Tiede and colleagues report 5 cases of patients experiencing the triad of thromboembolism, thrombocytopenia, and anti–platelet factor 4 (PF4) antibodies after vaccination with the Oxford-AstraZeneca adenoviral vector COVID-19 vaccine (ChAdOx1 nCoV-19). They highlight the unusual thrombotic presentation and distinct laboratory features. They further describe treatment for this novel syndrome, now most commonly referred to as vaccine-induced thrombotic thrombocytopenia (VITT).
Key Points
Patients presented with a broad spectrum of unusual thromboembolic manifestations after AZD1222 exposure.
A triad of thromboembolic events, thrombocytopenia, and anti-PF4 autoantibodies was characteristic of the disease.
Visual Abstract
Abstract
We report 5 cases of prothrombotic immune thrombocytopenia after exposure to the ChAdOx1 vaccine (AZD1222, Vaxzevria) against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Patients presented 5 to 11 days after first vaccination. The spectrum of clinical manifestations included cerebral venous sinus thrombosis, splanchnic vein thrombosis, arterial cerebral thromboembolism, and thrombotic microangiopathy. All patients had thrombocytopenia and markedly elevated D-dimer. Autoantibodies against platelet factor 4 (PF4) were detected in all patients, although they had never been exposed to heparin. Immunoglobulin from patient sera bound to healthy donor platelets in an AZD1222-dependent manner, suppressed by heparin. Aggregation of healthy donor platelets by patient sera was demonstrated in the presence of buffer or AZD1222 and was also suppressed by heparin. Anticoagulation alone or in combination with eculizumab or intravenous immunoglobulin (IVIG) resolved the pathology in 3 patients. Two patients had thromboembolic events despite anticoagulation at a time when platelets were increasing after IVIG. In summary, an unexpected autoimmune prothrombotic disorder is described after vaccination with AZD1222. It is characterized by thrombocytopenia and anti-PF4 antibodies binding to platelets in AZD1222-dependent manner. Initial clinical experience suggests a risk of unusual and severe thromboembolic events.
Introduction
The Oxford-AstraZeneca vaccine AZD1222 is projected to play a vital role in combating the COVID-19 pandemic.1 The vaccine is less expensive and easier to store than other vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).2 Published interim results of 4 randomized controlled trials in the United Kingdom, Brazil, and South Africa indicated a favorable safety profile,3 and the vaccine is now licensed in more than 100 countries. Since 15 March 2021, reports of an unexpected accumulation of thrombotic events in association with thrombocytopenia have emerged and resulted in a temporary suspension of inoculation in several European countries.4 We report our initial experience in 5 cases, providing insight into disease pathology, its broad spectrum of clinical manifestations, and response to therapy.
Study design
This consecutive single-center cohort includes all patients presenting to Hannover Medical School between 8 March and 4 April with known or suspected thromboembolic events and thrombocytopenia within 2 weeks after vaccination with AZD1222. Demographic and clinical characteristics are presented in Table 1. Detailed case descriptions are provided in the supplemental Information provided on the Blood Web site. Patients or representatives gave informed consent before publication. Anti–platelet factor 4 (PF4) autoantibodies were assessed with enzyme-linked immunosorbent assay (ELISA) and chemiluminescent immunoassay (CLIA). Detailed methods including flow cytometry and modified heparin-induced platelet aggregation assays are described in the supplemental Information.
Table 1.
Baseline characteristics, thromboembolic events, treatment overview, and outcome
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Age (y), sex | 63, female | 67, female | 41, female | 61, female | 61, female |
| Day of vaccination | 25 Feb 2021 | 15 Mar 2021 | 18 Mar 2021 | 15 Mar 2021 | 26 Mar 2021 |
| Hospital admission (day after vaccination) | 8 Mar 2021 (11) |
23 Mar 2021 (8) |
23 Mar 2021 (5) |
24 Mar 2021 (9) |
4 Apr 2021 (9) |
| Thromboembolic events (day of hospital stay) | CVST, TMA (1) |
Arterial cerebral embolism (1) |
TIA (1) |
SVT (6) |
Arterial cerebral thrombosis (1) Popliteal artery thrombosis (8) |
| Symptoms on admission | Headache, somnolence, dysphasia, right-sided hemiparesis, and arterial hypertension | Headache | Headache, diplopia | Fatigue | Headache, dysarthria, left-sided hemiplegia, conjugated gaze palsy |
| Imaging results | Left transverse and sigmoid sinus thrombosis Left temporal bleeding |
Cortical infarctions and aortic arch thrombi | No pathology | No pathology on admission. SVT day 6 after admission |
Right internal carotid and middle cerebral artery (M1) thrombosis. Right MCA territory infarction with hemorrhagic transformation |
| Platelet count on admission (/nL) | 27 | 40 | 105 | 12 | 62 |
| D-dimer on admission (mg/L) | >35.2 | >35.2 | 22.4 | >35.2 | >35.2 |
| Treatment | Heparin and eculizumab | Argatroban and IVIG | Argatroban | Argatroban, IVIG, alteplase (day 6), an eculizumab (day 7) | Argatroban and IVIG |
| Outcome | Recovering | Recovered | Recovered | Recovering | Recovering |
Patient 4 was first admitted to a remote hospital and transferred to Hannover Medical School on day 7 after admission. Please refer to supplemental Table 1 for additional laboratory parameters on admission and during follow-up.
TIA, transitory ischemic attack.
Results and discussion
Presenting characteristics
The patients were women between 41 and 67 years of age and presented 5 to 11 days after their first vaccination with AZD1222 (2.5 × 1010 particles). Patient 1 had cerebral venous sinus thrombosis (CVST) and progressive signs of thrombotic microangiopathy (TMA) including Coombs-negative hemolytic anemia, abundant schistocytes, and renal failure. Patient 2 had multiple cortical emboli and aortic arch thrombi. Patient 3 presented with headache and visual disturbance but had no abnormal imaging results. Patient 4 initially presented to a remote hospital without signs of thrombosis but developed a thromboembolic event during the first week. Patient 5 presented with cerebral artery thrombosis.
Platelet counts were reduced, and D-dimer was markedly elevated in all patients (comprehensive laboratory data presented in supplemental Table 1). None of the patients had previously been exposed to heparin or previous COVID-19. On admission, all patients were SARS-CoV-2 negative by polymerase chain reaction test.
Anti-PF4 antibodies
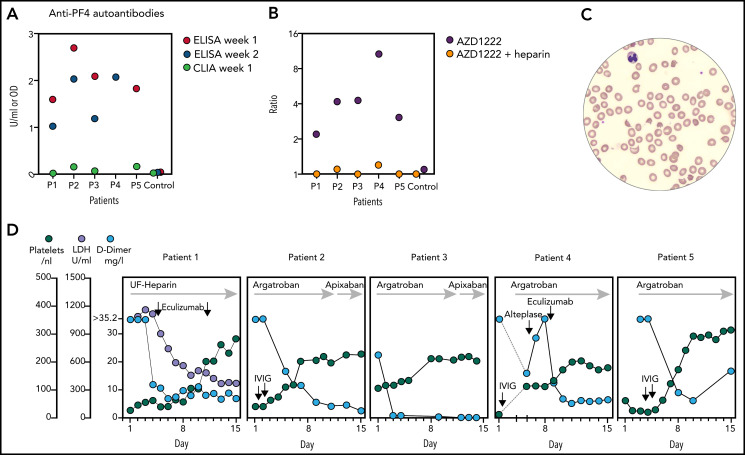
Because the clinical picture reminded of heparin-induced thrombocytopenia (HIT), patients were tested for anti-PF4 antibodies, but CLIA results were negative. Samples were retested by ELISA and proved strongly positive in all patients (Figure 1A). Reactivity was inhibited by heparin in all samples. Sera induced aggregation of normal donor platelets independent of heparin (supplemental Figure 5). AZD1222 induced binding of patient immunoglobulin to healthy donor platelets (Figure 1B).
Figure 1.
Anti-PF4 autoantibodies were detected in all samples by ELISA but not CLIA. (A) ELISA reactivity was inhibited to optical density <0.3 by heparin in all samples (2 U/mL, data not shown). (B) Binding of antibodies from patient sera to healthy donor platelets in the presence of AZD1222 was suppressed by heparin. Fluorescent intensity ratio of AZD1222 (or AZD1222 + heparin 100 U/mL) to buffer is shown. (C) Schistocytes in blood smear high power field in patient 1, indicative of TMA, on day 4. (D) Course of selected laboratory markers over time. X-axes show days of hospital stay. Y-axes on the left are for all panels.
Treatment and initial outcomes
All patients received anticoagulation with full-dose unfractionated heparin (patient 1) or argatroban (patients 2-5). No further treatment was given to patient 3, who recovered quickly. Patients 1, 2, and 5 received a dexamethasone pulse for 4 days.
Intravenous immunoglobulin (IVIG; 1 g/kg on 2 consecutive days) was given in patients 2, 4, and 5 according to ad hoc recommendations by the German, Austrian, and Swiss Society on Thrombosis and Haemostasis (GTH).5 Patient 2 had a favorable outcome with no further events. Patient 4 developed extensive splanchnic vein thrombosis (SVT), when platelets had recovered to 115/nL on day 6. Patient 5 had a popliteal artery occlusion, when platelets had recovered to 172/nL on day 8.
Eculizumab was given in patient 1 (900 mg weekly) because of TMA and renal failure. Signs of TMA resolved, and platelets recovered over the next week. Eculizumab was also given as a salvage therapy in patient 4 because of a severe thromboembolic event after IVIG and while on anticoagulation. She quickly improved and had no further events. Additional information on treatment and outcome is provided in the supplemental Information and Figure 1D.
Discussion
Reviewing our experience with this new kind of prothrombotic complication after vaccination, it is strikingly similar to spontaneous autoimmune HIT. This is an ultrarare disorder occurring in patients never exposed to heparin, and polyanionic substances of bacterial origin have been implicated in its pathogenesis.6 Although it remains to be demonstrated how AZD1222 could provoke anti-PF4 autoantibody formation, our observation of vaccine-dependent binding of patient antibodies to healthy donor platelets suggests its direct involvement.
Some of our observations may be important for clinical practice. The triad of thromboembolic events, thrombocytopenia, and anti-PF4 antibodies appears to be characteristic of the syndrome. This triad was also reported in other cases registered by German medical regulatory agencies.7 The GTH expert committee currently suggests to screen for anti-PF4 antibodies if thromboembolic events or thrombocytopenia occurred within 2 weeks after vaccination.5 Our experience indicates that such antibodies can be detected by HIT immunoglobulin G (IgG) ELISA of at least 2 manufacturers but not by the HIT IgG CLIA that is often used for screening by clinical laboratories.
The choice of anticoagulant requires careful consideration. Heparin is strictly avoided in patients with classical HIT8 but was apparently harmless in our first patient. In fact, we did not see evidence of heparin-enhancing platelet aggregation in our patients. However, caution is required because the polyanion heparin could possibly increase the aggregation potential of anti-PF4 antibodies in some patients, even if antibodies were not caused by heparin itself. Argatroban appears to be a safe and effective alternative.
The effect of IVIG is uncertain according to our initial experience. It was suggested by the GTH expert panel because of its reported efficacy in autoimmune HIT.5,9,10 Monomeric IgG in high concentrations can block Fc γ receptor 2A (FcγR2A), thereby inhibiting platelet activation and aggregation by PF4/anti-PF4 immune complexes.11 Platelet counts improved, but 2 of 3 treated patients in our series developed a new major thromboembolic event.
Eculizumab is perhaps an alternative but its effect is also uncertain. FcγR2A-dependent platelet activation (addressed by IVIG) may not be the only prothrombotic mechanism in this syndrome. PF4/anti-PF4 immune complexes activate the complement cascade.12 We saw the full picture of TMA in our first patient, reminiscent of atypical hemolytic uremic syndrome (AHUS). Eculizumab (but not anticoagulation alone or plasma exchange) appeared to have reversed the pathology in this patient, suggesting that it was caused by uncontrolled complement activation, just like in AHUS.13 Evidence of complement consumption was noted in patient 4, who received eculizumab because IVIG and anticoagulation had failed. A striking improvement of SVT was noted after eculizumab in this patient.
Our series indicates a broad spectrum of disease severity, similar to classical HIT. The presenting symptoms ranged from very mild to unusually severe. Awareness and early treatment can possibly prevent complications as suggested by the example of patient 3, who presented with mild symptoms already on day 5 and improved without sequelae after anticoagulation. As the knowledge about this syndrome continues to unfold, we suggest a high level of suspicion and prompt testing in patients with thrombocytopenia or signs of thromboembolism after vaccination.
Despite the suggestive temporal relationship between vaccination and the prothrombotic immune thrombocytopenia reported here, caution is required when drawing conclusions about the safety of AZD1222 in the general population. There is a high level of uncertainty in estimating the incidence of this complication. EMA’s safety committee PRAC had 62 and 24 cases of CVST and SVT, respectively, reported to its drug safety database as of 22 March 2021. Most cases were from the United Kingdom, where ∼ 25 million people received the vaccine at this time.14 The risks of COVID-19, including its own risk of thromboembolic complications, appear to be greater than the unexpected thromboembolic risk of the vaccine suggested by this and other emerging reports.15-17
Supplementary Material
Footnotes
For original data, please contact the corresponding authors.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.T. and A.G. wrote the paper; A.T., A.G., J.K.K. and F.D. analyzed clinical data; U.J.S., A.C., S.W., and R.B. performed laboratory analysis; and F.D. performed imaging analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andreas Tiede, Hannover Medical School, Department of Hematology, Hemostasis, Oncology and Stem Cell Transplantation, Carl Neuberg Strasse 1, 30625 Hannover, Germany; e-mail: tiede.andreas@mh-hannover.de; or Arnold Ganser, Hannover Medical School, Department of Hematology, Hemostasis, Oncology and Stem Cell Transplantation, Carl Neuberg Strasse 1, 30625 Hannover, Germany; e-mail: ganser.arnold@mh-hannover.de.
References
- 1.Mallapaty S, Callaway E.. What scientists do and don’t know about the Oxford-AstraZeneca COVID vaccine. Nature. 2021; 592(7852):15-17. [DOI] [PubMed] [Google Scholar]
- 2.Putter JS. Immunotherapy for COVID-19: evolving treatment of viral infection and associated adverse immunological reactions. Transfus Apheresis Sci. 2021; 60(2):103093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voysey M, Clemens SAC, Madhi SA, et al. ; Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paul Ehrlich Institute . Temporary suspension of vaccination with COVID-19 vaccine AstraZeneca. https://www.pei.de/EN/newsroom/hp-news/2021/210315-pei-informs-temporary-suspension-vaccination-astra-zeneca.html. Accessed 15 March 2021.
- 5.German, Austrian and Swiss Thrombosis and Hemostasis Society . GTH statement on vaccination with the AstraZeneca COVID-19 vaccine. https://gth-online.org/wp-content/uploads/2021/03/GTH_Stellungnahme_AstraZeneca_engl._3_24_2021.pdf. Accessed 28 March 2021.
- 6.Greinacher A, Selleng K, Warkentin TE.. Autoimmune heparin-induced thrombocytopenia. J Thromb Haemost. 2017;15(11):2099-2114. [DOI] [PubMed] [Google Scholar]
- 7.Paul Ehrlich Institute . COVID-19 vaccine AstraZeneca safety assessment. https://www.pei.de/DE/newsroom/hp-meldungen/2021/210319-covid-19-impfstoff-astrazeneca-ergebnis-sicherheitsbewertung-impfstoff-ist-sicher.html. Accessed 28 March 2021.
- 8.Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018; 2(22):3360-3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arcinas LA, Manji RA, Hrymak C, Dao V, Sheppard JI, Warkentin TE.. Autoimmune heparin-induced thrombocytopenia and venous limb gangrene after aortic dissection repair: in vitro and in vivo effects of intravenous immunoglobulin. Transfusion. 2019;59(6):1924-1933. [DOI] [PubMed] [Google Scholar]
- 10.Dougherty JA, Yarsley RL.. Intravenous immune globulin (IVIG) for treatment of autoimmune heparin-induced thrombocytopenia: a systematic review. Ann Pharmacother. 2021;55(2):198-215. [DOI] [PubMed] [Google Scholar]
- 11.Warkentin TE. High-dose intravenous immunoglobulin for the treatment and prevention of heparin-induced thrombocytopenia: a review. Expert Rev Hematol. 2019;12(8):685-698. [DOI] [PubMed] [Google Scholar]
- 12.Cines DB, Yarovoi SV, Zaitsev SV, et al. Polyphosphate/platelet factor 4 complexes can mediate heparin-independent platelet activation in heparin-induced thrombocytopenia. Blood Adv. 2016;1(1):62-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brodsky RA. Complement in hemolytic anemia. Blood. 2015;126(22):2459-2465. [DOI] [PubMed] [Google Scholar]
- 14.European Medicines Agency . AstraZeneca’s COVID-19 vaccine: EMA finds possible link to very rare cases of unusual blood clots with low blood platelets. https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood. Accessed 15 April 2021.
- 15.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination [published online ahead of print 9 April 2021]. N Engl J Med., doi:10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S.. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination [published online ahead of print 9 April 2021]. N Engl J Med., doi:10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination [published online ahead of print 16 April 2021]. N Engl J Med., doi:10.1056/NEJMoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.