Abstract
Introduction and objectives
Severe asthma management during the coronavirus disease 2019 (COVID-19) pandemic is a challenge and will continue to be, at least in the next few months, as herd immunity is still a mirage. A lot has to be learned about how COVID-19 affects underlying diseases, and severe asthma is no exception.
Methods
Narrative review of papers available until February 2021 in PubMed and Google Scholar, relating severe asthma and COVID-19. Four main research topics were reviewed: SARS-CoV-2 infection: immunology and respiratory pathology; interrelationship of severe asthma endotypes and COVID-19 disease mechanisms; severe asthma epidemiology and COVID-19; and biologics for severe asthma in the context of COVID-19.
Results
COVID-19 disease mechanisms start with upper respiratory cell infection, and afterwards several immunological facets are activated, contributing to disease severity, namely cell-mediated immunity and antibody production. Although infrequent in the COVID-19 course some patients develop a cytokine storm that causes organ damage and may lead to acute respiratory distress syndrome or multiorgan failure. Regarding severe asthma endotypes, type2-high might have a protective role both in infection risk and disease course. There is conflicting data regarding the epidemiological relationship between COVID-19 among severe asthma patients, with some studies reporting increased risk of infection and disease course, whereas others the other way round. Biologics for severe asthma do not seem to increase the risk of infection and severe COVID-19, although further evidence is needed.
Conclusions
Globally, in the era of COVID-19, major respiratory societies recommend continuing the biologic treatment, preferably in a self-home administration program.
Keywords: Antibodies, Monoclonal, Asthma, Covid-19, SARS-CoV-2
Introduction
During the coronavirus disease 2019 (COVID-19) pandemic, severe asthma management is a challenge and will continue to be at least in the following months. Despite the recent approval and use of COVID-19 vaccines, the milestone of herd immunity is yet away from reality worldwide.1 COVID-19 is caused due to the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and has caused a substantial increase in hospitalizations for pneumonia with multiorgan disease.2 COVID-19 first emerged in December 20192 and, by the end of 2020, has affected almost every country in the globe, resulting in more than 79 million cases and more than 1.9 million deaths.3 It is not clear why the clinical presentation may be so distinct, ranging from mild and even asymptomatic to severe clinical conditions such as pneumonia, acute respiratory distress syndrome (ARDS), organ dysfunction and death.4
Endemic human coronaviruses present a high homology with SARS-CoV-2.5 Cross-reactivity exists between these coronaviruses and may explain less severe COVID-19 as reported.6 Early in infection, SARS-CoV-2 targets cells, such as nasal and bronchial epithelial cells and pneumocytes. Subsequently, the viral inflammatory response is generated, consisting of innate and adaptive immunity (comprising humoral and cell-mediated immunity)2 , 7 The pathogenesis of COVID-19 results from an abnormal host response or overreaction of the immune system in some patients with unknown etiology.7 From a theoretical perspective, asthmatic patients should have increased susceptibility for SARS-CoV-2 infection and to severe COVID-19 due to a deficient antiviral immune response and the tendency for exacerbation elicited by common respiratory viruses.8 At the beginning of the pandemia, the inclusion of asthmatic patients and patients with other chronic lung diseases in a high-risk population for SARS-CoV-2 infection was based more on common sense than on scientific evidence.9 Available data at the moment has not shown consistently an expected increased burden of asthmatic individuals among COVID-19 patients.8
Severe asthma represents 3–10% of the nearly 400 million asthmatics worldwide but is associated with increased mortality and hospitalization, reduced quality of life and increased healthcare costs.10 Authorities and physicians are still learning how COVID-19 affects underlying diseases, and severe asthma is not an exception. Even though respiratory viruses are among the most common triggers for asthma exacerbations, not all of these viruses affect patients equally. Available data about whether patients with asthma are at higher risk of being infected with SARS-CoV-2 or having severe forms of the disease is somewhat conflicting.
In the last months, several papers have been published about the relationship between asthma and COVID-19 but a recent review about the particularities and novelties of severe asthma is lacking. Taking into consideration the complexity of severe asthma pathophysiology and the growing knowledge about COVID-19, the authors aim to review four different research topics about the possible interactions between these disease entities:
-
•
SARS-CoV-2 infection: immunology and respiratory pathology.
-
•
Interrelationship of severe asthma endotypes and COVID-19 disease mechanisms.
-
•
Severe asthma epidemiology and COVID-19.
-
•
Biologics for severe asthma in the context of COVID-19.
Methods
The author team generated the topics mentioned above before initiating the review. To answer these questions, a search was performed on PubMed and Google Scholar for papers relating to severe asthma and COVID-19 until February 2020. The search strategy was structured to include terms for “severe asthma” AND “COVID-19″ OR “SARS-CoV-2″. Data was then narratively synthesized by the research topic. Due to the emerging nature of evidence in this field, a broad approach to inclusion was followed, without any study type restriction. All the references judged to have relevant information about prespecified questions were included.
Results
SARS-CoV-2 infection: immunology and respiratory pathology
Upon entry into the host, the inhaled SARS-CoV-2 is likely to bind to epithelial cells in the nasal cavity and start replicating.11 Angiotensin-converting enzyme-2 (ACE2) is the primary receptor for both SARS-CoV-2 and SARS-CoV.11 The cellular protease TMPRSS2 also appears vital for SARS-CoV-2 cell entry.12 There is local propagation of the virus but a limited innate immune response.11 At this stage, the virus can be detected by nasal swabs and although the viral burden may be low, these individuals are infectious.11 The virus propagates and migrates down the respiratory tract along the conducting airways, and a more robust innate immune response is triggered.11 For about 80% of the infected patients, the disease will be mild and mostly restricted to the upper and conducting airway.13 This phase would be the result of the infection itself. Unfortunately, about 20% of the infected patients will progress and develop pulmonary infiltrates and some of these might develop very severe disease.13 The virus reaches the lung's gas exchange units and infects alveolar type II cells with high surface expression of ACE2 receptors. The virus rapidly disseminates through peripheral blood to other organs like the heart, kidney, liver, spleen, etc14 , 15 The cytokine storm generated damages the organs or may lead to ARDS or multiorgan failure in COVID-19.16, 17, 18 Human SARS-CoV-2 infection involves the innate immune response, T and B cell immunity and antiviral neutralizing antibodies.
Interrelationship of severe asthma endotypes and COVID-19 disease mechanisms
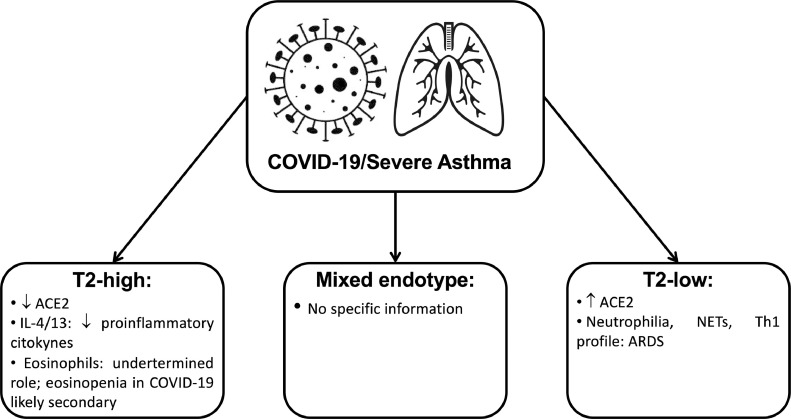
The current severe asthma approach relies on disease phenotypes identification, but these do not necessarily relate to or give insights into the underlying pathogenetic mechanisms described by disease endotypes.19 Based on the major immune-inflammatory pathway involved, type2-high, type2-low and mixed endotypes are described for severe asthma.19
Type2-high asthma / COVID-19
Type-2 immune responses predominantly mediate the majority of the disease. The type-2 immune response involves T helper (Th) 2 cells, type-2 B cells, group 2 innate lymphoid cells, type-2 macrophages, IL-4-secreting nature killer and natural killer T cells, basophils, eosinophils, and mast cells.8 A variety of cytokines produced by the immune system and epithelial cells contribute to the regulatory network.8 For example, IL-4 and IL-13 have essential roles in allergen-specific IgE production and accumulation of Th2 cells and eosinophils in local tissues, as well as epithelial barrier regulation. At the same time, IL-5, IL-9, and IL-13 contribute to eosinophilia and mucus production.8
Allergic asthmatic subjects seem to be less likely to be infected by the SARS-CoV-2, which could be due to different factors.20 The first hypothesis might be related to the anti-inflammatory effect of the inhaled corticosteroids (ICS) and their negative impact on the cytokine storm elicited by the virus.20 This effect might be boosted by increased compliance with asthma treatment driven by COVID-19 fear.20 Individuals with allergic asthma have confirmed reduced ACE2 expression21 and in vitro experiments showed that IL-13 also reduced the ACE2 expression.21 Interestingly, ICS were associated with lower expression of ACE2 and TMPRSS2 after adjustment for asthma severity and may explain the low prevalence of asthma among COVID-19 patients in some studies.22 This fact may justify why data about the relationship between asthma and susceptibility to SARS-CoV-2 is contradictory, as compliance with ICS might be an unconsidered confounding factor.22 Furthermore, some ICS have less peripheral airway deposition and might affect less ACE2 expression of type 2 pneumocytes.22 Another critical point is the smoking status of the patients.22 ACE2 expression in bronchial biopsies was found to be higher in smokers, suggesting an enhancing effect on SARS-CoV-2 entry into lung cells.22
Certain aspects of the type-2 immune response, including type 2 cytokines (IL- 4, IL-13, etc.) and accumulation of eosinophils, might provide potential protective effects against COVID-19 due to its anti-inflammatory effects.8 , 23 For example, IL-4 can suppress type-1 immune response.8 This effect is obtained not only by suppression of Th1 cells development but also by the inhibition in the production of multiple pro-inflammatory cytokines associated with type-1 immune response, including IL-1β, TNF-α, IL-6, and IL-12.8 It has also been shown that IL-13 has immunoregulatory effects through inhibiting the secretion of pro-inflammatory cytokines (IL-1α, IL-1β, IL-6, and TNF-α) and chemokines (IL-8, MIP-1α and MIP-1β, and monocyte chemotactic protein-3).8 Additionally, IL-13 mediated type-2 inflammation has a significant role in ACE2 downregulation and TMPRSS2 upregulation.7 , 22 Furthermore, IL-9 reduces TNF-α and IL-10, but increases the secretion of TGF-β on LPS-activated monocytes.8 It is possible, that predominance of type 2 cytokines might counteract the accumulation of pro-inflammatory cytokines, including the pathogenesis of COVID-19.8
Eosinophils cause tissue damage by releasing many toxic proteins and other preformed pro-inflammatory mediators after degranulation processes.24 Eosinophils can actively promote type 2 immune responses by producing a range of immunoregulating cytokines and other factors.24 Although eosinophils may have protective effects on different viral infections, their role in SARS-CoV-2 is incompletely understood and may even be absent as the antiviral activity has not yet been demonstrated in humans.21 , 25 Many COVID-19 patients present eosinopenia, although it is not reported in all cohorts21 , 24 This phenomenon is probably secondary and not directly contributing to disease course.21 , 24 The immune mechanism of eosinopenia in COVID-19 remains unclear. Still, it is probably multifactorial, involving inhibition of the eosinophil life cycle's main steps, apoptosis induced by type 1 IFN during the acute infection or relation to eosinophil consumption by eosinophil antiviral actions.24 Increased tissue migration is unlikely because eosinophils infiltration was not found in pulmonary tissue of COVID-19 patients, but further research is needed.21 An important fact is that eosinophil levels improved in patients before discharge, suggesting that eosinophil resolution may be an indicator of improvement of clinical condition.24
Type-2 low asthma / COVID-19
Type2-low severe asthma is a distinct endotype with relevant features such as increased severity and remodeling and less satisfactory response to anti-inflammatory treatment.19 The understanding of these disease mechanisms lags far behind type2-high endotype.19 Several pathways were evaluated, such as the dysregulated innate immune response, including intrinsic neutrophil abnormalities, the inflammasome pathway and the activation of the IL-17 pathway.19 In severe asthma with predominantly neutrophilic phenotype, transcobalamin-1, metalloproteinase (MMP) 9, mucins, and oxidative stress responses are upregulated.19 Many severe asthma facets are mechanistically associated with Th17 cell-derived cytokines and other immune factors that mediate neutrophilic influx to the airways and remodeling.19 TGF-β1 is a pivotal mediator involved in airway remodeling that correlates with enhanced Th17 activity and is essential for Th17 differentiation and IL-17A production. IL-17A can reciprocally enhance activation of TGF-β1 signaling pathways, whereas combined Th1/Th17 or Th2/Th17 immune responses additively impact severity.19 Type2-low and IFN-high individuals were found to express a high level of ACE2, and this might increase the risk of SARS-CoV-2 infection22 but further evidence is needed to corroborate this finding.
Neutrophils are one of the predominant lungs infiltrating leukocytes in severe COVID-19 and neutrophilia predicts poor clinical outcomes.21 Post-mortem analysis of lung samples from COVID-19 patients showed neutrophil infiltration in pulmonary capillaries and neutrophil extravasation into the alveolar space, and the inflammatory profile observed in lungs from COVID-19 patients is Th1, Th17 phenotype.21 , 24 In experimental models of smoke-induced acute respiratory distress, a Th17/neutrophilic syndrome, ACE2 was upregulated.26 A type 2-low endotype can be an aggravating factor to COVID-19 severity.27
Under neutrophil-activating conditions, such as those occurring during systemic inflammation, neutrophil extracellular traps (NETs) can be released.28 Although this is a way to trap pathogens, NET formation is linked to pulmonary diseases, particularly ARDS.28 Severe COVID-19 conditions with uncontrollable progressive inflammation presumably induce intense crosstalk between neutrophils releasing NETs and IL-1β secretion from macrophages.28
Mixed endotypes asthma / COVID-19
At the present moment, we could not find any information relating to mixed endotypes of severe asthma and COVID-19 and this is an area of particular research interest.
Fig. 1 is a summary of the main evidence about severe asthma endotypes and COVID-19 mechanisms:
Figure 1.
Summary of the main evidence about severe asthma endotypes and COVID-19 disease mechanisms. Legend: ACE2: angiotensin-converting enzyme 2 receptor; COVID-19: coronavirus disease 2019; IL: interleukin; NETs: neutrophil extracellular traps; Th1: T Helper 1 cell.
Severe asthma epidemiology and COVID-19
The literature search yielded 56 records and after identifying and removing duplicates 35 articles remained. After screening for relevant titles/abstracts, 11 publications were considered to report original epidemiological data. Four of these publications were related to Severe Asthma Registries.
In the Belgian Severe Asthma Registry29 only 14 out of 676 patients (2.1%) had SARS-CoV-2 infection confirmed by a positive PCR or serology testing. These findings indicated a lower incidence than in the general population (5.1%). There was no statistically difference in COVID-19 incidence between patients treated or untreated with biologics. No deaths were attributed to COVID-19 on this cohort of severe asthma patients.
In the Severe Asthma Network in Italy (SANI)30, Heffler et al. reported an incidence of 26 confirmed or highly suspected infections out of 1504 patients (1.73%). The majority (n = 21) were under biologics: 15 on anti-IL-5 and six on anti-IgE. As previously described, due to the higher proportion of COVID-19 infections with anti-IL-5 treatments, the authors speculated about the role of these monoclonal antibodies (mAbs) in the COVID-19 course. It should be noted that this finding was not seen in the Belgian registry.29 In the Italian cohort, the related mortality was 7.7%, lower than in the general Italian population (14.5%).
In the Italian Registry of Severe Asthma (IRSA)31, a different Italian severe asthma registry, seven subjects out of 558 (1.25%) contracted COVID-19. The hospitalization rate in COVID‐19 infected patients with severe asthma was similar to the general population, and no deaths were reported. The proportion of COVID-19 in patients on biologics was 5.43% compared to 0% on subjects treated with ICS‐LABA alone, but no statistical difference was found.
Eger et al.32 found a frequency of nine out of 634 (1.4%) patients with COVID-19 among severe asthma patients on biologics in the Dutch Severe Asthma Registry RAPSODI. From these, five patients needed intensive care and one patient died. In the group of 73 patients not prescribed any biological, one (1.73%) was diagnosed with COVID-19. In this study, the proportion of COVID-19 related hospitalization, intubation, and death was 1.26% in the patients under biologics, which is a number 4.5 times higher than in the Dutch population within the same age category (0.28%). It must be considered that RAPSODI patients under biologics had a higher prevalence of obesity than the general population (30% versus 15%).
Rial J. et al.33 conducted a multicentre study in nine centres from the Spanish Severe Asthma Network. Among 545 adult severe asthma patients under biological treatment, 35 (6.4%) had COVID-19, a proportion similar to the Spanish seroprevalence (5.2%) at the time of the study. Eight patients were hospitalized and one patient died. No statistical differences were found between the frequency of COVID-19 in patients under anti-IgE (5.32%) compared to the subjects treated with anti-IL-5 (7.4%).
Table 1 presents a summary of the epidemiological data regarding COVID-19 in the European severe asthma registries and the Spanish Severe Asthma Network.
Table 1.
– Epidemiological data regarding COVID-19 in the European severe asthma registries.
| Author | Study population | Deadline of the study | Number of participants | Proportion of COVID-19+ patients |
Proportion of deaths on COVID-19+ patients |
||
|---|---|---|---|---|---|---|---|
| Severe asthma | General population | Severe asthma | General population | ||||
| Hanon et al. (29) | Belgian Severe Asthma Registry | 8th July 2020 | n = 676 | 2.1% | 5.1% | 0% | 15.3%1 |
| Heffler et al. (30) | Severe Asthma Network in Italy (SANI) | 19th June 2020 | n = 1504 | 1.73% | 0.39%1 | 7.7% | 14.5% |
| Antonicelli et al. (31) | Italian Registry of Severe Asthma (IRSA) – until 18th May 2020 | 18th May 2020 | n = 558 | 1.25% | 0.39%1 | 0% | 14.2% |
| Eger et al. (32) | Dutch Severe Asthma Registry (RAPSODI) | 30th April 2020 | n = 634 | 1.4% | 0.231 | 1.25% | 0.28% |
| Rial J et al. (33) | Spanish Asthma Network (nine centers) | June 2020 | n = 545 | 6.4% | 5.2% | 0.18% | 11.13 |
1- Data not reported by the authors. Calculated using the data provided by World Health Organization (https://covid19.who.int/region/euro/country) at the time of the deadline of the study period.
Caminati et al.34 reviewed the medical records of patients admitted to COVID-19 Units of six Italian cities (n = 2000). From the 42 asthmatic patients identified, patients on GINA 4/5 and those not adequately treated were considered at higher risk. Hauron-Diaz et al.35, in a sample of 80 severe asthma patients followed in the Allergy Service of Infanta Leonor University Hospital (Madrid, Spain), SARS-CoV-2 infection was confirmed in three (3.75%) patients. None of the patients required intensive care. Chibba et al.36 conducted a retrospective study that assessed the risk of hospitalization associated with asthma and/or inhaled corticosteroid use in patients with COVID-19. The proportion of asthmatic patients using ICS plus long-acting beta2-agonists admitted to an intensive care unit was higher (57.9%) than those using ICS alone. Kow et al.37 suggest that this finding may indicate that those with more severe disease have worse outcomes. According to the health analytics platform OpenSafely38 that covers 40% of all patients in England, severe asthma (defined as asthma with recent use of an oral corticosteroid) was associated with COVID-19 related death after adjusting for sex and age (hazard ratio: 1.13; 95%: 1.01–1.26).
Calmes D. et al.39 conducted a study to evaluate if obstructive diseases were risk factors for intensive care unit stay and death due to COVID-19. Twenty out of 57 (35%) asthmatic patients included were under a high dose of inhaled steroids, and 2 (3%) were taking oral steroids daily. In this study, inhaled and oral corticosteroid treatment were not identified as risk factors.
Biologics for severe asthma in the context of COVID-19
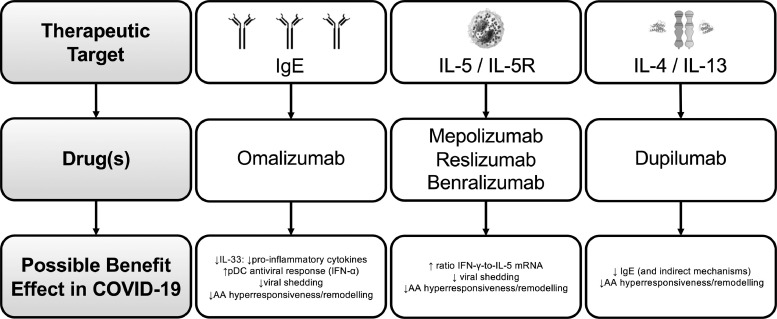
There are now five approved biologic agents for severe asthma: one anti-IgE – omalizumab, two anti-IL-5 - mepolizumab and reslizumab, one anti-IL-5 receptor alpha (Rα) – benralizumab - and the anti-IL-4Rα dupilumab.40
Omalizumab is a humanized monoclonal antibody that selectively binds to human IgE, preventing its high-affinity receptor.41 Regarding anti-IL-5/IL-5-R, their major effect is the reduction or depletion of tissue and peripheral blood eosinophils.41 Mepolizumab and reslizumab are humanized anti–IL-5 mAbs that bind circulating IL-5 with high affinity and prevent binding of IL-5 to its receptor.41 Benralizumab is a humanized afucosylated mAb that binds to the alpha subunit of the human IL-5Rα, specifically expressed on the surface of eosinophils and basophils.41 Dupilumab is a mAb that attaches to the alpha subunit of IL-4/13 receptors and promotes signaling after binding to the IL-4Rα subunit.41
MAbs targeting type-2 inflammation is likely to reduce the risk of COVID-19 mediated severe asthma exacerbations by reducing baseline airway inflammation and possibly through specific antiviral properties.20 Omalizumab, cross-linking IgE, would lead to lower type 1 IFN production.20 Mepolizumab, reslizumab and benralizumab, act by increasing the ratio of IFN-γ-to-IL-5 mRNA, which is associated with lower viral shedding and faster disease clearance.20 Finally, IL-4 is crucial for antibody switching to IgE, and IL-13 is a Th2 cytokine involved in airway hyperresponsiveness and remodeling; both of them are involved in susceptibility and clearance of viral infections affecting lower airways.20
Fig. 2 shows a summary of the potential main benefits of mAbs for severe asthma treatment in COVID-19:
Figure 2.
Summary of the potential main benefits of monoclonal antibodies for severe asthma treatment in COVID-19 Legend: AA: airways Ig: immunoglobulin; IFN: interferon; IL: interleukin; pDC: plasmacytoid dendritic cells.
From another perspective, it is theoretically reasonable that mAbs targeting type-2 asthma inflammation can be associated with increased risk for COVID-19 (in terms of infection or severity). However, early reports did not show this clearly.42 Available data did not show consistently significant differences among patients treated with different mAbs in a large cohort study.42 Even though, contradictorily, in the Severe Asthma Network in Italy (SANI), patients treated with anti-IL-5 mAbs had a considerably higher proportion of SARS-CoV-2 (71%) compared to those treated with anti-IgE (29%)30 and this possible effect about increased disease severity in patients treated with biologics for type-2 inflammation has been further debated in another recent study32. Although the number of cases was too small to draw any definitive conclusions, the authors speculated that different mAbs could have specific and distinct impact on antiviral immune response, as suggested for anti-IgE as protective for other viral infections.30 Furthermore, the authors also proposed that the consequence of eosinopenia induced by anti-IL-5 agents might be a risk factor for more severe COVID-19. Currently, no large series of severe asthmatics treated with biologicals infected by COVID-19 have been published, so the ideas about the role of mAbs in modulating the risk of COVID-19 are speculations and need further evidence.30 Assuming the previously stated notion that eosinopenia associated with COVID-19 is likely to be a secondary phenomenon, this concern about biological drug-induced eosinopenia may not be relevant.25 Comprehensive and large-scale investigations are expected to elucidate further the interactions between COVID-19 and type-2 high severe asthma.
Treatment with omalizumab might protect from severe forms of COVID-1943. Omalizumab was shown to enhance antiviral immunity via downregulation of the high-affinity IgE receptor on plasmacytoid dendritic cells, essential for antiviral immune responses.43 Cases of omalizumab patients who contracted COVID-19 have been recorded, and no increased susceptibility to severe disease or asthma exacerbations was observed43 ,.44 The PROSE study showed that in severe asthmatics, omalizumab treatment decreases the duration of viral infections, viral shedding and the risk of respiratory viral illnesses.45 Another study indicated that omalizumab treatment augments plasmacytoid dendritic cells IFN-α responses and attenuates the FcεRIα protein expression induced by these cells, reducing the susceptibility to virus-induced asthma exacerbations.46 Besides, IL-33 levels decrease after omalizumab treatment47 , 48 and this interleukin is important for the production of pro-inflammatory cytokines (including IL-6, IL-1β, TNF-α, MCP-1, and prostaglandin D2).49 All this data suggests a potential effect of omalizumab on antiviral responses.8 It would be interesting to explore whether previous or concurrent use of omalizumab might have protective effects on COVID-19 infection, either on duration, severity or both.8
In those on mepolizumab therapy, a very recent publication reports the outcomes of four severe asthmatic patients with COVID-19 while receiving treatment with mepolizumab, from different centres (UK, Italy and North America).50 Only one patient (who had recognized risk factors for admission and death from COVID-19) required hospitalization and ventilatory support but recovered without evidence of long-term respiratory consequences.50 Four published case reports describe six patients contracting SARS-CoV-2 while receiving benralizumab treatment for severe eosinophilic asthma. The range of symptoms experienced by each patient varied, but all of them recovered.51, 52, 53 These reports add to the debate about whether patients with eosinophil targeting therapies might have an unaltered outcome in COVID-19. As previously mentioned, evidence of severe asthma registries still poses this into question.32 Considering the reported evidence, it is clear that more data is needed. Until then, and for the moment, in severe eosinophilic asthma the expert recommendations are to continue biologic therapy unchanged.54
Case reports of severe asthma patients under dupilumab show no association with negative impact in COVID-19.55 , 56 However, as previously mentioned, dupilumab will block IL-4, which is fundamental for the differentiation of Th2 by IL-6 and this might shift the balance Th1/Th2 towards Th1 and facilitate INFγ production.57 The differentiation of Th2 by IL-6 is dependent on endogenous production of IL-4 whose activity is significantly reduced by dupilumab.57 This mechanism plays a central role in the “cytokine storm” damaging lung in COVID-19 patients57 but the clinical consequences are still a matter of debate32 , 56 On the other hand, due to the mechanism of action of dupilumab, drug discontinuation could be associated with higher susceptibility toward infections, even if there is no evidence supporting this hypothesis regarding SARS-CoV-2.57 Considering available evidence at the moment, treatment with dupilumab should not be stopped during the COVID-19 pandemic.
Globally, in the era of COVID-19, major respiratory societies (Global Initiative for Asthma, European Respiratory Society, British Thoracic Society and American Academy of Allergy, Asthma & Immunology) recommend continuing the biologic treatment, preferably in a self-administered home program.
Discussion
Evidence about COVID-19 is rapidly evolving, and data connecting asthma and COVID-19 is also trendy. A recent systematic review and meta-analysis about asthma and COVID-19 suggested that the prevalence of asthma among COVID-19 patients is similar to the global prevalence of asthma.58 Another interesting conclusion from this study is that people with asthma have a lower risk than those without asthma for acquiring COVID-19 and have similar clinical outcomes.58 Despite the increasing number of published studies about COVID-19 and asthma, the knowledge gap for severe asthma persists due to its particularities. The relationship between COVID-19 and severe asthma is nowadays still a matter for debate as conflicting evidence is published. In the following months with the burden of COVID-19 increasing in terms of mortality and healthcare resources utilization, severe asthma patients will face a hard challenge.3
The COVID-19 pandemic disrupted science in 2020 with a sharp increase in articles on all subjects being submitted to scientific journals and severe asthma was not an exception.59 This vortex of global research has mixed consequences.60 Positives include the higher provision of open access to COVID-19 studies, increased collaboration, expedited governance and ethics approvals of new clinical studies, and broader use of preprints.60 But many challenges have become evident.60 Before the pandemic, it was estimated that up to 85% of research was wasted because of poor questions, poor study design, regulation inefficiency, and no or insufficient reporting of results.60 Many of these problems are amplified in COVID-19 research, with time pressures and inadequate research infrastructure contributing. This might also contribute to the previously discrepancies about COVID-19 impact on asthma and severe asthma in particular.60 , 61 At the present moment, no definitive conclusions can be drawn as many confounding factors might have influenced available evidence.61 Interestingly, as time goes by, several aspects apart from the disease itself start to be shown about the impact of COVID-19 pandemic on severe asthma patients. A recent study concluded that during this period, severe asthma patients were significantly more impacted by the pandemic with increased rates of unemployment and difficulty in getting asthma meds compared to those with non-severe asthma.62
Recent publications have discussed the concept of a long-COVID syndrome that is common and independent from the severity of the acute COVID-19 syndrome.63 This syndrome is more frequent in women and may not be directly attributable to the effect of SARS-CoV-2 but rather an interaction of biopsychosocial effects.63 A recently published meta-analysis studied the frequency of potential respiratory symptoms in COVID-19 patients.64 Fatigue (52%), dyspnea (37%), chest pain (16%) and cough (14%) were the most frequently reported persistent symptoms among COVID-19 pneumonia survivors.64 Another recently published meta-analysis described that COVID-19 patients with pneumonia have long consequences in lung function and the most important one is the diffusion capacity affection.65 How these pathophysiological and symptomatic changes in long-COVID interact with severe asthma is still uncertain.
Anticipating that there are at least several months of pandemic still ahead and that SARS-CoV-2 will be circulating for a longer time, severe asthma patients need proper answers about their disease management in this context. Large-scale, preferably multinational real-life studies with detailed information on asthma phenotype and medication usage in patients with a confirmed diagnosis of COVID-19 would be an ideal next step to further build on this new evidence.61 One critical point is that the most relevant evidence about severe asthma and COVID-19 comes from studies published in mid-2020 and as the number of patients increases the data becomes more robust. From our point of view, apart from the previously discussed aspects, during this COVID-19 pandemic several questions for both patients and physicians involved in severe asthma management are at the forefront of everyone's mind, namely:
-
•
What are the long-term effects of past COVID-19 infection on asthma disease progression?
-
•
What is the real dual role of steroids used for severe asthma management in COVID-19 (cytokine blocking versus induction of coronavirus replication)?
-
•
What are the possible interactions between mAbs directed for COVID-19 treatment and mAbs for severe asthma treatment?
Conclusions
Severe asthma management during the COVID-19 pandemic is now a challenge that will continue in the near future until herd immunity is reached. COVID-19 disease mechanisms affect severe asthma patients and definitely, the disease endotypes might confer different responses. The immunological consequences of SARS-CoV-2 infection are broad and complex, contributing to the increasing complexity of severe asthma knowledge. Regarding severe asthma endotypes, type2-high seems to have a protective role both in SARS-CoV-2 infection and COVID-19 course. On the other hand, type-2 low seems to confer an increased risk of infection and severe disease forms. From a theoretical point of view, although this idea might be defensible further robust evidence is needed to corroborate it. Biologics for severe asthma do not seem to increase the risk of infection and severe COVID-19 and might even be protective, although further evidence is needed. Globally, in the era of COVID-19, major respiratory societies recommend continuing the biologic treatment, preferably in a self-administrated home program.
Funding
No funding to declare.
Declaration of Competing Interest
João Gaspar Marques has received research grants from AstraZeneca and honorarium as speaker/consultant from AstraZeneca, Novartis, Sanofi and TEVA. Mafalda van Zeller has received honorarium as speaker/consultant from AstraZeneca, GlaxoSmithKline, Novartis and TEVA. Pedro Carreiro Martins has received research grants from AstraZeneca and honorarium as speaker/consultant from AstraZeneca, GlaxoSmithKline, Novartis and Sanofi. Cláudia Chaves Loureiro has received honorarium as speaker/consultant from AstraZeneca, GlaxoSmithKline, Novartis, Sanofi and TEVA.
Acknowledgments
This paper would not have been possible without the tremendous effort of all healthcare professionals that produced scientific evidence at the same time that are managing COVID-19 in the wards and emergency rooms all over the World. To all of them our sincere gratitude.
References
- 1.Anderson R.M., Vegvari C., Truscott J., Collyer B.S. Challenges in creating herd immunity to SARS-CoV-2 infection by mass vaccination. Lancet. 2020;396(10263):1614–1616. doi: 10.1016/S0140-6736(20)32318-7. Nov 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. Aug 25. [DOI] [PubMed] [Google Scholar]
- 3.Organization WH. Coronavirus disease (COVID-19) epidemiological update. Accessed 31 of December 2020, 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 4.Parasher A. COVID-19: current understanding of its pathophysiology, clinical presentation and treatment. Postgrad Med J. 2021;97(1147):312–320. doi: 10.1136/postgradmedj-2020-138577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mateus J., Grifoni A., Tarke A., Sidney J., Ramirez S.I., Dan J.M., et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science. 2020;370(6512):89–94. doi: 10.1126/science.abd3871. Oct 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sagar M., Reifler K., Rossi M., Miller N.S., Sinha P., White L.F., et al. Recent endemic coronavirus infection is associated with less-severe COVID-19. J Clin Invest. 2021;131(1):e143380. doi: 10.1172/JCI143380. Jan 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mortaz E., Tabarsi P., Varahram M., Folkerts G., Adcock I.M. The immune response and immunopathology of COVID-19. Front Immunol. 2020;11:2037. doi: 10.3389/fimmu.2020.02037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu S., Zhi Y., Ying S. COVID-19 and asthma: reflection during the pandemic. Clin Rev Allergy Immunol. 2020;59(1):78–88. doi: 10.1007/s12016-020-08797-3. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patrucco F., Benfante A., Villa E., Principe S., Scichilone N., Solidoro P. Severe asthma and COVID-19: lessons from the first wave. J Asthma. 2020;16:1–7. doi: 10.1080/02770903.2020.1861622. Dec. [DOI] [PubMed] [Google Scholar]
- 10.Agache I., Akdis C.A., Akdis M., Canonica G.W., Casale T., Chivato T., et al. EAACI biologicals guidelines-recommendations for severe asthma. Allergy. 2021;76(1):14–44. doi: 10.1111/all.14425. Jan. [DOI] [PubMed] [Google Scholar]
- 11.Mason R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J. 2020;55(4) doi: 10.1183/13993003.00607-2020. , 2000607 Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. Apr 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. Apr 7. [DOI] [PubMed] [Google Scholar]
- 14.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. Mar 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agache I. Severe asthma phenotypes and endotypes. Semin Immunol. 2019;46 doi: 10.1016/j.smim.2019.101301. Dec. [DOI] [PubMed] [Google Scholar]
- 20.Patrucco F., Villa E., Foci V., Benfante A., Bellocchia M., Solidoro P. Severe asthma at COVID-19 time: what's new on biologic therapies. Minerva Med. 2012;(1):112–117. doi: 10.23736/S0026-4806.20.06727-0. Jun 19. [DOI] [PubMed] [Google Scholar]
- 21.Sokolowska M., Lukasik Z.M., Agache I., Akdis C.A., Akdis D., Akdis M., et al. Immunology of COVID-19: mechanisms, clinical outcome, diagnostics, and perspectives-A report of the European Academy of Allergy and Clinical Immunology (EAACI) Allergy. 2020;75(10):2445–2476. doi: 10.1111/all.14462. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozturk A.B., Caglayan B. Angiotensin converting enzyme-2 (ACE2) receptors, asthma and severe COVID-19 infection risk. Eur Ann Allergy Clin Immunol. 2020;52(6):282–285. doi: 10.23822/EurAnnACI.1764-1489.169. Nov. [DOI] [PubMed] [Google Scholar]
- 23.Hughes-Visentin A., Paul A.B.M. Asthma and COVID-19: what do we know now. Clin Med Insights Circ Respir Pulm Med. 2020;14 doi: 10.1177/1179548420966242. 1179548420966242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodrigo-Munoz J.M., Sastre B., Canas J.A., Gil-Martinez M., Redondo N., Del Pozo V. Eosinophil response against classical and emerging respiratory viruses: COVID-19. J Investig Allergol Clin Immunol. 2021;31(2):94–107. doi: 10.18176/jiaci.0624. Jun 16. [DOI] [PubMed] [Google Scholar]
- 25.Lindsley A.W., Schwartz J.T., Rothenberg M.E. Eosinophil responses during COVID-19 infections and coronavirus vaccination. J Allergy Clin Immunol. 2020;146(1):1–7. doi: 10.1016/j.jaci.2020.04.021. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Branco A., Sato M.N., Alberca R.W. The possible dual role of the ACE2 receptor in asthma and coronavirus (SARS-CoV2) infection. Front Cell Infect Microbiol. 2020;10 doi: 10.3389/fcimb.2020.550571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maes T., Bracke K., Brusselle G.G., et al. Inhaled corticosteroids and COVID-19. Am J Respir Crit Care Med. 2020;202(6):900–902. doi: 10.1164/rccm.202006-2129LE. Sep 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veras F.P., Pontelli M.C., Silva C.M., Toller-Kawahisa J.E., de Lima M., Nascimento D.C., et al. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J Exp Med. 2020;217(12) doi: 10.1084/jem.20201129. Dec 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanon S., Brusselle G., Deschampheleire M., Louis R., Michils A., Peche R., et al. COVID-19 and biologics in severe asthma: data from the Belgian Severe Asthma Registry. Eur Respir J. 2020;56 doi: 10.1183/13993003.02857-2020. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heffler E., Detoraki A., Contoli M., Papi A., Paoletti G., Malipiero G., et al. COVID-19 in Severe Asthma Network in Italy (SANI) patients: clinical features, impact of comorbidities and treatments. Allergy. 2021;76(3):887–892. doi: 10.1111/all.14532. Aug 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antonicelli L., Tontini C., Manzotti G., Ronchi L., Vaghi A., Bini F., et al. Severe asthma in adults does not significantly affect the outcome of COVID-19 disease: results from the Italian Severe Asthma Registry. Allergy. 2021;76(3):902–905. doi: 10.1111/all.14558. Aug 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eger K., Hashimoto S., Braunstahl G.J., Brinke A.T., Patberg K.W., Beukert A., et al. Poor outcome of SARS-CoV-2 infection in patients with severe asthma on biologic therapy. Respir Med. 2020;177 doi: 10.1016/j.rmed.2020.106287. Dec 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rial M.J., Valverde M., Del Pozo V., Gonzalez-Barcala F.J., Martinez-Rivera C., Munoz X., et al. Clinical characteristics in 545 patients with severe asthma on biological treatment during the COVID-19 outbreak. J Allergy Clin Immunol Pract. 2021;9(1):487–489. doi: 10.1016/j.jaip.2020.09.050. June 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caminati M., Vultaggio A., Matucci A., Senna G., Almerigogna F., Bagnasco D., et al. Asthma in a large COVID-19 cohort: prevalence, features, and determinants of COVID-19 disease severity. Respir Med. 2020;176 doi: 10.1016/j.rmed.2020.106261. Nov 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haroun-Diaz E., Vazquez de la Torre M., Ruano F.J., Somoza Alvarez M.L., Alzate D.P., Gonzalez P.L., et al. Severe asthma during the COVID-19 pandemic: clinical observations. J Allergy Clin Immunol Pract. 2020;8(8):2787–2789. doi: 10.1016/j.jaip.2020.06.033. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chhiba K.D., Patel G.B., Vu T.H.T., Chen M.M., Guo A., Kudlaty E., et al. Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID-19. J Allergy Clin Immunol. 2020;146(2):307–314. doi: 10.1016/j.jaci.2020.06.010. Aug 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kow C.S., Capstick T., Hasan S.S. Are severe asthma patients at higher risk of developing severe outcomes from COVID-19? Allergy. 2021;76(3):959–960. doi: 10.1111/all.14589. Oct 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calmes D., Graff S., Maes N., Frix A.N., Thys M., Bonhomme O., et al. Asthma and COPD are not risk factors for ICU stay and death in case of SARS-CoV2 infection. J Allergy Clin Immunol Pract. 2021;9(1):160–169. doi: 10.1016/j.jaip.2020.09.044. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Global Initiative for Asthma. Global strategy for asthma management and prevention. Updated 2020. http://ginasthma.org/
- 41.Caminati M., Bagnasco D., Rosenwasser L.J., Vianello A., Senna G. Biologics for the treatments of allergic conditions: severe asthma. Immunol Allergy Clin North Am. 2020;40(4):549–564. doi: 10.1016/j.iac.2020.07.003. Nov. [DOI] [PubMed] [Google Scholar]
- 42.Rial M.J., Valverde M., Del Pozo V., González-Barcala F.J., Martínez-Rivera C., Muñoz X., et al. Clinical characteristics in 545 patients with severe asthma on biological treatment during the COVID-19 outbreak. J Allergy Clin Immunol Pract. 2021;9(1):487–489. doi: 10.1016/j.jaip.2020.09.050. Oct 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lommatzsch M., Stoll P., Virchow J.C. COVID-19 in a patient with severe asthma treated with Omalizumab. Allergy. 2020;75(10):2705–2708. doi: 10.1111/all.14456. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dominguez-Ortega J., Lopez-Carrasco V., Barranco P., Ifim M., Luna J.A., Romero D., et al. Early experiences of SARS-CoV-2 infection in severe asthmatics receiving biologic therapy. J Allergy Clin Immunol Pract. 2020;8(8):2784–2786. doi: 10.1016/j.jaip.2020.06.027. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Esquivel A., Busse W.W., Calatroni A., Togias A.G., Grindle K.G., Bochkov Y.A., et al. Effects of omalizumab on rhinovirus infections, illnesses, and exacerbations of asthma. Am J Respir Crit Care Med. 2017;196(8):985–992. doi: 10.1164/rccm.201701-0120OC. Oct 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gill M.A., Liu A.H., Calatroni A., Krouse R.Z., Shao B., Schiltz A., et al. Enhanced plasmacytoid dendritic cell antiviral responses after omalizumab. J Allergy Clin Immunol. 2018;141(5):1735–1743. doi: 10.1016/j.jaci.2017.07.035. May 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yalcin A.D., Uzun R. Anti-IgE significantly changes circulating interleukin-25, vitamin-D and interleukin-33 levels in patients with allergic asthma. Curr Pharm Des. 2019;25(35):3784–3795. doi: 10.2174/1381612825666190930095725. [DOI] [PubMed] [Google Scholar]
- 48.Huang Y.C., Weng C.M., Lee M.J., Lin S.M., Wang C.H., Kuo H.P. Endotypes of severe allergic asthma patients who clinically benefit from anti-IgE therapy. Clin Exp Allergy. 2019;49(1):44–53. doi: 10.1111/cea.13248. Jan. [DOI] [PubMed] [Google Scholar]
- 49.Moulin D., Donze O., Talabot-Ayer D., Mezin F., Palmer G., Gabay C. Interleukin (IL)-33 induces the release of pro-inflammatory mediators by mast cells. Cytokine. 2007;40(3):216–225. doi: 10.1016/j.cyto.2007.09.013. Dec. [DOI] [PubMed] [Google Scholar]
- 50.Azim A., Pini L., Khakwani Z., Kumar S., Howarth P. Severe acute respiratory syndrome coronavirus 2 infection in those on mepolizumab therapy. Ann Allergy Asthma Immunol. 2021;126(4):436–440. doi: 10.1016/j.anai.2021.01.006. Jan 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Renner A., Marth K., Patocka K., Pohl W. COVID-19 in a severe eosinophilic asthmatic receiving benralizumab - a case study. J Asthma. 2020:1–3. doi: 10.1080/02770903.2020.1781165. Jun 18. [DOI] [PubMed] [Google Scholar]
- 52.Renner A., Marth K., Patocka K., Idzko M., Pohl W. COVID-19 in two severe asthmatics receiving benralizumab: busting the eosinophilia myth. ERJ Open Res. 2020;6(4):457–2020. doi: 10.1183/23120541.00457-2020. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garcia-Moguel I., Diaz Campos R., Alonso Charterina S., Fernandez Rodriguez C., Fernandez Crespo J. COVID-19, severe asthma, and biologics. Ann Allergy Asthma Immunol. 2020;125(3):357–359. doi: 10.1016/j.anai.2020.06.012. Sep 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klimek L., Pfaar O., Worm M., Eiwegger T., Hagemann J., Ollert M., et al. Use of biologicals in allergic and type-2 inflammatory diseases during the current COVID-19 pandemic: position paper of Arzteverband Deutscher Allergologen (AeDA)(A), Deutsche Gesellschaft fur Allergologie und Klinische Immunologie (DGAKI)(B), Gesellschaft fur Padiatrische Allergologie und Umweltmedizin (GPA)(C), Osterreichische Gesellschaft fur Allergologie und Immunologie (OGAI)(D), Luxemburgische Gesellschaft fur Allergologie und Immunologie (LGAI)(E), Osterreichische Gesellschaft fur Pneumologie (OGP)(F) in co-operation with the German, Austrian, and Swiss ARIA groups(G), and the European Academy of Allergy and Clinical Immunology (EAACI)(H) Allergol Select. 2020;4:53–68. doi: 10.5414/ALX02166E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhalla A., Mukherjee M., Radford K., Nazy I., Kjarsgaard M., Bowdish D.M.E., et al. Dupilumab, severe asthma airway responses, and SARS-CoV-2 serology. Allergy. 2021;76(3):957–958. doi: 10.1111/all.14534. Aug 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tanabe N., Matsumoto H., Hamada S., Ito I., Hirai T. Dupilumab maintenance therapy in an asthmatic patient with coronavirus disease 2019 pneumonia. Allergol Int. 2021;70(2):274–276. doi: 10.1016/j.alit.2020.10.005. Nov 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patruno C., Stingeni L., Fabbrocini G., Hansel K., Napolitano M. Dupilumab and COVID-19: what should we expect? Dermatol Ther. 2020;33(4):e13502. doi: 10.1111/dth.13502. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sunjaya A.P., Allida S.M., Di Tanna G.L., Jenkins C. Asthma and risk of infection, hospitalization, ICU admission and mortality from COVID-19: systematic review and meta-analysis. J Asthma. 2021:1–22. doi: 10.1080/02770903.2021.1888116. Feb 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Else H. How a torrent of COVID science changed research publishing - in seven charts. Nature. 2020;588(7839):553. doi: 10.1038/d41586-020-03564-y. Dec. [DOI] [PubMed] [Google Scholar]
- 60.Glasziou P.P., Sanders S., Hoffmann T. Waste in covid-19 research. BMJ. 2020;369:m1847. doi: 10.1136/bmj.m1847. May 12. [DOI] [PubMed] [Google Scholar]
- 61.Eger K., Bel E.H. Asthma and COVID-19: do we finally have answers? Eur Respir J. 2021;57:2004451. doi: 10.1183/13993003.04451-2020. Dec 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arora N., Lowe D., Sarsour N., Jaffee H., Eftekhari S., Carpenter L.M., et al. Asthma care during COVID-19: differences in attitudes and expectations between physicians and patients. J Asthma. 2021 doi: 10.1080/02770903.2021.1887214. Feb 24. [DOI] [PubMed] [Google Scholar]
- 63.Sykes D.L., Holdsworth L., Jawad N., Gunasekera P., Morice A.H., Crooks M.G. Post-COVID-19 symptom burden: what is long-COVID and how should we manage it? Lung. 2021;1999:113–119. doi: 10.1007/s00408-021-00423-z. Feb 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cares-Marambio K., Montenegro-Jimenez Y., Torres-Castro R., Vera-Uribe R., Torralba Y., Alsina-Restoy X., et al. Prevalence of potential respiratory symptoms in survivors of hospital admission after coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Chron Respir Dis. 2021;18 doi: 10.1177/14799731211002240. Jan-Dec14799731211002240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Torres-Castro R., Vasconcello-Castillo L., Alsina-Restoy X., Solis-Navarro L., Burgos F., Puppo H., et al. Respiratory function in patients post-infection by COVID-19: a systematic review and meta-analysis. Pulmonology. 2020 doi: 10.1016/j.pulmoe.2020.10.013. Nov 25. [DOI] [PMC free article] [PubMed] [Google Scholar]