Abstract
Objective:
A recent 3-month double-blind, placebo-controlled study demonstrated efficacy and safety of pediatric prolonged-release melatonin (PedPRM) for insomnia in children with autism spectrum disorder. This study examined the long-term effects of PedPRM treatment on sleep, growth, body mass index, and pubertal development.
Method:
Eighty children and adolescents (2–17.5 years of age; 96% with autism spectrum disorder) who completed the double-blind, placebo-controlled trial were given 2 mg, 5 mg, or 10 mg PedPRM nightly up to 104 weeks, followed by a 2-week placebo period to assess withdrawal effects.
Results:
Improvements in child sleep disturbance and caregiver satisfaction with child sleep patterns, quality of sleep, and quality of life were maintained throughout the 104-week treatment period (p < .001 versus baseline for all). During the 2-week withdrawal placebo period, measures declined compared with the treatment period but were still improved compared with baseline. PedPRM was generally safe; the most frequent treatment-related adverse events were fatigue (6.3%), somnolence (6.3%), and mood swings (4.2%). Changes in mean weight, height, body mass index, and pubertal status (Tanner staging done by a physician) were within normal ranges for age with no evidence of delay in body mass index or pubertal development.
Conclusion:
Nightly PedPRM at optimal dose (2, 5, or 10 mg nightly) is safe and effective for long-term treatment in children and adolescents with autism spectrum disorder and insomnia. There were no observed detrimental effects on children’s growth and pubertal development and no withdrawal or safety issues related to the use or discontinuation of the drug.
Clinical trial registration information:
Efficacy and Safety of Circadin in the Treatment of Sleep Disturbances in Children With Neuro-development Disabilities; https://clinicaltrials.gov/; NCT01906866.
Keywords: autism, melatonin, sleep
The prevalence of insomnia in children and adolescents with autism spectrum disorder (ASD) is high compared with typically developing peers and estimated at 50% to 80%.1 The most frequent problems are difficulty falling asleep (approximately 40%) and maintaining sleep (approximately 35%).2–4 An accumulating body of evidence demonstrates both short- and long-term negative consequences of poor sleep in children, including inattention, hyperactivity, irritability, poor memory, poorer school performance, anxiety, depression, and poorer cardiometabolic health in early adolescence.5–7 Sleep problems in children with ASD are particularly challenging to their families and associated with increased maternal distress, parental sleep disruption, and poor quality of life of caregivers.8 The improvement of sleep duration and onset in children with ASD is among their families’ priorities for research and treatment development.9,10
Clinical guidelines recommend sleep hygiene and/or behavioral intervention as the first-line treatment, but if this fails, there are no medications approved by the Food and Drug Administration for the treatment of pediatric insomnia.11 Melatonin, a hormone produced by the pineal gland during the night, is an endogenous sleep promoter and regulator of the circadian clock in humans. Some evidence suggests impaired regulation of melatonin production in children with ASD.12 In certain neurogenetic disorders (eg, Smith-Magenis syndrome [SMS]), melatonin production is abnormally shifted to the daytime hours.13 Exogenous melatonin has been found to be effective for sleep problems in ASD.1,14 Melatonin replacement therapy in ASD and SMS is therefore a rational etiological approach.
Whereas the ongoing need of using melatonin in the treatment of sleep problems in children with ASD is recommended as part of good clinical practice,1 data are needed to support evidence-based clinical recommendations regarding the duration and safety of long-term melatonin use in these children. Pediatric prolonged-release melatonin (PedPRM) is an oral solid preparation of prolonged-release melatonin designed to mimic the physiological secretion profile of melatonin providing sustained plasma levels for 8 to 10 hours. Because the prolonged-release properties of the preparation are lost if crushed, a pediatric-appropriate 3-mm-diameter, film-coated tasteless and odorless prolonged-release melatonin mini-tablet (Slenyto; Neurim Pharmaceuticals Ltd., Tel Aviv, Israel) was developed, which can be more easily swallowed and better tolerated by young children. PedPRM has recently been licensed by the European Medicines Agency for use in pediatric populations with ASD and SMS.
A randomized, double-blind (DB), placebo-controlled, parallel-group, multicenter (European Union and United States) study of PedPRM for 13 weeks (2 mg with an optional dose escalation to 5 mg after 3 weeks) in children (N = 25; age range 2–17.5 years) with ASD and SMS with or without ADHD comorbidity demonstrated that PedPRM was efficacious and safe compared with placebo for treatment of insomnia.14 The main benefits of PedPRM demonstrated in the DB trial were that total sleep time (TST) increased: participants slept on average 57.5 minutes longer at night with PedPRM compared with 9.14 minutes with placebo (p = .034). Sleep latency also improved, decreasing by 39.6 minutes on average with PedPRM and 12.5 minutes with placebo (p = .011), without causing earlier wakeup time.14 In addition, externalizing behavior in children and caregivers’ quality of life improved significantly (p = .021 and p = .010, respectively, compared with placebo treatment).15 Completers of the DB phase entered a prospective 91 weeks of open-label (OL) PedPRM treatment and finally 2 weeks of placebo to evaluate withdrawal effects.
Once all participants in the OL phase completed 39 weeks of follow-up (week 54), the 1-year data were summarized and published,16 while 80 participants continued for an additional 52 weeks of PedPRM treatment and 2 weeks placebo to complete the study. In this article, we report on the benefits and risks of PedPRM treatment (2 mg, 5 mg, or 10 mg daily up to 2 years of continuous use) and discontinuation, including impact of treatment on child sleep, growth, and puberty and caregivers’ sleep and quality of life, thus providing clinicians with evidence-based data relating to PedPRM effectiveness and safety.
METHOD
Participants
Participants included children and adolescents (2–17.5 years of age) with confirmed physician-diagnosed ASD according to DSM-IV/DSM-5 or ICD-10 criteria or SMS and a minimum of 3 months of impaired sleep, defined as ≤6 hours of continuous sleep and/or ≥0.5-hour sleep latency from light off 3 out of 5 nights per week for 2 weeks based on parent reports and patient medical history as described.14,16 The trial complied with the principles of the Declaration of Helsinki (1989) and standards of good clinical practices and was carried out between December 1, 2013, and February 28, 2018, in 14 centers in the United States and 10 centers in Europe. All caregivers gave written informed consent before participation and participants ≥6 years of age (according to country regulations) gave written informed consent in addition to their caregivers.
Study Procedures
The study design is illustrated in Figure 1A. Parents of children who did not have a documented history of sleep hygiene and behavioral intervention at screening received education in behavioral sleep interventions provided through a standardized pamphlet14,17 and were asked to implement the outlined strategies for 4 weeks. Participants who were still eligible were then entered into the study. The study comprised a 2-week single-blind placebo run-in (baseline), followed by a 13-week randomized DB PedPRM (mini-tablet) or placebo taken nightly after the evening meal, 30 to 60 minutes before bedtime. Completers entered a 91-week OL PedPRM treatment period (according to the final DB dose) followed by a 2-week single-blind placebo run-out (withdrawal period), for a total of 108 weeks of study medication.
FIGURE 1.
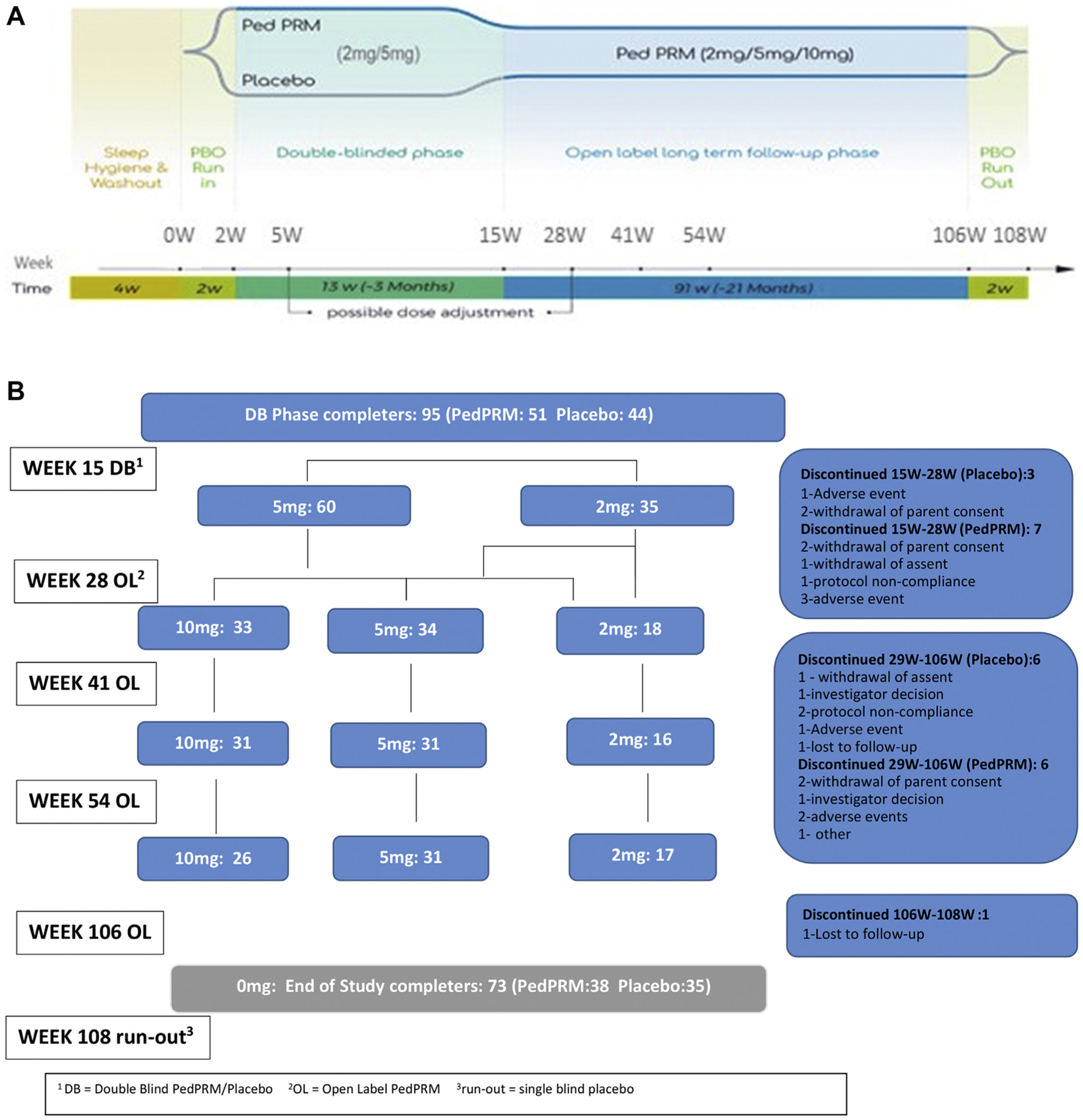
Overall Study Design, Patient Disposition, and Dose Breakdown for Participants
Note: CSDI = Composite Sleep Disturbance Index; DB = double-blind; OL = open-label; PBO = placebo; PRM = prolonged-release melatonin; SND = sleep and nap diary; TST = total sleep time.
The starting dose was 2 mg PedPRM (or placebo equivalent) with optional dose escalation from 2 to 5 mg after 3 weeks of DB treatment (week 5 in the trial) and from 2 to 5 mg or from 5 to 10 mg/day after 13 weeks of OL treatment (week 28) if participants failed to improve TST and/or sleep latency by at least 60 minutes from baseline. Optional decrease in dose was allowed at all times during the study, based on investigator decision (Figure 1B).
Sleep variables, reported by parent/caregiver, were assessed using a validated sleep and nap diary (SND) that has been used in previous trials including a previous pediatric immediate-release melatonin trial.17,18 The SND was to be completed every morning by the parent/caregiver at home for 14 days before each visit throughout the 13-week DB period and the first 39 weeks of the OL period. The a priori defined primary efficacy endpoint was the changes from baseline in mean TST over the 14 days by the end of the DB period. The primary secondary endpoint was the change from baseline in mean sleep latency. Other secondary sleep variables were the changes from baseline in mean duration of wake after sleep onset, mean number of awakenings, and mean longest sleep episode, all from the SND.
Child sleep was assessed at each visit throughout the 2-year study using the Composite Sleep Disturbance Index (CSDI), which scores the frequency and duration of the participant’s sleep habits over the previous month (6 habits: settling at bedtime, sleep induction, waking up during the night, resettling, early wake time, and co-sleeping with caregivers; scored 0–2; total score range 0–12).19 Caregiver measures included the Pittsburgh Sleep Quality Index (PSQI) score (total score >5 is considered significant sleep disturbances; total score range 0–21),20,21 5-item World Health Organization Well-Being Index (WHO-5) (covers positive mood, vitality, and general interests; total score range 1–25),22 and a separate CSDI item that records satisfaction with the child’s sleep patterns rated from 1 to 5, all of which were recorded at each visit.
Safety was monitored throughout the study, using standard clinical trials methods and definitions (treatment-emergent signs and symptoms [TESS],23 adverse events, vital signs, and physical examination). Epilepsy and health status were also assessed.
Child development was assessed in children ≥8 years of age using Tanner pubertal staging done by a physician, body mass index (BMI) percentiles (obesity), and z-scores. The Tanner scores consist of 3 scores for boys and 3 for girls, describing genitals, testicles, and pubic hair in boys and breasts, pubic hair, and menarche in girls. Results in our population were compared with the general Dutch population to assess pubertal development.23
Statistical Methods
Changes from baseline were analyzed using paired t tests for all observed cases (significance level < .05). Safety analyses were performed on all randomly assigned participants who took at least 1 dose of study medication.
RESULTS
Study Population
Of 119 randomly assigned and treated participants, 95 completed the DB phase (week 15) (51 of the PedPRM-treated and 44 of the placebo-treated groups, mean [SD] age 9 [4.2] years, range 2–17 years, 74.7% male participants). Completers entered the OL phase with PedPRM; 74 completed the treatment (week 106), and 73 completed the run-out phase (70 had ASD [95.9%] and 3 [4.1%] had SMS) (Figure 1B). In the DB phase, significantly more participants discontinued in the placebo group than PedPRM-treated group; the most common reasons for discontinuation were withdrawal of parent consent mainly because of personal reasons (n = 6) and adverse events (n = 6).
Treatment adherence was close to 100% throughout the study. Principal investigators reported that children were able to swallow the mini-tablets without crushing, thus confirming acceptability and suitability of 3-mm-diameter mini-tablets for preschoolers ≥2 years of age.24
Efficacy
The change from baseline in CSDI score after 13 weeks of treatment significantly correlated with the changes in TST recorded in the SND (Spearman’s rank correlation −0.375; p < .001) regardless of whether receiving the active drug or placebo (Supplemental Figure S1, available online).14 The changes in CSDI and caregiver variables for participants originally assigned to the 2 randomization groups by the end of the OL phase (week 106) are depicted in Table 1. There were no notable differences in outcomes at week 106 between participants originally assigned to placebo when given PedPRM treatment for 91 weeks (placebo group) or participants originally assigned to PedPRM and given PedPRM for 104 weeks (PedPRM group), thus allowing us to combine the groups (Figure 2). The mean (SE) changes from baseline in CSDI sleep disturbance, caregiver’s satisfaction with child’s sleep patterns, and WHO-5 quality of life for the combined PedPRM and placebo groups (n = 74) in the follow-up phase are presented in Figure 2. For completeness, data on this combined population from week 28 and week 5414,16 are also depicted (yellow outline). The same results were obtained when looking only at the 73 completers of the OL period. Increase in efficacy was observed between week 28 and week 54 that followed the dose optimization (at week 28) allowing some children to escalate to 10 mg/day. Improvements in CSDI (decrease), in caregiver satisfaction with child’s sleep patterns (increase), and WHO-5 (increase) were significant over baseline values and maintained until the end of PedPRM treatment.
TABLE 1.
Changes From Baseline in Child Sleep and Caregiver Variables at Week 106 in Pediatric Prolonged-Release Melatonin (PedPRM) and Placebo Groups
| Variable | PedPRM (n = 38)a | Placebo (n = 35)a | ||
|---|---|---|---|---|
| Mean change from baselineb | (95% CI) | Mean change from baselineb | (95% CI) | |
| CSDI (scale of 0–12) | −3.18 | (−4.49, −1.86) | −3.59 | (−4.86, −2.31) |
| pc | < .001 | < .001 | ||
| CSDI caregiver satisfaction (scale of 1–5) | 1.79 | (1.35,2.24) | 1.82 | (1.40,2.25) |
| pc | < .001 | < .001 | ||
| WHO-5c (scale of 0–25) | 3.44 | (1.51,5.36) | 1.52 | (−0.55, 3.58) |
| pc | .001 | .145 | ||
| PSQI (scale of 0–21) | −1.26 | (−2.46, −0.07) | −1.88 | (−3.28, −0.48) |
| pc | .039 | .010 | ||
Note: CSDI = Composite Sleep Disturbance Index; PSQI = Pittsburgh Sleep Quality Index; WHO-5 = 5-item World Health Organization Well-Being Index.
Participants in the PedPRM randomized group had 104 weeks of continuous treatment, and participants in the placebo group had 91 weeks of continuous PedPRM treatment.
Baseline = 2 weeks single-blind placebo run-in before randomization.
Paired t test for the change from baseline levels, significance at p < .05.
FIGURE 2.
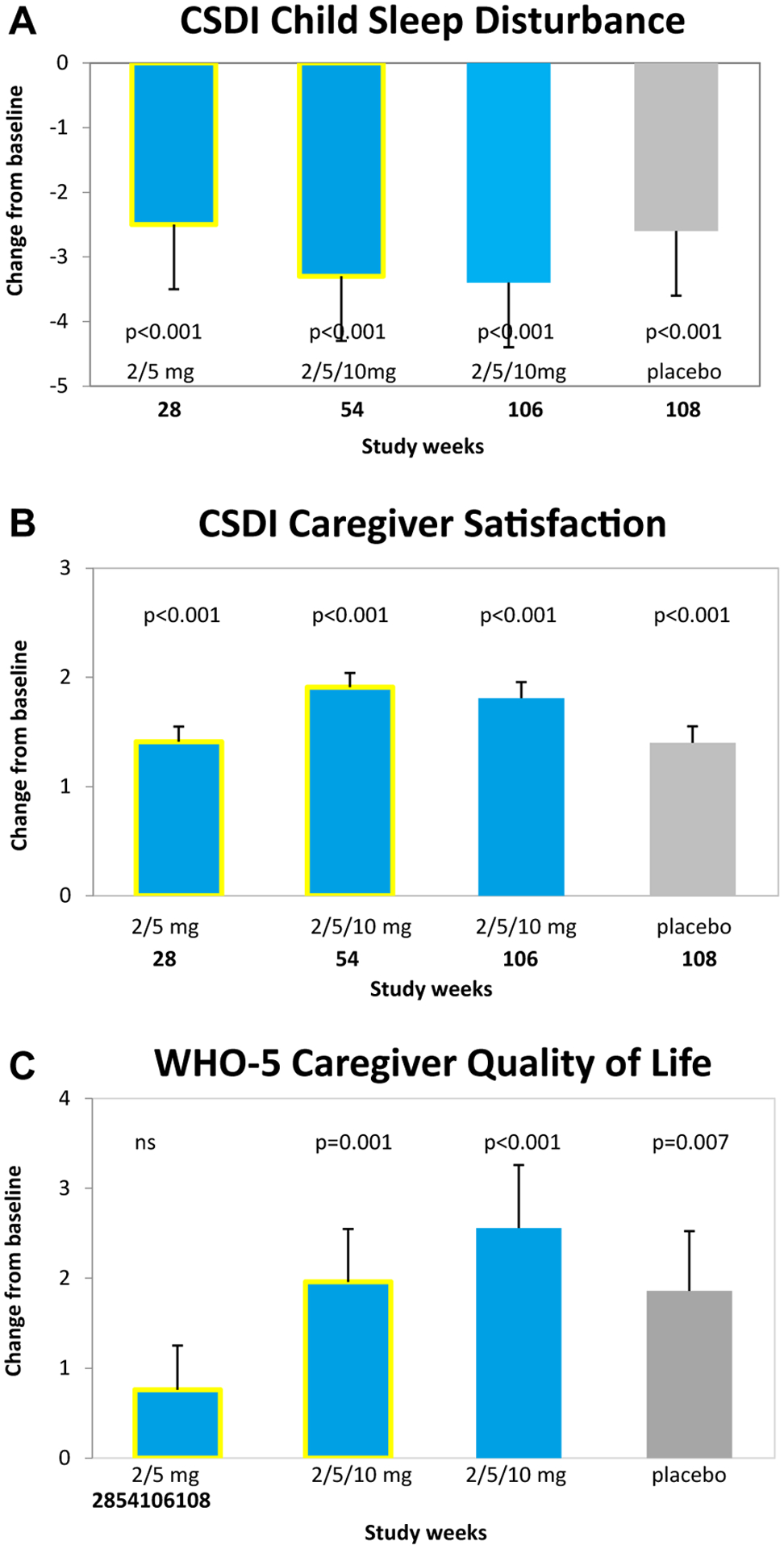
Effects of Pediatric Prolonged-Release Melatonin (PedPRM) Treatment on Child and Caregiver Parameters
Note: Effects of PedPRM (2, 5, or 10 mg/day) for 28 weeks,15 54 weeks,15 and 106 weeks and placebo withdrawal (2 weeks) on (A) CSDI child sleep disturbance, (B) CSDI caregiver satisfaction, and (C) WHO-5 caregiver quality of life. Data from 106 weeks and 2 weeks withdrawal are mean (SE) change from baseline in the combined PedPRM and placebo population. CSDI = Composite Sleep Disturbance Index; PSQI = Pittsburgh Sleep Quality Index; WHO-5 = 5-item World Health Organization Well-Being Index.
After the 2-weeks run-out on placebo (week 108), the treatment effects decreased but remained significantly better compared with baseline (Figure 2). Similarly, mean (SE) change from baseline in caregiver PSQI for the combined PedPRM and placebo groups (n = 74) at week 106 improved −1.55 (0.448) (p = .001) showing maintenance of beneficial effect from values seen a year before (week 54).16 The effects declined during run-out on placebo. Nevertheless, significant improvements in PSQI were still reported at withdrawal (not shown). Diagnosis (ASD with or without attention-deficit/hyperactivity disorder or SMS) and comedication (eg, stimulants) did not affect PedPRM efficacy outcomes (data not shown).
Safety
Mean time of PedPRM treatment in the entire study was 517.8 days (range 3–666 days) in the PedPRM group and 545.5 days (range 80–659 days) in the placebo group. By week 106, 23% (17/74) of participants used 2 mg/day, 42% (31/74) used 5 mg/day, and 35% (26/74) used 10 mg/day PedPRM (mean daily dose 6.06 mg/day). No particular traits in optimal dose used, such as age, comedication, diagnosis, or symptom severity, were noticed.
During the DB phase, 1 participant in the PedPRM group had a dose reduction from 5 mg to 2 mg owing to an unacceptable increase in daytime fatigue, and 2 participants in the placebo group had unscheduled dose decreases owing to unacceptable behavioral changes. In the 91-week OL phase, 6 participants had unscheduled dose decreases owing to unacceptable increases in daytime fatigue; in 4 of 6 participants, the increases in daytime fatigue occurred shortly after dose escalation and resolved by decreasing the dose to that used before the dose escalation. One patient had a dose decrease because of another reason, and 1 patient had a dose decrease because the treatment effect was reduced at the highest (10 mg) dose.
Treatment-Emergent Adverse Events
No deaths were reported during any phase of the study. The most commonly reported severe treatment-emergent adverse events (TEAEs) in both randomization groups were agitation, fatigue, and mood swings (Table S1, available online). In the placebo group, 1 participant temporarily discontinued owing to 2 serious adverse events (pneumonia and viral respiratory tract infection) and 1 nonserious adverse event (tachypnea).
Overall, TEAEs were reported by 51 (85.0%) participants in the PedPRM group and 50 (76.9%) participants in the placebo-treated group during the DB period and by 80 (84.2%) participants (PedPRM and placebo groups) during the 91-week OL phase (Table S2, available online). Most of these TEAEs were similar between groups and known symptoms in children with ASD (eg, agitation, mood swings) or generally in children (eg, upper respiratory tract infection, cough, dyspnea, vomiting). In the DB period, somnolence was significantly more common in the PedPRM-treated group than placebo-treated group (p = .044), and headaches were more common in the PedPRM-treated group than placebo-treated group but not significantly (p = .29). In the PedPRM group, 1 participant discontinued use owing to nonserious adverse events (fatigue, agitation, and stereotypies). During the OL phase, 24 participants reported 31 somnolence events for a rate of 0.19 events per participant for 1 year of treatment (Table S2, available online). This rate of 0.19 translates into less than 1 event per participant per 5 years of treatment. Six participants had unscheduled dose decreases owing to unacceptable increases in daytime fatigue. Most of these events occurred shortly after dose escalation, with resolution by decreasing the dose to that used before the dose escalation.
Treatment-Related Adverse Events
The rate of adverse events considered by the clinician to be treatment-related adverse events per participant per 1 year PedPRM treatment decreased from 1.87 in the DB phase to 0.078 in the OL phase (Table S3 available online). The most commonly reported treatment-related adverse events in the OL period were somnolence, fatigue, and mood swings (Table S3, available online).
No noticeable changes were found in vital signs at any time point during the study. There were no differences from baseline in the physical examination except for BMI and pubertal state (detailed under “Child Growth Parameters”), which were within normal ranges for their age.
A history of seizures was present in 16 participants (12.8%). Four participants in the PedPRM group and 3 in the placebo group had received a diagnosis of epilepsy before the study. Two of these participants experienced absences seizures during the OL phase: 1 participant experienced 2 nonserious seizures of 1-minute duration each, and the other experienced one 1-minute mild seizure. Two participants experienced new-onset seizures: 1 experienced a nonserious absence seizure under placebo (DB), and the other experienced 2 generalized tonic-clonic seizures of 1-minute duration recorded at week 54 and week 106 (OL) as nonserious adverse events, moderate in severity, and unlikely to be related to study treatment.
Treatment-Emergent Signs and Symptoms
TESS events were based on the TESS questionnaire; all events were intermittent. In the 13-week DB period, moderate/severe somnolence was more commonly reported with PedPRM treatment (26 participants) than placebo treatment (12 participants; p = .005) and occurred most commonly within a short time after dose escalation. Headaches (mostly mild/moderate) were also more commonly reported with PedPRM treatment (23 participants) than placebo treatment (9 participants; p = .015). There were no other notable differences between the treatment groups for TESS. During the 91-week OL period, moderate/severe somnolence was reported by 33 participants (26 from the PedPRM group and 7 from the placebo group). None of the TESS symptoms became more prevalent at week 108 (placebo run-out) compared with the PedPRM treatment weeks indicating no signs of withdrawal symptoms following discontinuation.
Child Growth Parameters
There were no significant differences between PedPRM and placebo groups for weight, height, or BMI in the DB phase. The mean (SE) difference in BMI between PedPRM and placebo groups after the 13-week DB treatment was −0.21 (0.151) (p = .16). BMI z-score had increased by 0.008 ± 0.3087 in the PedPRM group compared with 0.065 ± 0.4279 in the placebo group with mean treatment difference (PedPRM-placebo) of −0.055 (95% CI −0.198, 0.088) (p = .445), indicating no significant difference between the treatment groups.
The mean (SE) change from baseline in BMI after the 91-week OL phase for the whole group was 1.67 (0.278) (95% CI 1.12, 2.23) (p < .001). The mean (SE) change from baseline in BMI z-score for the whole group after the 91-week OL phase was 0.47 (0.085) (95% CI 0.30, 0.64) (p < .001). At week 106, the mean BMI z-score, which takes into account the age and sex of the child, was approximately 1.163 for the PedPRM group, 1.072 for the placebo group, and 1.1 (range −2.39 to 3.55) for the total group, which is considered within the normal range.25
Tanner Assessment of Pubertal Development
More participants in the placebo group were preadolescent compared with the PedPRM group (Tanner assessments) reflecting a slightly lower age at study entry (mean age 8.4 years versus 9.0 years) (Table 2). After 2 years, 31 participants ≥8 years of age provided data, and 13 were reluctant to do so. The SD scores at week 106 for the PedPRM and placebo groups were within the normal range for their age (Table 2 and Figure 3). At week 15, change from baseline for SD scores of pubic hair, breast, and genitalia development were similar between the PedPRM and placebo-treated groups.14 At week 106, the mean change from baseline in SD score of pubic hair, breast, and genitalia development increased by almost 1 to 1.5 points in both randomization groups. Four children with high BMI tended to mature somewhat earlier. In addition, most participants >8 years of age had shifted Tanner stage higher than their baseline (Figure 3). No delay in pubertal development was evident.
TABLE 2.
Pubertal Development and Change From Baseline in Mean Standard Deviation Scores at Week 106 in Children ≥8 Years of Age Treated With Pediatric Prolonged-Release Melatonin (PedPRM)
| SDS | PedPRM group | Placebo group | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | |||||
| Pubic hair growth | 0.881 (1.11), n = 19 | −0.43 to 3.04 | 1.323 (0.998), n = 12 | −0.43 to 2.63 | ||||
| Breast development | 0.709 (1.16), n = 7 | −0.12 to 3.14 | NA | NA | ||||
| Genitalia development | 0.692 (0.96), n = 12 | −0.55 to 2.12 | 1.205 (0.8), n = 12 | −0.55 to 2.11 | ||||
| Change from baseline | Mean (SD) | Range | (95%CI) | P | Mean (SD) | Range | (95%CI) | P |
| Pubic hair growth | 1.09 (1.24), n = 16 | 0.0 to 3.40 | (0.49, 1.69) | < .0001 | 1.55(1.11), n = 11 | 0.0 to 2.85 | (0.85, 2.35) | < .001 |
| Breast development | 1.78 (1.70), n = 5 | 0.0 to 3.54 | (0.21,3.36) | < .001 | NA | NA | NA | NA |
| Genitalia development | 0.74 (1.09), n = 11 | 0.0 to 2.99 | (0.05, 1.43) | < .001 | 11.30 (1.00), n = 11 | 0.0 to 2.48 | (0.67, 1.94) | < .001 |
Note: NA = not applicable; SDS = standard deviation score.
FIGURE 3.
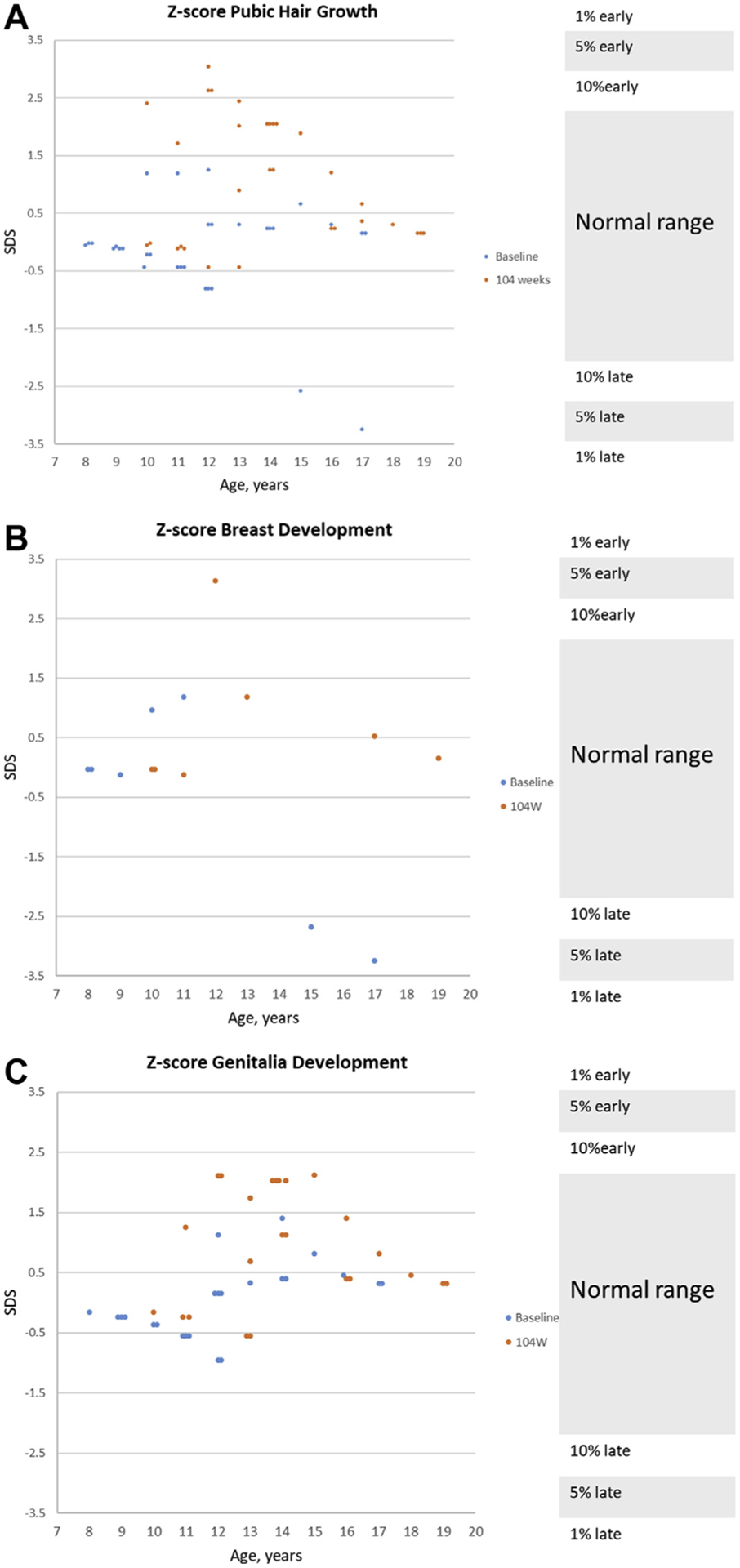
Effects of Continuous Pediatric Prolonged-Release Melatonin (PedPRM) Treatment on Pubertal Development
Note: Effects of continuous PedPRM treatment (104 weeks PedPRM, 91 weeks placebo) on (A) male and female participant pubic hair growth by age (n = 31), (B) female participant breast development by age (n = 7), and (C) male participant genitalia development by age (n = 24). The individual standard deviation scores (SDS) at baseline (week 2, blue) and end of PedPRM treatment (week 106, red) are depicted. Normal ranges are marked on the right-hand y-axis.39
DISCUSSION
This study strongly supports the longer-term effectiveness and safety of PedPRM for insomnia in children with ASD. The CSDI provides evidence on several aspects of child sleep disturbances: settling at bed time, sleep induction, waking up during the night, resettling, early wake time, and co-sleeping with caregivers. Changes in CSDI were significantly correlated with TST. The improvement in sleep CSDI was maintained throughout the 2 years under the optimal dose (2, 5, or 10 mg nightly) and so were the benefits to caregivers.
The pharmacological activity of PedPRM weans off after stopping the active treatment. With PedPRM, similar to prolonged-release melatonin in participants with insomnia ≥55 years of age,26,27 participants did not show any emerging symptoms or worsening of insomnia during the run-out period, suggesting that discontinuation is not associated with withdrawal effects or rebound insomnia.
Treatment compliance and acceptance in children with ASD, many of whom have swallowing difficulties and are sensitive to smell, was excellent without the need to crush or dissolve the mini-tablets (which would negate the prolonged-release properties) supporting the use of the tasteless and odorless mini-tablets in this population.
There were no notable effects of long-term PedPRM on vital signs or measures of child growth, and no unexpected safety issues were reported. Adverse effects were few and generally mild, with fatigue and somnolence emerging as the main treatment-related TEAEs. Somnolence was usually reported only once by a participant at some time during the treatment period, most commonly within a short time after dose escalation, and was much less common in the OL phase (Table S2, available online). Fatigue was usually reported shortly after dose escalation and resolved by decreasing the dose to that used before the dose escalation. Treatment-related somnolence and fatigue most probably reflected the pharmacological effect of residual daytime melatonin secondary to the excessive dose. It is possible that some of these participants were poor metabolizers of CYP1A2 enzyme and developed daytime somnolence or fatigue owing to melatonin accumulation.28 However, gradual loss of effect of exogenous melatonin, which is to be expected with poor CYP1A2 metabolism,28 was observed in only 1 participant on a 10-mg dose and resolved by decreasing the dose to the 5 mg used before dose escalation. Future studies measuring daytime melatonin levels in children with ASD before and after such treatment could elucidate whether somnolence and/or fatigue events were related to melatonin accumulation.
Children and adolescents with ASD can have more frequent or more severe mood changes than typically developing teenagers,29 and as this is an open-label study, the mood swings, though considered treatment-related, may in fact be due to the underlying disorder.
In the DB period, there were no seizures on PedPRM treatment, and there was 1 new-onset absence seizure on placebo. During the 91-week OL period, 1 participant experienced 2 absence seizures, and 1 participant had a new-onset absence seizure, all considered by the clinicians unlikely to be related to study treatment. There has been only 1 short report in the literature30 describing changes in seizure frequency with melatonin (the source of the melatonin impurities profile was not disclosed). Seizures were not reported as an adverse effect in a systematic review and meta-analysis on the safety of melatonin in ASD that included 35 published studies28 or in a retrospective review of melatonin usage in 107 children with ASD, including 21 with seizures.31 In summary, PedPRM treatment was not associated with new-onset or worsening seizures.
It has been suggested that melatonin is involved in the modulation of human sexual maturation,32 but clinical experience related to this issue has yielded inconclusive and sometimes conflicting results.33,34 An association of pineal tumors and precocious puberty was first hypothesized in 1898 when Heubner described the first case, but this has not been unequivocally demonstrated since.35 Decline in melatonin production after puberty suggested that puberty stage may mediate the decline of melatonin, or the decrease in melatonin amplitude may be an indicator of pubertal progression.36 However, despite supplemental melatonin use, 30 female participants and 15 male participants with chronic sleep-onset insomnia showed normal onset of puberty.37 Furthermore, a study of 3- to 10-year-old children38 receiving supplemental melatonin for 14 weeks showed no alterations in estrogen, testosterone, follicle-stimulating hormone, luteinizing hormone, or prolactin with treatment. The results of the present study show no delay in sexual maturation with PedPRM in 31 participants 8 to 17 years of age after 2 years of continuous use, and no child was delayed in sexual maturation.
A limitation in this study was the OL design. With respect to growth and pubertal development, we relied on published statistics, which may vary by location and genetic origin. Another limitation was missing data from 13 participants who declined to be assessed. Nevertheless, the fact that the placebo randomized group had 3 months less exposure and did not show more rapid growth or pubertal development than the PedPRM group supports that there were no major effects on child development and sexual maturation.
In conclusion, melatonin treatment should be considered only when sleep hygiene (including minimizing blue light in the evening) and behavioral interventions have been tried and were not successful. The long-term safety and unique efficacy profiles indicate that PedPRM provides significant benefits for insomnia in children and adolescents with ASD.
Supplementary Material
Acknowledgments
This project was supported by Neurim Pharmaceuticals. The project described was also supported by Clinical and Translational Sciences Awards (CTSA) Program award No. UL1 TR002243 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health. The medication used in the trial (Slenyto [PedPRM]) is a product of Neurim Pharmaceuticals.
Mr. Breddy served as the statistical expert for this research.
The authors would like to thank the study participants and their families for their cooperation and commitment. The authors thank all members of the trial team for their active contribution. The authors express their sincere gratitude to Moshe Laudon, PhD, of Tel Aviv University, for developing the investigational drug and to Amnon Katz, PhD, of the Hebrew University of Jerusalem, for his help with the study design and research. Moreover, the authors are grateful to all investigators that participated in the study.
Footnotes
Disclosure: Dr. Malow has served as an investigator and paid consultant for Neurim Pharmaceuticals, Vanda Pharmaceuticals, Janssen Pharmaceuticals, and Rockmelon. Dr. Findling has served as an investigator, has received research support, has acted as a paid consultant, and/or has served on a speaker’s bureau for Aevi, Akili, Alcobra, Allergan, Amerex, the American Academy of Child and Adolescent Psychiatry, American Psychiatric Press, Arbor, Bracket, Daiichi-Sankyo, Epharma Solutions, Forest, Genentech, Insys, Ironshore, KemPharm, Lundbeck, Merck, the National Institutes of Health, Neurim, Noven, Nuvelution, Otsuka, the Patient-Centered Outcomes Research Institute, Pfizer, Physicians Postgraduate Press, Roche, Sage, Shire (a Takeda company), Sunovion, Supernus Pharmaceuticals, Syneurx, Teva, TouchPoint, Tris, and Validus. Drs. Schroder and Maras have served as investigators and paid consultants for Neurim Pharmaceuticals. Dr. Nir has reported being an employee of Neurim Pharmaceuticals. Dr. Zisapel has reported being the founder and Chief Scientific Officer of Neurim Pharmaceuticals. Dr. Gringras has served as an investigator and paid consultant for Neurim Pharmaceuticals. Mr. Breddy has served as a paid consultant for Neurim Pharmaceuticals.
REFERENCES
- 1.Cuomo BM, Vaz S, Lee EAL, Thompson C, Rogerson JM, Falkmer T. Effectiveness of sleep-based interventions for children with autism spectrum disorder: a meta-synthesis. Pharmacotherapy. 2017;37:555–578. [DOI] [PubMed] [Google Scholar]
- 2.Krakowiak P, Goodlin-Jones B, Hertz-Picciotto I, Croen LA, Hansen RL. Sleep problems in children with autism spectrum disorders, developmental delays, and typical development: a population-based study. J Sleep Res. 2008;17:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taira M, Takase M, Sasaki H. Sleep disorder in children with autism. Psychiatry Clin Neurosci. 1998;52:182–183. [DOI] [PubMed] [Google Scholar]
- 4.Elrod MG, Hood BS. Sleep differences among children with autism spectrum disorders and typically developing peers: a meta-analysis. J Dev Behav Pediatr. 2015;36:166–177. [DOI] [PubMed] [Google Scholar]
- 5.Cespedes Feliciano EM, Quante M, Rifas-Shiman SL, Redline S, Oken E, Taveras EM. Objective sleep characteristics and cardiometabolic health in young adolescents. Pediatrics. 2018;142:e20174085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Millman RP; Working Group on Sleepiness in Adolescents/Young AdultsAAP Committee on Adolescence. Excessive sleepiness in adolescents and young adults: causes, consequences, and treatment strategies. Pediatrics. 2005;115:1774–1786. [DOI] [PubMed] [Google Scholar]
- 7.Mindell JA, Emslie G, Blumer J, et al. Pharmacologic management of insomnia in children and adolescents: consensus statement. Pediatrics. 2006;117:e1223–1232. [DOI] [PubMed] [Google Scholar]
- 8.Devnani PA, Hegde AU. Autism and sleep disorders. J Pediatr Neurosci. 2015;10:304–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McConachie H, Livingstone N, Morris C, et al. Parents suggest which indicators of progress and outcomes should be measured in young children with autism spectrum disorder. J Autism Dev Disord. 2018;48:1041–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Center for Drug Evaluation and Research; US Food and Drug Administration. The voice of the patient: a series of reports from the US Food and Drug Administration’s patient-focused drug development initiative: autism. Available at: https://www.fda.gov/media/111099/download. Accessed April 27, 2020.
- 11.Badin E, Haddad C, Shatkin JP. Insomnia: the sleeping giant of pediatric public health. Curr Psychiatry Rep. 2016;18:47. [DOI] [PubMed] [Google Scholar]
- 12.Kotagal S, Broomall E. Sleep in children with autism spectrum disorder. Pediatr Neurol. 2012;47:242–251. [DOI] [PubMed] [Google Scholar]
- 13.De Leersnyder H Inverted rhythm of melatonin secretion in Smith-Magenis syndrome: from symptoms to treatment. Trends Endocrinol Metab. 2006;17:291–298. [DOI] [PubMed] [Google Scholar]
- 14.Gringras P, Nir T, Breddy J, Frydman-Marom A, Findling RL. Efficacy and safety of pediatric prolonged-release melatonin for insomnia in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2017;56:948–957.e944. [DOI] [PubMed] [Google Scholar]
- 15.Schroder CM, Malow BA, Maras A, et al. Pediatric prolonged-release melatonin for sleep in children with autism spectrum disorder: impact on child behavior and caregiver’s quality of life. J Autism Dev Disord. 2019;49:3218–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maras A, Schroder CM, Malow BA, et al. Long-term efficacy and safety of pediatric prolonged-release melatonin for insomnia in children with autism spectrum disorder. J Child Adolesc Psychopharmacol. 2018;28:699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gringras P, Gamble C, Jones AP, et al. Melatonin for sleep problems in children with neurodevelopmental disorders: randomised double masked placebo controlled trial. BMJ. 2012;345:e6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wade AG, Crawford G, Ford I, et al. Prolonged release melatonin in the treatment of primary insomnia: evaluation of the age cut-off for short- and long-term response. Curr Med Res Opin. 2011;27:87–98. [DOI] [PubMed] [Google Scholar]
- 19.Quine L Sleep problems in children with mental handicap. J Ment Defic Res. 1991; 35(Pt 4):269–290. [DOI] [PubMed] [Google Scholar]
- 20.Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53:737–740. [DOI] [PubMed] [Google Scholar]
- 21.Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–1173. [DOI] [PubMed] [Google Scholar]
- 22.Bech P, Olsen LR, Kjoller M, Rasmussen NK. Measuring well-being rather than the absence of distress symptoms: a comparison of the SF-36 Mental Health subscale and the WHO-Five Well-Being Scale. Int J Methods Psychiatr Res. 2003;12:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nilsson ME, Koke SC. Defining treatment-emergent adverse events with the medical dictionary for regulatory activities (MedDRA). Drug Inf J. 2001;35:1289–1299. [Google Scholar]
- 24.Thomson SA, Tuleu C, Wong IC, Keady S, Pitt KG, Sutcliffe AG. Minitablets: new modality to deliver medicines to preschool-aged children. Pediatrics. 2009;123: e235–238. [DOI] [PubMed] [Google Scholar]
- 25.Must A, Anderson SE. Body mass index in children and adolescents: considerations for population-based applications. Int J Obes (Lond). 2006;30:590–594. [DOI] [PubMed] [Google Scholar]
- 26.Lemoine P, Nir T, Laudon M, Zisapel N. Prolonged-release melatonin improves sleep quality and morning alertness in insomnia patients aged 55 years and older and has no withdrawal effects. J Sleep Res. 2007;16:372–380. [DOI] [PubMed] [Google Scholar]
- 27.Luthringer R, Muzet M, Zisapel N, Staner L. The effect of prolonged-release melatonin on sleep measures and psychomotor performance in elderly patients with insomnia. Int Clin Psychopharmacol. 2009;24:239–249. [DOI] [PubMed] [Google Scholar]
- 28.Rossignol DA, Frye RE. Melatonin in autism spectrum disorders: a systematic review and meta-analysis. Dev Med Child Neurol. 2011;53:783–792. [DOI] [PubMed] [Google Scholar]
- 29.Arnold LE, Aman MG, Cook AM, et al. Atomoxetine for hyperactivity in autism spectrum disorders: placebo-controlled crossover pilot trial. J Am Acad Child Adolesc Psychiatry. 2006;45:1196–1205. [DOI] [PubMed] [Google Scholar]
- 30.Sheldon SH. Pro-convulsant effects of oral melatonin in neurologically disabled children. Lancet. 1998;351:1254. [DOI] [PubMed] [Google Scholar]
- 31.Anderson G Linking the biological underpinnings of depression: role of mitochondria interactions with melatonin, inflammation, sirtuins, tryptophan catabolites, DNA repair and oxidative and nitrosative stress, with consequences for classification and cognition. Prog Neuropsychopharmacol Biol Psychiatry. 2018;80(Pt C):255–266. [DOI] [PubMed] [Google Scholar]
- 32.Silman RE, Leone RM, Hooper RJ, Preece MA. Melatonin, the pineal gland and human puberty. Nature. 1979;282:301–303. [DOI] [PubMed] [Google Scholar]
- 33.Srinivasan V, Spence WD, Pandi-Perumal SR, Zakharia R, Bhatnagar KP, Brzezinski A. Melatonin and human reproduction: shedding light on the darkness hormone. Gynecol Endocrinol. 2009;25:779–785. [DOI] [PubMed] [Google Scholar]
- 34.Zwart TC, Smits MG, Egberts TCG, Rademaker CMA, van Geijlswijk IM. Long-term melatonin therapy for adolescents and young adults with chronic sleep onset insomnia and late melatonin onset: evaluation of sleep quality, chronotype, and lifestyle factors compared to age-related randomly selected population cohorts. Healthcare (Basel). 2018; 6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kitay JI. Pineal lesions and precocious puberty: a review. J Clin Endocrinol Metab. 1954; 14:622–625. [DOI] [PubMed] [Google Scholar]
- 36.Crowley SJ, Acebo C, Carskadon MA. Human puberty: salivary melatonin profiles in constant conditions. Dev Psychobiol. 2012;54:468–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Geijlswijk IM, Mol RH, Egberts TC, Smits MG. Evaluation of sleep, puberty and mental health in children with long-term melatonin treatment for chronic idiopathic childhood sleep onset insomnia. Psychopharmacology (Berl). 2011;216:111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malow B, Adkins KW, McGrew SG, et al. Melatonin for sleep in children with autism: a controlled trial examining dose, tolerability, and outcomes. J Autism Dev Disord. 2012; 42:1729–1737;author reply 1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Buuren S Puberty Plot Web Application Puberty Plot S-plus package. TNO Quality of Life. Copyright 2009. TNO. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


