Abstract
As the coronavirus disease 2019 continues to spread globally, its culprit, the severe acute respiratory syndrome coronavirus 2 has been brought under scrutiny. In addition to inhalation transmission, the possible fecal-oral viral transmission via water/wastewater has also been brought under the spotlight, necessitating a timely global review on the current knowledge about waterborne viruses in drinking water treatment system – the very barrier that intercepts waterborne pathogens to terminal water users. In this article we reviewed the occurrence, concentration methods, and control strategies, also, treatment performance on waterborne viruses during drinking water treatment were summarized. Additionally, we emphasized the potential of applying the quantitative microbial risk assessment to guide drinking water treatment to mitigate the viral exposure risks, especially when the unregulated novel viral pathogens are of concern. This review paves road for better control of viruses at drinking water treatment plants to protect public health.
Keywords: Drinking water treatment, Waterborne viruses, Conventional and emerging disinfection processes
Graphical abstract
1. Introduction
Supplying sufficient and clean drinking water has remained challenging in many countries and regions (Roberson, 2011; Kaushal, 2016; Soller et al., 2019). Among different water pollutants, waterborne pathogens, particularly viruses, pose a lasting threat to human health and well-being (Fenwick, 2006; Gerba et al., 2017). With inadequate sewage disinfection and poor hygiene, water-transmitted viral pathogens can find their pathway to potable water and cause human diseases. On the average, 829,000 people die from diarrhea as a result of unsafe drinking water and sanitization (World Health Organization). In 1990–2018, 303 typhoid and paratyphoid fever outbreaks were reported with 180,940 affected cases (Kim et al., 2019). Since the late 2019, emerging COVID-19, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has widely spread across the world and making all the countries and territories at the virus risk (Lu et al., 2020). Although SARS-CoV-2 transmission majorly occurs via surface contact and inhalation of viral droplets and aerosols (Deng et al., 2020), the viral fragment was recently found in excreta (van Doremalen et al., 2020; World Health Organization, 2020), causing a concern on the possible viral pollution in water and wastewater. For example, “minuscule traces” of SARS-CoV-2 were detected in 4 of 27 non-potable water samples (La Rosa et al., 2020). Therefore, special attention has been paid to removal and/or inactivation of waterborne viruses in potable water (Agence France-Presse, 2020).
Since the 19th century, techniques for concentration and detection of viruses have developed. Viruses can enter into a drinking water supply system through different routes and then increase microbial risk once if they are insufficiently mitigated. At drinking water treatment plants (DWTP), a physical and chemical treatment train builds barrier for alleviation of waterborne viruses. Efforts have been made to optimize virus detection and inactivation methods over the drinking water treatment. However, these studies principally focused on particular viruses, but the understanding of removal and/or inactivation of waterborne viruses in a drinking water system remains limited. In addition, quantitative microbial risk assessment (QMRA) is generally applied to assessment of the health risks resulting from exposure to specific viruses, while little endeavors aimed to evaluate the key steps in controlling microbial risk in drinking water treatment.
This article provides a critical review on the state-of-art knowledge regarding different aspects of waterborne viruses in drinking water treatment systems, including their sources, occurrence, concentration/detection techniques, and reduction technologies. Moreover, QMRA as a tool to guide drinking water treatment in term of mitigating potential viral exposure risk, especially for unregulated viral pathogens, are discussed.
2. Sources and occurrence of waterborne viruses in drinking water supply system
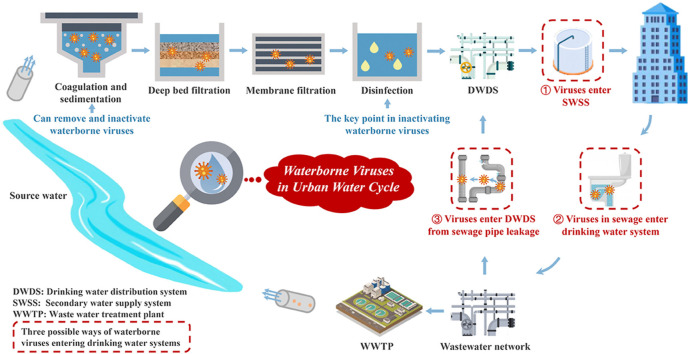
A virus is an acellular infectious particle, typically with negative charges, consisting of a nucleic acid core and a surrounding protein coat. Viruses are customarily called obligate intracellular parasites, because these infectious agents function only inside living cells. Viral sources for drinking water are diverse, such as untreated or insufficiently treated sewage, polluted stormwater runoff, and municipal solid waste. They can enter into drinking water systems at different phases (e.g., water sources, treatments, distribution, and storage). Among the different sources, wastewater is a major one (Boehm et al., 2016; Pickering et al., 2018; Luan et al., 2020). Viruses in untreated municipal wastewater typically range within 105–107 genome copies (GC) L−1 (Albinana-Gimenez et al., 2006). Substantial viruses can be excreted from both symptomatic and asymptomatic infection cases. Patients with symptomatic infection may even excrete viruses over several weeks after infection according to clinical observations (Albinana-Gimenez et al., 2006; Wu et al., 2020; Yeo et al., 2020). Traditional primary and secondary wastewater treatments can achieve 3–4 logs reduction of viruses (Sidhu et al., 2018). Some viruses may remain in the wastewater effluent and later enter and pollute natural receiving water bodies with the discharged wastewater. Virus is of particular concern when the treated wastewater flows into drinking water sources or inadequate viral removals occur at a purposeful or unplanned water reuse scenario. As is shown in Table S1, human adenovirus (HAdV) was reported to exhibit high resistance to different tertiary and advanced treatments at wastewater reclamation plants (Prado et al., 2019). At a de facto reuse situation, in which raw water contains a high fraction of wastewater effluent from upstream communities, infection risk of viruses (e.g., Norovirus (NV)) may violate local drinking water benchmarks (Lim et al., 2017). When the water body serves as both receiving water of the waste water treatment plant and source water of DWTP, free chlorine disinfection in DWTP plays an important role in providing sufficient removal of viruses (Sokolova et al., 2015). Thus, in the case that surface water is influenced by upstream sources, disinfection is important in controlling the microbial risks caused by waterborne viruses.
Another major virus pollution route occurs to water in a drinking water distribution system (DWDS) or during storage. Pressure transients in pipelines can cause the contamination in a secondary water supply system. Meanwhile, when soil or groundwater adjacent to a DWDS is polluted (e.g., by septic tank leaking and polluted stormwater infiltration), viruses can flow through damaged pipe walls and joints to make pollution (Teunis et al., 2010; Rodriguez et al., 2012; McGinnis et al., 2018; Kauppinen et al., 2019). On the other hand, still water stored in municipal water towers or indoor water tanks, when lacks protection of sufficient disinfectants, tends to be secondary pollution by viruses (Zhou et al., 2019).
Characteristics of several waterborne viruses typically found in drinking water are summarized in Table S2. The fecal-oral route represents a dominant transmission route from polluted water to human (e.g., consumption of contaminated drinking water), followed by direct contact with tainted water. Gerba et al. (2017) reported that infected people may excrete 1010 to 1012 viral particles per gram and/or mL of stool, suggesting that more abundant viruses can be released from infected individuals during clinical infections. Once virus enters into engineered drinking water systems, engineering interventions should be taken for abatement of it.
3. Methods for concentration and detection of viruses
Because viral concentration in water samples may vary within a large range (1–100,000 GC L−1 for Adenovirus (AdV), Hepatitis A virus (HAV), Coxsackievirus (CV), and Rotavirus (RV)), a two-step concentration procedure is frequently needed for recovery of viruses before detection for the samples with low viral concentrations, in which sample volumes are reduced from 10 to 1000 L to several milliliters (Project, 2020). As to viral detection technologies, cell culture is considered as the gold standard for detecting infectious Enterovirus (EV). It is derived from dispersed cells collected from original tissues and disaggregated by enzymatic, mechanical or chemical techniques, and provides large numbers of cells suitable for virus isolation, facilitated control of contamination with antibiotics and clean-air equipment (Leland and Ginocchio, 2007). Recently, molecular biotechnology has been widely used to detect and evaluate the occurrence of viruses in water. Advances in the virus concentration and detection technologies facilitate fundamental research and engineering practices on waterborne viruses in engineered water systems.
3.1. Methods for virus concentration
Performance and applicability of different virus concentration procedures has been documented (Haramoto et al., 2018; Bofill-Mas and Rusiñol, 2020). Most of previous efforts focused on investigations on virus adsorption-elution (VIRADEL), size-exclusion, and coagulation/flocculation processes. The commonly applied virus concentration techniques are as follows. (1) Electronegative filters, such as nylon membranes and mixed cellulose ester filters. Electronegative filtration based on VIRADEL is the earliest viral concentration method, in which viruses are adsorbed to the membrane surface via electrostatic attraction by pH adjustment and subsequent elution from the surface. Addition of a specific salt (e.g., MgCl2) or acid is needed to achieve the attraction between negatively charged viruses and the membrane. Despite difficulties in pH regulation, the technique is widely adopted for accomplishing a relatively high recovery in detection of EV (Cashdollar and Wymer, 2013). Haramoto et al. (2009) developed an electronegative filter along with Mg and Al for AdV in 250- or 500-mL water samples, which could achieve the mean recovery yields of 186%, 80%, 167%, 15%, and 39% for MilliQ water, tap water, bottled water, river water, and pond water, respectively. (2) Electropositive filters, such as glass-fiber filters and nano-aluminum filters, which attract the opposite charged viruses in the aquatic environment. It should be noted that the elution step, rather than the filtration itself, has the more important impact on the recovery rate (Bofill-Mas and Rusiñol, 2020). Their performance, advantages, and restrictions are available in detail elsewhere (Cashdollar and Wymer, 2013). (3) Ultrafiltration (UF), a size exclusion-based concentration procedure, which traps viruses larger than the membrane pore size, is suitable for small volume of low-turbidity samples. Recovery rates of the foremtioned concentration techniques rely on the viral type, water matrix constituents, and sample volume (Cashdollar and Wymer, 2013). Holowecky et al. (2009) used various UF cartridges to concentrate microorganisms, including MS2 and phi-X174 in drinking water samples with averaged recoveries ranging within 52–88% for MS2 and 55–95% for phi X174. (4) Viral flocculation/precipitation with organic/inorganic flocculants followed by subsequent sedimentation. This economical and practical technique is generally applied for small volume samples (Bofill-Mas and Rusiñol, 2020).
A secondary concentration is generally needed for a more manageable sample volume, thus the concentration applied in primary concentration and secondary concentration are different. The techniques frequently used for the secondary concentration of viral eluates include aqueous polymer two-phase separation, aluminum hydroxide precipitation – hydroextraction, adsorption-elution, organic flocculation, UF, and ultracentrifugation (Ikner et al., 2012). Presently, the methods of UF and electronegative filtration combined with polyethylene glycol precipitation are the most widely used for viruses in surface water. Beef extract and glycine, individually or in combination, are the most common eluents (Bofill-Mas and Rusiñol, 2020). Gilgen et al. (1997) developed a three-step isolation procedure for detection of EV, RV, and HAV, which combined filtration with a positively charged nylon membrane, UF and clean-up of the viral RNA with a silica-based membrane. The technique exhibited high sensitivity in measurement of waterborne viruses in wastewater.
3.2. Methods for virus detection
Cell culture method has long been applied for detection of infectious viruses. However, this detection technique likely underestimates the concentrations of infectious viruses at least by 2–3 orders of magnitude. For HAdV-2 in cell culture preparations, an estimated 57% of the aggregates contain 26 or more virions and 35% ranged from 51 to 150 virions (Kahler et al., 2016; Gerba et al., 2017; Gerba and Betancourt, 2019). The rapid development in molecular biological techniques such as quantitative polymerase chain reaction (qPCR) allows for more accurate quantification of virus genomes in water (Haramoto et al., 2018). The technique amplifies specific nucleic acid fragments in vitro. It can be used to detect viruses that are difficult to culture in cells or form plaques (Vinje, 2015). Additionally, assays can be modified to the desired specificity toward broad detection of an entire virus clade depending on the chosen target DNA/RNA sequences (Haramoto et al., 2018). Relying on RNA-dependent DNA polymerase, the reverse transcription-polymerase chain reaction (RT-PCR) begins with viral nucleic acid extraction, and then reverse transcription of RNA into complementary DNA (Carter et al., 2020). However, PCR-based methods are not suitable for distinguishing infectious and inactivated viruses (Bhattacharya et al., 2004). To overcome the issue, an integrated cell culture-polymerase chain reaction (ICC-PCR) technique is developed. ICC-PCR involves inoculation of the concentrated sample onto cell culture media followed by RT-PCR analysis. It has been applied to detection of infectious viruses in waters (Ming et al., 2011; Tao et al., 2016; Haramoto et al., 2018; Gerba and Betancourt, 2019), and validation of disinfection performance (Li et al., 2009; Guo et al., 2018).
Currently, fecal indicator bacteria (FIB), such as Escherichia coli (EC), are broadly used as an index for fecal contamination (Kato et al., 2018). However, an increasing attention has been paid to shortcomings of the traditional indices for EV and protozoan contamination. EC responds differently towards environmental stress and water treatment processes from protozoa and viral pathogens, which both are more resistant to chemical disinfectants (Ryu et al., 2010). Occurrence of waterborne-diseases outbreaks were reported with negative coliform results in water treatment systems (Puig et al., 1994; Taylor et al., 2001; Vivier et al., 2004). These studies reveal the lack of correlation between the detection of FIB and the presence of EV (Ebdon et al., 2012; Diston et al., 2015). Consequently, reliance on existing bacterial indicators cannot completely guarantee microbial safety of drinking water. Researchers have proposed to use EV as an indicator for human sewage contamination in aquatic environment with a full consideration of microbial factors, such as geographic distribution, resistance to disinfection (e.g., high temperature, chlorine, and low pH), behavior comparison, and molecular signals as well as persistence in water. Several alternative human-specific viral markers, such as human polyomaviruses (Albinana-Gimenez et al., 2009; Hewitt et al., 2013; Rachmadi et al., 2016), AdV (Albinana-Gimenez et al., 2009; Hewitt et al., 2013), and bacteriophages MS2 (Hsu et al., 2002; Tree et al., 2003) are commonly applied in lab-scale studies. Since water quality and testing schemes are site-specific, microbial safety of water should be systematically evaluated in response to local conditions. To this end, QMRA has been mainstreamed in drinking water supply systems as part of a paradigm shift in the drinking water industry towards water safety planning and risk-based system assessment (Hamouda et al., 2018).
4. Abatement of waterborne viruses
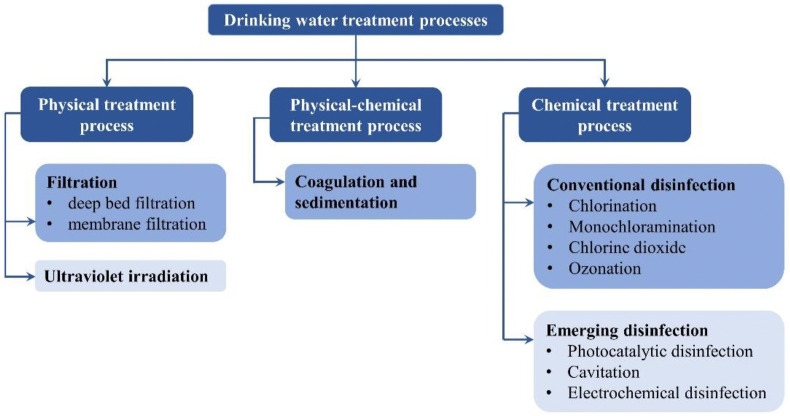
As shown in Fig. 1 , a drinking water treatment system consisting of different physical and chemical treatment units, so can provide multiple barriers to alleviation of virus in water. Abatement of waterborne viruses at different treatment processes is reviewed below.
Fig. 1.
Drinking water treatment processes in reduction of waterborne viruses.
4.1. Coagulation and sedimentation
The main purpose of coagulation and ensuing sedimentation is removing turbidity. Over the coagulation, viruses can be integrated into flocs through incorporation of developing flocs (charge neutralization or inter-particle bridging) and/or adsorption to the surface of formed flocs (sweep flocculation) (Heffron and Mayer, 2016). Hydrolyte species form during the addition of coagulants for destabilization of colloids by overcoming the repulsive force between colloidal particles. These flocs together with viruses can subsequently be removed by gravity-driven settling or filtration from the water (Tanneru and Chellam, 2012; Tanneru et al., 2013; Kreissel et al., 2014; Shirasaki et al., 2016a). Meanwhile, the intermediate polymers formed during hydrolysis of the aluminum coagulants exert a greater attraction on the viruses, making the viruses inactive or non-infectious (Matsui et al., 2003).
Aluminum and iron salts are two extensively used coagulants (Shirasaki et al., 2016b). Factors affecting the reduction of viruses in the coagulation process include pH, the types and doses of coagulants. The effect of pH on viral removal is primarily related to the isoelectric point of virus. After FeCl3 is dosed, pH significantly declines due to hydrolysis, thus enhancing the neutralization of EV surface charge and improving their removal efficiency. Compared with traditional coagulation, enhanced coagulation with an extremely high coagulant dose presents as a relatively highly effective way in alleviation of waterborne viruses. Of note, viruses incorporation into growing aluminum hydroxide and coprecipitation during charge neutralization are principal mechanisms during coagulation, while the adsorption of viruses to the formed aluminum hydroxide flocs plays a limited role (Shirasaki et al., 2016a).
Virus removal over coagulation relies on coagulant species. For ferrous-based coagulants, virus inactivation may occur when Fe (II) is oxidized (Heffron et al., 2019). Abbaszadegan et al. (2007) documented that enhanced coagulation with increased ferric chloride dose and/or pH adjustment showed higher removal for viruses. The maximum removal of 2.58 logs for AdV-4, 2.32 logs for MS2, 1.75 logs for PRD1, and 1.52 logs for Phi-X174 were achieved at 40 mg L−1 FeCl3 (real water, pH = 5 to 6, 21 °C). At the similar experimental conditions, the maximum reduction was 3 logs for Coxsackievirus B6 (CV B6), 1.75 logs for Echovirus 12, 0.36 log for MS2, and 1.3 logs for Phi-X174 (real water, pH = 5–6.5) (Mayer et al., 2008). It was concluded that coagulation process could reduce viruses by 0.5–7 logs, with a typical reduction of approximately 3 logs (Heffron and Mayer, 2016).
4.2. Physical disinfection
4.2.1. Filtration
Filtration applied at water treatment plants comprise of deep bed filtration and membrane filtration. In a deep bed filtration, water passes through a porous structure, in which treated water get through and the solid particles are intercepted and attracted. Among different deep bed filtration processes, rapid sand filtration (RSF) is the most widely adopted. As shown in Table 1 , the removal efficiency of viruses by RSF depends on physical and chemical characteristics and/or shape of viruses. For example, the removal of JC polyomavirus by RSF was lower than that of EV (0.49 ± 0.57 vs 1.26 ± 0.25 logs) (real water, pH = 6.0–8.0, 25–35 °C) (Asami et al., 2016). Generally, the combination of coagulation and filtration presents a higher removal efficiency for viruses. For example, Albinana-Gimenez et al. (2009) found that the total removal of JC polyomavirus after the coagulation and RSF processes was 4.56 logs.
Table 1.
Reduction of waterborne viruses during coagulation and filtration.
| Treatment Process | Virus | Log reduction | Coagulant and dose (mg L−1 Al or mg L−1 Fe) | pH | Turbidity | DOC (mg L−1) | Reference |
|---|---|---|---|---|---|---|---|
| Co | Poliovirus | 1.1 | AlCl3, 1.08 | 6.0 | 1.5 (NTU) | 0.9 | Shirasaki et al. (2016a) |
| 2.1 | AlCl3, 2.70 | 6.0 | 40.5 (NTU) | 2.2 | |||
| 1.8 | PACl, 1.08 | 8.0 | 1.5 (NTU) | 0.9 | |||
| Coxsackievirus | 1.4 | AlCl3, 2.70 | 7 | 40.5 (NTU) | 2.2 | ||
| 2.8 | PACl, 2.70 | 7 | 40.5 (NTU) | 2.2 | |||
| EC | MS2 | 0.36 | FeCl3, 13.8 | 6.5 | 9.18 (NTU) | 4.24 | Mayer et al. (2008) |
| Poliovirus | 2.5 | FeCl3, 13.8 | 6.5 | 22.2 (NTU) | 4.54 | ||
| Echovirus 12 | 1.0 | FeCl3, 13.8 | 6.5 | 22.2 (NTU) | 4.54 | ||
| Coxsackievirus B-6 | 2.75 | FeCl3, 13.8 | 6.5 | 22.2 (NTU) | 4.54 | ||
| JC polyomavirus | 2.3 | Alum, 7.12–9.47 | 7.06–7.17 | 51–67 (FAU) | |||
| Co, RSF | NV virus-like particles | 2.8 | FeCl3, 2.24 | 5.8 | 0.63 (NTU) | 0.76 | Shirasaki et al. (2010) |
| NV virus-like particles | 1.1 | PACl, 1.08 | 6.8 | 0.63 (NTU) | 0.76 | ||
| Qβ | 1.6 | PACl, 1.08 | 6.8 | 0.63 (NTU) | 0.76 | ||
| MS2 | 3.5 | FeCl3, 2.24 | 5.8 | 0.63 (NTU) | 0.76 | ||
| Co, MF | MS2 | 1.7 | FeCl3, 5 | 6.3 | 100 (NTU) approximately | Zhu et al. (2005b) | |
| 1.1 | FeCl3, 5 | 7.3 | 100 (NTU) approximately | ||||
| 0.9 | FeCl3, 5 | 8.3 | 100 (NTU) approximately | ||||
| Co, S, membrane filtration (0.4 μm) | MS2 | 2.2 | FeCl3, 4.48 | 7.3 | 2.5 (NTU) | 1.0 | Shirasaki et al. (2016b) |
Treatment process abbreviations: Co = coagulation; EC = enhanced coagulation; S = sedimentation; RSF = rapid sand filtration.
Pressure-driven membrane filtration can be subdivided into microfiltration (MF), UF, nanofiltration (NF), and reverse osmosis (RO). Due to screening, bridging and electricstatic attraction, suspensions and certain pathogens in water can be trapped on the membrane during water filtration. Physical sieving or adsorption, cake layer formation, and fouling state of the membrane phenomena enhance the capture of viruses for MF and UF membrane filtration processes. However, MF and UF membranes cannot fully remove viruses owning to the relatively large pore size (Fritzmann et al., 2007). Their performance in the reduction of viruses fluctuates with the operating conditions and likely fail after the damage of the membrane materials. It is reported that MF membrane could only achieve 0.5 log reduction of MS2 (synthetic fresh water, pH = 8.3) (Zhu et al., 2005a).
In contrast, NF and RO membranes can almost completely remove viruses because the viral particles can not to pass through the membrane materials. RO membranes exploit the osmotic pressure across a semi-permeable membrane to separate bacteria and viruses from drinking water, so the aggregation of viruses, protein content in the suspension and adsorption to the membrane material would influence the removal efficiency (Ideno et al., 2020). In view of the formation of undesirable biofilms on the membrane surface and prohibitive membrane cleaning and replacement (Tran et al., 2007; Stoica et al., 2018), RO membranes are not recommended for microorganism removal.
During the sedimentation and filtration processes, viruses, Giardia, Cryptosporidium and other pathogenic microorganisms are transferred from water-to-water treatment residuals (i.e., sludge). The sludge should be carefully managed and treated, particularly amid an epidemic.
4.2.2. Ultraviolet irradiation
Ultraviolet (UV) disinfection gains a growing interest in the water industry because the disinfection has proven for efficient inactivation of (oo)cysts of Cryptosporidium and Giardia in water. The UV disinfection process involves passing water through the tubes emitting UV lights, which can directly damage the viral nucleic acids (DNA/RNA) and render the genome nonreplicable, so that bacteria and viruses lose their viability and reproduction ability (Simonet and Gantzer, 2006; Wigginton et al., 2012; Sigstam et al., 2013). UV disinfection is commonly applied in the United States and Europe to address odor or DBPs issues (US EPA, 2005; Richardson et al., 2007). At the most cases, the UV disinfection technologies are based on continuous-wave monochromatic low-pressure (LP) and polychromatic medium-pressure (MP) UV systems. The LP mercury vapor lamps have a major wavelength output (85%) at 254 nm, while MP UV emits high-intensity UV spectrum between 200 nm and 400 nm (Oguma et al., 2002). Ye et al. (2018) observed a statistically decrease in the concentration of RT-qPCR target region (3–500 bp regions) during UV disinfection at 254 nm. There was no statistical difference in the approximated reaction rate constant of the Phi6 genome (0.063 ± 0.012 cm2 mJ−1) from that of Phi6 inactivation (0.067 ± 0.005 cm2 mJ−1) (P > 0.05), indicating that the genome reactions initiated the Phi6 inactivation.
The UV fluence requirements for achieving a virus inactivation by 1–4 logs with the monochromatic UV radiation based on inactivation rate constant k (cm2 mJ−1) was illustrated in detail elsewhere (Hijnen et al., 2006). The data showed that AdV was a limiting virus during UV disinfection. It has been studied that AdV-40 required over 150 mJ cm−2 for 3 logs inactivation and over 200 mJ cm−2 for 4 logs inactivation (buffered, disinfectant demand-free water, pH = 7, 22–25 °C) (Thurston-Enriquez et al., 2003b). A cell culture mRNA RT-PCR assay was developed to detect and quantify the inactivation of AdV with UV disinfection. Results showed a dose-dependent tendency in the infection loss and indicated difference in resistance using different inactivity assay (Ko et al., 2005). A similar study reported that LP and MP UV disinfections were equally effective in damaging the genome, but MP UV was more effective in inactivating AdV in cell culture, rather than in DNA damage induction (Eischeid et al., 2009).
4.3. Chemical disinfection
4.3.1. Inactivation kinetics of the microorganisms
Inactivation is defined as a decrease in the concentration of culturable microorganism N resulted from exposure to a certain concentration disinfectant within a specific exposure time t, thus causing the damage to the viral genome and/or the viral capsids. The inactivation kinetics can be described by the first-order disinfection model of Chick and Watson (Chick, 1908), that is, the proportion of viable microorganisms decreases exponentially with time:
where is the inactivation rate constant (dimensionless), is the microbial concentration after contact time, C is the disinfectant concentration, n is the coefficient of dilution (dimensionless). The Ct values are the product of the remaining disinfectant dose C and the contact time t, and act as an essential parameter for practical disinfection performance and disinfection system design.
4.3.2. Chlorination
Chlorination is currently the most used disinfection method at DWTP due to simple operation, technical viability, and low costs (Lim et al., 2010; Luo et al., 2020; Rachmadi et al., 2020). Hypochlorous acid (HOCl) and hypochlorite ion form through addition of chlorine-based products into the water. Inactivation of pathogenic microorganisms via free chlorine can be accomplished by destroying viral capsid protein and inhibiting the genome replication (Fuzawa et al., 2019). It is reported that free chlorine treatment caused extensive damage in genome and capsid protein with little or no detectable intact capsid protein remaining after 5 logs inactivation of MS2 (5 mM phosphate buffered saline, pH 7.4, at room temperature). The substantial genome damage resulted from treatments that inhibited replication functions, and the extensive capsid protein degradation resulted in significant loss in protein-mediated binding or injection functions (Wigginton et al., 2012). Different from many other chemical disinfecting agents, chlorine can maintain a lasting disinfection activity due to its slow decay rate, thus being used in DWDS and water storage systems for preventing microbial regrowth (World Health Organization, 1996; Xiao et al., 2020).
Several attempts were made to determine the chlorination efficiencies for waterborne viruses. Table 2 presents the Ct values estimated for 4 logs reduction of non-enveloped viruses from the efficiency factor Hom model, which is applied to describe microbial disinfection kinetics in real waters (Haas and Joffe, 1994). The virus disinfectant efficiency depends heavily on temperature and pH (Lim et al., 2010). At similar conditions, poliovirus (PV), CV B and Echovirus showed high resistance to chlorine disinfectants. At the same time, at pH 9 or greater, the required Ct values for virus inactivation increased significantly than at pH 7 or lower, showing a strong pH-reliance for EV-1, EV-12, CV B6, Coxsackievirus B5 (CV B5), and PV-1 (Black et al., 2009; Cromeans et al., 2010). Increase in chlorine dose showed little difference in required Ct values to achieve 4 logs reduction for RV (Xue et al., 2013). As for increase in temperature, the Ct values dropped from 0.435 mg × min L−1 to 0.183 mg × min L−1 if raised the temperature from 5 to 20 °C (Lim et al., 2010).
Table 2.
Required disinfectant dose for 4 log reduction of waterborne viruses with efficiency factor Hom model.
| Chemical Disinfection process | Virus | CT values (mg min L−1) | pH | Disinfectant dose (mg L−1) | Temperature (°C) | Water matrix | R2 | Reference |
|---|---|---|---|---|---|---|---|---|
| Chlorination | Adenovirus-2 | 1.65 | 8 | 2.7 | 25–26 | BDFa | 0.96 | Girones et al. (2014) |
| 0.15 | 7 | 0.2 | 5 | BCDFb | NKc | Loeb et al. (2018) | ||
| 0.27 | 8 | 0.2, | 5 | NK | ||||
| Adenovirus-40 | 0.22 | 6 | 0.17 | 5 | BDF | 0.99 | Thurston-Enriquez et al. (2003a) | |
| 0.75 | 7 | 0.17 | 5 | 0.99 | ||||
| 0.24 | 8 | 0.17 | 5 | 0.99 | ||||
| poliovirus-1 | 6.36 | 6 | 0.5 | 5 | 0.99 | |||
| 5.3 | 7.5 | 1.0 | 5 | NK | Black et al. (2009) | |||
| 22.3 | 9 | 1.0 | 5 | NK | ||||
| Coxsackievirus group B-3 | 2.9 | 7 | 0.2 | 5 | BCDF | NK | Cromeans et al. (2010) | |
| 1.7 | 8 | 0.2 | 5 | NK | ||||
| Coxsackievirus group B -5 | 11.5 | 7.5 | 1.0 | 5 | BDF | NK | Black et al. (2009) | |
| 22.9 | 9 | 1.0 | 5 | NK | ||||
| Coxsackievirus group B 6 | 7.4 | 7 | 0.2 | 5 | BCDF | NK | Cromeans et al. (2010) | |
| 10 | 8 | 0.2 | 5 | NK | ||||
| Echovirus-1 | 6.2 | 7.5 | 1.0 | 5 | BDF | NK | Black et al. (2009) | |
| 16.6 | 9 | 1.0 | 5 | NK | ||||
| Echovirus-12 | 7.4 | 7.5 | 1.0 | 5 | NK | |||
| 32.3 | 9 | 1.0 | 5 | NK | ||||
| Rotavirus | 5.55 | 7.2 | 0.4 | 20 | BODFd | 0.9838 | Xue et al. (2013) | |
| 5.59 | 7.2 | 0.6 | 20 | 0.9803 | ||||
| MS2 | 0.435 | 7.2 | 0.174 | 5 | 0.999 | Lim et al. (2010) | ||
| 0.183 | 7.2 | 0.172 | 20 | 1.000 | ||||
| Monochloramination | Adenovirus-2 | 1500 | 7 | 0.2 | 5 | BCDF | NK | Cromeans et al. (2010) |
| 2300 | 8 | 0.2, | 5 | NK | ||||
| Echovirus-1 | 42 | 7 | 0.2 | 5 | NK | |||
| Echovirus-11 | 1500 | 7 | 0.2 | 5 | NK | |||
| 1400 | 8 | 0.2 | 5 | NK | ||||
| Coxsackievirus group B-3 | 500 | 7 | 1 | 5 | NK | |||
| 420 | 8 | 1 | 5 | NK | ||||
| Coxsackievirus group B-5 | 900 | 7 | 1 | 5 | NK | |||
| 1100 | 8 | 1 | 5 | NK | ||||
| Chlorine dioxide | Enterovirus 71 | 6.27 | 7.2 | 0.5 | 20 | Buffered water | 0.9950 | Jin et al. (2013) |
| 4.24 | 7.2 | 2.0 | 20 | 0.9860 | ||||
| 9.68 | 7.2 | 1.5 | 4 | 0.9949 | ||||
| 4.79 | 7.2 | 1.5 | 36 | 0.9993 | ||||
| Rotavirus | 2.47 | 7.2 | 0.1 | 20 | BODF | 0.9757 | Xue et al. (2013) | |
| 1.21 | 7.2 | 0.2 | 20 | 0.9698 | ||||
| MS2 | 0.418 | 7.2 | 0.174 | 5 | 0.999 | Lim et al. (2010) | ||
| 0.138 | 7.2 | 0.178 | 20 | 0.999 | ||||
| 0.48 | 7.2 | 0.5 | 20 | Buffered water | 0.9950 | Jin et al. (2013) | ||
| Ozonation | Adenovirus-40 | 0.06 | 7 | 0.30 | 5 | distilled water | 0.99 | Thurston-Enriquez et al. (2005) |
| 0.07 | 7 | 0.49 | 5 | 0.98 |
BDF = buffered demand-free water.
BCDF = buffered chlorine-demand-free water.
NK=Not known.
BODF= Buffered oxidant demand-free water.
Although free chlorine is efficient in controlling EV, it has limited successes for some other viruses. Keswick et al. (1985) attempted to inactivate NV in a suspension with 3.75 mg L−1 free chlorine, but the suspension remained infectious after 30 min of contacting. On the other hand, the formation of DBPs during the chlorine disinfection is a undesirable but unavoidable issue with increased human-health risks of bladder cancer and adverse reproductive outcomes (US EPA, 2005; Richardson et al., 2007). Attention has been gradually paid to mutagenicity, carcinogenicity, and teratogenicity of DBPs produced from chlorination. Alternative disinfectants have been attempted to meet the regulations of DBPs (Gall et al., 2016). It is of the essence to implement a holistic control strategy for establishing multiple barriers (e.g., water source protection, physical processes for mitigation of the required disinfectant dose).
4.3.3. Monochloramination
Monochloramine, practically generated from the reactions of ammonia with aqueous chlorine, was first applied in the United States and Canada in 1917. Thereafter, the disinfecting agent was widely used in 1930s due to less odor caused by residual chlorine. Hypochlorite capable of being slowly released from monochloramine acts as the active ingredient. Therefore, monochloramine requires a longer contact time than chlorine to achieve the same disinfection efficiency.
HAdV was known to be resistant to monochloramine disinfection. Interestingly, HAdV treated by monochloramine could bind to the host cells, but genome replication and early and late mRNA transcription were inhibited (Sirikanchana et al., 2008; Gall et al., 2016). Cromeans et al. (2010) found that the Ct values of 900 mg × min L−1 and 1500 mg × min L−1 were required to achieve 4 logs inactivation of CV B5 and Echoviruses 11 (buffered chlorine-demand-free water, pH = 7, 5 ± 0.2 °C) respectively, HAdV-2, EV-11, and CV B5 showed resistance to monochloramination, increase in pH resulted in higher Ct values required. It is consistent with results in another study (Black et al., 2009). To achieve 4 logs removal for HAdV, the Ct values required for monochloramine at pH = 9 were nearly 13000 mg × min L−1 at 5 °C and over 5000 mg × min L−1 at 15 °C (Gall et al., 2016). It was noted that Ct values of monochloramine recommended for HAdV recommended by the US EPA may still be insufficient (Cromeans et al., 2010; Kahler et al., 2011).
4.3.4. Chlorine dioxide
Chlorine dioxide (ClO2) is a high-efficiency and low-cost disinfectant as an alternative for chlorine (Wigginton et al., 2012; Jin et al., 2013). It does not only exhibit an excellent disinfection performance, but also effectively achieve decolorization and deodorization, while generating less undesired DBPs. However, as a highly unstable chemical species, ClO2 needs to be on-site synthesized, making its application costly and inconvenient in practices.
Extensive coat protein and assembly protein degradation in MS2 were observed over ClO2 disinfection that resulted in a significant loss in protein-mediated binding or injection functions (Wigginton et al., 2012). Rather, ClO2 made no damage to the genome and replication function, in accordance with the reports of previous studies (Stewart et al., 2008; Sigstam et al., 2013). Thus, ClO2 is considered as a promising agent in activating viruses with genome repair mechanisms such as double-stranded DNA viruses (Wigginton et al., 2012). During the inactivation of infectivity of HAdV, the destruction of the antigenicity and damaging of the 5′nontranslated regions (the sequence from bp 1 to 671) of the genome occurred, suggesting that ClO2 reacted with viral capsid protein to inhibit HAV from attaching to the host cells or uncoating or penetrating (Li et al., 2004).
Efforts were made to investigate the inactivation efficiencies of ClO2 for different waterborne viruses, including PV, CV, Echovirus, and HAdV. In comparison with chlorine, ClO2 exhibited a better viruses inactivation performance (Lim et al., 2010; Xue et al., 2013). The required Ct values for 4 logs reduction of EV 71 dropped from 6.27 mg × min L−1 to 4.24 mg × min L−1 when the ClO2 dose increased from 0.5 min L−1 to 2.0 min L−1 (Jin et al., 2013). Also, the study of Xue et al. (2013) came to similar conclusions for RV inactivation. As to the influence of temperature, increase in temperature from 4 to 36 °C resulted in drops in the required Ct values from 9.68 mg × min L−1 to 4.79 mg × min L−1 for EV 71, and MS2 showed a similar trend (Jin et al., 2013). Also, an extended HOM model was developed with literature data (Schijven et al., 2019). As listed in Table 2, the inactivation results of ClO2 on RV were superior to those with chlorine. But the similar findings were not observed for MS2 inactivation, suggesting that effective inactivation of viruses relies heavily on selection of an appropriate disinfectant species in practice.
Chlorite and chlorate are the primary DBPs likely formed during ClO2 disinfection. High dose of ClO2 can result in neurotoxicity. To minimize the formation of chlorite and chlorate, ClO2 is mostly applied in a pre-oxidation in combination with a post-chlor(am)ination as the disinfection step.
4.3.5. Ozonation
Ozone (O3) is one of the strongest disinfectants suitable for almost all types of waterborne pathogens (Wolf et al., 2018). Beside pathogenic inactivation, O3 is adapted for taste and odor control as well as chemical oxidation of pollutants in drinking water. Shin and Sobsey (2003) documented that most EV (e.g., RV and HAV) were effectively inactivated by O3 with a CT99 (concentration × exposure time to achieve 99% inactivation of a microorganism) value much below 1 mg × min L−1.
Studies on inactivation of waterborne viruses with O3, such as EV, NV (Shin and Sobsey, 2003), PV (Shin and Sobsey, 2003; Thurston-Enriquez et al., 2005), HAdV (Thurston-Enriquez et al., 2005), and bacteriophage (Finch and Fairbairn, 1991; Shin and Sobsey, 2003), indicate that ozonation can serve as a highly effective disinfection process (Finch and Fairbairn, 1991; Shin and Sobsey, 2003; Wolf et al., 2018). Particularly, O3 is also effective against chlorine-resistant viruses such as AdV. A previous study revealed that O3 exposure reduced viral infectivity by lipid peroxidation and subsequent damage in lipid envelope and protein shell in HAdV-2, while the disrupting of the 5′-non-coding region (5′-NCR) of the PV-1 genome was observed in PV-1 during ozonation. These findings suggest that a wide range of viral types can be inactivated over O3 treatment (Sigmon et al., 2015).
However, the application of O3 in practice is restricted by its instability and limited solubility in water. The instability requires on-site synthesis of O3. Ozonation process is commonly used in conjunction with chlorine disinfection to ensure continuous disinfection in the DWTP. As the O3 concentration changes greatly in the contact tank, the Ct values when employing O3 disinfection should be calculated according to the actual designing parameters and operating conditions. As the disturbance in the solution greatly shorten the half-life of O3, the way of O3 charging should minimize the agitation (Dong et al., 2018a, 2018b).
4.4. Emerging disinfection technologies
4.4.1. Photocatalytic disinfection
Through the excitation of light, the semiconductor photocatalyst activate oxygen in gas or aquatic phase to produce a series of reactive substances, such as singlet oxygen, superoxide radicals, hydrogen peroxide (H2O2), hydroxyl radicals (·OH), and perhydroxyl radicals. These active substances effectively inactivate pathogens in the aquatic environment, thus serving as an alternative for conventional disinfection.
The key to applicability of the photocatalytic disinfection lies in the development of efficient and cost-effective photocatalysts. As the most popular photocatalyst, TiO2 has been utilized for the purification of water contaminated with inorganic and organic chemicals (Lee et al., 2011). However, the reaction kinetics of TiO2 limit the utilization in water disinfection. In view of this, researchers have been devoted to improving the photocatalytic inactivation efficiency of TiO2 and the utilization rate of solar energy for inactivation of viruses. Liga et al. (2011) attempted to enhance the antiviral activity of TiO2 in UV radiation with photocatalytic silver-doped titanium dioxide nanoparticles (nAg/TiO2), the synergistic effect between TiO2 and nAg promoted the inactivation rate for more than 5 times, and the increase in the generation of ·OH was a primary cause for inactivation of viruses. Another interesting research focused on the modification of TiO2 photocatalyst with SiO2, the modification of silica greatly increased the viruses adsorption density to the catalyst, thus the photocatalytic inactivation rate of the material to the MS2 was increased by 170% in the case of reducing the production of ·OH (Liga et al., 2013). This effective, simple, and cost-effective way of synthesis makes photocatalysis valuable in practice of drinking water disinfection. Another limitation of the application of TiO2 photocatalyst is the requirement of UV radiation, which accounted for only 4% of the solar radiation (Zhang et al., 2019). Therefore, studies have been carried out to modify TiO2 to enhance the visible light photocatalytic activity, including heavy metal deposition (Li et al., 2011), ion doping, and coupling TiO2 with other materials to improve photo-response and photocatalytic efficiency (Zhao et al., 2016).
In addition to TiO2-based photocatalysts, research on improving photocatalytic performance of the photocatalysts could be also extended to other metal oxides. The incorporation of graphene oxide sheet into the WO3 film formed a graphene-tungsten oxide composite thin films with sheetlike surface morphology and caused a nearly complete destruction in MS2 of the protein and a sharp increase in the RNA efflux after 3 h of irradiation, and maintained stable catalytic performance within 60 h (Akhavan et al., 2012).
4.4.2. Cavitation
Hydrodynamic cavitation (HC) and acoustic cavitation (AC) are common forms of cavitation. HC serves as a more cost-effective way of disinfection compared with AC, yet the cost of cavitation remains higher than conventional chlorination and ozonation process (Holkar et al., 2019). In the case of HC, the local pressure drops below the saturated pressure in the liquid, resulting in the formation, growth, and collapse of cavities (Suslick et al., 1997). The cellular damage in cavitation is caused by chemical reactions (generation of ·OH) and physical mechanisms (shock waves, pressure gradients, shear forces, and extreme local temperatures of 5000 K) (Arrojo et al., 2008). Cavitation is applied typically in combination with other disinfection processes, for example, ozonation with cavitation effectively lowered the dose of O3, reduced the size of the reactor and increased the inactivation rate of coliform bacteria and EC in lab-scale tests (Jyoti and Pandit, 2004; Sumikura et al., 2007). However, the mechanism of cavitation for inactivation of viruses remains unclear. It is known that heat and high pressure caused inactivation of viruses and that ·OH were associated with viral capsid proteins damage. Taking into account the local high pressure (Kovac et al., 2012), the high temperature condition formed (Duizer et al., 2004), and the ·OH generated during cavitation, the cavitation process could be regarded as a promising way for inactivation of viruses (Hawkins and Davies, 2001; Kosel et al., 2017; Zupanc et al., 2019).
It has been confirmed that extreme conditions occurred with shock waves, liquid microjets, and high shear forces during cavitation, resulting in physical damage to bacteria, however, few studies investigated viral inactivation with cavitation. Kosel et al. (2017) exposed MS2 to HC for about 1 h in the Venturi constriction, resulting in 4.8 logs reduction of viral infectivity in HC reactor with a sample volume of 1 L. To evaluate HC as a step-in water treatment for reduction of RV, a similar venturi cavitation chamber with a pulsating system was applied for disinfection, and observed a 75% reduction of the detected RV genomic RNA by RT-qPCR. However, reduction in nucleic acid could not reveal the change in viral infectivity (Dular et al., 2016). On the other hand, HC reactors with a sample volume of several milliliters to several liters have only been built in lab-scale research, while the economic and technical applicability in practice is worthy of further investigation.
4.4.3. Electrochemical disinfection
Electrochemical method is recognized as a high-efficiency, environmentally friendly alternative disinfection method for the treatment of drinking water. The typical application of electrochemical disinfection is for swimming pool, seawater, and drinking water disinfection.
Generally speaking, the electrochemical disinfection process consists of two categories: direct oxidation at the anode or indirect oxidation to form intermediate products. When the direct oxidation at anode is applied, the inactivation is achieved by the electron transfer between the electrode and the target substance without toxic substances and other organic substances generation. For instance, the intracellular coenzyme A underwent electrochemical oxidation via an electric current passing through the EC-containing suspension by means of granular activated carbon or activated carbon fibers, thus resulted in eradication of EC (Okochi et al., 1997). After 15min of electrochemical treatment at 0.9 V, 5 logs reduction of infectivity of Feline calicivirus was achieved, and the peptides located in the viral particles were observed to be oxidized in lab-scale electrochemical flow-cell system (4 mM phosphate buffered saline, pH = 7.4, room temperature) (Shionoiri et al., 2015). There are four factors that take effects in the rate of electrochemical disinfection: the mass transfer rate, the concentration of the reactant, the adsorption capacity of the reactant on the electrode, the relationship between the electron transfer rate of the reactant adsorbed on the electrode surface and the electrode material, and the rate of the ·OH generation on the electrode surface.
On the other hand, the indirect oxidation at anode generally requires a concentrated saline solution, thus the molecules and ions (e.g., H2O and Cl−) accumulated around the electrode are oxidized to produce chlorine-active substances (e.g., Cl2, HOCl, and ClO3) and oxygen-active substances (e.g., ·OH, atomic oxygen [O(3 P)], H2O2, O3). The intermediate products play a vital role in transferring electrons between the target substance and the electrode surface to realize the oxidation and removal of the target substance (Panizza and Cerisola, 2005; Santana et al., 2005; Martinez-Huitle and Brillas, 2008; Yang et al., 2018; Jung et al., 2020). Since natural water contains chloride ions, indirect oxidation plays a more significant role in drinking water disinfection. Previous studies confirmed that hypochlorite production by electrolysis from very dilute chloride solutions could be applied for water disinfection purposes (Kraft et al., 1999). When an iridium-antimony-tin-coated titanium anode and direct current were applied for inactivating MS2 in solutions with sodium chloride addition, better disinfection could be achieved with increases in salt content, contact time, and applied current (Fang et al., 2006). Chlorine-active substances generated by electrochemical device on-site performed a faster inactivation rate of EC, Vibrio cholerae, Clostridium perfringens and MS2 than that of free chlorine. However, though the viruses present smaller in size and simpler constructed compared to bacteria, their resistance to electrochemical treatment is greater than that of bacteria, so it limits the applicability and needs further investigation (Drees et al., 2003).
The inactivation efficiency of the electrochemical disinfection system depends to a large extent on the target cell structure, electrode materials, electrolyte composition, microorganisms, and other experimental parameters (e.g., flow rate and current density). The electrochemically generated oxidants like chloride-active substances enhance the disinfection efficiency, while H2PO4 2−, HCO3 −, and CO3 2− have inhibitory effect on the deactivation of electrochemical process (Christensen et al., 2003). It is speculated that the excessive chlorine species produced by electrochemical system owns the same disadvantages as chlorine in drinking water disinfection.
5. Quantitative microbial risk assessment application
The application of QMRA has become a promising predictive model for risk assessment (Schijven et al., 2011; Enger et al., 2012). The four steps of QMRA describe the whole process from the scientific understanding of pathogens, the transportation in natural and engineering systems, as well as the routes of exposure and disease outbreaks. QMRA is applied for estimation of the microbial risk resulted from exposure to a particular pathogen and reveal the critical steps in risk exposure. Also, the reverse risk assessment could be applied to evaluate the effect of drinking water treatment to achieve acceptable limits of microbial risk (US EPA, 2017). Owing to the difference in occurrence and resistance to disinfectant of viruses in aquatic environment, details on the recovery, viability, and infectivity (and other microbial factors relevant to the exposure assessment) in QMRA were reported to various extents. It is recommended to apply QMRA for microbial risk assessment based on site-specific conditions to reveal the processes with high potential risk, for the purpose of risk mitigation. The estimation of microbial risk from exposure to pathogens relies on raw water indicators (e.g., pathogen concentration), treatment performance (e.g., effect of drinking water treatment system), and exposure route (Schijven et al., 2016; Hamouda et al., 2018). Here, we focus on the studies that examined the performance of drinking water treatments for removing and/or inactivating waterborne viruses, and the proposed reduction of waterborne viruses to achieve the accepted annual disease risk in DWTP in Table S3.
As mentioned above, there are mainly three ways for viruses to enter and pollute drinking water, among which the sewage discharge and reuse serves as the vital way. It was believed that scenarios incorporating considerable failure in drinking water treatments resulted in the risk level surpassing the acceptable limit (Mohammed and Seidu, 2019). In drinking water treatment system, chlorine disinfection played a most important role in controlling the microbial risk (Sokolova et al., 2015; Mohammed and Seidu, 2019), while a sub-optimal disinfection processes (particularly UV treatment) significantly increased the pathogenic infection risk, which emphasized the importance of the ability to disinfection continuously (Mohammed and Seidu, 2019). In addition, the reverse risk assessments were applied to evaluate the reduction target in drinking water treatment system by setting annual disease risk targets. Astrom et al. (2007) put forward that 6–7 logs removal for NV and 5–6 logs removal for EV would be necessary to keep the risk limit. While another reasearch confirmed that the average required reduction for NV was between 7.6 and 8.8 logs (Sokolova et al., 2015). As to the treatment effect of viruses in the drinking water treatment, the disinfection process has a vital role in assuring inactivation of waterborne viruses and the microbial safety of drinking water supply. It is necessary to assess the site-specific microbial risk posed by waterborne viruses timely to ensure microbial safety of drinking water.
6. Conclusions
This paper reviewed and emphasized the knowledge about waterborne viruses in drinking water treatment system, including the sources and occurrence of viruses, and their concentration, detection, and reduction, as well as the critical processes and objectives in controlling the microbial risk according to QMRA. Although much effort has been made to improve the waterborne viruses related treatments (including concentration, detection, and reduction technologies), further research is needed for controlling microbial risk posed by waterborne viruses. This review focused on the drinking water treatment system, the crucial barrier to prevent the transmission of waterborne viruses, particular the performance of conventional and emerging disinfection techniques. The summarized emerging disinfection techniques and issues with the current technologies offer opportunities to achieve tradeoff between effective reduction of viruses and the generation of harmful disinfection byproducts. For the existing treatment systems, QMRA was proposed to guide engineering improvements to confine annual disease risks to acceptable levels. The key messages from this review are listed as follows:
-
(1)
The microbial health risk posed by the waterborne viruses could be assessed timely according to local conditions with QMRA, thus the critical steps in microbial risk exposure could be revealed. Also, the reverse risk assessment is a good way of evaluating drinking water treatment effect in achieving acceptable limits of microbial risk.
-
(2)
Coagulation, sedimentation, and disinfection are crucial in controlling waterborne viruses, and coagulation and sedimentation processes take effect in the effect of subsequent disinfection. The effect of coagulation and sedimentation significantly impact the subsequent disinfection step. Indicator viruses or bacteriophages with various sensitivities are needed to investigate the performance and mechanisms of reducing virus during coagulation and sedimentation, for a better control of waterborne viruses in the subsequent disinfection with less disinfectant's addition.
Credit author statement
Chen Li: Investigation, writing and editing. Deng Yang: Methodology, reviewing and editing. Dong Shengkun: Reviewing and editing. Wang Hong: Methodology, reviewing and editing. Li Pan: Reviewing and editing. Zhang Huaiyu: Reviewing and editing. Chu Wenhai: Methodology, reviewing and editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (Nos. 51822808, 52000184), Shanghai Science and Technology Project (No. 20230714100), and the National Major Science and Technology Project of China (No. 2017ZX0201005).
Handling Editor: Derek Muir
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.chemosphere.2021.130728.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abbaszadegan M., Mayer B.K., Ryu H., Nwachuku N. Efficacy of removal of CCL viruses under enhanced coagulation conditions. Environ. Sci. Technol. 2007;41:971–977. doi: 10.1021/es061517z. [DOI] [PubMed] [Google Scholar]
- Agence France-Presse . 2020. “Miniscule Traces” of Novel Coronavirus Found in Paris' Non-potable Water: Report. [Google Scholar]
- Akhavan O., Choobtashani M., Ghaderi E. Protein degradation and RNA efflux of viruses photocatalyzed by graphene–tungsten oxide composite under visible light irradiation. J. Phys. Chem. C. 2012;116:9653–9659. [Google Scholar]
- Albinana-Gimenez N., Clemente-Casares P., Bofill-Mas S., Hundesa A., Ribas F., Girones R. Distribution of human polyomaviruses, adenoviruses, and hepatitis E virus in the environment and in a drinking-water treatment plant. Environ. Sci. Technol. 2006;40:7416–7422. doi: 10.1021/es060343i. [DOI] [PubMed] [Google Scholar]
- Albinana-Gimenez N., Miagostovich M.P., Calgua B., Huguet J.M., Matia L., Girones R. Analysis of adenoviruses and polyomaviruses quantified by qPCR as indicators of water quality in source and drinking-water treatment plants. Water Res. 2009;43:2011–2019. doi: 10.1016/j.watres.2009.01.025. [DOI] [PubMed] [Google Scholar]
- Arrojo S., Benito Y., Tarifa A.M. A parametrical study of disinfection with hydrodynamic cavitation. Ultrason. Sonochem. 2008;15:903–908. doi: 10.1016/j.ultsonch.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Asami T., Katayama H., Torrey J.R., Visvanathan C., Furumai H. Evaluation of virus removal efficiency of coagulation-sedimentation and rapid sand filtration processes in a drinking water treatment plant in Bangkok, Thailand. Water Res. 2016;101:84–94. doi: 10.1016/j.watres.2016.05.012. [DOI] [PubMed] [Google Scholar]
- Astrom J., Petterson S., Bergstedt O., Pettersson T.J., Stenstrom T.A. Evaluation of the microbial risk reduction due to selective closure of the raw water intake before drinking water treatment. J. Water Health. 2007;5(Suppl. 1):81–97. doi: 10.2166/wh.2007.139. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S.S., Kulka M., Lampel K.A., Cebula T.A., Goswami B.B. Use of reverse transcription and PCR to discriminate between infectious and non-infectious hepatitis A virus. J. Virol. Methods. 2004;116:181–187. doi: 10.1016/j.jviromet.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Black S., Thurston J.A., Gerba C.P. Determination of Ct values for chlorine of resistant enteroviruses. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2009;44:336–339. doi: 10.1080/10934520802659653. [DOI] [PubMed] [Google Scholar]
- Boehm A.B., Wang D., Ercumen A., Shea M., Harris A.R., Shanks O.C., Kelty C., Ahmed A., Mahmud Z.H., Arnold B.F., Chase C., Kullmann C., Colford J.M., Luby S.P., Pickering A.J. Occurrence of host-associated fecal markers on child hands, household soil, and drinking water in rural Bangladeshi households. Environ. Sci. Technol. Lett. 2016;3:393–398. [PMC free article] [PubMed] [Google Scholar]
- Bofill-Mas S., Rusiñol M. Recent trends on methods for the concentration of viruses from water samples. Current Opinion in Environmental Science & Health. 2020;16:7–13. doi: 10.1016/j.coesh.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter L.J., Garner L.V., Smoot J.W., Li Y., Zhou Q., Saveson C.J., Sasso J.M., Gregg A.C., Soares D.J., Beskid T.R., Jervey S.R., Liu C. Assay techniques and test development for COVID-19 diagnosis. ACS Cent. Sci. 2020:591–605. doi: 10.1021/acscentsci.0c00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashdollar J.L., Wymer L. Methods for primary concentration of viruses from water samples: a review and meta-analysis of recent studies. J. Appl. Microbiol. 2013;115:1–11. doi: 10.1111/jam.12143. [DOI] [PubMed] [Google Scholar]
- Chick H. An investigation of the laws of disinfection. J. Hyg. 1908;8:92–158. doi: 10.1017/s0022172400006987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen P.A., Curtis T.P., Egerton T.A., Kosa S.A.M., Tinlin J.R. Photoelectrocatalytic and photocatalytic disinfection of E. coli suspensions by titanium dioxide. Appl. Catal. B Environ. 2003;41:371–386. [Google Scholar]
- Cromeans T.L., Kahler A.M., Hill V.R. Inactivation of adenoviruses, enteroviruses, and murine norovirus in water by free chlorine and monochloramine. Appl. Environ. Microbiol. 2010;76:1028–1033. doi: 10.1128/AEM.01342-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y., Li B., Nadagouda M.N., Tarabara V.V. Virus monitoring and removal in natural and built systems. J. Environ. Eng. 2020;146 [Google Scholar]
- Diston D., Sinreich M., Zimmermann S., Baumgartner A., Felleisen R. Evaluation of molecular- and culture-dependent MST markers to detect fecal contamination and indicate viral presence in good quality groundwater. Environ. Sci. Technol. 2015;49:7142–7151. doi: 10.1021/acs.est.5b00515. [DOI] [PubMed] [Google Scholar]
- Dong S., Li J., Kim M.-H., Cho J., Park S.-J., Nguyen T.H., Eden J.G. Deactivation of Legionella Pneumophila in municipal wastewater by ozone generated in arrays of microchannel plasmas. J. Phys. D Appl. Phys. 2018;51 [Google Scholar]
- Dong S., Massalha N., Plewa M.J., Nguyen T.H. The impact of disinfection Ct values on cytotoxicity of agricultural wastewaters. Ozonation vs. chlorination. Water Res. 2018;144:482–490. doi: 10.1016/j.watres.2018.07.065. [DOI] [PubMed] [Google Scholar]
- Drees K.P., Abbaszadegan M., Maier R.M. Comparative electrochemical inactivation of bacteria and bacteriophage. Water Res. 2003;37:2291–2300. doi: 10.1016/S0043-1354(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Duizer E., Bijkerk P., Rockx B., De Groot A., Twisk F., Koopmans M. Inactivation of caliciviruses. Appl. Environ. Microbiol. 2004;70:4538–4543. doi: 10.1128/AEM.70.8.4538-4543.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dular M., Griessler-Bulc T., Gutierrez-Aguirre I., Heath E., Kosjek T., Krivograd Klemencic A., Oder M., Petkovsek M., Racki N., Ravnikar M., Sarc A., Sirok B., Zupanc M., Zitnik M., Kompare B. Use of hydrodynamic cavitation in (waste)water treatment. Ultrason. Sonochem. 2016;29:577–588. doi: 10.1016/j.ultsonch.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Ebdon J.E., Sellwood J., Shore J., Taylor H.D. Phages of Bacteroides (GB-124): a novel tool for viral waterborne disease control? Environ. Sci. Technol. 2012;46:1163–1169. doi: 10.1021/es202874p. [DOI] [PubMed] [Google Scholar]
- Eischeid A.C., Meyer J.N., Linden K.G. UV disinfection of adenoviruses: molecular indications of DNA damage efficiency. Appl. Environ. Microbiol. 2009;75:23–28. doi: 10.1128/AEM.02199-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enger K.S., Nelson K.L., Clasen T., Rose J.B., Eisenberg J.N. Linking quantitative microbial risk assessment and epidemiological data: informing safe drinking water trials in developing countries. Environ. Sci. Technol. 2012;46:5160–5167. doi: 10.1021/es204381e. [DOI] [PubMed] [Google Scholar]
- Fang Q., Shang C., Chen G. MS2 inactivation by chloride-assisted electrochemical disinfection. J. Environ. Eng. 2006;132:13–22. [Google Scholar]
- Fenwick A. Waterborne infectious diseases--could they be consigned to history? Science. 2006;313:1077–1081. doi: 10.1126/science.1127184. [DOI] [PubMed] [Google Scholar]
- Finch G.R., Fairbairn N. Comparative inactivation of poliovirus type 3 and MS2 coliphage in demand-free phosphate buffer by using ozone. Appl. Environ. Microbiol. 1991;57:3121–3126. doi: 10.1128/aem.57.11.3121-3126.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzmann C., Löwenberg J., Wintgens T., Melin T. State-of-the-art of reverse osmosis desalination. Desalination. 2007;216:1–76. [Google Scholar]
- Fuzawa M., Araud E., Li J., Shisler J.L., Nguyen T.H. Free chlorine disinfection mechanisms of rotaviruses and human norovirus surrogate tulane virus attached to fresh produce surfaces. Environ. Sci. Technol. 2019;53:11999–12006. doi: 10.1021/acs.est.9b03461. [DOI] [PubMed] [Google Scholar]
- Gall A.M., Shisler J.L., Mariñas B.J. Inactivation kinetics and replication cycle inhibition of adenovirus by monochloramine. Environ. Sci. Technol. Lett. 2016;3:185–189. [Google Scholar]
- Gerba C.P., Betancourt W.Q. vol. 8. 2019. (Assessing the Occurrence of Waterborne Viruses in Reuse Systems: Analytical Limits and Needs). Pathogens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba C.P., Betancourt W.Q., Kitajima M. How much reduction of virus is needed for recycled water: a continuous changing need for assessment? Water Res. 2017;108:25–31. doi: 10.1016/j.watres.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilgen M., Germann D., Lüthy J., Hübner P. Three-step isolation method for sensitive detection of enterovirus, rotavirus, hepatitis A virus, and small round structured viruses in water samples. Int. J. Food Microbiol. 1997;37:189–199. doi: 10.1016/s0168-1605(97)00075-5. [DOI] [PubMed] [Google Scholar]
- Girones R., Carratala A., Calgua B., Calvo M., Rodriguez-Manzano J., Emerson S. Chlorine inactivation of hepatitis E virus and human adenovirus 2 in water. J. Water Health. 2014;12:436–442. doi: 10.2166/wh.2014.027. [DOI] [PubMed] [Google Scholar]
- Guo X., Wang S., Zhao C.L., Li J.W., Zhong J.Y. An integrated cell absorption process and quantitative PCR assay for the detection of the infectious virus in water. Sci. Total Environ. 2018;635:964–971. doi: 10.1016/j.scitotenv.2018.04.223. [DOI] [PubMed] [Google Scholar]
- Haas C.N., Joffe J. Disinfection under dynamic conditions: modification of hom's model for decay. Environ. Sci. Technol. 1994;28:1367–1369. doi: 10.1021/es00056a028. [DOI] [PubMed] [Google Scholar]
- Hamouda M.A., Jin X., Xu H., Chen F. Quantitative microbial risk assessment and its applications in small water systems: a review. Sci. Total Environ. 2018;645:993–1002. doi: 10.1016/j.scitotenv.2018.07.228. [DOI] [PubMed] [Google Scholar]
- Haramoto E., Katayama H., Utagawa E., Ohgaki S. Recovery of human norovirus from water by virus concentration methods. J. Virol. Methods. 2009;160:206–209. doi: 10.1016/j.jviromet.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Haramoto E., Kitajima M., Hata A., Torrey J.R., Masago Y., Sano D., Katayama H. A review on recent progress in the detection methods and prevalence of human enteric viruses in water. Water Res. 2018;135:168–186. doi: 10.1016/j.watres.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Hawkins C.L., Davies M.J. Generation and propagation of radical reactions on proteins. Biochim. Biophys. Acta. 2001;1504:196–219. doi: 10.1016/s0005-2728(00)00252-8. [DOI] [PubMed] [Google Scholar]
- Heffron J., Mayer B.K. Emerging investigators series: virus mitigation by coagulation: recent discoveries and future directions. Environ. Sci.: Water Research & Technology. 2016;2:443–459. [Google Scholar]
- Heffron J., McDermid B., Maher E., McNamara P.J., Mayer B.K. Mechanisms of virus mitigation and suitability of bacteriophages as surrogates in drinking water treatment by iron electrocoagulation. Water Res. 2019;163 doi: 10.1016/j.watres.2019.114877. [DOI] [PubMed] [Google Scholar]
- Hewitt J., Greening G.E., Leonard M., Lewis G.D. Evaluation of human adenovirus and human polyomavirus as indicators of human sewage contamination in the aquatic environment. Water Res. 2013;47:6750–6761. doi: 10.1016/j.watres.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Hijnen W.A., Beerendonk E.F., Medema G.J. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: a review. Water Res. 2006;40:3–22. doi: 10.1016/j.watres.2005.10.030. [DOI] [PubMed] [Google Scholar]
- Holkar C.R., Jadhav A.J., Pinjari D.V., Pandit A.B. Cavitationally driven transformations: a technique of process intensification. Ind. Eng. Chem. Res. 2019;58:5797–5819. [Google Scholar]
- Holowecky P.M., James R.R., Lorch D.P., Straka S.E., Lindquist H.D. Evaluation of ultrafiltration cartridges for a water sampling apparatus. J. Appl. Microbiol. 2009;106:738–747. doi: 10.1111/j.1365-2672.2008.04019.x. [DOI] [PubMed] [Google Scholar]
- Hsu F.C., Shieh Y.S., Sobsey M.D. Enteric bacteriophages as potential fecal indicators in ground beef and poultry meat. J. Food Protect. 2002;65:93–99. doi: 10.4315/0362-028x-65.1.93. [DOI] [PubMed] [Google Scholar]
- Ideno S., Takahashi K., Yusa K., Sakai K. Quantitative PCR evaluation of parvovirus B19 removal via nanofiltration. J. Virol. Methods. 2020;275 doi: 10.1016/j.jviromet.2019.113755. [DOI] [PubMed] [Google Scholar]
- Ikner L.A., Gerba C.P., Bright K.R. Concentration and recovery of viruses from water: a comprehensive review. Food Environ Virol. 2012;4:41–67. doi: 10.1007/s12560-012-9080-2. [DOI] [PubMed] [Google Scholar]
- Jin M., Shan J., Chen Z., Guo X., Shen Z., Qiu Z., Xue B., Wang Y., Zhu D., Wang X., Li J. Chlorine dioxide inactivation of enterovirus 71 in water and its impact on genomic targets. Environ. Sci. Technol. 2013;47:4590–4597. doi: 10.1021/es305282g. [DOI] [PubMed] [Google Scholar]
- Jung E., Shin H., Hooch Antink W., Sung Y.-E., Hyeon T. Recent advances in electrochemical oxygen reduction to H2O2: catalyst and cell design. ACS Energy Letters. 2020:1881–1892. [Google Scholar]
- Jyoti K.K., Pandit A.B. Ozone and cavitation for water disinfection. Biochem. Eng. J. 2004;18:9–19. [Google Scholar]
- Kahler A.M., Cromeans T.L., Metcalfe M.G., Humphrey C.D., Hill V.R. Aggregation of adenovirus 2 in source water and impacts on disinfection by chlorine. Food Environ Virol. 2016;8:148–155. doi: 10.1007/s12560-016-9232-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler A.M., Cromeans T.L., Roberts J.M., Hill V.R. Source water quality effects on monochloramine inactivation of adenovirus, coxsackievirus, echovirus, and murine norovirus. Water Res. 2011;45:1745–1751. doi: 10.1016/j.watres.2010.11.026. [DOI] [PubMed] [Google Scholar]
- Kato R., Asami T., Utagawa E., Furumai H., Katayama H. Pepper mild mottle virus as a process indicator at drinking water treatment plants employing coagulation-sedimentation, rapid sand filtration, ozonation, and biological activated carbon treatments in Japan. Water Res. 2018;132:61–70. doi: 10.1016/j.watres.2017.12.068. [DOI] [PubMed] [Google Scholar]
- Kauppinen A., Pitkanen T., Al-Hello H., Maunula L., Hokajarvi A.M., Rimhanen-Finne R., Miettinen I.T. Two drinking water outbreaks caused by wastewater intrusion including sapovirus in Finland. Int. J. Environ. Res. Publ. Health. 2019;16 doi: 10.3390/ijerph16224376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal S.S. Increased salinization decreases safe drinking water. Environ. Sci. Technol. 2016;50:2765–2766. doi: 10.1021/acs.est.6b00679. [DOI] [PubMed] [Google Scholar]
- Keswick B.H., Satterwhite T.K., Johnson P.C., DuPont H.L., Secor S.L., Bitsura J.A., Gary G.W., Hoff J.C. Inactivation of Norwalk virus in drinking water by chlorine. Appl. Environ. Microbiol. 1985;50:261–264. doi: 10.1128/aem.50.2.261-264.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Lee K.S., Pak G.D., Excler J.L., Sahastrabuddhe S., Marks F., Kim J.H., Mogasale V. Spatial and temporal patterns of typhoid and paratyphoid fever outbreaks: a worldwide review, 1990-2018. Clin. Infect. Dis. 2019;69:S499–S509. doi: 10.1093/cid/ciz705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko G., Cromeans T.L., Sobsey M.D. UV inactivation of adenovirus type 41 measured by cell culture mRNA RT-PCR. Water Res. 2005;39:3643–3649. doi: 10.1016/j.watres.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Kosel J., Gutierrez-Aguirre I., Racki N., Dreo T., Ravnikar M., Dular M. Efficient inactivation of MS-2 virus in water by hydrodynamic cavitation. Water Res. 2017;124:465–471. doi: 10.1016/j.watres.2017.07.077. [DOI] [PubMed] [Google Scholar]
- Kovac K., Diez-Valcarce M., Raspor P., Hernandez M., Rodriguez-Lazaro D. Effect of high hydrostatic pressure processing on norovirus infectivity and genome stability in strawberry puree and mineral water. Int. J. Food Microbiol. 2012;152:35–39. doi: 10.1016/j.ijfoodmicro.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Kraft A., Stadelmann M., Blaschke M., Kreysig D., Sandt B., Schroder F., Rennau J. Electrochemical water disinfection - Part I: hypochlorite production from very dilute chloride solutions. J. Appl. Electrochem. 1999;29:861–868. [Google Scholar]
- Kreissel K., Bosl M., Hugler M., Lipp P., Franzreb M., Hambsch B. Inactivation of F-specific bacteriophages during flocculation with polyaluminum chloride - a mechanistic study. Water Res. 2014;51:144–151. doi: 10.1016/j.watres.2013.12.026. [DOI] [PubMed] [Google Scholar]
- La Rosa G., Bonadonna L., Lucentini L., Kenmoe S., Suffredini E. Coronavirus in water environments: occurrence, persistence and concentration methods - a scoping review. Water Res. 2020;179 doi: 10.1016/j.watres.2020.115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Kim J., Choi W. TiO2Photocatalysis for the redox conversion of aquatic pollutants. Aquatic Redox Chemistry. 2011:199–222. [Google Scholar]
- Leland D.S., Ginocchio C.C. Role of cell culture for virus detection in the age of technology. Clin. Microbiol. Rev. 2007;20:49–78. doi: 10.1128/CMR.00002-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Gu A.Z., He M., Shi H.C., Yang W. UV inactivation and resistance of rotavirus evaluated by integrated cell culture and real-time RT-PCR assay. Water Res. 2009;43:3261–3269. doi: 10.1016/j.watres.2009.03.044. [DOI] [PubMed] [Google Scholar]
- Li J.W., Xin Z.T., Wang X.W., Zheng J.L., Chao F.H. Mechanisms of inactivation of hepatitis A virus in water by chlorine dioxide. Water Res. 2004;38:1514–1519. doi: 10.1016/j.watres.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Li M., Noriega-Trevino M.E., Nino-Martinez N., Marambio-Jones C., Wang J., Damoiseaux R., Ruiz F., Hoek E.M. Synergistic bactericidal activity of Ag-TiO(2) nanoparticles in both light and dark conditions. Environ. Sci. Technol. 2011;45:8989–8995. doi: 10.1021/es201675m. [DOI] [PubMed] [Google Scholar]
- Liga M.V., Bryant E.L., Colvin V.L., Li Q. Virus inactivation by silver doped titanium dioxide nanoparticles for drinking water treatment. Water Res. 2011;45:535–544. doi: 10.1016/j.watres.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Liga M.V., Maguire-Boyle S.J., Jafry H.R., Barron A.R., Li Q. Silica decorated TiO2 for virus inactivation in drinking water--simple synthesis method and mechanisms of enhanced inactivation kinetics. Environ. Sci. Technol. 2013;47:6463–6470. doi: 10.1021/es400196p. [DOI] [PubMed] [Google Scholar]
- Lim K.-Y., Wu Y., Jiang S.C. Assessment of Cryptosporidium and norovirus risk associated with de facto wastewater reuse in Trinity River, Texas. Microbial Risk Analysis. 2017;5:15–24. [Google Scholar]
- Lim M.Y., Kim J.M., Ko G. Disinfection kinetics of murine norovirus using chlorine and chlorine dioxide. Water Res. 2010;44:3243–3251. doi: 10.1016/j.watres.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Loeb S., Li C., Kim J.H. Solar photothermal disinfection using broadband-light absorbing gold nanoparticles and carbon black. Environ. Sci. Technol. 2018;52:205–213. doi: 10.1021/acs.est.7b04442. [DOI] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan X., Liu X., Fang C., Chu W., Xu Z. Ecotoxicological effects of disinfected wastewater effluents: a short review of in vivo toxicity bioassays on aquatic organisms. Environ. Sci.: Water Research & Technology. 2020;6:2275–2286. [Google Scholar]
- Luo P., Wang F., Krasner S.W., Fang C., Chen S., Chu W. Emerging investigator series: formation of brominated haloacetamides from trihalomethanes during zero-valent iron reduction and subsequent booster chlorination in drinking water distribution. Environ. Sci.: Water Research & Technology. 2020;6:1244–1255. [Google Scholar]
- Martinez-Huitle C.A., Brillas E. Electrochemical alternatives for drinking water disinfection. Angew Chem. Int. Ed. Engl. 2008;47:1998–2005. doi: 10.1002/anie.200703621. [DOI] [PubMed] [Google Scholar]
- Matsui Y., Matsushita T., Sakuma S., Gojo T., Mamiya T., Suzuoki H., Inoue T. Virus inactivation in aluminum and polyaluminum coagulation. Environ. Sci. Technol. 2003;37:5175–5180. doi: 10.1021/es0343003. [DOI] [PubMed] [Google Scholar]
- Mayer B.K., Ryu H., Abbaszadegan M. Treatability of U.S. Environmental Protection Agency contaminant candidate list viruses: removal of coxsackievirus and echovirus using enhanced coagulation. Environ. Sci. Technol. 2008;42:6890–6896. doi: 10.1021/es801481s. [DOI] [PubMed] [Google Scholar]
- McGinnis S., Spencer S., Firnstahl A., Stokdyk J., Borchardt M., McCarthy D.T., Murphy H.M. Human Bacteroides and total coliforms as indicators of recent combined sewer overflows and rain events in urban creeks. Sci. Total Environ. 2018;630:967–976. doi: 10.1016/j.scitotenv.2018.02.108. [DOI] [PubMed] [Google Scholar]
- Ming H.X., Zhu L., Zhang Y. Rapid quantification of infectious enterovirus from surface water in Bohai Bay, China using an integrated cell culture-qPCR assay. Mar. Pollut. Bull. 2011;62:2047–2054. doi: 10.1016/j.marpolbul.2011.07.024. [DOI] [PubMed] [Google Scholar]
- Mohammed H., Seidu R. Climate-driven QMRA model for selected water supply systems in Norway accounting for raw water sources and treatment processes. Sci. Total Environ. 2019;660:306–320. doi: 10.1016/j.scitotenv.2018.12.460. [DOI] [PubMed] [Google Scholar]
- Oguma K., Katayama H., Ohgaki S. Photoreactivation of Escherichia coli after low- or medium-pressure UV disinfection determined by an endonuclease sensitive site assay. Appl. Environ. Microbiol. 2002;68:6029–6035. doi: 10.1128/AEM.68.12.6029-6035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okochi M., Lim T.K., Nakamura N., Matsunaga T. Electrochemical disinfection of drinking water using an activated-carbon-fiber reactor capable of monitoring its microbial fouling. Appl. Microbiol. Biotechnol. 1997;47:18–22. doi: 10.1007/s002530050882. [DOI] [PubMed] [Google Scholar]
- Panizza M., Cerisola G. Application of diamond electrodes to electrochemical processes. Electrochim. Acta. 2005;51:191–199. [Google Scholar]
- Pickering A.J., Ercumen A., Arnold B.F., Kwong L.H., Parvez S.M., Alam M., Sen D., Islam S., Kullmann C., Chase C., Ahmed R., Unicomb L., Colford J.M., Jr., Luby S.P. Fecal indicator bacteria along multiple environmental transmission pathways (water, hands, food, soil, flies) and subsequent child diarrhea in rural Bangladesh. Environ. Sci. Technol. 2018;52:7928–7936. doi: 10.1021/acs.est.8b00928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado T., de Castro Bruni A., Barbosa M.R.F., Garcia S.C., de Jesus Melo A.M., Sato M.I.Z. Performance of wastewater reclamation systems in enteric virus removal. Sci. Total Environ. 2019;678:33–42. doi: 10.1016/j.scitotenv.2019.04.435. [DOI] [PubMed] [Google Scholar]
- Project G.W.P. SPECIFIC EXCRETED PATHOGENS: ENVIRONMENTAL AND EPIDEMIOLOGY ASPECTS. 2020. Global water pathogen ProjectPART THREE. [Google Scholar]
- Puig M., Jofre J., Lucena F., Allard A., Wadell G., Girones R. Detection of adenoviruses and enteroviruses in polluted waters by nested PCR amplification. Appl. Environ. Microbiol. 1994;60:2963–2970. doi: 10.1128/aem.60.8.2963-2970.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachmadi A.T., Kitajima M., Kato T., Kato H., Okabe S., Sano D. Required chlorination doses to fulfill the credit value for disinfection of enteric viruses in water: a critical review. Environ. Sci. Technol. 2020;54:2068–2077. doi: 10.1021/acs.est.9b01685. [DOI] [PubMed] [Google Scholar]
- Rachmadi A.T., Torrey J.R., Kitajima M. Human polyomavirus: advantages and limitations as a human-specific viral marker in aquatic environments. Water Res. 2016;105:456–469. doi: 10.1016/j.watres.2016.09.010. [DOI] [PubMed] [Google Scholar]
- Richardson S.D., Plewa M.J., Wagner E.D., Schoeny R., Demarini D.M. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. Mutat. Res. 2007;636:178–242. doi: 10.1016/j.mrrev.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Roberson J.A. What's next after 40 years of drinking water regulations? Environ. Sci. Technol. 2011;45:154–160. doi: 10.1021/es101410v. [DOI] [PubMed] [Google Scholar]
- Rodriguez R.A., Gundy P.M., Rijal G.K., Gerba C.P. The impact of combined sewage overflows on the viral contamination of receiving waters. Food Environ Virol. 2012;4:34–40. doi: 10.1007/s12560-011-9076-3. [DOI] [PubMed] [Google Scholar]
- Ryu H., Mayer B., Abbaszadegan M. Applicability of quantitative PCR for determination of removal efficacy of enteric viruses and Cryptosporidium by water treatment processes. J. Water Health. 2010;8:101–108. doi: 10.2166/wh.2009.208. [DOI] [PubMed] [Google Scholar]
- Santana M.H.P., Faria L.A.D., Boodts J.F.C. Electrochemical characterisation and oxygen evolution at a heavily boron doped diamond electrode. Electrochim. Acta. 2005;50:2017–2027. [Google Scholar]
- Schijven J., Foret J.M., Chardon J., Teunis P., Bouwknegt M., Tangena B. Evaluation of exposure scenarios on intentional microbiological contamination in a drinking water distribution network. Water Res. 2016;96:148–154. doi: 10.1016/j.watres.2016.03.057. [DOI] [PubMed] [Google Scholar]
- Schijven J., Teunis P., Suylen T., Ketelaars H., Hornstra L., Rutjes S. QMRA of adenovirus in drinking water at a drinking water treatment plant using UV and chlorine dioxide disinfection. Water Res. 2019;158:34–45. doi: 10.1016/j.watres.2019.03.090. [DOI] [PubMed] [Google Scholar]
- Schijven J.F., Teunis P.F., Rutjes S.A., Bouwknegt M., de Roda Husman A.M. QMRAspot: a tool for Quantitative Microbial Risk Assessment from surface water to potable water. Water Res. 2011;45:5564–5576. doi: 10.1016/j.watres.2011.08.024. [DOI] [PubMed] [Google Scholar]
- Shin G.A., Sobsey M.D. Reduction of Norwalk virus, poliovirus 1, and bacteriophage MS2 by ozone disinfection of water. Appl. Environ. Microbiol. 2003;69:3975–3978. doi: 10.1128/AEM.69.7.3975-3978.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shionoiri N., Nogariya O., Tanaka M., Matsunaga T., Tanaka T. Capsid protein oxidation in feline calicivirus using an electrochemical inactivation treatment. J. Hazard Mater. 2015;283:410–415. doi: 10.1016/j.jhazmat.2014.09.049. [DOI] [PubMed] [Google Scholar]
- Shirasaki N., Matsushita T., Matsui Y., Marubayashi T. Effect of aluminum hydrolyte species on human enterovirus removal from water during the coagulation process. Chem. Eng. J. 2016;284:786–793. [Google Scholar]
- Shirasaki N., Matsushita T., Matsui Y., Marubayashi T. Effect of coagulant basicity on virus removal from water by polyferric chloride. J. Water Supply Res. Technol. - Aqua. 2016;65:322–329. [Google Scholar]
- Shirasaki N., Matsushita T., Matsui Y., Oshiba A., Ohno K. Estimation of norovirus removal performance in a coagulation-rapid sand filtration process by using recombinant norovirus VLPs. Water Res. 2010;44:1307–1316. doi: 10.1016/j.watres.2009.10.038. [DOI] [PubMed] [Google Scholar]
- Sidhu J.P.S., Sena K., Hodgers L., Palmer A., Toze S. Comparative enteric viruses and coliphage removal during wastewater treatment processes in a sub-tropical environment. Sci. Total Environ. 2018;616–617:669–677. doi: 10.1016/j.scitotenv.2017.10.265. [DOI] [PubMed] [Google Scholar]
- Sigmon C., Shin G.-A., Mieog J., Linden K.G. Establishing surrogate–virus relationships for ozone disinfection of wastewater. Environ. Eng. Sci. 2015;32:451–460. [Google Scholar]
- Sigstam T., Gannon G., Cascella M., Pecson B.M., Wigginton K.R., Kohn T. Subtle differences in virus composition affect disinfection kinetics and mechanisms. Appl. Environ. Microbiol. 2013;79:3455–3467. doi: 10.1128/AEM.00663-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonet J., Gantzer C. Inactivation of poliovirus 1 and F-specific RNA phages and degradation of their genomes by UV irradiation at 254 nanometers. Appl. Environ. Microbiol. 2006;72:7671–7677. doi: 10.1128/AEM.01106-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirikanchana K., Shisler J.L., Marinas B.J. Effect of exposure to UV-C irradiation and monochloramine on adenovirus serotype 2 early protein expression and DNA replication. Appl. Environ. Microbiol. 2008;74:3774–3782. doi: 10.1128/AEM.02049-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolova E., Petterson S.R., Dienus O., Nystrom F., Lindgren P.E., Pettersson T.J. Microbial risk assessment of drinking water based on hydrodynamic modelling of pathogen concentrations in source water. Sci. Total Environ. 2015;526:177–186. doi: 10.1016/j.scitotenv.2015.04.040. [DOI] [PubMed] [Google Scholar]
- Soller J.A., Eftim S.E., Nappier S.P. Comparison of predicted microbiological human health risks associated with de Facto, indirect, and direct potable water reuse. Environ. Sci. Technol. 2019;53:13382–13389. doi: 10.1021/acs.est.9b02002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart D.J., Napolitano M.J., Bakhmutova-Albert E.V., Margerum D.W. Kinetics and mechanisms of chlorine dioxide oxidation of tryptophan. Inorg. Chem. 2008;47:1639–1647. doi: 10.1021/ic701761p. [DOI] [PubMed] [Google Scholar]
- Stoica I.M., Vitzilaiou E., Lyng Røder H., Burmølle M., Thaysen D., Knøchel S., van den Berg F. Biofouling on RO-membranes used for water recovery in the dairy industry. Journal of Water Process Engineering. 2018;24:1–10. [Google Scholar]
- Sumikura M., Hidaka M., Murakami H., Nobutomo Y., Murakami T. Ozone micro-bubble disinfection method for wastewater reuse system. Water Sci. Technol. 2007;56:53–61. doi: 10.2166/wst.2007.556. [DOI] [PubMed] [Google Scholar]
- Suslick K.S., Mdleleni M.M., Ries J.T. Chemistry induced by hydrodynamic cavitation. J. Am. Chem. Soc. 1997;119:9303–9304. [Google Scholar]
- Tanneru C.T., Chellam S. Mechanisms of virus control during iron electrocoagulation--microfiltration of surface water. Water Res. 2012;46:2111–2120. doi: 10.1016/j.watres.2012.01.032. [DOI] [PubMed] [Google Scholar]
- Tanneru C.T., Rimer J.D., Chellam S. Sweep flocculation and adsorption of viruses on aluminum flocs during electrochemical treatment prior to surface water microfiltration. Environ. Sci. Technol. 2013;47:4612–4618. doi: 10.1021/es400291e. [DOI] [PubMed] [Google Scholar]
- Tao C.W., Hsu B.M., Kao P.M., Huang W.C., Hsu T.K., Ho Y.N., Lu Y.J., Fan C.W. Seasonal difference of human adenoviruses in a subtropical river basin based on 1-year monthly survey. Environ. Sci. Pollut. Res. Int. 2016;23:2928–2936. doi: 10.1007/s11356-015-5501-8. [DOI] [PubMed] [Google Scholar]
- Taylor M.B., Cox N., Vrey M.A., Grabow W.O. The occurrence of hepatitis A and astroviruses in selected river and dam waters in South Africa. Water Res. 2001;35:2653–2660. doi: 10.1016/s0043-1354(00)00551-0. [DOI] [PubMed] [Google Scholar]
- Teunis P.F., Xu M., Fleming K.K., Yang J., Moe C.L., Lechevallier M.W. Enteric virus infection risk from intrusion of sewage into a drinking water distribution network. Environ. Sci. Technol. 2010;44:8561–8566. doi: 10.1021/es101266k. [DOI] [PubMed] [Google Scholar]
- Thurston-Enriquez J.A., Haas C.N., Jacangelo J., Gerba C.P. Chlorine inactivation of adenovirus type 40 and feline calicivirus. Appl. Environ. Microbiol. 2003;69:3979–3985. doi: 10.1128/AEM.69.7.3979-3985.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston-Enriquez J.A., Haas C.N., Jacangelo J., Gerba C.P. Inactivation of enteric adenovirus and feline calicivirus by ozone. Water Res. 2005;39:3650–3656. doi: 10.1016/j.watres.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Thurston-Enriquez J.A., Haas C.N., Jacangelo J., Riley K., Gerba C.P. Inactivation of feline calicivirus and adenovirus type 40 by UV radiation. Appl. Environ. Microbiol. 2003;69:577–582. doi: 10.1128/AEM.69.1.577-582.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran T., Bolto B., Gray S., Hoang M., Ostarcevic E. An autopsy study of a fouled reverse osmosis membrane element used in a brackish water treatment plant. Water Res. 2007;41:3915–3923. doi: 10.1016/j.watres.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Tree J.A., Adams M.R., Lees D.N. Chlorination of indicator bacteria and viruses in primary sewage effluent. Appl. Environ. Microbiol. 2003;69:2038–2043. doi: 10.1128/AEM.69.4.2038-2043.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US EPA . 2005. National Service Center for Environmental Publications (NSCEP): Membrane Filtration Guidance Manual. [Google Scholar]
- US EPA . 2017. Drinking Water Requirements for States and Public Water Systems: Long Term 2 Enhanced Surface Water Treatment Rule Documents. [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinje J. Advances in laboratory methods for detection and typing of norovirus. J. Clin. Microbiol. 2015;53:373–381. doi: 10.1128/JCM.01535-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier J.C., Ehlers M.M., Grabow W.O.K. Detection of enteroviruses in treated drinking water. Water Res. 2004;38:2699–2705. doi: 10.1016/S0043-1354(01)00433-X. [DOI] [PubMed] [Google Scholar]
- Wigginton K.R., Pecson B.M., Sigstam T., Bosshard F., Kohn T. Virus inactivation mechanisms: impact of disinfectants on virus function and structural integrity. Environ. Sci. Technol. 2012;46:12069–12078. doi: 10.1021/es3029473. [DOI] [PubMed] [Google Scholar]
- Wolf C., von Gunten U., Kohn T. Kinetics of inactivation of waterborne enteric viruses by ozone. Environ. Sci. Technol. 2018;52:2170–2177. doi: 10.1021/acs.est.7b05111. [DOI] [PubMed] [Google Scholar]
- World Health Organization, Water, Sanitation and Hygiene (WASH).
- World Health Organization . 1996. Fact Sheets on Environmental Sanitation. [Google Scholar]
- World Health Organization . 2020. Water, Sanitation, Hygiene, and Waste Management for the COVID-19 Virus: Interim Guidance. [Google Scholar]
- Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., Yin H., Xiao Q., Tang Y., Qu X., Kuang L., Fang X., Mishra N., Lu J., Shan H., Jiang G., Huang X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. The Lancet Gastroenterology & Hepatology. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao R., Duan Y., Chu W. The effectiveness of household water treatment and safe storage in improving drinking water quality: a disinfection by-product (DBP) perspective. J. Water Supply Res. Technol. - Aqua. 2020;69:785–806. [Google Scholar]
- Xue B., Jin M., Yang D., Guo X., Chen Z., Shen Z., Wang X., Qiu Z., Wang J., Zhang B., Li J. Effects of chlorine and chlorine dioxide on human rotavirus infectivity and genome stability. Water Res. 2013;47:3329–3338. doi: 10.1016/j.watres.2013.03.025. [DOI] [PubMed] [Google Scholar]
- Yang S., Verdaguer-Casadevall A., Arnarson L., Silvioli L., Čolić V., Frydendal R., Rossmeisl J., Chorkendorff I., Stephens I.E.L. Toward the decentralized electrochemical production of H2O2: a focus on the catalysis. ACS Catal. 2018;8:4064–4081. [Google Scholar]
- Ye Y., Chang P.H., Hartert J., Wigginton K.R. Reactivity of enveloped virus genome, proteins, and lipids with free chlorine and UV254. Environ. Sci. Technol. 2018;52:7698–7708. doi: 10.1021/acs.est.8b00824. [DOI] [PubMed] [Google Scholar]
- Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal–oral transmission of SARS-CoV-2 possible? The Lancet Gastroenterology & Hepatology. 2020;5:335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Li Y., Shuai D., Shen Y., Wang D. Progress and challenges in photocatalytic disinfection of waterborne Viruses: a review to fill current knowledge gaps. Chem. Eng. J. 2019;355:399–415. [Google Scholar]
- Zhao Z., Zhang W., Lv X., Sun Y., Dong F., Zhang Y. Noble metal-free Bi nanoparticles supported on TiO2 with plasmon-enhanced visible light photocatalytic air purification. Environ. Sci.: Nano. 2016;3:1306–1317. [Google Scholar]
- Zhou X., Kong D.G., Li J., Pang B.B., Zhao Y., Zhou J.B., Zhang T., Xu J.Q., Kobayashi N., Wang Y.H. An outbreak of gastroenteritis associated with GII.17 norovirus-contaminated secondary water supply system in wuhan, China, 2017. Food Environ Virol. 2019;11:126–137. doi: 10.1007/s12560-019-09371-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B., Clifford D.A., Chellam S. Comparison of electrocoagulation and chemical coagulation pretreatment for enhanced virus removal using microfiltration membranes. Water Res. 2005;39:3098–3108. doi: 10.1016/j.watres.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Zhu B., Clifford D.A., Chellam S. Virus removal by iron coagulation-microfiltration. Water Res. 2005;39:5153–5161. doi: 10.1016/j.watres.2005.09.035. [DOI] [PubMed] [Google Scholar]
- Zupanc M., Pandur Z., Stepisnik Perdih T., Stopar D., Petkovsek M., Dular M. Effects of cavitation on different microorganisms: the current understanding of the mechanisms taking place behind the phenomenon. A review and proposals for further research. Ultrason. Sonochem. 2019;57:147–165. doi: 10.1016/j.ultsonch.2019.05.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.