Abstract
Background
The benefit of IVIG (Intravenous Immunoglobulin) therapy for COVID-19 remains controversial. We performed a meta-analysis to investigate the efficacy of IVIG treatment in patients with COVID-19.
Methods
We searched articles from Web of Science, PubMed, Embase, the Cochrane Library, MedRxiv between 1 January 2020 and February 17, 2021. We selected randomized clinical trials and observational studies with a control group to assess the efficiency of IVIG in treating patients with COVID-19. Subjects were divided into ‘non-severe’, ‘severe’ and ‘critical’ three subgroups based on the information of the study and the World Health Organization (WHO) definition of severity. We pooled the data of mortality and other outcomes using either a fixed-effect model or a random-effects model.
Results
Our meta-analysis retrieved 4 clinical trials and 3 cohort studies including 825 hospitalized patients. The severity of COVID-19 is associated with the efficiency of IVIG. In critical subgroup, IVIG could reduce the mortality compared with the control group [RR = 0.57 (0.42–0.79, I2 = 025%). But there was no significant difference in the severe or non-severe subgroups.
Conclusion
IVIG has demonstrated clinical efficacy on critical ill patients with COVID-19. There may be a relationship between the efficacy of IVIG and the COVID-19 disease severity. Well-designed clinical trials to identify the clinical and biochemical characteristics in COVID-19 patients’ population that could benefit from IVIG are warranted in the future.
Keywords: COVID-19, IVIG, Efficacy, Meta-analysis
1. Introduction
In 2019, a novel coronavirus disease (COVID-19) which caused by the severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) has aroused an outbreak worldwide. COVID-19 has been diagnosed in 110.7 million cases and over 2.4 million deaths since the start of the pandemic [1]. The high infection rate and mortality pose an unprecedented challenge to clinicians. Numerous clinical trials, retrospective studies and observational studies about SARS-Cov-2 disease are underway, and we found some effective antiviral agents against the infection but the lack of standardized therapy makes the condition worse. In parallel with the development of new antiviral agents and vaccines in this disease, it is necessary to research the efficacy of existing therapeutic such as Intravenous immunoglobulin (IVIG).
IVIG is a kind of blood product from healthy donors containing a polyclonal IgG antibody. Known for its anti-inflammatory reactions, it has been used to treat patients with inflammatory diseases including Kawasaki diseases, multiple sclerosis and so on [2]. The SARS-Cov-2 virus is a member of coronavirus family. Based on the experience of treating previous coronavirus diseases such as severe acute respiratory syndrome (SARS) [3], Middle East respiratory syndrome (MERS) [4] and swine-origin influenza virus (SOIV) H1N1 [5], though no sufficient clinical data, it might be believed that IVIG could be used in COVID-19 patients and be one of worthwhile therapeutic options. The controversy over the efficacy of IVIG for improving in clinical symptoms and mortality, nevertheless, has been occurring with the increasing number of COVID-19 patients [6], [7], [8]. There still exist types of studies which have evaluated the efficiency of IVIG in patients with COVID-19 so far [9], [10]. A meta-analysis to review these evidences is greatly essential for the use of IVIG for COVID-19. We performed a meta-analysis by selecting literature from five databases to synthesize the results of well-done randomized clinical trial (RCTs) and observational studies to tested the significance of IVIG therapy and give some advice for clinical treatment.
2. Methods
This meta-analysis was described by the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA-P) statement [11]. The protocol for this study has been registered in the International Prospective Register of Systematic Reviews (PROSPERO, CRD42021238498).
2.1. Search strategy
Two reviewers (Yun Li and Xuan Cheng) searched Web of Science, PubMed, Embase, the Cochrane Library, MedRxiv for clinical trials and observational studies. A combination of search terminologies (“Coronavirus Disease 2019” OR “COVID-19” OR “SARS-Cov-2” OR “2019-nCoV Diseases” OR “COVID 19 Virus Infection”) AND (“IVIG” OR “immunoglobulin” OR “IVIG”). Search were down on February 17, 2021. The search was imposed restriction on the date between 1.1 2020 to 2.17 2021. No restriction on the geographical location or language of the studies.
2.2. Selection criteria
Inclusion criteria included:
(1) Study types: Clinical trials and observational studies which patients were divided into treatment group using IVIG and the control group not using IVIG.
(2) Patients: Patients with lab-confirmed COVID-19, aged 18 years and above.
(3) Outcome: The primary outcome measure was defined as mortality. The secondary outcome measures were mechanical ventilation need, length of hospital stay (day) and length of Intensive Care Unit (ICU) stay (day).
Exclusion criteria included:
(1) Studies with insufficient data;
(2) Studies with no control group;
(3) Review articles, viewpoints, editorials, and expert opinion.
2.3. Study selection and data extraction
Two reviewers (Huai-rong Xiang, Wen-wen Luo) independently screened the titles and abstracts of the studies to retrieve articles and extracted data based on the inclusion and exclusion criteria. Any discrepancies would be resolved through discussion (with a third author, if necessary).
Each included article was thoroughly reviewed, the following data and information were extracted:
(1) First author, publication date, region and study type.
(2) Treatment plan (including IVIG dosage, frequency and duration).
(3) Outcome indicators (including 28-mortality, the length of hospital or ICU stays).
Data was extracted and entered to a pre-defined and piloted Microsoft excel database.
2.4. Risk of bias assessment
Two reviewers (Qi-zhi Zhang and Xuan Cheng) independently evaluated the quality of this literature. In case of any disagreement, the third reviewer (Yun Li) consulted for reconciling any difference of opinion. The new Cochrane risk of bias tool [12] was used to evaluate the methodological quality of the RCTs, which includes five domains: ‘random sequence generation’, ‘concealment of allocation schemes’, ‘blinding’, ‘completeness of outcome data’, and ‘selective reporting’. For each domain, risk of bias judgements is ‘high’, ‘unclear’ or ‘low’. The Newcastle-Ottawa Scale (NOS) [13] was to evaluate the quality of the non-RCTs which comprises patient selection, comparability between two groups, and research results three components. The total score is 9 which was divided into three categories: (a) high risk (1–3); (b) some concerns (4–6); (c) low risk (7–9).
2.5. Statistical analysis
We conducted a subgroup to test the impact of COVID-19 disease severity using the World Health Organization definition of severity [14].
Critical ill group: defined by the criteria for: (1) Acute respiratory distress syndrome (ARDS), sepsis, septic shock, or other conditions that would normally require the provision of life-sustaining therapies such as mechanical ventilation (invasive or non-invasive) or vasopressor therapy. Severe group: defined by any of: (1) Oxygen saturation < 93% on room air. (2) Respiratory rate > 30 breaths/min in adults (3) PaO2/FiO2 ≤ 300 mmHg. Non-severe group: defined as absence of any criteria for severe or critical COVID-19.
All statistical analysis was conducted by RevMan 5.4 software. Statistical heterogeneity was evaluated by the I-squared (I2) test. According to the Cochran's Handbook for the systematical reviews of intervention, I2 value: 0% to 40% may represent not important heterogeneity, 40% to 75% represent moderate heterogeneity, 75% to 100% represent considerable level of heterogeneity. A fixed-effect model was used if I2<50%. When I2 value>60%, heterogeneity was considered significant, and the random-effect model was applied. The select indicators were included dichotomous data and continuous data. We used relative risk (RR) for dichotomous data and mean difference (MD) or standard mean difference (SMD) for continuous data with 95% confidence interval (95%CI).
We computed missing means and standard deviations (SDs) from medians, ranges (minimum to maximum), and interquartile ranges (IQRs) using the methods proposed by Hozo et al. [15] and Wan et al. [16].
3. Results
3.1. Description of the results
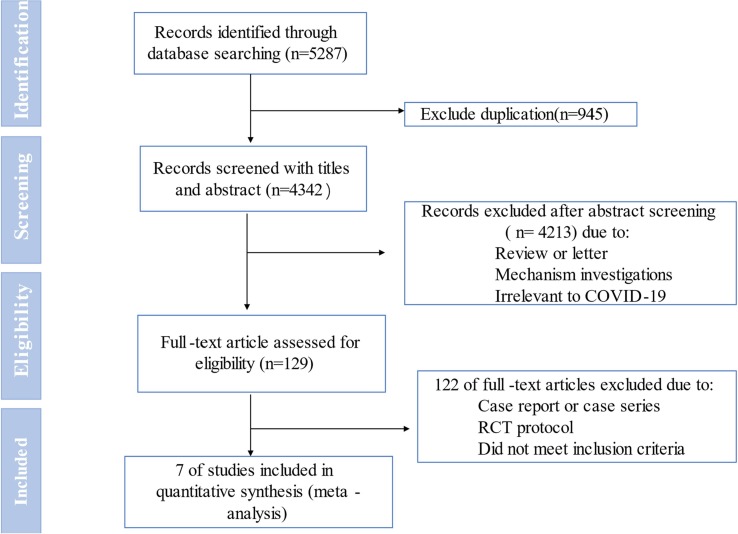
As shown in Fig. 1 , the total search process yielded 5287 records. Following removing duplicates publications 4342 studies were remained. After screening of titles and abstracts we excluded 4213 studies as these include reviews, commentaries, mechanism researches, and irrelevant to COVID-19. After comprehensively screening 129 full text, only 7 eligible studies were included. (RCTs: n = 4, Retrospective cohort studies (RCSs): n = 3) including 825 patients [17], [18], [19], [20], [21], [22], [23]. See Table 1
Fig. 1.
Flowchart of literature search study selection.
Table 1.
Characteristics of the studies included in the meta‑analysis.
| Frist author, year | Type of study | country | Type of disease | Sample (male) |
Treatment |
Summary | ||
|---|---|---|---|---|---|---|---|---|
| intervention | control | intervention | control | |||||
| Gharebaghi 2020 | RCT | Iran | Severe; A PCR-confirmed COVID-19 diagnosis, involvement of > than 30% of both lungs (ground-glass opacity) in high-resolution computed tomography (HRCT) (confirmed by two radiologists), O2saturation(satO2) of < 90%, and a lack of adequate response to initial treatment including at least both one antiviral and one chloroquine-class drug. |
30 (21) | 29 (20) | IVIG (human) flebo gamma 5% DIF GRIFOLS, four vials of 5 gm5 IVIG daily for three consecutive days; receive the same treatments as were introduced initially | Placebo; receive the same treatments as were introduced initially | 1. Improve their clinical outcome including serum creatinine, white blood cell which were higher in the control group 2. Reduce mortality rate. 3. Longer duration of hospitalization in the treatment group. |
| Sakoulas 2020 | RCT | USA | Severe: SpO2 < 96% on > 4 L O2 by nasal cannula APACHE II: (7, 7.5) |
16(10) | 17(10) | IVIG 0.5 g/Kg for 3 days and SOC; All received methylprednisolone | Receive any treatment not part of a RCT at the time of enrollment | 1. Lower rate of mechanical ventilation; 2.Shorter median hospital length of stay; 3. Shorter median ICU stay; 4. Greater improvement in PaO2/FiO2 at 7 days. |
| Tabarsi 2021 | RCT | Iran | Severe Breaths/min ≥ 30, SpO2 ≤ 93% PaO2/ FiO2 ≤ 300 mmHg. |
52(40) | 32(25) | 400 mg/kg daily for three doses IVIG, premedicated with 500 mg Acetaminophen, 100 mg Hydrocortisone, and 25 mg Diphenhydramine 30 min before the injection. SOC: oxygen and fluid support, lopinavir/ ritonavir (200/50 mg, Hetero labs), two tablets twice a day, and hydroxychloroquine (Tehran-Daru) 200 mg two times daily. |
SOC | 1. No significant difference between the two groups in terms of mortality rate and the need for mechanical ventilation; 2. The length of hospital stay was lower for the control group than that of the intervention group; 3. Positive relationship between the time from hospital admission to IVIG initiation and the length of stay in the hospital and ICU among the survivors. |
| Raman 2021 | RCT | India | Non-severe: Pneumonia were defined as: body temperature ≥ 38.0℃ or PaO2/ FiO2 100–300 mmHg or respiratory rate > 24/min and oxygen saturation 90–93% on room air or lung involvement confirmed with chest X-ray. |
50 (14) | 50 (19) | daily received immunoglobulin 0.4 g/kg body weight for 5 days SOC: Azithromycin; Lopinavir/ritonavir; Piperacillin + Tazobactam; Acetaminophen and Pantocid. |
SOC | 1. Reduce the number of days to clinical improvement, 2. Reduce the duration of use of mechanical ventilation; duration of hospitalization and length of stay in intensive coronary care unit from day 0 to 28. 3. Increased the proportion of patients with negative RT-PCR on day 14. |
| Shao 2020 | RCS | China | Severe , andcritical ill | 174 (112) | 151(77) | NM | NM | 1. The total duration of disease was longer in the IVIG group 2. Only in patients with critical type, IVIG could significantly reduce the 28-day mortality, decrease the inflammatory response and improve some organ functions; 3.The application of IVIG in the early stage (admission ≤ 7 days) with a high dose (>15 g per day) exhibited significant reduction in 60-day mortality in the critical-type patients. |
| Esen 2021 | RCS | Turkey | Critical (a) respiratory rate > 30/min, (b) signs of dyspnea and respiratory distress. (c)SpO2 < 90% and PaO2 < 70 mmHg, despite nasal oxygen support of > 5 L/min. (d) PaO2/FiO2 < 300 (mild acute respiratory distress syndrome (ARDS). |
51 (37) | 42 (31) | a dose 30 g/day IVIG for five consecutive days SOC: hydroxychloroquine (800 mg loading dose, LD; 400 mg/day maintenance dose, MD for 5 days); favipiravir (3200 mg LD; 1200 mg/day MD for 5 days), azithromycin (500 mg LD; 250 mg/day MD for 5 days), oseltamivir (150 mg/day for 5 days), tocilizumab or anakinra depending on inflammatory markers, methylprednisolone (200 mg/day), high dose vasopressors in case of septic shock and vitamin C (6 g/day i.v. for 7 days). |
SOC | IVIG group significantly prolonged median survival time and reduced plasma levels of C-reactive protein. |
| Huang 2021 | RCS | China | Non-severe; (1) Respiratory distress, respiratory rates ≥ 30/min; (2) Pulse oxygen saturation ≤ 93% in the resting state; (3) Oxygenation index ≤ 300 mmHg; (4) Require mechanical ventilation; (5) Shock; (6) Combined with other organ failures and needed treatment in ICU. |
45(23) | 90(50) | IVIG SOC: Corticosteroids, Chinese Medicine, Hydroxychloroquine, Thymosin α, Arbidol, Lopinavir/Ritonavir |
SOC | In non-severe patients with COVID-19, no benefit was observed with IVIG therapy beyond standard therapy. |
Note: IVIG: intravenous immunoglobulin; SOC: standard of care; NM: not mention.
According to the inclusion criteria of the article and the characteristic of patients at baseline, we divided subjects into ‘non-severe’ (two studies), ‘severe’ (four studies) and ‘critical’ (two studies) three subgroups to address heterogeneity. Due to the Shao et al. study provided both severe and critical patients’ outcomes, we defined the data of critical type patients as Shao et al. 2020 (a), and of the severe type patients as Shao et al. 2020(b).
3.2. Quality assessment
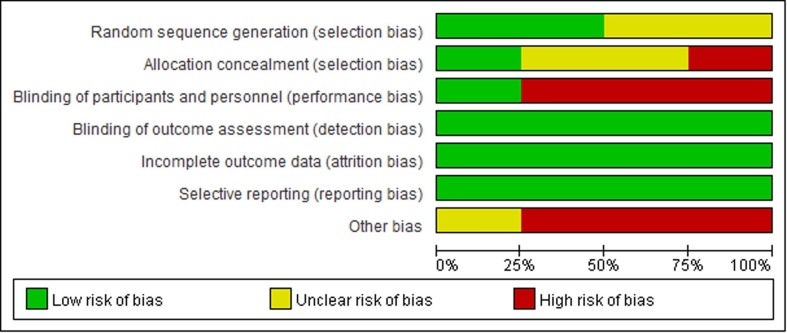
The quality of the included RCTs is shown in the Fig. 2 . Two RCTs [18], [20] provided the methods for the random sequence generation. Only one RCT [19] provided the blinding of participants and personnel and blinding of outcome assessment. All three retrospective cohort studies have adjusted the confounders in the analysis and the range of score is 5–6. See Table 2 . Overall, the quality of the seven included studies was low.
Fig. 2.
Risk of bias assessment of the included studies in the systematic review using the new Cochrane risk of bias tool for randomized controlled trials.
Table 2.
The quality of included Cohort Studies.
| Study | Representativeness of the Exposed Cohort | Selection of the Non-Exposed Cohort | Ascertainment of Exposure | Demonstration That Outcome of Interest Was Not Present at Start of Study | Comparability of Cohorts on the Basis of the Design or Analysis | Assessment of Outcome | Was Follow-Up Long Enough for Outcomes to Occur | Adequacy of Follow Up of Cohorts | Total |
|---|---|---|---|---|---|---|---|---|---|
| Shao 2020 | ★ | ★ | ★★ | ★ | ★ | ★ | 6 | ||
| Esen 2021 | ★ | ★ | ★★ | ★ | ★ | ★ | 6 | ||
| Huang 2021 | ★ | ★ | ★★ | ★ | ★ | 5 |
3.3. Mortality
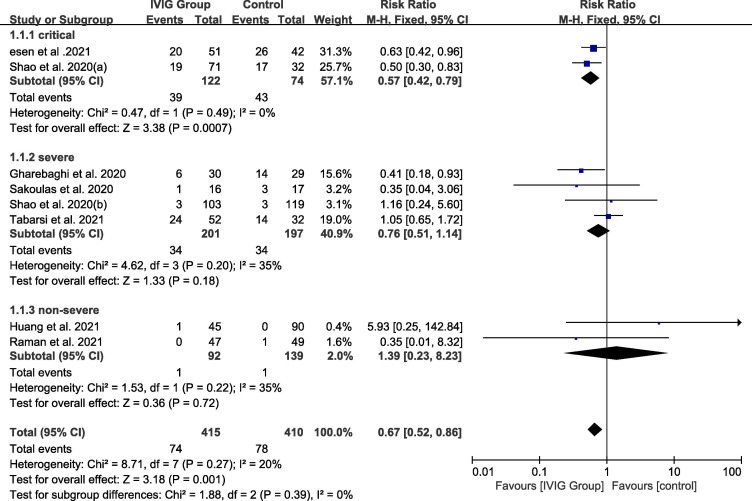
For critical subgroup, the pooled RR was 0.57 (95% CI: 0.42–0.79, I2 = 0%) which showed the mortality of critical ill patients treated with IVIG was lower compared with the control group. The RR for severe subgroup was 0.76 (95% CI: 0.51–1.14, I2 = 35%) and for non-severe subgroup was 1.39 (95%CI: 0.23–8.23., I2 = 20%) which both showed no significant difference. Fig. 3
Fig. 3.
Efficacy of IVIG on mortality in COVID-19 patients. Weights are from fixed-effects analysis. Note: CI = confidence interval, IVIG = intravenous immunoglobulin.
3.4. Duration of hospitalization
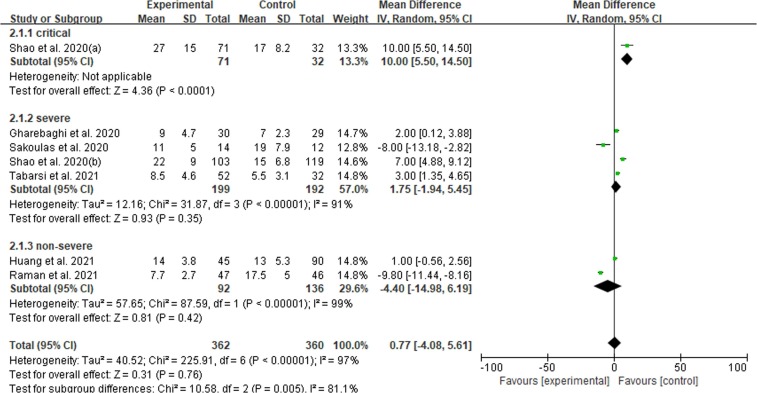
There were six articles reported on the length of hospitalization. For critical subgroup, MD = 10.00 (95%CI: 5.50 – 14.50) which indicated IVIG therapy could prolong the length of hospital stay but only one study available. For severe subgroup the result [MD = 1.75 (−1.94 – 5.45)] demonstrated that IVIG could increase the length of duration in hospitalization but no statistical significance difference. In non-severe subgroup, the pooled MD = −4.40 (−14.98 – 6.19, I2 = 99%). See Fig. 4 .
Fig. 4.
Efficacy of IVIG on duration of hospitalization in COVID-19 patients. Weights are from fixed-effects analysis. Note: CI = confidence interval, IVIG = intravenous immunoglobulin.
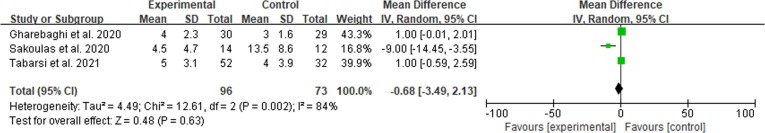
3.5. Duration of ICU
We only tested the impact of IVIG use on duration of ICU in severe patients because of the limited number of articles. The use of IVIG compared to the control group did not impact the length of hospitalizations. MD = −0.68 (−3.49 – 2.13, I2 = 84%). See Fig. 5 .
Fig. 5.
Efficacy of IVIG on duration of ICU in severe COVID-19 patients. Weights are from fixed-effects analysis. Note: CI = confidence interval, IVIG = intravenous immunoglobulin.
4. Discussion
This is the first meta-analysis included 4 RCTs and 3 RCSs investigating the effect of IVIG on clinical outcomes of different severity types patients diagnosed with COVID-19. We analyzed effectiveness of IVIG from these three aspects: mortality, length of duration in hospital or ICU. And our analysis suggested that IVIG may have an impact, with a low level of certainty, in reducing the rate of mortality and prolong the hospital stay in critical patients. We believed that there may be a strong relationship between the efficacy of IVIG and the COVID-19 disease severity.
There was a discrepancy between the random-effect results and fixed-effect results of IVIG versus control group when assessing the length of hospital day. Though the fixed-effect results provided good results that IVIG could increasing the hospital stay in critical and severe subgroup and reducing the days in non-severe subgroup, we still chosen the random-effect meta-analysis results due to the high heterogeneity. In random-effect meta-analysis, the critical subgroup analysis conducted that IVIG could prolong the time of hospitalization while the severe subgroup also showed a slightly trend on prolonging the days in hospital but no statistical significance. Theoretically, if IVIG pose an effective impact, the length of the hospital or ICU stay would be shorter than control group. One possible explanation is that the longer duration of hospitalization in IVIG group may be due to the higher survival rate in IVIG group than control group. So another term, there are more patients survived in IVIG group while similar condition patients in control group had been died earlier. In spite of it all, there are numerous influencing factors to affect the results such as time of the IVIG administration, the function of standard of care and so on. Hence the results should be interpreted with great caution.
The insufficient effects of IVIG therapy in the study may potentially be results of dosage, time of IVIG administration, treatment details (including IVIG use, types and doses of IVIG, and other treatments). In another study, Cao et al. [24] suggests that high-dose of IVIG (0.3–0.5 g per kg weight for five doses) combined corticosteroids could perform a fine outcome and be a valid and safe immunotherapy in COVID-19 patients. All the RCTs we included provided a high dosage which are consistent with Cao et al study’s dosage. In Xie et al study [25], the mortality rate of patients with COVID-19 is associated with received IVIG time which suggested that administered early on IVIG treatment. Application of IVIG within 48 h of ICU could significantly decrease mortality, the use of mechanical ventilation, shortened duration of ICU and length of hospital stay. Herth et al. [26] also confirmed this viewpoint which early administration of IVIG was correlated with shorter length of hospital duration, while late administration of IVIG was related to longer hospital duration.
Early research demonstrated that after contracting the virus, in the first week, the viral RNA of SARS-Cov2 will reached its highest point, and in the second week, most patients produced anti-viral antibodies against COVID-19, which indicates IVIG should be adopted as early as possible when the patients were diagnosed and hospitalized [27]. It shows that IVIG may be an effective therapeutic intervention when administered early. However, considering the high price and side effect, IVIG was used usually to severe or critical ill patients.
In previous studies, it has suggested that severe cytokine storm has been found to be related to increased death rates in critical COVID-19 patients [28]. IVIG showed multiple effects in enhancing the pro-inflammatory cells activation, and indirectly suppressing T-cell and B-cell proliferation by cytokines [29], [30], [31], [32]. Based on other case series [33], [34] that patients treated with IVIG, the results appear to be favorable and consistent which supported IVIG might be beneficial for covid-19 patients especially for severe or critical ill patients. Pei et al. [7] reported an opposite meta- analysis result that IVIG were not associated with reducing mortality in this population. The potential reason may be that neither the retrospective studies nor the available RCTs was included in their pooled results that lead to the quality of the analysis was low. Not only did they not adjust the confounders according the patients’ baseline characteristics, but the heterogeneity also was very high.
There are some limitations on our meta-analysis. First, the clinical outcomes of the IVIG therapy couldn’t quite represented the abilities to treat COVID-19 patients. Cellular and molecular assessment including the rate of cleaning virus and serum cytokines concentration could evaluate the effect of immunoglobulin more accurately and visually. Second, the analysis was included only four RCTs with small sample sizes. Only one trial was blinded which may subject to bias. Third, the benefits of IVIG are difficult to evaluate when it is coupled with corticosteroids or the recombinant modified IL-1 receptor antagonist, Tocilizumab. Lastly, the dose and timing of IVIG treatment in different center in cohort studies may not be consistent. We still need more RCTs or at least observational studies with a control group to confirm the results of the study.
5. Conclusion
In conclusion, based on low quality evidences, IVIG has demonstrated clinical efficacy on critical ill patients with COVID-19 on reducing the rate of mortality and increasing the length of hospitalization. There may be a strong relationship between the efficacy of IVIG and the COVID-19 disease severity. Well-designed clinical trials to identify the clinical and biochemical characteristics in COVID-19 patients’ population that could benefit from IVIG are warranted in the future.
6. Authors' contributions**a
Wenxin Peng, and Huairong Xiang designed this study; Xuan Cheng and Yun Li ran the searched strategy; Huairong Xiang and Wenwen Luo selected articles and extracted data; Qizhi Zhang and Xuan Cheng evaluate the quality of the literature. Huairong Xiang wrote the manuscript, Xuan Cheng, Yun Li, Wenwen Luo and Qizhi Zhang edited. All listed authors reviewed and approved the final manuscript.
Acknowledgments
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2021.107732.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Coronavirus disease (COVID-2019) situation reports. Geneva: World Health Organization; 2020. Available: https://www.who.int/publications/m/item/weekly-epidemiological-update---23-february-2021. [accessed 1 March 2021].
- 2.Perez E.E., Orange J.S., Bonilla F., et al. Update on the use of immunoglobulin in human disease: A review of evidence. J. Allergy Clin. Immunol. 2017;139(3s):S1–s46. doi: 10.1016/j.jaci.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 3.Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3(9):e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mustafa S., Balkhy H., Gabere M.N. Current treatment options and the role of peptides as potential therapeutic components for Middle East Respiratory Syndrome (MERS): A review. J. Infect. Public Health. 2018;11(1):9–17. doi: 10.1016/j.jiph.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luke T.C., Casadevall A., Watowich S.J., et al. Hark back: passive immunotherapy for influenza and other serious infections. Crit. Care Med. 2010;38(4 Suppl):e66–e73. doi: 10.1097/CCM.0b013e3181d44c1e. [DOI] [PubMed] [Google Scholar]
- 6.Tzilas V., Manali E., Papiris S., et al. Intravenous Immunoglobulin for the Treatment of COVID-19: A Promising Tool. Respiration. 2021;99(12):1087–1089. doi: 10.1159/000512727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pei L., Zhang S., Huang L., et al. Antiviral agents, glucocorticoids, antibiotics, and intravenous immunoglobulin in 1142 patients with coronavirus disease 2019: a systematic review and meta-analysis. Pol. Arch. Intern. Med. 2020;130(9):726–733. doi: 10.20452/pamw.15543. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J., Yang Y., Yang N., et al. Effectiveness of intravenous immunoglobulin for children with severe COVID-19: a rapid review. Ann. Transl. Med. 2020;8(10):625. doi: 10.21037/atm-20-3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daneshpazhooh M., Soori T., Isazade A., et al. Mucous membrane pemphigoid and COVID-19 treated with high-dose intravenous immunoglobulins: a case report. #N/A. 2020;31(5):446–447. doi: 10.1080/09546634.2020.1764472. [DOI] [PubMed] [Google Scholar]
- 10.Sheianov M.V., Udalov Y.D., Ochkin S.S., et al. Pulse Therapy With Corticosteroids and Intravenous Immunoglobulin in the Management of Severe Tocilizumab-Resistant COVID-19: A Report of Three Clinical Cases. Cureus. 2020;12(7):e9038. doi: 10.7759/cureus.9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D., Liberati A., Tetzlaff J., et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6(1000097) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins J.P., Altman D.G., Gotzsche P.C., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343 doi: 10.1136/bmj.d5928. d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 14.COVID-19 Clinical management: living guidance. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1.
- 15.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wan X., Wang W., Liu J., et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.R R.S., Barge V.B., Darivenula A.K., et al. A Phase II Safety and Efficacy Study on Prognosis of Moderate Pneumonia in COVID-19 patients with Regular Intravenous Immunoglobulin Therapy. J. Infect. Dis. 2021 doi: 10.1093/infdis/jiab098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.G. Sakoulas, M. Geriak, R. Kullar, et al. Intravenous Immunoglobulin (IVIG) Significantly Reduces Respiratory Morbidity in COVID-19 Pneumonia: A Prospective Randomized Trial. medRxiv. 2020:2020.2007.2020.20157891.
- 19.Gharebaghi N., Nejadrahim R., Mousavi S.J., et al. The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial. BMC Infect. Dis. 2020;20(1):786. doi: 10.1186/s12879-020-05507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tabarsi P., Barati S., Jamaati H., et al. Evaluating the effects of Intravenous Immunoglobulin (IVIg) on the management of severe COVID-19 cases: A randomized controlled trial. Int. Immunopharmacol. 2021;90(107205) doi: 10.1016/j.intimp.2020.107205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C., Fei L., Li W., et al. Efficacy Evaluation of Intravenous Immunoglobulin in Non-severe Patients with COVID-19: A Retrospective Cohort Study Based on Propensity Score Matching. Int. J. Infect. Dis.: IJID: off. Publ. Int. Soc. Infect. Dis. 2021 doi: 10.1016/j.ijid.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esen F., Ozcan P.E., Orhun G., et al. Effects of adjunct treatment with intravenous immunoglobulins on the course of severe COVID-19: results from a retrospective cohort study. Curr. Med. Res. Opin. 2021:1–14. doi: 10.1080/03007995.2020.1856058. [DOI] [PubMed] [Google Scholar]
- 23.Shao Z., Feng Y., Zhong L., et al. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID-19: a multicenter retrospective cohort study. Clin. Transl. Immunol. 2020;9(10) doi: 10.1002/cti2.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.W. Cao, X. Liu, T. Bai, et al. High-Dose Intravenous Immunoglobulin as a Therapeutic Option for Deteriorating Patients With Coronavirus Disease 2019, Open Forum. Infect. Dis., 7 (3) (2020) ofaa102. [DOI] [PMC free article] [PubMed]
- 25.Xie Y., Cao S., Dong H., et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J. Infect. 2020;81(2):318–356. doi: 10.1016/j.jinf.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herth F.J.F., Sakoulas G., Haddad F. Use of Intravenous Immunoglobulin (Prevagen or Octagam) for the Treatment of COVID-19: Retrospective Case Series. Respiration. 2020;99(12):1145–1153. doi: 10.1159/000511376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.A. Saghazadeh, N. Rezaei, Towards treatment planning of COVID-19: Rationale and hypothesis for the use of multiple immunosuppressive agents: Anti-antibodies, immunoglobulins, and corticosteroids, Int. Immunopharmacol., 84 (2020) 106560. [DOI] [PMC free article] [PubMed]
- 28.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hagiwara J., Yamada M., Motoda N., et al. Intravenous Immunoglobulin Attenuates Cecum Ligation and Puncture-Induced Acute Lung Injury by Inhibiting Apoptosis of Alveolar Epithelial Cells. J. Nippon Med. Sch. 2020;87(3):129–137. doi: 10.1272/jnms.JNMS.2020_87-303. [DOI] [PubMed] [Google Scholar]
- 30.Sun X., Wang T., Cai D., et al. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev. 2020;53:38–42. doi: 10.1016/j.cytogfr.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.M.S. Maddur, J. Vani, P. Hegde, et al. Inhibition of differentiation, amplification, and function of human TH17 cells by intravenous immunoglobulin, J. Allergy Clin. Immunol., 127 (3) (2011) 823–830.e821-827. [DOI] [PubMed]
- 32.Maddur M.S., Kaveri S.V., Bayry J. Circulating Normal IgG as Stimulator of Regulatory T Cells: Lessons from Intravenous Immunoglobulin. Trends Immunol. 2017;38(11):789–792. doi: 10.1016/j.it.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki Y., Tanino Y., Nikaido T., et al. Severe Coronavirus Disease 2019 That Recovered from Respiratory Failure by Treatment That Included High-dose Intravenous Immunoglobulin. Int. Med. (Tokyo Japan) 2021;60(3):457–461. doi: 10.2169/internalmedicine.6326-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carannante N., Fiorentino G., Corcione A., et al. Administration of Immunoglobulins in SARS-CoV-2-Positive Patient Is Associated With Fast Clinical and Radiological Healing: Case Report. Front. Med. (Lausanne) 2020;(7):388. doi: 10.3389/fmed.2020.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.