Abstract
A case of Rickettsia sibirica subspecies sibirica BJ-90 infection in China was identified by metagenomic analysis of an eschar biopsy specimen and confirmed by nested PCR. Seroprevalence of spotted fever group Rickettsia was ≈17.4% among the local population. This report highlights the threat of rickettsioses to public health in the Qinghai–Tibet Plateau.
Keywords: Rickettsiosis, Rickettsia sibirica subsp. sibirica BJ-90, Qinghai–Tibet Plateau, metagenomic sequencing, tickborne diseases, China, vector-borne infections, bacteria
Rickettsia, mainly transmitted by ticks, are a group of obligate gram-negative bacteria that cause mild to life-threatening rickettsioses. Two main groups of Rickettsia have been described on the basis of genetic differences and pathology, spotted fever group (SFG) and typhus group (TG). In China, 5 members of SFG have been identified in human cases (1–4), and 7 kinds of Rickettsia have been detected from ticks or animals in the Qinghai–Tibet Plateau, including R. heilongjiangensis, R. raoultii, R. slovaca, and R. sibirica, which are known to be pathogenic to humans (5–7). However, clinical cases have not been reported. Thus, rickettsioses are probably neglected by local physicians and public health officers. We report a severe case of R. sibirica subspecies sibirica BJ-90 infection in this region.
A 50-year-old herdsman from Zhamashi, Qinghai Province, China, was hospitalized on July 13, 2018, because of intensive intermittent headache, anorexia, and chest tightness. On his fifth day of sheep shearing (designated as day 1), a blood-fed tick had been found on his head. The tick was removed by hand but its mouth parts remained in the man’s scalp. The next day, he became ill with fever, myalgia, itchiness, and asthenia. On day 5, his symptoms intensified and included severe intermittent headaches, which lasted for ≈10 minutes at each onset; high fever, up to 39.5°C; and fatigue, palpitation, nausea, and vomiting. Erythematous rashes appeared on his trunk, all 4 limbs, and the area behind the ears. Because signs of neurologic dysfunction, including confusion, drowsiness, and delirium appeared, he sought care at Qilian County Hospital on day 9, where he was treated for infectious endocarditis for 3 days before transfer to Qinghai State Hospital. During his visit at the Qinghai State Hospital, he was conscious and alert. Erythematous macules were observed over his trunk, elbow, and lower limbs. A 1.5 × 1.1 cm2 black eschar was visible at his right posterior occipital bone area; no tenderness was reported (Appendix Figure 1). The eschar was surgically excised on day 16. No lymphadenopathy was found.
Alterations of the patient’s blood biochemistry included increased neutrophils (88.5% [reference 45%–75%]); decreased lymphocytes (9.3% [reference 20%–50%]), eosinophils (0% [reference 0.4%–8%]), and monocytes (1.9% [reference 3%–10%]); elevated creatine kinase–MB (42 U/L [reference 0–25 U/L]) and lactate dehydrogenase (445 U/L [reference 110–245 U/L]); and highly increased C-reactive protein (97.1 mg/dL [reference 0–5 mg/dL]), procalcitonin (0.433 ng/dL [reference 0–0.046 ng/dL]), D-dimers (12.28 μg/mL [reference 0–1.5 μg/mL]), fibrinogen degradation products (25 μg/mL [reference 0–5 μg/mL]), and β-microglobulin (4.5 μg/mL [reference 0.8–1.8 μg/mL]). The patient was prescribed levofloxacin lactate (0.5 g/d for 6 d). His symptoms subsided, and he was discharged on day 20.
On the basis of tick-bite history and the triad clinical characteristics (fever, rash, and eschar), nested PCR targeting the rickettsial citrate synthase conserved gene (gltA) was performed by using the eschar DNA as a template (Appendix). The 547-bp amplicon sequence shared 100% identity to R. sibirica 246, R. sibirica subsp. sibirica BJ-90, and R. sibirica subsp. mongolitimonae HA-91. The eschar DNA was sequenced by next-generation sequencing (BGI Genomics, https://www.bgi.com). A total of 21.6 Gb clean data were recovered from the high-throughput sequencing. Human reads (accounting for 99.9%) were filtered out. The remaining reads were mapped on the genome of R. sibirica 246 (GenBank accession no. AABW0100000). Rickettsial unique reads (n = 266) were analyzed against Refseq (https://www.ncbi.nlm.nih.gov/refseq; taxid 766). Most (226/266 [85%]) reads were 100% identical to R. sibirica subsp. sibirica BJ-90, whereas 213/266 (80%) were identical to R. sibirica 246, indicating that the Rickettsia was closest to R. sibirica subsp. sibirica BJ-90 (Table; Appendix Figure 2). Partial sequences of outer membrane protein A, outer membrane protein B, 17 kDa lipoprotein, surface cell antigen 1, and surface cell antigen 4 were amplified with specific primers. Phylogenetic trees showed that the Qinghai sequences clustered with R. sibirica subsp. sibirica BJ-90 (Figure; Appendix Figure 3). On the basis of next-generation sequencing data and PCR results, we concluded that the causative agent of the patient’s infection is closely related to R. sibirica subsp. sibirica BJ-90.
Table. Homology of 266 identified rickettsial unique reads shared by Rickettsia species, China*.
| % Identity | No. reads |
|||
|---|---|---|---|---|
| BJ-90 | R. sibirica 246† | HA-91‡ | R. heilongjiangensis‡ | |
| 100 | 226 | 213 | 153 | 48 |
| 99 | 25 | 35 | 76 | 65 |
| 98 | 6 | 9 | 19 | 46 |
| 1–97 | 9 | 9 | 17 | 102 |
| 0 | 0 | 0 | 1 | 5 |
*BJ-90, R. sibirica subsp. sibirica BJ-90; HA-91, R. sibirica subsp. mongolitimonae HA-91. †No significant difference (p = 0.35) compared with BJ-90 by Wilcoxon ranked nonparametric test. ‡Significant difference (p<0.05) compared with BJ-90 by Wilcoxon ranked nonparametric test.
Figure.
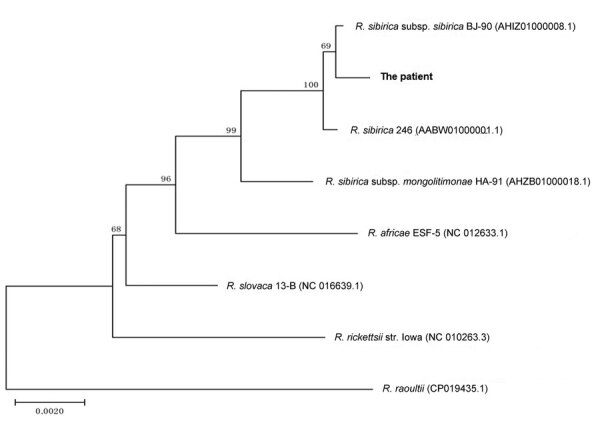
Phylogenetic analysis of concatenated nucleotide sequences from Rickettsia species collected in 2018 from eschar DNA from a patient in Qinghai Tibet Plateau, China (boldface), and reference sequences. A phylogenetic tree was constructed on the basis of the concatenated partial gltA, ompA, ompB, 17 kDa, sca1, and sca4 nucleotide sequences by using the neighbor-joining method with 1,000 bootstrap replicates. Numbers >70 indicate the bootstrapping value. GenBank accession numbers listed in Appendix Table 3 . Scale bar represents nucleotide substitutions.
We evaluated serum samples from the patient and persons from his surrounding community. Antibodies against R. rickettsii (SFG) and R. typhi (TG) were determined by indirect immunofluorescence assay. IgG titers of the patient’s paired serum samples on day 13 (1:128) and day 167 (1:4,096)) against SFG were increased by >4-fold, suggesting a recent infection with SFG. Approximately 17.4% (4/23) of the serum samples from the local community were positive for SFG, and 4.3% (1/23) were positive for TG (Appendix Table 1), indicating a high seroprevalence of SFG and co-circulation of TG in the region.
Because of the treating physicians’ unawareness of the prevalence of rickettsioses, the patient’s illness was misdiagnosed and incorrectly treated. In light of the fatal cases of R. sibirica subsp. sibirica infection recently documented in Russia and China (8–10), our report highlights the risk for rickettsial diseases among the public in the Qinghai–Tibet Plateau region and the urgent need for a large-scale seroepidemologic survey.
Additional information about severe case of rickettsiosis identified by metagenomic sequencing, China.
Acknowledgments
We thank Pierre Rivailler for analyzing the metagenomic sequences and reviewing this manuscript.
This study was supported by the National Science and Technology Major Projects on Infectious Disease Control and prevention (grant no. 2018ZX10714-002) and Development of Capacity for Pathogen Detection (grant no. 13103110200015003) from the National Institute for Communicable Disease Control and Prevention at the China Centers for Disease Control and Prevention.
Biography
Dr. Teng is a research associate at the National Institute for Communicable Disease Control and Prevention, China Centers for Disease Control and Prevention. His research interests include the detection and isolation of rickettsia and the epidemiology of rickettsioses.
Footnotes
Suggested citation for this article: Zhongqiu T, Shi Y, Peng Y, Zhang Y, Luo X, Lun X, et al. Severe case of rickettsiosis identified by metagenomic sequencing, China. Emerg Infect Dis. 2021 May [date cited]. https://doi.org/10.3201/eid2705.203265
These authors contributed equally to this article.
References
- 1.Merhej V, Angelakis E, Socolovschi C, Raoult D. Genotyping, evolution and epidemiological findings of Rickettsia species. Infect Genet Evol. 2014;25:122–37. 10.1016/j.meegid.2014.03.014 [DOI] [PubMed] [Google Scholar]
- 2.Fang LQ, Liu K, Li XL, Liang S, Yang Y, Yao HW, et al. Emerging tick-borne infections in mainland China: an increasing public health threat. Lancet Infect Dis. 2015;15:1467–79. 10.1016/S1473-3099(15)00177-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J, Hu W, Wu T, Li HB, Hu W, Sun Y, et al. Japanese spotted fever in eastern China, 2013. Emerg Infect Dis. 2018;24:2107–9. 10.3201/eid2411.170264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdad MY, Abou Abdallah R, Fournier PE, Stenos J, Vasoo S. A concise review of the epidemiology and diagnostics of rickettsioses: Rickettsia and Orientia spp. J Clin Microbiol. 2018;56:e01728–17. 10.1128/JCM.01728-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han R, Yang J, Niu Q, Liu Z, Chen Z, Kan W, et al. Molecular prevalence of spotted fever group rickettsiae in ticks from Qinghai Province, northwestern China. Infect Genet Evol. 2018;57:1–7. 10.1016/j.meegid.2017.10.025 [DOI] [PubMed] [Google Scholar]
- 6.Ying L, Zeng-kui L, Gang C, Ming K, Dao-xin L, Yan-ming Z. Identification and phylogenetic analysis of spotted fever group Rickettsia isolated from Qinghai province [in Chinese]. Chin J Vet Sci. 2014;34:1956–61. [Google Scholar]
- 7.Jian Y, Li J, Adjou Moumouni PF, Zhang X, Tumwebaze MA, Wang G, et al. Human spotted fever group Rickettsia infecting Yaks (Bos grunniens) in the Qinghai-Tibetan Plateau area. Pathogens. 2020;9:E249. 10.3390/pathogens9040249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li H, Fu XY, Jiang JF, Liu RX, Li R, Zheng YC, et al. Severe illness caused by Rickettsia sibirica subspecies sibirica BJ-90 infection, China. Emerg Microbes Infect. 2017;6:e107. 10.1038/emi.2017.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rudakov N, Samoylenko I, Shtrek S, Igolkina Y, Rar V, Zhirakovskaia E, et al. A fatal case of tick-borne rickettsiosis caused by mixed Rickettsia sibirica subsp. sibirica and “Candidatus Rickettsia tarasevichiae” infection in Russia. Ticks Tick Borne Dis. 2019;10:101278. 10.1016/j.ttbdis.2019.101278 [DOI] [PubMed] [Google Scholar]
- 10.Jia N, Jiang JF, Huo QB, Jiang BG, Cao WC. Rickettsia sibirica subspecies sibirica BJ-90 as a cause of human disease. N Engl J Med. 2013;369:1176–8. 10.1056/NEJMc1303625 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional information about severe case of rickettsiosis identified by metagenomic sequencing, China.


