Abstract
Objectives
To critically appraise the current evidence available from animal studies pertaining to the effectiveness of platelet-rich plasma (PRP) in accelerating orthodontic tooth movement.
Materials and Methods
Electronic searches of nine databases were conducted up to June 2020, followed by a hand search of the reference list of eligible studies. The study design required was prospective controlled animal studies. The primary outcome assessed was the rate of orthodontic tooth movement. The secondary outcome evaluated was histological changes after PRP application. Following study retrieval and selection, relevant data were extracted. Risk-of-bias (RoB) assessment was done using the Systematic Review Center for Laboratory Animal Experimentation's Risk of Bias Tool (SYRCLE's Risk of Bias Tool). Two review authors conducted the work of searching, study selection, and quality assessment independently and in duplicate.
Results
Of 193 studies, 5 animal studies were included in this systematic review. Three studies found a positive correlation between PRP injection and tooth movement acceleration, along with corresponding histological changes. Two studies detected no significant difference in tooth movement rate after PRP application.
Conclusions
Based on the current limited evidence, the efficacy of PRP on tooth movement acceleration remains debatable. More well-designed randomized controlled trials involving humans are called for to obtain more clinically significant conclusions.
Keywords: Orthodontics, Tooth movement techniques, Platelet-rich plasma, Acceleration
INTRODUCTION
Orthodontic treatment often requires 2 years and even longer for extraction cases and other complicated cases.1 Side effects including caries, periodontal disease, and root resorption are more common in prolonged treatment. Therefore, accelerating tooth movement and shortening the treatment time could be of great importance.
Many techniques have been developed to reduce the duration of treatment. Surgical-assisted approaches such as micro-osteoperforation and piezocision have been proven to be clinically effective.2,3 However, they require surgical injury to bone and tissue.4 Research concerning the effects of biological agents, including prostaglandin, parathyroid hormone, and vitamin D3, on tooth movement acceleration has shown conflicting results.5 However, the use of hormones or other allogenic products can result in irreversible systemic effects.6
Platelet-rich plasma (PRP), an autologous concentration of platelets in a minute volume of plasma, is a rich source of autologous growth factors and cytokines.7 Since these growth factors and cytokines can stimulate both osteoblastic and osteoclastic activity, PRP can thus interfere with the alveolar bone-remodeling process.8,9 The successful application of PRP has been reported in oral surgery and regenerative dentistry.10,11 These characteristics support the potential for PRP to accelerate orthodontic tooth movement, which is caused by the gradual modeling and remodeling of the surrounding alveolar bone. Liou12 reported that PRP achieved an accelerating effect in different types of tooth movements clinically. Recently, more studies have been conducted to confirm the effectiveness of PRP on orthodontic tooth movement acceleration, mostly in animal models. However, no systematic review has been performed to summarize and critically analyze the studies conducted. This systematic review aimed to evaluate the available knowledge, comprehensively, from animal studies regarding the influence of PRP injection on the rate of orthodontic tooth movement.
MATERIALS AND METHODS
Protocol and Registration
This review was registered in the PROSPERO database under protocol CRD42020188837, in accordance with the PRISMA checklist of systematic reviews and meta-analyses.13 This review was conducted in accordance with the Cochrane Handbook for Systematic Review of Interventions and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).14,15
Eligibility Criteria
The PICOS (population, intervention, comparison, outcome, study design) format was used to formulate the clinical question with defined inclusion and exclusion criteria (Table 1). Experimental prospective controlled animal studies involving healthy animals under active orthodontic treatment were included. The primary outcome assessed was the rate of orthodontic tooth movement, and the secondary outcome was histological changes after PRP application.
Table 1.
Eligibility Criteria for the Present Systematic Review
| Domain |
Inclusion Criteria |
Exclusion Criteria |
| Participants | Healthy animal subjects undergoing active orthodontic tooth movement | In vitro or ex vitro studies, in silico study, human studies |
| Intervention | Local injection of platelet-rich plasma, with or without activation agents | Simultaneous injection of agents other than platelet-rich plasma and activation agents |
| Comparison | Placebo intervention or no intervention | |
| Outcome | Primary outcome: quantitative data regarding the rate of orthodontic tooth movement (ie, the amount of tooth movement in a specific period of time) measured by various ways Secondary outcome: histological changes | Qualitative assessments regarding the rate of orthodontic tooth movement Inadequate definition of outcomes |
| Study design | Experimental prospective controlled animal study | Noncomparative studies Reviews, systematic reviews and meta-analyses, opinions and editorials |
Information Source and Search Strategy
PubMed, Cochrane Central Register of Controlled Trials (CENTRAL), Embase, Web of Science, China National Knowledge Infrastructure (CNKI), China Biology Medicine disc (CBM), System for Information on Grey Literature in Europe (SIGLE), Clinical Trial.gov, and ProQuest Dissertations & Theses were searched without language or time restrictions until June 2020. The details of the database search are summarized in Table 2. The reference lists of the eligible studies were also checked for additional relevant studies. This process was conducted independently and in duplicate.
Table 2.
Search Strategy for Each Database
| Database |
Search Strategy |
| PubMed | (orthodontics[Mesh] OR orthodontic*) AND (Tooth movement techniques[Mesh] OR tooth movement OR movement OR move OR moving OR retract*) AND (platelet-rich plasma [Mesh] OR platelet concentrate OR platelet gel OR PRP OR platelet) |
| Embase, Web of Science, CENTRAL | (orthodontics OR orthodontic*) AND (Tooth movement techniques OR tooth movement OR movement OR move OR moving OR retract*) AND (platelet-rich plasma OR platelet concentrate OR platelet gel OR PRP OR platelet) |
| Proquest | orthodontic*, tooth movement, platelet-rich plasma |
| CNKI, CBM, Clinical Trial.gov, SIGLE | tooth movement, platelet-rich plasma |
Study Selection
The study selection process was done by two reviewers independently and in duplicate. First, the titles and abstracts of articles were assessed for eligibility. Then, full-text reports were considered for articles that seemed to have met the inclusion criteria.
Data Collection and Data Items
Relevant data from the included studies were extracted by two reviewers in previously developed and piloted forms: author and year, study design, subject characteristics, orthodontic procedures, the injections applied in both the intervention group and control group, intervals of outcome assessment, and the details of PRP application.
Risk of Bias in Individual Studies
The risk of bias (RoB) of the included studies was assessed using the Systematic Review Center for Laboratory Animal Experimentation's Risk of Bias tool (SYRCLE's Risk of Bias Tool).16 The summary of RoB within a study was assessed according to Higgins and Green.17 This process was done independently and in duplicate by two authors.
Summary Measures and Synthesis of Results
Random effects meta-analysis was planned to determine pooled estimates and relative effects of PRP on the rate of tooth movement. However, the differences in the study design and interventions employed precluded quantitative data synthesis.
RoB Across Studies and Additional Analyses
Analyses for small-study effects, publication bias, and exploratory subgroup analyses were planned if an adequate number of studies were identified. The quality of evidence from the retrieved studies was assessed using the Grading of Recommendation Assessment, Development, and Evaluation (GRADE) approach.18
RESULTS
Study Selection
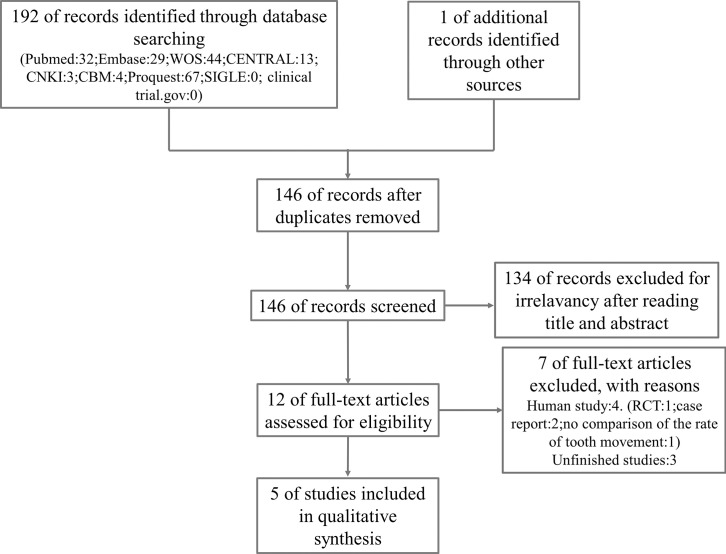
A total of 192 studies were initially identified through database searching, and 1 study was obtained by manual search. After removing duplicates, 146 studies remained. After reading the titles and abstracts, 12 studies remained for further assessment. Eventually, 5 studies were included in the qualitative analysis.19–23 Procedures of the electronic search are presented in Figure 1.
Figure 1.
Systematic search and selection strategy.
Study Characteristics
The characteristics of each included study are presented in Table 3. Among the five included studies, three studies used a split-mouth design.19,20,23 Apart from the comparison of a PRP injection group with a noninjection group, Akbulut et al.22 also compared a PRP group with a platelet-poor plasma (PPP) group, and Güleç et al.19 compared a high-platelet-concentration injection group with a moderate-platelet-concentration injection group. Orthodontic tooth movement was induced by the placement of coil springs, except for one study,21 in which tooth movement was initiated by placing a rubber separator between incisors. The concentrations of platelets in PRP in the included studies ranged from 2.45 to 6.6 times that of the whole blood. The details of the PRP application are presented in Table 4.
Table 3.
Characteristics of Included Studiesa
| Study ID |
Study Design |
Animals (Age/Weight) |
Orthodontic Procedure |
Study Groups |
Measurement Time |
Outcome |
| Rashid et al. 2017 | Split-mouth design | Six male mongrel dogs/11–15 mo, 13–17 kg | Maxillary second premolars were extracted A NiTi spring was stretched between the first premolar ligature tie and the TAD placed between maxillary the third and fourth premolars bilaterally Force:150 g | Group A: test group (25 units of PRP with 25 units of thrombin-CaCl2 solution) Group B: control group (50 units of thrombin-CaCl2) | 9 wk | Distance of tooth movement; histological findings |
| Akbulut et al. 2019 | Three arm | 54 Wistar male albino rats/6–8 wk, 150–200 g | Closed-coil springs were ligated to the maxillary incisors and first molars with stainless steel ligature wires Force: 50 g | Group A: PRP group (0.1 mL of PRP) Group B: PPP group (0.1 mL of PPP) Group C: control group (no injection) | 1, 3, 7, and 14 d | Distance of tooth movement; osteoblast and osteoclast cell counts, and ALP, TRAP, and TGF-β expressions |
| Güleç et al. 2017 | Two-arm, split-mouth design | 81 young adult Sprague-Dawley rats/9–10 wk, 348 ± 29 g | Superelastic closed-coil springs were placed between the maxillary molars and incisors Force: 40 cN | Group A: hPRP group (0.01 mL) Group B: mPRP group (0.01 mL) Group C: hPRP control group (no injection) Group D: mPRP control group (no injection) | 3, 7, 14, 21, and 60 d | Distance of tooth movement; bone content of interradicular space; alveolar bone volume; osteoclastic activity |
| Nakornnoi et al. 2019 | Two-arm, split-mouth design | 23 male New Zealand white rabbits/3–4 mo, 2.5–3 kg | Light-type nickel titanium closed-coil spring was placed between the maxillary first premolar and incisor on each side Force: 100 g | Group A: test group (0.5 mL L-PRP) Group B: control group (normal saline solution) Group C: blank control group (no injection) | 0, 3, 7, 14, 21, 28 d | Distance of tooth movement; osteoclast numbers; histological findings |
| Sufarnap et al. 2017 | Two arm | 24 young Guinea pigs/2–3 mo, 250–400 g | Power-O (Ormco) as a rubber separator between central incisors after PRP injection | Group A: test group (0.5 mL PRP) Group B: control group (no injection) | 6, 9, 12, 24 d | Distance of tooth movement |
ALP indicates alkaline phosphatase; hPRP, high platelet concentration injection group; L-PRP, leukocyte-platelet-rich plasma; mPRP, moderate platelet concentration injection group; PPP, platelet-poor plasma; PRP, indicates platelet-rich plasma; NiTi, nickel titanium; TAD, temporary anchorage device; TGF-β, transforming growth factor–β; TRAP, tartrate-resistant acid phosphatase.
Table 4.
Details of the Application of PRPa
| Study ID |
Source of PRP |
PRP Concentration |
Injection Method |
Injection Sites |
Dose |
Use of Activation Agent |
Injection Interval |
| Rashid et al. 2017 | Autologous | Unclear | Intraligamental injection | Middle of the distal side, distobuccal side, distopalatal side followed by buccal and palatal injections at the first premolar | 25 units | CaCl2 and thrombin | 0, 21, and 42 d |
| Akbulut et al. 2019 | Donor | PRP: 3617 × 103/μL PPP: 23 × 103/μL | Submucosal injection | Buccal vestibular mucosa next to the distal root of the maxillary right first molars | 0.1 mL PRP | Unclear | Injections were done only once at day 0 |
| Güleç et al. 2017 | Donor | hPRP: 2593.2 ± 257 × 103 platelets/μL mPRP: 1220.4 ± 154 × 103 platelets/μL | Submucosal injection | Buccal vestibular mucosa next to the mesial root of the right first molar | 0.01 mL PRP | Unclear | Injections were done only once at day 0 |
| Nakornnoi et al. 2019 | Autologous | PRP: platelet: 2314.44 ± 570.82 × 103 cells/μL Leukocytes: 6.87 ± 2.29 × 103 cells/μL | Submucosal injection | Buccal and lingual areas of the first maxillary premolar | 0.5 mL L-PRP | Unclear | Injections were done only once at day 0 |
| Sufarnap et al. 2017 | Donor | PRP: 507 × 103/μL | Unclear | Unclear | 0.5 mL PRP | Unclear | PRP was injected before rubber separator placement |
hPRP indicates high platelet concentration injection group; L-PRP, leukocyte-platelet-rich plasma; mPRP, moderate platelet concentration injection group; PPP, platelet-poor plasma; PRP, platelet-rich plasma.
RoB Within Studies
Two studies19,21 were determined to be at high RoB, and three studies20,22,23 were of unclear RoB. The methods of sequence generation, allocation concealment, blinding of caregivers and/or investigators during the intervention, and random outcome assessment were generally inadequately reported. Dropouts in one study were clearly outlined.21 Finally, there was no sufficient information to determine categorically the presence of any additional problems that could possibly increase the RoB. The RoB assessment of the included studies is presented in Table 5.
Table 5.
Summary of Risk-of-Bias Assessment According to the SYRCLE RoB Tool
| Signaling Questionsa |
Summary |
||||||||||
| 1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
||
| Rashid et al. 2017 | |||||||||||
| Akbulut et al. 2019 | |||||||||||
| Güleç et al. 2017 | |||||||||||
| Nakornnoi et al. 2019 | |||||||||||
| Sufarnap et al. 2017 | |||||||||||
Signaling questions: 1: Was the allocation sequence adequately generated and applied? 2: Were the groups similar at baseline or were they adjusted for confounders in the analysis? 3: Was the allocation adequately concealed? 4: Were the animals randomly housed during the experiment? 5: Were the caregivers and investigators blinded to the intervention that each animal received? 6: Were animals selected at random for outcome assessment? 7: Was the outcome assessor blinded? 8: Were incomplete outcome data adequately addressed? 9: Are reports of the study free of selective outcome reporting? 10: Was the study apparently free of other problems that could result in high risk of bias?
:low risk of bias; :unclear:high risk of bias.
Effect of PRP on the Rate of Orthodontic Tooth Movement
Three studies supported the effectiveness of PRP injection in accelerating the rate of orthodontic tooth movement. Güleç et al.19 reported that, on day 21, the amount of tooth movement induced by high concentrations of PRP was 1.7 times more than that on the control side and 1.4 times more than that induced by moderate concentrations of PRP. Rashid et al.20 also reported a higher percentage of mean changes in tooth movement than the control group, with a percentage change ratio of 2.13:1. Nakornnoi et al.23 found greater cumulative tooth movement in the L-PRP group than in the control group at all observation times, and a significantly greater rate of tooth movement was observed in the L-PRP group in the first 14 days (0–7 days: control side: 0.94 ± 0.09 mm, intervention side: 1.04 ± 0.05 mm; 7–14 days: control side: 0.45 ± 0.12 mm, intervention side: 0.58 ± 0.09mm, P < .05).
However, the other two studies found that PRP injection was not efficient in tooth movement acceleration. Akbulut et al.22 discovered that the amount of molar mesialization was less in the PRP group than that in the control group on day 3 (PRP group: 0.287 ± 0.176 mm, PPP group: 0.482 ± 0.128 mm, control group: 0.625 ± 0.028 mm, P = .01), and no significant difference was observed among the groups on days 1, 7, or 14. Sufarnap et al.21 found that tooth movement was similar with or without PRP injection on days 6, 9, 12, and 24 after rubber separator placement.
Histological Analysis
Histologic analysis was performed in four studies. Two studies19,22 reported similar results between the PRP and non-PRP group. One study found that the number of osteoclasts and osteoblasts and the results of the immunohistochemistry evaluations with or without PRP injection were similar from day 1 to day 14.22 Surprisingly, despite an increased rate of tooth movement in the PRP groups, Güleç et al.19 reported that the osteoclast counts in both high concentrations of PRP and medium concentrations of PRP were greater than the controls (12.01 ± 0.56 cells/mm2 and 14.05 ± 0.46 cells/mm2, respectively) only on day 3 and less than controls at all other observational days. In contrast, Rashid et al.20 and Nakornnoi et al.23 observed higher osteoclastic activity following PRP/L–PRP injection. Rashid et al.20 found significantly higher numbers of osteoblasts, osteoclasts, and cementoblasts in the PRP group as compared with the control group (osteoblasts: 16.2 ± 1.30 vs 12.0 ± 0.12 cells/10 μm2, P = .017; osteoclasts: 7.2 ± 1.30 vs 2.8 ± 0.84 cells/10 μm2, P = .006; cementoblasts: 21.8 ± 1.30 vs 14.4 ± 1.82 cells/10 μm2, P = .004). Nakornnoi et al.23 found a much greater number of osteoclasts in the L-PRP group than in the control group on day 7 (10.6 ± 2.07 vs 7.4 ± 2.30 cells) and day 14 (16.2 ± 3.03 vs 11.6 ± 3.04 cells), but there was no significant difference on day 28 (4.2 ± 1.78 vs 3.8 ± 1.48 cells).
RoB Across Studies and Additional Analysis
It was not possible to conduct analyses for small-study effects, publication bias, or subgroup analyses. The quality of evidence of the rate of orthodontic tooth movement assessed in the systematic review was very low. The RoB, inconsistency, indirectness, and imprecision were rated as serious. The reasons were that two studies were of high RoB, inconsistent results were presented in the included studies, the results could not be directly applied to human clinical settings, and the number of subjects included was limited. Publication bias was unlikely in the retrieved studies.
DISCUSSION
Recently, there has been an increased interest in research focused on tooth movement acceleration without the use of invasive surgical procedures. PRP is considered a potential tooth movement acceleration agent. According to the results of this review, three studies showed a positive correlation between local injection of PRP/L-PRP and acceleration of orthodontic tooth movement.19,20,23 Their results were in agreement with those of El-Timamy et al.,24 who showed PRP to be clinically feasible and effective in accelerating the rate of tooth movement in early stages of tooth movement.25 Two studies reported no beneficial effects of PRP injection on the rate of tooth movement.21,22
Histological analysis is beneficial for observing the effect of PRP on osteoblast and osteoclast activity, which regulates bone regeneration and thus regulates orthodontic tooth movement. Greater numbers of osteoclasts and osteoblasts were reported in two studies,20,23 whereas one study22 reported no significant difference in the cell counts at the early stage (14 days). Güleç et al.19 found that the osteoclast counts in the PRP injection groups were less than those of the control group at all observation times except day 3, despite recording an increased rate of tooth movement. Previous studies have shown conflicting results in terms of bone remodeling. Most studies favor the idea that PRP can stimulate both osteoblast and osteoclast precursor cells to divide and differentiate and promote bone regeneration,26–28 while others found that PRP did not improve bone regeneration.29,30 Future studies are needed to confirm the effect of PRP on bone remodeling.
Despite receiving substantial attention, there is still a lack of standardization in PRP treatment protocols. According to Marx,7 autologous blood was critical for achieving effective outcomes with the use of PRP, whereas the use of donor animal blood platelets could cause an overt immune reaction and lead to false-negative results. In addition, differences in the spinning techniques can cause different concentrations of platelets, leukocytes, and growth factors in PRP.31 Therefore, the heterogeneity of this systematic review might be partially attributable to the differences in the methods for PRP production, as three of the animal studies19,21,22 applied homologous blood from donors and only one study20 claimed to use the spinning technique as recommended by Marx.
There has also been no consensus on the optimal concentration of PRP. A minimum platelet concentration of 1 × 106 platelets/μL was defined by Marx.32 Sufarnap et al.21 used PRP at a concentration of 507 × 103 platelets/μL, less than the lower limit. In addition, some studies reported that high concentrations of PRP suppressed the viability and proliferation of alveolar bone cells and could be harmful for the healing process.33,34 Therefore, it is important to identify therapeutic concentration ranges for effective and harmless PRP application.
In the included studies, Nakornnoi et al.23 used L-PRP instead of pure PRP, claiming that leukocytes could lead to an initial burst release of proinflammatory growth factors, initiating cellular and molecular events in the early phase after orthodontic force application.35 However, whether to include leukocytes in the injected concentration has been a controversial topic. Yin et al.25 reported that pure PRP was more effective than L-PRP in terms of osteogenic differentiation of bone marrow stem cells in rats. Xu et al.36 also found that L-PRP could activate NF-κB and result in adverse effects on cartilage regeneration. Additional clinical trials are needed to justify leukocyte inclusion.
This was the first systematic review to evaluate the effect of PRP on orthodontic tooth movement acceleration. However, the limitations cannot be ignored. The foremost limitation of this review was that all data were drawn from animal experiments and cannot be directly extrapolated to humans. The studies included were of an unclear or even high RoB, and the overall quality was very low. In addition, quantitative analysis was not performed because of the significant heterogeneity in the designs of the included studies. Therefore, more high-quality studies with stricter designs are needed to draw more clinically significant conclusions.
CONCLUSIONS
Based on the current evidence, the effectiveness of PRP on orthodontic tooth movement acceleration cannot be confirmed.
It seems that the effect of PRP on the rate of tooth movement is affected by the standards of PRP acquisition, PRP concentrations, and the methods of PRP application.
More well-designed randomized controlled trials involving humans are needed to draw more clinically significant conclusions.
ACKNOWLEDGMENT
This systematic review was funded by the National Natural Science Foundation of China (81671021).
REFERENCES
- 1. .Fisher MA, Wenger RM, Hans MG. Pretreatment characteristics associated with orthodontic treatment duration. Am J Orthod Dentofacial Orthop. 2010;137:178–186. doi: 10.1016/j.ajodo.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 2. .Alikhani M, Raptis M, Zoldan B, et al. Effect of micro-osteoperforations on the rate of tooth movement. Am J Orthod Dentofacial Orthop. 2013;144:639–648. doi: 10.1016/j.ajodo.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 3. .Yi J, Xiao J, Li Y, Li X, Zhao Z. Efficacy of piezocision on accelerating orthodontic tooth movement: a systematic review. Angle Orthod. 2017;87:491–498. doi: 10.2319/01191-751.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. .Chang J, Chen PJ, Dutra EH, Nanda R, Yadav S. The effect of the extent of surgical insult on orthodontic tooth movement. Eur J Orthod. 2019;41:601–608. doi: 10.1093/ejo/cjz006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. .Nimeri G, Kau CH, Abou-Kheir NS, Corona R. Acceleration of tooth movement during orthodontic treatment—a frontier in orthodontics. Prog Orthod. 2013;14:42. doi: 10.1186/2196-1042-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. .Mangal U. Influence of platelet rich plasma on orthodontic tooth movement: a review. Biomed Pharmacol J. 2017;10:1463–1468. [Google Scholar]
- 7. .Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489–496. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 8. .Suaid FF, Carvalho MD, Santamaria MP, et al. Platelet-rich plasma and connective tissue grafts in the treatment of gingival recessions: a histometric study in dogs. J Periodont. 2008;79:888–895. doi: 10.1902/jop.2008.070339. [DOI] [PubMed] [Google Scholar]
- 9. .Andrade I, Taddei SRA, Souza PEA. Inflammation and tooth movement: the role of cytokines, chemokines, and growth factors. Semin Orthod. 2012;18:257–269. [Google Scholar]
- 10. .Xu J, Gou L, Zhang P, Li H, Qiu S. Platelet-rich plasma and regenerative dentistry. Aust Dent J. 2020;65:131–142. doi: 10.1111/adj.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. .Franchini M, Cruciani M, Mengoli C, et al. The use of platelet-rich plasma in oral surgery: a systematic review and meta-analysis. Blood Transf. 2019;17:357–367. doi: 10.2450/2019.0177-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. .Liou EJW. The development of submucosal injection of platelet rich plasma for accelerating orthodontic tooth movement and preserving pressure side alveolar bone. APOS Trends Orthod. 2016;6:5–11. [Google Scholar]
- 13. .Shamseer L. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation (vol 350, g7647, 2015) BMJ. 2016;354:1. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 14. .Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 15. .Higgins JPT, Thomas J, Chandler J, Cumpston M, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.0 (updated July 2019). London: Cochrane Collaboration; 2019. Available at: www.training.cochrane.org/handbook.
- 16. .Hooijmans CR, Rovers MM, de Vries RBM, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCL's risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:9. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. .Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:9. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. .Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64:380–382. doi: 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 19. .Güleç A, Bakkalbaşı B, Cumbul A, Uslu Ü, Alev B, Yarat A. Effects of local platelet-rich plasma injection on the rate of orthodontic tooth movement in a rat model: a histomorphometric study. Am J Orthod Dentofacial Orthop. 2017;151:92–104. doi: 10.1016/j.ajodo.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 20. .Rashid A, ElSharaby FA, Nassef EM, Mehanni S, Mostafa YA. Effect of platelet-rich plasma on orthodontic tooth movement in dogs. Orthod Craniofac Res. 2017;20:102–110. doi: 10.1111/ocr.12146. [DOI] [PubMed] [Google Scholar]
- 21. .Sufarnap E, Sofyanti E, Ilyas S. The effect of platelet-rich plasma to orthodontic tooth movement. In: PV Abbot, Tseng PSK, Porto I, Lestari W., editors. Proceedings of the International Dental Conference of Sumatera Utara 2017 Vol 8. Paris, France: Atlantis Press; 2017. pp. 80–83. [Google Scholar]
- 22. .Akbulut S, Yagci A, Yay AH, Yalcin B. Experimental investigation of effects of platelet-rich plasma on early phases of orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2019;155:71–79. doi: 10.1016/j.ajodo.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 23. .Nakornnoi T, Leethanakul C, Samruajbenjakun B. The influence of leukocyte-platelet-rich plasma on accelerated orthodontic tooth movement in rabbits. Korean J Orthod. 2019;49:372–380. doi: 10.4041/kjod.2019.49.6.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. .El-Timamy A, El Sharaby F, Eid F, El Dakroury A, Mostafa Y, Shakr O. Effect of platelet-rich plasma on the rate of orthodontic tooth movement: a split-mouth randomized trial. Angle Orthod. In press. [DOI] [PMC free article] [PubMed]
- 25. .Yin W, Qi X, Zhang Y, et al. Advantages of pure platelet-rich plasma compared with leukocyte- and platelet-rich plasma in promoting repair of bone defects. J Transl Med. 2016;14:73. doi: 10.1186/s12967-016-0825-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. .Gerard D, Carlson ER, Gotcher JE, Jacobs M. Effects of platelet-rich plasma at the cellular level on healing of autologous bone-grafted mandibular defects in dogs. J Oral Maxillofac Surg. 2007;65:721–727. doi: 10.1016/j.joms.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 27. .Thorwarth M, Wehrhan F, Schultze-Mosgau S, Wiltfang J, Schlegel KA. PRP modulates expression of bone matrix proteins in vivo without long-term effects on bone formation. Bone. 2006;38:30–40. doi: 10.1016/j.bone.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 28. .Otero L, Carrillo N, Calvo-Guirado JL, Villamil J, Delgado-Ruiz RA. Osteogenic potential of platelet-rich plasma in dental stem-cell cultures. Br J Oral Maxillofac Surg. 2017;55:697–702. doi: 10.1016/j.bjoms.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 29. .Casati MZ, Gurgel BCD, Goncalves PF, et al. Platelet-rich plasma does not improve bone regeneration around peri-implant bone defects: a pilot study in dogs. Int J Oral Maxillofacial Surg. 2007;36:132–136. doi: 10.1016/j.ijom.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 30. .Kilic SC, Gungormus M, Parlak SN. Histologic and histomorphometric assessment of sinus-floor augmentation with beta-tricalcium phosphate alone or in combination with pure-platelet-rich plasma or platelet-rich fibrin: a randomized clinical trial. Clin Implant Dent Relat Res. 2017;19:959–967. doi: 10.1111/cid.12522. [DOI] [PubMed] [Google Scholar]
- 31. .Oudelaar BW, Peerbooms JC, in't Veld RH, Vochteloo AJH. Concentrations of blood components in commercial platelet-rich plasma separation systems: a review of the literature. Am J Sports Med. 2019;47:479–487. doi: 10.1177/0363546517746112. [DOI] [PubMed] [Google Scholar]
- 32. .Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10:225–228. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 33. .Yamaguchi R, Terashima H, Yoneyama S, Tadano S, Ohkohchi N. Effects of platelet-rich plasma on intestinal anastomotic healing in rats: PRP concentration is a key factor. J Surg Res. 2012;173:258–266. doi: 10.1016/j.jss.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 34. .Choi BH, Zhu SJ, Kim BY, Huh JY, Lee SH, Jung JH. Effect of platelet-rich plasma (PRIP) concentration on the viability and proliferation of alveolar bone cells: an in vitro study. Int J Oral Maxillofacial Surg. 2005;34:420–424. doi: 10.1016/j.ijom.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 35. .Alhashimi N, Frithiof L, Brudvik P, Bakhiet M. Orthodontic tooth movement and de novo synthesis of proinflammatory cytokines. Am J Orthod Dentofacial Orthop. 2001;119:307–312. doi: 10.1067/mod.2001.110809. [DOI] [PubMed] [Google Scholar]
- 36. .Xu ZL, Yin WJ, Zhang YL, et al. Comparative evaluation of leukocyte- and platelet-rich plasma and pure platelet-rich plasma for cartilage regeneration. Sci Rep. 2017;7:14. doi: 10.1038/srep43301. [DOI] [PMC free article] [PubMed] [Google Scholar]