [PubMed] [Google Scholar]
- 44. Haber SN. Corticostriatal circuitry. Dialogues Clin Neurosci. 2016;18(1):7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kegeles LS, Abi-Dargham A, Frankle WG, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 2010;67(3):231–239. [DOI] [PubMed] [Google Scholar]
- 46. Farde L, Wiesel FA, Halldin C, Sedvall G. Central D2-dopamine receptor occupancy in schizophrenic patients treated with antipsychotic drugs. Arch Gen Psychiatry. 1988;45(1):71–76. [DOI] [PubMed] [Google Scholar]
- 47. Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10(1):79–104. [DOI] [PubMed] [Google Scholar]
- 48. Théberge J, Al-Semaan Y, Jensen JE, et al. Comparative study of proton and phosphorus magnetic resonance spectroscopy in schizophrenia at 4 Tesla. Psychiatry Res. 2004;132(1):33–39. [DOI] [PubMed] [Google Scholar]
- 49. Javitt DC, Duncan L, Balla A, Sershen H. Inhibition of system A-mediated glycine transport in cortical synaptosomes by therapeutic concentrations of clozapine: implications for mechanisms of action. Mol Psychiatry. 2005;10(3):275–287. [DOI] [PubMed] [Google Scholar]
- 50. Melone M, Vitellaro-Zuccarello L, Vallejo-Illarramendi A, et al. The expression of glutamate transporter GLT-1 in the rat cerebral cortex is down-regulated by the antipsychotic drug clozapine. Mol Psychiatry. 2001;6(4):380–386. [DOI] [PubMed] [Google Scholar]
- 51. Tanahashi S, Yamamura S, Nakagawa M, Motomura E, Okada M. Clozapine, but not haloperidol, enhances glial D-serine and L-glutamate release in rat frontal cortex and primary cultured astrocytes. Br J Pharmacol. 2012;165(5):1543–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mouchlianitis E, McCutcheon R, Howes OD. Brain-imaging studies of treatment-resistant schizophrenia: a systematic review. Lancet Psychiatry. 2016;3(5):451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hermann D, Weber-Fahr W, Sartorius A, et al. Translational magnetic resonance spectroscopy reveals excessive central glutamate levels during alcohol withdrawal in humans and rats. Biol Psychiatry. 2012;71(11):1015–1021. [DOI] [PubMed] [Google Scholar]
- 54. Mon A, Durazzo TC, Meyerhoff DJ. Glutamate, GABA, and other cortical metabolite concentrations during early abstinence from alcohol and their associations with neurocognitive changes. Drug Alcohol Depend. 2012;125(1-2):27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Thoma R, Mullins P, Ruhl D, et al. Perturbation of the glutamate-glutamine system in alcohol dependence and remission. Neuropsychopharmacology. 2011;36(7):1359–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Snyder J, Wilman A. Field strength dependence of PRESS timings for simultaneous detection of glutamate and glutamine from 1.5 to 7T. J Magn Reson. 2010;203(1):66–72. [DOI] [PubMed] [Google Scholar]
- 57. Rotaru D, Tsivaka D, Lythgoe D. Effects of outer volume saturation RF pulses and chemical shift displacement on MRS data. ISMRM 27th Annual Meeting and Exhibition. Montreal, Canada; 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.