Abstract
Breast cancer is a growing health issue globally and accounts as a second most cause of mortality. Natural products have been a fundamental of health care for long. Plants derived natural products have gained considerable attention over synthetic medicines, since they are safe and non-toxic. Krameria lappacea (Dombey) Burdet and B.B. Simpson plant belonging to Krameriaceae family, has been known for its beneficial effects against diseases. Herein, firstly, cytotoxic potential of petroleum ether (KLH), chloroform (KLC), ethyl acetate (KLEA), and ethanolic (KLET) extracts of K. lappacea was screened against MCF-7 cells exposed to 10–1000 μg/mL for 24 h. Secondly, the most cytotoxic extract (KLH) was used to explore the mechanisms of cytotoxicity in MCF-7 cells. MCF-7 cells were treated with KLH at 250–1000 μg/mL to measure the oxidative stress markers (glutathione (GSH) and lipid peroxidation (LPO)) and reactive oxygen species (ROS) generation. Further, loss of mitochondrial membrane potential (MMP) and caspase-3 and -9 enzyme activities were studied. The viability of MCF-7 cells were decreased from 44% to 90% for KLH, from 7% to 71% for KLEA, from 39% to 80% for KLC, and from 3% to 81% for KLET, respectively at 250–1000 μg/mL as observed by MTT assay. An increase of 91% in LPO and 2.2-fold in ROS generation and a decrease of 59% in GSH and 68% in MMP levels at 1000 μg/mL showed that KLH induced MCF-7 cell death via oxidative stress and elevated level of ROS generation which further leads to mitochondrial membrane dysfunction and activation of caspase enzymes. The findings of this study provide a mechanistic insight on anticancer efficacies of K. lappacea extracts against MCF-7 cells and support the use of it for the treatment of breast cancer diseases.
Keywords: Krameria lappacea (family: Krameriaceae), MCF-7, Anticancer activity, Oxidative stress, ROS generation, Loss of MMP, Caspase activities
1. Introduction
According to world health organization (WHO), breast cancer is a growing health issue globally and is the second most cause of mortality. Approximately 1 in 10 women is diagnosed with breast cancer at some stage of life (Caffarel et al., 2016). As per the International Agency for Research on Cancer (IARC) and Globocan 2018 data, breast cancer accounted for 0.62 million deaths besides 2.08 million new cases (approximately 11.6% of all types of cancer recorded) (Bray et al., 2018). If the current trends are to be believed, the mortality troll is expected to rise to a nerve-racking high of 6.99 million by 2040 (Ferlay et al., 2018). According to epidemiological reports, the incidence of breast cancer is continuously rising in the developing and developed countries (Rice and Whitehead, 2006). Although an extensive research effort has been made to understand breast cancer and find new ways to combat it. Nonetheless, the available treatments mainly include chemotherapy, involved the use of cytotoxic agents. However, the role of chemotherapy is still uncertain (Middleton et al., 2018). Although it has shown effective in some cases, but its usage is often followed by risk factors or side effects that may vary from short term to life threatening (Middleton et al., 2018). The main reason of side effects associated with chemotherapy is the lack of specificity of drugs for the cancer cells (Weinberg and Chandel, 2015). Natural products have been a fundamental of health care for long. It has been observed that almost 80% of population generally relies on natural medicine systems for primary health care (Ekor, 2014). Complementary and alternative remedies are commonly recognized in most developed countries like Europe, Australia, and North America (Braun et al., 2010, Anquez-Traxler, 2011). The main factors for the resurrection of the community attention in alternative and complementary remedies are the claims on the efficacy and safety of plant medicines, low cost, non-toxic nature, and improvement in the quality with developed technology (Bandaranayake, 2006). Over the past years, out of all the health care services carried in China, nearly 40% of population uses herbal medicines, while in Australia, Canada, United States, Belgium, and France by 48%, 70%, 42%, 38%, and 75%, respectively (Foster et al., 2000, WHO, 2002). This great surge and alleged patient satisfaction with herbal medicines fabricates a demand for new herbal products with high efficacy. Krameria lappacea (Dombey) Burdet and B.B. Simpson (syn. Krameria triandra Ruiz & Pavon), belonging to the family Krameriaceae, is a hemiparasite native to South America (Simpson, 1989). The plant is popularly known as “raiz de dientes” due to its traditional use of chewing stick for cleaning and strengthening teeth. In European medicine, K. lappacea was familiarized in the 18th century for the treatment of stomach ailments, nose bleeds, oropharyngeal inflammation, and menstrual problems (Simpson, 1991, Carini et al., 2002). It is also used for stomatitis, gingivitis, and pharyngitis treatment (Ratanhiae radix, 2009). The K. lappacea extracts as well as pure compounds are reported to possess anti-microbial, antioxidant, and photo-protective activities (Stahl and Ittel, 1981, Arnone et al., 1988, Facino et al., 1997, Carini et al., 2002, Baumgartner et al., 2011). In one of the studies, Arroyo et al. (2005) has reported the cytotoxic activity of aqueous extract of K. lappacea root in combination of Annona muricata leaves extract against human breast (MCF-7), lung (H-460), and central nervous system (SF-268) cancer cells in vitro. The leaves and roots of K. lappacea have also been reported for their in vivo therapeutic utilization on female reproductive function and health (Bussmann and Glenn, 2010). Aqueous extract of K. lappacea have also been documented to possess anti-inflammatory effect and safety on rats (Femández et al., 2007). The secondary metabolites of K. lappacea mainly include lignan derivatives and oligomeric proanthocyanidins. The isolated lignan derivatives from K. lappacea roots contribute to its anti-inflammatory activity (Baumgartner et al., 2011) and the procyanidines exerted antimicrobial and astringent effects (Scholz and Rimpler, 1989). Further phytochemicals, lignans, and procyanidins are well documented for their anticancer potential (Lee, 2017, Calado et al., 2018). Benzofuran derivative (DPPB), isolated from K. lappacea have also been reported to increase the endothelial nitric oxide synthase (eNOS) and NO availability in cultured endothelial cells (Ladurner et al., 2012). Our literature survey revealed that despite of rich lignin and procyanidin content in K. lappacea, no scientific report on its potential anti-cancer mechanisms has been published yet. Hence, the present investigation was aimed to investigate the cytotoxic potential of various extracts viz. petroleum ether (KLH), chloroform (KLC), ethyl acetate (KLEA), and ethanolic (KLET) extracts of K. lappacea root against human breast cancer cell line (MCF-7) and its possible mechanisms involved in cancer cell death.
2. Materials and methods
2.1. Materials
Cell culture medium, fetal bovine serum (FBS), trypsin, antibiotics, and trypan blue were obtained from Gibco, USA. All other chemicals and solvents were purchased from Sigma. The consumables were procured from Nunc.
2.2. Plant material and extraction
The roots of K. lappacea were purchased from the local market of Riyadh, Kingdom of Saudi Arabia. The identity of the plant material was confirmed by a taxonomist at King Saud University, Riyadh. For the preparation of extracts, the roots were broken to smaller pieces and subjected to sequential solvent extraction by maceration with petroleum ether, chloroform, ethyl acetate, and ethanol at room temperature accompanied by agitation. The respective extracts were obtained by drying the filtrates at 40 ℃ in a rotary evaporator. The obtained extracts were stored at 4 °C until further analysis. The extracts were diluted in dimethyl sulfoxide (DMSO) for bioassays.
2.3. Cell culture
MCF-7 (human breast cancer cell line) was used to assess the cytotoxic potential of petroleum ether (KLH), chloroform (KLC), ethylacetate (KLEA), and ethanolic (KLET) extracts of K. lappacea. The cell line was obtained from American Type Culture Collection (ATCC, Manassas, USA). The culture was facilitated by FBS (10%), NaHCO3 (0.2%), and antibiotic solution (1 mL per 100 mL of culture medium). The MCF-7 cells were cultivated at 37 °C in a CO2 incubator. Further, cell viability was analyzed using trypan blue assay (Pant et al., 2001), and the cells exhibiting more than 98% viability were used for further experiments.
2.4. MTT assay for cytotoxicity assessments
In a 96-well culture plate, the MCF-7 cells with a density of 1 × 104 cells /well were seeded. After incubation for 24 h, 100 μL of each extracts viz. KLH, KLC, KLEA, and KLET, diluted in DMEM (concentration range 10–1000 μg/mL) were added to the wells. The control sets having only culture medium were also set up parallel. The plates were again incubated for 24 h in a CO2 incubator. After incubation period, the cell viability was assessed by the colorimetric MTT assay as earlier reported (Mosmann, 1983). A 10 μL of MTT solution (5 mg/mL) was added to each well and incubated plates for 4 h at 37 °C in a CO2 incubator. Supernatant was then removed and DMSO was added to dissolve the MTT formazan. Finally, the absorbance of developed purple color was read at 550 nm.
2.5. NRU assay for cytotoxicity assessments
The assessment of cytotoxicity by NRU assay was achieved following earlier reported protocol (Borenfreund and Puerner, 1984). The concentrations of each plant extracts (KLH, KLC, KLEA, and KLET) ranging from 10 to 1000 μg/mL was added to 96-well culture plate having a cell density of 1 × 104 MCF-7 cells/well. After 24 h incubation, the wells were washed with PBS. Then, neutral red solution (50 μg/mL) was added to each well and incubated further for 3 h. The cells were washed with 1% CaCl2 and 0.5% H.CHO solution. Then, ethanol (50%) and acetic acid (1%) solution was added to each well for the extraction of dye. The absorbance was then read at 550 nm and compared with the controls.
2.6. Morphological analysis
The variations in the morphological characteristics of the MCF-7 cells after treatment with different extracts of K. lappacea (KLH, KLC, KLEA, and KLET) at varying concentrations (10–1000 μg/mL) was observed under light microscope at 20 × . After treatment of cells with K. lappacea extracts and incubation for 24 h, the images were grabbed and analyzed.
2.7. Glutathione and lipid peroxidation analysis
Glutathione content and lipid peroxidation level were assessed by commercially available kits (Cayman Chemicals). Briefly, MCF-7 cells were cultured in 6-well plates and exposed to 0, 250, 500, and 1000 μg/mL of KLH for 24 h. Control and treated sets were then washed with PBS and cells were scrapped. The scrapped cells were sonicated and homogenate was centrifuged at 3000 rpm for 15 min. The supernatant was then collected and assays were achieved following the instruction provided with the kits.
2.8. ROS measurement
ROS production was done using DCF-DA probe. Briefly, MCF-7 cells (1 × 104 cells/well) were cultured in black bottom 96-well culture plate for 24 h and were exposed to KLH (0, 250, 500, and 1000 μg/mL) for 24 h. Then cells were incubated with 20 μM of DCF-DA dye for 1 h. The fluorescence of DCF was measured at 485/528 excitation/emission using microplate fluorometer. In a parallel experiment, intracellular ROS production was also examined under a fluorescence microscope at 20× objective lens (magnification power = 200×) (Al-Oqail et al., 2019).
2.9. Loss of MMP
MMP was visualized by using Rhodamine-123 fluorescent dye (Al-Oqail et al., 2019). MCF-7 cells (2 × 104 cells/well) seeded in 24-well plate were exposed to KLH (0, 250, 500, and 1000 μg/mL) for 24 h. Afterward, cells were incubated with 10 μg/mL Rh-123 dye for 1 h at 37 °C. Then, after washing with PBS, images were grabbed under fluorescence microscope at 20× objective lens (magnification power = 200×). Also, the fluorescence intensity of control and exposed cells were measured by using microplate reader at an excitation and emission of 485 and 530 nm, respectively.
2.10. Caspase activities
Caspase-3 and -9 activities were determined by colorimetric assay kits (BioVision, USA). In brief, MCF-7 cells were exposed to 250, 500, and 1000 μg/mL KLH for 24 h. Then treated and exposed cells were harvested and lysed. Further, the experiment was achieved as per the instruction provided with kits.
2.11. Statistical analysis
The data were analyzed using one-way ANOVA with Dunnett’s post-hoc test. The statistically significance level was set at p < 0.05. The results are stated as the mean ± S.D. from three independent experiments.
3. Results
3.1. Assessments of cytotoxicity of K. lappacea extracts by MTT assay
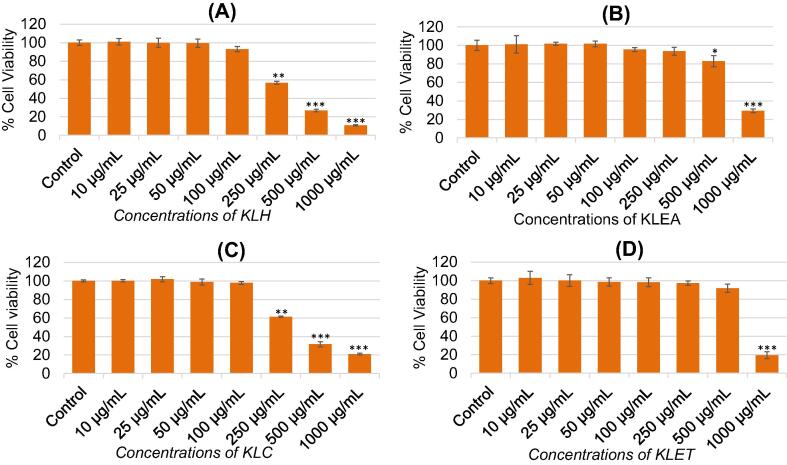
The response of cytotoxic effects of petroleum ether (KLH), ethyl acetate (KLEA), chloroform (KLC), and ethanol (KLET) extracts of K. lappacea against MCF-7 cells are given in Fig. 1A–D. Following the concentrations increase from 10 to 1000 μg/mL of various extracts of K. lappacea, the cell viability of MCF-7 cells was decreased from 44% to 90% for KLH, from 7% to 71% for KLEA, from 39% to 80% for KLC, and from 3% to 81% for KLET, respectively at 250–1000 μg/mL (Fig. 1A–D). As shown in figures, the cell viability of MCF-7 was recorded as 56%, 26%, and 10% for KLH; 93%, 83%, and 29% for KLEA; 61%, 31%, and 20% for KLC; and 97%, 91%, and 19% for KLET at 250, 500, and 1000 μg/mL, respectively by MTT assay. The decline in cell viability was observed in a concentration dependent and were significant at p < 0.05. The IC50 values obtained by MTT assay for KLH, KLEA, KLC, and KLET were 289 μg/ml, 706 μg/ml, 322 μg/ml, and 620 μg/ml, respectively.
Fig. 1.
Cytotoxic potential of Krameria lappacea extracts on MCF-7 cells measured by MTT assay. Cells were exposed to different concentrations (10–1000 μg/mL) of various extracts for 24 h. (A) Petroleum ether extract (KLH). (B) Ethyl acetate extract (KLAE). (C) Chloroform extract (KLC). (D) Ethanol extract (KLET). Results are expressed as the mean ± S.D. of three independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001 Vs Control.
3.2. Assessment of cytotoxicity of K. lappacea extracts by NRU assay
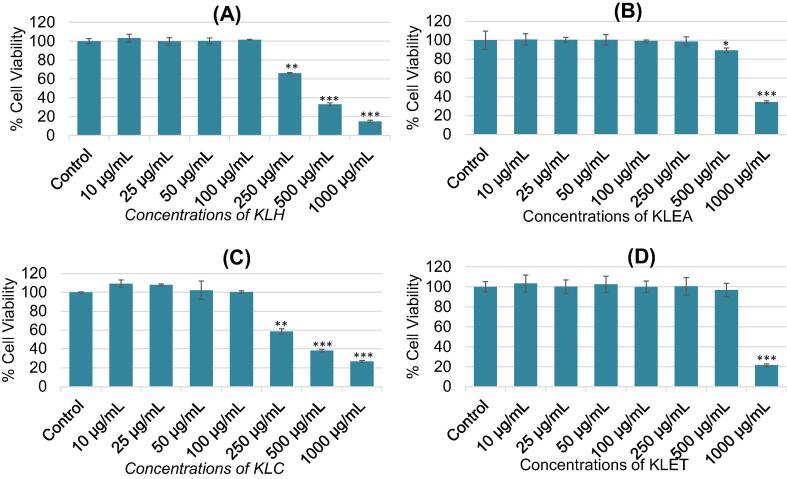
The Fig. 2 A–D summarized the effects of various extracts of K. lappacea as evaluated by NRU assay. Corresponding to MTT results, similar cytotoxic response was also observed in MCF-7 cells by NRU assay after the treatment of different concentrations of various extracts of K. lappacea. At an increasing concentration of KLH, KLEA, KLC, and KLET extracts of K. lappacea from 10 to 1000 μg/mL, the cell viability decreased from 35% to 86% for KLH, from 2% to 66% for KLEA, from 42% to 74% for KLC, and from 0 to 79% for KLET, respectively at 250–1000 μg/mL (Fig. 2A–D). The NRU assay also revealed similar cytotoxic responses of KLH, KLC, KLEA, and KLET on MCF-7 cells as observed by MTT assay. At 250, 500, and 1000 μg/mL, the viability of cells was recorded as 65%, 32%, and 14% for KLH; 98%, 89%, and 34% for KLEA; 58%, 38%, and 26% for KLC; and 100%, 96%, and 21% for KLET, respectively. Significant decreases were observed at p < 0.5.
Fig. 2.
Cytotoxic potential of Krameria lappacea extracts on MCF-7 cells measured by NRU assay. Cells were exposed to different concentrations (10–1000 μg/mL) of various extracts for 24 h. (A) Petroleum ether extract (KLH). (B) Ethyl acetate extract (KLAE). (C) Chloroform extract (KLC). (D) Ethanol extract (KLET). Results are expressed as the mean ± S.D. of three independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001 Vs Control.
3.3. Morphological changes induced by exposure of K. lappacea in MCF-7 cells
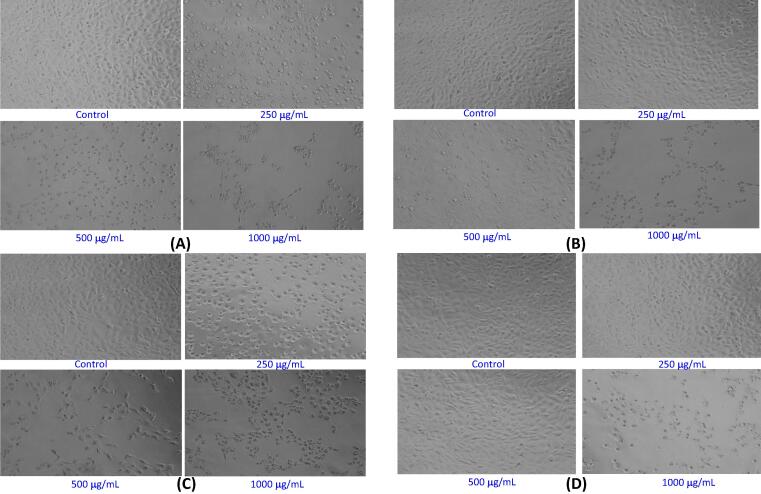
The changes in the morphological features of the MCF-7 cells upon the exposure to various concentrations of extracts (KLH, KLC, KLEA, and KLET) of K. lappacea in varying concentrations are depicted in Fig. 3 A–D.
Fig. 3.
Morphological characteristics of MCF-7 cells after the exposure of different concentrations (250–1000 μg/mL) of extracts for 24 h. (A) Petroleum ether extract (KLH). (B) Ethyl acetate extract (KLAE). (C) Chloroform extract (KLC). (D) Ethanol extract (KLET). Images were grabbed at 20× magnification.
3.4. Glutathione and lipid peroxidation analysis
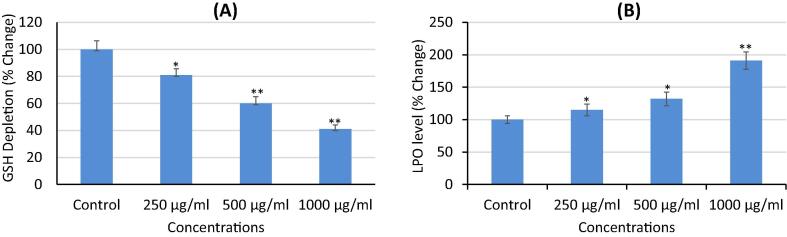
The results of the GSH and LPO levels are shown in Fig. 4A and B, respectively. As depicted in Fig. 4A, the KLH-treatment exhibited a 19%, 40%, and 59% of reduction at 250, 500, and 1000 μg/mL, respectively in GSH level (p < 0.01). However, exposure of KLH at 250, 500, and 1000 μg/mL significantly increased LPO level to 15%, 32%, and 91%, respectively (Fig. 4A).
Fig. 4.
Oxidative stress markers (A) Glutathione (GSH) depletion. (B) Lipid peroxidation (LPO) level in MCF-7 cells after the treatment of 250, 500, and 1000 μg/mL of petroleum ether extract (KLH) for 24 h. Results are expressed as the mean ± S.D. of three independent experiments. *p < 0.05 and **p < 0.01 Vs Control.
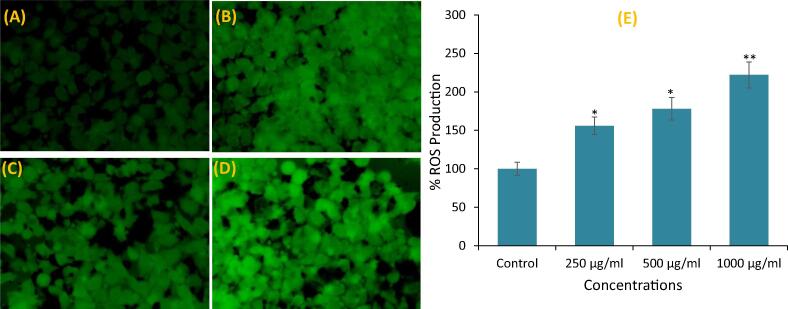
3.5. ROS measurement
The ROS production in MCF-7 exposed to KLH was found to be increased in a concentration-dependent manner (Fig. 5A-E). Fluorescence microscopic observation clearly revealed that KLH exposed MCF-7 cells exhibited dose-dependent increase in the intensity of green fluorescence as compared to untreated control (Fig. 5A-D). Highest ROS production was observed at 1000 μg/mL, where it was increased by 2.2-fold as compared to untreated control (Fig. 5E).
Fig. 5.
Intercellular ROS production in MCF-7 cells after the exposure of petroleum ether extract (KLH) for 24 h. (A) Control (B) 250 μg/mL (C) 500 μg/mL (D) 1000 μg/mL (E) Histogram showing percent ROS production in MCF-7 cells. Results are expressed as the mean ± S.D. of three independent experiments. *p < 0.01 and **p < 0.001 Vs Control.
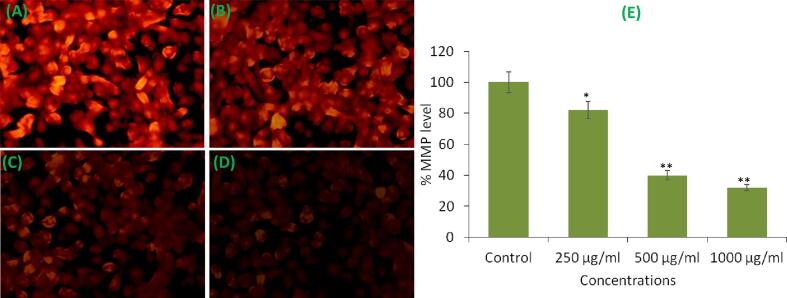
3.6. Loss of MMP
Loss of MMP is one of the mechanisms of cell death. The findings of this study indicated that the level of MMP was significantly decreased in MCF-7 cells following the exposure of KLH (Fig. 6A-E). The fluorescence intensity of Rh-123 dye was dose-dependently decreased as observed under fluorescence microscope (Fig. 6A-D). Further, the fluorescence intensity was also decreased by up to 68% at 1000 μg/mL of KLH as compared to untreated control (Fig. 6E). These results indicate that KLH induced MCF-7 cell death by decreasing the MMP level.
Fig. 6.
Mitochondrial membrane potential (intensity of Rh-123 dye) in MCF-7 cells after the exposure of petroleum ether extract (KLH) for 24 h. (A) Control (B) 250 μg/mL (C) 500 μg/mL (D) 1000 μg/mL (E) Histogram showing percent decline in the MMP level. Results are expressed as the mean ± S.D. of three independent experiments. *p < 0.01 and **p < 0.001 Vs Control.
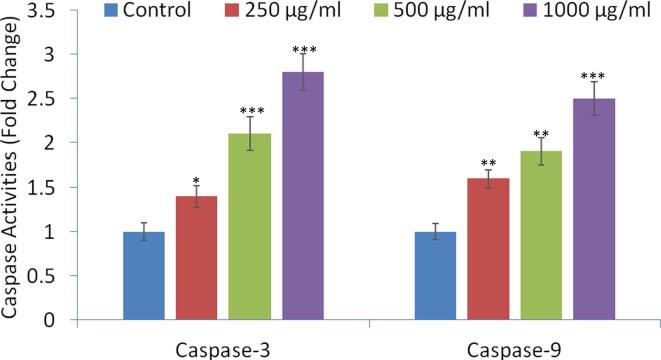
3.7. Caspase activities
The results of the caspase activities are shown in Fig. 7. The colorimetric enzymatic assay showed that KLH treatment significantly (p < 0.001) increase the level of caspase-3 by up to 1.4-, 2.1-, and 2.8-fold at 250, 500, and 1000 μg/mL, respectively as compared to untreated control. While, the enzyme level of caspase-9 was increased by up to 1.6-, 1.9-, and 2.5-fold (p < 0.001) at 250, 500, and 1000 μg/mL, respectively (Fig. 7).
Fig. 7.
Caspase-3 and caspase-9 activities in MCF-7 cells after the exposure of petroleum ether extract (KLH) at 0, 250, 500, and 1000 μg/mL concentrations for 24 h. Results are expressed as the mean ± S.D. of three independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001 Vs Control.
4. Discussion
Plants or plant derived ingredients are recognized in the management of different chronic diseases, like cancer (Forni et al., 2019). A variety of plant extracts have been proven to be useful against a different cancer cells (Shokrzadeh et al., 2010, Sodde et al., 2015). It has also been reported that plant extracts can induce cancer cell death through apoptosis or necrosis pathways (Aydemir et al., 2015). In spite of the constant development and involvement of present therapeutic approaches in the medical technology, breast cancer is gradually moving in to the class of long-lasting diseases. K. lappacea, one of the medicinally important plants of Krameriaceae family has also been documented for its beneficial effects (Simpson, 1991, Carini et al., 2002). However, cytotoxic/antiproliferative potential of K. lappacea against human breast cancer cell line (MCF-7) have not been studied yet. Hence, this study was carried out to screen the in vitro cytotoxic effects of different root bark extracts viz. petroleum ether (KLH), chloroform (KLC), ethyl acetate (KLEA), and ethanolic (KLET) extracts of K. lappacea against human breast carcinoma cell line (MCF-7). The anticancer activities of KLH, KLC, KLEA, and KLET were measured using MTT assay, NRU assay, and morphological analysis. MTT and NRU assays have been employed in this study, since they are simple, reliable, sensitive, and have been used for measuring the cytotoxicity and anticancer effects of plant extracts (Bacanli et al., 2017, Tihauan et al., 2020). MTT assay measures the conversion of mitochondrial dehydrogenase enzyme into the purple formazan in live cells (Mosmann, 1983), whereas, NRU assay measures the lysosomal integrity of live cells (Borenfreund and Puerner, 1984). These endpoints revealed that all the extracts exhibited a dose-dependent cytotoxicity in MCF-7 cells. Both MTT and NRU assays exhibited maximum cytotoxic effects on MCF-7 cells exposed to KLH followed by KLC and KLEA. The KLET was found to be less effective in comparison to the other tested extracts. The cytotoxic activity of KLET was found only at highest concentrations i.e. 1000 μg/mL, whereas other extracts have shown significant cytotoxicity even at 250 and 500 μg/mL of extracts. The lower concentrations such as 100 μg/mL and below did not produce any notable cytotoxic effects on MCF-7 cells exposed for 24 h. Therefore, the extract (KLH) showing most cytotoxic effects was selected for further analysis to explore the mechanisms of cytotoxicity in MCF-7 cells. These results were also supported by morphological analysis of MCF-7 treated with KLH, KLC, KLEA, and KLET. The light microscopic images revealed that MCF-7 exposed to K. lappacea extracts lost their normal growth, become rounded, shrinkage, and inhibited the cell growth. All these characteristics showed that cells were undergoing apoptosis (Brady, 2004). Similarly, Rahman et al. (2013), has also showed that Curcuma zedoaria-induced apoptosis in MCF-7 cells through morphological changes in MCF-7 cells. The tested doses of KLH, KLC, KLEA, and KLET were selected on the basis of earlier reports because the cytotoxic potential of various plant extracts have been tested in this concentration range in different studies (Akhir et al., 2011, Fabri et al., 2015). The cytotoxicity on primary human hepatocytes and the anti-proliferative activities of aerial part of K. lappacea against four human hepatocarcinoma cell lines has been reported earlier (Carraz et al., 2015). The results of this study were also supported by Vikas et al. (2019), who has showed that petroleum ether extracts of Annona squamosa extracts exhibited maximum cytotoxicity among the four tested extracts i.e. petroleum, chloroform, ethyl acetate, and methanol against various cancer cell lines such as nasopharyngeal (KB), lung (A-549), breast (MCF-7), and leukemia (K-562). In another study, Galium verum petroleum ether extract exhibited higher cytotoxicity over chloroform extract in human liver HepG2 and human colon HT-29 cancer cell lines (Pashapour et al., 2020). It has also been reported that more significant cytotoxicity of petroleum ether than the chloroform extract observed might be due to the existence of non-polar compounds in the extract (Sajjadi et al., 2015, Chen et al., 2016, Vaghora and Shukla, 2016). Therefore, it can be assumed that the differential cytotoxic/anticancer activities of K. lappacea observed in this study may be existence of non-polar compounds in the extracts. Our results are also in agreement with the other reports showing the cytotoxic effects of numerous plant extracts on MCF-7 (human breast cancer cells) (Khalifa et al., 2013, Singh et al., 2019, Chimplee et al., 2019). Further, the underlying mechanism of KLH induced MCF-7 cell death was subsequently explored. In this regard, the MCF-7 cells were exposed to 250–1000 μg/mL of KLH for 24 h, and oxidative stress markers (GSH and LPO), ROS generation, MMP, and caspase enzyme activities were studied. ROS raised LPO level is known to decrease endogenous antioxidants such as glutathione (GSH) (Widyaningsih et al., 2017). The elevated level of ROS has been reported to be involved in the cancer cell death (NavaneethaKrishnan et al., 2019). The results of this studies showed that the treatment of KLH at 250–1000 μg/mL significantly increased the level of LPO and decreased the GSH content in a concentration dependent manner. It has also been reported in previous studies that administration of plant extracts increased LPO and decreased GSH levels in cancer cells (Al-Oqail et al., 2019). It has also been documented that stimulation of plant extracts could produce excessive ROS generation which leads to oxidative and apoptotic cancer cell death (Marvibaigi et al., 2016). As observed in this study, the KLH treatment was found to increase the ROS production dose-dependently. These results indicated the role of oxidative stress and ROS generation in the cell death induced by KLH in MCF-7 cells. Loss of MMP has been found to be associated with the induction of apoptosis (Ly et al., 2003). Therefore, herein Rh-123 fluorescent staining was performed to assess whether KLH exposure causes apoptosis in MCF-7 cells by decreasing the MMP level. As depicted in Fig. 6, a dose-dependent decrease in loss of MMP level was observed after KLH treatment. This indicated that KLH caused mitochondrial dysfunction which leads to apoptotic pathway. Since apoptosis pathway is the furthermost commonly induced type of cell death shown by plant extracts (Fulda, 2010), therefore the capability of KLH-induced apoptosis was examined to explore the potential mechanism of action. The caspase-3 and caspase-9 enzyme activities, markers of apoptosis induction (Elmore, 2007) was measured in MCF-7 cells exposed to 250–1000 μg/mL of KLH for 24 h. The results showed that KLH treatment significantly increased the level of caspase-3 and -9 enzyme activities by up to 2.8- and 2.5-fold at 1000 μg/mL, respectively. These results are consistent with the previous reports that showed plant extracts or natural products can increase the enzyme activities which can induce caspase-dependent apoptosis in human breast cancer cells (Panicker et al., 2020) as well as in other cell types (Abdullah et al., 2017).
5. Conclusions
This investigation for the first time, demonstrated the cytotoxic potential of various root bark extracts of K. lappacea viz. KLH, KLC, KLEA, and KLET against human breast carcinoma cell line (MCF-7). The results exhibited a dose-dependent cytotoxic potential of extracts with the increasing concentrations as examined by MTT assay, NRU assay, and morphological characteristics analysis. In contrasts, KLH was found to produce more cytotoxic activity than other tested extracts in MCF-7 cells. Moreover, results also showed that KLH induced MCF-7 cell death via elevated level of ROS and oxidative stress which further leads to mitochondrial membrane dysfunction and activation of caspase enzymes. The findings of this study support the use of K. lappacea for the possible treatment of breast cancer diseases.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This research project was supported by a grant from the “Research Centre of the Female Scientific and Medical Colleges”, Deanship of Scientific Research, King Saud University.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdullah M. The value of caspase-3 after the application of Annona muricata leaf extract in COLO-205 colorectal cancer cell line. Gastroenterol. Res Prac. 2017;2017:4357165. doi: 10.1155/2017/4357165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhir N.A. Cytotoxicity of aqueous and ethanolic extracts of Ficus deltoidea on human ovarian carcinoma cell line. J. Adv. Med. Med. Res. 2011;2:397–409. [Google Scholar]
- Al-Oqail M.M. Corn Silk (Zea mays L.) Induced Apoptosis in Human Breast Cancer (MCF-7) Cells via the ROS-Mediated Mitochondrial Pathway. Oxid. Med. Cell. Longev. 2019:9789241. doi: 10.1155/2019/9789241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anquez-Traxler C. The legal and regulatory framework of herbal medicinal products in the European Union: a focus on the traditional herbal medicines category. Drug Inf. J. 2011;45:15–23. [Google Scholar]
- Arnone A. Isolation and structure determination of new active neolignans and norneoligans from Ratanhia. Gazz. Chim. Ital. 1988;118:675–682. [Google Scholar]
- Arroyo J. Actividad citotóxica in vitro de la mezcla de Annona muricata y Krameria lappacea sobre células cancerosas de glándula mamaria, pulmón y sistema nervioso central. Rev. Peru. Med. Exp. Salud Pública. 2005;22:247–253. [Google Scholar]
- Aydemir E.A. Cytotoxic and apoptotic effects of Ebenus boissieri Barbey on human lung cancer cell line A549. Pharmacogn. Mag. 2015;11(Suppl 1):S37–S45. doi: 10.4103/0973-1296.157679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacanli M. Assessment of cytotoxicity profiles of different phytochemicals: comparison of neutral red and MTT assays in different cells in different time periods. Turk. J. Pharm. Sci. 2017;14(2):95. doi: 10.4274/tjps.07078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandaranayake, W.M., 2006. Quality control, screening, toxicity, and regulation of herbal drugs, In: Ahmad, I., Aqil, F., Owais, M. (Eds.), Modern Phytomedicine. Turning Medicinal Plants into Drugs. Wiley-VCH GmbH & Co. KGaA, Weinheim, pp. 25–57.
- Baumgartner L. Lignan derivatives from Krameria lappacea roots inhibit acute inflammation in vivo and pro-inflammatory mediators in vitro. J. Nat. Prod. 2011;74:1779–1786. doi: 10.1021/np200343t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenfreund E., Puerner J.A. A simple quantitative procedure using monolayer cultures for cytotoxicity assays. J. Tissue Cult. Meth. 1984;9:7–9. [Google Scholar]
- Brady H.J., editor. Apoptosis Methods and Protocols. Humana Press; Totowa, NJ, USA: 2004. [Google Scholar]
- Braun L.A. Perceptions, use and attitudes of pharmacy customers on complementary medicines and pharmacy practice. BMC Complement. Altern. Med. 2010;10:38. doi: 10.1186/1472-6882-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Bussmann R.W., Glenn A. Medicinal plants used in Northern Peru for reproductive problems and female health. J. Ethnobiol. Ethnomed. 2010;6(1):30. doi: 10.1186/1746-4269-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffarel, M.M. et al., 2016. Molecular biology of breast cancer. In: eLS. Chichester. John Wiley & Sons, Ltd., UK, pp. 1–9.
- Calado A. The Effect of Flaxseed in Breast Cancer: A Literature Review. Front. Nutr. 2018;5:4. doi: 10.3389/fnut.2018.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carini M. Antioxidant and photoprotective activity of a lipophilic extract containing neolignans from Krameria triandra roots. Planta Med. 2002;68:193–197. doi: 10.1055/s-2002-23167. [DOI] [PubMed] [Google Scholar]
- Carraz M. Antiproliferative activity and phenotypic modification induced by selected Peruvian medicinal plants on human hepatocellular carcinoma Hep3B cells. J. Ethnopharmacol. 2015;166:185–199. doi: 10.1016/j.jep.2015.02.028. [DOI] [PubMed] [Google Scholar]
- Chen R. The Hedyotis diffusa Willd. (Rubiaceae): a review on phytochemistry, pharmacology, quality control and pharmacokinetics. Molecules. 2016;21(6):710. doi: 10.3390/molecules21060710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimplee S. Anti–breast cancer potential of frullanolide from Grangea maderaspatana plant by inducing apoptosis. Oncol. Lett. 2019;17(6):5283–5291. doi: 10.3892/ol.2019.10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014;4(177):1–10. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabri R.L. Antimicrobial, antioxidant and cytotoxicity potential of Manihot multifida (L.) Crantz (Euphorbiaceae) An. Acad Bras. Cienc. 2015;87(1):303–311. doi: 10.1590/0001-3765201520130251. [DOI] [PubMed] [Google Scholar]
- Facino R.M. A rapid screening by liquid chromatography/mass spectrometry and fast-atom bombardment tandem mass spectrometry of phenolic constituents with radical scavenging activity, from Krameria triandra roots. Rapid Commun. Mass Spectrom. 1997;11:1303–1308. [Google Scholar]
- Femández A. Efecto antiinflamatorio in vitro y seguridad en ratas del extracto acuosos atomizado de la raíz de Krameria lappacea (ratania) root. Ciencia e Investigación. 2007;10(2):65–70. [Google Scholar]
- Ferlay, J. et al., 2018. Global Cancer Observatory: Cancer Today. International Agency for Research on Cancer, Lyon, France. Available from: https://gco.iarc.fr/today.
- Forni C. Beneficial role of phytochemicals on oxidative stress and age-related diseases. Biomed Res. Int. 2019;2019:8748253. doi: 10.1155/2019/8748253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D.F. Alternative medicine use in older Americans. J. Am. Geriatr. Soc. 2000;48:1560–1565. doi: 10.1111/j.1532-5415.2000.tb03864.x. [DOI] [PubMed] [Google Scholar]
- Fulda S. Modulation of apoptosis by natural products for cancer therapy. Planta Med. 2010;76(11):1075–1079. doi: 10.1055/s-0030-1249961. [DOI] [PubMed] [Google Scholar]
- Khalifa N.S. In vitro cytotoxic and antioxidant activities of some plant extract on different human cancer cell lines, Egypt. J. Exp. Biol. (Bot) 2013;9(1):137–144. [Google Scholar]
- Ladurner A. 2-(2, 4-dihydroxyphenyl)-5-(E)-propenylbenzofuran promotes endothelial nitric oxide synthase activity in human endothelial cells. Biochem. Pharmacol. 2012;84(6):804–812. doi: 10.1016/j.bcp.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. Cancer Chemopreventive Potential of Procyanidin. Toxicol. Res. 2017;33(4):273–382. doi: 10.5487/TR.2017.33.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly J.D. The mitochondrial membrane potential (Δψ m) in apoptosis; an update. Apoptosis. 2003;8(2):115–128. doi: 10.1023/a:1022945107762. [DOI] [PubMed] [Google Scholar]
- Marvibaigi M. Antioxidant activity and ROS-dependent apoptotic effect of Scurrula ferruginea (Jack) danser methanol extract in human breast cancer cell MDA-MB-231. PLoS ONE. 2016;11(7) doi: 10.1371/journal.pone.0158942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton J. Chemotherapy-exacerbated breast cancer metastasis: A paradox explainable by dysregulated adaptive-response. Int. J. Mol. Sci. 2018;19(11):3333. doi: 10.3390/ijms19113333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- NavaneethaKrishnan S. ROS-mediated cancer cell killing through dietary phytochemicals. Oxid. Med. Cell. Longev. 2019;2019:9051542. doi: 10.1155/2019/9051542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicker N.G. Organic extracts from Cleome droserifolia exhibit effective caspase-dependent anticancer activity. BMC Compl. Med. Therap. 2020;20(1):1–3. doi: 10.1186/s12906-020-2858-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant A.B. In vitro cytotoxicity evaluation of plastic biomedical devices. Hum. Exp. Toxicol. 2001;20(8):412–417. doi: 10.1191/096032701682692919. [DOI] [PubMed] [Google Scholar]
- Pashapour S. The cytotoxicity of the chloroform and petroleum ether fractional extracts of Galium verum L. in HepG2 and HT29 cell lines. J. Kermanshah Univ. Med. Sci. 2020;24(2) [Google Scholar]
- Rahman S.N. In vitro morphological assessment of apoptosis induced by antiproliferative constituents from the rhizomes of Curcuma zedoaria. Evid. Based Complementary Altern. Med. 2013;2013 doi: 10.1155/2013/257108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratanhiae radix . second ed. Georg Thieme Verlag; Stuttgart: 2009. ESCOP Monographs Supplement 2009; pp. 213–216. [Google Scholar]
- Rice S., Whitehead S.A. Phytoestrogens and breast cancer - promoters or protectors? Endocr. Relat. Cancer. 2006;13(4):995–1015. doi: 10.1677/erc.1.01159. [DOI] [PubMed] [Google Scholar]
- Sajjadi S.E. Cytotoxic effect of Cousinia verbascifolia Bunge against OVCAR-3 and HT-29 cancer cells. J. Herbmed. Pharmacol. 2015;4(1):15–19. [Google Scholar]
- Scholz E., Rimpler H. Proanthocyanidins from Krameria triandra root. Planta Med. 1989;55:379–384. doi: 10.1055/s-2006-962032. [DOI] [PubMed] [Google Scholar]
- Shokrzadeh M. Cytotoxicity of hydro-alcoholic extracts of Cucurbita pepo and Solanum nigrum on HepG2 and CT26 cancer cell lines. Pharmacogn. Mag. 2010;6(23):176. doi: 10.4103/0973-1296.66931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson B.B. New York Botanical Garden Press; NY: 1989. Krameriaceae Flora Neotropica, 49; pp. 1–108. [Google Scholar]
- Simpson B.B. The past and present uses of rhatany (Krameria, Krameriaceae) Econ. Bot. 1991;45:397–409. [Google Scholar]
- Singh R.K. Cytotoxic and apoptotic inducing activity of Amoora rohituka leaf extracts in human breast cancer cells. J. Ayurveda Integr. Med. 2019;S0975–9476(18):30720–30724. doi: 10.1016/j.jaim.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodde V.K. Cytotoxic activity of Macrosolen parasiticus (L.) Danser on the growth of breast cancer cell line (MCF-7) Pharmacogn. Mag. 2015;11(Suppl 1):S156–S160. doi: 10.4103/0973-1296.157719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl E., Ittel I. Neue, lipophile benzofuranderivate aus Ratanhiawurzel. Planta Med. 1981;42(06):144–154. doi: 10.1055/s-2007-971618. [DOI] [PubMed] [Google Scholar]
- Tihauan B.-M. Experimental in vitro cytotoxicity evaluation of plant bioactive compounds and phytoagents: a review. Rom. Biotechnol. Lett. 2020;25(4):1832–1842. [Google Scholar]
- Vaghora B., Shukla V. Impact of different phytochemical classes and Ayurvedic plants in battle against cancer. Skin. 2016;13:14. [Google Scholar]
- Vikas B. Cytotoxicity profiling of Annona Squamosa in cancer cell lines. Asian Pac. J. Cancer Prev. 2019;20(9):2831–2840. doi: 10.31557/APJCP.2019.20.9.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg S.E., Chandel N.S. Targeting mitochondria metabolism for cancer therapy. Nat. Chem. Biol. 2015;11(1):9–15. doi: 10.1038/nchembio.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2002. Traditional Medicine Strategy (2002-2005). WHO/EDM/TRM/2002.1. Geneva, Switzerland: World Health Organization.
- Widyaningsih W. Protection by ethanolic extract from Ulva lactuca L. against acute myocardial infarction: antioxidant and antiapoptotic activities. Malaysian. J Med. Sci. 2017;24(6):39–49. doi: 10.21315/mjms2017.24.6.5. [DOI] [PMC free article] [PubMed] [Google Scholar]