Abstract
Objective
Ozone therapy has tremendous therapeutic potential owing to its antiviral, anti-inflammatory and antioxidant properties, and potential to improve oxygenation. A pilot clinical trial was conducted to evaluate the safety and efficacy of intravenous ozonised saline treatment in patients with moderate COVID-19 pneumonia.
Patients and Methods
10 patients were administered 200 ml freshly prepared ozonised saline intravenously over 1 h once a day for 8 days along with standard medical treatment. Clinical symptoms were monitored everyday and laboratory biomarkers, radiological findings at 1,3,6,10 days. Telephonic follow up was done for all after discharge till Day 14. 7 out of 10 patients required oxygen supplementation at recruitment.
Results
There was severe adverse event recorded in the study group. All patients improved from moderate to mild category in average 8 days and were discharged in average 9.7 days. None deteriorated to severe stage. All clinical symptoms resolved within 6 days and oxygen supplementation requirement reduced to none within 4.1 days. There was statistically significant reduction in CRP (p = 0.003), D-Dimer (p = 0.049), IL6 (p = 0.002) and statistically significant improvement (p = 0.001) in SpO2/FiO2 ratio. Change in LDH was borderline statistically not significant (p = 0.058). All patients showed significant resolution of bilateral interstitial infiltrates at the end of 10 days.
Conclusion
Resolved clinical symptoms, improved oxygenation, clearance of infiltrates on Chest X-ray and improvement in biomarkers in a short period with non-progression of the disease showed that IV ozonised saline therapy was safe and effective to prevent disease progression in COVID-19, making it an effective novel therapeutic tool.
Keywords: Ozonised saline, Covid-19, SARS-CoV-2, Antiviral, Coronavirus, Oxygenation
1. Introduction
COVID-19 is a respiratory illness caused due to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) [1], [2]. It has led to a global pandemic with a significant mortality rate. Clinically, Covid-19 presents with cough, breathlessness, fever, fatigue, gastrointestinal symptoms, muscle aches, pneumonia, and even death. These symptoms manifest primarily due to a ‘cytokine storm’ induced by the SARS-CoV-2 [3]. In severe cases, it may also lead to systemic inflammation leading to pulmonary fibrosis and systemic organ failure [4], [5].
Currently, the treatment available is symptomatic with general supportive and critical care as there are no approved definitive therapies against SARS-CoV-2. Hypoxia being the main pathology, [6] supplemental oxygen remains the mainstay treatment. Initially, hydroxychloroquine and azithromycin were advised but they were not very effective in severe cases. Antivirals such as oseltamivir, lopinavir- ritonavir were prescribed. Newer antivirals like favipiravir and remdesivir were introduced in the market for achieving better response. Steroids are also advised to curb the inflammation. Tocilizumab is being used to decrease IL6 levels which are exponentially raised in Covid-19 [7]. Despite all these measures the world is struggling with high mortality in Covid-19. Researchers are investigating novel multimodal therapeutic approaches for combating this illness [8].
Ozone therapy has tremendous potential for treatment of COVID-19 due to its properties of improved oxygenation, antioxidant, antiviral and anti-inflammatory [9]. Ozone is a paradoxical oxidative agent which can stimulate endogenous antioxidant pathways. It also enhances energy and oxygen supply through blood to tissues, thus reversing hypoxia [10]. It is a powerful anti-inflammatory agent that helps to curb the cytokine storm, thus reducing inflammation [11]. It is also an effective antimicrobial, and is known to reduce virulent activity in a preclinical HPV study [12], as well as in clinical studies treating HIV, [13] herpes zoster, [14] Hepatitis B, [15], [16], [17], [18] and Hepatitis C [19], [20]. It is also used as a treatment for diabetes [21] and respiratory disorders such as asthma [22] and chronic obstructive pulmonary disorder (COPD) [23]. Due to its promising ability in viral infections, pulmonary diseases and well-known safety there are several ongoing clinical trials investigating the role of ozone in treating COVID-19 [24], [25], [26], [27]. Recent studies published by Shah et al, [28] Franzini et al [29] and Schwartz et al [30] showed significant positive results in Covid-19 patients.
We conducted this clinical trial to evaluate the safety and efficacy of ozone therapy in Covid-19. This unique study shows serial objective data measures throughout 10 days revealing changes happening during the course of ozone therapy. Total 10 patients with confirmed diagnosis of Covid-19 in moderate stage were given IV ozonized saline as an adjuvant along with standard treatment. Patients were monitored for any major or minor adverse events.
2. Material and Methods
2.1. Study design
This clinical trial is a pilot study conducted on 10 patients diagnosed with Covid-19 pneumonia confirmed on qRt-PCR. (Fig. 1 ) The primary aim of this study was to evaluate the safety and efficacy of intravenous ozonized saline treatment in moderate stage Covid-19. The study was conducted at NeuroGen Brain and Spine Institute (Dedicated Covid Hospital), Navi Mumbai, India from 23 July 2020 to 13 August 2020 and was registered with the Clinical Trial Registry- India (CTRI) as CTRI/2020/07/026671.
Fig. 1.
Study Flow Chart.
2.2. Patient selection
Patient selection was based on principles of the World Medical Association Helsinki Declaration for Ethical Principles for medical research involving human subjects. The protocol of the trial was reviewed and approved by the Institutional Ethics Committee of NeuroGen Brain and Spine Institute, Navi Mumbai, Ozone Forum of India and National Institute of Naturopathy, Pune. A signed written informed consent was obtained from all the patients and their relatives before enrolling them in the clinical trial.
An independent data safety and monitoring board (DSMB) was constituted to monitor any adverse events.
2.2.1. Inclusion criteria
The Inclusion Criteria of the trial was (a) patients confirmed to be COVID-19 positive by quantitative real time polymerase chain reaction (qRT-PCR) assay, (b) age between 18 and 65 years (c) patients who give written informed consent and (d) patients that meet any one of the criteria of moderate stage according to Table 1
Table 1.
Risk stratification of Covid-19 patients for inclusion in the trial.
| SCORE | SLIGHT | SCORE | MODERATE | SCORE | SERIOUS | |
|---|---|---|---|---|---|---|
| Temperature | 1 | <37.5 °C | 2 | >37.5–38 °C | 3 | >38 °C |
| Dry Cough | 1 | <3 per min | 2 | 3–6 per min | 3 | >6 per min |
| P/F Ratio | ||||||
| PaO2/FiO2 | 1 | >300 | 4 | 300–200 | 6 | <100 |
| SpO2 in ambient AIR | 1 | >96% | 2 | <96%->88% | 3 | <88% |
| Chest X-ray | 0 | NEGATIVE | 3 | INTERSTITIAL | 6 | INTERSTITIAL + PULMONARY CONSOLIDATION |
| Breath Frequency (FR) | 0 | <12 RR/FR | 2 | >12- >20 RR/FR | 3 | >30 RR/FR |
| Total | 4 | 15 | 24 |
Slight risk: <5; Moderate risk: between 6 and 15; Serious risk: between 16 and 24
2.2.2. Exclusion criteria
The exclusion criteria of the trial was mild or severe stage according to Table 1, G6PD deficiency, Hyperthyroidism, Bleeding disorders (any coagulopathies), chronic uncontrolled illnesses such as uncontrolled diabetes, uncontrolled hypertension chronic renal failure, chronic liver disease, chronic neurodegenerative disorders, chronic heart conditions (chronic heart failure, chronic angina, previous myocardial infarction), malignancies, etc., Pregnant and lactating women, Patients on immunosuppressant drugs, Patients with a history of organ transplantation, Patients who are participating in other clinical trials.
2.3. Intervention protocol
2.3.1. Standard treatment
All patients received standard medication which included antibiotics (cephalosporin, ivermectin, doxycycline), one antiviral (Lopinavir/ritonavir or Favipiravir or Remdisivir), low molecular weight heparin, methylprednisolone, vitamin supplements (vitamin E, vitamin C, zinc, vitamin D, multivitamins) and antacid (pantoprazole). Vitamin C was administered twice a day with an interval of atleast 6 h before and after the administration of ozonised saline. Other medicines were administered for symptomatic relief such as paracetamol for fever, antitussive for cough, etc. Supplemental oxygen for patients who had oxygen saturation (SpO2) below 95% on room air. None of the patients received Tocilizumab.
2.3.2. Screening assessment
21 patients were screened and scored based on the risk stratification Table 1. Their medical history was recorded. Their clinical examination was conducted in detail. Serological, biochemical and hematological tests (G6PD, TSH, Complete blood count, Serum electrolytes test, Liver function test, Renal function test (BUN, creatinine), Serum HbA1C, HIV, HCV and HBV, Troponin, Serum Creatine Kinase (CPK), Serum Procalcitonin, ABG, Electrocardiogram and Chest X-ray were conducted.
10 patients who met the inclusion and exclusion criteria were included in the clinical trial. On these 10 patients alongwith the above tests, specific inflammatory biomarkers such as CRP, IL6, D-Dimer, Ferritin, LDH were conducted on Day 1, 3, 6, 10 before administration of ozonized saline.
2.3.3. Administration of ozonised saline
The ozone gas was generated from an ozone generator for medical use (O3-Ozonics generator, Ozone Forum of India), which is automated and standardized for time, volume and concentration. The ozone generator was manufactured by Ozonics exclusively for Ozone Forum (India). It has a stable and precise ozone concentration variable from 5 mcg/mL to 60 mcg/mL, (tested and certified using ozone analyser). All material in contact with ozone were of medical standards. Feed gas used was pure medical grade oxygen with a variable flow.
Ozone-oxygen mixture was bubbled in the saline at 5mcg/ml concentration at 500 ml/min flow of oxygen for 20 min this achieved 2 μgN/mL dose of ozone at intravenous administration of the solution. For ozonated saline, Ozone was bubbled in 200 ml 0.45% NaCl in a glass bottle for 20 min to saturate saline with Ozone. 200 ml ozonised saline was then immediately administered intravenously over 1 h at 60 drops per minute. This dose was repeated for each patient once in a day for 8 days. Freshly prepared ozonised saline was administered in each patient daily.
2.3.4. Study monitoring
Clinical parameters were measured daily by the clinical staff from Day 1 to discharge. Laboratory parameters, Chest X-ray and electrocardiograms were performed before the intervention (Day 1) followed by Day 3, 6 and 10. Data was recorded in the case record form and electronically.
2.4. Outcome measures
The primary outcome measures of this clinical trial were as follows:
-
a.
Time to recovery on 8-point ordinal scale
-
b.
Time to Resolution of symptoms (Fever, cough, shortness of breath)
-
c.
PAO2/FiO2
-
d.
Chest X-ray
-
e.
Inflammatory Biomarkers: D-Dimer, C- reactive Protein, IL-6, Ferritin, LDH
-
f.
Percentage of patients declining to serious stage at the end of 14 days
-
g.
Percentage of patients improving to mild stage at the end of 14 days
The secondary outcome measures of this clinical trial were as follows:
-
a.
Oxygen saturation index: SpO2 / FiO2
-
b.
Total blood count: WBC, Neutrophil-Lymphocyte Ratio (NLR), Hemoglobin, RBC
-
c.
Liver Function Test: SGOT, SGPT, Total and Direct Bilirubin
-
d.
Renal Function Test: BUN Creatinine
-
e.
Serum electrolytes
-
f.
Cardiac Function: Troponin and ECG
-
g.
Percentage Mortality
-
h.
Cumulative incidence of serious adverse events
-
i.
Duration of hospitalization
-
j.
Duration of oxygen use
-
k.
Change in SOFA score
All the outcome measures were conducted at Day 1,3,6 and 10. Telephonic follow up was done for all the patients after discharge till they completed Day 14.
2.4.1. Adverse event monitoring
All patients were monitored for major and minor adverse events during treatment, their hospital stay duration and till completion of trial duration i.e. 14 days. Major AE monitored were allergic reaction, anaphylactic reaction, any immediate AE after IV administration of ozonised saline, acute respiratory failure requiring mechanical ventilation, myocardial infarct, heart failure, secondary infection, pulmonary embolism, stroke, arrhythmias, liver failure, renal failure, life- threatening adverse events and death. Minor adverse events monitored were hematoma, local infection, pain at the site of injection, bleeding at the site of injection, thrombophlebitis, transiently raised LFT and headache. (Table 6)
Table 6.
Table representing occurrence of any adverse event.
| Adverse event | No. of patients that showed the adverse events |
|---|---|
| Major | |
| Allergic reaction | 0 |
| Anaphylactic reaction | 0 |
| Immediate AE after IV administration of ozonised saline | 0 |
| Acute respiratory failure requiring mechanical ventilation | 0 |
| Myocardial infarct | 0 |
| Heart failure | 0 |
| Secondary infection | 0 |
| Pulmonary embolism | 0 |
| Stroke | 0 |
| Arrhythmias | 0 |
| Liver failure | 0 |
| Renal Failure | 0 |
| Life- threatening adverse events | 0 |
| Death | 0 |
| Minor | |
| Hematoma | 0 |
| Thrombophlebitis | 0 |
| Local Infection | 0 |
| Pain at the site of injection | 4 |
| Bleeding at the site of injection | 0 |
| Dilutional Hyponatremia | 1 |
| Transiently raised LFT | 8 |
| Headache | 1 |
2.4.2. Data analysis
A detailed analysis was performed to study the outcome of the intervention. Neutrophil-Lymphocyte Ratio, SpO2/FiO2 ratio, CRP, IL-6, Ferritin, D-Dimer and LDH were analysed. Median of their values was calculated and a line graph with standard error was plotted to study the overall trend in the study population. Resolution of clinical symptoms was evaluated by calculating frequency of symptoms present on Day 1,3,6 and 10 and average number of days taken for resolution of symptoms. Patients chest X-rays were scored using an ordinal scale signifying degree of lung involvement and severity of condition.
2.4.3. Statistical analysis
Paired T-Test was performed on the lab parameters (CRP, IL6, D-Dimer and LDH) and oxygen saturation index SpO2/FiO2. Chest X-ray scores before and after intervention were compared using Wilcoxon sign rank test at the end of 10 days.
It was performed in IBM SPSS statistic version 20 and P < 0.05 was considered significant.
3. Results
3.1. Demographic data
10 patients with confirmed diagnosis of moderate Covid-19 were included in this trial. Out of these 9 were males and 1 was female. Age range of the study population was 25 to 59 years with average age as 36.2 years. (Table 2 )
Table 2.
Demographic Data of the clinical trial.
| Total | 10 | |
|---|---|---|
| Gender | Male | 9 |
| Female | 1 | |
| Mean Age | Range: 25 to 59 years | 36.2 |
| Comorbidities | Diabetes | 1 |
| Hypertension | 0 | |
| Kidney disease | 1 | |
| Smoker/Tobacco chewer | 2 | |
| Malignancy | 0 | |
| Cardiac disorders | 0 | |
| Supplemental Oxygen required at admission | 7 | |
| qRT-PCR positive | 10 | |
| Abnormal ECG | 0 |
3.2. Primary outcome measure
To evaluate efficacy of ozone therapy we calculated average number of days for parameters such as discharge from the hospital, recovery on 8 point ordinal scale, resolution of symptoms (cough and shortness of breath), oxygen use, resolution of inflammatory biomarkers, normalization of Oxygen saturation index, significant resolution on Chest X-Ray findings and change in severity of disease. (Table 3 ) None of the 10 patients showed worsening in disease severity. Moreover, on an average in 8 days, all 10 patients improved from moderate to mild category. All patients were discharged on an average in 9.7 days. It was observed that patients took only short period for resolution of their symptoms i.e. cough and shortness of breath. The oxygen requirement declined fast and duration of oxygen use was short. There was a drastic early decline seen in inflammatory biomarkers such as CRP, D-Dimer, IL6. NLR, Ferritin and LDH also showed significant reduction. (Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8 )
Table 3.
Average number of days for resolution of outcome measures.
| Parameters | Average no. days | |
|---|---|---|
| Time to Discharge | 9.7 | |
| Time to Recovery on 8-point ordinal scale (1–3) | 5.7 | |
| Time to Resolution of symptoms | Cough | 5.1 |
| Shortness of breath | 3.6 | |
| Duration of O2 use | 4.1 | |
| Time to resolution* of Inflammatory Biomarkers: | C- reactive Protein | 3 |
| IL-6 | 5.3 | |
| D- Dimer | 6.3 | |
| LDH# | 5 | |
| Time to normalization of Oxygen saturation index (SPO2 / FiO2) | 4.1 | |
| Time to change of SOFA score | 7.4 | |
| Time to significant resolution on Chest X-Ray findings | 8.1 | |
| Time to change from Moderate to severe risk | None progressed to severe | |
| Time to change from Moderate to Mild risk | 8 | |
| * Resolution was considered as half of the baseline values or within the normal range | ||
| # Except one patient reducing to normal and one patient near normal |
Fig. 3.
Graph representing change in CRP levels after ozone therapy.
Fig. 4.
Graph representing change in IL6 levels after ozone therapy.
Fig. 5.
Graph representing change in NLR after ozone therapy.
Fig. 6.
Graph representing change in Ferritin levels after ozone therapy.
Fig. 7.
Graph representing change in LDH levels after ozone therapy.
Fig. 8.
Graph representing change in D-Dimer levels after ozone therapy.
3.3. Clinical symptoms resolution
Overall mortality rate in this study population was zero. The frequency of occurrence of clinical symptoms in the 10 patients and average days for these symptoms to resolve was calculated. (Table 4 , Fig. 2) All patients showed resolution of all the symptoms by Day 10 except 1 patient who had slight inspiratory pleuritic pain which resolved on Day 11. Cough was the last symptom to resolve. Majority symptoms including shortness of breath of all patients resolved by Day 6. Before completion of Day 14, all patients were clinically stable and discharged home.
Table 4.
Resolution of clinical symptoms after ozone therapy.
| Symptom | Number of patients out of 10 showing the specific symptom |
Average number of days for resolution of symptoms | |||
|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 6 | Day 10 | ||
| Cough | 10/10 | 9/10 | 4/10 | 0/10 | 5.1 |
| Sputum | 4/10 | 4/10 | 2/10 | 0/10 | 5.5 |
| Shortness of breath | 8/10 | 7/10 | 0/10 | 0/10 | 3.6 |
| Sore throat | 2/10 | 2/10 | 0/10 | 0/10 | 3 |
| Chest pain | 3/10 | 2/10 | 2/10 | 1/10 | 4 |
| Diarrhea | 1/10 | 0/10 | 0/10 | 0/10 | 1 |
| GI bleeding | 0/10 | 0/10 | 0/10 | 0/10 | 0 |
| Loss of appetite | 7/10 | 6/10 | 0/10 | 0/10 | 3.5 |
| Loss of Taste | 7/10 | 5/10 | 0/10 | 0/10 | 2.8 |
| Loss of Smell | 4/10 | 4/10 | 0/10 | 0/10 | 3.5 |
| Generalized weakness/Fatigue | 3/10 | 2/10 | 0/10 | 0/10 | 3.3 |
| Giddiness | 3/10 | 1/10 | 0/10 | 0/10 | 1.3 |
| Headache | 2/10 | 2/10 | 0/10 | 0/10 | 2.5 |
| Muscle aches and Pain | 1/10 | 0/10 | 0/10 | 0/10 | 2 |
Fig. 2.
Frequency of clinical symptoms after ozone therapy.
3.4. Laboratory parameters
For all laboratory parameters, we calculated the median to demonstrate the trend, as the range of the values recorded in all the patients was varied and large. Procalcitonin (PCT) levels were normal in all patients throughout the study duration.
3.4.1. C-Reactive Protein
Median of values of serum C-Reactive protein (CRP) was calculated for all patients at Day 1, 3, 6 and 10 and plotted on a line graph. The normal range of CRP was 0–6 mg/l. Majority of the study population showed elevated levels of CRP on Day 1 demonstrating increased inflammation and disease severity. However, after intervention CRP levels reduced significantly at Day 3 and continued to reduce further. (Fig. 3) This trend indicated improvement in inflammation along with improved disease severity.
3.4.2. Interleukin 6 (IL 6)
IL 6 is an inflammatory marker for cytokine storm and its measurements indicate disease severity. In this study population, median of IL 6 values was calculated and was found to be elevated on Day 1 and Day 3 whereas, the values normalized at Day 6. (Fig. 4)
3.4.3. Neutrophil-Lymphocyte ratio (NLR)
Neutrophil-Lymphocyte ratio is an inflammatory biomarker and was used to study the systemic inflammation in the patients. Median of NLR was calculated for each patient and a line graph was plotted. Improvement was recorded in NLR. It was found that NLR increased on Day 3 and gradually reduced thereafter. (Fig. 5)
3.4.4. Ferritin
Serum ferritin levels are inflammatory markers and are closely related to the severity of COVID-19. The normal range of ferritin in males is 30–220 ng/ml and in females is 20–110 ng/ml. In our study population, it was observed that majority of the patients had high levels of ferritin on Day 1 which increased on Day 3 and eventually reduced at day 6 and onwards. However, the levels of ferritin had not become normal till day 10. (Fig. 6)
-
1.
Lactic acid dehydrogenase (LDH):Serum LDH was used as a potential marker for evaluating clinical severity and monitoring the response of ozone therapy in COVID-19. The normal range of LDH was 230–450 u/l. All patients showed elevated levels of LDH on Day 1. However, the levels reduced to normal on Day 3 and onwards. This indicates improved clinical severity and a positive response to the treatment. (Fig. 7)
-
2.
D-Dimer: D-dimer levels correlate with disease severity and are a reliable prognostic marker. On calculating the median of all values on day 1, 3, 6 and 10, it was observed that the majority of patients had elevated levels of D-Dimer on day1. Whereas, these levels normalized at Day 3 and onwards. (Fig. 8)
-
3.
Oxygen saturation index
-
(A)
SpO2/FiO2: Median of SpO2/FiO2 ratios of all patients on Day 1, 3, 6 and 10 were calculated and a line graph was plotted. It was observed that overall, on Day 1 and Day 3 all patients had low SpO2/FiO2 ratio which normalized on Day 6 and onwards. (Fig. 9) This indicated improved oxygenation and reduced disease severity.
-
(B)
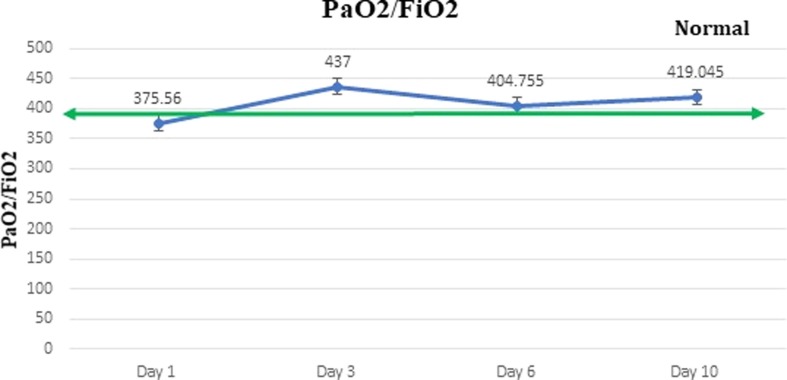
PaO2/FiO2: Median of PaO2/FiO2 ratios of all patients on Day 1, 3, 6 and 10 were calculated and a line graph was plotted. It was observed that on Day 1 patients had low PaO2/FiO2 ratio which normalized on Day 3 and onwards. (Fig. 10 ) This indicated improved oxygenation and reduced disease severity.
Fig. 9.
Graph representing change in SpO2/FiO2 levels after ozone therapy.
Fig. 10.
Graph representing change in PaO2/FiO2 levels after ozone therapy.
3.5. Results of radiological findings
All the patients showed bilateral interstitial infiltrates at the time of admission. Median score was 3 suggesting interstitial infiltration without powder thickening. Although there was no change observed in the X-ray findings of patients at Day 3, 1 patient showed complete resolution on Day 6 and 1 patient showed complete resolution on day 10. (Fig. 11 ) All patients improved radiologically showing statistically significant reduction (p = 0.004) in the median score from 3 to 1 at the end of 10 days.
Fig. 11.
Chest X - ray images showing resolution of the lung opacities after ozone therapy.
3.6. Statistical analysis
Paired t-test was performed on IL6, CRP, D-Dimer, LDH and SpO2/FiO2. Serum ferritin levels showed large variability in the values which was evident with standard deviation > mean ferritin levels at assessment (Mean = 454.89, SD = 580.00). Therefore, statistical analysis could not be performed for Ferritin.
It was found that there was a statistically significant reduction in IL6, CRP and D-Dimer and statistically significant improvement in SPO2/FiO2 ratio after intervention. However, change in LDH was borderline statistically significant. (Table 5 )
Table 5.
Table representing Pre and Post values of IL6, CRP, D-Dimer, LDH and SpO2/FiO2 and Mean, Standard Deviation, standard Error and P-value.
| Outcome Parameter | IL6 |
CRP |
D-dimer |
LDH |
SPO2/ FIO2 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Patient 1 | 61.68 | 28.65 | 14 | 6.2 | 142.59 | 136.94 | 340 | 573 | 461.9 | 466.66 |
| Patient 2 | 121.18 | 2.33 | 57.4 | 10.2 | 814.17 | 100 | 635 | 547 | 466.66 | 466.66 |
| Patient 3 | 60.35 | 6.19 | 51 | 6.2 | 536.88 | 330 | 353 | 356 | 256 | 466.66 |
| Patient 4 | 164.94 | 11.22 | 43.3 | 2.1 | 2488 | 540 | 564 | 361 | 259.45 | 466.66 |
| Patient 5 | 82.01 | 13.7 | 67.2 | 6 | 809.93 | 100 | 1421 | 539 | 215 | 471.4 |
| Patient 6 | 276.78 | 30.105 | 142 | 8.8 | 609.64 | 340 | 589 | 471 | 217.78 | 471.43 |
| Patient 7 | 215.51 | 71.36 | 78 | 13 | 832.53 | 210 | 709 | 419 | 184.9 | 466.66 |
| Patient 8 | 37.9 | 2.66 | 10.6 | 1.2 | 646.27 | 10 | 587 | 407 | 452.38 | 466.66 |
| Patient 9 | 59.19 | 1.5 | 35.9 | 5.2 | 50 | 200 | 662 | 252.2 | 264.86 | 466.66 |
| Patient 10 | 52.13 | 3.53 | 153 | 11.3 | 20 | 360 | 384 | 301 | 215.55 | 466.66 |
| Mean | 113.17 | 17.12 | 65.24 | 7.02 | 695.00 | 232.69 | 624.40 | 422.62 | 299.45 | 467.61 |
| Standard Deviation | 80.76 | 21.77 | 48.28 | 3.82 | 704.94 | 158.88 | 309.46 | 108.68 | 113.72 | 2.00 |
| Standard error | 25.54 | 6.88 | 15.27 | 1.21 | 222.92 | 50.24 | 97.86 | 34.37 | 35.96 | 0.63 |
| P Value | 0.002* | 0.003* | 0.049* | 0.058# | 0.001* | |||||
$ Borderline statistically significant
* Statistically significant
3.7. Adverse event monitoring
All patients were monitored for major and minor adverse events (AE). (Table 6 ) None of the 10 patients had any major AEs. However, 4 out 10 patients had mild pain at the site of injection, 1 patient complained of a headache which resolved spontaneously. 8 out of 10 patients had transient elevation of liver enzyme SGPT (patients were also on antivirals). This elevation could be due to antivirals or ozone or combination of both. It is difficult to pin-point any one of these. However, it is noteworthy that the elevation was only transient and resolved with discontinuation of antiviral drugs. 1 patient who had borderline low sodium levels on admission developed mild hyponatremia which resolved with salt tablets.
4. Discussion
4.1. Pathogenesis of Covid-19
COVID-19 is a rapidly spreading infectious disease caused by SARS-CoV-2. SARS-CoV-2 is an enveloped, positive-stranded RNA virus with nucleocapsid, and enters host cells via the ACE2 and Transmembrane Serine Protease 2 (TMPRSS2) receptors. These receptors are widely distributed on the human cells surface, especially the alveolar type II cells (AT2) and capillary endothelium, as well as intestinal enterocytes, heart, liver, kidneys [31]. Evidence suggests that the mortality in COVID-19 patients is associated to “cytokine storm” induced by the virus. There is an influx of various immune cells such as macrophages, neutrophils, and T cells from the circulation into the site of infection. This triggers an exaggerated, uncontrolled release of cytokines, leading to an excessive inflammatory response. The “cytokine storm” results from a sudden acute increase in circulating levels of different pro-inflammatory cytokines including IL-6, IL-1, TNF- α, and interferon [32]. Lung inflammation is one consequence of the cytokine storm that can progress into acute lung injury or its more severe form ARDS [33]. ARDS leading to low oxygen saturation levels is a major cause of mortality in COVID-19. COVID-19 infection may also lead to pulmonary edema, decreased gas exchange, acute respiratory distress syndrome, sepsis and septic shock, multiple organ failure including acute kidney injury, acute cardiac injury, liver damage, etc. which may eventually lead to death [31]. In moderate stage, hypoxia is a major concern due to accumulation of inflammatory exudate, collapse of alveoli and inflamed airways [34]. As a result, the SpO2 and PaO2 levels in infected patients is reduced. Evidence suggests that SARS-CoV2 can also trigger oxidative stress. Some authors suggest that the onset of severe lung injury in Covid-19 infected patients depends on degree of activation of the oxidative stress [35]. Covid-19 patients also develop coagulopathy which is characterized by mild thrombocytopenia, slight prolongation of the prothrombin time, high levels of D-dimer, and elevated levels of fibrinogen, factor VIII, and von Willebrand factor. The levels of D-dimer, a breakdown product of cross-linked fibrin, correlate with disease severity and predict the risk of thromboembolism, the need for ventilatory support, and mortality in Covid-19 [36].
4.2. Natural history of progression of Covid-19
According to WHO reports, 80% cases experience mild illness, 20% progress to moderate stage out of which 5% become critically ill. Previous studies in Covid-19 have also shown elevated levels of biomarkers such as CRP, IL6, D-Dimer, Ferritin, LDH [37].
Current therapy consists of antibiotics, antiviral therapy, anti-coagulants and systemic corticosteroids, which may have a limited effect on a fatal outcome [38]. Mild Covid-19 can be managed successfully however, patients with moderate disease require oxygen therapy and may deteriorate rapidly to severe stage. As a result, the focus of any treatment for Covid-19 should be to arrest the disease progression along with alleviating the symptoms. This will decrease the need of mechanical ventilatory support and mortality.
4.3. Clinical trial
This clinical trial was conducted to evaluate the safety and efficacy of IV ozonized saline as an adjuvant treatment in Covid-19. Ozone therapy has been extensively employed for treating various chronic diseases like infected wounds, circulatory disorders, geriatric conditions, macular degeneration, viral diseases, rheumatism/arthritis, cancer, severe acute respiratory syndrome and AIDS [39], [40], [41]. Therapeutic effects of ozone therapy are attributed to its oxygenating, immunomodulatory, anti-inflammatory, oxidation balancing and antiviral properties [42].
4.4. Medical ozone therapy
Medical Ozone is a mixture of 0.5–5% ozone and 99.5–95% oxygen. Ozone therapy has evolved as a promising therapeutic alternative in various conditions owing to its disinfectant effect and its capacity to transport and release oxygen into tissues. Systemically, ozone can be administered via (a) Major and Minor autohemotherapy with ozonised blood (b) IV ozonised saline (c) Rectal insufflation. In this clinical trial, ozonised saline was used as it is safe, avoids the inconvenience of extracorporeal blood ozonation, has a systemic reach with minimum patient contact and the dose can be controlled and kept constant. Use of ozonised saline is a common practice developed by the Russian school of ozone therapy in the city of Nizhny Novgorod (Volga Federal District) and was approved by the Ministry of Health of the Russian Federation in the early 80 s of the last century. This method consists of saturating the saline solution with a mixture of oxygen-ozone and administering it intravenously to the patient [43].
4.5. Correlation of study results with mechanism of action of ozone therapy.
Studies suggest that ozone targets the cysteines in the spike proteins of SARS-CoV2. It oxidizes the glycoprotein of its membrane, transforming it from the reduced form to the oxidized form. The virus can enter and infect the cells only when the glycoprotein is in the reduced form. Thus, ozone blocks the viral fusion with the ACE2 receptor. Ozone may also block virus attachment to the ACE2 receptor by upregulating the production of nitric oxide which inhibits viral replication of SARSCoV2 [44]. Ozone may also alter the reproductive cycle of the virus by damaging the capsid through peroxidation [45].
4.6. Anti-inflammatory
Ozone when administered in therapeutic dosages activate nuclear factor erythroid 2-related factor 2 (Nrf2) which inhibits the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway. NF-κB pathway induces the production of pro-inflammatory interleukins in inflamed tissues. TNFα, IFNγ, IL1β, IL6, IL8 [46]. Thus, blocking the pathway downregulates the release of proinflammatory cytokines and stimulates anti-inflammatory cytokines IL-4 and IL-10. This curbs the cytokine storm and may aid in restoration of normal functioning of the inflamed tissues and modulation of several anti-inflammatory cytokines [47], [48], [49]
In this clinical trial, disease severity and progression was evaluated by serial measurement of CRP, IL6, NLR, Ferritin and LDH which are inflammatory biomarkers and are raised in Covid-19.
CRP levels correlate with inflammation and also with lung lesions caused due to Covid-19 [50], [51]. In our study group, patients showed elevated CRP levels at baseline which almost normalized for all the patients by Day 10. (Fig. 3) This decline in CRP levels suggests anti-inflammatory effect of ozone therapy.
Increasing levels of IL-6 are hallmark of progression of Covid-19 and cytokine storm [52]. Our findings showed a decreasing trend in levels of IL6. IL6 levels of all patients improved on an average in 5.3 days. (Fig. 4) Improved IL6 levels may be due to the effect of ozone on NLRP3 inflammasome [53] Also the dramatic decline of IL6 correlated with resolution of clinical symptoms. This signifies that ozone has curbed the cytokine storm.
NLR is the proportion of neutrophils to lymphocytes measured in routine complete blood count (CBC) tests. An elevated NLR is associated with the prognosis of systemic inflammation in Covid-19. In a study conducted by Ciccullo et al, a higher NLR at hospital admission was associated with a more severe outcome indicating a correlation between hyperinflammatory state and COVID-19 pathogenesis [54]. In this clinical trial, NLR transiently increased after intervention and started to reduce thereafter. (Fig. 5) Decrease in NLR could be attributed to the anti-inflammatory effect of ozone therapy.
Hyperferritinemia i.e. increased levels of ferritin, is also associated with inflammation caused due to SARS-CoV-2 infection, and therefore, ferritin can be used to predict disease severity and the extent of the cytokine storm in infected patients [55]. In this study, ferritin levels reduced after ozone therapy but did not completely normalize till Day 10. Ferritin levels are shown to have a time lag behind CRP levels in Covid-19. Hence, we believe that with extended time these levels would have also normalized. (Fig. 6)
4.7. Anti-oxidant effects
Ozone therapy has the potential to modulate cytotoxity and exhibit cytoprotective effect by modulation of the endogenous antioxidant pathways including NF-κB/Nrf2 pathways. The main mechanism is related to the modulation of the oxidative stress which leads to re-equilibration of the cellular redox state which is an important process for inhibition of viral replication [56]. Viral infection causes oxidative stress by producing harmful free radicals ROS [57]. These are neutralized by body’s antioxidant defense mechanism. Ozone stimulates endogenous antioxidant pathways and enhances antioxidant enzymes like superoxide dismutase, glutathione peroxidase, catalase, etc. This controls the cellular damage in Covid-19 [49].
Increased LDH levels are associated with cellular damage caused due to tissue breakdown in COVID-19 [58] . In this clinical trial, patients showed decrease in levels of LDH after intervention which indicates reduction in cellular damage and validates the therapeutic effects of ozone therapy. (Fig. 7)
4.8. Improved oxygenation
Ozone therapy may improve oxygenation and blood circulation in the ischemic tissues by improving oxygen metabolism, increasing intra-erythrocytic 2,3-DPG level and of some prostacyclins such as PGI2. Due to increased 2,3-DPG level there is a shift in the dissociation curve of HbO2 to the right [49]. This causes increased delivery of oxygen to the tissues at cellular level. This was evident from the results of this study which showed rapid increase in SpO2/FiO2 ratio and PaO2/FiO2 ratio. (Fig. 9,10) Also, supplemental oxygen requirement of patients declined due to this improved oxygenation.
4.9. Improved coagulopathy
Ozone in therapeutic dosage is a hypocoagulant. This property reduces clot formation in Covid-19 .[59] D-dimer is commonly elevated in patients with COVID-19 [60], [61]. In this study, D-Dimer levels at baseline were elevated and began to normalize at Day 3. (Fig. 8) Evidence suggests that ozone has a positive effect on thromboembolism, or the co-morbid vascular and thrombotic disorders associated with COVID-19 consequently aiding in decrease of D-Dimer levels [30]. This contributes to overall clinical improvement and recovery seen in the study.
4.10. Antimicrobial l/Antiviral effect
Ozone also has antiviral effects. In vitro, it inhibits viral life cycles in lipid-enveloped viruses. Peroxidation of viral lipoproteins as well as glycoproteins stoichiometrically interferes in host-virus contact. This in turn reduces cytopathic virulence [62], [63]. Ozone may also oxidise free viral components, thus reducing viral load [64]. Ozone therapy has shown attenuation of viral infections like herpes by reducing the presence of discoid cells, shortening the prescription course and improving the efficacy, while relieving pain [14], [65] in hepatitis B, [15], [16], [17], [18] and HIV [13]. A review by Mawsouf et al. of a decade’s worth of HCV treatment by ozone therapy revealed a dramatic reduction in the viral load, indicating the potential of ozone therapy in combating viral infections [19]. Ozone is also bactericidal and prevents secondary infection [66]. In this trial, Procalcitonin (PCT) levels, marker or secondary infection, were normal in all patients throughout the study duration.
4.11. Clinical symptom resolution
All the patients receiving ozone treatment also showed significant improvement in the clinical condition. They recovered completely and were discharged from the hospital after an average duration of 9.7 days. All the patients recruited in the study had Moderate COVID-19 at enrolment however it improved to Mild COVID-19 in an average duration of 8 days. None of these patients progressed to Severe COVID-19. This clinical recovery can be attributed to several physiological changes such reduction in inflammatory response, seen as reduced levels of CRP (Fig. 3), IL-6 (Fig. 4), Ferritin (Fig. 6) and LDH (Fig. 7); reduction in thromboembolism and ischemia as evidenced by declining levels of D-Dimer (Fig. 8) and improved oxygenation seen as improved PaO2/FiO2 and oxygen saturation (SPO2) attributed to ozone therapy.
All the clinical symptoms presented by the patients were typical of COVID-19 [67], [68] Sore throat, diarrhea, muscle aches and pain, giddiness, headache and loss of taste recovered earliest i.e. within 3 days. Loss of appetite, loss of smell, generalized weakness, chest pain, shortness of breath took a little longer time to resolve i.e. 6 days. Cough being the last symptom to resolve. (Table 4) Many of the previous reports have shown patients that develop dyspnea may progress to severe stage that requires mechanical ventilation [69]. However, none of the patients in our trial required mechanical ventilation and dyspnea resolved in average 4 days.
4.12. Radiological resolution
All the patients at the time of admission showed areas of ground glass opacification bilaterally in chest X-rays, majority in the peripheral regions while some in lower and mid regions. The findings of chest X-ray in our patients were consistent with previous reports that showed bilateral infiltrates, more in the periphery and lower lobes [70], [71]. These reports also state that the lung involvement and severity of X-ray findings increases as the disease progresses. In this trial, radiological abnormalities were found to have resolved significantly in an average of 8.1 days post ozone therapy. Earliest resolution was seen in some patients within 6 days of starting the intervention.
The above findings suggest that early resolution of pulmonary infiltrates by ozone therapy may help to prevent fibrosis in these patients. Additionally, declined disease severity and normalization of oxygen saturation index suggests ceasation of cytokine storm, antiviral effect and improved oxygenation due to ozone therapy.
4.13. Adverse events
No major adverse events related to procedure or intervention were recorded. The minor adverse events such as pain at bleeding site and headache eventually subsided. Transient increase in LFT was recorded in 8 out of 10 patients. This elevation could be due to antivirals or ozone or combination of both. It is difficult to pin-point any one of these. However, it is noteworthy that the elevation was only transient and resolved with discontinuation of antiviral drugs. Hence, we recommend monitoring LFT when combination treatment of ozone and antivirals is administered. 1 patient who had borderline low sodium levels on admission developed mild dilutional hyponatremia which resolved with salt tablets.
Limitation: The limitation of the study was that it was conducted on a small sample size of 10 patients. Also, the gender distribution of the study population was not balanced. There was only 1 female included in the study. This study did not include patients with chronic uncontrolled illnesses.
5. Conclusion
In this clinical trial, clinical outcomes, laboratory biomarkers and radiological findings showed that IV ozonised saline therapy with standard treatment was safe and effective in moderate stage COVID-19 patients. No major adverse events were noted. Ozone therapy has anti-inflammatory, antioxidant, antiviral, and improved oxygenation ability which can alleviate deleterious effects of Covid-19. Owing to the anti-inflammatory and anti-viral mechanism of ozone, it can further be explored as remdesivir sparing and steroid sparing treatment strategy for Covid-19. The trial results, resolution of clinical symptoms and improved oxygenation in a short period with non-progression of disease makes this an effective therapeutic tool. Significant clearance of infiltrates on Chest X-ray corroborates the clinical improvement. The remarkable early decline of CRP. IL-6 and D-dimer which are the hallmarks of COVID-19 disease, provides objective evidence to introduce ozone therapy as an adjuvant to the standard treatment of COVID-19.
Data Availability Statement
Most of the data supporting the findings has been incorporated in the manuscript. Additional data of this study is available from the corresponding author upon request.
Funding Statement
The study was funded by Bisleri Charitable Trust
CRediT authorship contribution statement
Alok Sharma: Conceptualization, Supervision, Visualization, Methodology, Resources, Writing - review & editing. Mili Shah: Methodology, Funding acquisition, Writing - review & editing. Satya Lakshmi: Methodology. Hemangi Sane: Methodology, Project Administration, Supervision, Writing - review & editing. Jignasha Captain: Methodology. Nandini Gokulchandran: Project administration. Pallavi Khubchandani: Methodology. Pradeep. M.K: Methodology. Prakash Gote: Investigation. Balaji Tuppekar: Investigation. Pooja Kulkarni: Data curation, Investigation, Writing - original draft Preparation. Amruta Paranjape: Data curation, Investigation, Formal analysis. Radhika Pradhan: Investigation. Ritu Varghese: Investigation. Sushil Kasekar: Investigation. Vivek Nair: Investigation. Ummeammara Khanbande: Investigation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Tang D., Comish P., Kang R. The hallmarks of COVID-19 disease. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao Z., Zheng Z., Wu K., Junhua Z. Immune environment modulation in pneumonia patients caused by coronavirus: SARS-CoV, MERS-CoV and SARS-CoV-2. Aging (Albany NY). 2020;12:7639–7651. doi: 10.18632/aging.103101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li S.-W., Wang C.-Y., Jou Y.-J., Yang T.-C., Huang S.-H., Wan L., et al. SARS coronavirus papain-like protease induces Egr-1-dependent up-regulation of TGF-β1 via ROS/p38 MAPK/STAT3 pathway. Sci Rep. 2016;6:25754. doi: 10.1038/srep25754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahmudpour M., Roozbeh J., Keshavarz M., Farrokhi S., Nabipour I. COVID-19 cytokine storm: The anger of inflammation. Cytokine. 2020;133 doi: 10.1016/j.cyto.2020.155151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kashani K.B. Hypoxia in COVID-19: Sign of Severity or Cause for Poor Outcomes. Mayo Clin Proc. 2020;95:1094–1096. doi: 10.1016/j.mayocp.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atal S., Fatima Z. IL-6 Inhibitors in the Treatment of Serious COVID-19: A Promising Therapy? Pharmaceut Med. 2020;34:223–231. doi: 10.1007/s40290-020-00342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jean S.S., Lee P.I., Hsueh P.R. Treatment options for COVID-19: The reality and challenges. J Microbiol Immunol Infect. 2020;53:436–443. doi: 10.1016/j.jmii.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alessandra G., Andrea M., Giacomo R., et al. Ozone Therapy as a Possible Option in COVID-19 Management. Frontiers in Public Health. 2020;8:417. doi: 10.3389/fpubh.2020.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng L., Meng W., Li D., Qiu D., Wang S., Liu H. The effect of ozone on hypoxia, hemolysis and morphological change of blood from patients with aortic dissection (AD): a preliminary in vitro experiment of ozonated autohemotherapy for treating AD. Am J Transl Res. 2018;10:1829–1840. [PMC free article] [PubMed] [Google Scholar]
- 11.Bocci V., Larini A., Micheli V. Restoration of normoxia by ozone therapy may control neoplastic growth: a review and a working hypothesis. J Altern Complement Med. 2005;1:257–265. doi: 10.1089/acm.2005.11.257. [DOI] [PubMed] [Google Scholar]
- 12.Peirone C., Mestre V.F., Medeiros-Fonseca B., et al. Ozone therapy prevents the onset of dysplasia in HPV16-transgenic mice-A pre-clinical efficacy and safety analysis. Biomed Pharmacother. 2018;104:275–279. doi: 10.1016/j.biopha.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 13.Cespedes-Suarez J., Martin-Serrano Y., Carballosa-Peña M.R., Dager-Carballosa D.R. The immune response behavior in HIV-AIDS patients treated with Ozone therapy for two years. J Ozone Ther. 2019;2 [Google Scholar]
- 14.Huang J., Xiang Y., Gao L., Pan Y., Lu J. Topical ozone therapy: An innovative solution to patients with herpes zoster. J Cent South Univ (Med Sci). 2018;43:168–172. doi: 10.11817/j.issn.1672-7347.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Jiao XJ, Peng X. [Clinilal study of medical ozone therapy in chronic hepatitis B of 20 patients]. Zhonghua Shi Yan He Lin Chuang Bing Du XueZaZhi. 2008; 22: 484-485. [PubMed]
- 16.Chernyshev A.L., Filimonov R.M., Karasev A.V., Neronov V.A., Maksimov V.A. Combined treatment including ozone therapy of patients with viral hepatitis. Vopr Kurortol Fizioter Lech Fiz Kult. 2008;19–22 [PubMed] [Google Scholar]
- 17.Neronov V.A. Experience with the use of ozone for the treatment of chronic viral hepatitis. Vopr Kurortol Fizioter Lech Fiz Kult. 2009;14–7 [PubMed] [Google Scholar]
- 18.Cespedes-Suarez J, Martin-Serrano Y, Carballosa-Peña MR, Dager-CarballosaDR . Response of patients with chronic Hepatitis B in one year of treatment with Major Autohemotherapy. J Ozone Ther. 2018:2.
- 19.Mawsouf M.N., Tanbouli T.T., Viebahn-Hänsler R. Ozone Therapy in Patients with Viral Hepatitis C: Ten Years’ Experience. Ozone Sci Eng. 2012;34:451–458. [Google Scholar]
- 20.Zaky S., Kamel S.E., Hassan M.S., et al. Preliminary results of ozone therapy as a possible treatment for patients with chronic hepatitis C. J Altern Complement Med. 2011;17:259–263. doi: 10.1089/acm.2010.0016. [DOI] [PubMed] [Google Scholar]
- 21.Bocci V., Zanardi I., Huijberts M.S., Travagli V. It is time to integrate conventional therapy by ozone therapy in type-2 diabetes patients. Ann Transl Med. 2014;2:117. doi: 10.3978/j.issn.2305-5839.2014.07.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernández-Rosales F.A., Calunga-Fernández J.L., Turrent-Figueras J., Menéndez-Cepero S., Montenegro-Perdomo A. Ozone therapy effects on biomarkers and lung function in asthma. Arch Med Res. 2005;36:549–554. doi: 10.1016/j.arcmed.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 23.Borrelli E., Bocci V. Oxygen Ozone Therapy in the Treatment of Chronic Obstructive Pulmonary Disease: An Integrative Approach. Am J Clin Exp Med. 2014;2:9–13. [Google Scholar]
- 24.Guangjian N, Hongzhi Y. Clinical study for ozonated autohemotherapy in the treatment of Novel Coronavirus Pneumonia (COVID-19). ChiCTR2000030165. Academy of Medical Engineering and Translational Medicine, Tianjin University.2020-02-24. Available at: http://www.chictr.org.cn/showproj.aspx?proj=49947.
- 25.Linlin H, Xiangdong C. A randomized controlled trial for the efficacy of ozonated autohemotherapy in the treatment of Novel Coronavirus Pneumonia (COVID-19). ChiCTR2000030006. Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. 2020-02-19. Available at: http://www.chictr.org.cn/showproj.aspx?proj=49737.
- 26.Huiling H, Tong X. A multicenter randomized controlled trial for ozone autohemotherapy in the treatment of novel coronavirus pneumonia (COVID-19). ChiCTR2000030102. Tianjin Huanhu Hospital. 2020-02-23. Available at: http://www.chictr.org.cn/showproj.aspx?proj=49747.
- 27.Poscia, R. Oxygen-Ozone as Adjuvant Treatment in Early Control of COVID-19 Progression and Modulation of the Gut Microbial Flora (PROBIOZOVID). University of Rome Sapienza - Policlinico Umberto I Rome (Italy). ClinicalTrials.gov identifier (NCT number): NCT04366089.
- 28.Shah Mili, Captain Jignasha, Vaidya Vidyadhar, et al. Safety and efficacy of ozone therapy in mild to moderate COVID-19 patients: A phase 1/11 randomized control trial (SEOT study) International Immunopharmacology. 2021;91 doi: 10.1016/j.intimp.2020.107301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franzini M., Valdenassi L., Ricevuti G., Chirumbolo S., Depfenhart M., Bertossi D., Tirelli U. Oxygen-ozone (O2–O3) immunoceutical therapy for patients with COVID-19. Preliminary evidence reported. International Immunopharmacology. 2020;8 doi: 10.1016/j.intimp.2020.106879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz A, Martínez-Sánchez G, Menassa de Lucía A, Mejía Viana S, Alina Mita C. Complementary Application of the Ozonized Saline Solution in Moderate and Severe Patients with Pneumonia Covid-19: Efficacy and Tolerability. Preprints 2020, 2020060233.
- 31.Hoffmann M, Kleine-Weber H, Krüger N, Müller M, Drosten C, Pöhlmann S. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. bioRxiv. 2020 (Preprint).
- 32.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze M.G. Into the Eye of the Cytokine Storm. Microbiol Mol Biol Rev MMBR. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teo J. Early Detection of Silent Hypoxia in Covid-19 Pneumonia Using Smartphone Pulse Oximetry. J Med Syst. 2020;44:134. doi: 10.1007/s10916-020-01587-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ntyonga-Pono M.P. COVID-19 infection and oxidative stress: an under-explored approach for prevention and treatment? Pan Afr Med J. 2020;35:12. doi: 10.11604/pamj.2020.35.2.22877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan N.C., Weitz J.I. COVID-19 coagulopathy, thrombosis, and bleeding. Blood, The Journal of the American Society of Hematology. 2020;136:381–383. doi: 10.1182/blood.2020007335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terpos E., Ntanasis-Stathopoulos I., Elalamy I., Kastritis E., Sergentanis T.N., Politou M., Psaltopoulou T., Gerotziafas G., Dimopoulos M.A. Hematological findings and complications of COVID-19. Am J Hematol. 2020;95:834–847. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tobaiqy M., Qashqary M., Al-Dahery S., Mujallad A., Hershan A.A., Kamal M.A., Helmi N. Therapeutic management of patients with COVID-19: a systematic review. Infection Prevention in Practice. 2020;2 doi: 10.1016/j.infpip.2020.100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holmes J. Clinical reversal of root caries using ozone, double-blind, randomised, controlled 18-month trial. Gerodontology. 2003;20:106–114. doi: 10.1111/j.1741-2358.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- 40.Hernández F., Menéndez S., Wong R. Decrease of blood cholesterol and stimulation of antioxidative response in cardiopathy patients treated with endovenous ozone therapy. Free Radical Biol Med. 1995;19:115–119. doi: 10.1016/0891-5849(94)00201-t. [DOI] [PubMed] [Google Scholar]
- 41.Clavo B., Pérez J.L., López L., Suárez G., Lloret M., Rodríguez V., Macías D., Santana M., Morera J., Fiuza D., Robaina F., Günderoth M. Effect of ozone therapy on muscle oxygenation. J Altern Complement Med. 2003;9:251–256. doi: 10.1089/10755530360623365. [DOI] [PubMed] [Google Scholar]
- 42.Martínez-Sánchez G., Schwartz A., Donna V.D. Potential Cytoprotective Activity of Ozone Therapy in SARS-CoV-2/COVID-19. Antioxidants (Basel). 2020;9:389. doi: 10.3390/antiox9050389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwartz A. Ozonized Saline Solution (O3SS): Scientific Foundations. Ozone Therapy Global Journal. 2016;6:121–129. [Google Scholar]
- 44.Akerström S., Gunalan V., Keng C.T., Tan Y.J., Mirazimi A. Dual effect of nitric oxide on SARS-CoV replication: viral RNA production and palmitoylation of the Sprotein are affected. Virology. 2009;395:1–9. doi: 10.1016/j.virol.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fernández-Cuadros M.E., Albaladejo-Florín M.J., Peña-Lora D., Álava-Rabasa S., Pérez-Moro O.S. Ozone (O3) and SARS-CoV-2: Physiological Bases and Their Therapeutic Possibilities According to COVID-19 Evolutionary Stage. SN Compr Clin Med. 2020:1–9. doi: 10.1007/s42399-020-00328-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galiè M., Covi V., Tabaracci G., Malatesta M. The Role of Nrf2 in the Antioxidant Cellular Response to Medical Ozone Exposure. Int J Mol Sci. 2019;20(16) doi: 10.3390/ijms20164009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baeza J., Cabo J.R., Gómez M., et al. WFOTs Review on Evidence Based Ozone Therapy. World Federation of Ozone Therapy. 2015:116. [Google Scholar]
- 48.Bocci V., Valacchi G. Nrf2 activation as target to implement therapeutic treatments. Front Chem. 2015;3:4. doi: 10.3389/fchem.2015.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sagai M., Bocci V. Mechanisms of Action Involved in Ozone Therapy: Is healing induced via a mild oxidative stress? Med Gas Res. 2011;1:29. doi: 10.1186/2045-9912-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garg K., Barfield M.E., Pezold M.L., Sadek M., Cayne N.S., Lugo J., Maldonado T.S., Berland T.L., Rockman C.B., Jacobowitz G.R. Arterial thromboembolism associated with COVID-19 and elevated D-dimer levels. J Vasc Surg Cases Innov Tech. 2020;17:348–351. doi: 10.1016/j.jvscit.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chalmers S., Khawaja A., Wieruszewski P.M., Gajic O., Odeyemi Y. Diagnosisand treatment of acute pulmonary inflammation in critically ill patients: the role of inflammatory biomarkers. World J Crit Care Med. 2019;8:59. doi: 10.5492/wjccm.v8.i5.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ulhaq Z.S., Soraya G.V. Interleukin-6 as a potential biomarker of COVID-19 progression. Med Mal Infect. 2020;50:382–383. doi: 10.1016/j.medmal.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rowen R.J. Ozone and oxidation therapies as a solution to the emerging crisis in infectious disease management: a review of current knowledge and experience. Med Gas Res. 2019;9:232–237. doi: 10.4103/2045-9912.273962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ciccullo A., Borghetti A., Zileri Dal Verme L., Tosoni A., Lombardi F., Garcovich M., Biscetti F., Montalto M., Cauda R., Di Giambenedetto S. GEMELLI AGAINST COVID Group. Neutrophil-to-lymphocyte ratio and clinical outcome in COVID-19: a report from the Italian front line. Int J Antimicrob Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.106017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu Z., McGoogan J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 56.Bocci V. (2010) The Clinical Application of Ozonetherapy. In: OZONE. Springer, Dordrecht.
- 57.Inal M., Dokumacioglu A., Ozcelik E., Ucar O. The effects of ozone therapy and coenzyme Q(1)(0) combination on oxidative stress markers in healthy subjects. Ir J Med Sci. 2011;180:703–707. doi: 10.1007/s11845-011-0675-7. [DOI] [PubMed] [Google Scholar]
- 58.Chen C., Zhu Y.H., Huang J.A. Clinical evaluation of potential usefulness of serum lactate dehydrogenase level in follow-up of small cell lung cancer. Journal of cancer research and therapeutics. 2018;14:336. doi: 10.4103/0973-1482.168994. [DOI] [PubMed] [Google Scholar]
- 59.Qiu J., Hs Chen. Efficacy and safety of ozone therapy administered by autologous blood transfusion for acute ischemic stroke: study protocol for a multi-center open-label large-sample parallel randomized controlled trial. Asia Pac J Clin Trials Nerv Syst Dis. 2016;1:37–42. [Google Scholar]
- 60.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou X., Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Y., Gao W., Guo W., Guo Y., Shi M., Dong G., Ge Q., Zhu J., Lu J. Prominent coagulation disorder is closely related to inflammatory response and could be as a prognostic indicator for ICU patients with COVID-19. J Thromb Thrombolysis. 2020;1–8 doi: 10.1007/s11239-020-02174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elvis A.M., Ekta J.S. Ozone therapy: A clinical review. J Nat Sci Biol Med. 2011;2:66–70. doi: 10.4103/0976-9668.82319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gulmen S., Kurtoglu T., Meteoglu I., Kaya S., Okutan H. Ozone therapy as an adjunct to vancomycin enhances bacterial elimination in methicillin resistant Staphylococcus aureus mediastinitis. J Surg Res. 2013;185:64–69. doi: 10.1016/j.jss.2013.05.085. [DOI] [PubMed] [Google Scholar]
- 64.Bocci V., Travagli V., Zanardi I. The failure of HIV vaccines: a new autovaccine may overcome some problems. Med. Hypothesis. 2009;72:662–664. doi: 10.1016/j.mehy.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 65.Bassi P., Sbrascini S., Mattassi R., D'Angelo F., Franchina A. Ozone in the treatment of herpes zoster. Riv Neurobiol. 1982;28:328–333. [PubMed] [Google Scholar]
- 66.Giuliani G., Ricevuti G., Galoforo A., Franzini M. Microbiological aspects of ozone: bactericidal activity and antibiotic/antimicrobial resistance in bacterial strains treated with ozone. Ozone Therapy. 2018;3:7971. [Google Scholar]
- 67.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., Ma K., Xu D., Yu H., Wang H., Wang T., Guo W., Chen J., Ding C., Zhang X., Huang J., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368 doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu J., Zhang S., Wu Z., Shang Y., Dong X., Li G., Zhang L., Chen Y., Ye X., Du H., Liu Y., Wang T., Huang S., Chen L., Wen Z., Qu J., Chen D. Clinical outcomes of COVID-19 in Wuhan, China: a large cohort study. Ann Intensive Care. 2020;10:99. doi: 10.1186/s13613-020-00706-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. The lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu J., Yu H., Zhang S. The indispensable role of chest CT in the detection of coronavirus disease 2019 (COVID-19) European journal of nuclear medicine and molecular imaging. 2020;47:1638–1639. doi: 10.1007/s00259-020-04795-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang Guoquan, Gong Tao, Wang Guangbin, Wang Jianwen, Guo Xinfu, Cai Erpeng, Li Shirong, Li Xiaohu, Yongqiang Yu., Lin Liangjie. Timely Diagnosis and Treatment Shortens the Time to Resolution of Coronavirus Disease (COVID-19) Pneumonia and Lowers the Highest and Last CT Scores From Sequential Chest CT. American Journal of Roentgenology. 2020;215:367–373. doi: 10.2214/AJR.20.23078. [DOI] [PubMed] [Google Scholar]