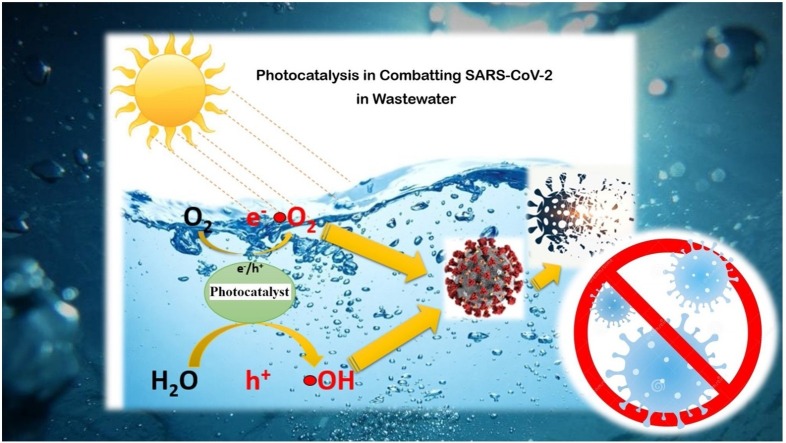
Graphical abstract
Keywords: Photocatalysis, Wastewater, Virus, SARS-CoV-2, COVID-19
Abstract
Photocatalytic technology offers powerful virus disinfection in wastewater via oxidative capability with minimum harmful by-products generation. This review paper aims to provide state-of-the-art photocatalytic technology in battling transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in wastewater. Prior to that, the advantages and limitations of the existing conventional and advanced oxidation processes for virus disinfection in water systems were thoroughly examined. A wide spectrum of virus degradation by various photocatalysts was then considered to understand the potential mechanism for deactivating this deadly virus. The challenges and future perspectives were comprehensively discussed at the end of this review describing the limitations of current photocatalytic technology and suggesting a realistic outlook on advanced photocatalytic technology as a potential solution in dealing with similar upcoming pandemics. The major finding of this review including discovery of a vision on the possible photocatalytic approaches that have been proven to be outstanding against other viruses and subsequently combatting SARS-CoV-2 in wastewater. This review intends to deliver insightful information and discussion on the potential of photocatalysis in battling COVID-19 transmission through wastewater.
1. Introduction
Novel coronavirus disease 2019 (COVID-19) has been declared a pandemic in most countries since the end of March 2020 and has affected 216 countries causing over 2 billion deaths as of 24 January 2021. This COVID-19 is an illness caused by the new coronavirus emerging in Wuhan, Hubei Province, China, in early December 2019. On 11 March 2020, the World Health Organization declared COVID-19 a pandemic [1]. Since the effects of this novel coronavirus are close to those of extreme acute respiratory syndrome (SARS), it has been named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [2]. It is believed that this virus was developed from bats and then moved into other mammalian hosts before jumping to humans. COVID-19 is the third zoonotic epidemic of the twenty-first century, after SARS (2002–2003) and Middle East respiratory syndrome (MERS, 2012) [3].
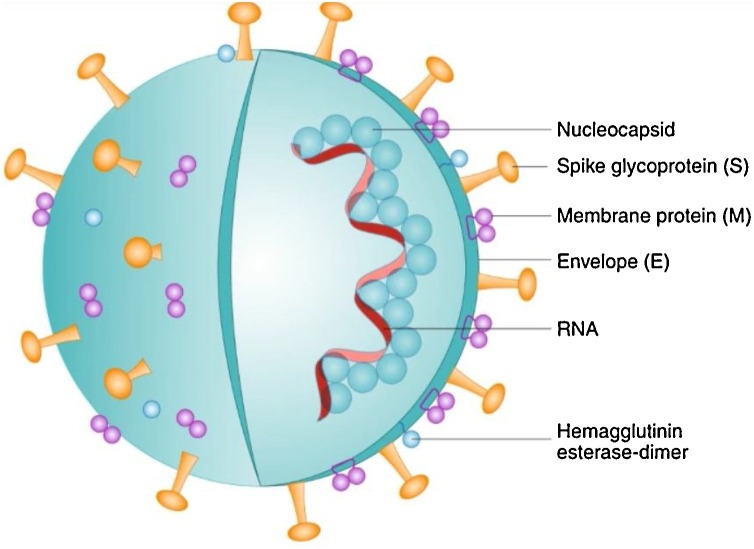
COVID-19 virus is a positive-stranded ribonucleic acid (RNA) virus with a crown-like appearance under an electron microscope due to the presence of spike glycoproteins on the envelope (Fig. 1 ) [4]. In addition, the COVID-19 virus is similar to the SARS virus in 2003 but spread faster. Illness caused by COVID-19 is mostly respiratory disease, while symptoms can be cough, fever, nausea, and diarrhoea [5]. The main route of transmission of the COVID-19 virus is inhalation by person-to-person transmission. Based on its symptoms, the transmission can also occur through respiratory droplets and aerosol transmission. For this reason, researchers have focused on various types of transmission, including wastewater, due to the probability that the virus may affect the wastewater from the hospital waste, such as human faecal from infected persons [6].
Fig. 1.
Schematic diagram of COVID-19 virus structure [4].
Many contaminants can be found in wastewater, especially those discharged from the hospital, such as pharmaceutical residues, chemical substances, radioisotopes, and microbial pathogens [7]. Notably, various viruses have been found in the hospital wastewater such as adenoviruses, hepatitis A (HAV), and polioviruses. This is according to the media that can be the reason for the viruses to be transmitted, which is water media. Since 2003, drainage plumbing systems have been deemed a possible mechanism for spreading the SARS-CoV-1 coronavirus into the sewage system for coronavirus-infected populations living in apartment buildings [8,9]. The SARS-CoV-2 virus, like SARS-CoV-1, can be transmitted by aerosols or microscopic water droplets [10]. In fact, according to van Rowan et al. (2020) [11], the SARS-CoV-2 and SARS-CoV-1 viruses have comparable stability in aerosols on the surface. Viruses may remain viable and contagious on surfaces (for a few days) and in aerosols depending on the inoculum shed (for hours) [11].
To curb and alleviate infectious drinking water, water purification technologies have advanced significantly in the last century [[12], [13], [14], [15], [16]]. The number of waterborne diseases outbreaks, including cholera and typhoid, has declined because of drinking water disinfection. Water disinfection is the elimination, deactivation, or destruction of pathogenic microorganisms from water. Microorganisms are killed or made inactive, putting an end to their development and reproduction. Sterilisation is a disinfection-related process. During the sterilisation process, all present microorganisms, both dangerous and harmless, are destroyed [17].
Researchers in the Netherlands were the first to detect COVID-19 viruses in the hospital wastewater [18]. Later, Wu et al. [19] also found COVID-19 viruses in the hospital wastewater that is believed transmitted from the human faecal matter of infected person. As in many countries in this world, several studies detected the existence of the COVID-19 virus in the human faecal of COVID-19 patients with or without gastrointestinal symptoms [20]. It should be noted that in some countries, they are not discharged properly. These hazardous contaminants, especially viruses, can represent chemical, biological, and physical risks for public and environmental health.
Conventionally, virus disinfection in water and wastewater divided into two main categories which are physical and chemical methods. Physical disinfection including through heating, adsorption and filtration. Virus will be physically removed from water based on size exclusion. However, due to their small sizes and unique properties of the viruses, they are difficult to remove and deactivate. While, chemical disinfection involved the usage of chemicals to disinfect the virus. Chlorination technique is one the most common technique for virus disinfection that using chlorine gas, chloramines or hypochlorite solution [[21], [22], [23], [24]]. Previous study reported chlorination could remove SARS-CoV-1 efficiently [25]. Unfortunately, chlorination was opposed due to generation of mutagenic and carcinogenic disinfection by products. Likewise, chlorination also imparts unpleasant tastes and odours to the water. In battling the pandemic, the performance and environmental safety should be taken into serious consideration.
As the current published research of pandemic COVID-19 is focusing on the occurrence, detection, and transmission of viruses in the environment [[26], [27], [28], [29]], this paper aims to provide state-of-the-art technology in battling transmission of SARS-CoV-2 in wastewater via photocatalytic degradation. The concept and limitation of the existing conventional and current advanced oxidation processes for virus disinfection in water systems were reviewed extensively. A broad range of virus degradation by various photocatalysts was also deliberated to understand the possible mechanism to deactivate this deadly virus. At the end of this review, the challenge and future perspectives are included to provide the limitation of current photocatalytic and suggest a reasonable outlook on developing advanced photocatalytic technology as a promising alternative in handling similar forthcoming pandemics. To the best of our knowledge, this is the first systematic review that delivers insightful information and discussion on the potential of photocatalysis in battling COVID-19 transmission through wastewater.
2. Virus disinfection via conventional methods
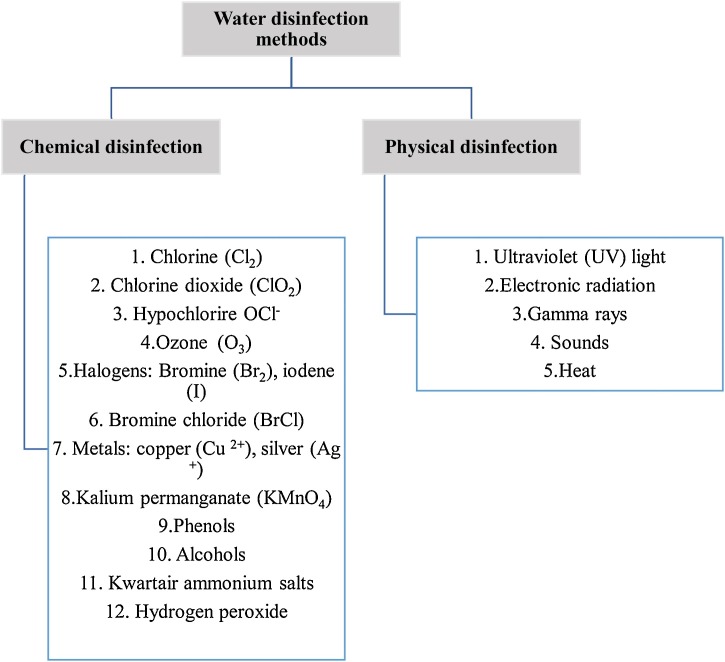
Physical or chemical disinfectants may be used for disinfection. Chemical pollutants of water, which act as carbohydrates or habitat for microorganisms, are often extracted by the disinfectant. Disinfectants should do more than just destroy bacteria. Disinfectants must also have a residual effect, which ensures that they must stay present in the water after disinfection. After disinfection, a disinfectant can deter pathogenic microorganisms from emerging in the pipes and recontaminating the water. Fig. 2 shows the classification of water disinfection methods [17].
Fig. 2.
Classification of water disinfection methods.
Many wastewater treatment methods aim to inactivate pathogenic microorganisms that are toxic to marine organisms. Recent tests, however, have shown that even after water treatment, a measurable number of pathogens existed in effluents. In hospitals and treatment centres, chlorine is one of the most used disinfectants [30]. In addition to destroying most microbial communities, this detergent has a detrimental influence on the ecosystem and may be hazardous to marine organisms if it reaches waterways [31].
Chlorination can also result in nitrosodimethylamine (NDMA) development, which has been linked to human cancer. The chlorination process will react with organic matter, producing trihalometanes (THMs), a carcinogenic compound [30]. Conventional filtration methods for wastewater treatment are unsuccessful at eliminating micropollutants like viruses. Furthermore, certain bacteria have been discovered to be immune to such chemical materials [32]. These results are in line with the findings of Al-Gheethi et al., who discovered the existence of viable microbial cells even after the treatment phase [33].
The use of solar-based disinfection (SODIS) technology, especially in water and wastewater disinfection, is a promising approach. This system is ideal because of the abundance of high solar radiation, low capital expense, and long-term feasibility. Meanwhile, nanotechnology’s use in wastewater treatment has also been recorded in the literature. Noman et al., for example, looked at how bimetallic bionanoparticles inactivated antibiotic-resistant E. coli (Gram-negative) and Staphylococcus aureus (Gram-positive) bacteria seeded in greywater [34]. According to the inactivation mechanism, the bacterial cells were inactivated due to disruption to the bacterial cell wall’s carbohydrates and protein structures [35]. The C—C bonds of the functional groups found in the bacterial cell wall were broken. The combination of SODIS and nanotechnology could result in a novel disinfection method for inactivating human viruses. The most popular nanoparticles used for wastewater disinfection are ZnO, which are more efficient when exposed to sunlight, theoretically increasing antiviral activity [36].
As specified by ISO 13408-1, sterility assurance level (SAL) is used to characterise the killing efficiency of a treatment process, with the treatment process being very efficient if the SAL is very poor. Log reduction accepted by the United States Environmental Protection Agency (USEPA) is a term widely used to measure the efficiency of disinfection processes [37]. SAL is generally written as 10n. Based on the pathogen’s initial concentration, a 103 or 106 value is most often used for sterilisation. The log reduction is (101), which represents a 90 % reduction in the microbial community. The microbial population falls from one million (106) to almost zero, or a reduction of 99.9999 %, when disinfection with a 6-log reduction (106) is used [38].
A kill rate of 99.99 % is measured by a four-log reduction. The growth of inactive bacteria should be calculated as an indication for inactive viruses to ensure that the removal of treated effluent is healthy. If no growth can be seen in the culture medium after incubation, these cells are designated as destroyed [39]. However, the capacity of microbial cells to resuscitate can be affected by the storage conditions of disinfected samples. Salmonella, S. aureus, and Enterococcus faecalis were resuscitated in sewage samples treated with solar disinfection (SODIS) for 6 h and deposited at 37 °C for 4 days. The capacity of the disinfection process to damage the bacteria’s cell walls can enable cells to survive. In this situation, a pathogen growth potential (PGP) bioassay must be performed [40]. Table 1 summarises the water disinfection process, with reviews of the advantages and disadvantages.
Table 1.
Summary of water disinfection methods.
| Methods | Advantages | Limitations | References |
|---|---|---|---|
| Chlorine gas |
|
|
[21] |
| Chlorination (sodium hypochlorite solution) |
|
|
[22] |
| Chlorination (solid calcium hypochlorite) |
|
|
[23] |
| Chloramines |
|
|
[24] |
| Ozonation |
|
|
[41] |
| Ultraviolet (UV) light |
|
|
[42] |
| Photocatalytic disinfection |
|
|
[43] |
3. Virus disinfection via an advanced oxidation process
Advanced oxidation processes are a recently discovered technology for the disinfection of viruses in polluted wastewater by generating reactive oxygen species such as hydroxyl radicals as an oxidising agent to treat harmful pathogens. The generation of radicals may be initiated by primary oxidants such as hydrogen peroxide, ozone, sources of energy (UV light, ultrasonic and heat), or catalysts such as titania, iron oxide, or other semiconductors. Hydroxyl radicals are known to rapidly and unselectively bombard organic molecules. The generated radicals degrade the organic molecules found on the virus cell wall and directly deteriorate the virus pathogens. The advanced oxidation processes include ozonation, ultrasound, Fenton process, and photocatalysis, have been effectively and efficiently utilised in treating virus-contaminated water. The methods offer other diverse approaches to the production of hydroxyl radicals that make it more flexible and therefore provide a better approach to complying with strict guidelines during wastewater treatment.
Ozonation is a common technique in virus disinfection from wastewater that utilises three oxygen atoms (O3) called ozone into the water. Ozone presents in the form of gas, which is one of the most potent oxidising agents. When ozone is dissolved in water, it produces a broad spectrum of reactive oxygen radicals (ROS) that could oxidise organic materials in virus membranes, which destroys the cell wall and leads to cell bursting, causing immediate degradation of the viruses [44]. Previous studies reported that the virus could be removed from wastewater by ozonation [45,46]. Furthermore, ozone is highly reactive and must be directly used after onsite generation and difficult to store [47]. Ozonation in wastewater could also result in the formation of harmful by-products such as aldehydes, carboxylic acids, and bromate when reacting with dissolved organic matter and bromide [45].
Ultrasound is a longitudinal wave with frequencies in the range of 20–106 kHz [48]. This frequency is above the human hearing range (20 Hz to 20 kHz) but below the mega-sonic range (>600 kHz) [49]. This technique has attracted wide attention in water and sewage system due to simplicity, high decomposition speed, and zero secondary pollution. Ultrasound is evaluated as one of the AOP for the degradation of pollutants in the water system due to the formation of free hydroxyl radicals with oxidising capabilities [50,51]. Ultrasound technique was reported to disinfect microorganisms and viruses by several mechanisms based on acoustic cavitation [49,52,53]. Cavitation is the phenomenon of microbubbles or cavities forming, growing, and collapsing in a liquid in extremely short time intervals (milliseconds) [49]. The mechanism involved are: (1) the viruses could be chemically disinfected by bombarded with hydroxyl radicals generated via ultrasound, (2) the virus could be physically attacked by the high temperature and pressure that results from the momentum of bubble collapse that can kill the viruses, and (3) shear forces that persuaded by microstreaming that could damage the virus. Ultrasound technique can be applied as a stand-alone process or integrated with other disinfection techniques such as chlorination, heating, ozonation, and UV irradiation [53,54]. However, the utilisation of ultrasound technique in water treatment requires high operating and maintaining costs due to the high energy consumption and replacement of instruments like the ultrasound probe, which continue to be managed by the ultrasonic activity itself [55].
The Fenton process is the reaction between aqueous ferrous ions and hydrogen peroxide to generate hydroxyl radicals in acidic conditions. Fenton’s reagent was first discovered in 1894 by Henry John Horstman Fenton through the oxidation of tartaric acid by activating hydrogen peroxide into hydroxyl radicals and hydroxide ions by Fe2+ [56]. Later only in the late 1960s, the Fenton process successfully destroyed hazardous organic pollutants in wastewater by radical oxidation and flocculation [56]. This process is considered one of the most effective AOP in removing organic pollutants and microbial disinfection in wastewater application. The main benefits of this process in disinfection treatment are: (1) both reagents (iron and hydrogen peroxide) are non-toxic and cheap, (2) harmless by-products generation as in chlorination and ozonation, and (3) no mass transfer limitation. However, there are several limitations suffered by the Fenton process, such as (1) high consumption of hydrogen peroxide, (2) strict pH range, (3) sludge generation, and (4) the accumulation of ferric sludge that could affect the oxidation performance. Therefore, several modifications to Fenton reagent were made to overcome the limitation of the traditional Fenton reagent, such as integration with external energy and development of heterogeneous Fenton process to ensure efficient and sustainable water purification [57]. Nieto-Juarez et al. investigated the inactivation of MS2 virus by iron hydroxide mediated Fenton-like process under the sunlight and in the dark [58]. They found viruses can be physically removed from water as well as inactivated by adsorption and a particle-mediated photo-Fenton-like process using heterogeneous Fenton-like processes.
According to the above description, photocatalytic disinfection is the most effective procedure for water treatment. Mass transfer limitations must be minimised for efficient TiO2 water treatment because photocatalytic degradation occurs primarily on the surface of TiO2. Organic pollutants adsorb poorly on TiO2 surface due to their low affinity for organic pollutants (particularly hydrophobic organic pollutants), resulting in slow photocatalytic degradation rates. As a result, pollutant targeting around TiO2 nanoparticles to improve photocatalytic performance must be considered [59].
Furthermore, due to the instability of the nanosized particle, TiO2 nanoparticles can aggregate, obstructing light incidence on the active centres and reducing catalytic activity. However, it should be noted that those small particles can experience greater scattering, which may reduce their photocatalytic activity compared to larger particles. Furthermore, one major practical obstacle for the slurry method is to extract the nanosized TiO2 particles from the treated water, which is both an economic and a safety issue [60]. Previous studies have used the following countermeasures to resolve the weaknesses of TiO2 dependent photocatalysis:
1. Changes to the TiO2 catalyst to allow visible light to be used [61].
2. Catalyst synthesis should be designed to produce catalysts with well-defined crystal structures, high affinity for different organic pollutants, and smaller particle sizes [62].
3. Design and develop a second generation TiO2 catalyst with high separation efficiency and the ability to be recovered and regenerated [63].
These modifications and advancements aim to improve photocatalytic performance, complete organic pollutant degradation, visible light absorption, stability, reproducibility, and TiO2 recycle and reuse capabilities. The following section will focus on photocatalytic action as a disinfection process for water treatment.
4. Virus disinfection via photocatalysis
Photocatalysts are semiconductor oxides that serve as heterogeneous catalysts in the presence of electromagnetic radiation. They act as a medium to decompose living or non-living microstructures accumulated on any surface or suspended in liquid or gases and come into contact with a solid surface. Photocatalysts may also use photocatalytic reactions to break the water and create hydrogen. The phenomenon mentioned above occurs due to a process known as photocatalytic oxidation and reduction [60]. The photocatalytic process involves three main stages: (1) formation of photoinduced charge carrier, (2) separation of charge carrier and distribution to the surface of the photocatalyst, and (3) oxidation and reduction reaction on the surface of the photocatalyst [13].
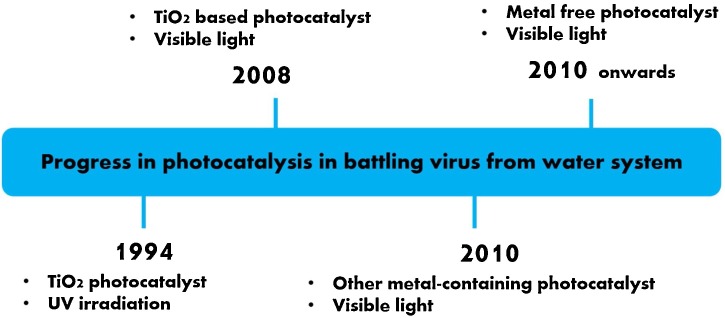
As illustrated in Fig. 3 , Zhang et al. [64] suggested the progress on photocatalysis in battling virus from the water system. Virus disinfection in water by photocatalysis was pioneered by Sierka and Sjogren in 1994 [44]. They found MS2 viruses successfully disinfected by TiO2 photocatalyst under UV irradiation. In 2008, most research works focused on developing TiO2-based photocatalyst that can deactivate viruses under visible light irradiation. Later, the potential of various metals other than TiO2, such as iron oxide [65], silver [66], alumina [66], and copper oxide [67], was investigated for virus disinfection under visible light. Since then, metal-free photocatalyst like carbon-based photocatalyst with antiviral properties was further explored to obtain cheap, safe, and sustainable materials for viruses disinfection in the water.
Fig. 3.
Progress in photocatalysis in battling virus from the water system. Reproduced with permission from Elsevier, [64].
4.1. Performance evaluation on virus disinfection via photocatalysis
Earlier, TiO2 photocatalyst has shown great potential as a solution for sewage and wastewater treatment because it is non-toxic, cheap, and abundantly available. TiO2 photocatalysts successfully deactivated viruses like phage MS2, bacteriophage Qβ, phage f2, murine norovirus, and human adenovirus [[68], [69], [70], [71], [72]]. Viruses disinfection by photocatalyst could overcome the drawbacks of the conventional disinfection methods, such as the generation of harmful by-products and utilisation of large volumes of chemicals. However, there are main limitations suffered by TiO2 photocatalyst, which are lower bandgap, poor capability for carrier charge separation that results in incompetent exploitation of visible light, and low photodegradation performances. Later, coupling TiO2 photocatalyst with other metals such as manganese (Mn) [73], palladium [74], silver oxide (AgO), copper [75,76] and copper oxide [67] to form heterojunction photocatalyst could further enhance the photocatalytic activities on virus degradation through visible light irradiation.
The technology of photocatalysis that employs carbon-based photocatalyst has attracted much attention due to the zero risk of metal leaching into the water system and optimum natural light-harvesting capability [[77], [78], [79]]. The non-metal photocatalysts that have been developed for virus disinfection include carbon-based materials such fullerene [80,81] carbon nanotube [82], carbon dot [83,84], and graphitic carbon nitride (g-C3N4) [78,85,86].
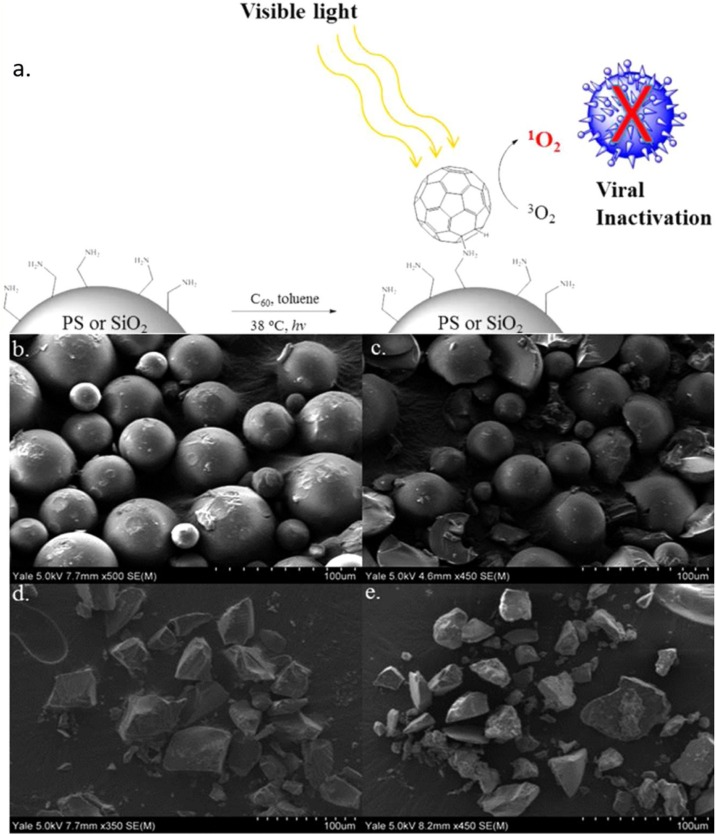
Fullerene (C60) is a spherical carbon-based molecule made up of carbon atoms kept together by sp2 hybridisation. Fullerenes have a peculiar three-dimensional structure and have high chemical stability. They also have a large specific surface area and strong electrical conductivity. Fullerene offers the generation of ROS under visible light due to its small gap between the highest occupied molecular orbital and lowest occupied molecular orbital, which is approximately 1.6–1.9 eV [87]. A previous study reported that the suspensions of polyhydroxylated fullerene successfully inactivated the non-enveloped viruses like MS2 bacteriophage, enterobacteria phage PRD1, and bacteriophage T7 under UVA irradiation [88]. Hotze et al., determined that the virus inactivation by fullerene material is mainly based on the production of singlet oxygen (1O2), one of the ROS by fullerene aggregates, and resistance of the viruses determined by the structural and composition of non-enveloped virus capsids. However, the potential of fullerene as a photocatalyst in wastewater treatment was limited to the aggregation of the nanoscale particles that reduces the photochemical properties. Therefore, immobilisation of fullerene appeared as the most practical approach to retain the photoactivity in the aqueous system. Moor et al. managed to immobilise fullerene on silica gel and polystyrene resin by the simple nucleophilic addition of a primary amine across a [6,6] fullerene double bond and followed by proton transfer under mild condition as shown in Fig. 4 (a) [80]. Based on the SEM images in Fig. 4(b–e), Moor et al., suggested the surface of PS resin and silica gel were covered by monolayer coverage of fullerene and did not exhibit significant aggregation. The immobilisation of fullerene on solid materials also promoted the production of 1O2 in the water under visible light irradiation and deactivated MS2 bacteriophages without exhibiting significant loss of photocatalytic activity after repeated cycles. Besides that C60 fullerene, the immobilised C70 fullerene on MCM-41 also displays antiviral properties towards MS2 in the water system under visible light irradiation [81].
Fig. 4.
(a) Schematic illustration on immobilization of fullerene on PS or SiO2 and SEM images of (b) neat PS resin, (c) C60 coated on PS resin, (d) neat SiO2 gel and (e) C60 coated on SiO2 gel [80].
In contrast, g-C3N4 can be synthesised directly from earth-rich, low-cost precursors rich in nitrogen, e.g., heating the melamine [79]. The g-C3N4 consists of organic elements such as carbon, nitrogen, and hydrogen and has the ability to break water and produce hydrogen under visible light irradiation, making it a non-metal catalyst and widely utilised for water treatment [[89], [90], [91]]. To date, g-C3N4 was documented for having antimicrobial and antivirus properties through photocatalytic degradation [92]. The bandgap of g-C3N4 is 2.7 eV that is appropriate for visible-light-driven photocatalyst with a conductive band of −1.1 eV and valence band of +1.6 eV, and normal hydrogen electrode as reference [93].
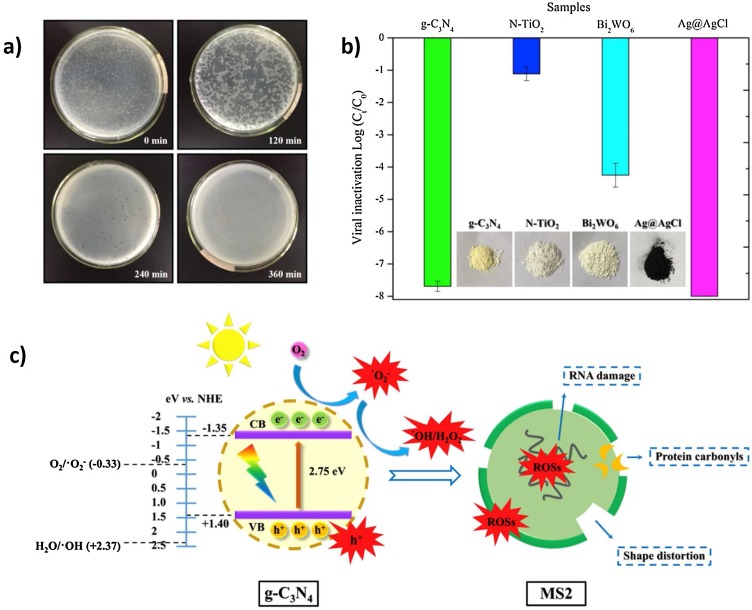
To evaluate the photocatalytic inefficient of g-C3N4 for virus disinfection under visible light irradiation, Li et al. used bacteriophage MS2 as a model virus [78]. As depicted in Fig. 5 (a), bacteriophage MS2 was completely inactivated within 360 min under visible light irradiation. The regrowth test was also conducted in the dark for 72 h. No visible plaques formed, indicating that g-C3N4 had inactivated the virus through the photocatalysis process. As shown in Fig. 5(b), Li et al. also compared the performance of g-C3N4 with other metal-based visible-driven photocatalysts such as nitrogen doped TiO2 (N-TiO2), Bi2WO6 and Ag@AgCl for viral deactivation. They revealed that more than 7-log of MS2 were deactivated by g-C3N4, whereas about 1-log and 4-log of MS2 were deactivated by N-TiO2 and Bi2WO6 photocatalyst, respectively. The Ag@AgCl recorded the highest inactivation of MS2, which resulted from the presence of biocidal silver ions and silver nanoparticles that performed as an electron sink to expedite charge separation and enhance the photon harvesting [94]. However, the application of silver-based photocatalyst is costly and may pose health risks in the treated water due to the dissolution of Ag+ and Ag nanoparticles. As depicted in Fig. 5(c), the degradation mechanism of MS2 by g-C3N4 under visible light irradiation mainly involved the oxidation damage on the surface protein of the virus by ROS that resulted in the leakage and shape distortion which finally led to the rapid destruction of genetic materials namely RNA and caused the viral death with no regrowth.
Fig. 5.
(a) Images of MS2 plaques formation before and after photocatalytic disinfection by g-C3N4 under visible light irradiation, (b) comparison of photocatalytic performance on MS2 inactivation under visible light irradiation, (c) schematic diagram of proposed mechanism on MS2 inactivation by g-C3N4 photocatalyst [78].
4.2. Mechanism of photocatalysis on virus disinfection
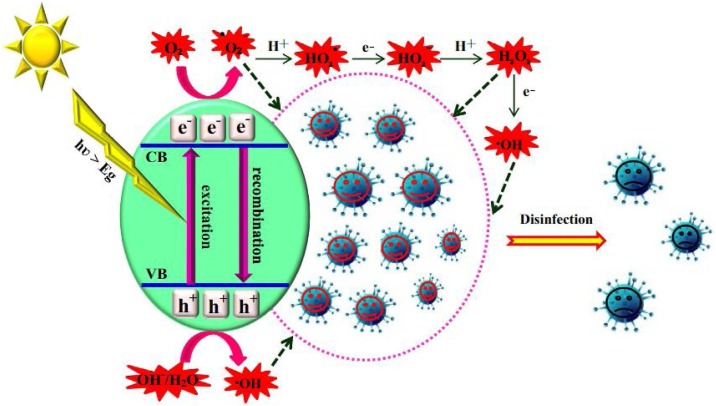
It was first to observe the photocatalysis mechanism by TiO2 to inactivate the virus by destroying the shell and/or capsid of viruses. As a result, genetic materials, minerals, and proteins were released inside the viruses and caused the virus to inactivate. Herein, it should be noted that the photocatalysis mechanism of the virus occurred on the surface of the film and can be explained by photodegradation of the protein capsid of the virus and subsequently efflux of the viral RNA enveloped by the protein layer. When virus reacts with the surface of the catalyst, active radicals such as O2 •‾, HOO•, and HO• formed and oxidised the C—H bonds and degraded viruses [95]. It is well known that most photocatalysts are semiconductor materials. The photocatalytic process based on semiconductor materials can be briefly described as shown in Fig. 6 . When the semiconductor is irradiated by light, photogenerated electron (e−) and hole (h+) are generated and react with other substances to form ROS, including •OH, H2O2, H+, and •O2 −, and these ROS participate in the photocatalytic degradation bacteria process. Afterwards, reactive oxygen species attack the cell membrane. The coenzyme A on the cell membrane is damaged, resulting in inhibition of respiration dependent on the intact cell membrane, reduction or loss of cellular respiration activity, and eventually cause cell death.
Fig. 6.
Virus inactivation process through photocatalysis [95].
To view the potential of photocatalysis as an alternative solution for battling COVID-19, understanding the real mechanism of deactivation and destroying the microorganisms, especially coronaviruses, during photocatalytic disinfection is vitally important. It might be helpful to develop a more efficient and powerful photocatalyst by (1) designing the morphology according to virology, (2) hybridising or functionalising with transition metals ions, and (3) fabricating composites or heterogeneous photocatalyst for efficient energy utilisation and recovery.
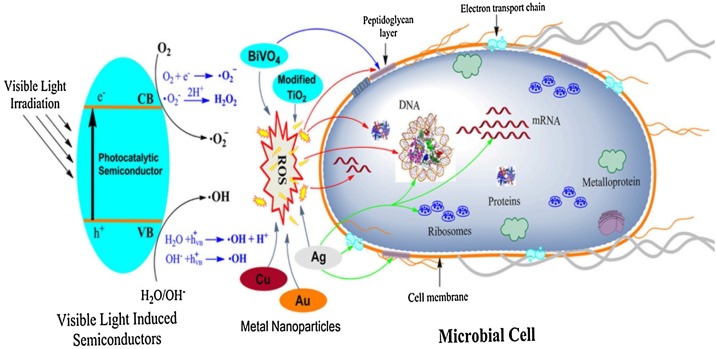
Typically, microorganisms contain outer membrane, peptidoglycan, and cytoplasmic membranes responsible for structural integrity and retention. The membranes surround an internal liquid-based cytoplasmic matrix that is comprised of genetic material and biochemical systems. Based on the complexity of the microorganisms, the complete mechanism of their degradation by photocatalytic remains partially known. As illustrated in Fig. 7 , Regmi et al. reviewed the possible mechanism of photocatalytic degradation by semiconductor and nanoparticles photocatalyst on microbial cells in wastewater environment involving (1) oxidative stress induction; where the excess generation of ROS leads to peroxidation of the lipid membrane and protein attack that depresses the activity of some periplasmic enzyme and directly interact and damage the genetic materials, (2) metal ion release; the metal ions could exempt from semiconductor photocatalyst then passed through the cell membrane and directly reacted with the functional groups of nucleic acid and protein such as −COOH, –NH, and –SH, before finally destroying them, (3) non-oxidative mechanism; reducing the critical cellular metabolism such as amino acid, protein, nucleotide, and carbohydrate metabolism without oxidative stress induction [96].
Fig. 7.
Schematic illustration of the proposed mechanisms of microbial disinfection by difference semiconductor photocatalysts through activation of semiconductor by visible light, then generation of ROS by various semiconductors followed by the release of metal ions targets generic materials like mRNA, deoxyribonucleic acid (DNA), and ribosomes (The blue color arrow indicates targets of bismuth vanadate, BiVO4. The green color arrow indicates targets of Ag nanoparticle) [96].
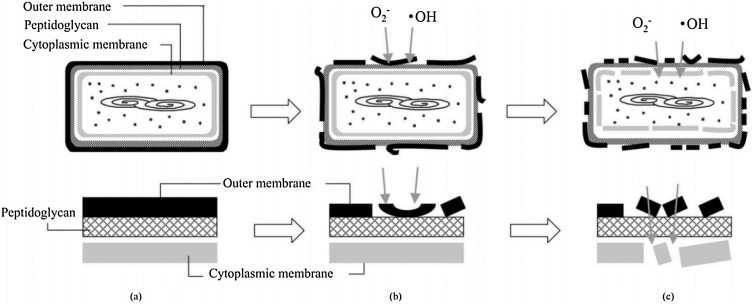
Nevertheless, only the first mechanism has gained the interest of the researchers, which involved the generation of ROS that plays a major role in virus disinfection. Researchers strongly agreed that the main degradation of microorganism is initiated by the prolonged ROS attack results on the damage of the cell wall, followed by the cytoplasmic membrane and direct attack of intracellular components comprising genetic materials within the microorganism as depicted in Fig. 8 [97].
Fig. 8.
Schematic diagram of the disinfection of E. coli by photoactivation of TiO2 photocatalyst; (a) before disinfection, (b) ROS attack results in damage of the outer membrane cell wall, (c) prolonged ROS attack results in degradation of peptidoglycan, cytoplasmic membrane and direct DNA damage [97].
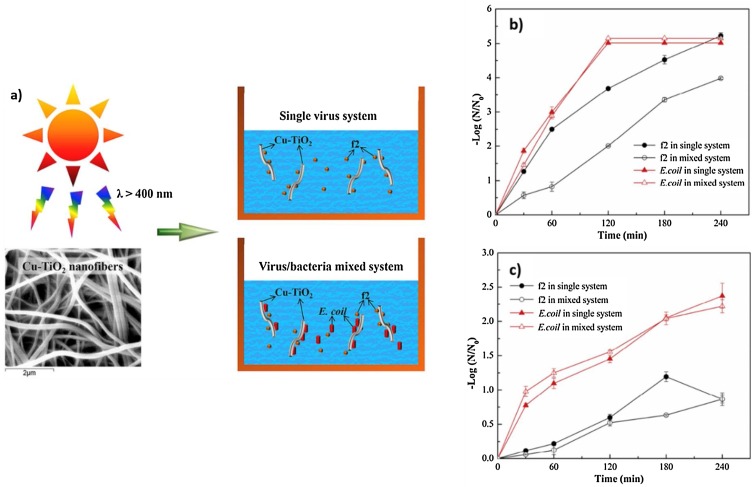
Viruses and their host bacteria typically co-exist in actual water conditions. Therefore, the efficiency of photocatalytic inactivation in the mixed system of virus/bacteria is of practical importance. For instance, Zheng et al. fabricated Cu–TiO2 nanofibres for the removal of virus bacteriophage f2 and bacteria E. coli 285 [75] (Fig. 9 (a)). They compared the inactivation of both virus and bacteria in a single and mixed systems with the presence and absence of source light. Under visible light, both E. coli and bacteriophage f2 were inactivated completely within 240 min, as shown in Fig. 9(b). This indicates bacteriophage f2 is more resistant to photocatalytic oxidation in the virus/bacteria mixed system than E.coli 286. However, in the virus/bacteria mixed system with the absence of source light, the removal efficiency of bacteriophage f2 decreased significantly compared to that in the single virus system, as depicted in Fig. 9(c). It can be assumed that free ROSs play an important role in phage f2 inactivation and in the bulk phase.
Fig. 9.
(a) Photocatalysis activity of Cu-TiO2 nanofibers in single virus system and virus/bacteria mixed system, (b) photocatalytic performance under visible light irradiation, and (c) photocatalytic performance without light irradiation [75].
Virus disinfection in the water system by photocatalysis is also affected by the presence of natural organic molecules. Besides bacteria, the actual water system also contains natural organic matters (NOMs) such as nucleic acids, carbohydrates, and proteins. Their presence could quench the generated ROS and serve as disinfectants [98]. Therefore, the disinfection of viruses must also consider the presence of NOMs. To further explore this situation, Cheng et al. utilised humic acid as NOM representative and Cu–TiO2 nanofibers as a photocatalyst [76]. They found that when the concentration of humic acid was increased from 0 to 15 mg/L, the removal efficiency of bacteriophage f2 declined from 5.00- to 1.89-log. This is due to the presence of humic acid that prevented the viral photocatalytic efficiency of the photocatalyst. It could be inferred that the presence of humic acid also dramatically reduces its stability. Particularly, for the practical application of virus photocatalytic disinfection technology, the existence of NOMs is a non-negligible problem, and more relevant research should be conducted to gain a better understanding of this subject.
5. Challenges and future perspectives
The impressive performance of the virus inactivation through the photocatalysis process in water, as summarised in Table 2 , has proven the capability of photocatalysis to disinfect various harmful viruses. However, there are several challenges and barriers in the disinfection of viruses by photocatalysis, especially in battling them in wastewater. First, the difficulty in recovery of the suspended photocatalyst from the solution. Because of the possible toxicity of nanosized photocatalysts and the photocatalysts functionalised with other carcinogen substances, the powdery-shaped and suspended nanosized photocatalysts must be removed before the treated water is reused or released into the environment The photocatalytic disinfection of the virus could also be more harmful if the released photocatalyst has not gone through a complete reaction, where disinfection has still not occurred, and the photocatalyst is still holding the adsorbed harmful viruses. Second, in real water applications, the nanosized photocatalysts are brittle and prone to aggregation. Aggregation of photocatalyst could hinder the active surface area and reduce the photocatalytic performance. Therefore, those barriers must be overcome if photocatalytic degradation is to be used independently in wastewater treatment.
Table 2.
Summary of virus inactivation through photocatalysis in water.
| Photocatalyst | Viruses | Light source | Virus inactivation efficiency | References |
|---|---|---|---|---|
| TiO2 | Phage MS2 | UV | 2.8-log in 65 min | [44] |
| TiO2 | Bacteriophage Qβ | UV | 3.5-log in 2 min | [69] |
| TiO2 | Phage MS2 | 18 W black light blue (BLB) lamp | 1.8-log in 180 min | [68] |
| TiO2 films | Influenza virus H9N2 | UV | 4-log in 150 min | [100] |
| TiO2 | Phage f2 | 6 W black light lamp | 6-log in 15 min | [70] |
| TiO2 | MS-2 bacteriophage | 4 W BLB lamp | 2-log in 109 min | [71] |
| TiO2 | Phage f2 | 4 W UV | 5−6-log in 160 min | [101] |
| TiO2 | Murine norovirus | UV | 3.3-log in 24 h | [72] |
| TiO2 P25 | Human adenovirus | UV | 0.49-log in 14.3 min | [98] |
| Palladium-modified nitrogen-doped titanium oxide fiber (TiON/PdO) | Phage MS2 | Xe arc lamp | 1.2-log in 60 min | [74] |
| Cu-TiO2 nanofibers | Bacteriophage f2 | Xe lamp | 4.0-log in 120 min | [75] |
| Cu-TiO2 nanofibers | Bacteriophage f2 | Xe lamp | > 5-log in 240 min | [76] |
| Mn-TiO2 | Phage MS2 | 150 W Xe ozone-free lamp | 4-log in 60 min | [73] |
| TiO2/CuO films | Phage T4 | 40 W UVA lamp | 9.9-log in 180 min | [67] |
| SiO2-TiO2 | Phage MS2 | 8 W UVA lamp | 5-log in 1.8 min | [102] |
| nAg/TiO2 | Phage MS2 | 8 W UVA lamp | 9.9-log in 180 min | [103] |
| Ag-AgI/ Al2O3 | Human rotavirus Wa | Visible | 3.2-log in 40 min | [66] |
| Pt-WO3 | Influenza virus H1N1 | Visible | > 5.5-log in 120 min | [104] |
| FeO | Phage MS2 | Simulated solar | 5-log in 30 min | [65] |
| g-C3N4 | Phage MS2 | 300 W Xe lamp | 8-log in 300 min | [78] |
| C60/SiO2 | Phage MS2 | UV | 3.55-log in 75 min | [80] |
| Fluorescent | 2.8-log in 75 min | |||
| C70/SiO2 | Phage MS2 | Sunlight | 4.4-log in 90 min | [81] |
| Visible | 4.35-log in 90 min | |||
| Rh-SrTiO3 | Phage Qβ | Vis | 5-log in 120 min | [105] |
| g-C3N4 with H2O2 | Human adenoviruses | Visible | 2.6-log in 150 min | [85] |
| g-C3N4 | Phage MS2 | Visible | 8.0-log in 240 min | [86] |
| g-C3N4/ expanded perlite | Phage MS2 | Visible | 5.8-log in 420 min | [92] |
To address the above challenges, immobilising the photocatalyst into porous or floating substrate could solve the recovery and agglomeration issues of the suspended photocatalysts in the aqueous system [13,67,92]. The porous substrate could be an organic or inorganic membrane leading to the development of a bifunctional photocatalytic membrane that acts as a filter and photocatalyst in the same chamber. However, incorporating the photocatalyst in the substrate might result in the sedimentation of the photocatalyst at the bottom of the substrate. This condition could hinder the maximum light utilisation from activating the photocatalyst. Hence, modification or functionalisation of the photocatalyst and the substrate can be adapted to guarantee the photocatalyst remains at the top surface of the substrate. Other than that, utilisation of translucent or transparent substrate might solve the source light utilisation.
Besides that, the application of electrospun nanofibrous photocatalyst also attracted much attention in virus disinfection in water systems due to their super porosity and high surface area to volume ratio [74,76,99]. Nevertheless, electrospun nanofibrous photocatalysts are broadly acknowledged as brittle and fragile, and they can collapse easily due to their large pore size. This condition makes them not suitable to be used in water treatment for long-term application. Post-treatment after fabrication of nanofibrous photocatalytic could enhance the flexibility and mechanical strength of the nanofibrous photocatalyst.
Apart from that, viruses were reported to have stronger resistance towards photocatalytic disinfection than other microbes such as bacteria due to their differences in structural and geometric properties [75]. Unlike bacteria, viruses are the smallest and tiniest germ that can spread easily through the air that can cause various diseases with specifically targeted cells. For example, when a certain virus gets inside a host, it can hijack the specific cellular machinery like blood, respiratory system, liver, or other organs to generate clones of itself, overtaking more cells, continuing to reproduce, and finally destroying the targeted cells and harming the body. In the case of COVID-19, when SARS-CoV-2 gets inside the human body, it specifically attacks the upper and the lower respiratory system like sinuses, nose, throat, windpipe, and lungs before damaging the organs and terminating the whole body. The recent finding through protein simulation revealed that the active SARS-CoV-2 spike protein is more stable than the active SARS-CoV-1 spike protein [106]. In comparison, SARS-CoV-1 moves faster, active, and nonactive, requiring a longer time to attach to human cell because of their instability. Meanwhile, SARS-CoV-2 are more stable and ready to attack which makes the COVID-19 much easily transmitted among human compared to MERS and SARS-CoV-1. The detection of the variant of SARS-CoV-2 through mutation also increases global awareness for improving current wastewater treatment.
Therefore, it is urgently crucial to develop an advanced remedy to deactivate the targeted viruses, especially SARS-CoV-2 in the water system. Development of smart or intelligent photocatalytic membrane could specifically exclude, adsorb, and photocatalytically degrade the virus according to their structural and geometric properties. By integrating stimuli-responsive materials that act as automatic doors by flexible adjustment of pore sizes and surface properties in response to the size or biological properties of the targeted viruses, the intelligent or smart membrane could overcome the bottlenecks of current photocatalytic technology.
6. Conclusion
In conclusion, there has been a significant rise in evidence suggesting the presence of pathogenic novel SARS-CoV-2 in wastewater. Throughout this review, the articles on various viruses disinfection by photocatalyst were evaluated extensively. It is discovered that in the presence of electromagnetic radiation, photocatalysts degrade any microorganism that has spread through surfaces or polluted the air, including the current deadly COVID-19 virus. There are only a few photocatalysts that operate successfully in the presence of UV radiation at the moment. The scientific work on improving the performance of photocatalysts in the presence of visible spectrum radiation, such as solar radiation, is still ongoing. However, the photocatalysts’ stability and protection must be assured before being used in the public domain. If these limitations are overcome, photocatalysis can become a more effective weapon in the fight against the virus’s spread. It is hoped that photocatalysts will be commercialised and adopted on a wide scale in the near future to clean up contamination and kill deadly species like coronavirus.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported/funded by the Ministry of Science, Technology and Innovation, Malaysia under the International Collaboration Fund (IF0120I1176). The authors also would like to acknowledge the financial support provided by Universiti Teknologi Malaysia under Professional Development Research Management grant, R.J130000.7113.04E66, Ministry of Higher Education, Malaysia under HICoE grant, R.J090301.7851.4J433.
References
- 1.He Y., Sutton N.B., Rijnaarts H.H.H., Langenhoff A.A.M. Degradation of pharmaceuticals in wastewater using immobilized TiO2 photocatalysis under simulated solar irradiation. Appl. Catal. B Environ. 2016;182:132–141. doi: 10.1016/j.apcatb.2015.09.015. [DOI] [Google Scholar]
- 2.Shakil M.H., Munim Z.H., Tasnia M., Sarowar S. COVID-19 and the environment: a critical review and research agenda. Sci. Total Environ. 2020;745 doi: 10.1016/j.scitotenv.2020.141022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gogniat G., Dukan S. TiO2 photocatalysis causes DNA damage via fenton reaction-generated hydroxyl radicals during the recovery period. Appl. Environ. Microbiol. 2007;73:7740–7743. doi: 10.1128/AEM.01079-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Florindo H.F., Kleiner R., Vaskovich-Koubi D., Acúrcio R.C., Carreira B., Yeini E., Tiram G., Liubomirski Y., Satchi-Fainaro R. Immune-mediated approaches against COVID-19. Nat. Nanotechnol. 2020;15:630–645. doi: 10.1038/s41565-020-0732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal–oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol. Hepatol. 2020;5:335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A., Diaz G., Cohn A., Fox L., Patel A., Gerber S.I., Kim L., Tong S., Lu X., Lindstrom S., Pallansch M.A., Weldon W.C., Biggs H.M., Uyeki T.M., Pillai S.K. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Achak M., Alaoui Bakri S., Chhiti Y., M’Hamdi Alaoui F.E., Barka N., Boumya W. SARS-CoV-2 in hospital wastewater during outbreak of COVID-19: a review on detection, survival and disinfection technologies. Sci. Total Environ. 2021;761 doi: 10.1016/j.scitotenv.2020.143192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X.W., Li J.S., Jin M., Zhen B., Kong Q.X., Song N., Xiao W.J., Yin J., Wei W., Wang G.J., Si B.Y., Guo B.Z., Liu C., Ou G.R., Wang M.N., Fang T.Y., Chao F.H., Li J.W. Study on the resistance of severe acute respiratory syndrome-associated coronavirus. J. Virol. Methods. 2005;126:171–177. doi: 10.1016/j.jviromet.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markel T.A., Gormley T., Greeley D., Ostojic J., Wise A., Rajala J., Bharadwaj R., Wagner J. Hats off: a study of different operating room headgear assessed by environmental quality indicators. J. Am. Coll. Surg. 2017;225:573–581. doi: 10.1016/j.jamcollsurg.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Clegg T.A., Doyle M., Ryan E., More S.J., Gormley E. Characteristics of Mycobacterium bovis infected herds tested with the interferon-gamma assay. Prev. Vet. Med. 2019;168:52–59. doi: 10.1016/j.prevetmed.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Rowan N.J., Laffey J.G. Unlocking the surge in demand for personal and protective equipment (PPE) and improvised face coverings arising from coronavirus disease (COVID-19) pandemic – implications for efficacy, re-use and sustainable waste management. Sci. Total Environ. 2021;752 doi: 10.1016/j.scitotenv.2020.142259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nasir A.M., Goh P.S., Abdullah M.S., Cheer N.B., Ismail A.F. Adsorptive nanocomposite membranes for heavy metal remediation: recent progresses and challenges. Chemosphere. 2019;232:96–112. doi: 10.1016/j.chemosphere.2019.05.174. [DOI] [PubMed] [Google Scholar]
- 13.Nasir A.M., Jaafar J., Aziz F., Yusof N., Norhayati W., Salleh W., Ismail A.F., Aziz M. A review on floating nanocomposite photocatalyst: fabrication and applications for wastewater treatment. J. Water Process Eng. 2020;36:101300. doi: 10.1016/j.jwpe.2020.101300. [DOI] [Google Scholar]
- 14.Nasir A.M., Md Nordin N.A.H., Goh P.S., Ismail A.F. Application of two-dimensional leaf-shaped zeolitic imidazolate framework (2D ZIF-L) as arsenite adsorbent: kinetic, isotherm and mechanism. J. Mol. Liq. 2018;250 doi: 10.1016/j.molliq.2017.12.005. [DOI] [Google Scholar]
- 15.Nasir A.M., Goh P.S., Ismail A.F. Highly adsorptive polysulfone/hydrous iron-nickel-manganese (PSF/HINM) nanocomposite hollow fiber membrane for synergistic arsenic removal. Sep. Purif. Technol. 2019;213 doi: 10.1016/j.seppur.2018.12.040. [DOI] [Google Scholar]
- 16.Nasir A.M., Goh P.S., Ismail A.F. Novel synergistic hydrous iron-nickel-manganese (HINM) trimetal oxide for hazardous arsenite removal. Chemosphere. 2018;200:504–512. doi: 10.1016/j.chemosphere.2018.02.126. [DOI] [PubMed] [Google Scholar]
- 17.Guzman Herrador B.R., de Blasio B.F., MacDonald E., Nichols G., Sudre B., Vold L., Semenza J.C., Nygård K. Analytical studies assessing the association between extreme precipitation or temperature and drinking water-related waterborne infections: a review. Environ. Heal. 2015;14:29. doi: 10.1186/s12940-015-0014-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environ. Sci. Technol. Lett. 2020;7:511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- 19.Wu F.Q., Xiao A., Zhang J.B., Gu X.Q., Lee W.L., Kauffman K., Hanage W.P., Matus M., Ghaeli N., Endo N., Duvallet C., Moniz K., Erickson T.B., Chai P.R., Thompson J., Alm E.J. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. MedRxi. 2020 doi: 10.1101/2020.04.05.20051540. 2020.04.05.20051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lahrich S., Laghrib F., Farahi A., Bakasse M., Saqrane S., El Mhammedi M.A. Review on the contamination of wastewater by COVID-19 virus : impact and treatment. Sci. Total Environ. 2021;751:142325. doi: 10.1016/j.scitotenv.2020.142325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barber L.B., Hladik M.L., Vajda A.M., Fitzgerald K.C., Douville C. Impact of wastewater infrastructure upgrades on the urban water cycle: reduction in halogenated reaction byproducts following conversion from chlorine gas to ultraviolet light disinfection. Sci. Total Environ. 2015;529:264–274. doi: 10.1016/j.scitotenv.2015.04.112. [DOI] [PubMed] [Google Scholar]
- 22.Cobankara F.K., Ozkan H.B., Terlemez A. Comparison of organic tissue dissolution capacities of sodium hypochlorite and chlorine dioxide. J. Endod. 2010;36:272–274. doi: 10.1016/j.joen.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 23.Liang J., Huang J., Zhang S., Yang X., Huang S., Zheng L., Ye M., Sun S. A highly efficient conditioning process to improve sludge dewaterability by combining calcium hypochlorite oxidation, ferric coagulant re-flocculation, and walnut shell skeleton construction. Chem. Eng. J. 2019;361:1462–1478. doi: 10.1016/j.cej.2018.10.143. [DOI] [Google Scholar]
- 24.Donnermair M.M., Blatchley E.R. Disinfection efficacy of organic chloramines. Water Res. 2003;37:1557–1570. doi: 10.1016/S0043-1354(02)00522-5. [DOI] [PubMed] [Google Scholar]
- 25.Han W., Zhang P.H., Cao W.C., Yang D.L., Taira S., Okamoto Y., Arai J.I., Yan X.Y. The inactivation effect of photocatalytic titanium apatite filter on SARS virus. Prog. Biochem. Biophys. 2004;31:982–985. [Google Scholar]
- 26.Mohan S.V., Hemalatha M., Kopperi H., Ranjith I., Kumar A.K. SARS-CoV-2 in environmental perspective: occurrence, persistence, surveillance, inactivation and challenges. Chem. Eng. J. 2021;405:126893. doi: 10.1016/j.cej.2020.126893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhatt A., Arora P., Prajapati S.K. Occurrence, fates and potential treatment approaches for removal of viruses from wastewater: a review with emphasis on SARS-CoV-2. J. Environ. Chem. Eng. 2020;8:104429. doi: 10.1016/j.jece.2020.104429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corpuz M.V.A., Buonerba A., Vigliotta G., Zarra T., Ballesteros F., Campiglia P., Belgiorno V., Korshin G., Naddeo V. Viruses in wastewater: occurrence, abundance and detection methods. Sci. Total Environ. 2020;745:140910. doi: 10.1016/j.scitotenv.2020.140910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel M., Kumar A., Pittman C.U., Mlsna T., Mohan D. Coronavirus (SARS-CoV-2) in the environment : occurrence, persistence, analysis in aquatic systems and possible management. Sci. Total Environ. 2021;765:142698. doi: 10.1016/j.scitotenv.2020.142698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crini G., Lichtfouse E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019;17:145–155. doi: 10.1007/s10311-018-0785-9. [DOI] [Google Scholar]
- 31.Chen G. Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 2004;38:11–41. doi: 10.1016/j.seppur.2003.10.006. [DOI] [Google Scholar]
- 32.Huang J.-J., Hu H.-Y., Tang F., Li Y., Lu S.-Q., Lu Y. Inactivation and reactivation of antibiotic-resistant bacteria by chlorination in secondary effluents of a municipal wastewater treatment plant. Water Res. 2011;45:2775–2781. doi: 10.1016/j.watres.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 33.Krasner S.W., Westerhoff P., Chen B., Rittmann B.E., Amy G. Occurrence of disinfection byproducts in United States wastewater treatment plant effluents. Environ. Sci. Technol. 2009;43:8320–8325. doi: 10.1021/es901611m. [DOI] [PubMed] [Google Scholar]
- 34.Sharshir S.W., Algazzar A.M., Elmaadawy K.A., Kandeal A.W., Elkadeem M.R., Arunkumar T., Zang J., Yang N. New hydrogel materials for improving solar water evaporation, desalination and wastewater treatment: a review. Desalination. 2020;491 doi: 10.1016/j.desal.2020.114564. [DOI] [Google Scholar]
- 35.McGuigan K.G., Conroy R.M., Mosler H.-J., du Preez M., Ubomba-Jaswa E., Fernandez-Ibañez P. Solar water disinfection (SODIS): a review from bench-top to roof-top. J. Hazard. Mater. 2012;235–236:29–46. doi: 10.1016/j.jhazmat.2012.07.053. [DOI] [PubMed] [Google Scholar]
- 36.Parsa S.M., Rahbar A., Koleini M.H., Davoud Javadi Y., Afrand M., Rostami S., Amidpour M. First approach on nanofluid-based solar still in high altitude for water desalination and solar water disinfection (SODIS) Desalination. 2020;491 doi: 10.1016/j.desal.2020.114592. [DOI] [Google Scholar]
- 37.Walls L.E., Velasquez-Orta S.B., Romero-Frasca E., Leary P., Yáñez Noguez I., Orta Ledesma M.T. Non-sterile heterotrophic cultivation of native wastewater yeast and microalgae for integrated municipal wastewater treatment and bioethanol production. Biochem. Eng. J. 2019;151 doi: 10.1016/j.bej.2019.107319. [DOI] [Google Scholar]
- 38.Singh R., Purohit S., Chacharkar M.P., Bhandari P.S., Bath A.S. Microbiological safety and clinical efficacy of radiation sterilized amniotic membranes for treatment of second-degree burns. Burns. 2007;33:505–510. doi: 10.1016/j.burns.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 39.Yung P.T., Ponce A. Fast sterility assessment by germinable-endospore biodosimetry. Appl. Environ. Microbiol. 2008;74:7669–7674. doi: 10.1128/AEM.01437-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geraghty M., Sheridan V., McCann M., Devereux M., McKee V. Synthesis and anti-Candida activity of copper(II) and manganese(II) carboxylate complexes: X-ray crystal structures of [Cu(sal)(bipy)]·C2H5OH·H2O and [Cu(norb)(phen)2]·6.5H2O (salH2=salicylic acid; norbH2=cis-5-norbornene-endo-2,3-dicarboxylic acid; bipy= Polyhedron. 1999;18:2931–2939. doi: 10.1016/S0277-5387(99)00201-6. [DOI] [Google Scholar]
- 41.von Gunten U. Ozonation of drinking water: part I. Oxidation kinetics and product formation. Water Res. 2003;37:1443–1467. doi: 10.1016/S0043-1354(02)00457-8. [DOI] [PubMed] [Google Scholar]
- 42.Lester Y., Sharpless C.M., Mamane H., Linden K.G. Production of photo-oxidants by dissolved organic matter during UV water treatment. Environ. Sci. Technol. 2013;47:11726–11733. doi: 10.1021/es402879x. [DOI] [PubMed] [Google Scholar]
- 43.Guo S., Zhu X., Zhang H., Gu B., Chen W., Liu L., Alvarez P.J.J. Improving photocatalytic water treatment through nanocrystal engineering: mesoporous nanosheet-assembled 3D BiOCl hierarchical nanostructures that induce unprecedented large vacancies. Environ. Sci. Technol. 2018;52:6872–6880. doi: 10.1021/acs.est.8b00352. [DOI] [PubMed] [Google Scholar]
- 44.Sjogren J.C., Sierka R.A. Inactivation of phage MS2 by iron-aided titanium dioxide photocatalysis. Appl. Environ. Microbiol. 1994;60:344–347. doi: 10.1128/aem.60.1.344-347.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Von Gunten U. Oxidation Processes in Water Treatment: Are We on Track? Environ. Sci. Technol. 2018;52:5062–5075. doi: 10.1021/acs.est.8b00586. [DOI] [PubMed] [Google Scholar]
- 46.Wolf C., Pavese A., von Gunten U., Kohn T. Proxies to monitor the inactivation of viruses by ozone in surface water and wastewater effluent. Water Res. 2019;166:115088. doi: 10.1016/j.watres.2019.115088. [DOI] [PubMed] [Google Scholar]
- 47.Torii S., Itamochi M., Katayama H. Inactivation kinetics of waterborne virus by ozone determined by a continuous quench flow system. Water Res. 2020;186:116291. doi: 10.1016/j.watres.2020.116291. [DOI] [PubMed] [Google Scholar]
- 48.Xu X., Cao D., Wang Z., Liu J., Gao J., Sanchuan M., Wang Z. Study on ultrasonic treatment for municipal sludge. Ultrason. Sonochem. 2019;57:29–37. doi: 10.1016/j.ultsonch.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 49.Doosti M.R., Kargar R., Sayadi M.H. Water treatment using ultrasonic assistance: a review. Ecology. 2012;2:96–110. [Google Scholar]
- 50.Camargo-Perea A.L., Rubio-Clemente A., Peñuela G.A. Use of ultrasound as an advanced oxidation process for the degradation of emerging pollutants in water. Water (Switzerland). 2020;12:1–23. doi: 10.3390/W12041068. [DOI] [Google Scholar]
- 51.Pétrier C. 2014. The Use of Power Ultrasound for Water Treatment. [DOI] [Google Scholar]
- 52.Gao S., Lewis G.D., Ashokkumar M., Hemar Y. Inactivation of microorganisms by low-frequency high-power ultrasound: 2. A simple model for the inactivation mechanism. Ultrason. Sonochem. 2014;21:454–460. doi: 10.1016/j.ultsonch.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 53.Luo K., Oh D.H. Inactivation kinetics of Listeria monocytogenes and Salmonella enterica serovar Typhimurium on fresh-cut bell pepper treated with slightly acidic electrolyzed water combined with ultrasound and mild heat. Food Microbiol. 2016;53:165–171. doi: 10.1016/j.fm.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 54.Li J., Suo Y., Liao X., Ahn J., Liu D., Chen S., Ye X., Ding T. Analysis of Staphylococcus aureus cell viability, sublethal injury and death induced by synergistic combination of ultrasound and mild heat. Ultrason. Sonochem. 2017;39:101–110. doi: 10.1016/j.ultsonch.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 55.Fetyan N.A.H., Salem Attia T.M. Water purification using ultrasound waves: application and challenges. Arab J. Basic Appl. Sci. 2020;27:194–207. doi: 10.1080/25765299.2020.1762294. [DOI] [Google Scholar]
- 56.Huang C.P., Dong C., Tang Z. Advanced chemical oxidation: its present role and potential future in hazardous waste treatment. Waste Manag. 1993;13:361–377. doi: 10.1016/0956-053X(93)90070-D. [DOI] [Google Scholar]
- 57.Giannakis S., Liu S., Carratalà A., Rtimi S., Bensimon M., Pulgarin C. Effect of Fe(II)/Fe(III) species, pH, irradiance and bacterial presence on viral inactivation in wastewater by the photo-Fenton process: kinetic modeling and mechanistic interpretation. Appl. Catal. B Environ. 2017;204:156–166. doi: 10.1016/j.apcatb.2016.11.034. [DOI] [Google Scholar]
- 58.Nieto-Juarez J., Pierzchala K., Sienkiewicz A., Kohn T. Inactivation of MS2 coliphage in Fenton and Fenton-like systems: role of transition metals, hydrogen peroxide and sunlight. Environ. Sci. Technol. 2010;44:3351–3356. doi: 10.1021/es903739f. [DOI] [PubMed] [Google Scholar]
- 59.Kusiak-Nejman E., Morawski A.W. TiO2/graphene-based nanocomposites for water treatment: a brief overview of charge carrier transfer, antimicrobial and photocatalytic performance. Appl. Catal. B Environ. 2019;253:179–186. doi: 10.1016/j.apcatb.2019.04.055. [DOI] [Google Scholar]
- 60.Kim H.G., Hwang D.W., Bae S.W., Jung J.H., Lee J.S. Photocatalytic water splitting over La2Ti2O7 synthesized by the polymerizable complex method. Catal. Letters. 2003;91:193–198. doi: 10.1023/B:CATL.0000007154.30343.23. [DOI] [Google Scholar]
- 61.Reza K.M., Kurny A.S.W., Gulshan F. Parameters affecting the photocatalytic degradation of dyes using TiO2: a review. Appl. Water Sci. 2017;7:1569–1578. doi: 10.1007/s13201-015-0367-y. [DOI] [Google Scholar]
- 62.Li Z., Ji S., Liu Y., Cao X., Tian S., Chen Y., Niu Z., Li Y. Well-defined materials for heterogeneous catalysis: from nanoparticles to isolated single-atom sites. Chem. Rev. 2020;120:623–682. doi: 10.1021/acs.chemrev.9b00311. [DOI] [PubMed] [Google Scholar]
- 63.Dong H., Zeng G., Tang L., Fan C., Zhang C., He X., He Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015;79:128–146. doi: 10.1016/j.watres.2015.04.038. [DOI] [PubMed] [Google Scholar]
- 64.Zhang C., Li Y., Shuai D., Shen Y., Wang D. Progress and challenges in photocatalytic disinfection of waterborne Viruses: a review to fill current knowledge gaps. Chem. Eng. J. 2019;355:399–415. doi: 10.1016/j.cej.2018.08.158. [DOI] [Google Scholar]
- 65.Giannakis S., Liu S., Carratalà A., Rtimi S., Talebi Amiri M., Bensimon M., Pulgarin C. Iron oxide-mediated semiconductor photocatalysis vs. Heterogeneous photo-Fenton treatment of viruses in wastewater. Impact of the oxide particle size. J. Hazard. Mater. 2017;339:223–231. doi: 10.1016/j.jhazmat.2017.06.037. [DOI] [PubMed] [Google Scholar]
- 66.Hu X., Hu C., Peng T., Zhou X., Qu J. Plasmon-induced inactivation of enteric pathogenic microorganisms with Ag-AgI/Al2O3 under visible-light irradiation. Environ. Sci. Technol. 2010;44:7058–7062. doi: 10.1021/es1012577. [DOI] [PubMed] [Google Scholar]
- 67.Ditta I.B., Steele A., Liptrot C., Tobin J., Tyler H., Yates H.M., Sheel D.W., Foster H.A. Photocatalytic antimicrobial activity of thin surface films of TiO2, CuO and TiO2/CuO dual layers on Escherichia coli and bacteriophage T4. Appl. Microbiol. Biotechnol. 2008;79:127–133. doi: 10.1007/s00253-008-1411-8. [DOI] [PubMed] [Google Scholar]
- 68.Cho M., Chung H., Choi W., Yoon J. Different inactivation behaviors of MS-2 phage and Escherichia coli in TiO2 photocatalytic disinfection. Appl. Environ. Microbiol. 2005;71:270–275. doi: 10.1128/AEM.71.1.270-275.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee S., Nakamura M., Ohgaki S. Inactivation of phage Qß by 254nm UV light and titanium dioxide photocatalyst. J. Environ. Sci. Heal. Part A. 1998;33:1643–1655. doi: 10.1080/10934529809376809. [DOI] [Google Scholar]
- 70.Zuo X., Chu X., Hu J. Effects of water matrix on virus inactivation using common virucidal techniques for condensate urine disinfection. Chemosphere. 2015;136:118–124. doi: 10.1016/j.chemosphere.2015.04.083. [DOI] [PubMed] [Google Scholar]
- 71.Cho M., Cates E.L., Kim J.-H. Inactivation and surface interactions of MS-2 bacteriophage in a TiO2 photoelectrocatalytic reactor. Water Res. 2011;45:2104–2110. doi: 10.1016/j.watres.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 72.Lee J., Zoh K., Ko G. Inactivation and UV disinfection of murine norovirus with TiO&sub&2&/sub& under various environmental conditions. Appl. Environ. Microbiol. 2008;74:2111. doi: 10.1128/AEM.02442-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Venieri D., Gounaki I., Binas V., Zachopoulos A., Kiriakidis G., Mantzavinos D. Inactivation of MS2 coliphage in sewage by solar photocatalysis using metal-doped TiO2. Appl. Catal. B Environ. 2015;178:54–64. doi: 10.1016/j.apcatb.2014.10.052. [DOI] [Google Scholar]
- 74.Li Q., Page M.A., Mariñas B.J., Jian K.S. Treatment of coliphage MS2 with palladium-modified nitrogen-doped titanium oxide photocatalyst illuminated by visible light. Environ. Sci. Technol. 2008;42:6148–6153. doi: 10.1021/es7026086. [DOI] [PubMed] [Google Scholar]
- 75.Zheng X., Shen Z., Cheng C., Shi L., Cheng R., Yuan D. Photocatalytic disinfection performance in virus and virus/bacteria system by Cu-TiO2 nanofibers under visible light. Environ. Pollut. 2018;237:452–459. doi: 10.1016/j.envpol.2018.02.074. [DOI] [PubMed] [Google Scholar]
- 76.Cheng R., Kang M., Shen Z., Shi L., Zheng X. Visible-light-driven photocatalytic inactivation of bacteriophage f2 by Cu-TiO2 nanofibers in the presence of humic acid. J. Environ. Sci. 2019;77:383–391. doi: 10.1016/j.jes.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 77.Chi L., Qian Y., Guo J., Wang X., Arandiyan H., Jiang Z. Novel g-C3N4/TiO2/PAA/PTFE ultrafiltration membrane enabling enhanced antifouling and exceptional visible-light photocatalytic self-cleaning. Catal. Today. 2019;335:527–537. doi: 10.1016/j.cattod.2019.02.027. [DOI] [Google Scholar]
- 78.Li Y., Zhang C., Shuai D., Naraginti S., Wang D., Zhang W. Visible-light-driven photocatalytic inactivation of MS2 by metal-free g-C3N4: virucidal performance and mechanism. Water Res. 2016;106:249–258. doi: 10.1016/j.watres.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 79.Alias N.H., Jaafar J., Samitsu S., Ismail A.F., Mohamed M.A., Othman M.H.D., Rahman M.A., Othman N.H., Nor N.A.M., Yusof N., Aziz F. Mechanistic insight of the formation of visible-light responsive nanosheet graphitic carbon nitride embedded polyacrylonitrile nanofibres for wastewater treatment. J. Water Process Eng. 2020;33:101015. doi: 10.1016/j.jwpe.2019.101015. [DOI] [Google Scholar]
- 80.Moor K.J., Kim J.H. Simple synthetic method toward solid supported C60 visible light-activated photocatalysts. Environ. Sci. Technol. 2014;48:2785–2791. doi: 10.1021/es405283w. [DOI] [PubMed] [Google Scholar]
- 81.Moor K.J., Valle D.C., Li C., Kim J.-H. Improving the visible light photoactivity of supported fullerene photocatalysts through the use of [C70] fullerene. Environ. Sci. Technol. 2015;49:6190–6197. doi: 10.1021/es505888d. [DOI] [PubMed] [Google Scholar]
- 82.Banerjee I., Douaisi M.P., Mondal D., Kane R.S. Light-activated nanotubeporphyrin conjugates as effective antiviral agents. Nanotechnology. 2012;23 doi: 10.1088/0957-4484/23/10/105101. [DOI] [PubMed] [Google Scholar]
- 83.Barras A., Pagneux Q., Sane F., Wang Q., Boukherroub R., Hober D., Szunerits S. High efficiency of functional carbon nanodots as entry inhibitors of herpes simplex virus type 1. ACS Appl. Mater. Interfaces. 2016;8:9004–9013. doi: 10.1021/acsami.6b01681. [DOI] [PubMed] [Google Scholar]
- 84.Sengupta J., Mustansar C. Carbon nanomaterials to combat virus : a perspective in view of COVID-19. Carbon Trends. 2021;2:100019. doi: 10.1016/j.cartre.2020.100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang C., Li Y., Wang C., Zheng X. Different inactivation behaviors and mechanisms of representative pathogens (Escherichia coli bacteria, human adenoviruses and Bacillus subtilis spores) in g-C3N4-based metal-free visible-light-enabled photocatalytic disinfection. Sci. Total Environ. 2021;755 doi: 10.1016/j.scitotenv.2020.142588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang C., Li Y., Zhang W., Wang P., Wang C. Metal-free virucidal effects induced by g-C3N4 under visible light irradiation: statistical analysis and parameter optimization. Chemosphere. 2018;195:551–558. doi: 10.1016/j.chemosphere.2017.12.122. [DOI] [PubMed] [Google Scholar]
- 87.Pan Y., Liu X., Zhang W., Liu Z., Zeng G., Shao B., Liang Q., He Q., Yuan X., Huang D., Chen M. Advances in photocatalysis based on fullerene C60 and its derivatives: Properties, mechanism, synthesis, and applications. Appl. Catal. B Environ. 2020;265:118579. doi: 10.1016/j.apcatb.2019.118579. [DOI] [Google Scholar]
- 88.Hotze E.M., Badireddy A.R., Chellam S., Wiesner M.R. Mechanisms of bacteriophage inactivation via singlet oxygen generation in UV illuminated fullerol suspensions. Environ. Sci. Technol. 2009;43:6639–6645. doi: 10.1021/es901110m. [DOI] [PubMed] [Google Scholar]
- 89.Hashimah N., Jaafar J., Samitsu S., Ismail A.F., Othman M.H.D., Rahman M.A., Hidayati N., Yusof N., Aziz F., Mohd T.A.T. Efficient removal of partially hydrolysed polyacrylamide in polymer-flooding produced water using photocatalytic graphitic carbon nitride nanofibres. Arab. J. Chem. 2020;13:4341–4349. doi: 10.1016/j.arabjc.2019.08.004. [DOI] [Google Scholar]
- 90.Sudhaik A., Raizada P., Shandilya P., Jeong D.Y., Lim J.H., Singh P. Review on fabrication of graphitic carbon nitride based efficient nanocomposites for photodegradation of aqueous phase organic pollutants. J. Ind. Eng. Chem. 2018;67:28–51. doi: 10.1016/j.jiec.2018.07.007. [DOI] [Google Scholar]
- 91.Hasija V., Raizada P., Sudhaik A., Sharma K., Kumar A., Singh P., Jonnalagadda S.B., Thakur V.K. Recent advances in noble metal free doped graphitic carbon nitride based nanohybrids for photocatalysis of organic contaminants in water: a review. Appl. Mater. Today. 2019;15:494–524. doi: 10.1016/j.apmt.2019.04.003. [DOI] [Google Scholar]
- 92.Zhang C., Li Y., Shuai D., Zhang W., Niu L., Wang L., Zhang H. Visible-light-driven, water-surface-floating antimicrobials developed from graphitic carbon nitride and expanded perlite for water disinfection. Chemosphere. 2018;208:84–92. doi: 10.1016/j.chemosphere.2018.05.163. [DOI] [PubMed] [Google Scholar]
- 93.Ong W., Tan L., Ng Y.H., Yong S., Chai S. Graphitic carbon nitride (g‑C3N4)‑based photocatalysts for artificial photosynthesis and environmental remediation : are we a step closer to achieving sustainability? Chem. Rev. 2016;116:7159–7329. doi: 10.1021/acs.chemrev.6b00075. [DOI] [PubMed] [Google Scholar]
- 94.Xia D., An T., Li G., Wang W., Zhao H., Keung P. Synergistic photocatalytic inactivation mechanisms of bacteria by graphene sheets grafted plasmonic Ag e AgX (X ¼ Cl, Br, I) composite photocatalyst under visible light irradiation. Water Res. 2016;99:149–161. doi: 10.1016/j.watres.2016.04.055. [DOI] [PubMed] [Google Scholar]
- 95.Habibi-Yangjeh A., Asadzadeh-Khaneghah S., Feizpoor S., Rouhi A. Review on heterogeneous photocatalytic disinfection of waterborne, airborne, and foodborne viruses: Can we win against pathogenic viruses? J. Colloid Interface Sci. 2020;580:503–514. doi: 10.1016/j.jcis.2020.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Regmi C., Joshi B., Ray S.K., Gyawali G. Understanding mechanism of photocatalytic microbial decontamination of environmental wastewater. Front. Chem. 2018;6:1–6. doi: 10.3389/fchem.2018.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Byrne J.A., Dunlop P.S.M., Hamilton J.W.J., Fernández-Ibáñez P., Polo-López I., Sharma P.K., Vennard A.S.M. A review of heterogeneous photocatalysis for water and surface disinfection. Molecules. 2015;20:5574–5615. doi: 10.3390/molecules20045574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guo B., Snow S.D., Starr B.J., Xagoraraki I., Tarabara V.V. Photocatalytic inactivation of human adenovirus 40: effect of dissolved organic matter and prefiltration. Sep. Purif. Technol. 2018;193:193–201. doi: 10.1016/j.seppur.2017.11.012. [DOI] [Google Scholar]
- 99.Nasir A.M., Awang N., Jaafar J., Ismail A.F., Othman M.H.D., Rahman M.A., Aziz F., Mat Yajid M.A. Recent progress on fabrication and application of electrospun nanofibrous photocatalytic membranes for wastewater treatment: a review. J. Water Process Eng. 2021;40:101878. doi: 10.1016/j.jwpe.2020.101878. [DOI] [Google Scholar]
- 100.Cui H., Jiang J., Gu W., Sun C., Wu D., Yang T., Yang G. Photocatalytic inactivation efficiency of anatase nano-TiO2 sol on the H9N2 avian influenza virus. Photochem. Photobiol. 2010;86:1135–1139. doi: 10.1111/j.1751-1097.2010.00763.x. [DOI] [PubMed] [Google Scholar]
- 101.Cheng R., Shen L., Wang Q., Xiang S., Shi L., Zheng X., Lv W. Photocatalytic membrane reactor (PMR) for virus removal in drinking water: effect of humic acid. Catalysts. 2018;8 doi: 10.3390/catal8070284. [DOI] [Google Scholar]
- 102.Jafry H.R., Liga M.V., Li Q., Barron A.R. Simple route to enhanced photocatalytic activity of P25 titanium dioxide nanoparticles by silica addition. Environ. Sci. Technol. 2011;45:1563–1568. doi: 10.1021/es102749e. [DOI] [PubMed] [Google Scholar]
- 103.Liga M.V., Bryant E.L., Colvin V.L., Li Q. Virus inactivation by silver doped titanium dioxide nanoparticles for drinking water treatment. Water Res. 2011;45:535–544. doi: 10.1016/j.watres.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 104.Takehara K., Yamazaki K., Miyazaki M., Yamada Y., Ruenphet S., Jahangir A., Shoham D., Okamura M., Nakamura M. Inactivation of avian influenza virus H1N1 by photocatalyst under visible light irradiation. Virus Res. 2010;151:102–103. doi: 10.1016/j.virusres.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 105.Yamaguchi Y., Usuki S., Kanai Y., Yamatoya K., Suzuki N., Katsumata K., Terashima C., Suzuki T., Fujishima A., Sakai H., Kudo A., Nakata K. Selective inactivation of bacteriophage in the presence of Bacteria by use of ground Rh-Doped SrTiO3 photocatalyst and visible light. ACS Appl. Mater. Interfaces. 2017;9:31393–31400. doi: 10.1021/acsami.7b07786. [DOI] [PubMed] [Google Scholar]
- 106.Kumar V.G., Ogden D.S., Isu U.H., Polasa A., Losey J., Moradi M. Differential dynamic behavior of prefusion spike proteins of SARS coronaviruses 1 and 2. BioRxiv. 2020;2019:1–42. doi: 10.1101/2020.12.25.424008. [DOI] [Google Scholar]