Abstract
The Severe acute respiratory syndrome may be caused by coronavirus disease which has resulted in a global pandemic. Polymorphisms in the population play a role in susceptibility to severity. We aimed to perform a systematic review related to the effect of single nucleotide polymorphisms in the development of severe acute respiratory syndrome (SARS). Twenty-eight eligible articles published were identified in PubMed, ScienceDirect, Web of Science, PMC Central and Portal BVS and additional records, with 20 studies performed in China. Information on study characteristics, genetic polymorphisms, and comorbidities was extracted. Study quality was assessed by the STrengthening the REporting of Genetic Association (STREGA) guideline. Few studies investigated the presence of polymorphisms in HLA, ACE1, OAS-1, MxA, PKR, MBL, E-CR1, FcγRIIA, MBL2, L-SIGN (CLEC4M), IFNG, CD14, ICAM3, RANTES, IL-12 RB1, TNFA, CXCL10/IP-10, CD209 (DC-SIGN), AHSG, CYP4F3 and CCL2 with the susceptibility or protection to SARS-Cov. This review provides comprehensive evidence of the association between genetic polymorphisms and susceptibility or protection to severity SARS-CoV. The literature about coronavirus infection, susceptibility to severe acute respiratory syndrome (SARS) and genetic variations is scarce. Further studies are necessary to provide more concrete evidence, mainly related to Covid-19.
Keywords: Coronavirus, Severe acute respiratory syndrome, Susceptibility, Genetic polymorphism
1. Introduction
Since December 2019, when the first cases of COVID-19 were described in Wuhan (China), the virus has rapidly spread to other parts of the world (Li et al., 2020) and it was considered by the World Health Organization (WHO) as one public health problem (Zheng et al., 2020; Carod Artal, 2020). Coronaviruses (CoVs) consist of an enveloped, positive-sense, single-stranded RNA viruses (genome size - 30 Kb), belonging to the family Coronaviridae and subfamily Coronavirinae (Fehr and Perlman, 2015) and has been associated with several clinical conditions that involved respiratory, enteric, hepatic, neurological, hypercoagulability and endotheliopathy symptoms and signs (Almqvist et al., 2020; Benvenuto et al., 2020; Hassan et al., 2020).
Previous outbreaks have reported an association between coronaviruses and severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS) and the Severe acute respiratory syndrome coronavirus 2 or SARS-CoV-2 (Rothan and Byrareddy, 2020). Severity ranges from asymptomatic infection or mild disease, characterized by dry cough, fever, dyspnea, and fatigue, to critical illness with respiratory failure, SARS and death (Dahan et al., 2020). In SARS, subjects show clinical inflammation, hemorrhage, alveolar edema, hyaline membrane formation typical of the exudative stage, fluid accumulation with consequent fibrosis pulmonary and lead to respiratory failure (Gralinski and Baric, 2015).
The pathophysiology of SARS-Cov-2 is related to the mechanism used by the virus to enter into the host cell by binding to the angiotensin-2 converting enzyme (ACE-2) which is expressed in various tissues. The spike protein (localized in virus membrane) is needed to bidding process, and it is also considered as a necessary factor for virulence and to promote specific tissue affinity or tissue tropism and infectivity (Du et al., 2009; Hoffmann et al., 2020; Li, 2016). The protein Spike is cleaved in the S2 domain, which is adjacent to the fusion peptide by the protease TMPRSS2, localized in the host cells, causing a structural modification capable of facilitating the entry of the virus in target cells (Hoffmann et al., 2020).
Single nucleotide polymorphism (SNP) can be localized in coding and non-coding regions and, it may be involved in various phenotypic characteristics including susceptibility, severity, resistance or protection to diseases (Shen et al., 1999). A polymorphism may have functional effects, resulting in modifications of the catalytic function, stability and/or level of expression of the protein (Kelada et al., 2003). Polymorphisms in MHC I and II had influence in the cell entry process by other CoVs (Vera S.F. Chan et al., 2006; Keicho et al., 2009; Wang et al., 2011) (Li et al., 2020, Keicho et al., 2009; Chan et al., 2006; Wang et al., 2011). According to Bender et al. (2020), link between a virus and the Toll-like receptor 7 (TRL7) / Toll-like receptor 8 (TLR8) may activate a pathway that stimulates the production of pro-inflammatory cytokines (Qin et al., 2020). Additionally, the angiotensin I converting enzyme enhances the synthesis of angiotensin II, which induces cell proliferation and enhances proinflammatory cytokines and metalloproteinases matrix (Sprague and Khalil, 2009). These events may be due to the viral and/or host genetic characteristics as described in studies with other coronaviruses (REFERENCIAS). Thus, the aim of this study was to analyze genetics profiles involved in the pathogenesis of the severe acute respiratory syndrome (SARS) in patients with coronavirus disease.
2. Material and methods
2.1. Design
This systematic review followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) (Moher et al., 2009). The systematic review is registered in PROSPERO: CRD42020178334.
2.2. Search strategy
The method PICOS (Santos et al., 2007) was used for definition of descriptors for assessing eligibility. In which participants were patients (P) with the severe acute respiratory syndrome and coronavírus; the intervention (I): genetics polymorphisms identification; compared to control groups (C): absence of coronavírus group; outcome (O): the presence or absence of the genetic polymorphism in the group of patients with coronavírus; Study design (S): a genetic association study. The keywords were used as follows: coronavirus, polymorphism genetic and severe acute respiratory syndrome. The databases PubMed, Web of Science, Science direct, PMC central and Virtual Health Library – VHL (Portal BVS - Portal Regional da BVS). To supplement the electronic search, references of all relevant studies were also screened for potential consideration.
2.3. Selection criteria
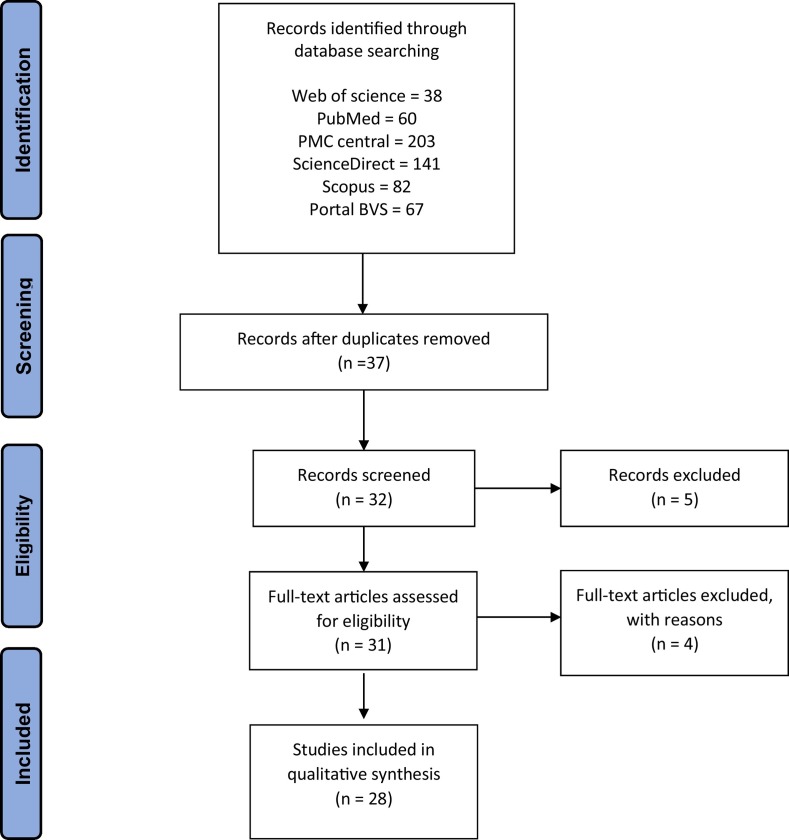
We researched observational studies carried out in humans that evaluated the associations between genetic polymorphisms and susceptibility to severe acute respiratory syndrome and coronavirus. The studies included in the systematic review were (1) articles that investigated genetic polymorphisms about coronavirus associated with severe acute respiratory syndrome, (2) original data, and (3) studies in English or Portuguese language. The exclusion criteria were (1) duplicated articles; (2) case, review, or articles with only an abstract and (3) articles with in vitro experiments. The full text was read by all authors, because studies could not be decided based only the title and abstract. The selected studies were established by the descriptors eligible for this review. Disagreements regarding to the inclusion of studies were resolved by a consensus (Fig. 1 ).
Fig. 1.
Flow diagram Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) illustrating the studies' selection process.
2.4. Data extraction and quality assessment
The selected articles were analyzed and data were extracted by two independent researchers in regards to the author, year, study design, country, design, sample size, genotyping method, gene, SNPs, outcomes, Hardy–Weinberg equilibrium (HWE) and comorbidities in SARS patients. Four authors (S.P.O.; A.C.M.S.; E.L.M.; L.C.S.) independently extracted data from all eligible studies on the collection forms according to a standard protocol. Two reviewers (BRCS and BBS) independently assessed the study design, methods and results of the genetic association studies. Methodological quality of each study was based on the STrengthening the REporting of Genetic Association (STREGA) guideline and the results were discussed to reach a consensus (Little et al., 2009). STREGA contains five main divisions, including genotyping methods and errors, population stratification, haplotype variation, HWE and replication, with a total of nine items to be evaluated. In all studies, the total score was measured by attributing one point for each item, with a high score indicating studies with better quality (range: 0–9, higher scores indicating higher overall quality).
3. Results
3.1. Overview of results
In the initial screening, we identified a total of 591 publications and posteriorly we remove the duplicates. A total of 39 papers were eligible and selected for full-text review based on the title and abstract information related with SARS and genetic polymorphism. The primary reasons for exclusion included: no full-text access, duplicates, reviews or metanalyses and no sufficient information about the theme (Fig. 1).
Finally, 28 eligible studies were included in this review, approaching the following 40 genes: Angiotensin-converting enzyme (ACE) (n = 3) (Chan et al., 2005c; Chiu et al., 2004; Itoyama et al., 2005, Itoyama et al., 2004); Mannose-binding lectin (MBL) (n = 4) (Ip et al., 2005; Tu et al., 2015; Yuan et al., 2005; Zhang et al., 2005); Human leukocyte antigen (HLA) (n = 3) (Keicho et al., 2009; Lin et al., 2003); MX dynamin like GTPase 1 (MxA) (n = 2) (He et al., 2006); Cluster of Differentiation 209 (CD209) (n = 1) (Chan et al., 2010); Tnf alpha (n = 2); Interferon (IFN) (n = 2); liver/lymph node-specific intracellular adhesion molecules-3 grabbing non-integrin (L-SIGN) (n = 2); erythrocyte-complement receptor Type1 (E-CR1) (n = 1) (Wang et al., 2005); 2′-5′-Oligoadenylate Synthetase 1 (OAS1) (n = 1) (He et al., 2006); cluster of differentiation 14 (CD14) (n = 1); toll like receptor 2 (TLR2) (n = 1); toll like receptor 4 (TLR4) (n = 1); Intercellular Adhesion Molecule 3 (ICAM3) (n = 1) (Chan et al., 2007); Fragment of IgE Receptor II (FCER2) (n = 1); C-X-C Motif Chemokine Ligand 10 (CXCL10) (n = 1) (Hsieh et al., 2010); Heme Oxygenase 1 (HMOX1) (n = 1) (Hsieh et al., 2010); Fibrinogen-like protein 2 (FGL2) (n = 1) (Hsieh et al., 2010); alpha 2-HS Glycoprotein (AHSG) (n = 1); Cytochrome P450 Family 3A (CYP4F3A) (n = 1); C-C Motif Chemokine Ligand 2 (CCL2) (n = 1); FcγRIIA (n = 1); Interleukin-10 (n = 1); Interleukin-12 (n = 1); mannose-binding protein-associated serine protease 2 (MASP-2) (n = 1) and Regulated upon Activation Normal T Cell Expressed and Presumably Secreted (RANTES) (n = 1).
Most studies included both male and female participants, however, male sex was more prevalent. Of the 27 articles, 26 were case-control studies and one cohort study. Methods for detecting polymorphisms included PCR-SSP (polymerase chain reaction-sequence specific of primers), RFLP for restriction enzyme (Restriction Fragment Length Polymorphism), qPCR (real-time PCR), sequencing-based typing (SBT), single specific primer-polymerase chain reaction (PCR-SSP) and sequence-specific oligonucleotides probes (PCR-SSOP) (Table 1 ). Few studies (Chan et al., 2005c; Itoyama et al., 2004; Lin et al., 2003; Wang et al., 2005; Zhang et al., 2005) reported the presence of SARS-Cov severity associated comorbidities as a chronic obstructive pulmonary disease, flu, coronary artery disease, cerebral vascular disease, cancer, diabetes mellitus, chronic kidney disease, cirrhosis, hepatitis C, Human Immunodeficiency Virus (HIV) and systemic arterial hypertension.
Table 1.
Characteristics of studies included in this review.
| Author, year |
Country |
Design |
Sample size |
Genotyping method |
Gene |
SNPs |
Outcomes |
HWE (Y/N) |
STREGA total score |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | |||||||||
| Lin et al., 2003 | Taiwan | Case–control | 37 | 190 | PCR-SSOP | HLA-class I and II | HLA-A, B and DRB | HLA-B*4601 and risk to SARS | NR | 15 |
| Itoyama et al., 2004 | Vietnam | Case–control | 44 | 153 | Conventional PCR | ACE1 | insertion/deletion (I/D) | progression of pneumonia (D allele) | NR | 15 |
| Chiu et al., 2004 | China | Case–control | 168 | 328 | Real-Time PCR | ACE2 | rs2285666, rs4646142, rs714205, rs2106809 and rs2074192 | No relation | Y | 18 |
| Chan et al., 2005a, Chan et al., 2005b, Chan et al., 2005c | China | Case–control | 140 | 326 | Conventional PCR | ACE | insertion/deletion (I/D) | No relation | Y | 18 |
| Hamano et al., 2005 | Vietnam | Case-control | 44 | 103 | PCR - RFLP | OAS-1 | rs2660 and | associated with SARS-CoV infection or development of SARS | N | 19 |
| rs3741981 | ||||||||||
| PCR - RFLP | MxA | −88 | N | |||||||
| PCR - RFLP | PKR | −168 | N | |||||||
| Ip et al., 2005 | China | Case- control | 569 | 1189 | Real- time -PCR | MBL | promoter (−221 X/Y) and exon 1 (codon 54 A/B) | susceptibility to SARS | NR | 20 |
| Itoyama et al., 2005 | Vietnam | Case- control | 44 | 103 | PCR - RFLP | ACE2 | rs2285666, rs4646140, rs25082, rs25424, rs4646165, rs2301693, rs2301692, rs30816, rs4646174, rs33121, rs33205, rs36655, rs38926, rs39663, rs39705 and rs39844 | No relation | NR | 18 |
| Wang et al., 2005 | China | Case–control | 54 | 212 | PCR - RFLP | E-CR1 | CR1 Hind III | Progression of SARS | Y | 18 |
| Yuan et al., 2005 | China | Case-control | 180 | 200 | PCR- SSP | FcγRIIA | R-H-131 | Susceptibility to SARS | NR | 18 |
| MBL2 | rs11003125, rs7096206, rs5030737, rs1800450 and rs1800451 | Susceptibility to SARS | NR | |||||||
| Zhang et al., 2005 | China | Case–control | 352 | 392 | Conventional PCR | MBL | -596, -550, -435, -427, -349, -336, -329 to >324, -221, -70, 4, 223, 230, 239 and 366 | B allele had an increased susceptibility to SARS-CoV infection | Y | 18 |
| Chan et al., 2006 | China | Case-control | 285 | 380 | Conventional PCR | L-SIGN (CLEC4M) | CLEC4M 69-nucleotide tandem repeats in exon 4 | homozygosity for L-SIGN and protective role | N | 15 |
| He et al., 2006 | China | Case-control | 66 | 64 | Conventional PCR | OAS1 | 3′UTR 347 locus of the exon 8 | susceptibility to SARS | NR | 15 |
| PCR - RFLP | MxA | - 88 | susceptibility to SARS | NR | ||||||
| Chong et al., 2006 | China | Case-control | 476 | 449 | RT-PCR | IFNG | rs2430561 | IFN-γ + 874 associated with susceptibility to SARS | Y | 15 |
| TNF-α | -308 | No relation | Y | |||||||
| IL-10 | -1082 and -592 | No relation | Y | |||||||
| Yuan et al., 2007 | China | Case-control | 152 | 198 | PCR - RFLP | CD14 | s2569190-159 | Genotype CC and risk | NR | 14 |
| TLR2 | 2180 and 2408 | No relation | NR | |||||||
| TLR4 | 12,874 and 13,174 | No relation | NR | |||||||
| Chan et al., 2007 | China | Case-control | 817 | 906 | Real-Time PCR | ICAM3 | rs2304237 (Asp143Gly) | Susceptibility of SARS | Y | 15 |
| Conventional PCR | FCER2 | rs4804773 (Trp62Arg); rs889182; rs1990975; rs2287868 and rs2303112. | No relation | Y | ||||||
| Ng et al., 2007 | China | Case-control | 495 | 578 | PCR | RANTES | rs2107538, rs2107538, rs2280789, rs8878, rs4859587 and rs10336 | RANTES -28 G allele was associated with SARS susceptibility in Hong Kong Chinese | Y | 18 |
| Khoo et al., 2008 | China | Case-control | 285 | 380 | PCR Multiplex | L-SIGN INF-α, INF-α, INF-β, INF-γ, INF-γ, IL1-α, IL1-α, IL1-β, IL-4, IL-6, iNOS, iNOS | Not described | No relation | NR | 18 |
| Tang et al., 2008 | China | Case- control | 115 | 296 | PCR- RFLP | IL-12 RB1 | + 705, + 1158, + 1196 and + 1664 | (CT e TT) increased risk of developing SARS | N | 20 |
| Wang et al., 2008a, Wang et al., 2008b | China | Case-control | 75 | 92 | PCR- SBT | TNF-alfa | −1031, −863, −857, −572, −308, −238, −204, −163 | CT genotype protective effect and TT genotype, CT and CC were found associated with a risk effect | NR | 16 |
| Xiong et al., 2008 | China | Case- control | 95 | 403 | PCR- SSP | HLA | HLA-A, HLA-B, HLA-DRB1 | No relation | N | 18 |
| Keicho et al., 2009 | Vietnam | Case-control | 62 | 50 | PCR | HLA classe I and class II | exons 2 and 3 of HLA -A, -B, and -C | HLA-DRB1*1202 with susceptibility to SARS | Y | 15 |
| exon 2 of HLA-DRB1 and -DQB1 | ||||||||||
| Wang et al., 2009 | China | Case-control | 376 | 523 | PCR | MASP2 | rs12711521, rs2261695, rs2273346 and rs7548659 | No relation | NR | 15 |
| Chan et al., 2010 | China | Case-control | 824 | 471 | Real-Time PCR | CD209 (DC-SIGN) | −336A (rs4804803) | No relation | Y | 18 |
| CXCL10/IP-10 | −938 | Protective | NR | 15 | ||||||
| HO-1 | −497 | No relation | NR | |||||||
| Fgl2 | +158 (rs2075761) | +158 T/* and Risk | NR | |||||||
| Hsieh et al., 2010 | China | Cohort | 824 | – | Real-Time PCR | CD209 (DC-SIGN) | −336A (rs4804803) | higher standardized LDH levels | Y | 15 |
| Ching et al., 2010 | China | Case-control | 792 | 418 | PCR | IFN | rs2071430 and rs17000900 | -88 T-positive and -123A-positive genotypes were significantly associated with decreased susceptibility to SARS coronavirus infection | Y | 16 |
| Zhu et al., 2011 | China | Case-control | 624 | 791 | PCR-RFLP | AHSG | rs2248690, rs2077119, rs4917, rs2593813 and rs4918 | rs2248690 AA and protection | Y | 16 |
| CYP4F3 | rs3794987, rs1159776, rs4646519 and rs1290625 | rs3794987 GG/AG and increased susceptibility to SARS | Y | |||||||
| Tu et al., 2015 | China | Case-control | 932 | 982 | PCR-RFLP | CCL2 | rs1024611 | risk of SARS-CoV infection | Y | 16 |
| MBL | Codon 54 variant (A > B) | risk of SARS-CoV infection | Y | |||||||
| rs1800450 | ||||||||||
| Ellinghaus et al., 2020 | Italian and Spanish | Case–control | 1760 | 2205 | Global Screening Array (GSA) | 3p21.31 | rs11385942 (SLC6A20, LZTFL1, CCR9, FYCO1, CXCR6 and XCR1) | association signal | NR | 20 |
| 9q34.2 | rs657152 (ABO blood group locus) | higher risk in blood group A to SARS-Cov-2 | NR | |||||||
3.2. Study quality assessment
Most of the selected studies met four of the nine items in the STREGA guideline. No study reached all nine items. However, one research (Tang et al., 2008) reported seven items, except for descriptions of laboratory/center where the genotyping was done and the statement of whether the study is the first report of a genetic association, a replication effort, or whether the assays were conducted simultaneously or in batch. Only one study (Yuan et al., 2007) described the following items: genotyping methods/platform and the numbers of individuals for whom genotyping was attempted and successful. The majority of the studies reported genotyping methods and platform and Hardy-Weinberg equilibrium (Table 2 ).
Table 2.
The quality of reporting using the STrengthening the REporting of Genetic Association (STREGA) guideline.
| Author, year |
Description of genotyping methods and errors |
Description of modeling population stratification |
Description of modeling haplotype Variation |
Statement of Whether Hardy-Weinberg Equilibrium was considered |
Statement of whether the study is the first report of a genetic association, a replication effort, or both |
Total score |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Genotyping methods and platforms | Error rates and call rates | Laboratory/center where the genotyping was done | Conducting genotypes simultaneously or in smaller batches | The numbers of individuals for whom genotyping was attempted and successful | ||||||
| Lin et al., 2003 | Y | N | N | N | Y | Y | N | N | N | 3 |
| Itoyama et al., 2004 | Y | N | N | N | Y | Y | N | N | N | 3 |
| Chiu et al., 2004 | Y | N | N | N | Y | Y | N | Y | N | 4 |
| Chan et al., 2005a, Chan et al., 2005b, Chan et al., 2005c | Y | N | N | N | Y | Y | N | Y | N | 4 |
| Hamano et al., 2005 | Y | N | N | Y | N | Y | Y | Y | N | 5 |
| Ip et al., 2005 | Y | N | Y | Y | N | Y | Y | Y | N | 6 |
| Itoyama et al., 2005 | Y | N | N | Y | Y | Y | N | N | N | 4 |
| Wang et al., 2005 | Y | N | N | N | Y | Y | N | Y | N | 4 |
| Yuan et al., 2005 | Y | Y | N | Y | N | N | Y | N | N | 4 |
| Zhang et al., 2005 | Y | N | N | N | Y | Y | N | Y | N | 4 |
| Chan et al., 2006 | Y | N | N | N | N | Y | N | Y | N | 3 |
| He et al., 2006 | Y | N | N | N | Y | Y | N | N | N | 3 |
| Chong et al., 2006 | Y | N | N | Y | N | N | N | Y | N | 3 |
| Yuan et al., 2007 | Y | N | N | N | Y | M | N | N | N | 2 |
| Chan et al., 2007 | Y | N | N | N | Y | Y | N | Y | N | 4 |
| Ng et al., 2007 | Y | Y | N | Y | N | Y | Y | Y | N | 6 |
| Khoo et al., 2008 | Y | N | N | Y | Y | N | N | Y | N | 4 |
| Tang et al., 2008 | Y | Y | N | Y | Y | Y | Y | Y | N | 7 |
| Wang et al., 2008a, Wang et al., 2008b | Y | N | N | Y | N | Y | N | Y | N | 4 |
| Xiong et al., 2008 | Y | N | N | Y | Y | Y | Y | N | N | 5 |
| Keicho et al., 2009 | Y | N | N | N | Y | M | N | Y | N | 3 |
| Wang et al., 2009 | Y | N | N | Y | N | N | N | Y | N | 3 |
| Chan et al., 2010 | Y | N | N | N | Y | Y | N | Y | N | 4 |
| Hsieh et al., 2010 | Y | N | N | N | N | Y | N | Y | N | 3 |
| Ching et al., 2010 | Y | Y | N | Y | N | Y | Y | Y | N | 6 |
| Zhu et al., 2011 | Y | N | N | N | Y | Y | N | Y | N | 4 |
| Tu et al., 2015 | Y | N | N | N | Y | Y | N | Y | N | 4 |
| Ellinghaus et al., 2020 | Y | N | Y | Y | Y | Y | N | Y | Y | 7 |
4. Discussion
4.1. Cytokines
Cytokines play a crucial role in the development of physiological functions, and their dysregulation or activation follows homeostatic changes, triggering the immunopathogenesis of several pathologies. Here, the cytokines interferon gamma (IFN-γ), tumor necrosis factor (TNF-α) and Interleukin-1 (IL-1) were investigated which were correlated with SARS in patients with covid-19. The interferon gamma (IFN-γ) is important in response immune. It activates monocytes and macrophages, participate in antiviral responses producing free radicals and pro-inflammatory cytokines, such as tumor necrosis factor (TNF-α) (Biron et al., 1999). Thus, IFN-γ and TNF-α play an important role in the antiviral response and the inflammatory process. In a study conducted by Chong and collaborators in 2006, the A allele of the IFNG + 874 polymorphism was associated with susceptibility to SARS in population. However, in that same study, no significant correlation was observed in the SNP TNFA-308 (Chong et al., 2006).
The mechanism by which the IFNG + 874A/T polymorphism influences susceptibility to SARS depends on its role in regulating IFN-γ production. It is possible that the high production of IFN-γ may impair their antiviral response against SARS-CoV, making these individuals more susceptible to viral infection, where the IFNG + 874A allele was significantly associated with a genetic risk factor for SARS (Chong et al., 2006). In another study conducted in the Chinese population, the IFNG + 874A allele was associated with susceptibility to SARS (Chong et al., 2006).
Type I interferons (alpha and beta), induced by virus infection, has an important role in the first line of defense, inducing intracellular antiviral proteins, such as 2′, 5′-oligoadenylate synthetase 1 (OAS-1), and myxovirus resistance-A (MxA) (Samuel, 2001). The 2′, 5′-oligoadenylate synthase (OAS) are known to mediate the antiviral response system by activating the RNA cleavage pathway. OAS silencing significantly decreased IL-1β, TNF-α and MCP-1 and had no effect on IL-10 secretion. OAS1, 2 and 3 restrict intracellular mycobacterial replication and increase the secretion of pro-inflammatory cytokines (Leisching, 2019). A case-control study in the Vietnamese population demonstrated that polymorphisms in (rs2660 and rs3741981) OAS-1 gene was associated with infection/development to SARS-CoV. Additionally, the polymorphism in the −88 position MxA gene was associated with hypoxemic status in the case group (Hamano et al., 2005).
TNF-alpha is a pro-inflammatory cytokine produced mainly by macrophages and lymphocytes (Arslan et al., 2010). The main physiological effect of TNF-α is to promote the immune and inflammatory response, recruiting neutrophils and monocytes (Vitale and de Ribeiro, 2007). TNF-α regulates the expression of neutrophil-endothelial cell adhesion molecules and chemokines, which recruit leukocytes (Makhatadze, 1998). A study relating TNF-α polymorphisms with interstitial pulmonary fibrosis in patients with SARS in China, showed that CT genotype at locus −204 was found associated with a protective effect on SARS compared to the TT genotypes [CI (95% CI) of 0.95 (0.90–0.99)] (Wang et al., 2008a).
In addition, interleukins act as an important component of pro-inflammatory and anti-inflammatory responses, producing and releasing proteins from the acute phase of hepatocytes and inducing symptoms such as fever. IL-12 is responsible for inducing other interleukins and acts in synergy with mainly with TNF-a (Moura et al., 2001). A study in Chinese population found that genetic variants in IL-12 were related to SARS susceptibility to (Tang et al., 2008). Investigations regarding cytokines and the identification of the functioning of the immune response in the face of need to be investigated should become crucial for studies on interventions considering immunotherapy in view of the severity of SARS-CoV infection. Thus, the innate and adaptive immune system takes multiple measures to respond to virus infection.
Toll-like receptors (TLRs).
Toll-like receptors (TLRs) have been conserved during evolution of innate immune receptors and expressed in various cells of the mammalian host. TLRs also play roles in the formation of pathogenic specific cellular and humoral immune responses (Kumar et al., 2009). The impairment of Toll-like receptors (TLRs) due to polymorphisms in TLR genes may alter immune response to a wide variety of microbial ligands, including virus. Polymorphisms in TLR2 and TLR4 have been linked to infectious diseases in human (Yuan et al., 2007). The TLR-Arg677Trp polymorphism was reported to be present in a study conducted with Korean lepromatous leprosy patients (Kang and Chae, 2001). This polymorphism has been also associated with tuberculosis in a Tunisian population (Ben-Ali et al., 2004). Yuan et al. (2007) demonstrated a possible link between CD14–159 CC genotype and severity of SARS-CoV infection.
4.1.1. Chemokines
Chemokines are proteins involved in immune responses, recruiting leukocytes, and they are divided into two subfamilies: CC and CXC based on the conserved cysteine motifs (Conti and DiGioacchino, 2001; Yadav et al., 2010). The Monocyte Chemoattractant Protein-1 (MCP-1), also known as CCL2, was the first CC chemokine discovered in humans. It is produced by a variety of cells, such as endothelial cells, macrophages, cytokines and growth factors. CCL2 chemokine is expressed in tissue during inflammation and it is important for antiviral response. In the viral infections, CCL2 has been associated with influenza (Lai et al., 2017) and HIV (Ansari et al., 2011).
The main inducers of MCP-1 expression are pro-inflammatory cytokines, such as Interleukin-1, Interleukin-4, Interleukin-6 and tumor necrosis factor alpha. Furthermore, it's can act as a monocytes recruiter during infection and activate T lymphocytes (Bianconi et al., 2018; Deshmane et al., 2009; Gschwandtner et al., 2019; Yadav et al., 2010). Genetic variations in the CCL2 gene are associated with diseases and its manifestations. A polymorphism in a promoter region of the CCL2 gene (−2518) was associated with Mycobacterium tuberculosis and pulmonary tuberculosis (Singh et al., 2014).
CXCL10, also known as interferon-γ-inducible protein 10 (IP-10), is an inflammatory chemokine secreted by monocytes, neutrophils, dendritic cells, lymphocytes and endothelial cells. Th1 cells attract to CXCL10 is categorized as Th1-chemokine (Lee et al., 2009; Oliviero, 2020; Zhao et al., 2018). CXCL10 is located on chromosome 4 in the band q21 (Liu et al., 2011b). CXCL10 has various functions including the induction of apoptosis, regulation of cell growth and, in the immune response, acts in the recruitment of leukocytes and also induce the production of Interleukin-8 (Lee et al., 2009; Liu et al., 2011a; Ragusa and Fallahi, 2017). In addition, CXCL10 may be highly expressed in various human diseases (Liu et al., 2011a). The GG genotype of the CXCL10 -135G/A (rs56061981) polymorphism decreased the expression of CXCL10 in T cells, which can alter the recruitment of mononuclear cells, contributing to susceptibility to tuberculosis (Singh et al., 2017).
RANTES (regulated in the activation of normal T cell expressed and secreted), also known as CCL5 is produced by platelets, macrophages, eosinophils, endothelium, and epithelial cells (Appay and Rowland-Jones, 2001; Marques et al., 2013; Trenchevska et al., 2015). RANTES gene is located in chromosome 17q11.2-q12 (An et al., 2002; Lwanira et al., 2015). An increase in the RANTES expression has been associated with several inflammatory processes, including the recruitment of untreated cells (Appay and Rowland-Jones, 2001; Levy, 2009). Also, CCL5 plays a role in the immune response to viral infection, including respiratory tract infections, such as influenza (Chan et al., 2005a, Chan et al., 2005b, Chan et al., 2005c; Marques et al., 2013). Several polymorphisms have been found in the RANTES gene such as −403G/A and the −28C/G located in the promoter region, which may alter RANTES function or influence its activity (Lwanira et al., 2015; Tahara et al., 2014).
These chemokines are involved in several diseases, including SARS. The involvement of CCL2 was demonstrated in the study of Tu et al., 2015, which showed that individuals who are predisposed to have amounts of CCL2 protein were more susceptible to the development of SARS-Cov infection and a combination of this protein and MBL polymorphisms may have a strong correlation with the susceptibility to SARS-CoV infection. Furthermore, the polymorphism in the −28 G allele was associated with the susceptibility to the infection, severe clinical outcomes and death from SARS (Ng et al., 2007). Functional polymorphisms in chemokines can influence its function and may be associated with severe clinical course.
4.1.2. Mannose-binding lectin (MBL)
MBL (mannose-binding lectin) is a component of the innate immune response. MBL is produced by the liver and plays an important role against bacterial and viral infections, activating the lectin pathway in the complement system. In humans, this protein is encoded by the MBL2 gene and deficiency in MBL serum levels are common, affecting 30% of the population (Heitzeneder et al., 2012). Polymorphisms in the MBL2 gene that decreased MBL serum levels may be important risk factors for neonatal sepsis and pneumonia (Özkan et al., 2012). Associations with SARS-CoV infection and the codon 54 variant have also been observed indicating an important role of MBL in the pathogenesis of this disease (Tu et al., 2015; Zhang et al., 2005).
Mannose-binding protein-associated serine protease 2 (MASP-2) is associated with MBL in serum and binds to carbohydrates that initiate the lectin complement pathway which is recognized as the third pathway for complement activation when activated with the MBL-MASP complex (Matsushita, 1996; Stover et al., 2001). This protease is encoded by the MASP-2 gene, which is located on chromosome 1p36.23–31 (Stover et al., 2001). It has been shown that polymorphism in this gene may affect the levels of MASP-2 in the serum and lead to susceptibility to some diseases such as tuberculosis and leprosy, caused by mycobacteria (Boldt et al., 2013; Chen et al., 2015) and in diseases caused by virus. Polymorphisms in the MASP-2 gene increased protease levels in a Brazilian population and were associated with susceptibility to HCV infection (Tulio et al., 2011). Another study carried out in Brazil showed an association with HTLV-1 infection (Coelho-dos-Reis et al., 2013). On the other hand, no association was found between coronavirus and MASP-2 polymorphisms in the infection of SARS-CoV in a Chinese population (Wang et al., 2009).
FcγRs (Fc gamma receptors) are a group of surface glycoproteins belonging to the immunoglobulin (Ig) superfamily that binds to the portion Fc of IgG, these receptors are comprised of two classes: activation and inhibition (Nimmerjahn and Ravetch, 2010). FcγRIIA is expressed in monocytes, macrophages, dendritic cells, eosinophils and neutrophils. This receptor is encoded by the FCGR2A gene and polymorphism in that gene can alter the FcγRIIA isoform which can be associated with susceptibility to different infections (Pleass and Woof, 2001). The FcγRIIA R131H polymorphism has been evaluated in many diseases (Oliveira et al., 2011). In relation to respiratory diseases, this polymorphism was associated with susceptibility and idiopathic severity of pulmonary fibrosis (Bournazos et al., 2010). In our review, only one study evaluated the FcγRIIA polymorphism with SARS-CoV infection, the homozygous RR showed an association with disease severity even when factors such as comorbidities were removed from the analysis, revealing that RR genotype is a risk factor for disease severity (Yuan et al., 2005).
4.1.3. Angiotensin-converting enzymes (ACE)
Angiotensin-converting enzymes are an important component of the Renin-angiotensin-aldosterone system, which plays an important role in SARS-COV and SARS-COV-2 infections (Alexandre et al., 2020). There are two types of this enzyme, Angiotensin-converting enzymes 1 (ACE 1) and Angiotensin-converting enzymes 2 (ACE2). The ACE1 cleaves the angiotensin I into angiotensin (Ang) II that binds to AT1R and promotes vasoconstriction and bronchoconstriction, increases vascular permeability, inflammation and fibrosis, resulting in the development of SARS in individuals infected by SARS-COV and SARS-COV2. ACE2 cleaves angiotensin I to angiotensin and angiotensin II to angiotensin, promoting vasodilation, decreased vascular permeability, inhibition of inflammation and fibrosis formation, blocking the effects of angiotensin II/ATR1 and protecting the host against SARS (Alexandre et al., 2020; Rossi et al., 2020). However, the role of ACE2 in SARS-COV (or SARS-COV-2) infection may be more complex because this membrane enzyme acts as a receptor to viral entry into the host cell, causing a detrimental effect on the contamination phase (Wang et al., 2008a, Wang et al., 2008b). Therefore, the expression of ACE 1 and ACE2 plays a fundamental role in COVID-19 infection (Tas et al., 2020). Polymorphisms in these genes may influence the levels of gene expression and the catalytic activity of their enzymes (Rigat et al., 1990). In addition to COVID-19, polymorphisms in the ACE2 gene have been associated with other diseases such as hypertension, stroke recurrence, Type 2 Diabetes Mellitus (Devaux et al., 2020, Wu et al., 2017).
In our review, three studies evaluated the association between polymorphisms in the ACE1 or ACE2 genes and susceptibility to SARS (Chan et al., 2005b; Itoyama et al., 2005, Itoyama et al., 2004). Two studies evaluated the ACE1 insertion/deletion (I/D) polymorphism and showed controversial results. Itoyama et al. (2004) showed that the D allele was significantly higher in the hypoxemic group than in the non-hypoxic group. However, Chan et al., 2005a, Chan et al., 2005b, Chan et al., 2005c found no relationship between the ACE1 insertion/deletion (I/D) polymorphism and susceptibility to SARS or the need for intensive care in patients with SARS. This inconsistency may be attributed to: (1) differences in the selection of study groups. Itoyama et al. (2004) divided SARS cases into two groups: non-hypoxia or hypoxemia. While Chan et al., 2005a, Chan et al., 2005b, Chan et al., 2005c categorized subjects with severe disease into patients who developed SARS and patients who required admission to the intensive care unit (ICU); (2) the small sample size in the study conducted in Vietnam. Previous reports have shown that individuals with DD genotype has a two-fold increase in ACE levels when compared to II genotype. Therefore, further studies with this and other polymorphisms in the gene ACE1 are needed to better understand the susceptibility of SARS in patients with coronavirus.
In Vietnamese Population an association between SNPs in the ACE2 gene and susceptibility to SARS-COV was observed. This study found 19 SNPs, of these, 13 were new SNPs in the literature. None of these SNPs were associated with susceptibility to SARS-COV. However, the small sample size may have influenced the result (Itoyama et al., 2005). The lack of studies involving polymorphisms in the ACE2 gene in susceptibility to SARS-COV is evident. Therefore, it is necessary to conduct further studies on this topic.
4.1.4. Human leukocyte antigen system (HLA)
The HLA exhibits more than 27,258 known alleles (Institute, M.E.B, n.d.) and molecules resulting from the expression of these genes are also known as MHC (Major Histocompatibility Complex). The HLA gene is found on chromosome 6, in the short arm, and for educational purposes, these genes are grouped into 3 groups, I, II and III genes (Dausset, 1984; Goldberg and Rizzo, 2015).
Class I is located in the most distant region, encoding histocompatibility molecules, classic heavy chains, such as HLA-A, B and C molecules, having an excellent polymorphic characteristic. Class II is located in the most centromeric portion of the MHC region and encodes two chains that form the functional heterodimers: HLA-DR, HLA-DQ, HLA-DP, HLA-DM and HLA-DO, being recognized for its influence on the control immune response. Expression in class II is more restricted when compared to class I, and expression in antigen-presenting cells, such as macrophages and Langerhans cells is predominant. Class III genes are located between class I and I and, even included in the MHC complex, they do not encode histocompatibility molecules and encode other molecules that may or may not be part of the immune system (Goldberg and Rizzo, 2015; Nepom and Erlich, 1991).
In the immune aspect, the action of HLA depends directly on cellular configuration to develop its activity of presenting antigens. Class I cells have extracellular domains, in their heavy chain, that contain amino acid residues, forming peptides that will later be presented to the CD4 T lymphocyte. These molecules are expressed on the surfaces of the nucleated cells. The class II molecules present on the surfaces of some types of cells, such as macrophages and dendritic cells, have 2 domains with amino acid residues that after being processed, join the binding grooves, stabilizing and presenting them to the CD4 T lymphocytes. The variation of amino acids in the regions of the HLA molecules binds and presents different peptides, being recognized by different T lymphocytes. Evidences suggest that this variation is due to the different responses of subjects when they come into contact with foreign antigens (Fernandes et al., 2003).
Each HLA molecule is able to bind widely to various types of peptides (Colten, 1985). The association between HLA class I and the severity of SARS was evidenced by an important increase in the odds ratio of HLA-B* 4601 in the group of SARS patients who died or were intubated compared to other groups (Lin et al., 2003). In this study, HLA-class I and II alleles were typed by PCR-SSOP and included 37 cases of probable SARS, 28 patients with fever later excluded as probable SARS and 101 uninfected health professionals who were exposed or possibly exposed to the coronavirus SARS. A set of 190 healthy unrelated normal Taiwanese was used as a control group.
On the other hand, in the study by Keicho et al. (2009) between HLA class II genes, HLA-DRB1*12 there was a positive association and HLA-DRB1*13 showed a negative association when the SARS group was compared with the contacts. When patients contact and non-contacts were compared together as a single control group, HLA-DRB1*1202 showed a significant association, under the dominant model, it was the most strongly associated with SARS. The study population was composed by unrelated Vietnamese patients: 44 SARS patients, 103 staff members from the same hospital who had contact with patients, and 50 healthy individuals with no history of contact with SARS patients.
A study conducted in Chinese population, included 95 individuals recovered from SARS (Xiong et al., 2008), found no association between the HLA -A,-B and -DRB1 alleles with the development of SARS. The authors suggest that other factors such as relative population density, frequency and extent of travel by affected individuals and the rapid introduction and adoption of protective measures may have influenced the restricted spread of SARS.
4.1.5. Cluster of differentiation
Immune responses to pathogens are identified by Pattern recognition receptors (PRRs) (Amarante-Mendes et al., 2018; Lee and Kim, 2007). Polymorphisms in this gene may consecutively affect the immunity of the host, entry and replication of the virus (Geijtenbeek et al., 2009). In this review we identified that polymorphisms in cluster of differentiation 209 (CD209/DC-SIGN), cluster of differentiation 14 (CD14), liver/lymph node-specific intracellular adhesion molecules-3 grabbing non-integrin (L-SIGN), cell-specific intercellular adhesion molecule and dendrites (ICAM-3) and fibrinogen-like protein 2 (FGL2) have been associated with SARS in patients with coronavirus.
The CD209 gene is located on chromosome 19p13.2–3 and the polymorphism of DC-SIGN (CD209 9) in promoter region −336A>G (rs4804803) affects Sp1 transcription factor and was reported influencing in the transcription activity of CD209 in vitro (Liu et al., 2003). It1s was investigated in study included, and this molecule is relevant in the early interaction of some pathogens with dendritic cells specially viruses (Sakuntabhai et al., 2005). This polymorphism was not related with susceptibility to SARS (Chan et al., 2010). However, the A/A genotype of this variant was related to increase serum levels of lactate dehydrogenase (LDH). This enzyme is released into the extracellular environment during cellular damage and it was associated with inflammation in infections (Glick, 1969).
ICAM-3 (cell-specific intercellular adhesion molecule and dendrites) is a linked to mannose expressed on the surface of dendritic cells and is a molecule relevant for initiating the immune responses measured by T cells (Fawcett et al., 1992; Montoya et al., 2002). L-SIGN is homolog to DC-SIGN (CD209) belongs to calcium-dependent lectin and expression is restricted to lymph node endothelial cells, sinusoidal endothelial cells of the placenta and liver (Braet and Wisse, 2002). In a research conducted in a Chinese population, the homozygosity for L-SIGN was linked to a protective role to SARS susceptibility (Chan et al., 2006). A correlation between homozygous ICAM3 Gly143 (rs2304237) polymorphism and higher LDH levels and lower total white blood cell counts in SARS Chinese patients (Chan et al., 2007).
LDH is an enzyme that catalyzes the conversion of pyruvate to lactate with subsequent conversion to NADH and NAD + (Hsu and Sabatini, 2008). Increased levels can lead to injury and organ failure, influencing the clinical outcomes of patients with COVID-19 (Henry et al., 2020). Investigations into the role of LDH levels in COVID-19 infection concluded that there was a significant increase in patients with disease severity and pneumonia (Henry et al., 2020, Hsu and Sabatini, 2008), contributing to the dynamics of mortality. In addition, the decrease in blood components can culminate in hypoxic conditions in critically ill patients.
The differentiation antigen cluster 14 (CD14) is a receptor with multiple inflammatory functions and it is located on the surface of macrophages and monocytes (Mendel et al., 2014). CD14 associated with lipopolysaccharide (LPS) receptor pathway through the TLR4, culminate in the release of proinflammatory cytokines (Fernández-Real et al., 2003), triggering inflammation reaction which causing the endothelial damage, disturbance of immunologic function and vascular smooth muscle cells proliferation (De Tena et al., 2005). In Chinese population with coronavirus and SARS, the C/C genotype of −159 C>T SNP (rs2569190) in CD14 gene was more frequent in patients with SARS, suggesting that enhanced viral toxicity due to decreased antiviral response leading to disease severity (Yuan et al., 2007).
Fibrinogen-like protein-2 (FGL2) is regulated by CD4+ T cells and has immunosuppressive and inflammatory properties (Lin et al., 2017). This protein was investigated as a candidate biomarker to predict the severity of the disease in viral infections (Luft et al., 2018; Zhu et al., 2005). In patients with SARS due to coronavirus infection, the +158 T/* (rs2075761) polymorphism was described as a risk of disease severity (Hsieh et al., 2010). Studies have previously shown that Fgl2?? influences the inhibition of dendritic cells maturation and progression of B cells apoptosis and macrophages (Joller et al., 2014; Liu et al., 2010).
4.2. Others polymorphisms
Other polymorphisms were identified in order to trace knowledge about the genetic background of patients with SARS-COV infection: Alpha 2-HS Glycoprotein (AHSG); Cytochrome P450 Family 3A (CYP4F3A) and protein kinase R (PKR). Alpha 2-HS Glycoprotein (AHSG) and Cytochrome P450 Family 3A (CYP4F3A) have been studied in the Chinese population with SARS-CoV during 2003 epidemic. The authors concluded that AA genotype of the SNP rs2248690 of the AHSG gene was correlated with protection for SARS, which is related to the elevation of serum levels of this liver glycoprotein. This is vital for the deactivation of macrophages in view of the immune response, which suggests that polymorphisms in this gene are relevant to determine a possible role for resistance to SARS-CoV infection (Zhu et al., 2011).
A recent study conducted in the Spanish and Italian population carried out a study of genomic association in the Spanish population in order to be able to track SNPs related to the severity of the disease and consecutively with respiratory failure. It was concluded that in locus 3p21.31, the genes SLC6A20, LZTFL1, CCR9, FYCO1, CXCR6 and XCR1 were related to SARS-CoV-2. The 9q34.2 locus present in the ABO blood group, demonstrated that people with blood group A are 1.45 times more likely to develop severe SARS-CoV-2 when compared to blood group O (Ellinghaus et al., 2020).
5. Conclusion
In conclusion, our systematic review emphasized the association of polymorphisms with susceptibility or protection to SARS-COV infection. The study of genetic polymorphisms related to SARS-COV has been explored in the literature. Therefore, new studies with this proposal may contribute to a better understanding of the disease susceptibility, mainly of COVID-19 (SARS-COV-2) which has been current focus on global health, considering clinical syndromes associated: mild disease, uncomplicated pneumonia, severe pneumonia, SARS, sepsis and septic shock. Additional studies, including best study designs, implications polymorphisms on the profile clinic (presence of comorbidities) and severity (including asymptomatic population; classification of patient severity), laboratorial parameters (blood count, coagulation profile, including renal and liver function, creatine kinase, lactate dehydrogenase, electrolytes, myocardial enzymes, cytokines, serum ferritin and procalcitonin), genetic polymorphism and prolonged mechanical ventilation and genic expression are needed to enhance the prediction of these results leading to a better understanding of the interaction between genes and outcome of clinic SARS-COV.
Declaration of competing interest
-
1.
All authors have participated in (a) conception and design, or analysis and interpretation of the data; (b) drafting the article or revising it critically for important intellectual content; and (c) approval of the final version.
-
2.
This manuscript has not been submitted to, nor is under review at, another journal or other publishing venue.
-
3.
The authors have no affiliation with any organization with a direct or indirect financial interest in the subject matter discussed in the manuscript
-
4.
The following authors have affiliations with organizations with direct or indirect financial interest in the subject matter discussed in the manuscript.
References
- Alexandre J., Cracowski J., Richard V., Bouhanick B. Renin-angiotensin-aldosterone system and COVID-19 infection. Ann. Endocrinol. (Paris) 2020 doi: 10.1016/j.ando.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almqvist J., Granberg T., Tzortzakakis A., Klironomos S., Kollia E., Öhberg C., Martin R., Piehl F., Ouellette R., Ineichen B.V. Neurological manifestations of coronavirus infections - a systematic review. Ann. Clin. Transl. Neurol. 2020 doi: 10.1002/acn3.51166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarante-Mendes G.P., Adjemian S., Branco L.M., Zanetti L.C., Weinlich R., Bortoluci K.R. Pattern recognition receptors and the host cell death molecular machinery. Front. Immunol. 2018 doi: 10.3389/fimmu.2018.02379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An P., Nelson G.W., Wang L., Donfield S., Goedert J.J., Phair J., Vlahov D., Buchbinder S., Farrar W.L., Modi W., O’Brien S.J., Winkler C.A. Modulating influence on HIV/AIDS by interacting RANTES gene variants. Proc. Natl. Acad. Sci. U. S. A. 2002;99:10002–10007. doi: 10.1073/pnas.142313799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari A.W., Heiken H., Meyer-Olson D., Schmidt R.E. CCL2: a potential prognostic marker and target of anti-inflammatory strategy in HIV/AIDS pathogenesis. Eur. J. Immunol. 2011;41:3412–3418. doi: 10.1002/eji.201141676. [DOI] [PubMed] [Google Scholar]
- Appay V., Rowland-Jones S.L. RANTES: a versatile and controversial chemokine. Trends Immunol. 2001 doi: 10.1016/S1471-4906(00)01812-3. [DOI] [PubMed] [Google Scholar]
- Arslan N., Erdur B., Aydin A. Hormones and cytokines in childhood obesity. Indian Pediatr. 2010 doi: 10.1007/s13312-010-0142-y. [DOI] [PubMed] [Google Scholar]
- Ben-Ali M., Barbouche M.R., Bousnina S., Chabbou A., Dellagi K. Toll-like receptor 2 Arg677Trp polymorphism is associated with susceptibility to tuberculosis in Tunisian patients. Clin. Diagn. Lab. Immunol. 2004;11:625–626. doi: 10.1128/CDLI.11.3.625-626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender A.T., Tzvetkov E., Pereira A., Wu Y., Kasar S., Przetak M.M., Vlach J., Niewold T.B., Jensen M.A., Okitsu S.L. TLR7 and TLR8 differentially activate the IRF and NF-κB pathways in specific cell types to promote inflammation. ImmunoHorizons. 2020;4:93–107. doi: 10.4049/immunohorizons.2000002. [DOI] [PubMed] [Google Scholar]
- Benvenuto D., Giovanetti M., Salemi M., Prosperi M., De Flora C., Junior Alcantara L.C., Angeletti S., Ciccozzi M. The global spread of 2019-nCoV: a molecular evolutionary analysis. Pathog. Glob. Health. 2020;114:64–67. doi: 10.1080/20477724.2020.1725339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianconi V., Sahebkar A., Atkin S.L., Pirro M. The regulation and importance of monocyte chemoattractant protein-1. Curr. Opin. Hematol. 2018 doi: 10.1097/MOH.0000000000000389. [DOI] [PubMed] [Google Scholar]
- Biron C.A., Nguyen K.B., Pien G.C., Cousens L.P., Salazar-Mather T.P. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- Boldt A.B.W., Goeldner I., Stahlke E.R.S., Thiel S., Jensenius J.C., de Messias-Reason I.J.T. Leprosy association with low MASP-2 levels generated by MASP2 haplotypes and polymorphisms flanking MAp19 exon 5. PLoS One. 2013;8 doi: 10.1371/journal.pone.0069054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bournazos S., Grinfeld J., Alexander K.M., Murchison J.T., Wallace W.A., McFarlane P., Hirani N., Simpson A.J., Dransfield I., Hart S.P. Association of FcγRIIa R131H polymorphism with idiopathic pulmonary fibrosis severity and progression. BMC Pulm. Med. 2010;10 doi: 10.1186/1471-2466-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braet F., Wisse E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Comp. Hepatol. 2002 doi: 10.1186/1476-5926-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carod Artal F.J. Complicaciones neurológicas por coronavirus y COVID-19. Rev. Neurol. 2020;70(1) doi: 10.33588/rn.7009.2020179. [DOI] [PubMed] [Google Scholar]
- Chan M.C.W., Cheung C.Y., Chui W.H., Tsao G.S.W., Nicholls J.M., Chan Y.O., Chan R.W.Y., Long H.T., Poon L.L.M., Guan Y., Peiris J.S.M. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir. Res. 2005;6:1–13. doi: 10.1186/1465-9921-6-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A., Tang N.L., Hui D.S., Chung G.T., Wu A.K., Chim S.S., Chiu R.W., Lee N., Choi K.W., Sung Y.M., Chan P.K., Tong Y.K., Lai S.T., Yu W.C., Tsang O., Lo D. 2005. Absence of Association Between Angiotensin Converting Enzyme Polymorphism and Development of Adult Respiratory Distress Syndrome in Patients With Severe Acute Respiratory Syndrome: A Case Control Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.C.A., Tang N.L.S., Hui D.S.C., Chung G.T.Y., Wu A.K.L., Chim S.S.C., Chiu R.W.K., Lee N., Choi K.W., Sung Y.M., Chan P.K.S., Tong Y.K., Lai S.T., Yu W.C., Tsang O., Lo Y.M.D. Absence of association between angiotensin converting enzyme polymorphism and development of adult respiratory distress syndrome in patients with severe acute respiratory syndrome: a case control study. BMC Infect. Dis. 2005;5 doi: 10.1186/1471-2334-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Vera S.F., Chan K.Y.K., Chen Y., Poon L.L.M., Cheung A.N.Y., Zheng B., Chan K.-H., Mak W., Ngan H.Y.S., Xu X., Screaton G., Tam P.K.H., Austyn J.M., Chan L.-C., Yip S.-P., Peiris M., Khoo U.-S., Lin C.-L.S. Homozygous L-SIGN (CLEC4M) plays a protective role in SARS coronavirus infection. Nat. Genet. 2006;38:38–46. doi: 10.1038/ng1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.Y.K., Ching J.C.Y., Xu M.S., Cheung A.N.Y., Yip S., Yam L.Y.C., Lai S., Chu C., Wong A.T.Y., Song Y., Huang F., Liu W., Chung P.H., Leung G.M., Chow E.Y.D., Chan E.Y.T., Chan J.C.K., Ngan H.Y.S., Tam P., Chan L., Sham P., Chan V.S.F., Peiris M., Lin S.C.L., Khoo U. Association of ICAM3 genetic variant with severe acute respiratory syndrome. J. Infect. Dis. 2007;196:271–280. doi: 10.1086/518892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.Y.K., Xu M.S., Ching J.C.Y., So T.M.K., Lai S.T., Chu C.M., Yam L.Y.C., Wong A.T.Y., Chung P.H., Chan V.S.F., Lin C.L.S., Sham P.C., Leung G.M., Peiris J.S.M., Khoo U.S. CD209 (DC-SIGN) -336A>G promoter polymorphism and severe acute respiratory syndrome in Hong Kong Chinese. Hum. Immunol. 2010;71:702–707. doi: 10.1016/j.humimm.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Liang Y., Li W., Wang M., Hu L., Abuaku B.K., Huang X., Tan H., Wen S.W. Impact of MBL and MASP-2 gene polymorphism and its interaction on susceptibility to tuberculosis. BMC Infect. Dis. 2015;15:151. doi: 10.1186/s12879-015-0879-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching J.C., Chan K.Y., Lee E.H., Xu M.S., Ting C.K., So T.M., Sham P.C., Leung G.M., Peiris J.S., Khoo U.S., 2010. Significance of the myxovirus resistance A (MxA) gene -123C>a single-nucleotide polymorphism in suppressed interferon beta induction of severe acute respiratory syndrome coronavirus infection. J. Infect. Dis. 201 (12), 1899-1908. doi:10.1086/652799. [DOI] [PMC free article] [PubMed]
- Chiu R.W.K., Tang N.L.S., Hui D.S.C., Chung G.T.Y., Chim S.S.C., Chan K.C.A., Sung Y., Chan L.Y.S., Tong Y., Lee W., Chan P.K.S., Lo Y.M.D. ACE2 gene polymorphisms do not affect outcome of severe acute respiratory syndrome. Clin. Chem. 2004;50:1683–1686. doi: 10.1373/clinchem.2004.035436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong W.P., Ip W.K.E., Tso G.H.W., Ng M.W., Wong W.H.S., Law H.K.W., Yung R.W.H., Chow E.Y., Au K.L., Chan E.Y.T., Lim W., Peiris J.S.M., Lau Y.L. The interferon gamma gene polymorphism +874 A/T is associated with severe acute respiratory syndrome. BMC Infect. Dis. 2006;6 doi: 10.1186/1471-2334-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho-dos-Reis J.G., Passos L., Duarte M.C., Araújo M.G., Campi-Azevedo A.C., Teixeira-Carvalho A., Peruhype-Magalhães V., Trindade B.C., Dos Santos Dias R., Martins M.L., Carneiro-Proietti A.B., Guedes A.C., Gonçalves D.U., Martins-Filho O.A. Immunological profile of HTLV-1-infected patients associated with infectious or autoimmune dermatological disorders. PLoS Negl. Trop. Dis. 2013;7(7):e2328. doi: 10.1371/journal.pntd.0002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colten H.R. Complement. Springer; Berlin Heidelberg: 1985. Molecular genetics of the major histocompatibility linked complement genes; pp. 39–48. [DOI] [PubMed] [Google Scholar]
- Conti P., DiGioacchino M. MCP-1 and RANTES are mediators of acute and chronic inflammation. Allergy Asthma Proc. 2001 doi: 10.2500/108854101778148737. [DOI] [PubMed] [Google Scholar]
- Dahan S., Segal G., Katz I., Hellou T., Tietel M., Bryk G., Amital H., Shoenfeld Y., Dagan A. Ferritin as a marker of severity in COVID-19 patients: a fatal correlation. Isr. Med. Assoc. J. 2020;8:429–434. [PubMed] [Google Scholar]
- Dausset J. Vol. 46. Vox Sang; 1984. The Birth of MAC; pp. 235–237. [DOI] [PubMed] [Google Scholar]
- De Tena J.G., Kriszbacher I., Koppán M., Bódis J., Hansson G.K. Inflammation, atherosclerosis, and coronary artery disease [5] (multiple letters) N. Engl. J. Med. 2005 doi: 10.1056/NEJM200507283530425. [DOI] [PubMed] [Google Scholar]
- Deshmane S.L., Kremlev S., Amini S., Sawaya B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interf. Cytokine Res. 2009 doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux C.A., Rolain J.-M., Raoult D. ACE2 receptor polymorphism: Susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. J. Microbiol. Immunol. Infect. 2020;53(3):425–435. doi: 10.1016/j.jmii.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L., He Y., Zhou Y., Liu S., Zheng B.J., Jiang S. The spike protein of SARS-CoV - a target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009 doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinghaus D., Degenhardt F., Bujanda L., Buti M., Albillos A., Invernizzi P., Fernández J., Prati D., Baselli G., Asselta R., et al. Genomewide association study of severe Covid-19 with respiratory failure. N. Engl. J. Med. 2020;383:1522–1534. doi: 10.1056/NEJMoa2020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett J., Holness C.L.L., Needham L.A., Turley H., Gattert K.C., Mason D.Y., Simmons D.L. Molecular cloning of ICAM-3, a third ligand for LFA-1, constitutively expressed on resting leukocytes. Nature. 1992;360:481–484. doi: 10.1038/360481a0. [DOI] [PubMed] [Google Scholar]
- Fehr A.R., Perlman S. Coronaviruses: Methods and Protocols. Springer New York; 2015. Coronaviruses: an overview of their replication and pathogenesis; pp. 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes A.P.M., Maciel L.M.Z., Foss M.C., Donadi E.A. Como entender a associação entre o sistema HLA e as doenças auto-imunes endócrinas. Arq. Bras. Endocrinol. Metabol. 2003;47:601–611. doi: 10.1590/s0004-27302003000500015. [DOI] [Google Scholar]
- Fernández-Real J.M., Broch M., Richart C., Vendrell J., López-Bermejo A., Ricart W. CD14 monocyte receptor, involved in the inflammatory cascade, and insulin sensitivity. J. Clin. Endocrinol. Metab. 2003;88:1780–1784. doi: 10.1210/jc.2002-020173. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek T.B.H., den Dunnen J., Gringhuis S.I. Pathogen recognition by DC-SIGN shapes adaptive immunity. Future Microbiol. 2009 doi: 10.2217/fmb.09.51. [DOI] [PubMed] [Google Scholar]
- Glick J.H. Serum lactate dehydrogenase isoenzyme and total lactate dehydrogenase values in health and disease, and clinical evaluation of these tests by means of discriminant analysis. Am. J. Clin. Pathol. 1969;52:320–328. doi: 10.1093/ajcp/52.3.320. [DOI] [PubMed] [Google Scholar]
- Goldberg Anna Carla, Rizzo Luiz Vicente. MHC structure and function – antigen presentation. Part 1. Einstein (Sao Paulo) 2015 doi: 10.1590/S1679-45082015RB3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralinski L.E., Baric R.S. Molecular pathology of emerging coronavirus infections. J. Pathol. 2015;235:185–195. doi: 10.1002/path.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschwandtner M., Derler R., Midwood K.S. More than just attractive: how CCL2 influences myeloid cell behavior beyond chemotaxis. Front. Immunol. 2019 doi: 10.3389/fimmu.2019.02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamano E., Hijikata M., Itoyama S., Quy T., Phi N.C., Long H.T., Ha L.D., Van Ban V., Matsushita I., Yanai H., Kirikae F., Kirikae T., Kuratsuji T., Sasazuki T., Keicho N. Polymorphisms of interferon-inducible genes OAS-1 and MxA associated with SARS in the Vietnamese population. Biochem. Biophys. Res. Commun. 2005;329:1234–1239. doi: 10.1016/j.bbrc.2005.02.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan S.A., Sheikh F.N., Jamal S., Ezeh J.K., Akhtar A. Coronavirus (COVID-19): a review of clinical features, diagnosis, and treatment. Cureus. 2020;12 doi: 10.7759/cureus.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Feng D., de Vlas S.J., Wang H., Fontanet A., Zhang P., Plancoulaine S., Tang F., Zhan L., Yang H., Wang T., Richardus J.H., Habbema J.D.F., Cao W. Association of SARS susceptibility with single nucleic acid polymorphisms of OASI and MxA genes: a case-control study. BMC Infect. Dis. 2006;6 doi: 10.1186/1471-2334-6-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeneder S., Seidel M., Förster-Waldl E., Heitger A. Mannan-binding lectin deficiency - good news, bad news, doesn’t matter? Clin. Immunol. 2012 doi: 10.1016/j.clim.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Henry B.M., Aggarwal G., Wong J., Benoit S., Vikse J., Plebani M., Lippi G. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: a pooled analysis. Am. J. Emerg. Med. 2020;38(9):1722–1726. doi: 10.1016/j.ajem.2020.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181 doi: 10.1016/j.cell.2020.02.052. 271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh Y.H., Chen C.W.S., Schmitz S.F.H., King C.C., Chen W.J., Wu Y.C., Ho M.S. Candidate genes associated with susceptibility for SARS-coronavirus. Bull. Math. Biol. 2010;72:122–132. doi: 10.1007/s11538-009-9440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute, M.E.B., n.d. Numbers of HLA Alleles [WWW Document]. URL https://www.ebi.ac.uk/ipd/imgt/hla/stats.html (accessed 5.11.20).
- Hsu P.P., Sabatini D.M. Cancer cell metabolism: warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Ip W.K.E., Chan K.H., Law H.K.W., Tso G.H.W., Kong E.K.P., Wong W.H.S., To Y.F., Yung R.W.H., Chow E.Y., Au K.L., Chan E.Y.T., Lim W., Jensenius J.C., Turner M.W., Peiris J.S.M., Lau Y.L. Mannose-binding lectin in severe acute respiratory syndrome coronavirus infection. J. Infect. Dis. 2005;191(10):1697–1704. doi: 10.1086/429631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoyama S., Keicho N., Quy T., Phi N.C., Long H.T., Dang Ha L., Van Ban V., Ohashi J., Hijikata M., Matsushita I., Kawana A., Yanai H., Kirikae T., Kuratsuji T., Sasazuki T. ACE1 polymorphism and progression of SARS. Biochem. Biophys. Res. Commun. 2004;323:1124–1129. doi: 10.1016/j.bbrc.2004.08.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoyama S., Keicho N., Hijikata M., Quy T., Phi N.C., Long H.T., Ha L.D., Van Ban V., Matsushita I., Yanai H., Kirikae F., Kirikae T., Kuratsuji T., Sasazuki T. Identification of an alternative 5′-untranslated exon and new polymorphisms of angiotensin-converting enzyme 2 gene: lack of association with SARS in the Vietnamese population. Am. J. Med. Genet. 2005;136(A):52–57. doi: 10.1002/ajmg.a.30779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joller N., Lozano E., Burkett P.R., Patel B., Xiao S., Zhu C., Xia J., Tan T.G., Sefik E., Yajnik V., Sharpe A.H., Quintana F.J., Mathis D., Benoist C., Hafler D.A., Kuchroo V.K. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity. 2014;40:569–581. doi: 10.1016/j.immuni.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang T.J., Chae G.T. Detection of Toll-like receptor 2 (TLR2) mutation in the lepromatous leprosy patients. FEMS Immunol. Med. Microbiol. 2001;31:53–58. doi: 10.1111/j.1574-695X.2001.tb01586.x. [DOI] [PubMed] [Google Scholar]
- Keicho N., Itoyama S., Kashiwase K., Phi N.C., Long H.T., Ha L.D., Van Ban V., Hoa B.K., Le Hang N.T., Hijikata M., Sakurada S., Satake M., Tokunaga K., Sasazuki T., Quy T. Association of human leukocyte antigen class II alleles with severe acute respiratory syndrome in the Vietnamese population. Hum. Immunol. 2009;70:527–531. doi: 10.1016/j.humimm.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelada S.N., Eaton D.L., Wang S.S., Rothman N.R., Khoury M.J. The role of genetic polymorphisms in environmental health. Environ. Health Perspect. 2003;111:1055–1064. doi: 10.1289/ehp.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo U.S., Chan K.Y., Chan V.S., Ching J.C., Yam L., Chu C.M., Lai S.T., Wong T.Y., Tam P., Yip S.P., Leung G.M., Lin C.L., Peiris J.S. Role of polymorphisms of the inflammatory response genes and DC-SIGNR in genetic susceptibility to SARS and other infections. Hong Kong Med. J. 2008;14(Suppl 4):31–35. PMID: 18708672. [PubMed] [Google Scholar]
- Kumar H., Kawai T., Akira S. Toll-like receptors and innate immunity. Biochem. Biophys. Res. Commun. 2009 doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- Lai C., Wang K., Zhao Z., Zhang L., Gu H., Yang P., Wang X. C-C motif chemokine ligand 2 (CCL2) mediates acute lung injury induced by lethal influenza H7N9 virus. Front. Microbiol. 2017;8 doi: 10.3389/fmicb.2017.00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.S., Kim Y.-J. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu. Rev. Biochem. 2007;76:447–480. doi: 10.1146/annurev.biochem.76.060605.122847. [DOI] [PubMed] [Google Scholar]
- Lee E.Y., Lee Z.H., Song Y.W. CXCL10 and autoimmune diseases. Autoimmun. Rev. 2009 doi: 10.1016/j.autrev.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Leisching G.R. PI3-Kinase δγ catalytic isoforms regulate the Th-17 response in tuberculosis. Front Immunol. 2019;10:2583. doi: 10.3389/fimmu.2019.02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J.A. The unexpected pleiotropic activities of RANTES. J. Immunol. 2009;182:3945–3946. doi: 10.4049/jimmunol.0990015. [DOI] [PubMed] [Google Scholar]
- Li F. Structure, function, and evolution of coronavirus spike proteins. Annu. Rev. Virol. 2016 doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Lei P., Zeng B., Li Zongliang, Yu P., Fan B., Wang C., Li Zicong, Zhou J., Hu S., Liu H. Coronavirus disease (COVID-19): spectrum of CT findings and temporal progression of the disease. Acad. Radiol. 2020 doi: 10.1016/j.acra.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M., Tseng H.K., Trejaut J.A., Lee H.L., Loo J.H., Chu C.C., Chen P.J., Su Y.W., Lim K.H., Tsai Z.U., Lin R.Y., Lin R.S., Huang C.H. Association of HLA class I with severe acute respiratory syndrome coronavirus infection. BMC Med. Genet. 2003:4. doi: 10.1186/1471-2350-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Chen R., Jiang X., Wu X., Huang X., Dong X., Yang X., Lin X., Chen Xin, Chen Xiangrong, Huang Z. Elevated fibrinogen-like protein 2 in TNBS-induced colitis mice: association with Th17 and regulatory T cells. Mol. Med. Rep. 2017;16:3445–3454. doi: 10.3892/mmr.2017.7005. [DOI] [PubMed] [Google Scholar]
- Little J., Higgins J.P., Ioannidis J.P., Moher D., Gagnon F., von Elm E., Khoury M.J., Cohen B., Davey-Smith G., Grimshaw J., Scheet P., Gwinn M., Williamson R.E., Zou G.Y., Hutchings K., Johnson C.Y., Tait V., Wiens M., Golding J., van Duijn C., McLaughlin J., Paterson A., Wells G., Fortier I., Freedman M., Zecevic M., King R., Infante-Rivard C., Stewart A., Birkett N. STrengthening the REporting of genetic association studies (STREGA)—an extension of the STROBE statement. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Yu W., Liou L.Y., Rice A.P. Isolation and characterization of the human DC-SIGN and DC-SIGNR promoters. Gene. 2003;313:149–159. doi: 10.1016/s0378-1119(03)00674-7. [DOI] [PubMed] [Google Scholar]
- Liu Y., Xu S., Xiao F., Xiong Y., Wang X., Gao S., Yan W., Ning Q. The FGL2/fibroleukin prothrombinase is involved in alveolar macrophage activation in COPD through the MAPK pathway. Biochem. Biophys. Res. Commun. 2010;396:555–561. doi: 10.1016/j.bbrc.2010.04.145. [DOI] [PubMed] [Google Scholar]
- Liu M., Guo S., Hibbert J.M., Jain V., Singh N., Wilson N.O., Stiles J.K. CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor Rev. 2011 doi: 10.1016/j.cytogfr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Guo S., Stiles J.K. The emerging role of CXCL10 in cancer. Oncol. Lett. 2011 doi: 10.3892/ol.2011.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft O., Khattar R., Farrokhi K., Ferri D., Yavorska N., Zhang J., Sadozai H., Adeyi O., Chruscinski A., Levy G.A., Selzner N. Inhibition of the Fibrinogen-Like Protein 2:FcγRIIB/RIII immunosuppressive pathway enhances antiviral T-cell and B-cell responses leading to clearance of lymphocytic choriomeningitis virus clone 13. Immunology. 2018;154:476–489. doi: 10.1111/imm.12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lwanira C.N., Mukasa M.K., Swedberg G., Kironde F. Frequency of RANTES gene polymorphisms and their association with incidence of malaria: a longitudinal study on children in Iganga district, Uganda. Malar. J. 2015;14:341. doi: 10.1186/s12936-015-0875-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhatadze N.J. Tumor necrosis factor locus: genetic organisation and biological implications. Hum. Immunol. 1998;59:571–579. doi: 10.1016/s0198-8859(98)00056-1. [DOI] [PubMed] [Google Scholar]
- Marques R.E., Guabiraba R., Russo R.C., Teixeira M.M. Targeting CCL5 in inflammation. Expert Opin. Ther. Targets. 2013 doi: 10.1517/14728222.2013.837886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita M. The lectin pathway of the complement system. Microbiol. Immunol. 1996;40:887–893. doi: 10.1111/j.1348-0421.1996.tb01156.x. [DOI] [PubMed] [Google Scholar]
- Mendel I., Feige E., Yacov N., Salem Y., Levi I., Propheta-Meiran O., Shoham A., Ishai E., George J., Harats D., Breitbart E. VB-201, an oxidized phospholipid small molecule, inhibits CD14- and Toll-like receptor-2-dependent innate cell activation and constrains atherosclerosis. Clin. Exp. Immunol. 2014;175:126–137. doi: 10.1111/cei.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 2009;151(264–9):W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- Montoya M.C., Sancho D., Bonello G., Collette Y., Langlet C., He H.T., Aparicio P., Alcover A., Olive D., Sánchez-Madrid F. Role of ICAM-3 in the initial interaction of T lymphocytes and APCs. Nat. Immunol. 2002;3:159–168. doi: 10.1038/ni753. [DOI] [PubMed] [Google Scholar]
- Moura H.V. de, Pomerantzeff P.M.A., Gomes W.J. Síndrome da resposta inflamatória sistêmica na circulação extracorpórea: papel das interleucinas. Rev. Bras. Cir. Cardiovasc. 2001;16:376–387. doi: 10.1590/s0102-76382001000400010. [DOI] [Google Scholar]
- Nepom G.T., Erlich H. MHC class-II molecules and autoimmunity. Annu. Rev. Immunol. 1991 doi: 10.1146/annurev.iy.09.040191.002425. [DOI] [PubMed] [Google Scholar]
- Ng M.W., Zhou G., Chong W.P., Lee L.W.Y., Law H.K.W., Zhang H., Wong W.H.S., Fok S.F.S., Zhai Y., Yung R.W.H., Chow E.Y., Au K.L., Chan E.Y.T., Lim W., Peiris J.S.M., He F., Lau Y.L. The association of RANTES polymorphism with severe acute respiratory syndrome in Hong Kong and Beijing Chinese. BMC Infect. Dis. 2007;7:50. doi: 10.1186/1471-2334-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn F., Ravetch J.V. 2010. FcγRs in Health and Disease; pp. 105–125. [DOI] [PubMed] [Google Scholar]
- Oliveira C.R., Pereira L.I.A., Pereira A.J.C.S., Ferreira A.A., Crespo A.M.C., Silveira L.A. Allelic polymorphism of human fcγriia-h/r131 receptor in american tegumentary leishmaniasis. Int. J. Immunogenet. 2011;38:225–231. doi: 10.1111/j.1744-313X.2011.00997.x. [DOI] [PubMed] [Google Scholar]
- Oliviero Antonio, et al. COVID-19 pulmonary and olfactory dysfunctions: is the chemokine CXCL10 the common denominator? Neuroscientist. 2020;27(3):214–221. doi: 10.1177/1073858420939033. [DOI] [PubMed] [Google Scholar]
- Özkan H., Köksal N., Çetinkaya M., Klç Ş., Elebi S., Oral B., Budak F. Serum mannose-binding lectin (MBL) gene polymorphism and low MBL levels are associated with neonatal sepsis and pneumonia. J. Perinatol. 2012;32:210–217. doi: 10.1038/jp.2011.79. [DOI] [PubMed] [Google Scholar]
- Pleass R.J., Woof J.M. Fc receptors and immunity to parasites. Trends Parasitol. 2001 doi: 10.1016/S1471-4922(01)02086-4. [DOI] [PubMed] [Google Scholar]
- Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.S. Dysregulation of immune response in patients with Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragusa F., Fallahi P. IP-10 in occupational asthma: review of the literature and case-control study. Clin. Ter. 2017 doi: 10.7417/CT.2017.1998. [DOI] [PubMed] [Google Scholar]
- Rigat B., Hubert C., Alhenc-Gelas F., Cambien F., Corvol P., Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J. Clin. Invest. 1990;86:1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi G.P., Sanga V., Barton M. Potential harmful effects of discontinuing ACE-inhibitors and ARBs in COVID-19 patients. Elife. 2020 doi: 10.7554/eLife.57278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuntabhai A., Turbpaiboon C., Casadémont I., Chuansumrit A., Lowhnoo T., Kajaste-Rudnitski A., Kalayanarooj S.M., Tangnararatchakit K., Tangthawornchaikul N., Vasanawathana S., Chaiyaratana W., Yenchitsomanus P.T., Suriyaphol P., Avirutnan P., Chokephaibulkit K., Matsuda F., Yoksan S., Jacob Y., Lathrop G.M., Malasit P., Desprès P., Julier C. A variant in the CD209 promoter is associated with severity of dengue disease. Nat. Genet. 2005;37:507–513. doi: 10.1038/ng1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel C.E. Antiviral actions of interferons. Clin. Microbiol. Rev. 2001 doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos C.M.D.C., Pimenta C.A.D.M., Nobre M.R.C. A estratégia PICO para a construção da pergunta de pesquisa e busca de evidências. Rev Latino Am Enferm. 2007;15:2–5. doi: 10.1590/S0104-11692007000300023. [DOI] [Google Scholar]
- Shen L.X., Basilion J.P., Stanton J. Single-nucleotide polymorphisms can cause different structural folds of mRNA. Proc. Natl. Acad. Sci. U. S. A. 1999;96:7871–7876. doi: 10.1073/pnas.96.14.7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B., Chitra J., Selvaraj P. CCL2, CCL3 and CCL4 gene polymorphisms in pulmonary tuberculosis patients of South India. Int. J. Immunogenet. 2014;41:98–104. doi: 10.1111/iji.12085. [DOI] [PubMed] [Google Scholar]
- Singh B., Anbalagan S., Selvaraj P. Regulatory role of CCL5 (rs2280789) and CXCL10 (rs56061981) gene polymorphisms on intracellular CCL5 and CXCL10 expression in pulmonary tuberculosis. Hum. Immunol. 2017;78:430–434. doi: 10.1016/j.humimm.2017.03.008. [DOI] [PubMed] [Google Scholar]
- Sprague A.H., Khalil R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 2009 doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover C., Endo Y., Takahashi M., Lynch N.J., Constantinescu C., Vorup-Jensen T., Thiel S., Friedl H., Hankeln T., Hall R., Gregory S., Fujita T., Schwaeble W. The human gene for mannan-binding lectin-associated serine protease-2 (MASP-2), the effector component of the lectin route of complement activation, is part of a tightly linked gene cluster on chromosome 1p36.2-3. Genes Immun. 2001;2:119–127. doi: 10.1038/sj.gene.6363745. [DOI] [PubMed] [Google Scholar]
- Tahara T., SHIBATA T., Okubo M., Ishizuka T., Kawamura T., Yamashita H., Nakamura M., Nakagawa Y., Nagasaka M., Arisawa T., Ohmiya N., Hirata I. Effect of RANTES gene promoter genotypes in patients with ulcerative colitis. Biomed. Rep. 2014;2:602–606. doi: 10.3892/br.2014.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F., Liu W., Zhang F., Xin Z.-T., Wei M.-T., Zhang P.-H., Yang H., Ly H., Cao W.-C. IL-12 RB1 genetic variants contribute to human susceptibility to severe acute respiratory syndrome infection among Chinese. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tas S.K., Kirkik D., Işik M.E., Kalkanli N., Uzunoglu A.S., Uzunoglu M.S., Altunkanat D., Alragabi J.M.M., Tanoglu A. Role of ACE2 gene expression in Renin Angiotensin System and its importance in Covid-19: In Silico Approach. Brazilian Arch. Biol. Technol. 2020;63 e20200304. [Google Scholar]
- Trenchevska O., Sherma N.D., Oran P.E., Reaven P.D., Nelson R.W., Nedelkov D. Quantitative mass spectrometric immunoassay for the chemokine RANTES and its variants. J. Proteome. 2015;116:15–23. doi: 10.1016/j.jprot.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu X., Chong W.P., Zhai Y., Zhang H., Zhang F., Wang S., Liu W., Wei M., Siu N.H.O., Yang H., Yang W., Cao W., Lau Y.L., He F., Zhou G. Functional polymorphisms of the CCL2 and MBL genes cumulatively increase susceptibility to severe acute respiratory syndrome coronavirus infection. J. Inf. Secur. 2015;71:101–109. doi: 10.1016/j.jinf.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulio S., Faucz F.R., Werneck R.I., Olandoski M., Alexandre R.B., Boldt A.B., Pedroso M.L., de Messias-Reason I.J. MASP2 gene polymorphism is associated with susceptibility to hepatitis C virus infection. Hum Immunol. 2011;72(10):912–915. doi: 10.1016/j.humimm.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale R.F., de Ribeiro F.A.Q. The role of tumor necrosis factor-alpha (TNF-alpha) in bone resorption present in middle ear cholesteatoma. Braz. J. Otorhinolaryngol. 2007;73:117–121. doi: 10.1016/s1808-8694(15)31133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F.S., Chu F.L., Jin L., Li Y.G., Zhang Z., Xu D., Shi M., Wu H., Moulds J.M. Acquired but reversible loss of erythrocyte complement receptor 1 (CR1, CD35) and its longitudinal alteration in patients with severe acute respiratory syndrome. Clin. Exp. Immunol. 2005;139:112–119. doi: 10.1111/j.1365-2249.2005.02681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Wei M., Han Y., Zhang K., He L., Yang Z., Su B., Zhang Z., Hu Y., Hui W. Roles of TNF-alpha gene polymorphisms in the occurrence and progress of SARS-Cov infection: a case-control study. BMC Infect. Dis. 2008;8:27. doi: 10.1186/1471-2334-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Yan J., Shi Y., Li P., Liu C., Ma Q., Yang R., Wang X., Zhu L., Yang X., Cao C. Lack of association between polymorphisms of MASP2 and susceptibility to SARS coronavirus infection. BMC Infect. Dis. 2009;9:51. doi: 10.1186/1471-2334-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Yang P., Liu K., Guo F., Zhang Y., Zhang G., Jiang C. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008 doi: 10.1038/cr.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Chen R.-F., Liu J.-W., Lee I.-K., Lee C.-P., Kuo H.-C., Huang S.-K., Yang K.D. DC-SIGN (CD209) promoter −336 A/G polymorphism is associated with dengue hemorrhagic fever and correlated to DC-SIGN expression and immune augmentation. PLoS Negl. Trop. Dis. 2011;5 doi: 10.1371/journal.pntd.0000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.H., Li J.Y., Wang C., Zhang L.M., Qiao H. The ACE2 G8790A polymorphism: involvement in Type 2 diabetes mellitus combined with cerebral stroke. J. Clin. Lab. Anal. 2017;31(2) doi: 10.1002/jcla.22033. e22033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong P., Zeng X., Song M.S., Jia S.W., Zhong M.H., Xiao L.L., Lan W., Cai C., Wu X.W., Gong F.L., Wang W. Lack of association between HLA-A, -B and -DRB1 alleles and the development of SARS: a cohort of 95 SARS-recovered individuals in a population of Guangdong, southern China. Int. J. Immunogenet. 2008;35(1):69–74. doi: 10.1111/j.1744-313X.2007.00741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav A., Saini V., Arora S. MCP-1: Chemoattractant with a role beyond immunity: a review. Clin. Chim. Acta. 2010 doi: 10.1016/j.cca.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Yuan F.F., Tanner J., Chan P.K.S., Biffin S., Dyer W.B., Geczy A.F., Tang J.W., Hui D.S.C., Sung J.J.Y., Sullivan J.S. Influence of FcgammaRIIA and MBL polymorphisms on severe acute respiratory syndrome. Tissue Antigens. 2005;66:291–296. doi: 10.1111/j.1399-0039.2005.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan F.F., Boehm I., Chan P.K.S., Marks K., Tang J.W., Hui D.S.C., Sung J.J.Y., Dyer W.B., Geczy A.F., Sullivan J.S. High prevalence of the CD14-159CC genotype in patients infected with severe acute respiratory syndrome-associated coronavirus. Clin. Vaccine Immunol. 2007;14:1644–1645. doi: 10.1128/CVI.00100-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Zhou G., Zhi L., Yang H., Zhai Y., Dong X., Zhang X., Gao X., Zhu Y., He F. Association between mannose-binding lectin gene polymorphisms and susceptibility to severe acute respiratory syndrome coronavirus infection. J. Infect. Dis. 2005;192:1355–1361. doi: 10.1086/491479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Yang X., Zhang X., Yu Q., Zhao P., Wang J., Duan C., Li J., Johnson H., Feng X., Zhang H. IP-10 and RANTES as biomarkers for pulmonary tuberculosis diagnosis and monitoring. Tuberculosis. 2018;111:45–53. doi: 10.1016/j.tube.2018.05.004. [DOI] [PubMed] [Google Scholar]
- Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020 doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Wang Y., Zhang H., Liu X., Chen T., Yang R., et al. Genetic Variation of the human α-2-Heremans-Schmid Glycoprotein (AHSG) gene associated with the risk of SARS-CoV infection. PLoS ONE. 2011;6(8) doi: 10.1371/journal.pone.0023730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C.L., Yan W.M., Zhu F., Zhu Y.F., Xi D., Tian D.Y., Levy G., Luo X.P., Ning Q. Fibrinogen-like protein 2 fibroleukin expression and its correlation with disease progression in murine hepatitis virus type 3-induced fulminant hepatitis and in patients with severe viral hepatitis B. World J. Gastroenterol. 2005;11:6936–6940. doi: 10.3748/wjg.v11.i44.6936. [DOI] [PMC free article] [PubMed] [Google Scholar]