Abstract
Populations in the high-income countries of Western Europe are aging due to increased life expectancy. As the prevalence of diabetes and obesity has increased, so has the burden of kidney failure. To determine the global capacity for kidney replacement therapy and conservative kidney management, the International Society of Nephrology conducted multinational, cross-sectional surveys and published the findings in the International Society of Nephrology Global Kidney Health Atlas. In the second iteration of the International Society of Nephrology Global Kidney Health Atlas, we aimed to describe the availability, accessibility, quality, and affordability of kidney failure care in Western Europe. Among the 29 countries in Western Europe, 21 (72.4%) responded, representing 99% of the region’s population. The burden of kidney failure prevalence varied widely, ranging from 760 per million population (pmp) in Iceland to 1612 pmp in Portugal. Coverage of kidney replacement therapy from public funding was nearly universal, with the exceptions of Germany and Liechtenstein where part of the costs was covered by mandatory insurance. Fourteen (67%) of 21 countries charged no fees at the point of care delivery, but in 5 countries (24%), patients do pay some out-of-pocket costs. Long-term dialysis services (both hemodialysis and peritoneal dialysis) were available in all countries in the region, and kidney transplantation services were available in 19 (90%) countries. The incidence of kidney transplantation varied widely between countries from 12 pmp in Luxembourg to 70.45 pmp in Spain. Conservative kidney care was available in 18 (90%) of 21 countries. The median number of nephrologists was 22.9 pmp (range: 9.47–55.75 pmp). These data highlight the uniform capacity of Western Europe to provide kidney failure care, but also the scope for improvement in disease prevention and management, as exemplified by the variability in disease burden and transplantation rates.
Keywords: chronic kidney disease, dialysis, end-stage kidney disease, kidney failure, kidney registries, kidney transplantation
Compared with other world regions, Western Europe benefits from functional health care systems, established noncommunicable disease guidelines, higher health professional density, high availability of essential medicines, and more widespread universal health coverage.1,2 In line with this infrastructure, there has been a decline in premature mortality from the 4 major types of noncommunicable diseases (cardiovascular diseases, cancer, diabetes, and chronic respiratory diseases).3 However, large variability in mortality rates remains both between sexes and between countries. One of every 10 Europeans has chronic kidney disease (CKD); among contributing factors are the rising prevalence of diabetes and obesity, and dynamics associated with an aging population.4 In parallel with these changes, the prevalence of kidney replacement therapy (KRT; dialysis and kidney transplantation) is steadily growing and has led to an equally aging dialysis population.5 Since 1964, the European Renal Association-European Dialysis and Transplant Association has collected data on KRT modalities such as dialysis and transplantation via national and regional kidney registries from 36 countries in Europe and has charted the evolution of kidney failure (KF) epidemiology through the publication of annual reports6, 7, 8, 9, 10 and epidemiologic trends.11 As one of the world’s largest international registries of kidney care, it contributes substantially to enabling international comparisons.
In 2020, nephrology in Europe faced a number of new challenges in addition to the well-known changes in population demographics with aging population and workforce challenges. First and foremost, the global pandemic resulting from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus (referred to as coronavirus disease 2019 [COVID-19]) has a number of far-reaching complications including high rates of acute kidney injury (AKI),12 risk of transmission of infection among vulnerable in-center dialysis patients, and the suspension of transplant services to avoid additional immunosuppression at this time.13 Secondly, the ongoing war and consequent population movement in Syria and elsewhere in the Middle East, Africa, and South America have led to a rise in the number of refugees receiving KRT in European countries.14 In addition to ethical, financial, and legal implications, forced migration in this context may increase the diversity of kidney pathologies, physical and psychological comorbidities, and cultural nuances. Secondly, climate change may pose new problems, with an increasing number of heat waves in Europe15 and heat-related AKI as an important contributor to mortality in this setting.16 There is also a climate-driven risk of increased “tropical” infections, for example, poleward shifts of Aedes-borne virus distributions (particularly dengue, chikungunya, and Zika), with their associated AKI morbidity.17 Unexpected extreme weather conditions can also disrupt critical infrastructure (e.g., power and water supplies, transportation, and telecommunication services) required to deliver dialysis services.18 Lastly, Brexit may have ramifications for patients with kidney disease in terms of reciprocal health care, the supply of medical consumables, organ sharing across the European Union (EU) countries participating in the Eurotransplant and health care in Northern Ireland,19 as well as for European research and recruitment more broadly.19 However, it is likely that in the short-to-medium term, Brexit as a geopolitical issue will pale in comparison to the COVID-19 threat to globalization. With this landscape in mind, we use data from the second International Society of Nephrology Global Kidney Health Atlas (ISN-GKHA) survey to report on the availability, accessibility, affordability, and quality of KF care in Western Europe. However, we should emphasize that this is only a snapshot of disease burden in the region. The methodology for this research is described in detail elsewhere.20
Results
Results of this study are presented in tables and figures and broadly summarized into 2 categories: desk research (Tables 121, 22, 23, 24 and 225, 26, 27, 28, Figure 1, and Supplementary Table S1) and survey administration (Figure 2, Figure 3, Figure 4, Figure 5 and Supplementary Figures S1–S7).
Table 1.
Health finance, service delivery, and workforce prevalence in the 21 countries in Western Europe that participated in the ISN-GKHA survey21, 22, 23, 24
| Country | Area (km2) | Total population (2018) | GDP (PPP), $ billionsa | Total health expenditures, % of GDPa | Annual cost KRTb (US$) and out-of-pocket cost/% paid by patient from total costc |
||
|---|---|---|---|---|---|---|---|
| HD | PD | KT (first year) | |||||
| Global median [IQR]d | — | — | — | 6.5 [4.9–8.8] | 22,617 [14,882–49,690] | 20,524 [14,305–33,905] | 25,356 [15,913–43,901] |
| Western Europe median [IQR]d | — | — | 441.0 [266.0–2048.0] | 9.8 [8.7–10.6] | 60,037 [50,558–77,040] | 47,963 [30,248–60,816] | 54,342 [34,090–71,777] |
| Austria | 83,871 | 8,793,370 | 441.0 | 10.4 | 56,252/0 | 30,248/0 | 67,059/0 |
| Belgium | 30,528 | 11,570,762 | 529.2 | 10.0 | 67,512/0 | 61,643/0 | 38,451/0 |
| Denmark | 43,094 | 5,809,502 | 287.8 | 10.4 | 59,576/0 | 28,102/0 | 25,836/0 |
| Finland | 357,022 | 5,537,364 | 244.9 | 9.4 | 78,233/0 | 66,816/0 | 36,117/0 |
| France | 643,801 | 67,364,357 | 2856.0 | 11.1 | 85,436/1–25 | 69,516/1–25 | 114,220/1–25 |
| Germany | 357,022 | 80,457,737 | 4199.0 | 11.2 | 76,642 | 51,196 | 101,915 |
| Greece | 131,957 | 10,761,523 | 299.3 | 8.4 | 45,435/0 | 56,354/0 | 63,196/0 |
| Iceland | 572 | 343,518 | 18.2 | 8.6 | 73,320/1–25 | 17,155/1–25 | —/1–25 |
| Ireland | 70,723 | 5,068,050 | 353.3 | 7.8 | 60,498/0 | 36,926/0 | —/0 |
| Israel | 20,770 | 8,424,904 | 317.1 | 7.4 | 56,174/0 | 65,716/0 | —/0 |
| Italy | 2586 | 62,246,674 | 2317.0 | 9.0 | 46,912/0 | 26,254/0 | 71,461/0 |
| Liechtenstein | 160 | 38,547 | 5.0 | — | — | — | — |
| Luxembourg | 2586 | 605,764 | 62.1 | 6.0 | —/0 | —/0 | —/0 |
| Malta | 316 | 449,043 | 19.3 | 9.6 | —/0 | —/0 | —/0 |
| Netherlands | 41,543 | 17,151,228 | 924.4 | 10.7 | 103,187/1–25 | 67,974/1–25 | 71,882/1–25 |
| Norway | 323,802 | 5,372,191 | 381.2 | 10.0 | 50,847/0 | 19,061/0 | 33,414/0 |
| Portugal | 450,295 | 10,335,493 | 314.1 | 9.0 | 32,846/0 | 32,109/0 | 105,183/0 |
| Spain | 505,370 | 49,331,076 | 1778.0 | 9.2 | 56,602/0 | 39,414/0 | 45,487/0 |
| Sweden | 450,295 | 10,040,995 | 518.0 | 11.0 | 92,285/1–25 | 83,193/1–25 | —/1–25 |
| Switzerland | 41,277 | 8,292,809 | 523.1 | 12.1 | 67,800/1–25 | 47,963/1–25 | —/1–25 |
| United Kingdom | 243,610 | 65,105,246 | 2925.0 | 9.9 | 49,690/0 | 31,505/0 | 27,971/0 |
—, Data not reported/unavailable; GDP, gross domestic product; GKHA, Global Kidney Health Atlas; HD, hemodialysis; IQR, interquartile range; ISN, International Society of Nephrology; KRT, kidney replacement therapy; KT, kidney transplant; PD, peritoneal dialysis; PPP, purchasing power parity.
Estimates are in US$ 2017.
Detailed reference list on annual cost of KRT is available in the Supplementary Appendix.
Costs are in US$ 2016.
Median and interquartile ranges are calculated for the selected countries in the ISN-GKHA survey only.
Table 2.
Kidney replacement therapy and nephrology workforce statistics for the 21 countries in Western Europe that participated in ISN-GKHA survey25, 26, 27, 28
| Country | Treated KF (pmp) |
Prevalence of long-term dialysis (pmp) |
Long-term dialysis centers (pmp) |
Kidney transplantation (pmp) |
Nephrology workforce (pmp) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incidence | Prevalence | HD | PD | Total (HD + PD) | HD | PD | Incidence | Prevalence | Centers | Nephrologists | Nephrology trainees | |
| Global median [IQR]a | 142 [106–193] | 787 [522–1047] | 310.0 [99.0–597.0] | 25.0 [2.0–56.0] | 359.0 [112.0–636.0] | 4.5 [1.0–10.0] | 1.3 [0.4–2.5] | 14.0 [5.0–36.0] | 269.0 [66.0–468.0] | 0.4 [0.2–0.7] | 10.0 [1.2–22.9] | 1.4 [0.3–3.7] |
| Western Europe median [IQR]a | 131 [113–178] | 1038 [948–1261] | 477.5 [319.4–670.1] | 52.8 [43.5–67.4] | 518.4 [376.0–717.6] | 6.9 [4.5–10.1] | 2.3 [1.8–3.6] | 45.1 [32.2–51.9] | 547.9 [486.1–619.6] | 0.5 [0.4–0.8] | 22.9 [16.0–29.9] | 5.9 [3.1–9.3] |
| Austria | 129 | 1087 | 477.5 | 40.9 | 518.4 | 9.1 | 1.0 | 49.2 | 569.0 | 0.5 | 34.1 | 5.7 |
| Belgium | 182 | 1287 | 663.3 | 54.3 | 717.6 | 4.6 | 2.3 | 48.1 | 569.2 | 0.6 | — | — |
| Denmark | 131 | 958 | 364.5 | 94.7 | 459.2 | 2.3 | 2.3 | 45.1 | 499.2 | 0.5 | 25.8 | 4.5 |
| Finland | 100 | 909 | 291.6 | 69.7 | 361.3 | 5.4 | 3.6 | 43.6 | 547.9 | 0.2 | 14.5 | 1.8 |
| France | 173 | 1310 | 670.1 | 46.2 | 716.3 | 4.2 | 1.2 | 58.2 | 593.4 | 0.5 | 20.0 | 5.1 |
| Germany | — | — | 768.1 | 38.8 | 806.9 | 9.9 | 2.5 | 23.4 | — | 0.4 | 18.6 | 3.7 |
| Greece | 252 | 1319 | 1010.0 | 66.7 | 1076.7 | 15.8 | 2.8 | 15.7 | 242.1 | 0.4 | 55.8 | 7.4 |
| Iceland | 143 | 760 | 195.1 | 58.2 | 253.3 | 11.6 | 2.9 | 26.7 | 506.7 | 5.8 | 29.1 | — |
| Ireland | 88 | 827 | 310.1 | 44.3 | 354.4 | 4.5 | 2.0 | 40.0 | 473.0 | 0.2 | 9.5 | 5.9 |
| Israel | 193 | 1138 | 662.8 | 43.5 | 706.3 | 9.3 | 2.5 | 44.2 | 432.0 | 0.7 | 29.7 | 2.4 |
| Italy | 140 | 1236 | 738.8 | 78.3 | 817.1 | 9.9 | 3.6 | 37.8 | 419.0 | 0.7 | 48.2 | 8.0 |
| Liechtenstein | — | — | — | — | — | 25.9 | 25.9 | — | — | — | 51.9 | 0.0 |
| Luxembourg | — | — | 596.7 | 4.7 | 601.4 | 8.3 | 1.7 | 12.0 | — | — | 21.5 | — |
| Malta | — | — | — | — | — | 4.5 | 2.2 | 52.5 | — | 2.2 | 13.4 | 22.3 |
| Netherlands | 115 | 1038 | 323.2 | 52.8 | 376.0 | 4.4 | 2.3 | 57.6 | 661.8 | 0.5 | 17.5 | 2.3 |
| Norway | 111 | 962 | 232.5 | 49.9 | 282.4 | 4.7 | 4.3 | 51.7 | 679.6 | 0.2 | 27.9 | 18.6 |
| Portugal | 230 | 1612 | 871.3 | 48.1 | 919.4 | 10.2 | 1.7 | 51.4 | 693.0 | 0.7 | 27.5 | 9.7 |
| Spain | 141 | 1213 | 499.8 | 67.4 | 567.2 | 6.9 | 2.2 | 70.5 | 645.7 | 0.9 | 20.3 | 7.3 |
| Sweden | 116 | 987 | 319.4 | 88.6 | 408.0 | 6.8 | 4.2 | 47.9 | 579.0 | 0.4 | 22.9 | 9.0 |
| Switzerland | 97 | 937 | 395.1 | 40.2 | 435.3 | 12.1 | 7.2 | 42.4 | 501.9 | 0.7 | 30.4 | 5.1 |
| United Kingdom | 118 | 971 | 383.8 | 53.3 | 437.1 | 1.0 | 0.8 | 52.1 | 533.8 | 0.3 | 9.8 | 6.1 |
—, Data not reported/unavailable; GKHA, Global Kidney Health Atlas; HD, hemodialysis; IQR, interquartile range; ISN, International Society of Nephrology; KF, kidney failure; PD, peritoneal dialysis; pmp, per million population.
Median value and interquartile range are calculated for the selected countries in the ISN-GKHA survey only.
Figure 1.
Countries in the International Society of Nephrology (ISN) Western Europe region that participated in the ISN Global Kidney Health Atlas survey. NIS, Newly Independent States.
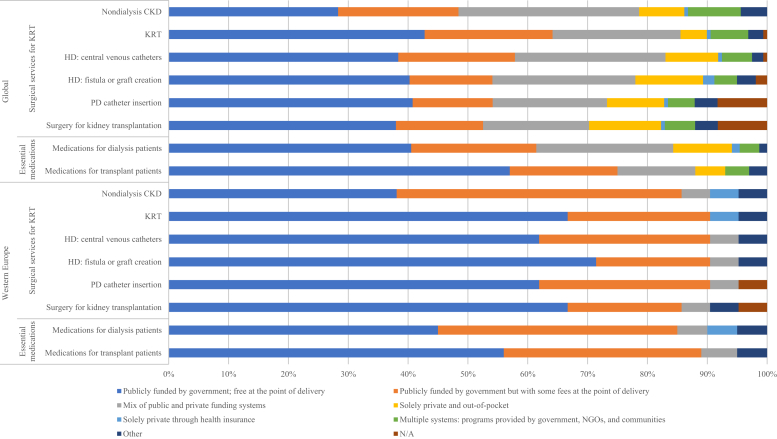
Figure 2.
Funding structures for nondialysis chronic kidney disease (CKD) and kidney replacement therapy (KRT) care. Values represent absolute number of countries in each category, expressed as a percentage of total number of countries. HD, hemodialysis; N/A, not provided; NGOs, nongovernmental organizations; PD, peritoneal dialysis.
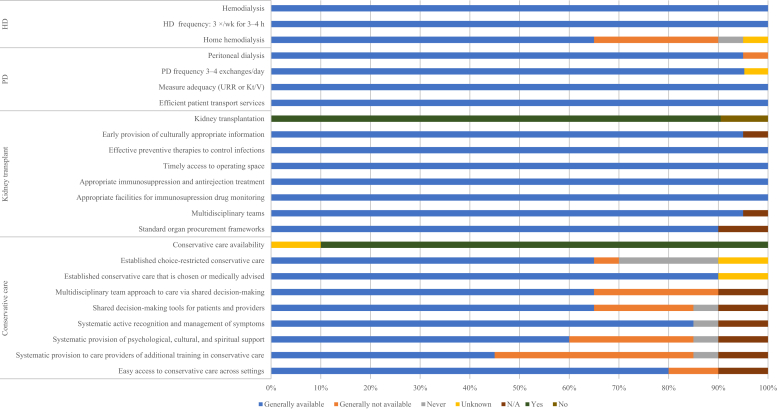
Figure 3.
Availability of choice in kidney replacement therapy or conservative care for patients with kidney failure. HD, hemodialysis; Kt/V, measure of dialysis adequacy; N/A, not provided; PD, peritoneal dialysis; URR, urea reduction ratio.
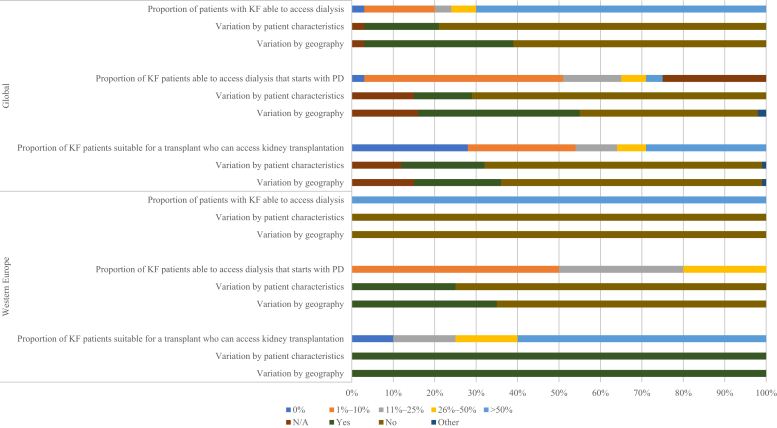
Figure 4.
Accessibility of kidney replacement therapy for patients with kidney failure (KF). N/A, not provided; PD, peritoneal dialysis.
Figure 5.
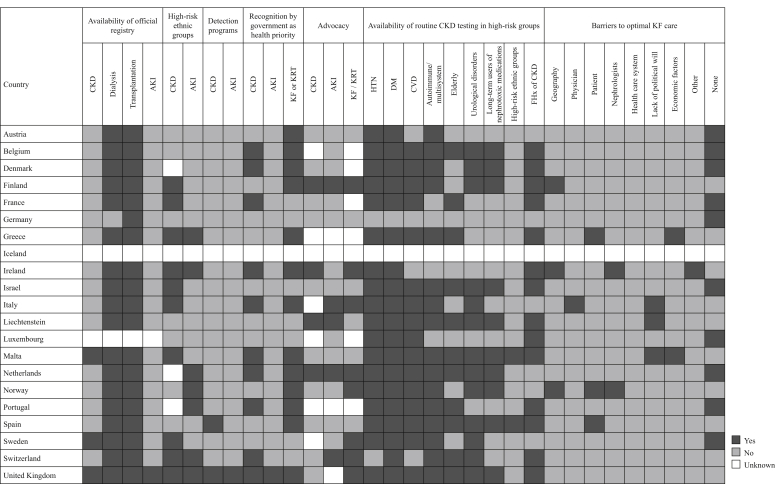
Country-level scorecard for registries, national policies, advocacy, and barriers to optimal kidney failure (KF) care in Western Europe. AKI, acute kidney injury; CKD, chronic kidney disease; CVD, cardiovascular disease; DM, diabetes mellitus; FHx, family history; HTN, hypertension; KRT, kidney replacement therapy.
Setting
Western Europe has had various historical (noncommunist vs. formerly communist countries), geographic, and economic (high- rather than middle-/low-income countries) definitions in the past. However, for the purpose of this analysis, we used the ISN regional definition, which includes continental Europe (Austria, Belgium, France, Germany, Ireland, Liechtenstein, Luxembourg, the Netherlands, Switzerland, and the United Kingdom), Scandinavia (Denmark, Finland, Iceland, Norway, and Sweden), and Mediterranean Europe (Andorra, Greece, Israel, Italy, Malta, Monaco, Portugal, San Marino, Spain, and the Vatican).29 This equated to a population of 433.7 million inhabitants living in predominantly industrialized, high-income countries. Germany (80.5 million), the United Kingdom (65.1 million), and France (67.4 million) are the most populous countries within Western Europe.30
Western Europe is one of the wealthiest regions of the world and continues to experience economic growth, as evidenced by an increase in the gross national income per capita from US$ 19,752 in 2000 to US$ 35,420 in 2018 and a total regional gross domestic product (GDP) of US$ 18.8 trillion, representing 22% of the global economy.30 Accordingly, the average life expectancy at birth has increased progressively to 80.9 years. However, despite faring better than many other regions in the world, income inequality still exists in Western Europe. For example, the Gini index, a statistical measure of income equality whereby 0 represents perfect equality and 100 represents perfect inequality, is 36 in the United Kingdom, 34 in Spain, and 33 in Greece.31 The EU Regional Human Development Index report also shows a clear northwest/southeast divide across EU regions for the overall index, which is a parameter composite of a country’s life expectancy, education index, and gross national income per capita.32 Within countries, differences exist among country regions as to performance in human development. This is especially the case for the United Kingdom, Spain, France, Italy, Germany, and Belgium. Capital city regions generally outperform non–capital city regions within countries.
Brief summary of the current state of kidney care in the region
The incidence of KF varies widely across Western European countries. According to the European Renal Association-European Dialysis and Transplant Association 2017 registry report based on data collected via national and regional kidney registries, the incidence of KRT for such patients in Europe was 127 per million population (pmp).10 Within Western European countries, this ranged from 97 pmp in Switzerland to 252 pmp in Greece. Nearly two-thirds of patients were male with a mean age of 65.3 years, and diabetic nephropathy was the underlying etiology in 23% of cases. Hemodialysis (HD) was the most frequent modality at the start of KRT, followed by peritoneal dialysis (PD) and then pre-emptive kidney transplant. In the Western Europe region, the prevalence of KRT was 854 pmp, ranging from 760 pmp in Iceland to 1965 pmp in Portugal. It has been proposed that varying socioeconomic factors, prevention programs, and real differences in CKD incidence may account for these differences.33
Characteristics of participating countries
Forty-six respondents representing 21 of the 29 (72.4 %) countries in the ISN’s Western Europe region completed the online questionnaire (Figure 1). The majority of respondents were nephrologists (n = 38, 85%), followed by policy makers (n = 4, 9%), other stakeholders (n = 2, 4%), non-nephrologist physicians (n = 1, 2%), and other health professionals (n = 1, 2%) with an overall response rate of 68.7%. Participating countries jointly represented a population of 433.1 million (99.9% of the total population in Western Europe). All participating countries were in the high-income category (n = 21, 100%), as were the 8 territories that did not participate in the survey (Andorra, the Channel Islands, the Faroe Islands, Gibraltar, Greenland, the Isle of Man, Monaco, and San Marino). As a proportion of GDP, health expenditures in participating countries ranged from 12.1% in Switzerland to 6% in Luxembourg (Table 1).21, 22, 23
Burden of CKD and KF in Western Europe
The average prevalence of CKD in Western Europe was 10.1% (95% confidence interval: 9.6, 10.6), ranging from 7.84% in Israel to 11.84% in Sweden. The highest proportions of deaths and disability-adjusted life years attributed to CKD were found in Israel, Austria, and Greece (Supplementary Table S1).
Data on the prevalence of KF in Western Europe were available for all participating countries, with the exception of Liechtenstein and Malta. The median prevalence of treated KF in Western Europe was 1038 pmp (interquartile range [IQR]: 948–1261), which is higher than the global median of 759 pmp. The countries with the highest prevalence were Portugal (1612 pmp), Greece (1319 pmp), and France (1310 pmp) (Table 2).25 The median number of new cases of treated KF in the region (131 pmp; IQR: 113-177.5) was lower than the global median (142 pmp). Greece, Portugal, and Israel had the highest incidences of KF in Western Europe (252 pmp, 230 pmp, and 193 pmp, respectively). In all Western European countries, HD was the most common dialysis modality (Table 2).25, 26, 27 However, PD accounted for a significant proportion of dialysis treatment in Iceland, Sweden, and Denmark (23%, 22%, and 21%, respectively). Patients living with a functioning kidney transplant comprised approximately two-thirds of all treated KF patients in Norway, Iceland, and the Netherlands (70.6%, 66.7%, and 63.8%, respectively). The country with the lowest prevalence of patients with KF living with kidney transplants was Greece (242.1 pmp; 18.4% of all patients with KF) (Table 2).25
Health finance and service delivery
In the vast majority of countries, costs of nondialysis CKD and KRT care were covered by public funding (Figure 2). Services were free at the point of delivery with no out-of-pocket costs for the patient in 67% of countries in Western Europe, compared with just 43% of countries around the world. Coverage of KRT costs by public funding (in whole or in part) was nearly universal in the region, with the exceptions of Germany and Liechtenstein, where part of the costs was covered by mandatory insurance.
Among the 21 participating countries, 18 (86%) had data available about the annual cost of dialysis. In the region, median annual costs (in USD) per person for maintenance HD ($60,037; IQR: $50,558–$77,040) and maintenance PD ($47,963; IQR: $30,248–$60,816) were well above global averages ($22,617 and $20,524, respectively) (Table 1).24 Data on the annual cost of kidney transplantation in the first year were available for 13 countries; costs per patient range from $27,971 in the United Kingdom to $114,220 in France. Patients in 14 countries (67%) paid no out-of-pocket costs for KRT, and patients in 5 countries (France, Iceland, the Netherlands, Sweden, and Switzerland) covered 1%–25% of these costs (Table 1).24 Fees were covered exclusively by private health insurance providers in Liechtenstein. A statutory health insurance model was used in Germany.
The organization and delivery of KF care was structured differently (no national framework) by regions in 4 countries (19%) and between adults and children in 3 countries (14%). In almost half of all countries, responsibility for KF care oversight rests with individual hospitals, trusts, or organizations (n = 10, 48%), followed by a national body (n = 7, 33%) and provincial or state level organizations (n = 7, 33%). Two countries (Germany and Liechtenstein) reported the organization and delivery of KF care as other.
Health workforce for nephrology care
Nephrologists are primarily responsible for KF care in Western Europe (n = 21, 100%), with some support from primary care physicians (n = 2, 10%) and nurse practitioners (n = 2, 10%) in some countries. The median number of nephrologists (22.9 pmp, IQR: 16.0–29.9) and the median number of nephrology trainees (5.9 pmp, IQR: 3.1–9.3) in Western Europe were much higher than the median numbers worldwide (9.95 and 1.4 pmp, respectively) (Table 2).28 There was, however, great variability within the region, with countries such as Greece, Italy, and Liechtenstein reporting more than 40 nephrologists pmp, whereas the United Kingdom and Ireland both reported less than 10 nephrologists pmp. The most commonly reported workforce shortages were for both nephrologists (43%, n = 9) and dialysis nurses (43%, n = 9), followed by surgeons for HD access (38%, n = 8) and vascular access coordinators (29%, n = 6) (Supplementary Figure S1). Germany, Ireland, and Malta reported shortages of 7 or more types of care providers, whereas Finland, France, Liechtenstein, Spain, and the United Kingdom did not report any workforce deficits.
Essential medications and health product access for KRT modalities
All countries in Western Europe had the capacity to provide long-term HD (Figure 3). The median number of HD centers was 6.9 pmp (n = 21, IQR: 4.5–10.1), with the highest densities in Liechtenstein and Greece (25.94 and 15.80 pmp, respectively) and the lowest in the United Kingdom and Denmark (0.95 and 2.32 pmp, respectively) (Table 2).25 Home HD was available in 65% (n = 13) of countries in the region. In only one-third of countries (n = 7), more than 50% of HD patients began treatment with functioning vascular access (fistula or graft) (Supplementary Figure S2). In half of all countries (n = 10), 11%–50% of patients started dialysis with a temporary dialysis catheter. PD was also widely available in all Western European countries (Figure 3). The median number of PD centers in the region was 2.3 pmp (n = 21, IQR: 1.8–3.6), which is above the global average. Liechtenstein and Switzerland had the highest PD capacity (centers pmp), whereas the United Kingdom and France had the lowest (Table 2).25,26 All countries in Western Europe were able to offer adequate frequency of PD exchanges (3–4 manual exchanges per day or equivalent cycles on automated PD), had the capacity to measure PD adequacy (via measurement of urea reduction ratio or Kt/V), and had efficient patient transport services available (Figure 3).
Kidney transplantation was available across most of the region (n = 19, 90%), with a regional median of 0.52 transplant centers pmp (n = 19, IQR: 0.4–0.8), just above the global median of 0.42 pmp (IQR: 0.20–0.72) (Table 2).25 Iceland and Malta had the highest capacity for transplantation (5.82 and 2.23 transplant centers pmp, respectively), whereas Finland had the lowest (0.18 transplant centers pmp). All countries with transplant capacity performed a combination of deceased and living donor kidney transplants, with the exception of Iceland, which performs living donor kidney transplants only. The vast majority of countries with kidney transplantation available had national transplant waitlists (89%, n = 17), whereas the rest had regional lists only. Most countries were able to provide early and culturally appropriate information about transplantation to patients (95%, n = 19) (Figure 3). All countries in Western Europe were able to provide effective preventive therapy to control infections, timely access to operating space, appropriate immunosuppression treatment, and appropriate facilities to monitor immunosuppression drugs consistently. A standard organ procurement framework was available in all countries that have the capacity for transplantation.
In all surveyed countries in Western Europe, at least half of all patients with KF were able to access dialysis, a proportion that does not vary due to regional capacity or individual patient characteristics (Figure 4). Among those able to access dialysis, only a small minority (1%–10%) began with PD in 50% of countries (n = 10); this proportion was affected by regional capacity and patient characteristics in 35% and 25% of countries, respectively. In 4 countries (Finland, Malta, Norway, and Sweden), 26%–50% of patients began with PD. In 60% of countries (n = 12), more than 50% of eligible patients were able to access transplantation services.
Conservative care (nondialytic management of KF) was available in the majority of countries (n = 18, 90%) in Western Europe when medically advised or chosen by the patient (Figure 3). Among those countries able to offer this service, 65% (n = 13) took a multidisciplinary approach to care via shared decision-making and had decision-making tools readily available for patients and providers. Most countries (85%, n = 17) had the capacity to systematically and actively recognize and manage symptoms. Twelve countries (60%) were able to provide psychological, cultural, and spiritual support, but only 9 countries (45%) had the resources to systematically train health care providers in conservative care. However, regional data for countries were not available, and conservative care accessibility may vary within countries.
Reporting of KRT quality indicators
Twenty countries were able to provide information about the adequacy of PD; 19 and 18 countries were able to provide the same information about HD and transplantation, respectively. The least-reported indicator of dialysis adequacy was patient-reported outcome measures, with almost one-third of countries reporting that this information was collected by fewer than 10% of centers (Supplementary Figure S3). Technique survival in HD patients was also less commonly measured by centers, as were delayed graft function and rejection rates in transplant recipients. Small solute clearance (e.g., Kt/V or creatinine clearance) was measured in >75% of centers in 95% of countries.
Health information systems, statistics, and national health policy
Most countries in Western Europe had official dialysis and transplantation registries (n = 18 and 19, respectively), few had CKD registries (n = 3, 14.3%), and only Malta and the United Kingdom had AKI registries (Figure 5). Iceland and Luxembourg had no registries at all, whereas only Malta and the United Kingdom had registries for every aspect of kidney care. Participation in registries was mandatory in 74% of countries (14 of 19) for kidney transplantation and 67% of countries for dialysis (12 of 18), but only 33% of countries (1 of 3) for CKD. Nearly all registries had national coverage and collect general information on the etiology of kidney disease, dialysis modality, or transplant source (Supplementary Figure S4). Patient outcome measures were not as consistently collected, with only 33% and 42% of hospitalizations recorded for dialysis and kidney transplant patients, respectively. Quality-of-life measures were very infrequently collected; however, mortality rates were recorded for almost all dialysis and transplant patients.
Routine testing for kidney disease is available to almost all patients with diabetes and hypertension in the region, but only in 50% of countries to long-term users of nephrotoxic medications and in 10% of countries to high-risk ethnic groups (despite 35% of countries identifying ethnic groups as being at high risk for CKD). Testing was available in 70% of countries for those with a family history of kidney disease (Figure 5). Only 1 and 2 countries had AKI and CKD detection programs, respectively. Services to treat and diagnose complications of KF were mostly available in the region (Supplementary Figure S5).
Only 55% of countries (n = 11) in Western Europe had national strategies to improve care for patients with CKD, including either CKD-specific strategies or those that had been incorporated into noncommunicable disease strategies, the former being more inclusive of all patients with kidney disease than the latter (Supplementary Figures S6 and S7). National CKD-specific policies were available in only 5 countries (25%). Recognition of kidney disease as a health priority at the government level was more common for KF, followed by CKD and AKI (65%, 50%, and 5% of countries, respectively). Advocacy groups for KF, CKD, and AKI existed in 35%, 20%, and 25% of countries, respectively. The most commonly cited barriers to optimal KF care were geography (15%), the individual patient (15%), and lack of political will (15%), followed by nephrologists (10%) and economic factors (10%) (Figure 5).
Discussion
Our study highlights several important aspects of KF care in Western Europe. The median prevalence of treated KF was higher than the global median, and there was variation between countries even in Western Europe. HD remains the main dialysis modality, and PD remains underutilized. There was also striking variation in the incidence of kidney transplantation between countries. In the majority of countries, public funding covered costs associated with nondialysis CKD and KRT care. Although the median number of nephrologists and nephrology trainees in Western Europe was higher than the global average, workforce shortages of nephrologists and other essential health care staff were still reported across the region. Most countries had dialysis and transplant centers, and official registries to capture dialysis and transplant activities, but very few had AKI or CKD detection programs. Only half of all countries in Western Europe had national strategies in place to improve CKD care.
All Western Europe is classified as a high-income region by the World Bank; however, there is substantial variation in treated KF incidence and prevalence rates. It has been proposed that varying prophylaxis programs, both in terms of primary prevention of CKD and how CKD progression is managed, as well as real differences in underlying CKD incidence may account for this variation,33 which persists even with stratification by diabetes, hypertension, and obesity.34 However, a decrease in KF incidence since 2001 has been noted among those between the ages of 60 and 69 in Denmark, from 400 ppm to 218 ppm, associated with a concurrent large increase in antihypertensive drug use, particularly drugs blocking the renin-angiotensin system.35 There is substantial heterogeneity internationally in the control of hypertension with poorer than expected blood pressure control in some European cohorts.36
The EVEREST study,37 a European Renal Association-European Dialysis and Transplant Association Registry initiative, identified GDP per capita and percentage of GDP spent on health care to be important in determining KRT incidence (i.e., there appears to be an 11% increase in KRT incidence associated with each 1% increase in GDP spent on health care), but some of the countries with the highest KF incidence such as Portugal, Greece, and Israel have some of the lowest health expenditures. In Europe, the experience has been that dialysis has a disproportionately high impact on public health expenditure in countries with a lower GDP that carries the risk that this money is deviated from other urgent needs such as CKD primary and secondary prevention.38 The risk of CKD progression can vary across countries even after accounting for the distributions of age, sex, comorbidities, and laboratory markers,39 and this variability has been demonstrated between Western European countries.40 Other factors that have been proposed to explain the wide variation in incidence include differences in genetic predisposition,41 birth weight,42 lifestyle factors such as exercise43 or dietary habits,44 and public health strategies to control hypertension,45 obesity,46 and tobacco consumption.47
Although PD was widely available in Western European countries, HD remained the most frequent dialysis modality used; only a small minority of patients began dialysis with PD. For certain candidates, PD has a number of advantages over HD, including preservation of residual kidney function,48 greater independence,49 and better initial survival.50 The availability of home HD varied among countries, even though in highly selected patients, it is associated with lower costs,51 less need for specialized personnel, better control of blood pressure, anemia, and phosphate levels,52 improved quality of life, and greater flexibility for patients compared with in-center dialysis.53
There was striking variation in the incidence of kidney transplantation amongst Western European countries, ranging from 12 pmp in Luxembourg to 70.45 pmp in Spain. With the exception of Iceland, Israel, and the Netherlands, the incidence of deceased donor kidney transplantation was much greater than that of living donor transplantation. Kidney transplantation is by far the most cost-effective KRT option due to a combination of prolonged survival, improved quality of life, and reduced medical costs after the first year.54 Funded by the EU, the Effect of Differing Kidney Disease Treatment Modalities and Organ Donation and Transplantation Practices on Health Expenditure and Patient Outcomes project was established in 2017 to examine the epidemiology and costs of different treatment modalities for KF, including the reasons behind the substantial variability in access to kidney transplantation.55 Some of the barriers appear to be fear of kidney rejection or graft failure, fear of surgery or medication, negative experiences with grafts (self or others), distrust of health care professionals, doing well on dialysis, religious opposition to transplantation, and costs, all of which underscore the need for improved patient education and communication.56 Better national health policies are clearly needed to improve the numbers. Different strategies may be required, depending on whether a country has a low level of deceased donor or living donor donation, or both. Countries with opt-out policies (or a practically defunct presumed consent system) such as Spain and Austria appear to have higher transplantation rates,57 suggesting that Western Europe may benefit from a global opt-out strategy. In an opt-out strategy, organ donation is the default option at the time of death, and so people must explicitly “opt out” of organ donation if they do not wish it, as opposed to an opt-in policy that necessitates explicit consent. However, in a more recent analysis of organ donation and transplantation rates in 35 countries, no significant difference was observed in rates of kidney (35.2 vs. 42.3, respectively), non-kidney (28.7 vs. 20.9, respectively), or total solid organ transplantation (63.6 vs. 61.7, respectively).58 This suggests that there are other barriers to organ donation that need to be addressed including education and infrastructure, and that opt-out policies alone are unlikely to be successful.59 Collaboration in broader programs such as Eurotransplant, Scandiatransplant, and the South Transplant Alliance may also improve transplant activity.60 Another option may be to widen the donor pool to include expanded criteria donors and non-heartbeat donors, which are both underutilized in Europe.
In 2019, on request of the European Commission, the European Kidney Health Alliance formulated a joint statement of recommendations on how to improve organ donation and transplantation within the EU.61 Their key recommendations were to mobilize political will to make organ donation and transplantation a priority, to improve legal and institutional framework, to streamline organization and invest in leadership at all levels, to allocate appropriate funds for organ donation and transplantation programs, to promote education and training across all stakeholders, to eradicate inequities in organ donation, to boost benchmarking, and to leverage research.
In the vast majority of countries, nondialysis CKD and KRT care were publicly funded, but out-of-pocket copayments varied. However, the annual costs of dialysis and kidney transplantation were more than twice as high in Western Europe as the global median. Overall, 2% of health expenditures are allocated to KRT, which is required for just 0.1% of the population.62 Promotion of more cost-effective forms of KRT (PD, home HD) or kidney transplantation may decrease financial pressure. Primary prevention of CKD by preventing underlying conditions such as diabetes mellitus and hypertension is also paramount.
Despite having more nephrologists and nephrology trainees in Western Europe compared with the rest of the world, there was much variation across the region, with countries such as Ireland and the United Kingdom both reporting less than 10 nephrologists pmp. Crucially, Ireland and the United Kingdom did not have particularly low numbers of nephrology trainees, suggesting that a lack of judicious workforce planning was central to these disparities. Other proposed contributing factors include declining interest in nephrology among trainees,63 over-reliance on foreign medical graduates,64 and erosion of nephrology practice scope by other specialists.65
Patient-reported outcome measures were very infrequently collected in Western European kidney units and registries compared with other parts of the world. Their importance in health care is increasingly recognized, as they can quantify a wide variety of health concepts that are relevant to the patient, such as quality of life, functional status, and symptom burden.66 Patients with advanced CKD often experience poor health-related quality of life and numerous physical and emotional disease-related symptoms.67 Routine collection of patient-reported outcome measures, as occurs in Australia and New Zealand,68 could improve symptom management, promote shared decision-making, and better address patients’ needs.
Very few countries had AKI or CKD registries, or corresponding detection programs. AKI registries not only enable better tracking of the epidemiology of AKI, its burden, associated mortality, and longer-term adverse kidney outcomes (i.e., subsequent development of CKD), but also facilitate temporal and regional comparisons.69 Similarly, CKD registries permit longitudinal study and analysis of outcomes, and can generate prediction factors influencing the prognosis, care patterns, and disparities in the delivery of care.70 They create opportunities for quality improvement and KRT planning. However, the corollary is that registries are very time-consuming and costly when potentially affected individuals cannot be easily identified, and thus their contribution must be weighed against their cost. Population-based screening for CKD is also controversial. Early detection of proteinuria in an unselected population has not been shown to be cost-effective.71 However, the See Kidney Disease (SeeKD) targeted screening project in Canada that screened adults with risk factors for CKD (i.e., diabetes, hypertension, vascular disease, family history of kidney problems, etc.) identified a high proportion of individuals with risk factors for CKD and a high prevalence of unrecognized CKD.72 Kidney Disease: Improving Global Outcomes (KDIGO) recommends that all countries should have a targeted CKD screening program;73 countries in Western Europe generally comply with this recommendation by routinely testing all patients with hypertension, diabetes, and vascular disease. More uniform testing of high-risk ethnic groups or those with a family history of CKD is needed, however. Only about half of all countries recognize CKD as a health priority and have national strategies to improve care, with few specific policies. National CKD strategies can help develop a consistent approach to address key risk factors in the prevention, detection, and management of kidney diseases to minimize their progression and complications.
The most commonly cited barriers to KF care were geography, the individual patient, and lack of political will (Figure 5). It has been shown that rural patients have less access to dialysis units and home dialysis therapies when compared with urban patients,74 and that that geographic location was associated with less frequent patient visits by dialysis providers.75 Patient-specific factors may include the absence of symptoms, dialysis fears, work-related concerns, socioeconomic circumstances, and cultural differences.76,77 Lastly, it is well recognized that improving global access to safe, sustainable, and equitable integrated KF care will require key stakeholders at governmental and policy maker level.78
In summary, the region is performing better in all aspects of kidney care overall compared with other regions, but improvements can be made through expansion of CKD prevention efforts and realignment of priorities (less emphasis on KRT provision and more on prevention efforts). There is also a need for better workforce planning, multidisciplinary teams, and telemedicine. Collection and reporting of quality indicators, particularly patient-reported outcomes, should be routinely incorporated into KF care. Health information systems should be expanded to prevent and manage KF. KF prevention and treatment should also be more broadly promoted by implementing policies, strategies, and advocacy programs, and mitigating barriers.
Disclosure
K-UE reports grants from Astra Zeneca, Bayer, Fresenius, and Vifor, during the conduct of the study; personal fees from Akebia, Bayer, Fresenius, and Vifor and grants from Amgen, Genzyme, and Shire, outside the submitted work. DWJ reports grants and personal fees from Baxter Healthcare and Fresenius Medical Care, travel sponsorship from Amgen, personal fees from Astra Zeneca, AWAK, and Ono, and grants from National Health and Medical Research Council of Australia, outside the submitted work. VJ reports grants from GlaxoSmithKline and Baxter Healthcare, provides scientific leadership to George Clinical, and consultancy fees for Biocon, Zudis Cadilla, and NephroPlus, all paid to his institution, outside the submitted work. ER reports personal fees and nonfinancial support from Alexion Pharmaceuticals, outside the submitted work. All the other authors declared no competing interests.
Acknowledgments
This article is published as part of a supplement supported by the International Society of Nephrology (ISN; grant RES0033080 to the University of Alberta).
The ISN provided administrative support for the design and implementation of the study and data collection activities. The authors were responsible for data management, analysis, and interpretation, as well as manuscript preparation, review, and approval, and the decision to submit the manuscript for publication.
We thank Kara Stephenson Gehman in International Society of Nephrology Global Kidney Health Atlas (ISN-GKHA)for carefully editing the English text of a draft of this manuscript. We thank Jo-Ann Donner, Coordinator at the ISN, for her prominent role and leadership in the manuscript management, editorial reviews, and submission process to Kidney International Supplements, Sandrine Damster, Senior Research Project Manager at the ISN, and Alberta Kidney Disease Network staff (Ghenette Houston, Sue Szigety, and Sophanny Tiv) for helping to organize and conduct the survey and for providing project management support. We also thank the ISN headquarters staff including the Executive Director, Charu Malik, and the Advocacy team. We also appreciate the support from the ISN’s Executive Committee, regional leadership, and Affiliated Society leaders at the regional and country levels for their help with the ISN-GKHA survey.
Footnotes
Table S1. Burden of chronic kidney disease and its risk factors in 21 countries in Western Europe that participated in the ISN-GKHA survey.
Figure S1. Shortages of kidney failure care providers identified by the 21 Western European countries that participated in the ISN-GKHA survey.
Figure S2. Proportion of patients starting dialysis with different forms of vascular access and adequate education.
Figure S3. Quality indicators monitored and reported by countries that participated in the ISN-GKHA survey.
Figure S4. Registry characteristics for countries that reported having 1 or more registries in the ISN-GKHA survey.
Figure S5. Availability of services to diagnose and treat complications of kidney failure.
Figure S6. National strategies available in countries.
Figure S7. Population covered under national noncommunicable disease and chronic kidney disease strategies.
Supplementary Appendix. Reference list for annual cost of kidney replacement therapy (for Table 1).
Supplementary Material
References
- 1.Bello A.K., Levin A., Tonelli M. International Society of Nephrology; Brussels, Belgium: 2017. Global Kidney Health Atlas: A report by the International Society of Nephrology on the current state of organization and structures for kidney care across the globe. [Google Scholar]
- 2.Rondeau E., Luyckx V.A., Anders H.J. Challenges and opportunities for nephrology in Western Europe. Kidney Int. 2019;95:1037–1040. doi: 10.1016/j.kint.2018.09.028. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization European health report 2018: more than numbers—evidence for all. https://www.euro.who.int/en/data-and-evidence/european-health-report/european-health-report-2018 Available at:
- 4.European Kidney Health Alliance Recommendations for sustainable kidney care; 2015. http://ekha.eu/wp-content/uploads/2016/01/EKHA-Recs-for-Sustainable-Kidney-Care-25.08.2015.pdf Available at:
- 5.Pippias M., Jager K.J., Kramer A. The changing trends and outcomes in renal replacement therapy: data from the ERA-EDTA Registry. Nephrol Dial Transplant. 2016;31:831–841. doi: 10.1093/ndt/gfv327. [DOI] [PubMed] [Google Scholar]
- 6.Kramer A., Stel V.S., Abad Diez J.M. Renal replacement therapy in Europe—a summary of the 2010 ERA-EDTA Registry Annual Report. Clin Kidney J. 2013;6:105–115. doi: 10.1093/ckj/sfs164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noordzij M., Kramer A., Abad Diez J.M. Renal replacement therapy in Europe: a summary of the 2011 ERA-EDTA Registry Annual Report. Clin Kidney J. 2014;7:227–238. doi: 10.1093/ckj/sfu007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pippias M., Stel V.S., Abad Diez J.M. Renal replacement therapy in Europe: a summary of the 2012 ERA-EDTA Registry Annual Report. Clin Kidney J. 2015;8:248–261. doi: 10.1093/ckj/sfv014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kramer A., Pippias M., Stel V.S. Renal replacement therapy in Europe: a summary of the 2013 ERA-EDTA Registry Annual Report with a focus on diabetes mellitus. Clin Kidney J. 2016;9:457–469. doi: 10.1093/ckj/sfv151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kramer A., Pippias M., Noordzij M. The European Renal Association–European Dialysis and Transplant Association (ERA-EDTA) Registry Annual Report 2016: a summary. Clin Kidney J. 2019;12:702–720. doi: 10.1093/ckj/sfz011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van de Luijtgaarden M.W., Jager K.J., Segelmark M. Trends in dialysis modality choice and related patient survival in the ERA-EDTA Registry over a 20-year period. Nephrol Dial Transplant. 2016;31:120–128. doi: 10.1093/ndt/gfv295. [DOI] [PubMed] [Google Scholar]
- 12.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. The Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyarsky B.J., Chiang T.P., Werbel W.A. Early impact of COVID-19 on transplant center practices and policies in the United States. Am J Transplant. 2020;20:1809–1818. doi: 10.1111/ajt.15915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Biesen W., Vanholder R., Vanderhaegen B. Renal replacement therapy for refugees with end-stage kidney disease: an international survey of the nephrological community. Kidney Int Suppl. 2016;6:35–41. doi: 10.1016/j.kisu.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.IPCC Climate change 2014: synthesis report. Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change. In: Core Writing Team, Pachauri RK, Meyer LA, eds. Geneva, Switzerland: IPCC; 2014. https://www.ipcc.ch/report/ar5/syr/ Available at:
- 16.Conti S., Masocco M., Meli P. General and specific mortality among the elderly during the 2003 heat wave in Genoa (Italy) Environ Res. 2007;103:267–274. doi: 10.1016/j.envres.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Ryan S.J., Carlson C.J., Mordecai E.A., Johnson L.R. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Negl Trop Dis. 2019;13 doi: 10.1371/journal.pntd.0007213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson A.H., Cohen A.J., Kutner N.G. Missed dialysis sessions and hospitalization in hemodialysis patients after Hurricane Katrina. Kidney Int. 2009;75:1202–1208. doi: 10.1038/ki.2009.5. [DOI] [PubMed] [Google Scholar]
- 19.Kmietowicz Z. Brexit and kidney disease: five minutes with…Fiona Loud. BMJ. 2018;363:k5099. doi: 10.1136/bmj.k5099. [DOI] [PubMed] [Google Scholar]
- 20.Bello A.K., Okpechi I.G., Jha V. Understanding distribution and variability in care organization and services for the management of kidney care across world regions. Kidney Int Suppl. 2021;11:e4–e10 [Google Scholar]
- 21.World Health Organization The Global Health Observatory; 2019. https://www.who.int/gho/en/ Available at:
- 22.Central Intelligence Agency The world factbook. Published 2019. https://www.cia.gov/the-world-factbook/ Available at:
- 23.World Bank GDP ranking; 2019. https://datacatalog.worldbank.org/dataset/gdp-ranking Available at:
- 24.van der Tol A., Lameire N., Morton R.L. An international analysis of dialysis services reimbursement. Clin J Am Soc Nephrol. 2019;14:84–93. doi: 10.2215/CJN.08150718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ERA-EDTA Registry ERA-EDTA Registry Annual Report 2017. https://www.era-edta.org/en/registry/publications/annual-reports/ Available at:
- 26.Jain A.K., Blake P., Cordy P., Garg A.X. Global trends in rates of peritoneal dialysis. J Am Soc Nephrol. 2012;23:533–544. doi: 10.1681/ASN.2011060607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.United States Renal Data System (USRDS) National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2018. 2018 USRDS Annual Data Report: epidemiology of kidney disease in the United States. [Google Scholar]
- 28.Bello A., Levin A., Lunney M. Current status of end-stage kidney disease care in world countries and regions—an international survey. BMJ. 2019;367:l5873. doi: 10.1136/bmj.l5873. [DOI] [PubMed] [Google Scholar]
- 29.International Society of Nephrology Western Europe: Represented countries; 2020. https://www.theisn.org/about-isn/governance/regional-boards/western-europe/#102 Available at:
- 30.World Bank European Union; 2020. https://data.worldbank.org/region/european-union Available at:
- 31.Organisation for Economic Co-operation and Development (OECD) Income inequality; 2020. https://data.oecd.org/inequality/income-inequality.htm Available at:
- 32.Hardeman S., Dijkstra L. The EU Regional Human Development Index; 2014. https://ec.europa.eu/jrc/en/publication/eur-scientific-and-technical-research-reports/eu-regional-human-development-index Available at:
- 33.Heaf J. Current trends in European renal epidemiology. Clin Kidney J. 2017;10:149–153. doi: 10.1093/ckj/sfw150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruck K., Stel V.S., Gambaro G. CKD prevalence varies across the European general population. J Am Soc Nephrol. 2016;27:2135–2147. doi: 10.1681/ASN.2015050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heaf J.G., Wehberg S. Reduced incidence of end stage renal disease among the elderly in Denmark: an observational study. BMC Nephrol. 2012;13:131. doi: 10.1186/1471-2369-13-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alencar de Pinho N., Levin A., Fukagawa M. Considerable international variation exists in blood pressure control and antihypertensive prescription patterns in chronic kidney disease. Kidney Int. 2019;96:983–994. doi: 10.1016/j.kint.2019.04.032. [DOI] [PubMed] [Google Scholar]
- 37.Caskey F.J., Kramer A., Elliott R.F. Global variation in renal replacement therapy for end-stage renal disease. Nephrol Dial Transplant. 2011;26:2604–2610. doi: 10.1093/ndt/gfq781. [DOI] [PubMed] [Google Scholar]
- 38.van der Tol A., Stel V.S., Jager K.J. A call for harmonization of European kidney care: dialysis reimbursement and distribution of kidney replacement therapies. Nephrol Dial Transplant. 2020;35:979–986. doi: 10.1093/ndt/gfaa035. [DOI] [PubMed] [Google Scholar]
- 39.Orlandi P.F., Huang J., Fukagawa M. A collaborative, individual-level analysis compared longitudinal outcomes across the International Network of Chronic Kidney Disease (iNETCKD) cohorts. Kidney Int. 2019;96:1217–1233. doi: 10.1016/j.kint.2019.07.024. [DOI] [PubMed] [Google Scholar]
- 40.Bruck K., Jager K.J., Zoccali C. Different rates of progression and mortality in patients with chronic kidney disease at outpatient nephrology clinics across Europe. Kidney Int. 2018;93:1432–1441. doi: 10.1016/j.kint.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 41.Kottgen A., Glazer N.L., Dehghan A. Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet. 2009;41:712–717. doi: 10.1038/ng.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silverwood R.J., Pierce M., Hardy R. Low birth weight, later renal function, and the roles of adulthood blood pressure, diabetes, and obesity in a British birth cohort. Kidney Int. 2013;84:1262–1270. doi: 10.1038/ki.2013.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stengel B., Tarver-Carr M.E., Powe N.R. Lifestyle factors, obesity and the risk of chronic kidney disease. Epidemiology. 2003;14:479–487. doi: 10.1097/01.EDE.0000071413.55296.c4. [DOI] [PubMed] [Google Scholar]
- 44.Chrysohoou C., Panagiotakos D.B., Pitsavos C. Adherence to the Mediterranean diet is associated with renal function among healthy adults: the ATTICA study. J Ren Nutr. 2010;20:176–184. doi: 10.1053/j.jrn.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 45.Jafar T.H., Stark P.C., Schmid C.H. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med. 2003;139:244–252. doi: 10.7326/0003-4819-139-4-200308190-00006. [DOI] [PubMed] [Google Scholar]
- 46.Bonnet F., Deprele C., Sassolas A. Excessive body weight as a new independent risk factor for clinical and pathological progression in primary IgA nephritis. Am J Kidney Dis. 2001;37:720–727. doi: 10.1016/s0272-6386(01)80120-7. [DOI] [PubMed] [Google Scholar]
- 47.Orth S.R., Stockmann A., Conradt C. Smoking as a risk factor for end-stage renal failure in men with primary renal disease. Kidney Int. 1998;54:926–931. doi: 10.1046/j.1523-1755.1998.00067.x. [DOI] [PubMed] [Google Scholar]
- 48.Marron B., Remon C., Perez-Fontan M. Benefits of preserving residual renal function in peritoneal dialysis. Kidney Int Suppl. 2008;108:S42–S51. doi: 10.1038/sj.ki.5002600. [DOI] [PubMed] [Google Scholar]
- 49.Bro S., Bjorner J.B., Tofte-Jensen P. A prospective, randomized multicenter study comparing APD and CAPD treatment. Perit Dial Int. 1999;19:526–533. [PubMed] [Google Scholar]
- 50.Heaf J.G., Lokkegaard H., Madsen M. Initial survival advantage of peritoneal dialysis relative to haemodialysis. Nephrol Dial Transplant. 2002;17:112–117. doi: 10.1093/ndt/17.1.112. [DOI] [PubMed] [Google Scholar]
- 51.Krahn M.D., Bremner K.E., de Oliveira C. Home dialysis is associated with lower costs and better survival than other modalities: a population-based study in Ontario, Canada. Perit Dial Int. 2019;39:553–561. doi: 10.3747/pdi.2018.00268. [DOI] [PubMed] [Google Scholar]
- 52.Wong B., Collister D., Muneer M. In-center nocturnal hemodialysis versus conventional hemodialysis: a systematic review of the evidence. Am J Kidney Dis. 2017;70:218–234. doi: 10.1053/j.ajkd.2017.01.047. [DOI] [PubMed] [Google Scholar]
- 53.Finkelstein F.O., Finkelstein S.H., Wuerth D. Effects of home hemodialysis on health-related quality of life measures. Semin Dial. 2007;20:265–268. doi: 10.1111/j.1525-139X.2007.00287.x. [DOI] [PubMed] [Google Scholar]
- 54.Wong G., Howard K., Chapman J.R. Comparative survival and economic benefits of deceased donor kidney transplantation and dialysis in people with varying ages and co-morbidities. PloS One. 2012;7 doi: 10.1371/journal.pone.0029591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jager K.J., Stel V.S., Branger P. The effect of differing kidney disease treatment modalities and organ donation and transplantation practices on health expenditure and patient outcomes. Nephrol Dial Transplant. 2018;33:560–562. doi: 10.1093/ndt/gfx082. [DOI] [PubMed] [Google Scholar]
- 56.Vanholder R., Stel V.S., Jager K.J. How to increase kidney transplant activity throughout Europe—an advocacy review by the European Kidney Health Alliance. Nephrol Dial Transplant. 2019;34:1254–1261. doi: 10.1093/ndt/gfy390. [DOI] [PubMed] [Google Scholar]
- 57.Shepherd L., O'Carroll R.E., Ferguson E. An international comparison of deceased and living organ donation/transplant rates in opt-in and opt-out systems: a panel study. BMC Med. 2014;12:131. doi: 10.1186/s12916-014-0131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arshad A., Anderson B., Sharif A. Comparison of organ donation and transplantation rates between opt-out and opt-in systems. Kidney Int. 2019;95:1453–1460. doi: 10.1016/j.kint.2019.01.036. [DOI] [PubMed] [Google Scholar]
- 59.Matesanz R., Dominguez-Gil B. Opt-out legislations: the mysterious viability of the false. Kidney Int. 2019;95:1301–1303. doi: 10.1016/j.kint.2019.02.028. [DOI] [PubMed] [Google Scholar]
- 60.Zivcic-Cosic S., Busic M., Zupan Z. Development of the Croatian model of organ donation and transplantation. Croat Med J. 2013;54:65–70. doi: 10.3325/cmj.2013.54.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.European Kidney Health Alliance—Thematic Network on Improving Organ Donation and Transplantation in the EU A shared vision for improving organ donation and transplantation in the EU; 2019. http://ekha.eu/wp-content/uploads/FINAL_Joint-Statement-of-the-Thematic-Network-on-Organ-Donation-and-Transplantation.pdf Available at:
- 62.Vanholder R., Annemans L., Brown E. Reducing the costs of chronic kidney disease while delivering quality health care: a call to action. Nat Rev Nephrol. 2017;13:393–409. doi: 10.1038/nrneph.2017.63. [DOI] [PubMed] [Google Scholar]
- 63.Parker M.G., Ibrahim T., Shaffer R. The future nephrology workforce: will there be one? Clin J Am Soc Nephrol. 2011;6:1501–1506. doi: 10.2215/CJN.01290211. [DOI] [PubMed] [Google Scholar]
- 64.Lane C.A., Brown M.A. Nephrology: a specialty in need of resuscitation? Kidney Int. 2009;76:594–596. doi: 10.1038/ki.2008.685. [DOI] [PubMed] [Google Scholar]
- 65.Berns J.S., Ellison D.H., Linas S.L., Rosner M.H. Training the next generation's nephrology workforce. Clin J Am Soc Nephrol. 2014;9:1639–1644. doi: 10.2215/CJN.00560114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tang E., Bansal A., Novak M., Mucsi I. Patient-reported outcomes in patients with chronic kidney disease and kidney transplant—Part 1. Front Med. 2017;4:254. doi: 10.3389/fmed.2017.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fukuhara S., Lopes A.A., Bragg-Gresham J.L. Health-related quality of life among dialysis patients on three continents: the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2003;64:1903–1910. doi: 10.1046/j.1523-1755.2003.00289.x. [DOI] [PubMed] [Google Scholar]
- 68.Morton R.L., Lioufas N., Dansie K. Use of patient-reported outcome measures and patient-reported experience measures in renal units in Australia and New Zealand: a cross-sectional survey study. Nephrology (Carlton) 2020;25:14–21. doi: 10.1111/nep.13577. [DOI] [PubMed] [Google Scholar]
- 69.Sawhney S., Fraser S.D. Epidemiology of AKI: utilizing large databases to determine the burden of AKI. Adv Chronic Kidney Dis. 2017;24:194–204. doi: 10.1053/j.ackd.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Larsson S., Lawyer P., Garellick G. Use of 13 disease registries in 5 countries demonstrates the potential to use outcome data to improve health care's value. Health Aff (Millwood) 2012;31:220–227. doi: 10.1377/hlthaff.2011.0762. [DOI] [PubMed] [Google Scholar]
- 71.Boulware L.E., Jaar B.G., Tarver-Carr M.E. Screening for proteinuria in US adults: a cost-effectiveness analysis. JAMA. 2003;290:3101–3114. doi: 10.1001/jama.290.23.3101. [DOI] [PubMed] [Google Scholar]
- 72.Galbraith L.E., Ronksley P.E., Barnieh L.J. The See Kidney Disease Targeted Screening Program for CKD. Clin J Am Soc Nephrol. 2016;11:964–972. doi: 10.2215/CJN.11961115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Levey A.S., Atkins R., Coresh J. Chronic kidney disease as a global public health problem: approaches and initiatives—a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72:247–259. doi: 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- 74.Rodriguez R.A., Hotchkiss J.R., O'Hare A.M. Geographic information systems and chronic kidney disease: racial disparities, rural residence and forecasting. J Nephrol. 2013;26:3–15. doi: 10.5301/jn.5000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Erickson K.F., Tan K.B., Winkelmayer W.C. Variation in nephrologist visits to patients on hemodialysis across dialysis facilities and geographic locations. Clin J Am Soc Nephrol. 2013;8:987–994. doi: 10.2215/CJN.10171012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Griva K., Seow P.S., Seow T.Y. Patient-related barriers to timely dialysis access preparation: a qualitative study of the perspectives of patients, family members, and health care providers. Kidney Med. 2020;2:29–41. doi: 10.1016/j.xkme.2019.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lenz O., Mekala D.P., Patel D.V. Barriers to successful care for chronic kidney disease. BMC Nephrol. 2005;6:11. doi: 10.1186/1471-2369-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harris D.C.H., Davies S.J., Finkelstein F.O. Increasing access to integrated ESKD care as part of universal health coverage. Kidney Int. 2019;95:S1–S33. doi: 10.1016/j.kint.2018.12.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.