Abstract
Background:
The impact of coronavirus disease 2019 (COVID-19) on maternal and newborn health is unclear. We aimed to evaluate the association between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection during pregnancy and adverse pregnancy outcomes.
METHODS:
We conducted a systematic review and meta-analysis of observational studies with comparison data on SARS-CoV-2 infection and severity of COVID-19 during pregnancy. We searched for eligible studies in MEDLINE, Embase, ClinicalTrials.gov, medRxiv and Cochrane databases up to Jan. 29, 2021, using Medical Subject Headings terms and keywords for “severe acute respiratory syndrome coronavirus 2 OR SARS-CoV-2 OR coronavirus disease 2019 OR COVID-19” AND “pregnancy.” We evaluated the methodologic quality of all included studies using the Newcastle–Ottawa Scale. Our primary outcomes were preeclampsia and preterm birth. Secondary outcomes included stillbirth, gestational diabetes and other pregnancy outcomes. We calculated summary odds ratios (ORs) or weighted mean differences with 95% confidence intervals (CI) using random-effects meta-analysis.
RESULTS:
We included 42 studies involving 438 548 people who were pregnant. Compared with no SARS-CoV-2 infection in pregnancy, COVID-19 was associated with preeclampsia (OR 1.33, 95% CI 1.03 to 1.73), preterm birth (OR 1.82, 95% CI 1.38 to 2.39) and stillbirth (OR 2.11, 95% CI 1.14 to 3.90). Compared with mild COVID-19, severe COVID-19 was strongly associated with preeclampsia (OR 4.16, 95% CI 1.55 to 11.15), preterm birth (OR 4.29, 95% CI 2.41 to 7.63), gestational diabetes (OR 1.99, 95% CI 1.09 to 3.64) and low birth weight (OR 1.89, 95% CI 1.14 to 3.12).
INTERPRETATION:
COVID-19 may be associated with increased risks of preeclampsia, preterm birth and other adverse pregnancy outcomes.
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and was declared a global pandemic in March 2020.1 Pregnant people and infants may be particularly susceptible to COVID-19 because the physiologic changes of pregnancy involve cardiorespiratory and immune systems, which may result in an altered response to SARS-CoV-2 infection in pregnancy. 2 Fetuses may be exposed to SARS-CoV-2 during critical periods of fetal development.3 The nature of the association between COVID-19 and pregnancy outcomes remains unclear, and meta-analyses involving patients with COVID-19 who are pregnant are limited. Previous reviews have focused mostly on prevalence estimates from case reports or case series that are difficult to interpret and potentially biased.4,5 A 2020 systematic review suggested that people who are pregnant did not have an increased risk of SARS-CoV-2 infection or symptomatic COVID-19, but they were at risk of severe COVID-19 compared with those who were not pregnant.5 However, this review included suspected COVID-19 cases in addition to confirmed cases.5 Although some recent observational studies have suggested that people with confirmed asymptomatic and symptomatic COVID-19,6–15 as well as mild and severe infections,6,8,9,15–22 may be at risk of adverse pregnancy outcomes, we are unaware of any systematic reviews that have comprehensively evaluated these data.
We performed a systematic review and meta-analysis of maternal, fetal and neonatal outcomes among pregnant patients with COVID-19. We aimed to determine the association between SARS-CoV-2 infection and adverse pregnancy outcomes, including preeclampsia, preterm birth and stillbirth.
Methods
This systematic review was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Data sources, search strategy and study selection
We performed a systematic search of MEDLINE, Embase, ClinicalTrials. gov, medRxiv and Cochrane databases up to Jan. 29, 2021, to identify observational studies with comparative data for people with COVID-19 who were pregnant (Appendix 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.202604/tab-related-content). Our search strategy followed the Peer Review of Electronic Search Strategies (PRESS) guidelines. We searched the databases using a combination of Medical Subject Headings (MeSH) terms and keywords for “severe acute respiratory syndrome coronavirus 2 OR SARS-CoV-2 OR 2019 novel coronavirus OR COVID-19” AND “pregnancy,” with language restricted to English abstracts. We also manually searched references cited in these articles to identify any additional studies.
Two investigators independently screened the titles and abstracts identified by the electronic searches, compared selected studies and resolved discrepancies by discussion.
We scrutinized and selected full-length articles of studies evaluating COVID-19 in pregnancy and maternal and infant outcomes that met the following inclusion criteria: observational study; population included pregnant people; SARS-CoV-2 infection was confirmed by a polymerase chain reaction (PCR) test or with codes for confirmed COVID-19 from the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10); comparisons included patients with COVID-19 versus those without COVID-19, patients with symptomatic versus those with asymptomatic COVID-19, or severe versus mild COVID-19; outcomes included maternal, fetal or neonatal morbidity and mortality; comparative data needed to calculate effect sizes were available; and methodologic quality assessment criteria in the Newcastle–Ottawa Scale suggested low or moderate risk of bias.23
We excluded studies that met at least 1 of the following exclusion criteria: reviews, case reports or case series; studies with no comparison data; and studies that included cases of infective pneumonia caused by other viral agents. If more than 1 study was published that involved the same cohort with identical outcomes, we included the report containing the most comprehensive information to avoid including the same data twice.
Case definition
We defined cases of COVID-19 as confirmed SARS-CoV-2 infection in a person who was pregnant. Controls without COVID-19 included pregnant people with negative PCR tests, those who were pregnant before the pandemic or those who were pregnant and asymptomatic early in the pandemic. We defined asymptomatic COVID-19 as a positive test result for SARS-CoV-2 in a patient who never developed symptoms of COVID-19 and symptomatic COVID-19 as a positive test result for SARS-CoV-2 with the development of fever, cough, shortness of breath, fatigue, or loss of taste or smell. We defined severe COVID-19 as the presence of dyspnea, respiratory rate at 30 breaths per minute or more and oxygen saturation at 93% or less on room air, or findings consistent with pneumonia.24 We defined mild COVID-19 as a positive test result for SARS-CoV-2 without development of severe symptoms. We classified cases of COVID-19 as symptomatic or asymptomatic and severe or mild. For symptomatic COVID-19, we included patients with any symptom regardless of severity. For severe COVID-19, we included patients with severe and critical symptoms, whereas mild COVID-19 included asymptomatic or mild cases.6,7,19
Outcomes
We selected outcomes based on clinical importance and availability in published studies. Our primary outcomes included preeclampsia and preterm birth. Our secondary outcomes were gestational diabetes, chorioamnionitis or intra-amniotic infection, cesarean delivery, abnormal liver function, lymphopenia, mechanical ventilation, admission to the intensive care unit (ICU), stillbirth (fetal loss at the 20th week of pregnancy or later), fetal distress, birth weight, low birth weight, gestational age at birth, admission to the neonatal ICU (NICU) and neonatal death.
Assessment of study quality
Two reviewers independently assessed the methodologic quality of each study using the Newcastle–Ottawa Scale.23 Cohort studies were evaluated for the following 3 domains: quality of selection of cohorts (4 stars), comparability of cohorts (2 stars) and assessment of outcome (3 stars). Case–control studies were assessed for quality of selection of cases and controls (4 stars), comparability of cases and controls (2 stars) and ascertainment of exposure (3 stars). We considered a total of 7 out of 9 stars to be a low risk of bias, 4–6 stars to be a moderate risk and less than 4 stars to be a high risk of bias (Appendix 2, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.202604/tab-related-content). In case of a disagreement, reviewers reached consensus by discussion with a third reviewer.
Data extraction and analysis
Two authors extracted data independently and in duplicate, with any discrepancy resolved by discussion. Data were extracted and statistical analysis carried out using Review Manager 5.4. We used unadjusted estimates for meta-analysis because most of the studies reported only data for raw outcomes. We used the Mantel–Haenszel method to combine data on dichotomous outcomes, and measures of effect are presented as odds ratios (ORs) with 95% confidence intervals (CIs). For continuous data, we calculated the sample size weighted mean difference (MD) when outcomes were measured the same way between studies. We assessed the associations between COVID-19 morbidity and pregnancy outcomes (patients with COVID-19 versus pregnant people with no SARS-CoV-2 infection). We also evaluated outcomes of COVID-19 severity in pregnant patients with confirmed infection (symptomatic versus asymptomatic and severe versus mild COVID-19).
We used forest plots to show individual point estimates (95% CIs) for each study, and a diamond to represent the pooled point estimate (95% CI) for each outcome of interest. We evaluated heterogeneity with the I2 statistic. If the I2 value was 40% or greater, we considered heterogeneity to be present.25 We pooled results using random-effects models. We performed a sensitivity analysis that excluded preprints and studies with moderate risk of bias and used fixed-effect models for outcomes from studies with small numbers of patients. We used funnel plots to assess publication bias.
Ethics approval
Ethics approval was not sought for this systematic review because the data were publicly available.
Results
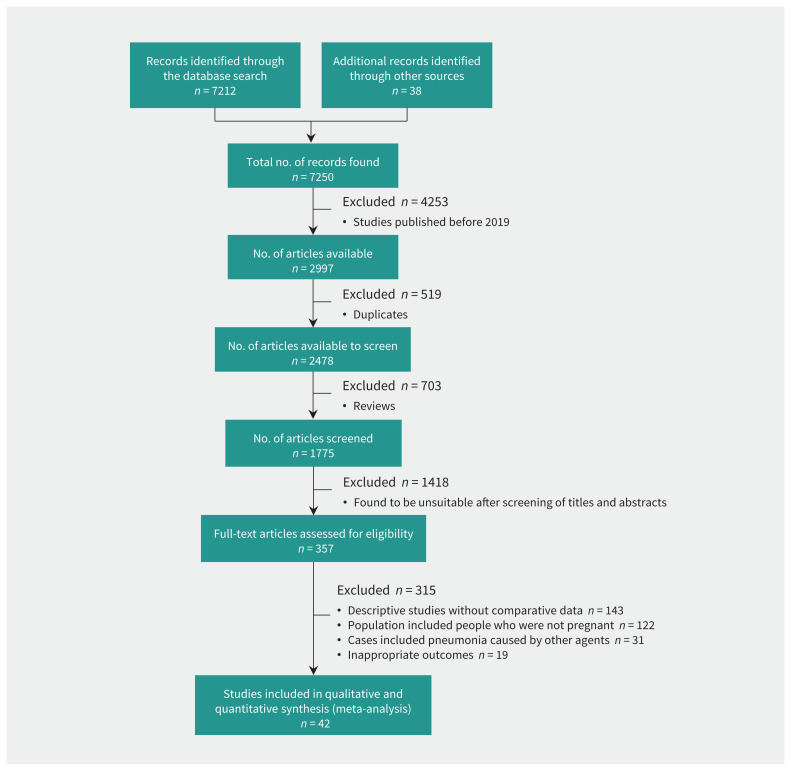
We found 7212 potentially relevant citations using our search strategy. The PRISMA flow diagram (Figure 1) summarizes the process of literature search and selection of studies. After screening titles and abstracts, we read 357 full-text articles. Forty-two observational studies7–15,17,19,21,22,26–54 involving 438 548 pregnant people met the inclusion criteria and were included in the systematic review and meta-analysis. Our assessment of the methodologic quality of each eligible study is summarized in Appendix 2. Ninety-five percent (40 out of 42) of the observational studies had an overall low risk of bias according to the Newcastle–Ottawa Scale, and 5% (2 out of 42) of the studies had moderate risk.
Figure 1:
Flow chart for the selection of studies.
Characteristics of the included studies are summarized in Appendix 3, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.202604/tab-related-content. Of the 42 studies, 16 were prospective cohorts,7,9,11,18,19,21,28,30,33,34,36,37,46,49–51 21 were retrospective cohorts8,10,12–14,17,27,29,32,35,38–45,48,53,54 and 5 were case–control studies.22,31,47,50,52 One prospective study resulted in 2 publications, 1 preprint study for positive versus negative SARS-CoV-2 infection34 and another for severe versus mild COVID-19.18 Cases of COVID-19 were confirmed by PCR testing. There were 28 studies of confirmed versus no SARS-CoV-2 infection in pregnancy.11–13,15,22,27–32,34,35,37,39–45,47–51,53,54 Twelve studies compared symptomatic versus asymptomatic COVID-19.7–15,46,48,52 There were 13 studies of severe versus mild COVID-19 in pregnancy.8,9,15,17–19,21,22,26,33,36,38,48
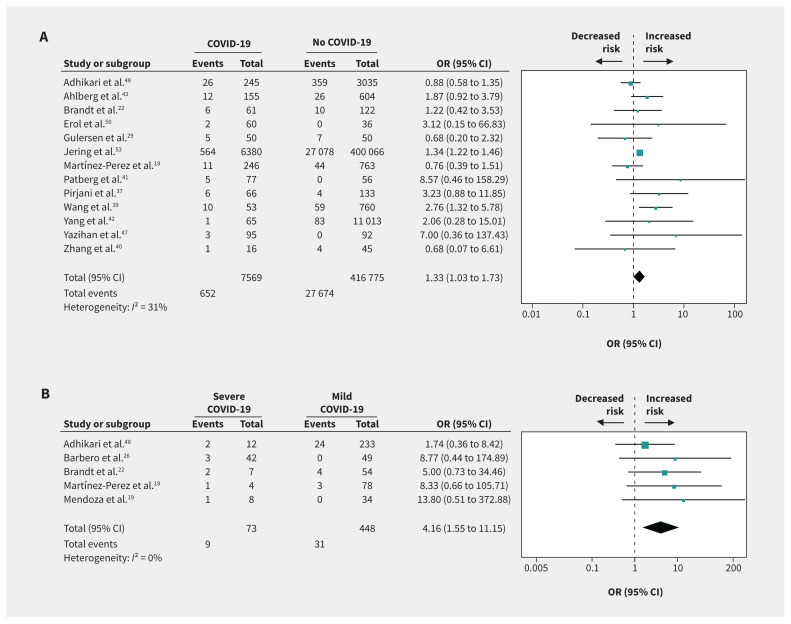
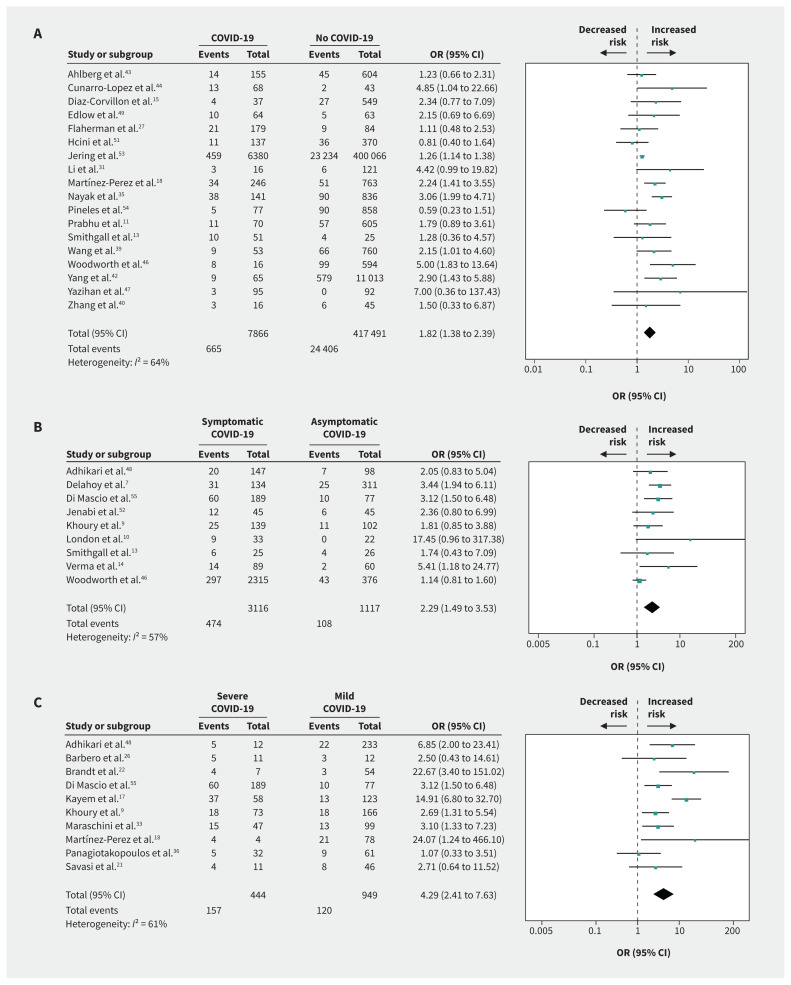
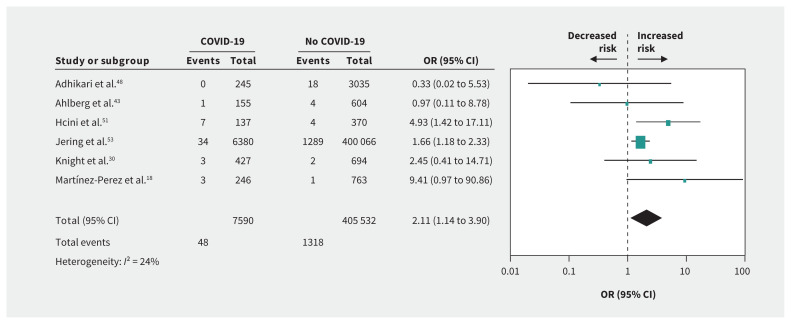
Compared with no infection, we found that SARS-CoV-2 infection in pregnancy was associated with preeclampsia (OR 1.33, 95% CI 1.03 to 1.73; I2 = 31%; based on 13 studies) (Figure 2A), preterm birth (OR 1.82, 95% CI 1.38 to 2.39; I2 = 64%; 18 studies) (Figure 3A), stillbirth (OR 2.11, 95% CI 1.14 to 3.90; I2 = 24%; 6 studies) (Figure 4), ICU admission (OR 4.78, 95% CI 2.03 to 11.25; I2 = 76%; 5 studies), lower birth weight (grams; mean difference −68.96, 95% CI −130.22 to −7.69; I2 = 29%; 13 studies) and NICU admission (OR 3.69, 95% CI 1.39 to 9.82; I2 = 94%; 10 studies) (Appendix 4, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.202604/tab-related-content). COVID-19 was not associated with gestational diabetes, cesarean delivery, postpartum hemorrhage or neonatal death compared with no COVID-19 (Table 1).
Figure 2:
Forest plots of summary crude odds ratios (ORs) and 95% confidence intervals (CIs) for the association between coronavirus disease 2019 (COVID-19) and preeclampsia. (A) Association between COVID-19 and preeclampsia (patients with COVID-19 versus patients without COVID-19). (B) Association between severe COVID-19 and preeclampsia (patients with severe versus mild COVID-19).
Figure 3:
Forest plots of summary crude odds ratios (ORs) and 95% confidence intervals (CIs) for the association between coronavirus disease 2019 (COVID-19) and preterm birth. (A) Association between COVID-19 and preterm birth (patients with COVID-19 versus no COVID-19). (B) Association between symptomatic COVID-19 and preterm birth (patients with symptomatic versus asymptomatic COVID-19). (C) Association between severe COVID-19 and preterm birth (patients with severe versus mild COVID-19).
Figure 4:
Forest plots of summary crude odds ratios (ORs) and 95% confidence intervals (CIs) for the association between coronavirus disease 2019 (COVID-19) and stillbirth.
Table 1:
Summary of pooled results using random-effects models
| Outcome | No. of studies | No. of participants | No. of events | Mean difference (95% CI) | OR (95% CI) | Heterogeneity, % |
|---|---|---|---|---|---|---|
| Patients with COVID-19 versus those without COVID-19 | ||||||
| Preeclampsia | 13 | 424 344 | 28 326 | 1.33 (1.03 to 1.73) | 31 | |
| Gestational diabetes | 13 | 425 890 | 40 567 | 1.03 (0.76 to 1.39) | 54 | |
| Fetal distress | 3 | 874 | 47 | 1.50 (0.64 to 3.53) | 8 | |
| Stillbirth | 6 | 413 122 | 1366 | 2.11 (1.14 to 3.90) | 24 | |
| Chorioamnionitis or intra-amniotic infection | 5 | 4368 | 433 | 0.85 (0.57 to 1.26) | 0 | |
| Admission to the ICU | 5 | 409 737 | 2012 | 4.78 (2.03 to 11.25) | 76 | |
| Cesarean delivery | 22 | 429 366 | 121 650 | 1.00 (0.82 to 1.23) | 78 | |
| Postpartum hemorrhage | 5 | 2981 | 355 | 0.89 (0.52 to 1.53) | 55 | |
| Preterm birth | 18 | 425 357 | 25 071 | 1.82 (1.38 to 2.39) | 64 | |
| Neonatal sex, male | 5 | 11 985 | 6369 | 0.97 (0.71 to 1.33) | 9 | |
| Gestational age at birth, wk | 13 | 4197 | −0.24 (−0.49 to 0.00) | 61 | ||
| Birth weight, g | 13 | 2973 | −68.96 (−130.22 to −7.69) | 29 | ||
| Low birth weight | 2 | 1054 | 678 | 2.32 (0.26 to 21.07) | 85 | |
| Admission to the NICU | 10 | 5675 | 785 | 3.69 (1.39 to 9.82) | 94 | |
| Neonatal death | 5 | 2838 | 1.10 (0.41 to 2.95) | 0 | ||
| Patients with symptomatic versus asymptomatic COVID-19 | ||||||
| Gestational hypertension or preeclampsia | 8 | 4122 | 333 | 1.20 (0.92 to 1.56) | 1 | |
| Gestational diabetes | 5 | 3767 | 256 | 1.12 (0.82 to 1.55) | 0 | |
| Mechanical ventilation | 3 | 1023 | 62 | 16.29 (3.88 to 68.47) | 0 | |
| Admission to the ICU | 4 | 1178 | 97 | 7.40 (0.48 to 114.24) | 76 | |
| Cesarean delivery | 9 | 4232 | 1406 | 1.57 (1.32 to 1.85) | 1 | |
| Preterm birth | 9 | 4233 | 582 | 2.29 (1.49 to 3.53) | 57 | |
| Admission to the NICU | 4 | 2365 | 248 | 3.47 (0.38 to 31.49) | 88 | |
| Neonatal death | 4 | 938 | 11 | 3.67 (0.88 to 15.29) | 0 | |
| Patients with severe versus mild COVID-19 | ||||||
| Preeclampsia | 5 | 521 | 40 | 4.16 (1.55 to 11.15) | 0 | |
| Gestational diabetes | 5 | 1140 | 105 | 1.99 (1.09 to 3.64) | 14 | |
| Abnormal liver function | 4 | 350 | 116 | 6.47 (2.60 to 16.09) | 50 | |
| Lymphopenia | 4 | 561 | 221 | 3.04 (1.93 to 4.79) | 0 | |
| Admission to the ICU | 5 | 757 | 70 | 15.46 (5.79 to 41.23) | 0 | |
| Mechanical ventilation | 5 | 962 | 97 | 19.31 (9.38 to 39.72) | 0 | |
| Cesarean delivery | 8 | 1138 | 452 | 2.58 (1.64 to 4.06) | 43 | |
| Preterm birth | 10 | 1393 | 277 | 4.29 (2.41 to 7.63) | 61 | |
| Gestational age at birth, wk | 8 | 709 | −3.50 (−5.96 to −1.03) | 91 | ||
| Low birth weight | 2 | 400 | 74 | 1.89 (1.14 to 3.12) | 0 | |
| Admission to the NICU | 5 | 729 | 160 | 3.95 (1.43 to 10.95) | 79 | |
| Neonatal death | 3 | 827 | 12 | 33.71 (5.18 to 219.44) | 0 | |
Note: CI = confidence interval, COVID-19 = coronavirus disease 2019, ICU = intensive care unit, NICU = neonatal intensive care unit, OR = odds ratio.
Compared with asymptomatic COVID-19, symptomatic COVID-19 in pregnancy was associated with increased risk of preterm birth (OR 2.29, 95% CI 1.49 to 3.53; I2 = 57%; based on 9 studies) (Figure 3B) and cesarean delivery (OR 1.57, 95% CI 1.32 to 1.85; I2 = 1%; 9 studies) (Appendix 4). Symptomatic COVID-19 was not associated with gestational diabetes (Table 1).
Compared with mild COVID-19, severe COVID-19 was strongly associated with preeclampsia (OR 4.16, 95% CI 1.55 to 11.15; I2 = 0%; based on 5 studies) (Figure 2C), preterm birth (OR 4.29, 95% CI 2.41 to 7.63; I2 = 61%; 10 studies) (Figure 3C), gestational diabetes (OR 1.99, 95% CI 1.09 to 3.64; I2 = 14%; 5 studies), ICU admission (OR 15.46, 95% CI 5.79 to 41.23; I2 = 0%; 5 studies), mechanical ventilation (OR 19.31, 95% CI 9.38 to 39.72; I2 = 0%; 5 studies), cesarean delivery (OR 2.58, 95% CI 1.64 to 4.06; I2 = 43%; 8 studies), low birth weight (OR 1.89, 95% CI 1.14 to 3.12; I2 = 0%; 2 studies) and NICU admission (OR 3.95, 95% CI 1.43 to 10.95; I2 = 79%; 5 studies) (Table 1, Appendix 4).
Sensitivity analyses produced similar results (Appendices 5 and 6, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.202604/tab-related-content). Funnel plots for preeclampsia and preterm birth did not suggest that the conclusions were affected by publication bias (Appendix 7, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.202604/tab-related-content).
Interpretation
We found that COVID-19 in pregnancy is associated with preeclampsia, stillbirth and preterm birth compared with no COVID-19. Symptomatic COVID-19 was associated with an increased risk of cesarean delivery and preterm birth compared with asymptomatic COVID-19. Compared with mild COVID-19, severe COVID-19 was strongly associated with preeclampsia, gestational diabetes, preterm birth and low birth weight. This meta-analysis of observational studies is unique in providing comparative data on COVID-19 morbidity during pregnancy. Our findings suggest that COVID-19 in pregnancy is associated with preeclampsia and preterm birth, and that severe COVID-19 can lead to considerable maternal and neonatal morbidity.
We selected studies with low-to-moderate risk of bias using strict quality assessment criteria.23 Although the number of publications on COVID-19 in pregnant people continues to increase, previous systematic reviews on COVID-19 in pregnancy have included mainly case reports and case series,4,5 or reviewed case reports for other types of coronavirus,55 describing the proportion of patients with clinical manifestations or pregnancy complications. These reviews recognized the lack of good-quality data in the early stage of the pandemic that are needed to draw unbiased conclusions. One of the earliest systematic reviews reported that there was no difference in the clinical characteristics of patients with COVID-19 who were pregnant compared with patients who were not pregnant.56 A living systematic review that included mainly case reports and case series,5 many of which used control groups of people who were not pregnant, or did not have a comparison group, reported that pregnant patients were less likely to have COVID-19 symptoms than those with COVID-19 who were not pregnant.5 Our meta-analysis of recent good-quality cohort studies with comparative data does not align with these previous reviews, and provides clear evidence that symptomatic or severe COVID-19 is associated with a considerable risk of preeclampsia, preterm birth and low birth weight.
The mechanisms underlying the association between COVID-19 and preeclampsia are unclear, but investigators have shown that SARS-CoV-2 may lead to renin–angiotensin system dysfunction and vasoconstriction by binding to angiotensin-converting enzyme 2 receptors.57 The hallmark of preeclampsia is a systematic endothelial dysfunction, which may share a common pathway with COVID-19 illness as the vascular effects of SARS-CoV-2 infection are increasingly recognized. One study found that people with severe COVID-19 who were pregnant acquired clinical manifestations similar to preeclampsia and were distinguishable by biomarker levels, including serum-soluble fms-like tyrosine kinase and placental growth factor.19 Some studies have shown that SARS-CoV-2 infection may create a proinflammatory state that is followed by systemic endothelial dysfunction and preeclampsia.58,59 Our finding is consistent with a 2020 study in Sweden that reported that pregnant people with COVID-19 had a higher prevalence of preeclampsia.43
Our meta-analysis also suggests that SARS-CoV-2 infection was associated with preterm birth, stillbirth and lower birth weight but not with cesarean delivery, compared with the absence of SARS-CoV-2 infection. We also found that severe COVID-19 was strongly associated with preterm birth and other adverse perinatal outcomes. Some of these excess risks could relate to preeclampsia, although SARS-CoV-2 infection may also cause exaggerated systemic inflammatory responses involved in the pathogenesis of preterm birth or a suboptimal environment for fetal growth and development. Placental fetal vascular malperfusion has been found in placental histopathologic findings in patients with COVID-19 at delivery, 41 which may contribute to fetal growth, stillbirth and preterm birth. A recent national quasi-experimental study in the Netherlands found that COVID-19 mitigation measures were associated with a reduced incidence of preterm birth.60
Lack of knowledge about SARS-CoV-2 infection in pregnancy has raised urgent questions among obstetricians and neonatologists about the risk of maternal, fetal and neonatal morbidity and mortality. There is an urgent need for evidence to guide clinical decisions. Our findings suggest that SARS-CoV-2 infection increases the risk of preeclampsia, stillbirth, preterm birth and NICU admission, and that severe COVID-19 illness in pregnancy is particularly problematic for adverse maternal, fetal and neonatal outcomes. Clinicians should be aware of these adverse outcomes when managing pregnancies in patients with COVID-19 and adopt effective strategies to prevent or reduce risks to patients and fetuses.
Limitations
We did not register our study with the International Prospective Register of Systematic Reviews (PROSPERO), and our literature search was restricted to publications in English. Although we included a comprehensive number of outcomes, we cannot rule out the possibility that some associations were spurious. Some of the included studies (14%) did not require a negative result for a PCR test to be included in the unexposed comparison group.22,32,37,40,42,47 We estimated unadjusted effect sizes, which may have overestimated risks. The largest study that had data on confounders suggested that associations between COVID-19 and pregnancy outcomes were somewhat attenuated after adjustment, although risks remained elevated for most outcomes.53 We also could not determine the clinical importance of some of the outcomes. For example, we cannot confirm that all patients admitted to the NICU required critical care. The reason for preterm birth was not clear, including if preterm birth was medically indicated or spontaneous. As the data were observational, we cannot eliminate the possibility of residual confounding.
Conclusion
We found that SARS-CoV-2 infection in pregnancy was associated with risks of preeclampsia, stillbirth, preterm birth and NICU admission. In addition, severe SARS-CoV-2 infection was strongly associated with preeclampsia and other adverse maternal and neonatal outcomes. Future studies are needed to collect more robust data to further validate or substantiate these findings, better understand the pathophysiologic pathways that explain these associations and identify effective strategies to prevent adverse outcomes in pregnant people with COVID-19.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Shu Qin Wei and Nathalie Auger contributed to the conception and design of the study. Shu Qin Wei and Marianne Bilodeau-Bertrand screened the articles and assessed the quality of the studies. Shu Qin Wei and Shiliang Liu performed the data extraction and analysis. Shu Qin Wei drafted the manuscript, and Nathalie Auger, Marianne Bilodeau-Bertrand and Shiliang Liu revised the manuscript critically for important intellectual content. All of the authors contributed to the interpretation of the data, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: This work was supported by a grant from the Canadian Institutes of Health Research (no. PJT-162300). Nathalie Auger has received a career award from the Fonds de recherche du Québec-Santé (no. 34695).
Data sharing: All relevant data are provided in the article and the appendices, and are available for use.
References
- 1.Wang C, Horby PW, Hayden FG, et al. A novel coronavirus outbreak of global health concern. Lancet 2020;395:470–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wastnedge EAN, Reynolds RM, van Boeckel SR, et al. Pregnancy and COVID-19. Physiol Rev 2021;101:303–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dashraath P, Wong JLJ, Lim MXK, et al. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am J Obstet Gynecol 2020;222:521–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huntley BJF, Huntley ES, Di Mascio D, et al. Rates of maternal and perinatal mortality and vertical transmission in pregnancies complicated by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a systematic review. Obstet Gynecol 2020;136:303–12. [DOI] [PubMed] [Google Scholar]
- 5.Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ 2020;370:m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breslin N, Baptiste C, Gyamfi-Bannerman C, et al. COVID-19 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM 2020;2:100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delahoy MJ, Whitaker M, O’Halloran A, et al. Characteristics and maternal and birth outcomes of hospitalized pregnant women with laboratory-confirmed COVID-19-COVID-NET, 13 states, March 1–August 22, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WAPM (World Association of Perinatal Medicine) Working Group on COVID-19. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection. Ultrasound Obstet Gynecol 2021;57:232–41. [DOI] [PubMed] [Google Scholar]
- 9.Khoury R, Bernstein PS, Debolt C, et al. Characteristics and outcomes of 241 births to women with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection at five New York City medical centers. Obstet Gynecol 2020;136:273–82. [DOI] [PubMed] [Google Scholar]
- 10.London V, McLaren R, Atallah F, et al. The relationship between status at presentation and outcomes among pregnant women with COVID-19. Am J Perinatol 2020;37:991–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prabhu M, Cagino K, Matthews KC, et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG 2020;127:1548–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakowicz A, Ayala AE, Ukeje CC, et al. Risk factors for severe acute respiratory syndrome coronavirus 2 infection in pregnant women. Am J Obstet Gynecol MFM 2020;2:100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smithgall MC, Liu-Jarin X, Hamele-Bena D, et al. Third-trimester placentas of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-positive women: histomorphology, including viral immunohistochemistry and in-situ hybridization. Histopathology 2020;77:994–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verma S, Bradshaw C, Auyeung NSF, et al. Outcomes of maternal–newborn dyads after maternal SARS-CoV-2. Pediatrics 2020;146:e2020005637. [DOI] [PubMed] [Google Scholar]
- 15.Díaz-Corvillón P, Mönckeberg M, Barros A, et al. Routine screening for SARS-CoV-2 in unselected pregnant women at delivery. PLoS One 2020;15:e0239887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen J, Vignaux O, Jacquemard F. Covid-19 in pregnant women: general data from a French National Survey. Eur J Obstet Gynecol Reprod Biol 2020;251: 267–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kayem G, Lecarpentier E, Deruelle P, et al. A snapshot of the COVID-19 pandemic among pregnant women in France. J Gynecol Obstet Hum Reprod 2020;49: 101826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martínez-Perez O, Vouga M, Cruz Melguizo S, et al. Association between mode of delivery among pregnant women with COVID-19 and maternal and neonatal outcomes in Spain. JAMA 2020;324:296–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendoza M, Garcia-Ruiz I, Maiz N, et al. Preeclampsia-like syndrome induced by severe COVID-19: a prospective observational study. BJOG 2020;127: 1374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onwuzurike C, Diouf K, Meadows AR, et al. Racial and ethnic disparities in severity of COVID-19 disease in pregnancy in the United States. Int J Gynaecol Obstet 2020;151:293–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Savasi VM, Parisi F, Patanè L, et al. Clinical findings and disease severity in hospitalized pregnant women with coronavirus disease 2019 (COVID-19). Obstet Gynecol 2020;136:252–8. [DOI] [PubMed] [Google Scholar]
- 22.Brandt JS, Hill J, Reddy A, et al. Epidemiology of coronavirus disease 2019 in pregnancy: risk factors and associations with adverse maternal and neonatal outcomes. Am J Obstet Gynecol 2020; S0002-9378:31134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wells G, Shea B, O’connell D, et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa: The Ottawa Hospital Research Institute; 2013. Available: www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 2020 Sept. 10). [Google Scholar]
- 24.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239–42. [DOI] [PubMed] [Google Scholar]
- 25.Deeks JJ, Higgins JPT, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions Version 6.1. Cochrane; 2020. Available: www.training.cochrane.org/handbook (accessed 2021 Jan. 22). [Google Scholar]
- 26.Barbero P, Mugüerza L, Herraiz I, et al. SARS-CoV-2 in pregnancy: characteristics and outcomes of hospitalized and non-hospitalized women due to COVID-19. J Matern Fetal Neonatal Med 2020. July 20 [Epub ahead of print];1–7. 10.1080/14767058.2020.1793320. [DOI] [PubMed] [Google Scholar]
- 27.Flaherman VJ, Afshar Y, Boscardin J, et al. Infant outcomes following maternal infection with SARS-CoV-2: first report from the PRIORITY study. Clin Infect Dis 2020. Sept. 18 [Epub ahead of print];ciaa1411. 10.1093/cid/ciaa1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffin I, Benarba F, Peters C, et al. The impact of COVID-19 infection on labor and delivery, newborn nursery, and neonatal intensive care unit: prospective observational data from a single hospital system. Am J Perinatol 2020;37: 1022–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gulersen M, Prasannan L, Tam Tam H, et al. Histopathologic evaluation of placentas after diagnosis of maternal severe acute respiratory syndrome coronavirus 2 infection. Am J Obstet Gynecol MFM 2020;2:100211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knight M, Bunch K, Vousden N, et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population-based cohort study. BMJ 2020;369:m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li N, Han L, Peng M, et al. Maternal and neonatal outcomes of pregnant women with COVID-19 pneumonia: a case-control study. Clin Infect Dis 2020;71:2035–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Chen H, Tang K, et al. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J Infect 2020. Mar. 4 [Epub ahead of print]. 10.1016/j.jinf.2020.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maraschini A, Corsi E, Salvatore MA, et al. Coronavirus and birth in Italy: results of a national population-based cohort study. Ann Ist Super Sanita 2020;56:378–89. [DOI] [PubMed] [Google Scholar]
- 34.Martinez-Perez O, Rodriguez PP, Hernandez MM, et al. The association between COVID-19 and preterm delivery: a cohort study with a multivariate analysis [preprint]. medRxiv 2020. Sept. 7. Available: 10.1101/2020.09.05.20188458 (accessed 2021 Jan. 20). [DOI]
- 35.Nayak AH, Kapote DS, Fonseca M, et al. Impact of the coronavirus infection in pregnancy: a preliminary study of 141 patients. J Obstet Gynaecol India 2020;70:256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panagiotakopoulos L, Myers TR, Gee J, et al. SARS-CoV-2 infection among hospitalized pregnant women: reasons for admission and pregnancy characteristics — eight U.S. health care centers, March 1–May 30, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1355–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pirjani R, Hosseini R, Soori T, et al. Maternal and neonatal outcomes in COVID-19 infected pregnancies: a prospective cohort study. J Travel Med 2020;27: taaa158. 10.1093/jtm/taaa158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vivanti AJ, Mattern J, Vauloup-Fellous C, et al. Retrospective description of pregnant women infected with severe acute respiratory syndrome coronavirus 2, France. Emerg Infect Dis 2020;26:2069–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang MJ, Schapero M, Iverson R, et al. Obstetric hemorrhage risk associated with novel COVID-19 diagnosis from a single-institution cohort in the United States. Am J Perinatol 2020;37:1411–6. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Jiang Y, Wei M, et al. Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province. Zhonghua Fu Chan Ke Za Zhi 2020;55: 166–71. [DOI] [PubMed] [Google Scholar]
- 41.Patberg ET, Adams T, Rekawek P, et al. COVID-19 infection and placental histopathology in women delivering at term. Am J Obstet Gynecol 2020. Oct. 19 [Epub ahead of print]; S0002-9378(20)31194-7. 10.1016/j.ajog.2020.10.020. [DOI] [Google Scholar]
- 42.Yang R, Mei H, Zheng T, et al. Pregnant women with COVID-19 and risk of adverse birth outcomes and maternal-fetal vertical transmission: a population-based cohort study in Wuhan, China. BMC Med 2020;18:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahlberg M, Neovius M, Saltvedt S, et al. Association of SARS-CoV-2 test status and pregnancy outcomes. JAMA 2020;324:1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cunarro-Lopez Y, Cano-Valderrama Ó, Pintado-Recarte P, et al. Maternal and perinatal outcomes in patients with suspected COVID-19 and their relationship with a negative RT–PCR result. J Clin Med 2020;9:3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rios-Silva M, Murillo-Zamora E, Mendoza-Cano O, et al. COVID-19 mortality among pregnant women in Mexico: a retrospective cohort study. J Glob Health 2020;10:020512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woodworth KR. Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy — SET-NET, 16 jurisdictions, March 29–October 14, 2020. MMWR Morb Mortal Wkly Rep 2021;69:1635–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yazihan N, Tanacan A, Erol SA, et al. Comparison of VEGF-A values between pregnant women with COVID-19 and healthy pregnancies and its association with composite adverse outcomes. J Med Virol 2021;93:2204–9. [DOI] [PubMed] [Google Scholar]
- 48.Adhikari EH, Moreno W, Zofkie AC, et al. Pregnancy outcomes among women with and without severe acute respiratory syndrome coronavirus 2 infection. JAMA Netw Open 2020;3:e2029256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edlow AG, Li JZ, Collier AY, et al. Assessment of maternal and neonatal SARS-CoV-2 viral load, transplacental antibody transfer, and placental pathology in pregnancies during the COVID-19 pandemic. JAMA Netw Open 2020;3:e2030455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Erol SA, Tanacan A, Anuk AT, et al. Evaluation of maternal serum afamin and vitamin E levels in pregnant women with COVID-19 and its association with composite adverse perinatal outcomes. J Med Virol 2021;93:2350–8. [DOI] [PubMed] [Google Scholar]
- 51.Hcini N, Maamri F, Picone O, et al. Maternal, fetal and neonatal outcomes of large series of SARS-CoV-2 positive pregnancies in peripartum period: a single-center prospective comparative study. Eur J Obstet Gynecol Reprod Biol 2021; 257: 11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jenabi E, Bashirian S, Khazaei S, et al. Pregnancy outcomes among symptomatic and asymptomatic women infected with COVID-19 in the west of Iran: a case–control study. J Matern Fetal Neonatal Med 2020. Dec. 15 [Epub ahead of print];1–3. 10.1080/14767058.2020.1861599. [DOI] [PubMed] [Google Scholar]
- 53.Jering KS, Claggett BL, Cunningham JW, et al. Clinical characteristics and outcomes of hospitalized women giving birth with and without COVID-19. JAMA Intern Med 2021. Jan. 15 [Epub ahead of print];e209241. 10.1001/jamainternmed.2020.9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pineles BL, Alamo IC, Farooq N, et al. Racial–ethnic disparities and pregnancy outcomes in SARS-CoV-2 infection in a universally tested cohort in Houston, Texas. Eur J Obstet Gynecol Reprod Biol 2020;254:329–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Di Mascio D, Khalil A, Saccone G, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM 2020;2:100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matar R, Alrahmani L, Monzer N, et al. Clinical presentation and outcomes of pregnant women with COVID-19: a systematic review and meta-analysis. Clin Infect Dis 2021;72:521–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gheblawi M, Wang K, Viveiros A, et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin–angiotensin system. Circ Res 2020;126:1456–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coronado-Arroyo JC, Concepción-Zavaleta MJ, Zavaleta-Gutiérrez FE, et al. Is COVID-19 a risk factor for severe preeclampsia? Hospital experience in a developing country. Eur J Obstet Gynecol Reprod Biol 2021;256:502–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Todros T, Masturzo B, Francia SD. COVID-19 infection: ACE2, pregnancy and preeclampsia. Eur J Obstet Gynecol Reprod Biol 2020;253:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Been JV, Ochoa LB, Bertens LCM, et al. Impact of COVID-19 mitigation measures on the incidence of preterm birth: a national quasi-experimental study. Lancet Public Health 2020;5:e604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]