Abstract
We present characteristics of infective endocarditis (IE) caused by Staphylococcus lugdunensis and compare with IE caused by Staphylococcus aureus and other CoNS, in the National Swedish Registry of IE (2008–2018). Thirty episodes of S. lugdunensis IE were registered, of which 21 cases affected native valves, and 7 patients were subjected to surgery. The mortality rate at 30 days was significantly higher for S. lugdunensis IE (20%, n = 6), than for IE caused by other CoNS (7%) or S. aureus (9%) p = 0.016. Septic embolisation was only reported in two cases (7%). The most common treatment was isoxazolyl penicillin (n = 18).
Introduction
Staphylococcus lugdunensis belongs to the group of CoNS and was first described in 1988 [1–3]. Since then, S. lugdunensis has attracted increasing interest as a cause of infective endocarditis (IE). It is known to infect native valves to a larger extent than other CoNS, and to have a clinical presentation more similar to S. aureus [4]. Several case reports indicate that S. lugdunensis can cause severe IE with rapid destruction of heart valves and massive septic embolisation [5–9], and many studies have emphasised the importance of early surgical intervention [7, 8, 10]. S. lugdunensis is also often susceptible to narrow-spectrum antibiotics including penicillin, and recent reports have indicated that benzyl penicillin may be a better alternative for susceptible isolates than standard treatment with isoxazolyl penicillin or vancomycin [11–13].
Our aim was to describe the clinical presentation of IE caused by S. lugdunensis, and to compare it with the clinical presentation of IE caused by other CoNS and S. aureus. A secondary aim was to present the antibiotic treatment of S. lugdunensis IE.
Material and methods
We retrospectively reviewed cases of IE caused by S. lugdunensis, S. aureus and other CoNS from the Swedish Registry of Infective Endocarditis between 2008 and 2018.
Cases were classified according to Duke’s criteria [14, 15]. If information about comorbidities were missing, we interpreted it as a negation of that condition. For other missing data, no imputations were made. Treatment delay was defined as days from onset of symptoms until start of IE treatment. Definite antibiotic treatment was defined as the antibiotic given more than 50% of the treatment time. Antibiotic susceptibility data were received by personal contact with the local microbiological department at each site. Antibiotic susceptibility testing was performed according to EUCAST guidelines [16].
Comparisons between groups were made with chi-squared test when testing categorical variables and Mann-Whitney U test for continuous data. Survival data were calculated with a Kaplan-Meier survival curve and log-rank test. Two-tailed p < 0.05 was regarded as statistically significant. Analyses were performed using the SPSS software, version 25 (SPSS, Armonk, NY, USA).
The study was approved by the Medical Ethics Committee (Institutional Review Board) of Lund University (Dnr 2017/1002).
Results and discussion
In total, we found 30 cases of IE caused by S. lugdunensis, 262 cases of IE caused by other CoNS and 1892 cases of IE caused by S. aureus. Clinical characteristics of the different groups are summarised in Table 1. Patients with S. lugdunensis IE were significantly older than patients with IE caused by S. aureus (73 vs 66 years, p = 0.01). When iv-drug users were excluded, the median age of S. aureus cases increased to 69.5 years, and the age difference between the groups was no longer significant (p = 0.44). In agreement with previous reports, 21 patients (70%) in the S. lugdunensis group had native valve IE, and the most common localisation was the aortic valve (60%) [7, 17]. The proportion of native valve IE in the S. lugdunensis group was similar to that in S. aureus IE, but significantly higher than for other CoNS (70% vs 35%, p = 0.0001).
Table 1.
Patient characteristics and outcome data
| Bacteria (n) |
S. lugdunensis n = 30 |
CoNS n = 262 |
P value CoNS vs S. lugdunensis |
S. aureus n = 1892 |
P value S. aureus vs S. lugdunensis |
|---|---|---|---|---|---|
| Background data | |||||
| Age (years); median (IQR) | 73 (65–84) | 72 (61–80) | 66 (45–79) | p = 0.01 | |
| Gender-female | 11 (37%) | 86 (33%) | 725 (38%) | ||
| Diabetes | 9 (30%) | 58 (22%) | 349 (18%) | ||
| Cancer last 5 years | 6 (20%) | 46 (18%) | 173 (9%) | p = 0.042 | |
| IV drug users | 0 (0%) | 9 (3%) | 448 (24%) | p = 0.002 | |
| Prosthetic valve | 8 (27%) | 115 (44%) | 255 (14%) | p = 0.037 | |
| Pacemaker/ICD | 1 (3%) | 74 (28%) | p = 0.031 | 324 (17%) | p = 0.046 |
| Native valve disease | 5 (17%) | 55 (21%) | 222 (12%) | ||
| Treatment delay, days median (IQR) | 9 (4-15) | 10 (3–26) | 5 (2-9) | p < 0.001 | |
| Dukes criteria | |||||
| Definite | 27 (90%) | 194 (74%) | 1544 (82%) | ||
| Possible | 3 (10%) | 67 (26%) | 338 (18%) | ||
| Localisation | |||||
| Aortic | 18 (60%) | 121 (46%) | 577 (31%) | p = 0.001 | |
| Mitral | 10 (33%) | 76 (29%) | 596 (32%) | ||
| Tricuspid | 1 (3%) | 22 (8%) | 441 (23%) | p = 0.01 | |
| Type of infection | |||||
| Prosthetic IE | 6(20%) | 110 (42%) | p = 0.02 | 245 (13%) | |
| Pacemaker/ ICD IE | 1(3%) | 48 (18%) | p = 0.01 | 179 (9%) | |
| Native valve IE | 21 (70%) | 90 (35%) | p = 0.0001 | 1103 (58%) | |
| Community acquired | 25 (83%) | 179 (68%) | 1543 (82%) | ||
| Outcome | |||||
| Antibiotic treatment, median days (IQR) | 31 (18–37) | 35 (28–42) | p = 0.046 | 30 (28-40) | |
| Embolisation | 2 (7%) | 62 (24%) | p = 0.033 | 907 (48%) | p < 0.001 |
| Surgical intervention | 7 (23%) | 111 (42%) | p = 0.044 | 455 (24%) | |
| Day of surgery, median (IQR) |
5 (1–9) |
12 (5-20) |
12 (7-23) |
||
|
Mortality at 30 days In-hospital mortality |
6 (20%)* 7 (23%) |
17(7%) 49 (19%) |
166 (9%) 268 (14%) |
||
| Day of death in hospital, Median (IQR) | 9 (8–23) | 36 (28–47) | p = 0.007 | 25 (14-39) | p = 0.016 |
Data are presented as number and (%) unless otherwise stated. Survival data calculated with Kaplan-Meier survival curve and log-rank test
ICD intracardiac device, IQR interquartile range
*p = 0.016
There was a significantly lower occurrence of septic embolisation in patients with S. lugdunensis IE (7%, n = 2), both compared with the S. aureus group (48%, p < 0.001) and the other CoNS group (24%, p = 0.033). This is in contrast to earlier published studies, reporting a high frequency of severe septic embolisation [5, 6, 8, 9]. This discrepancy could be a result of previous publication bias, unthorough clinical examination or failure to report correctly to the database registry. Speaking against the latter is that the embolisation frequency for S. aureus was in line with previously published data [18].
Moreover, earlier studies have reported a need of surgical intervention in a large proportion of cases [5, 7, 19], but only seven patients with S. lugdunensis IE (23%) underwent surgery in our cohort. This was similar to the S. aureus group (24%) but lower than for other CoNS cases (42% vs 23%, p = 0.044), which can probably be attributed to the high proportion of prosthetic valve IE in the other CoNS group.
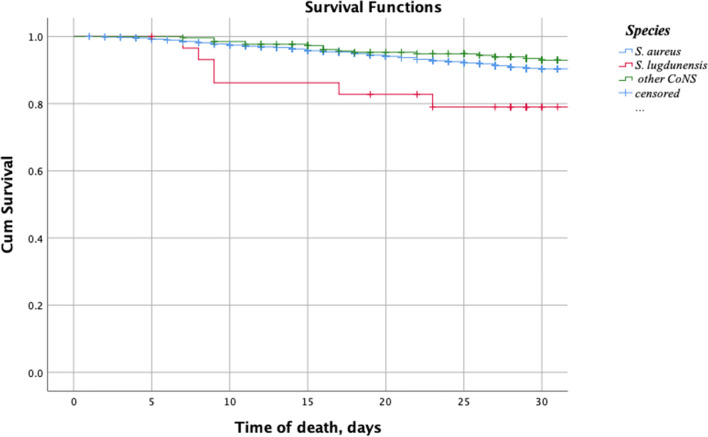
In-hospital mortality was comparable between the groups. However, death occurred after a median time of 9 days in the S. lugdunensis group, which was significantly earlier than both the groups of S. aureus (median 25 days, p = 0.016) and other CoNS (median 36 days, p = 0.007) as visualised in Fig. 1 and Table 1. This makes all-cause mortality at 30 days significantly higher in the S. lugdunensis group (20%, n = 6) compared with other CoNS (7%, n = 17) and S. aureus (9%, n = 166), p = 0.016. This indicates, as previously reported, that S. lugdunensis on some occasions can cause an aggressive form of IE, which supports the importance of early identification and early surgical intervention for this pathogen [7, 8, 10].
Fig. 1.
Kaplan-Meier plot survival after hospitalisation with IE caused by different staphylococci
The most common antibiotic treatment of S. lugdunensis IE, given to 18 cases (60%), was an isoxazolyl penicillin. All isolates were reported susceptible to isoxazolyl penicillin, except for two isolates where data were missing. Despite this, 7 patients (23%) received treatment with different antibiotic combinations, and only one of these patients had a prosthetic valve IE. Only one isolate was tested for penicillin G even though S. lugdunensis is known to have a conserved susceptibility to most antibiotics [5, 12, 20]. Recent research has suggested penicillin to be the preferred treatment of S. lugdunensis infections [12], and reliable methods for susceptibility testing are available [21].
The most important weakness of this study is the small number of S. lugdunensis IE cases. Given the few cases of S. lugdunensis IE, this study was underpowered to detect any small differences between the groups, and statistically significant differences have to be interpreted with caution. Even so, this is to our knowledge the largest S. lugdunensis IE cohort described, and the registry-based design of the study adds important knowledge about the clinical presentation of S. lugdunensis IE.
Acknowledgements
The authors wish to thank the Swedish Registry of Infective Endocarditis.
Funding
Open Access funding provided by Lund University. This work was funded by the Regional Research Funds, grant numbers 40231 and 40284; Skåne University Hospital Foundations, grant number 91202; MIMS Clinical Research Fellows, grant number 81226; and The Knut and Alice Wallenberg foundation, the Medical Faculty at Lund University and Region Skåne, grant number 81234.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Freney J, Brun Y, Bes M, Meugnier H, Grimont F, Grimont PAD, Nervi C, Fleurette J. Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., two species from human clinical specimens. Int J Syst Evol Microbiol. 1988;38(2):168–172. doi: 10.1099/00207713-38-2-168. [DOI] [Google Scholar]
- 2.Etienne J, Pangon B, Leport C, Wolff M, Clair B, Perronne C, Brun Y, Bure A. Staphylococcus lugdunensis endocarditis. Lancet. 1989;1(8634):390. doi: 10.1016/s0140-6736(89)91770-4. [DOI] [PubMed] [Google Scholar]
- 3.Fleurette J, Bes M, Brun Y, Freney J, Forey F, Coulet M, Reverdy ME, Etienne J. Clinical isolates of Staphylococcus lugdunensis and S. schleiferi: bacteriological characteristics and susceptibility to antimicrobial agents. Res Microbiol. 1989;140(2):107–118. doi: 10.1016/0923-2508(89)90044-2. [DOI] [PubMed] [Google Scholar]
- 4.Frank KL, Del Pozo JL, Patel R. From clinical microbiology to infection pathogenesis: how daring to be different works for Staphylococcus lugdunensis. Clin Microbiol Rev. 2008;21(1):111–133. doi: 10.1128/CMR.00036-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anguera I, Del Rio A, Miro JM, Matinez-Lacasa X, Marco F, Guma JR, Quaglio G, Claramonte X, Moreno A, Mestres CA, Mauri E, Azqueta M, Benito N, Garcia-de la Maria C, Almela M, Jimenez-Exposito MJ, Sued O, De Lazzari E, Gatell JM, Hospital Clinic Endocarditis Study G. Staphylococcus lugdunensis infective endocarditis: description of 10 cases and analysis of native valve, prosthetic valve, and pacemaker lead endocarditis clinical profiles. Heart. 2005;91(2):e10. doi: 10.1136/hrt.2004.040659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel R, Piper KE, Rouse MS, Uhl JR, Cockerill FR, 3rd, Steckelberg JM. Frequency of isolation of Staphylococcus lugdunensis among staphylococcal isolates causing endocarditis: a 20-year experience. J Clin Microbio. 2000;38(11):4262–4263. doi: 10.1128/JCM.38.11.4262-4263.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabe MA, Shrestha NK, Gordon S, Menon V. Staphylococcus lugdunensis: a rare but destructive cause of coagulase-negative staphylococcus infective endocarditis. Eur Heart J Acute Cardiovasc Care. 2014;3(3):275–280. doi: 10.1177/2048872614523350. [DOI] [PubMed] [Google Scholar]
- 8.Kyaw H, Raju F, Shaikh AZ, Lin AN, Lin AT, Abboud J, Reddy S. Staphylococcus lugdunensis endocarditis and cerebrovascular accident: a systemic review of risk factors and clinical outcome. Cureus. 2018;10(4):e2469. doi: 10.7759/cureus.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koh TW, Brecker SJ, Layton CA. Successful treatment of Staphylococcus lugdunensis endocarditis complicated by multiple emboli: a case report and review of the literature. Int J Cardiol. 1996;55(2):193–197. doi: 10.1016/0167-5273(96)02679-4. [DOI] [PubMed] [Google Scholar]
- 10.Farrag N, Lee P, Gunney R, Viagappan GM. Staphylococcus lugdunensis endocarditis. Postgrad Med J. 2001;77(906):259–260. doi: 10.1136/pmj.77.906.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank KL, Reichert EJ, Piper KE, Patel R. In vitro effects of antimicrobial agents on planktonic and biofilm forms of Staphylococcus lugdunensis clinical isolates. Antimicrob Agents Chemother. 2007;51(3):888–895. doi: 10.1128/AAC.01052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taha L, Stegger M, Söderquist B. Staphylococcus lugdunensis: antimicrobial susceptibility and optimal treatment options. Eur J Clin Microbiol Infect Dis: Off Publ Eur Soc Clin Microbiol. 2019;38(8):1449–1455. doi: 10.1007/s10096-019-03571-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kragsbjerg P, Bomfim-Loogna J, Törnqvist E, Söderquist B. Development of antimicrobial resistance in Staphylococcus lugdunensis during treatment-report of a case of bacterial arthritis, vertebral osteomyelitis and infective endocarditis. Clin Microbiol Infect. 2000;6(9):496–499. doi: 10.1046/j.1469-0691.2000.00103.x. [DOI] [PubMed] [Google Scholar]
- 14.Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96(3):200–209. doi: 10.1016/0002-9343(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 15.Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG, Jr, Ryan T, Bashore T, Corey GR. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2000;30(4):633–638. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 16.EUCAST (2019) EUCAST clinical breakpoint Table http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_9.0_Breakpoint_Tables.pdf. Accessed 201210
- 17.Liu PY, Huang YF, Tang CW, Chen YY, Hsieh KS, Ger LP, Chen YS, Liu YC. Staphylococcus lugdunensis infective endocarditis: a literature review and analysis of risk factors. J Microbiol Immunol Infect. 2010;43(6):478–484. doi: 10.1016/S1684-1182(10)60074-6. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez Guerrero ML, Gonzalez Lopez JJ, Goyenechea A, Fraile J, de Gorgolas M. Endocarditis caused by Staphylococcus aureus: a reappraisal of the epidemiologic, clinical, and pathologic manifestations with analysis of factors determining outcome. Medicine (Baltimore) 2009;88(1):1–22. doi: 10.1097/MD.0b013e318194da65. [DOI] [PubMed] [Google Scholar]
- 19.Zinkernagel AS, Zinkernagel MS, Elzi MV, Genoni M, Gubler J, Zbinden R, Mueller NJ. Significance of Staphylococcus lugdunensis bacteremia: report of 28 cases and review of the literature. Infection. 2008;36(4):314–321. doi: 10.1007/s15010-008-7287-9. [DOI] [PubMed] [Google Scholar]
- 20.Hellbacher C, Törnqvist E, Söderquist B. Staphylococcus lugdunensis: clinical spectrum, antibiotic susceptibility, and phenotypic and genotypic patterns of 39 isolates. Clin Microbiol Infect. 2006;12(1):43–49. doi: 10.1111/j.1469-0691.2005.01296.x. [DOI] [PubMed] [Google Scholar]
- 21.Hagstrand Aldman M, Påhlman LI. Evaluation of penicillin G susceptibility testing methods for Staphylococcus lugdunensis. J Antimicrob Chemother. 2020;75(5):1206–1211. doi: 10.1093/jac/dkaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]