Abstract
Salvia species have been traditionally used to improve cognition and have been proved to be a potential natural treatment for Alzheimer’s disease. Salvia fruticosa Mill. (Turkish sage or Greek sage) demonstrated to have anticholinergic effects in vitro.
The aim of this study was to understand the mechanism underlying the neuroprotective effects of S. fruticosa infusion and its representative compound rosmarinic acid, which was detected by LC-DAD-ESI-MS/MS. The protective effects of the S. fruticosa infusion (SFINF) and its major substance rosmarinic acid (RA) on amyloid beta 1–42 -induced cytotoxicity on SH-SY5Y cells together with p-GSK-3β activation were investigated. Their in vitro inhibitory effects against glycogen synthase kinase 3β, β-secretase, and casein kinase 1δ enzymes were also evaluated.
The results showed that treatment with the all tested concentrations, SFINF significantly decreased Aβ 1–42-induced cytotoxicity and exhibited promising in vitro glycogen synthase kinase 3β inhibitory activity below 10 µg/mL (IC50 6.52 ± 1.14 µg/mL), in addition to β-secretase inhibition (IC50 86 ± 2.9 µg/mL) and casein kinase 1δ inhibition (IC50 121.57 ± 4.00). The SFINF (100 µg/mL and 250 µg/mL) also activated the expression of p-GSK-3β in amyloid beta 1–42 treated SH-SY5Y cells.
The outcomes of this study demonstrated that the S. fruticosa infusion possessed activity to prevent amyloid beta 1–42 -induced neurotoxicity and provided proof that its mechanism may involve regulation of p-GSK-3β protein.
Keywords: Lamiaceae, Neurotoxicity, Molecular modeling, Neuroprotection, Rosmarinic acid
1. Introduction
Alzheimer's disease (AD) is a progressive and irreversible neurodegenerative disease with an increasing prevalence in aging populations. Amyloid beta (Aβ) aggregates and hyper-phosphorylated neurofibrillary tangles of tau represent the two main pathological hallmarks of AD (Atri, 2019). Although many pathological mechanisms have been proposed for AD, Aβ is thought to be one of the most important key molecules in the onset and progression of the disease. According to the amyloid cascade hypothesis, the increase in the Aβ fragment (1–42) (Aβ 1–42) causes oligomerization, deposition, and accumulation of Aβ. Then accumulated Aβ 1–42 results in oxidative stress and neuronal cell loss, one of the main causes of dementia with an inflammatory response by disturbing the homeostasis of the central nervous system (Busche and Hyman, 2020). The increase in β-secretase (BACE-1) levels leads to the generation and concentration of toxic forms of Aβ in the brain, resulting in neurodegeneration (Hu et al., 2010). The well-known kinase family members glycogen synthase kinase-3β (GSK-3β) and casein kinase-1δ (CK-1δ) have been recently shown in a variety of studies involving apoptotic neuronal cell death, Aβ 1–42 toxicity, and pathological accumulation of tau (Bjørklund et al., 2019, Eldar-Finkelman and Martinez, 2011). Since the key roles of these enzymes in the initiation and persistence of AD are understood, the search for compounds that act as BACE-1, GSK-3β, and CK-1δ inhibitors has become even more important.
Traditional and Complementary Medicine, especially treatment with medicinal plants has been an important healthcare component among some people who have limited facility with having difficulties in accessing modern medicine or who believe the effectiveness of the traditional medicine superior to modern medicine for many years (Hill et al., 2019, Jafari et al., 2020).
Numerous medicinal plants and their extracts containing several active constituents have been used to treat central nervous system (CNS)-related disorders. Among these, Salvia species are renowned for their reported favorable effects on memory failure, depression, and cerebral ischemia (Perry et al., 2003).
The genus Salvia (Lamiaceae) is represented by more than 900 species worldwide and 100 species in the Flora of Turkey (Celep et al., 2015). A comprehensive review about Salvia species gathered recent data on the biological activities, focusing on their anticholinesterase, anti-HIV, cytotoxic, antioxidant, antimicrobial, and antileishmanial activities (Wu et al., 2012). The main phytochemical groups, including caffeic acid monomers and dimers and other salvianolic acids; apigenin, luteolin, and their 6-hydroxylated derivatives; ursane-, oleanane-, and lupane-type triterpenes; and abietane- and neoclerodane-type diterpenes, of Salvia species are reported in broad and detailed reviews (Lu and Yeap Foo, 2002, Topçu, 2006).
Various in vitro and in vivo pharmacological studies, i.e., on anticholinesterase, anti-neuroinflammatory, antioxidative, Aβ degradation and Aβ producing secretase modulation activities, apoptotic mechanisms, and heavy metal binding properties, were performed to test the role of Salvia species in the treatment of AD (Howes and Houghton, 2003, Howes et al., 2003, Perry et al., 1999). The milestones of these studies were the proof of the effect of two Salvia species, S. officinalis L. (sage) and S. lavandula Alain (Spanish sage), on the improvement in cognitive functions when clinically tested in different types of AD (Akhondzadeh et al., 2003, Kennedy et al., 2011, Perry et al., 2018, Perry et al., 2003, Tildesley et al., 2003). Additionally, over the past 20 years, almost all of the Salvia species that grow in Turkey have been studied in terms of anticholinesterase activities, and it is demonstrated that the activities of these plants are due to their high content of phenolic compounds, especially phenolic acids along with some volatile constituents (Demirezer et al., 2015, Kolak Ufuk et al., 2009, Orhan et al., 2007, Orhan et al., 2012, Şenol et al., 2010). Among the species tested, S. fruticosa has been shown to be remarkably effective in acetylcholinesterase and butyrylcholinesterase inhibition (Şenol et al., 2011).
This background on Salvia species prompted us to investigate the neuroprotective effects of S. fruticosa, which is one of the most important herbal teas traditionally consumed throughout Mediterranean countries.
In the present study, to understand the neuroprotective effects of S. fruticosa infusion (SFINF) on the SHSY5Y cell line, different cell-based assays were performed in addition to examination of its in vitro enzyme (GSK-3β, CK-1δ, and BACE-1) inhibitory properties related to AD. Molecular docking studies of the predominant compounds, including rosmarinic acid (RA), were carried out in the active sites of GSK-3β to predict the most appropriate binding modes and support the experimental data
2. Materials and methods
2.1. Plant material, extract preparation, and determination of phenolic compounds
Aerial parts of S. fruticosa were collected from Antalya; Kemer, Küçükburun region, Pinus brutia forest, at 50 m, May 2013. Assoc. Prof. Dr. Mehmet Yavuz Paksoy identified the plants, and a voucher specimen (PAKSOY 1470) has been deposited in the Herbarium of Fırat University, Elazığ, Turkey. The dried and crude powdered aerial parts of S. fruticosa were immersed in 100 mL of boiling water for 5 min three times (each 10 g). After filtration, the infusions were combined and lyophilized until used for further assays (SFINF-yield 12%).
All the equipment and the chromatographic separation, elution, detection, and data collection methods used were the same as those described previously by Gürbüz et al. (2019).
2.2. Biological activity and molecular docking simulations
2.2.1. In vitro GSK-3β, CK-1δ, and BACE-1 inhibition
The method described by Baki et al. (2007) was used for the inhibition of GSK-3β. A Kinase-Glo Kit was used to measure the activity of the SFINF and RA versus CK-1δ and GSK-3β. The inhibitory activity was calculated on the basis of maximal activities measured in the absence of inhibitor. The BACE-1 assay, which was based on FRET methodology, was performed according to the manufacturer protocol available from Invitrogen (L0724). The IC50 was calculated as the concentration of each compound that reduces the enzymatic activity 50% with respect to that without inhibitors. The results were represented as the mean ± standard deviation from three independent experiments (n = 3).
2.2.2. Molecular docking
The chemical formulas of the three predominant compounds (RA, luteolin 7-O-glucuronide, and caffeic acid) were drawn in ChemBiodraw Ultra 12.0. The published crystal structure of GSK-3β complexed with the co-crystallized ligand AR-A014418 (N-(4-methoxybenzyl)-N′-(5-nitro-1,3-thiazol-2-yl)urea) was obtained from the Protein Data Bank (PDB code: 1Q5K) (Bhat et al., 2003). The compounds were docked into the catalytic site of the enzyme with Autodock 4.2 (Morris et al., 2009), integrated into LigandScout 4.2, using default parameters. All results obtained were ranked according to their binding affinities (kcal/mol) and the best docking pose of each compound was selected for further visual evaluation. The molecular docking figures were generated using Maestro (Schrödinger, 2019).
2.2.3. Neuroprotection
2.2.3.1. General
For the neuroprotection assays and Western blot analysis, the SH-SY5Y (neuroblastoma) cell line was purchased from the American Type Culture Collection (ATCC, CRL 2266) and cultivated in DMEM F-12, containing FBS 10%, 100 U/mL penicillin, and 100 µg/mL streptomycin. The cells were seeded on a 96-well plate, 96-well E-plate, and 6-well plate for the Sulforhodamine B assay, neuroprotection assay, and Western blot analysis, respectively. The amyloid beta peptide (1–42) was purchased from Anaspec (Cat: AS-20276), Sulforhodamine B was purchased from Santa Cruz (3520-42-1), and SB216763 was purchased from Sigma-Aldrich as a positive control for the biological assays.
2.2.3.2. Sulforhodamine B (SRB) cell viability assay
The SRB colorimetric method was used (Vichai and Kirtikara, 2006) after 24 h incubation of the SH-SY5Y cells with different concentrations of the SFINF (500, 250, 100, 50, 10 µg/mL) and RA (500, 250, 100, 50, 10 µM) to determine the nontoxic dose. Final absorbance was measured at 492 nm using a multifunctional microplate reader (BioTek Gen 5, USA). All the measurements were obtained at least in triplicate and IC50 values were calculated to assign nontoxic concentrations of the SFINF and RA (data not shown).
2.2.3.3. Real-time cell electronic sensing system
The neuroprotective effects of the SFINF on Aβ 1–42 treated and untreated SH-SY5Y cells were monitored with a real-time cell analyzer (RTCA; Roche Applied Science, Mannheim, Germany) as described by Hou et al. (2014) with slight modifications. A well-known GSK-3β inhibitor, SB216763, was used as a positive control. The RTCA monitors the impedance changes elicited by the cell index (CI) in a real-time manner, which enables concentration–activity curves to be maintained, dependent on the time point or period of time chosen. To adjust the ideal cell number in wells, 6250, 12500, 25000, and 50,000 cells/well were seeded. After half an hour of incubation at room temperature, E-plates were placed into the RTCA station. The electrical impedance was measured every 15 min by the RTCA associated software of the xCELLigence system as a dimensionless parameter termed CI. Based on the growth curve and CI values of the SH-SY5Y cells, 12,500 cells/well were selected for further analysis.
2.2.3.4. Preparation of Aβ 1–42
Briefly, the Aβ 1–42 peptide was dissolved in 1 mM in hexafluoroisopropanol (HPIF, Sigma-Aldrich) and aliquoted in sterile microcentrifuge tubes. The HPIF was then withdrawn under vacuum and stored at −20 °C. Aβ 1–42 peptide fibril formation was achieved based on the protocol explained by Dahlgren et al. (Dahlgren et al., 2002).
2.2.3.5. Cytotoxicity test of Aβ 1–42
First 12,500 cells/well were seeded in the 96-well E-plate and then 24 h post-seeding when the cells were in the log growth phase the cells were treated with different concentration of Aβ 1–42 (22, 11, 5, 2.5 µM) to determine the optimal Aβ 1–42 concentration for the assay and 22 µM Aβ 1–42 was selected for further studies (data not shown).
2.2.3.6. Effects of the SFINF and RA against Aβ 1–42 damage in SH-SY5Y cells
SH-SY5Y cells (12500 cells/well) were seeded in a 96-well E-plate. After complete adhesion for 24 h, the medium was replaced with fresh medium. The cells were treated with 22 µM Aβ 1–42 and nontoxic concentrations of the SFINF (250, 100, 50, and 10 µg/mL) and RA (50 µM and 100 µM) subsequently. Data points were collected in at least triplicate every 15 min throughout the duration of the experiments.
2.2.3.7. Western blot
Western blot analysis was carried out using crude lysates of SH-SY5Y human neuroblastoma cells. After being treated with the SFINF in 250 and 100 µg/mL concentrations for 24 h the cells were lysed in commercial RIPA lysis buffer (Santa Cruz sc-24948). The lysate was centrifuged at 4 °C for 30 min at 12000 rpm. The clear supernatant was collected and the total protein amount was determined by the Lowry method (Hartree, 1972). Next 30 µg of protein lysates were resolved on 10% sodium dodecyl sulfate–polyacrylamide (SDS-PAGE) gels and then electro-transferred onto polyvinylidene difluoride (PVDF) membrane. After being blocked with 5% nonfat milk in Tris-buffered saline (TBS, 0.1 M, pH 7.4) the membranes were incubated with primary antibodies, namely anti-GSK-3β (1:1000 dilution, Cell Signaling Technology (CST), 9315S), anti-Phospho-GSK-3β (Ser9) (1:1000 dilution, CST, 9336S), and anti-β actin (1:1000 dilution, CST, 4967S). After overnight incubation of the primary antibodies the membranes were washed three times for 15 min and were incubated with a secondary antibody, HRP-conjugated goat anti-rabbit secondary antibody (1:10.000 dilutions, Jackson Immunoresearch 111–035–144), for 2 h at room temperature. Protein bands were detected by enhanced chemiluminescence (ECL, Santa Cruz Biotechnology, CA, USA) on XO-MAT film. SB216763 was used as a positive control.
3. Results
3.1. Phenolic compounds of the SFINF
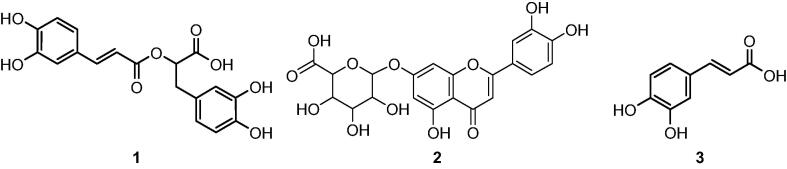
The chemical profile of the SFINF, which was determined by LC-DAD-ESI-MS/MS, is summarized in Table 1. The phytochemical examination demonstrated that the constituents attributed to the SFINF are in agreement with the published literature for S. fruticosa and for the genus Salvia (Koutsoulas et al., 2019, Vergine et al., 2019). Flavone and flavonol derivatives together with caffeic acid derivatives were detected as the main groups for the SFINF. The phytochemicals were determined from their retention times and observed [M−H]− ions with supportive UV spectra and MS/MS interpretation in comparison with the relevant literature. When considering the UV chromatogram of the SFINF, it is apparent that RA (1) is the major element of the SFINF. Thus, RA was selected as a major and representative molecule for the rest of the activity assays. The other predominant compounds are luteolin 7-O-glucuronide (2) and caffeic acid (3) (Fig. 1).
Table 1.
Phenolic compounds tentatively identified in the SFINF.
| Tentative identification | Rt (min) | [M−H]− | MS2 (m/z) | Ref. |
|---|---|---|---|---|
| Apigenin di-C-hexoside | 16.9 | 593 | 473, 383, 353 | (Dias et al., 2013) |
| Caffeic acid | 20.2 | 179 | 135 | std |
| Luteolin 7-O-glucuronide | 21.1 | 461 | 285, 175 | (Cvetkovikj et al., 2013) |
| Salvianolic acid E/B | 21.7 | 717 | 555, 519, 475, 359, 313, 295 | (Barros et al., 2013) |
| Isorhamnetin glucuronide | 21.9 | 491 | 315, 299 | (Spínola et al., 2015) |
| Caffeic acid derivative | 22.2 | 487 | 323, 179, 161, 133 | (Helmja et al., 2008) |
| Apigenin glucuronide | 22.6 | 445 | 269, 175, 113 | (Guimarães et al., 2013) |
| Isorhamnetin glucoside | 22.9 | 477 | 315, 300, 284, 175, 113 | (Kontogianni et al., 2013) |
| Isosalvianolic acid | 23.0 | 493 | 359, 313, 295, 197, 179, 161 | (Barros et al., 2013) |
| Rosmarinic acid | 23.06 | 359 | 197, 161, 135 | (Lu and Yeap Foo, 2002) |
| Luteolin | 26.9 | 285 | 133 | (Lu and Yeap Foo, 2002) |
| Isorhamnetin | 27.4 | 315 | 300, 297, 137 | (Lu and Yeap Foo, 2002) |
| Salvianolic acid F | 28.16 | 313 | 161, 133 | (Lu and Yeap Foo, 2002) |
Fig. 1.
The major compounds in the SFINF.
3.2. In vitro GSK-3β, BACE-1, and CK-1δ inhibitory activities
To understand the in vitro inhibitory effects of the SFINF, GSK-3β, BACE-1, and CK-1δ were selected as the key enzymes related to AD. In particular, the SFINF possessed promising GSK-3β inhibitory activity below 10 µg/mL (IC50 6.52 ± 1.14 µg/mL), followed by BACE-1 inhibition (IC50 86 ± 2.9 µg/mL) and CK-1δ inhibition (IC50 121.57 ± 4.00). Consequently, the SFINF had overall higher inhibitory activity on GSK-3β compared to the other two enzymes.
3.3. Molecular docking studies
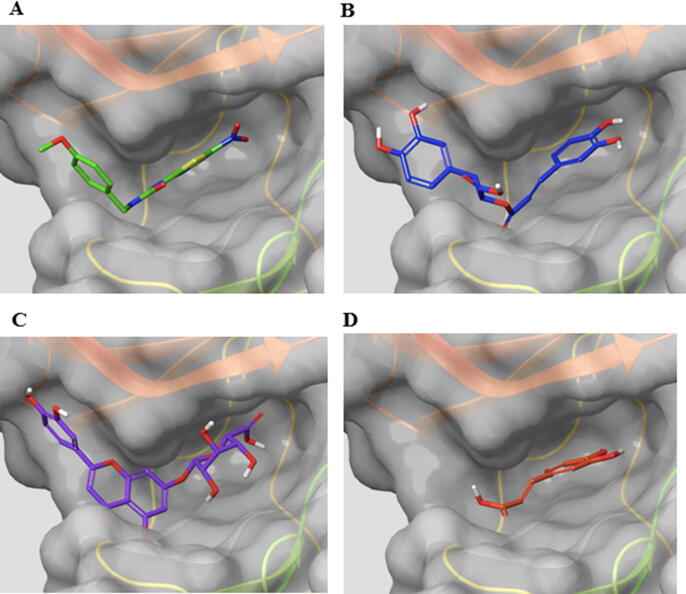
The SFINF was tested for its inhibitor activity on three different enzymes (GSK-3β, BACE-1, and CK-1δ) that play roles in the progression of AD and was found to be most effective as an inhibitor of GSK-3β. Therefore, molecular docking studies were performed to gain insights as to whether one of the predominant compounds in the SFINF is mainly responsible for GSK-3β inhibition. With this aim, three predominant compounds (RA, luteolin 7-O-glucuronide, and caffeic acid) were docked into the active pocket of the enzyme. We additionally re-docked the co-crystallized ligand (AR-A014418) present in the crystal structure of GSK-3β and compared the result with its original pose by using the root-mean-square deviation (RMSD) value. It was found to be 1.06 and in this way we also checked the reliability of our docking parameters.
The obtained docking results of the three compounds were ranked according to their estimated binding energies (kcal/mol) and each ligand with the best docking score (the lowest binding energy) was selected for further visual evaluation.
According to the docking poses provided in Fig. 2, caffeic acid partially occupied the binding pocket of the enzyme, as it is a less bulky compound compared to the others. It is not surprising that its binding energy was higher (-11.43 kcal/mol) than the values calculated for RA (-14.62 kcal/mol) and luteolin 7-O-glucuronide (-13.98 kcal/mol).
Fig. 2.
Binding modes of the co-crystallized inhibitor AR-A014418 (A), RA (B), luteolin 7-O-glucuronide (C), and caffeic acid (D) in the binding pocket of GSK-3β. Ligands are shown as green, blue, purple, and orange sticks for AR-A014418, RA, luteolin 7-O-glucuronide, and caffeic acid, respectively.
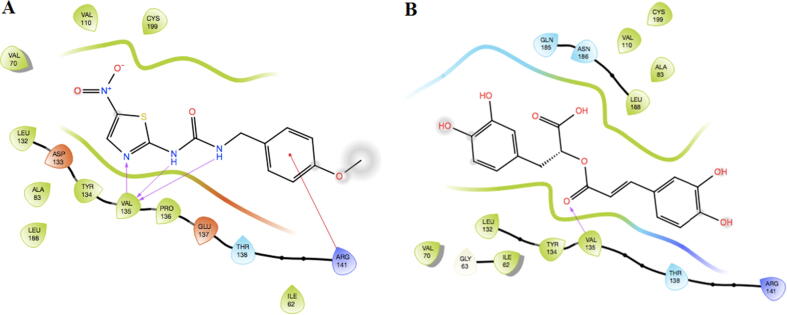
As RA has the best docking score with the lowest binding energy among those three compounds, we examined its binding mode to GSK-3β compared to the original ligand. We observed that RA has a similar orientation to AR-A014418 in the active site of GSK-3β (Fig. 3).
Fig. 3.
2D interactions of AR-A014418 (A) and RA (B) in the active site of GSK-3β.
Based on these findings, we decided to determine the GSK-3β inhibitory activity of RA as the major component of SFINF. However, RA did not inhibit GSK-3β effectively with IC50 > 100 µM. This result suggested that RA as the representative compound of the SFINF was not primarily responsible for the GSK-3β inhibition. We surmise that the synergistic effect of the compounds present in the SFINF can lead to this effect in the treatment of AD through GSK-3β inhibition.
3.4. Neuroprotective effect of SFINF extract on Aβ 1–42 neurotoxicity
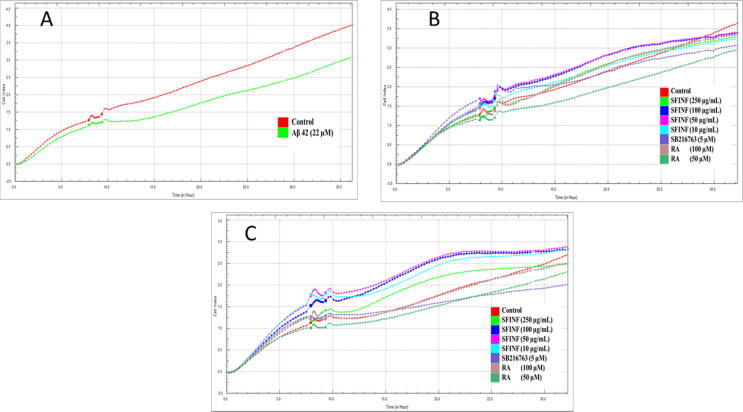
To determine the neuroprotective effects of different concentrations prepared from the SFINF and RA in the presence/absence of Aβ 1–42 on SH-SY5Y cells, an impedance-based cell profile assay was carried out. The results are shown in Fig. 4. Each colored trace shown in the figure represents the impedance of cells calculated from the RTCA. Fig. 4 shows that the treatment of SH-SY5Y cells with Aβ 1–42 for 36 h significantly suppressed cell proliferation as determined by the RTCA at a concentration of 22 µM (A). In the same figure all concentrations of the SFINF, 100 µM of RA, and the positive control stimulated cell proliferation in a nondose-dependent manner. The cells were shown to grow steadily within the dose range of 10–250 µg/mL. A dose of 50 µM RA exerted a slightly inhibitory effect on cell proliferation (B). All of the concentrations of the SFINF completely reversed the suppressive effect of Aβ 1–42 on cell proliferation and enhanced cell proliferation from 35% to 70% after incubating for 10 h. No significant differences were observed between 50 µM RA and 5 µM SB216763 neuroprotection when compared to the Aβ 1–42 treated control (C).
Fig. 4.
Dynamic monitoring of cytotoxic responses of Aβ 1–42 to cell proliferation. SH-SY5Y cells were treated with Aβ 1–42 at a concentration of 22 µM. Aβ 1–42 reduced the proliferation of SH-SY5Y cells to approximately 30% 24 h after the treatment (A). Dynamic monitoring of cytotoxic responses of samples to cell proliferation. SH-SY5Y cells were treated with different concentrations of the SFINF, RA, and SB216763. None of the tested samples reduced the cell proliferation of the cells prominently 24 h after treatment. SFINF (250 µg/mL) and RA (100 µM) caused a slight increment in the cell index after treatment (B). Dynamic monitoring of protective responses of samples to SH-SY5Y cells in the presence of 22 µM Aβ 1–42. SH-SY5Y cells were treated with Aβ 1–42 and different concentrations of the SFINF, RA, and SB216763 (C).
3.5. p-GSK-3β Activation
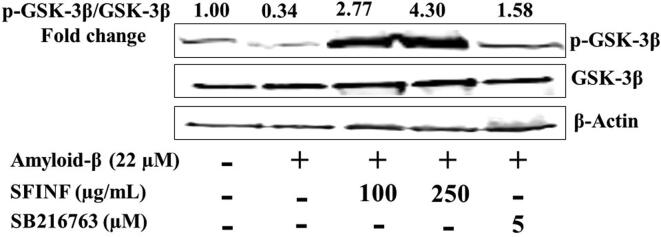
To investigate whether SFINF and Aβ 1–42 affect p-GSK-3β and GSK-3β expression in SH-SY5Y cells, Western blot analysis was performed. To evaluate activation, the p-GSK-3β to GSK-3β ratio was determined. Total protein lysate was separated by SDS-PAGE (25 µg/lane), electro-transferred to PVDF membrane, and probed with antibody to GSK-3β and p-GSK-3β. The decrease in the p-GSK-3β/GSK-3β ratio caused by Aβ 1–42 was reversed by the SFINF in a dose-dependent manner at 100 and 250 µg/mL (Fig. 5).
Fig. 5.
Effect of SFINF (100 and 250 µg/mL) on Aβ 1–42-induced GSK-3β activation. Characteristic Western blot analysis of p-GSK-3β and GSK-3β in SH-SY5Y cells; treated with Aβ 1–42 (22 µM) and in the presence/absence of the SFINF and positive control (SB216763).
4. Discussion
The aggregation of Aβ peptides from 40 to 42 amino acids is broadly accepted to be one of the main elements responsible for AD (Espargaró et al., 2017). The correlation between BACE-1, GSK-3β, and CK-1δ and the pathological accumulation of tau and formation of Aβ 1–42 represents an unconventional and promising approach for the prevention and therapy of AD. Consequently, the spotting of potential inhibitors of amyloid aggregation and related enzymes has recently attracted much interest.
S. fruticosa is widely distributed in Mediterranean countries and has a less bitter taste than S. officinalis. It is frequently consumed as an herbal tea prepared as an infusion, and also has economic value as a source of essential oil and as a crude drug for the local population.
The present study revealed for the first time that SFINF can protect SH-SY5Y cells against Aβ 1–42-induced toxicity. This conclusion is supported by the following evidence: 1) the SFINF prevented Aβ 1–42-mediated neurotoxicity in cultured SH-SY5Y cells; 2) the SFINF inhibited BACE-1, GSK-3β, and CK-1δ in vitro with a varying IC50 value under 125 µg/mL; 3) the SFINF increased phospho-GSK-3β levels; 4) a possible synergistic effect of the compounds present in the SFINF can lead to a neuroprotective effect through GSK-3β inhibition.
Herein, all of the concentrations of the SFINF completely reversed the suppressive effect of Aβ 1–42 on SH-SY5Y cell proliferation and enhanced cell proliferation from 35% to 70%, while there was no apparent change after the treatment with RA compared to the control.
To understand the neuroprotective mechanism, the SFINF was tested for its inhibitory effect on GSK-3β, BACE-1, and CK-1δ enzymes. The SFINF possessed promising GSK-3β inhibitory activity below 10 µg/mL (IC50 6.52 ± 1.14 µg/mL), followed by BACE-1 inhibition (IC50 86 ± 2.9 µg/mL) and CK-1δ inhibition (IC50 121.57 ± 4.00 µg/mL). Based on these results it can be suggested that moderate CK-1δ and BACE-1 inhibitory properties of SFINF in addition to the strong GSK-3β inhibitory potential play important roles in the protection of neurons from Aβ-induced toxicity and contribute to the anti-Alzheimer effects of the SFINF together. The in vitro GSK-3β inhibitory potential of the SFINF was also supported by the excessive expression of p-GSK-3β with the Western blot analysis and this effect was stronger than that of the well-known GSK-3β inhibitor SB216763. The phytochemical examination led to the separation and identification of thirteen compounds in the SFINF. Based on the UV chromatogram of the SFINF, RA was detected as the main compound in the extract, followed by luteolin 7-O-glucuronide and caffeic acid. Prompted by the molecular docking studies, we additionally tested RA solely for its in vitro GSK-3β inhibitory potential but its inhibition value suggested that, as the major component of the SFINF, RA is not responsible on its own for the strong GSK-3β inhibition effect of the infusion.
In a recent study, caffeic acid, the monomer of RA, and many other salvianolic acids were reported to decrease Aβ-induced tau phosphorylation (Sul et al., 2009). In other research, it was indicated that RA prohibits the fibrillization and decreases vibrational modes incorporated with the β sheet in tau protein linked to AD (Cornejo et al., 2017). While the complete mechanisms responsible for Aβ-mediated neurotoxicity remain unclear, various studies have shown that Aβ causes reactive oxygen species (ROS) generation and ROS induce DNA injury, enzyme oxidation, lipid peroxidation, and cell apoptosis. Thus, ROS also have been included in AD pathologies and are an important target in AD and related irregularities. Using antioxidants should be a rational and promising approach to alleviate oxidative-dependent and Aβ-caused cytotoxicity (Zhang et al., 2016). In addition to the enzyme inhibition properties of the SFINF, the phenolic pool of the SFINF with its well-known strong antioxidant effects can contribute to the neuroprotective effect.
Several cell-based studies revealed that S. officinalis and RA protected PC12 cells from Aβ 1–42 toxicity through different mechanisms (Iuvone et al., 2006), while radix S. miltiorrhiza has been proved to revise the Aβ 25–35-induced cytotoxicity in PC-12 cells (Zhou et al., 2011) in vitro. Shortly before it is demonstrated that different S. fruticosa extracts exhibited a pronounced neuroprotective effect against Aβ 25–35 toxicity in combination with their significant antioxidant potential (Ververis et al., 2020). Salvianolic acid B (SalB), one of the active antioxidant depsides from S. miltiorrhiza, has been shown to reduce Aβ 1–40 fibril formation and aggregation in addition to the protection of SH-SY5Y cells from Aβ 1–42-induced cell death in vitro (Durairajan et al., 2008, Tang and Zhang, 2001) and in vivo (Lee et al., 2013). Wang et al. (Wang et al., 2010) discovered that SalB exerted neuroprotective effects via anti-inflammatory properties on microglia cells. Furthermore, SalB can mediate the GABAergic neurotransmitter system to improve Aβ 25–35-induced memory impairment (Kim et al., 2011). Recently, it was demonstrated that SalB inhibits Aβ 40 and Aβ 42 generation modulating BACE-1 activity in SH-SY5Y-APPsw cells (Tang et al., 2016). In addition to salvianolic acids, many studies have demonstrated that flavonoids and their derivatives have numerous neuroprotective and neuro-inflammatory activities through various pathways related to AD (Balez et al., 2016, Rezai-Zadeh et al., 2008). More recently, some common flavonoids, i.e., kaempferol, myricetin, quercetin, and apigenin, together with phenolic compounds, i.e., curcumin and RA, were screened for Aβ 42 aggregation inhibitory potential in bacterial cells. In that study, apigenin and quercetin showed potent anti-aggregation activity, while RA displayed low and curcumin, a diarylheptanoid compound, showed moderate activity (Espargaró et al., 2017).
Supporting these related data in the literature, our study revealed that the SFINF protected SH-SY5Y cells from Aβ 1–42-induced neurotoxicity in vitro and, to the best of our knowledge, this is the first study to show this preventive effect in a real-time manner that can be basically linked to its GSK-3β inhibitory effect. Consequently, to interpret all the in vitro neurobiological activity results and the phytochemical data, it should be stated that RA not only itself but also phenolic compounds, especially flavones, and monomers and dimers of phenolic acid derivatives together may play a synergistic role in the anti-Alzheimer effects of the SFINF. The results of the present study validate the traditional use of Salvia species for cognitive disorders and highlight the mechanisms underlying the neuroprotective effects on Aβ 1–42-induced neurotoxicity via different mechanisms for the first time.
5. Conclusion
We investigated the neuroprotective effects and potential molecular mechanisms of Salvia fruticosa infusion and rosmarinic acid against Aβ 1–42 toxicity in cultured SH-SY5Y neurons. The SFINF exhibits significant protection against Aβ 1–42-induced cytotoxicity, being superior to rosmarinic acid. The additional ability of inhibiting glycogen synthase kinase 3β CK-1δ and β-secretase and increasing of p-GSK-3 beta protein levels makes the Salvia fruticosa infusion a promising multifunctional neuroprotective agent for further studies involving the development of new anti-Alzheimer agents from natural sources.
However, one of the limitations of the present study is that the phytochemical profile could change according to different ecosystems, which requires a standardization of the major compounds for repeatable qualitative and quantitative results and for repeatable in-vivo studies indeed. Another limitation is that the concentrations used in the biological assays are not of physiologic relevance and future studies should be performed considering blood–brain barrier permeability and the ADME properties of Salvia fruticosa infusion.
Author contributions
PG designed the main study and PG and AHD performed the neurobiological assays; MBY directed the optimum in vitro assay conditions and concentrations in cell culture related experiments; FG carried out the LC-MS/MS experiments; MGG performed and interpreted the molecular docking studies, MYP provided the plant material; PG prepared the manuscript; CP contributed to experimental consumables of in vitro enzyme assays and interpreted the results; LÖD proposed the plant material; MGG, MBY, and LÖD performed the critical readings.
Funding
The authors are indebted to the Research Foundation of Erciyes University (TCD-2012-4006, FCD-2018-7834) for financially supporting the project.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank Dr. Ana Martinez (CSIC-CIB) for her kind and valuable support concerning the enzyme assays. Also many thanks to Dr. Ahmet Cumaoğlu for processing and arranging the Western blotting figures for the manuscript. MGG would like to thank Prof. Dr. Gerhard Wolber for providing the license for LigandScout 4.2.
Footnotes
Peer review under responsibility of King Saud University.
References
- Akhondzadeh S., Noroozian M., Mohammadi M., Ohadinia S., Jamshidi A.H., Khani M. Salvia officinalis extract in the treatment of patients with mild to moderate Alzheimer's disease: a double blind, randomized and placebo-controlled trial. J. Clin. Pharm. Ther. 2003;28:53–59. doi: 10.1046/j.1365-2710.2003.00463.x. [DOI] [PubMed] [Google Scholar]
- Atri A. Current and future treatments in Alzheimer's disease, Seminars in neurology. Thieme Med. Publ. 2019:227–240. doi: 10.1055/s-0039-1678581. [DOI] [PubMed] [Google Scholar]
- Baki A., Bielik A., Molnár L., Szendrei G., Keserü G.M. A high throughput luminescent assay for glycogen synthase kinase-3 β inhibitors. Assay Drug Dev. Technol. 2007;5(1):75–84. doi: 10.1089/adt.2006.029. [DOI] [PubMed] [Google Scholar]
- Balez R., Steiner N., Engel M., Muñoz S.S., Lum J.S., Wu Y., Wang D., Vallotton P., Sachdev P., O’Connor M. Neuroprotective effects of apigenin against inflammation, neuronal excitability and apoptosis in an induced pluripotent stem cell model of Alzheimer’s disease. Sci. Rep. 2016;6:31450. doi: 10.1038/srep31450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros L., Duenas M., Dias M.I., Sousa M.J., Santos-Buelga C., Ferreira I. Phenolic profiles of cultivated, in vitro cultured and commercial samples of Melissa officinalis L. infusions. Food Chem. 2013;136:1–8. doi: 10.1016/j.foodchem.2012.07.107. [DOI] [PubMed] [Google Scholar]
- Bhat R., Xue Y., Berg S., Hellberg S., Ormö M., Nilsson Y., Radesäter A.-C., Jerning E., Markgren P.-O., Borgegård T. Structural insights and biological effects of glycogen synthase kinase 3-specific inhibitor AR-A014418. J. Biol. Chem. 2003;278:45937–45945. doi: 10.1074/jbc.M306268200. [DOI] [PubMed] [Google Scholar]
- Bjørklund G., Aaseth J., Dadar M., Chirumbolo S. Molecular targets in Alzheimer’s disease. Mol. Neurobiol. 2019;56:7032–7044. doi: 10.1007/s12035-019-1563-9. [DOI] [PubMed] [Google Scholar]
- Busche M.A., Hyman B.T. Synergy between amyloid-β and tau in Alzheimer’s disease. Nat. Neurosci. 2020;23:1183–1193. doi: 10.1038/s41593-020-0687-6. [DOI] [PubMed] [Google Scholar]
- Celep F., Dirmenci T., Güner Ö. Salvia hasankeyfense (Lamiaceae), a new species from Hasankeyf (Batman, South-eastern Turkey) Phytotaxa. 2015;227:289–294. [Google Scholar]
- Cornejo A., Aguilar Sandoval F., Caballero L., Machuca L., Muñoz P., Caballero J., Perry G., Ardiles A., Areche C., Melo F. Rosmarinic acid prevents fibrillization and diminishes vibrational modes associated to β sheet in tau protein linked to Alzheimer’s disease. J. Enzyme Inhib. Med. Chem. 2017;32:945–953. doi: 10.1080/14756366.2017.1347783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvetkovikj I., Stefkov G., Acevska J., Stanoeva J.P., Karapandzova M., Stefova M., Dimitrovska A., Kulevanova S. Polyphenolic characterization and chromatographic methods for fast assessment of culinary Salvia species from South East Europe. J. Chromatogr. A. 2013;1282:38–45. doi: 10.1016/j.chroma.2012.12.068. [DOI] [PubMed] [Google Scholar]
- Dahlgren K.N., Manelli A.M., Stine W.B., Baker L.K., Krafft G.A., LaDu M.J. Oligomeric and fibrillar species of amyloid-β peptides differentially affect neuronal viability. J. Biol. Chem. 2002;277:32046–32053. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- Demirezer L.Ö., Gürbüz P., Uğur E.P.K., Bodur M., Özenver N., Uz A., Güvenalp Z. Molecular docking and ex vivo and in vitro anticholinesterase activity studies of Salvia sp. and highlighted rosmarinic acid. Turk. J. Med. Sci. 2015;45:1141–1148. doi: 10.3906/sag-1404-42. [DOI] [PubMed] [Google Scholar]
- Dias M.I., Barros L., Dueñas M., Pereira E., Carvalho A.M., Alves R.C., Oliveira M.B.P.P., Santos-Buelga C., Ferreira I.C.F.R. Chemical composition of wild and commercial Achillea millefolium L. and bioactivity of the methanolic extract, infusion and decoction. Food Chem. 2013;141:4152–4160. doi: 10.1016/j.foodchem.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Durairajan S.S.K., Yuan Q., Xie L., Chan W.-S., Kum W.-F., Koo I., Liu C., Song Y., Huang J.-D., Klein W.L. Salvianolic acid B inhibits Aβ fibril formation and disaggregates preformed fibrils and protects against Aβ-induced cytotoxicty. Neurochem. Int. 2008;52:741–750. doi: 10.1016/j.neuint.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Eldar-Finkelman H., Martinez A. GSK-3 inhibitors: preclinical and clinical focus on CNS. Front. Mol. Neurosci. 2011;4 doi: 10.3389/fnmol.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espargaró A., Ginex T., Vadell M.d.M., Busquets M.A., Estelrich J., Muñoz-Torrero D., Luque F.J., Sabate R. Combined in vitro cell-based/in silico screening of naturally occurring flavonoids and phenolic compounds as potential anti-alzheimer drugs. J. Nat. Prod. 2017;80:278–289. doi: 10.1021/acs.jnatprod.6b00643. [DOI] [PubMed] [Google Scholar]
- Guimarães R., Barros L., Dueñas M., Calhelha R.C., Carvalho A.M., Santos-Buelga C., Queiroz M.J.R.P., Ferreira I.C.F.R. Nutrients, phytochemicals and bioactivity of wild Roman chamomile: a comparison between the herb and its preparations. Food Chem. 2013;136:718–725. doi: 10.1016/j.foodchem.2012.08.025. [DOI] [PubMed] [Google Scholar]
- Gürbüz P., Martinez A., Pérez C., Martínez-González L., Göger F., Ayran İ. Potential anti-Alzheimer effects of selected Lamiaceae plants through polypharmacology on glycogen synthase kinase-3β, β-secretase, and casein kinase 1δ. Ind. Crops Prod. 2019;138:111431. [Google Scholar]
- Hartree E.F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal. Biochem. 1972;48:422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Helmja K., Vaher M., Püssa T., Raudsepp P., Kaljurand M. Evaluation of antioxidative capability of the tomato (Solanum lycopersicum) skin constituents by capillary electrophoresis and high-performance liquid chromatography. Electrophoresis. 2008;29:3980–3988. doi: 10.1002/elps.200800012. [DOI] [PubMed] [Google Scholar]
- Hill J., Seguin R., Phanga T., Manda A., Chikasema M., Gopal S., Smith J.S. Facilitators and barriers to traditional medicine use among cancer patients in Malawi. PLoS ONE. 2019;14:e0223853. doi: 10.1371/journal.pone.0223853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X.-Q., Yan R., Yang C., Zhang L., Su R.-Y., Liu S.-J., Zhang S.-J., He W.-Q., Fang S.-H., Cheng S.-Y. A novel assay for high-throughput screening of anti-Alzheimer's disease drugs to determine their efficacy by real-time monitoring of changes in PC12 cell proliferation. Int. J. Mol. Med. 2014;33:543–549. doi: 10.3892/ijmm.2013.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes M.-J.R., Houghton P.J. Plants used in Chinese and Indian traditional medicine for improvement of memory and cognitive function. Pharmacol. Biochem. Behav. 2003;75:513–527. doi: 10.1016/s0091-3057(03)00128-x. [DOI] [PubMed] [Google Scholar]
- Howes M.-J.R., Perry N.S.L., Houghton P.J. Plants with traditional uses and activities, relevant to the management of Alzheimer's disease and other cognitive disorders. Phytother. Res. 2003;17:1–18. doi: 10.1002/ptr.1280. [DOI] [PubMed] [Google Scholar]
- Hu X., Zhou X., He W., Yang J., Xiong W., Wong P., Wilson C.G., Yan R. BACE1 deficiency causes altered neuronal activity and neurodegeneration. J. Neurosci. 2010;30:8819–8829. doi: 10.1523/JNEUROSCI.1334-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuvone T., De Filippis D., Esposito G., D'Amico A., Izzo A.A. The spice sage and its active ingredient rosmarinic acid protect PC12 cells from amyloid-β peptide-induced neurotoxicity. J. Pharmacol. Exp. Ther. 2006;317:1143–1149. doi: 10.1124/jpet.105.099317. [DOI] [PubMed] [Google Scholar]
- Jafari M., Wang Y., Amiryousefi A., Tang J. Unsupervised learning and multipartite network models: a promising approach for understanding traditional medicine. Front. Pharmacol. 2020;11:1319. doi: 10.3389/fphar.2020.01319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D.O., Dodd F.L., Robertson B.C., Okello E.J., Reay J.L., Scholey A.B., Haskell C.F. Monoterpenoid extract of sage (Salvia lavandulaefolia) with cholinesterase inhibiting properties improves cognitive performance and mood in healthy adults. J. Psychopharmacol. 2011;25:1088–1100. doi: 10.1177/0269881110385594. [DOI] [PubMed] [Google Scholar]
- Kim D.H., Park S.J., Kim J.M., Jeon S.J., Kim D.-H., Cho Y.-W., Son K.H., Lee H.J., Moon J.-H., Cheong J.H., Ko K.H., Ryu J.H. Cognitive dysfunctions induced by a cholinergic blockade and Aβ25–35 peptide are attenuated by salvianolic acid B. Neuropharmacology. 2011;61:1432–1440. doi: 10.1016/j.neuropharm.2011.08.038. [DOI] [PubMed] [Google Scholar]
- Kolak Ufuk H.I., Mehmet Öztürk, Fevzi Özgökçe, Gülaçtı Topçu, Ayhan Ulubelen. Antioxidant and anticholinesterase constituents of Salvia poculata. Turk. J. Chem. 2009;33:813–823. [Google Scholar]
- Kontogianni V.G., Tomic G., Nikolic I., Nerantzaki A.A., Sayyad N., Stosic-Grujicic S., Stojanovic I., Gerothanassis I.P., Tzakos A.G. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem. 2013;136:120–129. doi: 10.1016/j.foodchem.2012.07.091. [DOI] [PubMed] [Google Scholar]
- Koutsoulas A., Čarnecká M., Slanina J., Tóth J., Slaninová I. Characterization of phenolic compounds and antiproliferative effects of Salvia pomifera and Salvia fruticosa extracts. Molecules. 2019;24:2921. doi: 10.3390/molecules24162921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.W., Kim D.H., Jeon S.J., Park S.J., Kim J.M., Jung J.M., Lee H.E., Bae S.G., Oh H.K., Son K.H.H. Neuroprotective effects of salvianolic acid B on an Aβ25–35 peptide-induced mouse model of Alzheimer's disease. Eur. J. Pharmacol. 2013;704:70–77. doi: 10.1016/j.ejphar.2013.02.015. [DOI] [PubMed] [Google Scholar]
- Lu Y., Yeap Foo L. Polyphenolics of Salvia—a review. Phytochemistry. 2002;59:117–140. doi: 10.1016/s0031-9422(01)00415-0. [DOI] [PubMed] [Google Scholar]
- Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., Olson A.J. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comp Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orhan I., Kartal M., Naz Q., Ejaz A., Yilmaz G., Kan Y., Konuklugil B., Şener B., Iqbal Choudhary M. Antioxidant and anticholinesterase evaluation of selected Turkish Salvia species. Food Chem. 2007;103:1247–1254. [Google Scholar]
- Orhan I.E., Senol F.S., Ozturk N., Akaydin G., Sener B. Profiling of in vitro neurobiological effects and phenolic acids of selected endemic Salvia species. Food Chem. 2012;132:1360–1367. doi: 10.1016/j.foodchem.2011.11.119. [DOI] [PubMed] [Google Scholar]
- Perry E.K., Pickering A.T., Wang W.W., Houghton P.J., Perry N.S. Medicinal plants and Alzheimer's disease: from ethnobotany to phytotherapy. J. Pharm. Pharmacol. 1999;51:527–534. doi: 10.1211/0022357991772808. [DOI] [PubMed] [Google Scholar]
- Perry N., Menzies R., Hodgson F., Wedgewood P., Howes M.-J., Brooker H., Wesnes K., Perry E. A randomised double-blind placebo-controlled pilot trial of a combined extract of sage, rosemary and melissa, traditional herbal medicines, on the enhancement of memory in normal healthy subjects, including influence of age. Phytomedicine. 2018;39:42–48. doi: 10.1016/j.phymed.2017.08.015. [DOI] [PubMed] [Google Scholar]
- Perry N.S.L., Bollen C., Perry E.K., Ballard C. Salvia for dementia therapy: review of pharmacological activity and pilot tolerability clinical trial. Pharmacol. Biochem. Behav. 2003;75:651–659. doi: 10.1016/s0091-3057(03)00108-4. [DOI] [PubMed] [Google Scholar]
- Rezai-Zadeh K., Ehrhart J., Bai Y., Sanberg P.R., Bickford P., Tan J., Shytle R.D. Apigenin and luteolin modulate microglial activation via inhibition of STAT1-induced CD40 expression. J Neuroinflamm. 2008;5:41. doi: 10.1186/1742-2094-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrödinger, 2019. Maestro, New York.
- Spínola V., Pinto J., Castilho P.C. Identification and quantification of phenolic compounds of selected fruits from Madeira Island by HPLC-DAD–ESI-MSn and screening for their antioxidant activity. Food Chem. 2015;173:14–30. doi: 10.1016/j.foodchem.2014.09.163. [DOI] [PubMed] [Google Scholar]
- Sul D., Kim H.-S., Lee D., Joo S.S., Hwang K.W., Park S.-Y. Protective effect of caffeic acid against beta-amyloid-induced neurotoxicity by the inhibition of calcium influx and tau phosphorylation. Life Sci. 2009;84:257–262. doi: 10.1016/j.lfs.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Şenol F.S., Orhan I., Celep F., Kahraman A., Doğan M., Yilmaz G., Şener B. Survey of 55 Turkish Salvia taxa for their acetylcholinesterase inhibitory and antioxidant activities. Food Chem. 2010;120:34–43. [Google Scholar]
- Şenol F.S., Orhan I.E., Erdem S.A., Kartal M., Şener B., Kan Y., Celep F., Kahraman A., Dogan M. Evaluation of cholinesterase inhibitory and antioxidant activities of wild and cultivated samples of sage (Salvia fruticosa) by activity-guided fractionation. J. Med. Food. 2011;14:1476–1483. doi: 10.1089/jmf.2010.0158. [DOI] [PubMed] [Google Scholar]
- Tang M., Zhang J. Salvianolic acid B inhibits fibril formation and neurotoxicity of amyloid beta-protein in vitro. Acta Pharmacol. Sin. 2001;22:380–384. [PubMed] [Google Scholar]
- Tang Y., Huang D., Zhang M.-H., Zhang W.-S., Tang Y.-X., Shi Z.-X., Deng L., Zhou D.-H., Lu X.-Y. Salvianolic acid B inhibits Aβ generation by modulating BACE1 activity in SH-SY5Y-APPsw cells. Nutrients. 2016;8:333. doi: 10.3390/nu8060333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tildesley N.T.J., Kennedy D.O., Perry E.K., Ballard C.G., Savelev S., Wesnes K.A., Scholey A.B. Salvia lavandulaefolia (Spanish Sage) enhances memory in healthy young volunteers. Pharmacol. Biochem. Behav. 2003;75:669–674. doi: 10.1016/s0091-3057(03)00122-9. [DOI] [PubMed] [Google Scholar]
- Topçu G. Bioactive triterpenoids from Salvia species. J. Nat. Prod. 2006;69:482–487. doi: 10.1021/np0600402. [DOI] [PubMed] [Google Scholar]
- Vergine M., Nicolì F., Negro C., Luvisi A., Nutricati E., Accogli R.A., Sabella E., Miceli A. Phytochemical profiles and antioxidant activity of Salvia species from southern Italy. Rec. Nat. Prod. 2019;13:215. [Google Scholar]
- Ververis, A., Savvidou, G., Ioannou, K., Nicolaou, P., Christodoulou, K., Plioukas, M., 2020. Greek Sage Exhibits Neuroprotective Activity against Amyloid Beta-Induced Toxicity. Evidence-Based Complementary and Alternative Medicine 2020. [DOI] [PMC free article] [PubMed]
- Vichai V., Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006;1:1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- Wang S.-X., Hu L.-M., Gao X.-M., Guo H., Fan G.-W. Anti-inflammatory activity of salvianolic acid B in microglia contributes to its neuroprotective effect. Neurochem. Res. 2010;35:1029–1037. doi: 10.1007/s11064-010-0151-1. [DOI] [PubMed] [Google Scholar]
- Wu Y.-B., Ni Z.-Y., Shi Q.-W., Dong M., Kiyota H., Gu Y.-C., Cong B. Constituents from Salvia species and their biological activities. Chem. Rev. 2012;112:5967–6026. doi: 10.1021/cr200058f. [DOI] [PubMed] [Google Scholar]
- Zhang X.-Z., Qian S.-S., Zhang Y.-J., Wang R.-Q. Salvia miltiorrhiza: a source for anti-Alzheimer’s disease drugs. Pharm. Biol. 2016;54:18–24. doi: 10.3109/13880209.2015.1027408. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Li W., Xu L., Chen L. In Salvia miltiorrhiza, phenolic acids possess protective properties against amyloid β-induced cytotoxicity, and tanshinones act as acetylcholinesterase inhibitors. Environ. Toxicol. Pharmacol. 2011;31:443–452. doi: 10.1016/j.etap.2011.02.006. [DOI] [PubMed] [Google Scholar]