Abstract
Vaccines to prevent acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection elicit an immune neutralizing response. Some concerns have been raised regarding the safety of SARS-CoV-2 vaccines, largely based on case-reports of serious thromboembolic events after vaccination. Some mechanisms have been suggested which might explain the adverse cardiovascular reactions to SARS-CoV-2 vaccines. Different vaccine platforms are currently available which include live attenuated vaccines, inactivated vaccines, recombinant protein vaccines, vector vaccines, DNA vaccines and RNA vaccines. Vaccines increase the endogenous synthesis of SARS-CoV-2 Spike proteins from a variety of cells. Once synthetized, the Spike proteins assembled in the cytoplasma migrate to the cell surface and protrude with a native-like conformation. These proteins are recognized by the immune system which rapidly develops an immune response. Such response appears to be quite vigorous in the presence of DNA vaccines which encode viral vectors, as well as in subjects who are immunized because of previous exposure to SARS-CoV-2. The resulting pathological features may resemble those of active coronavirus disease. The free-floating Spike proteins synthetized by cells targeted by vaccine and destroyed by the immune response circulate in the blood and systematically interact with angiotensin converting enzyme 2 (ACE2) receptors expressed by a variety of cells including platelets, thereby promoting ACE2 internalization and degradation. These reactions may ultimately lead to platelet aggregation, thrombosis and inflammation mediated by several mechanisms including platelet ACE2 receptors. Whereas Phase III vaccine trials generally excluded participants with previous immunization, vaccination of huge populations in the real life will inevitably include individuals with preexisting immunity. This might lead to excessively enhanced inflammatory and thrombotic reactions in occasional subjects. Further research is urgently needed in this area.
Keywords: SARS-CoV-2, COVID-19, ACE2, Vaccines, Renin-angiotensin-aldosterone system, Thrombosis, Adverse event
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) rapidly spread across the world and killed more than 2.9 million individuals globally, with 137 million cases being confirmed by laboratory tests (April 16 2021, https://covid19.who.int/). Thus, there is an ongoing search for therapeutics finalized to block the transition from infection to severe forms of coronavirus disease 2019 (COVID-19).
Different therapeutic strategies are under scrutiny which include blockade of SARS-CoV-2 from binding to human cell receptors, prevention of the viral ribonucleic acid (RNA) synthesis and replication, the restoration of the host's innate immunity, and the modulation of the host's specific receptors or enzymes [1]. Nevertheless, vaccines to prevent SARS-CoV-2 infection are considered the most promising approach for curbing the pandemic.
To date, a total of seven SARS-CoV-2 vaccines are available across three different platforms [2], [3], [4] and as of 16 April 2021, a total of 734.121.870 vaccine doses have been administered (https://covid19.who.int/).
However, some concerns regarding the safety of SARS-CoV-2 vaccines have been recently raised, mostly based on scattered reports of thromboembolic events [5], [6], [7], [8].
To this regard, we aimed to summarize main mechanisms of SARS-CoV-2 vaccines and their potential interactions with the cardiovascular system.
2. Brief overview of approved vaccines in Europe
Vaccines for SARS-CoV-2 are being developed using several different platforms (Table 1 ). They include live attenuated vaccines, inactivated vaccines, recombinant protein vaccines, vector vaccines (replication-incompetent vector vaccines, replication-competent vector vaccines, and inactivated virus vector vaccines), deoxyribonucleic acid (DNA) vaccines, and RNA vaccines (Table 1).
Table 1.
Different platforms used to develop vaccines for SARS-CoV-2. For each platform, advantages and limitations are also reported.
| Platform | Development | Advantages | Limitations |
|---|---|---|---|
| Inactivated vaccine | Chemically inactivated virus | Stable; immune response targeting the Spike protein and other components of the virus | Integrity of the immunogenic particles must be maintained |
| Live attenuated vaccine | Genetically weakened versions of the wild-type virus | Stimulate humoral and cellular immunity to multiple components of the whole attenuated virus | Reversion to or recombination with the wild-type virus (nucleotide substitution during viral replication) |
| Recombinant protein vaccines | Composed of viral proteins that have been expressed in one of various systems | Safe; no live components of the virus | Memory is to be tested |
| Viral vector vaccine | Replication-incompetent or replication-competent viral vector expressing the target viral protein | Robust immune response | Potential integration of the viral genome into the host genome |
| DNA vaccine | Plasmid DNA that contain mammalian expression promotors and the target gene | High stable | Low immunogenicity |
| RNA vaccine | mRNA encoding for target viral proteins | No interactions with the recipient's DNA | To be maintained at very low temperatures |
Legend: DNA= deoxyribonucleic acid; RNA= ribonucleic acid; mRNA=messenger ribonucleic acid.
Main features of SARS-CoV-2 vaccines, including those approved by the European Medical Agency (EMA), are reported in Table 2 .
Table 2.
Main features of COVID-19 vaccines. From ref. [2, [9], [10], [11], 18, 19] and www.clinicaltrials.gov.
| Vaccine | Developer | Platform | Doses | Efficacy** |
|---|---|---|---|---|
| BNT162b2* | Pfizer/BioNTech | mRNA | 2 (3 weeks apart) | 95% |
| mRNA-1273* | Moderna | mRNA | 2 (4 weeks apart) | 94% |
| Ad26.COV2.S* | Janssen/Johnson &Johnson | DNA Adenovirus vector | 1 | 67% |
| CVnCoV | CureVAC | mRNA | 2 (4 weeks apart) | NA |
| ChAdOx1nCoV-19* | AstraZeneca/University of Oxford/Serum Institute of India | DNA Adenovirus vector | 2 (4/8 to 12 weeks apart) | 70% |
| NVX-CoV2373 | Novavax | Recombinant protein | 2 (3 weeks apart) | 89% |
| Gam-COVID-Vac (Sputnik V) | Gamaleya Institute | DNA Adenovirus vectors | 2 (3 weeks apart) | 92% |
Legend: *= vaccines authorised for use in the European Union (https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/covid-19-vaccines); **= efficacy against symptomatic/moderate/severe COVID-19 (see references for details); mRNA=messenger ribonucleic acid.
As detailed below, two main types of coronavirus disease (COVID-19) vaccines are approved for emergency use (messenger ribonucleic acid [mRNA] technology with lipid nanoparticle delivery systems and DNA technology with non-replicating recombinant adenovirus vector systems).
2.1. ChAdOx1nCoV-19
COVID-19 Vaxzeria (previously COVID-19 vaccine AstraZeneca) is a monovalent vaccine composed of a single recombinant, replication-deficient chimpanzee adenovirus (ChAdOx1) DNA vector encoding the S glycoprotein of SARS-CoV-2 (ChAdOx1-S, AZD1222) [2, 3]. The SARS-CoV-2 S immunogen in the vaccine is expressed in the trimeric pre-fusion conformation; the coding sequence has not been modified in order to stabilize the expressed S-protein in the pre-fusion conformation [2, 3].
The Vaxzevria vaccination course consists of two separate doses of 0.5 ml each. The second dose should be administered between 4 and 12 weeks after the first dose. Individuals who have received the first dose of COVID-19 Vaxzevria vaccine should receive the second dose of the same vaccine to complete the vaccination course. Each dose (as suspension for injection) of this vaccine contains not less than 2.5 × 108 infectious units of ChAdOx1-S. It is for intramuscular injection only, preferably in the deltoid muscle of the upper arm.
According to EMA indications, protection from COVID-19 starts from approximately 3 weeks after the first dose of Vaxzevria vaccine and individuals may not be fully protected until 15 days after the second dose is administered.
The clinical efficacy of Vaxzevria vaccine has been evaluated by an interim analysis of data from four ongoing blinded, randomised, controlled trials done across the UK, Brazil, and South Africa [9]. Participants aged 18 years and older were randomly assigned in a 1:1 ratio to ChAdOx1 nCoV-19 vaccine or control (meningococcal group A, C, W, and Y conjugate vaccine or saline).
The studies excluded participants with severe and/or uncontrolled cardiovascular, gastrointestinal, liver, renal, endocrine/metabolic disease, and neurological illnesses, as well as those with severe immunosuppression, pregnant women and participants with a known history of SARS-CoV-2 infection [9].
The primary efficacy analysis included symptomatic COVID-19 in previously seronegative participants with a nucleic acid amplification test-positive swab more than 14 days after a second dose of vaccine.
The overall efficacy was 70.4% (95% confidence interval [CI]: 54.8–80.6) in preventing symptomatic COVID-19 (131 confirmed COVID-19 cases among over 11,000 participants; 30 in the vaccine group and 101 in the control group) [9].
Starting from the 21th day after the first dose, 10 patients were hospitalized for COVID-19, all in the control arm. COVID-19 was severe in two of these patients and one patient died [9].
Similar results were obtained in a randomized, double-blind, placebo-controlled multicentre phase III trial assessing the safety, efficacy, and immunogenicity of AZD1222 compared to placebo for the prevention of COVID-19. Data from 32,449 participants across 88 trial centres in the US, Peru and Chile showed a vaccine efficacy against symptomatic COVID-19 equal to 76%; vaccine efficacy rose to 100% and 85% against severe or critical disease and hospitalization, and against symptomatic COVID-19 in participants aged 65 years and over, respectively [10].
2.2. BNT162b2
One dose of the Pfizer/BioNTech vaccine (0.3 mL) contains 30 micrograms of COVID-19 single-stranded, 5′-capped messenger RNA (mRNA) produced using a cell-free in vitro transcription from the corresponding DNA templates, encoding the viral Spike protein of SARS-CoV-2 [2]. Specifically, the mRNA is embedded in lipid nanoparticles. It codes for membrane-anchored, full-length Spike protein with two point mutations within the central helix. Mutation of these two amino acids to proline locks Spike protein in an antigenically preferred prefusion conformation [2]. This vaccine should be administered intramuscularly after dilution and the preferred site is the deltoid muscle of the upper arm [2].
In an ongoing multinational, placebo-controlled, observer-blinded, pivotal efficacy trial, subjects 16 years of age or older are randomized in a 1:1 ratio to receive two doses, 21 days apart, of either placebo or the BNT162b2 vaccine candidate [11].
Briefly, a total of 43,548 participants underwent randomization, of whom 43,448 received injections (21,720 with BNT162b2 and 21,728 with placebo). Overall, there were 8 cases of COVID-19 with onset at least 7 days after the second dose among participants assigned to receive BNT162b2 and 162 cases among those assigned to placebo (95% of efficacy, 95% credible interval: 90.3 to 97.6) [11]. Similar vaccine efficacy was also observed across subgroups defined by age, sex, race, ethnicity, baseline body-mass index, and comorbidities. The incidence of serious adverse events was low and was similar in the vaccine and placebo groups [11].
Of note, some observational investigations from various countries showed similar results [12], [13], [14], [15], [16].
2.3. mRNA-1273
The vaccine mRNA-1273 manufactured by Moderna is a lipid nanoparticle-encapsulated, nucleoside-modified mRNA based vaccine that encodes the SARS-CoV-2 S-2P antigen, consisting of the SARS-CoV-2 glycoprotein with a transmembrane anchor and an intact S1–S2 cleavage site [17]. The lipid nanoparticle capsule composed of four lipids was formulated in a fixed ratio of mRNA and lipid. The mRNA-1273 vaccine was provided as a sterile liquid for injection at a concentration of 0.5 mg per milliliter. Normal saline was used as a diluent to prepare the doses administered [17].
A phase 3 randomized, observer-blinded, placebo-controlled trial was conducted at 99 centers across the United States [18]. Overall, 30,420 subjects were randomly assigned in a 1:1 ratio to receive two intramuscular injections of mRNA-1273 (100 μg) or placebo 28 days apart. The primary end point was prevention of COVID-19 illness with onset at least 14 days after the second injection in participants who had not previously been infected with SARS-CoV-2 [18].
Symptomatic COVID-19 illness was confirmed in 185 participants in the placebo group and in 11 participants in the mRNA-1273 group (vaccine efficacy: 94.1%, 95% CI: 89.3 - 96.8%) [18].
Severe Covid-19 occurred in 30 participants, with one fatality; all the 30 cases were in the placebo group. Moderate, transient reactogenicity after vaccination occurred more frequently in the mRNA-1273 group. Serious adverse events were rare, and the incidence was similar in the two groups [18].
2.4. Ad26.COV2.S
The Janssen COVID-19 vaccine is a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector (DNA) encoding a full-length and stabilized SARS-CoV-2 Spike (S) protein. The vaccine was derived from the first clinical isolate of the Wuhan strain (Wuhan 2019; whole genome sequence, NC_045512) [19].
A Phase 1–2a trial [19] included healthy adults (N=805) aged between 18 and 55 years (cohort 1) and ≥65 years (cohort 3) who were randomly assigned to receive the Ad26.COV2.S vaccine at a dose of 5 × 1010 viral particles (low dose) or 1 × 1011 viral particles (high dose) per milliliter or placebo in a single-dose or two-dose schedule [19]. Neutralizing-antibody titers against wild-type virus were detected in 90% or more of all participants on day 29 after the first vaccine dose. A second dose provided an increase in the titer by a factor of 2.6 to 2.9 [19].
As reported by the Food and Drug Administration (FDA) [20], in a phase III efficacy trial recruiting 40,000 study participants aged 18 years and older, this vaccine was given as a single dose. It showed 66.9% efficacy (95% CI: 59.0–73.4) in preventing moderate, severe or critical COVID-19 (464 cases of which 116 in the vaccine group and 348 in the placebo group). Vaccine efficacy started at 28 days after vaccination and it was similar to that after 14 days [20].
3. Differences between platforms in perspectives
Vaccines currently approved or under scrutiny for human use have been developed using different advanced technologies (Table 1) [3, 4]. As aforementioned, vaccines currently approved by EMA use two different platforms (namely, non-replicating DNA viral vectors, and mRNA).
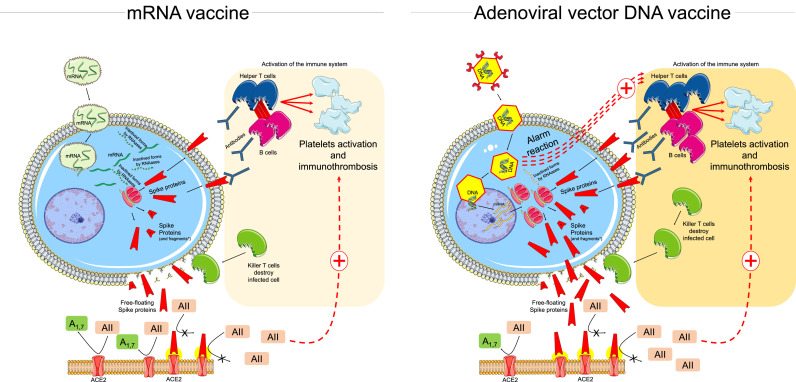
The main differences between these platforms are depicted in Fig. 1 .
Fig. 1.
Schematic mechanism of action of mRNA and adenoviral vector DNA vaccines and their potential cardiovascular interactions throughout the activation of the immune system and the interaction between free-floating Spike proteins and ACE2 (see text for details).
Legend: AII=angiotensin 2; A1,7=angiotensin1,7; ACE2=angiotensin converting ezyme 2 receptor; DNA= deoxyribonucleic acid; RNA= ribonucleic acid; mRNA=messenger ribonucleic acid.
The non-replicating viral vectors (a chimpanzee adenovirus for ChAdOx1nCoV-19) are carriers of a double-stranded gene coding for the viral Spike protein (Fig. 1, right panel). DNA is not as fragile as RNA, and the adenovirus's tough protein coat helps protecting the genetic material inside. Once inside the infected cell, the viral vector enters the nucleus where it produces the antigen through multiple mRNA molecules, without making copies of itself. The mRNA leaves the nucleus of the cell and begins assembling Spike proteins. Some of the Spike proteins which are produced in the cytoplasma along with a variable amount of Spike proteins broken into fragments migrate to the surface of the cell [3, 4].
The protruding Spike proteins are recognized by the immune system that triggers an immune response. Adenovirus vector elicits a specific immune response by the host. Nonetheless, the use of animal adenovirus may decrease the preexisting vector immunity observed using a human viral vector [4]. The early innate response to Adenovirus vector generally starts 1–3 hours to 1 day post-vaccination through cytokines/chemokines and immune cells, with final enhancement of the immune reactions against Spike proteins [21].
Furthermore, the viral vector vaccines also contain inherent adjuvant properties. Following injection, adenoviruses target innate immune cells (i.e. dendritic cells) and macrophages and stimulate innate immune responses by engaging multiple pattern-recognition receptors to induce type I interferon secretion with consequent delivery of both an antigenic and inflammatory signal to T cells in lymphonodes draining the injection site (activating T cells and mobilizing adaptive immunity) [22].
In case of viral vectors with DNA, an integration of the viral genome into the host genome has not been excluded [3].
RNA vaccines (conventional mRNA or self-amplifying mRNA) led to the expression of encoded proteins [3, 4]. They work on the strategy of using the host cell transcription machinery to produce the target proteins by making multiple copies of the protein from each mRNA template and induce adaptive immunity, eliciting B and T cell immune response (Fig. 1, left panel). The mRNA can serve as an immunogen, encoding the viral protein, and adjuvant, owing to intrinsic immunostimulatory properties of RNA. More specifically, upon entry into cells, mRNA is recognized by endosomal and cytosolic innate sensors that form a critical part of the innate immune response to viruses, resulting in cellular activation, and production of type I interferon and multiple inflammatory mediators [23, 24]. Of note, lipid nanoparticle carrier further protects the mRNA, can target delivery to lymphatics and promote protein translation in lymphonodes. Once in the lymphonodes, the lipid nanoparticle carrier is engulfed by dendritic cells, which subsequently produce and present the antigen to T cells for activation of the adaptive immune response [23, 24].
Nonetheless, the modification of the mRNA is needed to increases its stability [3], and its encapsulation into lipid nanoparticles improves the cellular delivery; the cytoplasmic localization of the translation and non-integration into the host genome are other advantages of the mRNA vaccine [3, 4]. Another obvious implication is that, compared to viral vectors which must enter the nucleus of a cell, the mRNA is only present in the cytoplasm and it is exposed to active RNA degradation systems (‘RNAase enzymes’) which are the first defense against RNA viruses by reducing the overall degree of translation procedures.
Like viral-vector vaccines, Spike proteins and their fragments produced by the infected-cells accommodate on their surface, being rapidly recognized by the host immune system with subsequent production of antibodies. Both vaccine types generate significant neutralizing antibody and virus-specific T cell responses [25, 26]. Specifically, the ability of mRNA and viral vector vaccines to promote intracellular production of Spike protein along with innate immune responses should prime both CD8+ and CD4+ T cells to differentiate into effector and memory subsets by the production of type I interferon [24, 27].
Furthermore, the second dose of these vaccines is associated with an enhancement of the inflammatory response deriving from short-term changes to innate cells like macrophages through a phenomenon called ‘trained immunity’, and/or from activation of memory T cells and B cells generated from the initial injection [24, 27]. It is not entirely clear how these vaccines mobilize the immune response, as well as the durability of protection [24].
4. Lessons from MERS and SARS-CoV-1
In the last few years, vaccines using DNA or mRNA as platform technology have generated a considerable interest for their potential role as health solutions. These types of vaccine induce humoral (neutralizing antibodies) and cellular immune responses and may avoid potential health risk of working with inactivated or attenuated pathogens. The processes of their development are generally more rapid than “conventional” techniques [28].
Data regarding the differences between platforms (see previous section) and their interaction with the host are mainly available from pre-clinical studies completed with SARS-CoV-1 and the Middle East Respiratory Syndrome coronavirus (MERS-CoV). Similar to SARS-CoV-2, the antigenic target for both SARS-CoV-1 and MERS vaccines was the large surface Spike protein. Several MERS and SARS-CoV-1 vaccine candidates have been developed and/or tested for efficacy, including viral vector-based vaccines constructed using viral vectors that express Spike protein [29], [30], [31]. Generally, these vaccine candidates have demonstrated their ability to induce immune responses and/or neutralizing antibodies [29], [30], [31]. Antibodies binding to the receptor binding domain (RBD) of the Spike protein showed the potential to prevent its interaction with the angiotensin-converting enzyme 2 (ACE2) and neutralize the viruses [29], [30], [31].
Nevertheless, animal studies of vaccines for SARS-CoV-1 and MERS-CoV also demonstrated that vaccination induces non-neutralizing antibodies that may mediate enhancement of virus infection (after challenge with wild-type virus) or cause harmful immune responses, such as inflammation, enhanced hepatitis, and eosinophilic lung inflammation [32], [33], [34], [35].
These experimental studies for SARS-CoV-1 and MERS-CoV had raised some concerns regarding an enhancement of the disease and the induction of virus-enhancing antibody and harmful immune responses with vaccination [36].
5. Vaccination and immunothrombosis
Some questions regarding the safety of COVID-19 vaccines have been recently raised and based on sparse reports of thromboembolic events, fatal in some cases, following Vaxzevria and Johnson & Johnson vaccine receipt [5], [6], [7], [8].
Taking into consideration all currently available evidence, including the advice from an ad hoc expert group, EMA has recently concluded that unusual thrombotic events should be listed as a side effect of Vaxzevria (formerly COVID-19 Vaccine AstraZeneca).
Specifically, the Pharmacovigilance Risk Assessment Committee (PRAC) of EMA carried out an in-depth review of 62 cases (18 fatal) of cerebral venous sinus thrombosis (CVST) and 24 cases of splanchnic vein thrombosis reported in the European Union drug safety database (EudraVigilance) as of March 22, 2021 [17]. However, the EMA concluded that “the reported combination of blood clots and low blood platelets is very rare, and the overall benefits of the vaccine in preventing COVID-19 outweigh the risks of side effects”.
In this context, a recent report of the Danish National Patient Registry evaluated the incidence of first-time cases of venous thromboembolism in the general adult population recorded from January 1, 2010, and November 30, 2018 [37]. All incidence rates were calculated by dividing the number of incident venous thromboembolisms during follow-up by the sum of person-years during follow-up and reported per 1000 person-years [37]. Subsequently, using these incidence rates for venous thromboembolism, the Authors estimated the number of cases that would be expected over the course of 1 week and 1 month, respectively, in a population with the same size as that having received the Oxford–AstraZeneca COVID-19 vaccine in Europe by March 10, 2021 [37]. This was done by rescaling the incidence rates to the weekly (7 days) and monthly level (30.5 days) per individual, and multiplying them by 5 million (i.e., size matching the approximate number of people having received the Oxford–AstraZeneca COVID-19 vaccine in Europe by March 10, 2021).
Incidence rates were calculated for the overall population and for the subgroup of Danes aged 18–64 years. This 18–64-year age group represents the age group in which the Oxford–AstraZeneca COVID-19 vaccine has predominantly been used in most European countries [37]. The incidence rate per 1000 person-years was 1.76 (95% CI: 1.75 – 1.78) for venous thromboembolism among Danes aged 18–99 years, and 0.95 (95% CI: 0.94 – 0.96) among Danes aged 18–64 years [37]. When restricting to deep vein thrombosis or pulmonary embolism, the incidence rate per 1000 person-years was 1.70 (95% CI: 1.68 – 1.71) among Danes aged 18–99 years and 0.91 (0.89 – 0.92) for those aged 18–64 years [37].
As detailed by the Authors [37], in a population of 5 million people this incidence would correspond to approximately 169 expected cases of venous thromboembolism per week, or 736 expected cases per month if based on the incidence rate among the 18–99-year-old Danes. Similarly, if estimated on the incidence rates among 18–64-year-old Danes, one would expect 91 cases of venous thromboembolism per week, or 398 cases per month.
In other words, these results remark the concept that the reported number of thromboembolic events among Europeans who have received the Oxford–AstraZeneca COVID-19 vaccine is not increased relative to the expected number estimated from incidence rates from the entire Danish population before the introduction of the vaccination program [37].
However, as stated by the Authors, the Danish data cannot rule out the possibility that some venous thromboembolic events reported in relation to the use of the vaccine are really caused by the vaccine [37].
Similarly, a joint Chronic Disease Center (CDC) and FDA Statement recommended a pause in the use of this vaccine out of an abundance of caution for the reported of 6 cases of rare and severe type of blood clot in individuals after receiving the Johnson & Johnson vaccine [6].
A plausible explanation for the combination of thrombotic events and low blood platelets count (<150,000/microL), observed as serious adverse event, is the immune response (‘immunothrombosis’) [38] following vaccination, which resembles a condition similar to the heparin-induced thrombocytopenia (HIT) [39, 40]. These events could be related to vaccine-induced autoantibodies against a PF4 platelet antigen (vaccine-induced prothrombotic immune thrombocytopenia), a reaction occurring 4 to 20 days after vaccination. A positive HIT antibody suggests such diagnosis [39, 40]. Most of the cases from Europe have occurred in women under age 55.
Although these events were very rare, their incidence and the similar pattern across different individuals raised some concerns for a causality relation with vaccine. Besides, the pathogenesis of hypercoagulability after vaccination remains poorly understood. An enhanced immunological reaction mimicking active COVID-19 may be postulated. Indeed, immunothrombosis is a peculiar pathogenic mechanism in COVID-19. As suggested by Bonaventura and co-workers, SARS-CoV-2 infection induces the activation of neutrophils and monocytes which may interact with platelets and the coagulation cascade potentially leading to intravascular clot formation in small and larger vessels [38].
6. Free-floating Spike proteins and ACE2 interactions
When a vaccinated cell dies or is destroyed by the immune system, the debris may release a large amount of Spike proteins and protein fragments (free-floating Spike proteins).
It is well known that SARS-CoV-2 uses ACE2 as a Trojan horse to invade target cells. Thus, interactions between free-floating Spike proteins and ACE2 of other cells are highly plausible mechanisms. As recently demonstrated for adenovirus-vectored vaccines, Spike proteins produced upon vaccination have the native-like mimicry of SARS-CoV-2 Spike protein's receptor binding functionality and prefusion structure [41].
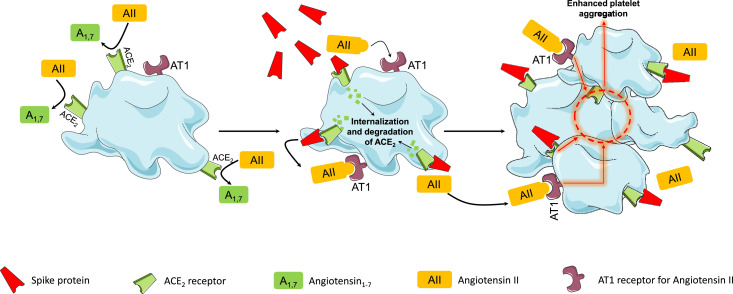
The native-like conformation of the Spike protein produced by vaccines has the potential to interact with ACE2, promote ACE2 internalization, and its degradation [42]. Of note, such phenomenon has been also observed in platelets [43]. Zhang and co-workers found that SARS-CoV-2 induced a time-dependent decrease in ACE2 levels in platelets, indicating the degradation of ACE2 upon ACE2 activation [43]. Spike protein induces a dose-dependent enhancement of platelet aggregation and adenosine triphosphate (ATP) release [43]. The subuni 1 of the Spike protein, but not subunit 2, is that binds to ACE2 of platelets thereby triggering platelet aggregation (Fig. 2 ) [43].
Fig. 2.
Effects on platelets of the interaction between ACE2 and free-floating Spike proteins (see text for details).
Legend: AII=angiotensin 2; A1,7=angiotensin1,7; ACE2=angiotensin converting enzyme 2 receptor.
The loss of ACE2 receptor activity from the external site of the cellular membrane, as mediated by the interaction between ACE2 and SARS-CoV-2 Spike proteins, leads to less angiotensin II inactivation and less generation of antiotensin1–7 [44, 45]. The imbalance between angiotensin II overactivity and of antiotensin1–7 deficiency may trigger inflammation, thrombosis, and other adverse reactions (Fig. 1) [44, 45]. In this context, it is not clear whether the interaction between free-floating Spike proteins and ACE2 may favor such imbalance and influence the potential adverse events following vaccination (Fig. 1).
Conclusions
SARS-CoV-2 vaccination is now offering the opportunity to come out of the current phase of the pandemic. Vaccines that elicit a sufficient neutralizing response should be able to offer protection against COVID-19 [46]. However, in addition to efficacy, safety is an important issue for any SARS-CoV-2 vaccine. Despite different platforms are currently available, most vaccines share the following common features: (a) they encode SARS-CoV-2 Spike proteins; (b) Spike proteins assembled by the infected cell migrate to the cell surface; (c) the protruding Spike proteins have a native-like conformation [41] and are recognized by the immune system to develop the immune response.
In this context, viral vector vaccines might trigger a further enhancement of the immune reactions against Spike proteins by engaging a stronger innate responses also mediated by cytokines, chemokines and immune cells [21]. Such immune reaction appears to mimic an active COVID-19 disease.
Previous studies dating back to MERS-CoV and SARS-CoV-1 infection showed that vaccines based on the full-length Spike protein of SARS-CoV may induce a strong immune inflammatory responses at various levels including the lung and the liver [29, [32], [33], [34]].
The picture is complicated by the evidence of an enhanced immune response to a single dose of SARS-CoV-2 mRNA vaccine in seropositive persons because of a previous exposure to SARS-CoV-2 [47]. In this setting, Kramer and co-workers recently evaluated the antibody responses in subjects with or without preexistent SARS-CoV-2 immunity (67 seronegative and 43 seropositive participants) who received their first Spike mRNA vaccine dose in 2020 (Pfizer vaccine or Moderna vaccine) [47]. Repeated sampling after the first dose indicated that the majority of seronegative participants had variable and relatively low SARS-CoV-2 immunoglobulin G (IgG) responses within 9 to 12 days after vaccination. In contrast, participants with SARS-CoV-2 antibodies at baseline before the first vaccine injection rapidly developed antibody titers 10 to 45 times as high as those of vaccinees without preexisting immunity [47]. Moreover, the antibody titers of the vaccinees without preexisting immunity increased by a factor of 3 after the second vaccine dose, and no increase in antibody titers was observed in the COVID-19 survivors who received the second vaccine dose [47]. Notably, these Authors extended their analysis by computing the frequency of systemic reactions including fatigue, headache, chills, muscle pain, fever, and joint pain after the first dose of vaccine in 148 seronegative and 82 seropositive participants. The vaccine recipients with preexisting immunity had a higher frequency and severity of systemic reactions than those without immunity [47].
These results open a debate on the applicability of clinical trials results to the real life [48]. Clinical trials which tested the efficacy and safety of SARS-CoV-2 vaccines [9, 11, 18, 19] generally included subjects who were negative, at entry, to SARS-CoV-2 infection. Indeed, a new positivity to SARS-CoV-2 infection was an end-point of these trials. When applying the results of clinical trials to the real life, we cannot exclude the possibility that the vaccination of a growing number of subjects from different Countries with preexisting immunity to SARS-Cov-2 may trigger unexpectedly intense, albeit very rare, inflammatory and thrombotic reactions in previously immunized and predisposed individuals.
The basic mechanisms involved in the above reactions require further research. For example, free-floating Spike proteins released by the destroyed cells previously targeted by vaccines may interact with ACE2 of other cells, thereby promoting ACE2 internalization and degradation [42]. This mechanism enhances platelet aggregation [43]. The interaction between ACE2 and free-floating Spike proteins also enhances the imbalance between angiotensin II overactivity and of antiotensin1–7 deficiency through the loss of ACE2 receptor activity, which may contribute to trigger inflammation, thrombosis, and other adverse reactions (Fig. 1).
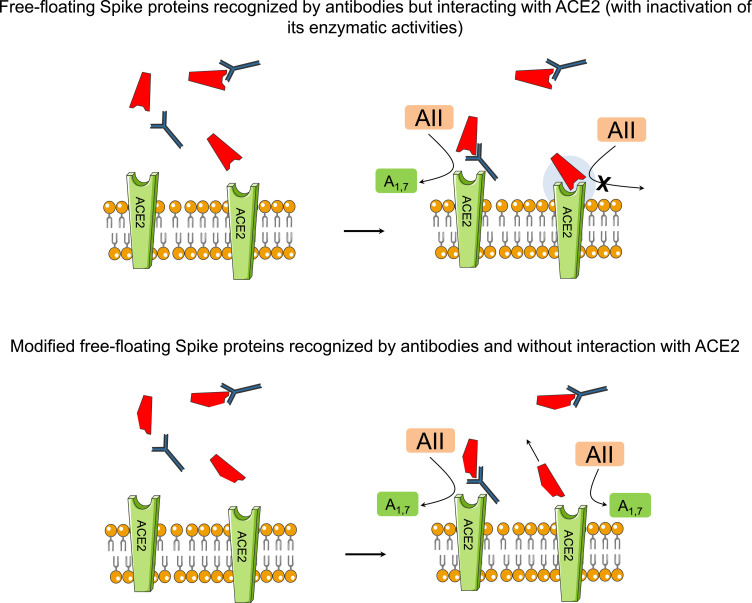
In this context, protein engineering approaches to identify binders to viral entry proteins may offer an alternative therapeutic strategy to ameliorate the potential detrimental effects of the interaction between ACE2 and Spike proteins (Fig. 3 ).
Fig. 3.
Use of vaccines with DNA templates or mRNA encoding mutated Spike proteins with conformational change as alternative therapeutic strategy to ameliorate the potential detrimental effects of the interactions between ACE2 and Spike proteins (see text for details).
Legend: AII=angiotensin 2; A1,7=angiotensin1,7; ACE2=angiotensin converting ezyme 2 receptor.
It has been suggested that the use of vaccines with DNA templates or mRNA encoding mutated Spike proteins with conformational change (from pre-fusion conformation to post-fusion conformation after interactions with ACE2, or change in RBD) might partly lose adherence to ACE2 receptors [49, 50].
In conclusion, the ideal SARS-CoV-2 vaccine candidate should possess a high immunogenicity, a well-established ability to induce effective immune responses through neutralizing antibodies, an almost complete protection against severe forms of COVID-19 and a good safety without important harmful responses. Given the high amount of active research in this area, it is conceivable that newer vaccines with these features with be developed in a near future.
Declaration of Competing Interest
None of the authors of this study has financial or other reasons that could lead to a conflict of interest.
Footnotes
None of the authors of this study has financial or other reasons that could lead to a conflict of interest.
References
- 1.Angeli F., Reboldi G., Verdecchia P. SARS-CoV-2 infection and ACE2 inhibition. J Hypertens. 2021 doi: 10.1097/HJH.0000000000002859. in press. [DOI] [PubMed] [Google Scholar]
- 2.Connors M., Graham B.S., Lane H.C., Fauci A.S. SARS-CoV-2 vaccines: much accomplished, much to learn. Ann Intern Med. 2021 doi: 10.7326/M21-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaur S.P., Gupta V. COVID-19 vaccine: a comprehensive status report. Virus Res. 2020;288 doi: 10.1016/j.virusres.2020.198114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakhiet M., Taurin S. SARS-CoV-2: targeted managements and vaccine development. Cytokine Growth Factor Rev. 2021;58:16–29. doi: 10.1016/j.cytogfr.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wise J. Covid-19: European countries suspend use of Oxford-AstraZeneca vaccine after reports of blood clots. BMJ. 2021;372:n699. doi: 10.1136/bmj.n699. [DOI] [PubMed] [Google Scholar]
- 6.Joint CDC and FDA Statement on Johnson & Johnson COVID-19 vaccine. https://www.fda.gov/news-events/press-announcements/joint-cdc-and-fda-statement-johnson-johnson-covid-19-vaccine, 2021. (Last access on date April 14, 2021).
- 7.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021 doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schultz N.H., Sorvoll I.H., Michelsen A.E., Munthe L.A., Lund-Johansen F., Ahlen M.T., et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021 doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ChAdOx1 nCoV-19/AZD1222; https://www.astrazeneca.com/content/astraz/media-centre/press-releases/2021/azd1222-us-phase-iii-primary-analysis-confirms-safety-and-efficacy.html, 2021. (Accessed on April 8, 2021).
- 11.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amit S., Regev-Yochay G., Afek A., Kreiss Y., Leshem E. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet. 2021;397(10277):875–877. doi: 10.1016/S0140-6736(21)00448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benenson S., Oster Y., Cohen M.J., Nir-Paz R. BNT162b2 mRNA Covid-19 vaccine effectiveness among health care workers. N Engl J Med. 2021 doi: 10.1056/NEJMc2101951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021 doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rinott E., Youngster I., Lewis Y.E. Reduction in COVID-19 patients requiring mechanical ventilation following implementation of a national COVID-19 vaccination program - Israel, December 2020-February 2021. MMWR Morb Mortal Wkly Rep. 2021;70(9):326–328. doi: 10.15585/mmwr.mm7009e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson M.G., Burgess J.L., Naleway A.L., Tyner H.L., Yoon S.K., Meece J., et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers - Eight U.S. Locations, December 2020-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(13):495–500. doi: 10.15585/mmwr.mm7013e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., et al. An mRNA vaccine against SARS-CoV-2 - preliminary report. N Engl J Med. 2020;383(20):1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadoff J., Gars M.Le, Shukarev G., Heerwegh D., Truyers C., de Groot A.M., et al. Interim results of a phase 1-2a trial of Ad26.COV2.S Covid-19 vaccine. N Engl J Med. 2021 doi: 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.FDA Briefing Document . Vaccines and related biological products advisory committee meeting. 2021. Janssen Ad26.COV2.S vaccine for the prevention of COVID-19.https://www.fda.gov/media/146217/download February 26. Accessed on April 8, 2021. [Google Scholar]
- 21.Collignon C., Bol V., Chalon A., Surendran N., Morel S., van den Berg R.A., et al. Innate immune responses to chimpanzee adenovirus vector 155 vaccination in mice and monkeys. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.579872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sayedahmed E.E., Elkashif A., Alhashimi M., Sambhara S., Mittal S.K. Adenoviral vector-based vaccine platforms for developing the next generation of influenza vaccines. Vaccines (Basel) 2020;8(4) doi: 10.3390/vaccines8040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teijaro J.R., Farber D.L. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021;21(4):195–197. doi: 10.1038/s41577-021-00526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sahin U., Muik A., Derhovanessian E., Vogler I., Kranz L.M., Vormehr M., et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(7830):594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 26.Widge A.T., Rouphael N.G., Jackson L.A., Anderson E.J., Roberts P.C., Makhene M., et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021;384(1):80–82. doi: 10.1056/NEJMc2032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao Y., Jeyanathan M., Haddadi S., Barra N.G., Vaseghi-Shanjani M., Damjanovic D., et al. Induction of autonomous memory alveolar macrophages requires T cell help and is critical to trained immunity. Cell, 2018;175(6):1634–1650. doi: 10.1016/j.cell.2018.09.042. e17. [DOI] [PubMed] [Google Scholar]
- 28.Liu M.A. A comparison of plasmid DNA and mRNA as vaccine technologies. Vaccines (Basel) 2019;7(2) doi: 10.3390/vaccines7020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du L., Tai W., Zhou Y., Jiang S. Vaccines for the prevention against the threat of MERS-CoV. Expert Rev Vaccines. 2016;15(9):1123–1134. doi: 10.1586/14760584.2016.1167603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faber M., Lamirande E.W., Roberts A., Rice A.B., Koprowski H., Dietzschold B., et al. A single immunization with a rhabdovirus-based vector expressing severe acute respiratory syndrome coronavirus (SARS-CoV) S protein results in the production of high levels of SARS-CoV-neutralizing antibodies. J Gen Virol. 2005;86(Pt 5):1435–1440. doi: 10.1099/vir.0.80844-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song F., Fux R., Provacia L.B., Volz A., Eickmann M., Becker S., et al. Middle East respiratory syndrome coronavirus spike protein delivered by modified vaccinia virus Ankara efficiently induces virus-neutralizing antibodies. J Virol. 2013;87(21):11950–11954. doi: 10.1128/JVI.01672-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Czub M., Weingartl H., Czub S., He R., Cao J. Evaluation of modified vaccinia virus Ankara based recombinant SARS vaccine in ferrets. Vaccine. 2005;23(17-18):2273–2279. doi: 10.1016/j.vaccine.2005.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaume M., Yip M.S., Kam Y.W., Cheung C.Y., Kien F., Roberts A., et al. SARS CoV subunit vaccine: antibody-mediated neutralisation and enhancement. Hong Kong Med J. 2012;18(Suppl 2):31–36. [PubMed] [Google Scholar]
- 34.Weingartl H., Czub M., Czub S., Neufeld J., Marszal P., Gren J., et al. Immunization with modified vaccinia virus Ankara-based recombinant vaccine against severe acute respiratory syndrome is associated with enhanced hepatitis in ferrets. J Virol. 2004;78(22):12672–12676. doi: 10.1128/JVI.78.22.12672-12676.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agrawal A.S., Tao X., Algaissi A., Garron T., Narayanan K., Peng B.H., et al. Immunization with inactivated middle east respiratory syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus. Hum Vaccin Immunother. 2016;12(9):2351–2356. doi: 10.1080/21645515.2016.1177688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang N., Jiang S., Du L. Current advancements and potential strategies in the development of MERS-CoV vaccines. Expert Rev Vaccines. 2014;13(6):761–774. doi: 10.1586/14760584.2014.912134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ostergaard S.D., Schmidt M., Horvath-Puho E., Thomsen R.W., Sorensen H.T. Thromboembolism and the Oxford-AstraZeneca COVID-19 vaccine: side-effect or coincidence? Lancet. 2021 doi: 10.1016/S0140-6736(21)00762-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonaventura A., Vecchie A., Dagna L., Martinod K., Dixon D.L., Van Tassell B.W., et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol. 2021 doi: 10.1038/s41577-021-00536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pai M, Grill A, Ivers N, et al. Vaccineinduced prothrombotic immune thrombocytopenia VIPIT following AstraZeneca COVID-19 vaccination. Science briefs of the Ontario COVID-19 science advisory table. 2021;1(17). https://doi.org/10.47326/ocsat.2021.02.17.1.0.
- 40.Updated GTH statement on vaccination with the AstraZeneca COVID-19 vaccine, as of March 22, 2021. https://gth-online.org/wp-content/uploads/2021/03/GTH_Stellungnahme_AstraZeneca_engl._3_22_2021.pdf, 2021. (Accessed on April 7, 2021).
- 41.Watanabe, Y., L. Mendonca, E.R. Allen, A. Howe, M. Lee, J.D. Allen, et al., Native-like SARS-CoV-2 spike glycoprotein expressed by ChAdOx1 nCoV-19/AZD1222 vaccine. bioRxiv, 2021. [DOI] [PMC free article] [PubMed]
- 42.Deshotels M.R., Xia H., Sriramula S., Lazartigues E., Filipeanu C.M. Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an angiotensin II type I receptor-dependent mechanism. Hypertension. 2014;64(6):1368–1375. doi: 10.1161/HYPERTENSIONAHA.114.03743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang S., Liu Y., Wang X., Yang L., Li H., Wang Y., et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J Hematol Oncol. 2020;13(1):120. doi: 10.1186/s13045-020-00954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verdecchia P., Cavallini C., Spanevello A., Angeli F. COVID-19: ACE2 centric infective disease? Hypertension. 2020;76(2):294–299. doi: 10.1161/HYPERTENSIONAHA.120.15353. [DOI] [PubMed] [Google Scholar]
- 45.Verdecchia P., Cavallini C., Spanevello A., Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020;76:14–20. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Addetia A., Crawford K.H.D., Dingens A., Zhu H., Roychoudhury P., Huang M.L., et al. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. J Clin Microbiol. 2020;58(11) doi: 10.1128/JCM.02107-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krammer F., Srivastava K., Alshammary H., Amoako A.A., Awawda M.H., Beach K.F., et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384(14):1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Treweek S., Zwarenstein M. Making trials matter: pragmatic and explanatory trials and the problem of applicability. Trials. 2009;10:37. doi: 10.1186/1745-6215-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coutard B., Valle C., de Lamballerie X., Canard B., Seidah N.G., Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res. 2020;176 doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mercurio I., Tragni V., Busto F., De Grassi A., Pierri C.L. Protein structure analysis of the interactions between SARS-CoV-2 spike protein and the human ACE2 receptor: from conformational changes to novel neutralizing antibodies. Cell Mol Life Sci. 2021;78(4):1501–1522. doi: 10.1007/s00018-020-03580-1. [DOI] [PMC free article] [PubMed] [Google Scholar]