Dear Editor,
Coronavirus disease 2019 (COVID-19) has similarities to systemic autoimmune conditions, including an association with increased incidence of autoantibodies [1–3], including those directed toward cytokines [4]. However, reports to date lack longitudinal assessments and have inadequate controls (i.e., comparison to different severities within COVID-19 or to healthy individuals). Moreover, previous studies reported the emergence of autoantibodies in severe respiratory and infectious disease [2, 5].
We performed an observational cohort study in which we prospectively enrolled adults with suspected COVID-19-associated acute respiratory failure on admission to the intensive care unit (ICU). Patients were classified based on SARS-CoV-2 testing by polymerase chain reaction (PCR). The primary clinical outcome was death in ICU within 3 months; secondary outcomes included in-hospital death and disease severity. Anti-nuclear antibodies (ANA), antigen-specific autoantibodies (sp-AAB), myositis-related autoantibodies and anti-cytokine autoantibodies (aC-AAB) were measured longitudinally. Patients did not receive any COVID-19-specific therapies since recruitment occurred before any were considered standard of care.
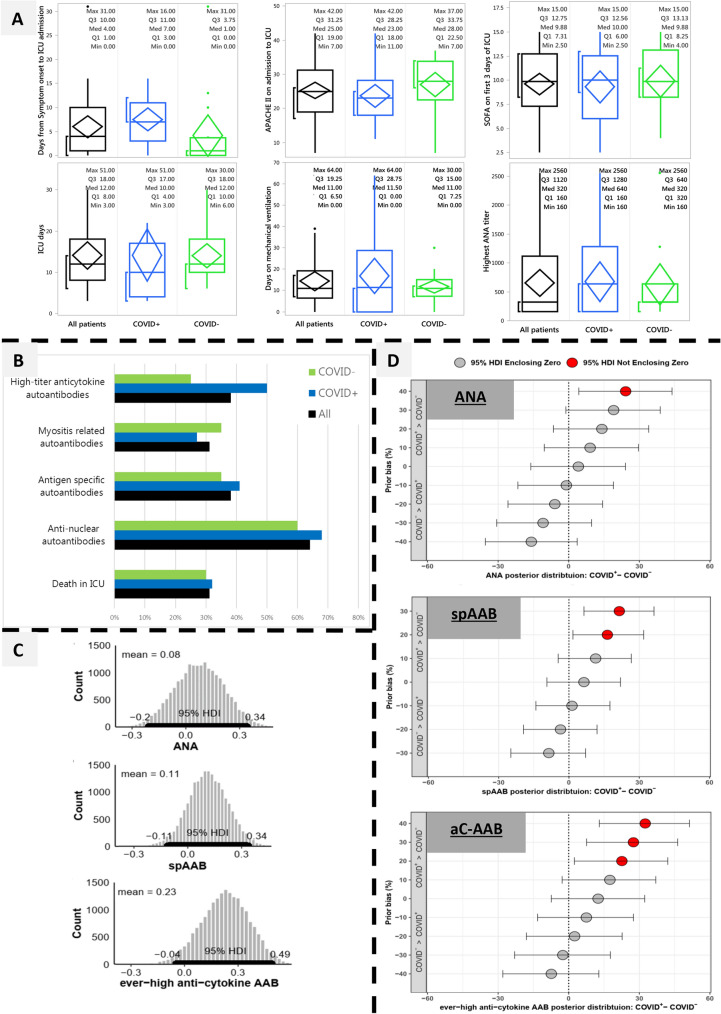
The 22 COVID+ and 20 COVID− patients had similar baseline characteristics (Supplement): 69% males, median age 60.5 years, mean APACHE II score 25.3. Sixty-four percent had ANA, 38% had sp-AAB, 31% had myositis-related autoantibodies, and 38% aC-AAB. Cytoplasmic dense and fine speckled ANA immunofluorescence patterns (AC20 and/or AC19) were significantly associated with worse clinical severity scores (Supplement). Although for some aC-AAB there were large absolute differences between the COVID+ and COVID−, overall, there were no statistically significant differences between the two cohorts for any of the autoantibodies (including anti-interferon autoantibodies[4]), their titers, staining patterns or their temporal development (Fisher’s exact test and ANOVA, false discovery rate q = 0.05; Fig. 1 and Supplement).
Fig. 1.
Autoantibody repertoire in COVID+ vs COVID−. aC-AAB anti-cytokine autoantibodies, ANA anti-nuclear antibodies, APACHE Acute Physiology and Chronic Health Evaluation (score), HDI high density interval, ICU intensive care unit, SOFA sequential organ failure assessment (score), sp-AAB antigen-specific autoantibodies. Panel A: Clinical and laboratory variables are on the Y axes for all patients (black, N = 42), COVID+ (blue, N = 22) and COVID− (green, N = 20). The horizontal line within the box represents the median. The ends of the box represent the 25th and 75th quantiles (i.e., 1st and 3rd quartiles). The lines extend up to the highest value within 1.5 times the interquartile range from the top and bottom of the box. Outliers beyond the lines are shown as dots. The diamond contains the mean value in the middle and encompasses the 95% confidence interval of the mean. The bracket outside of the box identifies the shortest half, which encompasses the densest 50% of the observations. None of the variables were significantly different (ANOVA with false discovery rate at q = 0.05; supplement). Panel B: Autoantibodies and death in the ICU are on the Y axis, with their percentages on the X axis. Color scheme and sample size are as for panel A. aC-AAB were classified into “ever-positive” and “ever-high positive” (see Supplemental information and methods); here, we present the more specific results using the higher titer threshold. None of the variables were significantly different (Fisher’s exact test with false discovery rate at q = 0.05; supplement). Panel C: The histograms represent the credible estimates of the posterior probability of the difference between the COVID+ and COVID− for the prevalence of ANA, sp-AAB and ever-high aC-AAB (from top to bottom). This is encapsulated in the 95% HDI, which is the shortest interval that encompasses 95% of the distribution (bold black line along the x-axis). Since the 95% HDI encloses zero, it is credible that there is no difference between the COVID+ and COVID−. Given the lack of previous literature on autoantibodies that compared COVID+ vs COVID− critically ill patients and given the previous literature on development of autoantibodies during severe respiratory disease, we used a non-informative prior. That is, there is no a priori assumption of a difference in the autoantibody prevalence between these two populations when deriving the posterior distributions from the existing data (Supplement). Panel D: These results are analogous to panel C, except that instead of using a non-informative prior, a variety of prior probabilities were tested sequentially. This prior probability sensitivity analysis serves to stabilize the results by testing a range of possible priors around the original estimates. The box and whiskers represent the 95% HDI at each level of prior bias. The Y axis shows the trialed a priori differences between the prevalence of COVID+ vs COVID−, at positive, equal (y = 0), or negative values. It illustrates the required levels of a priori differences in prevalence (%), and the resultant 95% HDI of posterior differences (%) that are compatible with the experimental observations, for ANA (top), sp-AAB (middle), and aC-AAB (bottom). For example, for our results to be compatible with a true difference in the prevalence of ANA between the COVID+ and COVID− patients, it would need to be assumed a priori that COVID+ patients have a 35% higher prevalence of ANA (HDI with a red circle). The simulations were performed on 5% intervals; for clarity, the results are shown at 10% intervals and quoted to the nearest 5%. See supplement for the detailed methods and a summarizing table of these results
Given the relatively small sample size, Bayesian analysis was performed to obtain credible estimates of the true differences between COVID+ and COVID− patients. This confirmed the results obtained with hypothesis testing (Fig. 1): no differences were found between COVID+ and COVID− for ANA, sp-AAB or aC-AAB. Sensitivity analysis showed that, based on our results, it would be necessary to assume a priori 15–35% higher prevalence of autoantibodies among the COVID+ for them to be credibly different from the COVID− (Fig. 1). Moreover, even if there were reasons for such assumptions, the resulting posterior mean differences would be on the order of only 15–25% higher autoantibodies among the COVID+ (Fig. 1 and Supplement).
We offer two biological and two methodological messages: (1) There was a spectrum of reactivity with high prevalence in the extensive panel of autoantibodies in patients with respiratory failure, an underappreciated phenomenon. Some autoantibodies associated with worse severity deserve longer-term study. (2) Patients with COVID-19-induced respiratory failure have similar autoantibody profiles as contemporaneous, comparably ill respiratory failure patients without COVID-19, suggesting that autoantibodies are a feature of acute respiratory failure regardless of COVID-19 status. Given our modest sample size, the possibility of true differences still exists, but these differences would likely be small. (3) Proper control groups are indispensable for appropriate interpretation of results, COVID-19 related or otherwise. (4) Bayesian analysis generated credible intervals of effect sizes providing important insights, beyond point estimates, for calibrating our clinical gestalt.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge the technical assistance of Haiyan Hou, Meifeng Zhang and Emily Walker in the MitogenDx Laboratory at the University of Calgary. We thank Marlene Santos, Gyan Sadhu, Imrana Khalid, and Sebastian Duncan, the research coordinators at St Michael’s Hospital Critical Care Research Unit. We are grateful to patients and families that have generously consented to the study. The members of the COVID-19 chapter of the “Longitudinal Biomarkers in Lung Injury” study group are: Robert Rottapel: Departments of Medicine and Immunology, University of Toronto, Toronto, ON, Canada, Division of Rheumatology, St. Michael’s Hospital, Toronto, ON, Canada; Claudia C. dos Santos: Critical Care Department, St. Michael’s Hospital, Toronto, ON, Canada, Interdepartmental Division of Critical Care, University of Toronto and Keenan Centre for Biomedical Research, St. Michael’s Hospital, Toronto, On, Canada; Alex P. Di Battista: Faculty of Kinesiology and Physical Education, University of Toronto, Toronto ON, Canada; Arthur S. Slutsky: Critical Care Department, St. Michael’s Hospital, Toronto, ON, Canada, Interdepartmental Division of Critical Care, University of Toronto and Keenan Centre for Biomedical Research, St. Michael’s Hospital, Toronto, On, Canada; Andrew J. Baker: Critical Care Department, St. Michael’s Hospital, Toronto, ON, Canada, Interdepartmental Division of Critical Care, University of Toronto and Keenan Centre for Biomedical Research, St. Michael’s Hospital, Toronto, On, Canada.
Author contributions
This report is part of the COVID-19 chapter of the Longitudinal biomarkers in lung injury study. AB and CDS are principal investigators; MJF, RR and AS are collaborators/co-investigators and UT is the research lead. RR, MJF, UT, and CDS conceived of the study; MJF and UT wrote the manuscript drafts; AS, AB, RR and CDS provided critique and technical guidance; UT and ADB performed the data analysis and creation of the figures. All authors edited the manuscript, through to the final version, read and approved the final submission.
Funding
St. Michael’s Hospital Foundation, internal competitive grant to AB and CDS. Autoantibody testing was provided as a gift in kind by MitogenDx (Calgary, AB, Canada).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflicts of interests
MJF is the Director of MitogenDx. MJF is a consultant for and received speaking honoraria from Inova Diagnostics Inc (San Diego, CA) and Werfen International (Barcelona, Spain). All the other authors have no disclosures to declare.
Ethical approval
This research was approved by the Research Ethics Boards of St Michael’s Hospital and performed in accordance with the Helsinki Declaration of 1975 as revised in 2013.
Informed consent
Informed consent was obtained from all patients or surrogate decision makers when available. Otherwise, as per the study’s protocol and as approved by the REB, given the minimal-risk nature of the study, a deferred consent model was invoked while continuing to pursue direct consent throughout the study period.
Study registration
NCT04747782—clinicaltrials.gov.
Footnotes
The members of the COVID-19 chapter of the “Longitudinal Biomarkers in Lung Injury” study group are listed in acknowledgements.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Marvin J. Fritzler, Email: fritzler@ucalgary.ca
On behalf of the COVID-19 chapter of the “Longitudinal Biomarkers in Lung Injury” study group:
Robert Rottapel, Claudia C. dos Santos, Alex P. Di Battista, Arthur S. Slutsky, and Andrew J. Baker
References
- 1.Vlachoyiannopoulos PG, Magira E, Alexopoulos H, Jahaj E, Theophilopoulou K, Kotanidou A, Tzioufas AG. Autoantibodies related to systemic autoimmune rheumatic diseases in severely ill patients with COVID-19. Ann Rheum Dis. 2020;79:1661–1663. doi: 10.1136/annrheumdis-2020-218009. [DOI] [PubMed] [Google Scholar]
- 2.Lerma LA, Chaudhary A, Bryan A, Morishima C, Wener MH, Fink SL. Prevalence of autoantibody responses in acute coronavirus disease 2019 (COVID-19) J Transl Autoimmun. 2020;3:100073. doi: 10.1016/j.jtauto.2020.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sacchi MC, Tamiazzo S, Stobbione P, Agatea L, De GP, Stecca A, et al. SARS-CoV-2 infection as a trigger of autoimmune response. Clin Transl Sci. 2020 doi: 10.1111/cts.12953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, Zhang Y, et al. Auto-antibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370:eabd4585. doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burbelo PD, Seam N, Groot S, Ching KH, Han BL, Meduri GU, et al. Rapid induction of autoantibodies during ARDS and septic shock. J Transl Med. 2010;8:97–98. doi: 10.1186/1479-5876-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.