Abstract
Mitochondria are the primary source of energy production in the brain thereby supporting most of its activity. However, mitochondria become inefficient and dysfunctional with age and to a greater extent in neurological disorders. Thus, mitochondria represent an emerging drug target for many age-associated neurological disorders. This review summarizes recent advances (covering from 2010 to May 2020) in the use of natural products from plant, animal, and microbial sources as potential neuroprotective agents to restore mitochondrial function. Natural products from diverse classes of chemical structures are discussed and organized according to their mechanism of action on mitochondria in terms of modulation of biogenesis, dynamics, bioenergetics, calcium homeostasis, and membrane potential, as well as inhibition of the oxytosis/ferroptosis pathway. This analysis emphasizes the significant value of natural products for mitochondrial pharmacology as well as the opportunities and challenges for the discovery and development of future neurotherapeutics.
Keywords: Natural products, mitochondrial dysfunction, oxytosis/ferroptosis, aging, neurological disorders, neuropharmacology, drug discovery
1. Introduction
Neurological disorders affect nearly 50 million people worldwide (WHO, 2017). However, there are no drugs for any of these conditions that are disease modifying in the sense that they slow down or revert the progression of the neuropathological process (Bellantuono, 2018; Gauthier, et al., 2016). Aging leads to progressive and detrimental changes in the brain and old age is the greatest risk factor for many neurological disorders such as Alzheimer’s (AD), Parkinson’s (PD), and Huntington’s diseases (HD) as well as stroke. Therefore, the aging process must be taken into account in order to understand the molecular and cellular basis of neurological disorders (Hou, et al., 2019; López-Otín, Blasco, Partridge, Serrano, & Kroemer, 2013). Many have argued that deficits in cerebral bioenergetics and metabolism associated with aging are central to the development of cognitive decline (Butterfield & Halliwell, 2019; Currais, 2015; Kapogiannis & Mattson, 2011; Schubert, 2005). Mitochondria are the primary source of energy in the brain, and their impairment has been implicated in the aging process and to a greater extent in neurological disorders (Chan, 2006; Nunnari & Suomalainen, 2012). However, relatively little progress has been made towards preventing mitochondrial dysfunction to promote cognition and brain health (Lin & Beal, 2006; Murphy & Hartley, 2018). It is therefore of crucial importance to investigate the detrimental changes that take place in mitochondria with aging as well as that are exacerbated by disease or injury and, based on this knowledge, to develop therapeutic interventions for neurological disorders.
Natural products, defined as small molecule compounds from natural sources such as plants, animals, and microorganisms, have been used for the treatment of human diseases for thousands of years and they have proven to be a valuable source of new drugs. According to the latest statistics of the US-FDA approved drugs, many prescription medicines in the clinic today are derived from natural products (Newman & Cragg, 2020). Over the past decade, the identification of natural products that target mitochondrial function has become an emerging field in drug discovery. An early review by Biasutto et al. drew attention to the mitochondrial effects of selected plant dietary compounds (Biasutto, Szabo’, & Zoratti, 2011). These natural products include potential therapeutics to stimulate mitochondrial biogenesis, modulate mitochondrial dynamics, improve mitochondrial bioenergetics and metabolism, sustain mitochondrial membrane potential and calcium homeostasis, and resolve imbalances in the mitochondrial redox status with aging. In the present review, we cover up-to-date research articles published within the last ten years on neuroprotective natural products from plant, animal, and microbial sources, with a specific focus on their reported bioactivities on mitochondria. In addition, some examples from our own research focused on natural product-based drug discovery for AD therapies are highlighted.
The literature search was performed with the SciFinder (Chemical Abstracts Service) and PubMed (National Institutes of Health) databases from January 2010 to May 2020, resulting in a total of 127 natural products spanning over 240 references on relevant topics. For clarity, the neuroprotective natural products are categorized into groups based on their structural classes and modes of action on mitochondria. Anticancer natural products that induce mitochondrial toxicity and apoptosis, as well as natural products that have no published reports for their positive effects on the nervous system were excluded from this review. In addition, biochemical/pharmacological investigations on natural product extracts whose bioactive chemical identities were not described were also excluded. Finally, we discuss our views on the potential use of these natural products in mitochondrial pharmacology and future drug development for the treatment of age-associated neurological disorders.
1.1. A key role for mitochondria in brain health
The human brain is a unique organ in that it consumes about 20% of the total oxygen and up to 25% of the total energy required by the body, yet it represents only 2% of the body mass (Bélanger, Allaman, & Magistretti, 2011; Schubert, 2005). The brain comprises a heterogeneous mixture of cell types including neurons, the main cell type responsible for neurotransmission, and glial cells (i.e., astrocytes, oligodendrocytes, and microglia), mostly involved in supporting brain functions. To fulfill the high energy demand required for synaptic transmission and other vital neuronal activities in the brain, neurons and glia rely on mitochondria, the “powerhouse” of the cell (Bélanger, et al., 2011; Harris, Jolivet, & Attwell, 2012).
Mitochondria are the primary source of adenosine triphosphate (ATP) which fuels energy-dependent biochemical reactions in the whole body, particularly in the brain. A majority of the ATP is produced by consuming glucose and oxygen via the Krebs cycle and oxidative phosphorylation (OXPHOS) within the brain mitochondria. Along with ATP biosynthesis, mitochondria play an essential role in numerous neuronal functions such as the maintenance of neuronal redox homeostasis, membrane potential and ion balance for synaptic signaling, exchange of neurotransmitters, as well as neuronal survival and death (Grimm & Eckert, 2017).
Owing to the multiple and crucial tasks that mitochondria execute in the central nervous system (CNS) to keep the brain functioning properly, mitochondrial dysfunction has been associated with almost every neurological disorder as well as with the aging process (Cunnane, et al., 2020; Lin & Beal, 2006). Although the underlying mechanisms by which mitochondrial dysfunction participates in such a wide range of age-related neurological disorders remain elusive, energy deficits with aging may contribute to neurodegeneration (Hou, et al., 2019; Lin & Beal, 2006) and ischemic stroke (Yousufuddin & Young, 2019), along with other neuropathological conditions, by disrupting neuronal activities in affected areas of the brain. Therefore, developing therapies to treat mitochondrial disturbances in age-associated neurological disorders is an attractive pursuit.
1.2. Mitochondrial dysfunction in age-associated neurological disorders
A growing body of preclinical and clinical evidence indicates that damage and dysregulation of mitochondrial structure and function in brain cells appear to be relevant to the pathogenesis of age-associated neurological disorders including AD, PD, HD, frontotemporal dementia (FTD), Lewy body dementia (LBD), amyotrophic lateral sclerosis (ALS), Friedreich’s ataxia (FRDA), as well as brain ischemia (Cunnane, et al., 2020; Lin & Beal, 2006; Yang, Mukda, & Chen, 2018). The high energetic activity of postmitotic neurons in the brain inevitably results in the progressive accumulation of toxic byproducts such as mitochondrial DNA (mtDNA) mutations, oxidative stress, and neurotoxic metabolites in mitochondria over time with aging (Terman, Kurz, Navratil, Arriaga, & Brunk, 2010). Therefore, neurons are believed to be more prone to mitochondrial pathologies (Grimm & Eckert, 2017). Since the incidence of many neurological disorders rises exponentially in the population above the age of 65 (WHO, 2017), it has been postulated that mitochondrial dysfunction could be one of the driving factors that contribute to the onset and progression of these disorders (Chan, 2006; Currais, 2015). Below, we discuss four common age-associated neurological disorders (AD, PD, HD, and stroke) and their associated mitochondrial pathology.
1.2.1. Alzheimer’s disease
With respect to clinical and post-mortem information in AD, it has been shown that the mitochondrial electron transport chain (ETC) complexes as well as the mitochondrial translocase of the outer membrane (TOM) and the translocase of the inner membrane (TIM) complexes show a significant reduction in activity, causing dysregulation of OXPHOS, ATP depletion, and accumulation of reactive oxygen species (ROS) in the brain tissues of AD patients (Devi, Prabhu, Galati, Avadhani, & Anandatheerthavarada, 2006; Pérez-Gracia, Torrejón-Escribano, & Ferrer, 2008; Reichmann, Fhirke, Hebenstreit, Schrubar, & Riederer, 1993). In addition, studies using in vitro and in vivo disease models have demonstrated a causative association between mitochondrial dysfunction and the disease. For instance, it has been shown that the toxic β-amyloid (Aβ) oligomers in neurons or amyloid deposits in transgenic AD mouse models can cause loss of the mitochondrial membrane potential (ΔΨm), increase ROS, trigger influx of mitochondrial Ca2+, lower ATP levels, and reduce the mitochondrial respiratory rate, which eventually results in memory loss and cognitive impairment (Behl, Davis, Lesley, & Schubert, 1994; Huang, et al., 2020; Perez Ortiz & Swerdlow, 2019).
1.2.2. Parkinson’s disease
Mitochondrial defects are well-known for their clinical relevance in PD and related parkinsonism (Langston, Ballard, Tetrud, & Irwin, 1983; Subramaniam & Chesselet, 2013). In cell and rodent models mimicking the pathological features of PD, neurotoxins like 6-hydroxydopamine (6-OHDA), 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), paraquat, and rotenone interfere with the mitochondrial ETC complexes, reduce ATP production, induce ROS production, and eventually lead to mitochondrial dysfunction and the irreversible damage of dopaminergic neurons (Blandini & Armentero, 2012; Dauer & Przedborski, 2003; Pienaar & Chinnery, 2013). These toxins achieve their neurotoxicity by distinct mechanisms due to their chemical variance, biological affinity, and cellular uptake pathways. For example, MPTP is first metabolized to 1-methyl-4-phenylpyridinium (MPP+) by monoamine oxidase B in astrocytes and then selectively transported by the dopamine transporter into dopaminergic neurons where MPP+ inhibits the mitochondrial complex I (Mizuno, Sone, & Saitoh, 1987). Rotenone targets mitochondria directly and is a systemic inhibitor of the mitochondrial complex I (Betarbet, et al., 2000). Paraquat enters the mitochondrial matrix driven by ΔΨm and is then reduced to monocation radicals by the mitochondrial complex I, where it subsequently triggers mitochondrial ROS production (Cochemé & Murphy, 2008). 6-OHDA is not a complex I inhibitor per se, and it primarily affects redox cycling and promotes ROS-induced neurotoxicity (Blum, et al., 2001). Regarding transgenic animal models of PD, the MitoPark mice were created by selective inactivation of mitochondrial transcription factor A (TFAM) in midbrain dopaminergic neurons (Ekstrand, et al., 2007). TFAM is a nuclear-encoded protein that translocates to mitochondria and controls vital parameters of mtDNA such as expression, copy number regulation or repair (Larsson, et al., 1998). The loss of TFAM in the MitoPark mice results in a dramatic disruption of the expression of genes encoded by mtDNA, such as cytochrome c oxidase, causing ETC deficiency. This model recapitulates key features of a sporadic PD-like phenotype in humans, including the progressive, age-dependent loss of dopaminergic neurons, particularly in the substantia nigra, and decline of motor function, along with the presence of intraneuronal inclusions such as mitochondrial protein and membrane components (Ekstrand & Galter, 2009; Ekstrand, et al., 2007). These inclusions in the mice do not contain α-synuclein and hence are different from the Lewy bodies observed in PD patients. However, recent findings on post-mortem PD brains have shown that Lewy bodies containing misfolded protein aggregates (i.e., α-synuclein) also include membranes from mitochondria (Shahmoradian, et al., 2019), suggesting a role for dysfunctional mitochondria in the formation of these inclusions and the progression of the disease in PD patients.
1.2.3. Huntington’s disease
Mitochondrial dysfunction also plays a role in the pathology of HD (Costa & Scorrano, 2012; Intihar, Martinez, & Gomez-Pastor, 2019). For instance, mitochondrial biogenesis has been shown to be compromised in HD. Transcriptional and protein levels of the peroxisome proliferator-activated receptor- γ coactivator 1α (PGC-1α), a key activator of mitochondrial biogenesis and respiration, are decreased in post-mortem samples from HD patients (Cui, et al., 2006; Johri, Chandra, & Flint Beal, 2013). Moreover, mutant huntingtin protein has the ability to trigger mitochondrial fission and a number of proteins controlling mitochondrial dynamics are altered in patients as well as in different models of HD (Costa, et al., 2010; Guo, et al., 2013; Shirendeb, et al., 2011; Song, et al., 2011). Finally, several studies reported impairment of mitophagy with huntingtin-induced neurotoxicity in both in vitro and in vivo models of HD (Franco-Iborra, et al., 2020; Guo, et al., 2016).
1.2.4. Brain ischemic/hemorrhagic stroke
Mitochondrial damage is one of the hallmarks in acute neurological disorders such as ischemic or hemorrhagic strokes (Campbell, et al., 2019; Yang, et al., 2018), where age is a major risk factor for the disorders (Yousufuddin & Young, 2019). Ischemic stroke deprives parts of the brain from glucose and oxygen while disturbing mitochondrial energetic and redox homeostasis and consequently leading to neuronal death (Campbell, et al., 2019; Yang, et al., 2018). In intracerebral hemorrhage, blood emerging from ruptured vessels causes immediate neuronal tissue destruction and secondary damage due to an excessive inflammatory response and an increase in ROS production (Qu, Chen, Hu, & Feng, 2016). It has been shown that exposure of neurons to hemoglobin can induce lipid peroxidation, free radical formation, and release of free iron, while causing necrotic, apoptotic and ferroptotic neuronal death (Zille, et al., 2017). In addition, defects in mitochondrial biogenesis and dynamics have been reported in rodent models of ischemic stroke associated with neuronal death (Ren, et al., 2019; Zhou, Wang, Li, Yu, & Zhao, 2018).
2. Natural product-based neurotherapeutics targeting mitochondrial dysfunction
As discussed above, extensive experimental and clinical investigations have revealed that aberrant brain alterations in mitochondria occur in neurological disorders. Thus, searching for neurotherapeutics that protect mitochondria in the CNS may be a promising approach for developing effective treatments (Andreux, Houtkooper, & Auwerx, 2013; Murphy & Hartley, 2018; Schubert, et al., 2018). Herein, we summarize the available literature from the past ten years on natural products from plant, animal, and microbial origins that have shown promising results for their neuroprotective effects on diverse aspects of mitochondrial biology.
2.1. Modulation of mitochondrial biogenesis
Mitochondrial biogenesis is a complex process that results in the generation of new mitochondria and involves over 150 proteins including transcription factors, enzymes, and receptors. Among them, PGC-1α, AMP-activated protein kinase (AMPK), and sirtuin-1 (SIRT1) are the three major cooperating players in mitochondrial biogenesis (Figure 1) (Cantó, et al., 2009; Herzig & Shaw, 2018; Lin, Handschin, & Spiegelman, 2005). PGC-1α is a positive regulator of mitochondrial biogenesis and metabolism. In mammalian cells, PGC-1α is activated sequentially by SIRT1 (deacetylation) and AMPK (phosphorylation). PGC-1α then binds to peroxisome proliferator-activated receptor- γ (PPARγ) and activates the nuclear respiratory factors (NRF-1 and NRF-2) to induce the expression of mitochondrial genes (e.g., proteins of the Krebs cycle and OXPHOS system). PGC-1α also up-regulates multiple nuclear-encoded proteins such as TFAM, DNA polymerase γ (POLG), Twinkle helicase, and the mitochondrial single stranded binding protein (mtSSB), which are responsible for the transcription, replication, and packaging of mtDNA in mitochondria (Scheibye-Knudsen, Fang, Croteau, Wilson, & Bohr, 2015) (Figure 1). Because mitochondrial biogenesis is impaired with aging and in associated neurological disorders (Whitaker, Corum, Beeson, & Schnellmann, 2016), identifying therapeutic compounds that induce mitochondrial biogenesis through modulation of the SIRT1-AMPK-PGC-1α pathway may be beneficial towards preventing pathology.
Figure 1.
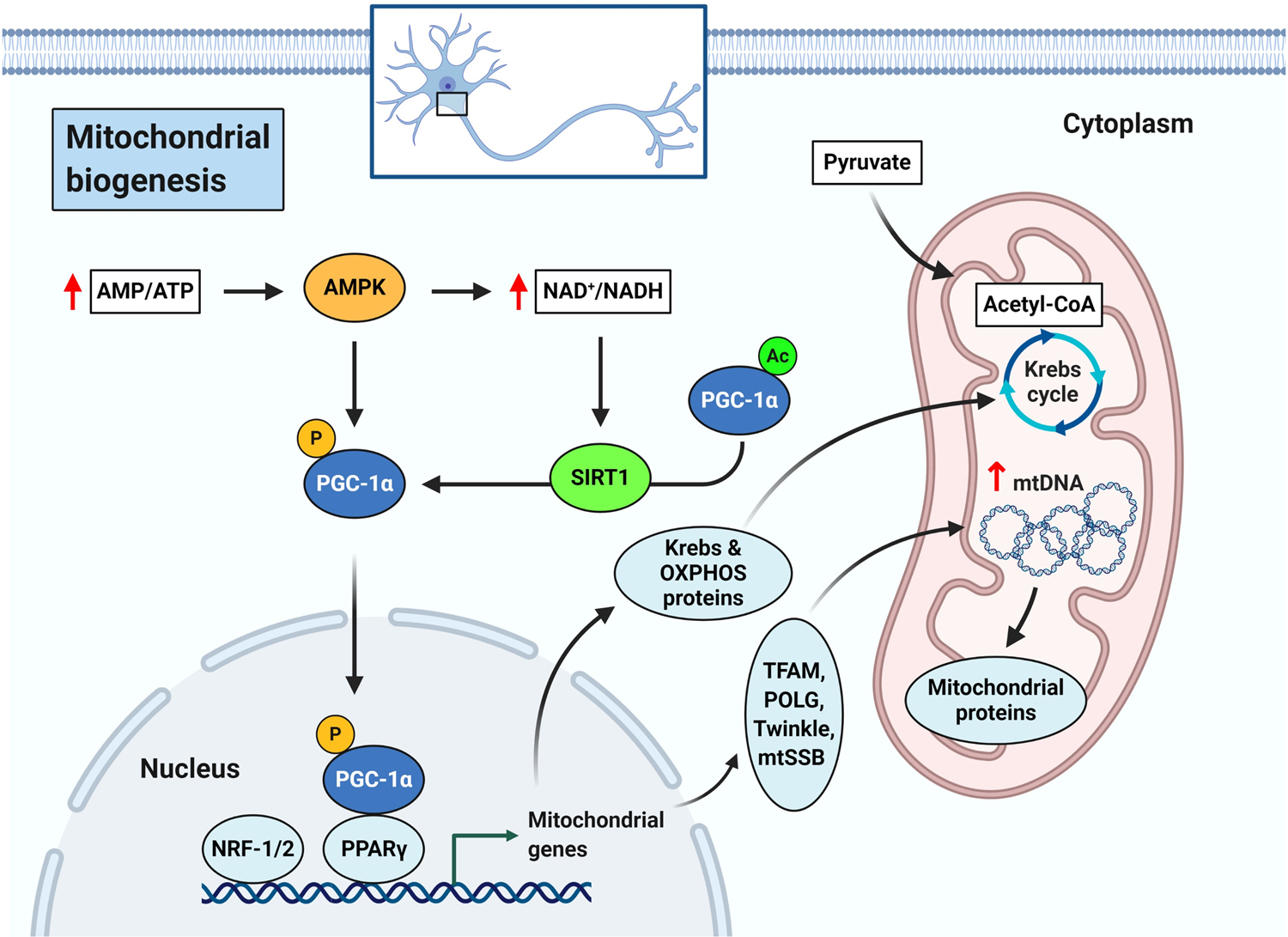
Schematic representation of mitochondrial biogenesis pathway.
Many natural products have been reported to modulate mitochondrial biogenesis and have shown efficacy in multiple in vitro and in vivo models of neuropathology (Table 1). One widely studied compound that has shown neuroprotection through modulation of mitochondrial biogenesis signaling is the polyphenol resveratrol from the berries of Vaccinium species and other plants. Resveratrol has been shown to stimulate mitochondrial biogenesis by activating the SIRT1-AMPK-PGC1-α axis in cell and animal models of AD (Porquet, et al., 2014), PD (Ferretta, et al., 2014), and Down’s syndrome (Valenti, et al., 2016). Resveratrol also prevented cerebral mtDNA deletion and cognitive impairment in the brains of senescence accelerated mice prone 8 (SAMP8), a model of age-associated dementia (Liu, Zhang, Yang, & He, 2012). The resveratrol dimer, ε-viniferin from Vitis vinifera, attenuated the neurotoxicity of the huntingtin protein and promoted mitochondrial biogenesis in cell models of HD (Fu, et al., 2012).
Table 1.
Neuroprotective natural products targeting mitochondrial dysfunction reported from January 2010 to May 2020
|
a Mode of action (MOA) on mitochondria | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compound name | Structural class | Biogenesis | Dynamics | Bioenergetics | ∆Ψm | [Ca2+]m | Oxytosis/ferroptosis | References |
| PlantSource | ||||||||
| Resveratrol | Stilbenoid | X | X | X | X | X | X | (Ferretta et al., 2014; Ishige et al., 2001; Kesherwani et al., 2013; Liu et al., 2012; Narayanan, Dave, Saul, & Perez-Pinzon, 2015; Peng, Tao, et al., 2016; Porquet et al., 2014; Valenti et al., 2016) |
| ε-Viniferin | Stilbenoid | X | X | (Fu et al., 2012) | ||||
| Artoindonesianin O | Stilbenoid | X | (Seo et al., 2014) | |||||
| Pterostilbene | Stilbenoid | X | (Wang, Liu et al., 2016) | |||||
| Curcumin | Diarylheptanoid | X | X | X | X | X | X | (Eckert et al., 2013; Liu et al., 2008; Liu et al., 2014; Rajasekar et al., 2013; Reddy et al., 2016; Seo et al., 2010; van der Merwe et al., 2017) |
| Tetrahydrocurcumin | Diarylheptanoid | X | (Vacek et al., 2018) | |||||
| Acerogenin A | Diarylheptanoid | X | (Lee et al., 2015) | |||||
| Juglanin C | Diarylheptanoid | X | (Yang et al., 2011) | |||||
| Genistein | Isoflavone | X | X | X | (Atlante et al., 2010; Rasbach & Schnellmann, 2008) | |||
| Daidzein | Isoflavone | X | X | X | (Atlante et al., 2010; Rasbach & Schnellmann, 2008) | |||
| Baicalein | Flavone | X | X | X | X | X | (Ishige et al., 2001; Li et al., 2012; Wang et al., 2017; Zhang et al., 2017) | |
| Luteolin | Flavone | X | X | X | (Ishige et al., 2001; Zhao, Yao-Yue, Qin, & Guo, 2012) | |||
| Nobiletin | Flavone | X | X | (Lee, Amarsanaa, et al., 2018) | ||||
| 7,8-Dihydroxyflavone | Flavone | X | (Chen, Chua, et al., 2011) | |||||
| Cudraflavone B | Flavone | X | (Lee et al., 2014) | |||||
| Quercetin | Flavonol | X | X | X | X | X | (Ay et al., 2017; Ishige et al., 2001; Liu et al., 2015) | |
| Fisetin | Flavonol | X | X | X | (Alikatte, Palle, Rajendra Kumar, & Pathakala, 2020; Ishige et al., 2001) | |||
| Galangin | Flavonol | X | (Ishige et al., 2001) | |||||
| Morin | Flavonol | X | (Ishige et al., 2001) | |||||
| Kaempferol | Flavonol | X | (Ishige et al., 2001) | |||||
| Myricetin | Flavonol | X | (Zhang et al., 2011) | |||||
| Epigallocatechin-3-gallate (EGCG) | Flavanol | X | X | X | X | X | X | (Chen et al., 2017; Chen et al., 2018; He et al., 2019; Valenti et al., 2013; Valenti et al., 2016) |
| Catechin/epicatechin | Flavanol | X | X | (Moreno-Ulloa et al., 2014) | ||||
| Theaflavic acid | Flavanol | X | (Li, Shi, et al., 2020) | |||||
| Procyanidin B2 3”-O-gallate | Flavanol | X | (Song et al., 2019) | |||||
| Procyanidin C1 | Flavanol | X | (Song, Lee, & Kang, 2019) | |||||
| Naringenin | Flavanone | X | X | (de Oliveira, Brasil, & Andrade, 2017) | ||||
| Sterubin | Flavanone | X | (Fischer et al., 2019; Maher, Fischer, et al., 2020) | |||||
| Liquiritigenin | Flavanone | X | X | (Jo et al., 2016; Yang, Park, & Song, 2013) | ||||
| Dihydromyricetin | Flavanonol | X | X | (Liu et al., 2016) | ||||
| Xanthohumol | Chalconoid (flavonoid) | X | (Wang, Ho, et al., 2020) | |||||
| Isoliquiritigenin | Chalconoid (flavonoid) | X | X | (Yang et al., 2012) | ||||
| Morachalcone A | Chalconoid (flavonoid) | X | (Wen et al., 2020) | |||||
| Morachalcone D | Chalconoid (flavonoid) | X | (Wen et al., 2020) | |||||
| Silibinin | Flavonolignan | X | X | X | (Geed et al., 2014; Schramm et al., 2018) | |||
| Isoorientin | Flavone glycoside | X | X | (Liang et al., 2016; Liang & Li, 2018; Ziqubu et al., 2020) | ||||
| Orientin | Flavone glycoside | X | (Yu, Wang, et al., 2015) | |||||
| Palhinoside D | Flavone glycoside | X | (Li et al., 2020) | |||||
| Puerarin | Isoflavone glycoside | X | X | (Zhu et al., 2016) | ||||
| Rutin | Flavonol glycoside | X | (Yu, Li, et al., 2015) | |||||
| Icariin | Flavonol glycoside | X | X | (Zeng et al., 2019; Zeng, Wang, et al., 2019) | ||||
| Isoquercitrin | Flavonol glycoside | X | (Carmona et al., 2020) | |||||
| Naringin | Flavanone glycoside | X | X | (Garabadu & Agrawal, 2020) | ||||
| Chrysanthemin | Anthocyanidin glycoside | X | (Ereminas et al., 2017) | |||||
| Mulberrofuran K | Lignan | X | (Xia et al., 2019) | |||||
| Pinoresinol | Lignan | X | (In et al., 2015) | |||||
| Rasidasin II | Neolignan | X | (Zhou, Yao, et al., 2018) | |||||
| Obovatol | Neolignan | X | X | (Yang, Lee, et al., 2013) | ||||
| Honokiol | Neolignan | X | X | (Yang, Lee, et al., 2013) | ||||
| Syringaresinol-4-O-β-D-glucopyranoside | Lignan glycoside | X | (Yang et al., 2020) | |||||
| Casuarinin | Ellagitannin | X | (Song et al., 2017) | |||||
| Coniferyl ferulate | Hydroxycinnamic acid | X | (Gong et al., 2020) | |||||
| Ferulic acid | Hydroxycinnamic acid | X | X | X | (Anis et al., 2020; Zafeer, Firdaus, Anis, & Mobarak Hossain, 2019) | |||
| Artepillin C | Hydroxycinnamic acid | X | (Takashima et al., 2019) | |||||
| Daphnetin | Coumarin | X | (Du et al., 2014) | |||||
| Auraptene | Terpenoid coumarin | X | (Jang et al., 2019) | |||||
| Hydroxytyrosol | Simple phenol | X | X | (Peng, Hou, et al., 2016; Zheng et al., 2015) | ||||
| Salidroside | Phenolic glycoside | X | X | (Barhwal et al., 2015) | ||||
| Gastrodin | Phenolic glycoside | X | X | (de Oliveira et al., 2018) | ||||
| α-Arbutin | Phenolic glycoside | X | (Ding et al., 2020) | |||||
| Butylphthalide | Polyketide | X | (Xiong et al., 2012) | |||||
| γ-Mangostin | Xanthonoid (polyketide) | X | (Wang, Li, et al., 2016) | |||||
| β-Lapachone | Naphthoquinone (polyketide) | X | (Lee, Ban, et al., 2018) | |||||
| Emodin | Anthraquinone (polyketide) | X | (Ahn et al., 2016) | |||||
| Chrysophanol | Anthraquinone (polyketide) | X | X | (Chae et al., 2017) | ||||
| Hyperforin | Polyketidic terpenoid | X | (Zolezzi et al., 2013) | |||||
| 5-Heptadecylresorcinol | Resorcinolic lipid (polyketide) | X | X | (Liu et al., 2020) | ||||
| ∆9-Tetahydrocannabinolic acid (∆9-THCA) |
Cannabinoid (polyketidic terpenoid) | X | (Nadal et al., 2017) | |||||
| ∆9-Tetrahydrocannabinol (THC) | Cannabinoid (polyketidic terpenoid) | X | X | X | (Schubert et al., 2019; Zeissler et al., 2016) | |||
| Cannabidiol (CBD) | Cannabinoid (polyketidic terpenoid) | X | X | (Schubert et al., 2019; Sun et al., 2017) | ||||
| Cannabinol (CBN) | Cannabinoid (polyketidic terpenoid) | X | (Schubert et al., 2019) | |||||
| Cannabichromene (CBC) | Cannabinoid (polyketidic terpenoid) | X | (Schubert et al., 2019) | |||||
| Linalool | Monoterpenoid | X | X | X | X | (Sabogal-Guáqueta et al., 2019) | ||
| Oleuropein | Iridoid glycoside (monoterpenoid) | X | X | (Kim et al., 2018; Park et al., 2017) | ||||
| Geniposide | Iridoid glycoside (monoterpenoid) | X | (Lv et al., 2014) | |||||
| Artemisinin | Sesquiterpenoid | X | X | (Lin et al., 2018; Yan et al., 2017) | ||||
| Daphne D | Sesquiterpenoid | X | (Wang, Liu et al., 2020) | |||||
| Linearol | Diterpenoid | X | X | X | (González-Burgos et al., 2016) | |||
| Sidol | Diterpenoid | X | X | X | (González-Burgos et al., 2016) | |||
| Andrographolide | Diterpenoid | X | (Yang & Song, 2014) | |||||
| Tanshinone I | Diterpenoid quinone | X | X | (de Oliveira, Schuck, & Bosco, 2017) | ||||
| Tanshinone IIa | Diterpenoid quinone | X | X | X | (Li et al., 2017) | |||
| Cryptotanshinone | Diterpenoid quinone | X | (Lee et al., 2020) | |||||
| Celastrol | Triterpenoid | X | (Deng et al., 2013) | |||||
| Asiatic acid | Triterpenoid | X | X | X | X | (Ding et al., 2018; Lee et al., 2012; Xu et al., 2012) | ||
| Tenuigenin | Triterpenoid | X | (Liang et al., 2011) | |||||
| Protopanaxadiol | Triterpenoid | X | X | (Bak et al., 2016) | ||||
| Glycyrrhizic acid | Triterpenoid saponin | X | X | (Rashedinia, Saberzadeh, Khosravi Bakhtiari, Hozhabri, & Arabsolghar, 2019) | ||||
| Ginsenoside Rb1 | Triterpenoid saponin | X | X | X | X | (Fernández-Moriano, González-Burgos, et al., 2017; Xu et al., 2019) | ||
| Ginsenoside Rg1 | Triterpenoid saponin | X | X | X | X | (Fernández-Moriano, González-Burgos, et al., 2017; Xu et al., 2019) | ||
| Bicelaphanol A | Trinorditerpenoid | X | (Wang et al., 2013) | |||||
| Astaxanthin | Carotenoid (tetraterpenoid) | X | (Lee et al., 2011) | |||||
| α-Tocopherol (vitamin E) | Terpenoid quinone | X | X | X | (Sanderson et al., 2015; Schubert, Kimura, & Maher, 1992) | |||
| Huperzine A | Sesquiterpenoid alkaloid | X | X | (Mao et al., 2016; Yang, Ye, et al., 2012) | ||||
| Tomatidine | Steroidal alkaloid (triterpenoid) | X | (Taveira et al., 2014) | |||||
| Berberine | Benzylisoquinoline alkaloid | X | X | (Yu et al., 2018) | ||||
| Neferine | Benzylisoquinoline alkaloid | X | X | (Wu et al., 2019) | ||||
| Fangchinoline | Benzylisoquinoline alkaloid | X | (Bao et al., 2019) | |||||
| Sanguinarine | Benzophenanthridine alkaloid | X | X | (Park et al., 2014) | ||||
| 3,3’-Diindolylmethane | Indole alkaloid | X | (Ito et al., 2017) | |||||
| Voacamine | Indole alkaloid | X | (Currais et al., 2014) | |||||
| Geissoschizine methyl ether | Indole alkaloid | X | X | (Sun et al., 2016) | ||||
| 3-Alkyl-1,4-benzoquinone | Fatty acid benzoquinone | X | (Madathil et al., 2012) | |||||
| Embelin | Fatty acid benzoquinone | X | X | X | (Rao et al., 2020) | |||
| AnimalSource | ||||||||
| Melatonin | Indole hormone | X | X | X | X | X | X | (Ansari Dezfouli et al., 2019; Carretero et al., 2009; Chuang et al., 2016; Herrera et al., 2007; Jou et al., 2010) |
| α-Lipoic acid | Fatty acid | X | X | X | (Jiang, Yin, Yao, Brinton, & Cadenas, 2013; Mehrotra et al., 2015) | |||
| 15-Deoxy- ∆12,14-prostaglandin J2 | Fatty acid | X | (Wappler et al., 2013) | |||||
| Progesterone | Steroid (triterpenoid) | X | (Grimm et al., 2014) | |||||
| Estradiol | Steroid (triterpenoid) | X | (Grimm et al., 2014) | |||||
| Allopregnanolone | Steroid (triterpenoid) | X | (Grimm et al., 2014; Wang, Yao, et al., 2020) | |||||
| Estrone | Steroid (triterpenoid) | X | (Grimm et al., 2014) | |||||
| Testosterone | Steroid (triterpenoid) | X | (Grimm et al., 2014) | |||||
| 3α-Androstanediol | Steroid (triterpenoid) | X | (Grimm et al., 2014) | |||||
| Dehydroepiandrosterone | Steroid (triterpenoid) | X | (Grimm et al., 2014) | |||||
| Ursodeoxycholic acid | Steroid (triterpenoid) | X | X | X | (Bell et al., 2018) | |||
| Ursocholanic acid | Steroid (triterpenoid) | X | X | (Mortiboys et al., 2013) | ||||
| Gracilin A | Norditerpenoid | X | (Leirós, Sánchez, et al., 2014) | |||||
| Makaluvamine J | Iminoquinone alkaloid | X | (Alonso et al., 2016) | |||||
| MicrobialSource | ||||||||
| Santacruzamate A | Peptide | X | X | (Chen, et al., 2019) | ||||
| Fumarprotocetraric acid | Depsidon (polyketide) | X | X | (Fernández-Moriano, Divakar, et al., 2017) | ||||
| Bikaverin | Naphthoquinone (polyketide) | X | (Nirmaladevi et al., 2014) | |||||
| Anhydroexfoliamycin | Naphthoquinone (polyketide) | X | (Leirós, Alonso, et al., 2014) | |||||
| Fusarubin | Naphthoquinone (polyketide) | X | (Choi et al., 2020) | |||||
| Evariquinone | Anthraquinone (polyketide) | X | (Song et al., 2018) | |||||
| Polyozellin | Terphenylquinone | X | (Yang & Song, 2015) | |||||
| Fischerin | Pyridone alkaloid | X | (Bang et al., 2019) | |||||
| Pontemazine B | Phenazine alkaloid | X | (Cha et al., 2015) | |||||
“X” denotes reported MOA on mitochondria.
Curcumin, a diarylheptanoid found in turmeric (Curcuma longa), has also shown promise for inducing mitochondrial biogenesis in several disease models. Curcumin stimulated the expression of the mitochondrial biogenesis genes PGC1-α, NRF-1, NRF-2, and TFAM in Aβ-treated human SH-SY5Y neuroblastoma cells (Reddy, et al., 2016). Another study showed that curcumin attenuated neuronal death and prevented cerebral ischemia/reperfusion injury with concomitant increases in mitochondrial mass and expression of the mitochondrial biogenesis regulators NRF-1 and TFAM in rat brains (Liu, et al., 2014).
A number of flavonoids belonging to different structural classes are potential simulators of mitochondrial biogenesis. The flavonol quercetin, found in many fruits and vegetables, was able to increase mitochondrial mass and mtDNA content in mouse dopaminergic MN9D cells as well as in the MitoPark mouse model of PD, where it stimulated expression of PGC-1α, TFAM, and cytochrome B that are responsible for mitochondrial biogenesis (Ay, et al., 2017). The flavanonol dihydromyricetin from Ampelopsis grossedentata has been shown to protect against neurodegeneration and memory impairment in rats subjected to cerebral hypoxia-ischemia, where it increased PGC-1α and TFAM expression in hippocampal neurons (Liu, et al., 2016). The flavone baicalein from Scutellaria baicalensis was found to stimulate the expression of PGC-1α, NRF-1, and TFAM in the substantia nigra of rotenone-treated PD rats and to improve their motor behavior (Zhang, et al., 2017). Epigallocatechin-3-gallate (EGCG), commonly found in green tea, is an ester of epigallocatechin and gallic acid. Research showed that EGCG promoted neuronal mitochondrial biogenesis by activating SIRT1/AMPK/PGC-1α signaling in hippocampal neural progenitor cells from the Ts65Dn mouse model of Down’s syndrome as well as in primary fibroblasts from Down’s syndrome patients (Valenti, et al., 2013).
Observations of induced mitochondrial biogenesis were also noted for plant-derived flavonoid glycosides. Isoorientin is a 6-C-glycosylflavone found in corn, passion flower, and Fenugreek seed. Isoorientin and its derivatives have been shown to protect from amyloid and tau toxicities in the SH-SY5Y neuronal cells and APP/PS1 mouse model of AD, where their neuroprotective mechanisms work in part due to the promotion of mitochondrial biogenesis (Liang, Zhang, Su, Williams, & Li, 2016; Ziqubu, et al., 2020). Icariin, a prenylated flavonol glucoside from Epimedium grandiflorum, protected against rotenone-induced loss of dopaminergic neurons in the rat substantia nigra through up-regulation of PGC-1α expression (Zeng, Wang, et al., 2019). Salidroside, a simple phenolic glucoside from Rhodiola rosea, has been shown to protect from hypoxia-induced neurodegeneration and memory impairment, where it was found to increase PGC-1α, AMPK and SIRT1 expression and mtDNA content in the rat hippocampus (Barhwal, Das, Kumar, Hota, & Srivastava, 2015).
β-Lapachone is a naphthoquinone that was isolated from the bark of the lapacho tree Tabebuia avellanedae. This compound prevented behavioral and cognitive impairments in the R6/2 mouse model of HD and enhanced mitochondrial biogenesis through up-regulation of SIRT1 and deacetylation of PGC-1α in neuronal stem cells in the R6/2 HD brain (Lee, Ban, Chung, Im, & Kim, 2018). Embelin is a naturally occurring p-benzoquinone isolated from berries and other fruits. A recent study showed that embelin induced mitochondrial biogenesis via activation of SIRT1 and protected from MPTP-induced neurotoxicity in cellular and mouse models of PD (Rao, Sharma, & Kalivendi, 2020).
Studies also showed that phytocannabinoids such as ∆9-tetrahydrocannabinol (Δ9-THC) from Cannabis sativa L. prevented cell death induced by MPP+ toxicity in the SH-SY5Y cell model of PD by stimulating the expression of PGC-1α, NRF-1, and TFAM, and increasing mtDNA content (Zeissler, et al., 2016). Δ9-Tetahydrocannabinolic acid (Δ9-THCA) was shown to rescue cell viability against mutant huntingtin protein induced toxicity in the STHdh and N2a neuronal cell models of HD, where it up-regulated the expression of PGC-1α and increased mitochondrial mass in cells (Nadal, et al., 2017).
In addition to phenolic compounds, plant-derived terpenoids were found to induce mitochondrial biogenesis. For example, the triterpenoid asiatic acid from Centella asiatica showed neuroprotective effects against glutamate, rotenone, and α-synuclein toxicities in SH-SY5Y cells, where it concomitantly stimulated the expression of PGC-1α and SIRT1 (Ding, et al., 2018; Xu, et al., 2012). The triterpenoid saponins such as ginsenosides Rb1 and Rg1 from Panax ginseng increased mtDNA content and mitochondrial function to protect from oxygen–glucose-deprivation/reperfusion (OGD/R)-induced injury, a stroke model, in mouse primary astrocytes (Xu, et al., 2019).
Alkaloids and related hormones were found to be potential stimulators of mitochondrial biogenesis as well. For instance, berberine, an isoquinoline alkaloid found in the plant Berberis species, can modulate the SIRT1/AMPK/PGC-1α pathway and improve cognitive function in aging rats (Yu, et al., 2018). Melatonin, an indole-type hormone (derived from tryptophan) mainly found in animals, has been shown to prevent amyloid neurotoxicity and memory loss in a rat AD model, where it promoted the expression of SIRT1 and TFAM in conjunction with increased mtDNA content in the hippocampus (Ansari Dezfouli, Zahmatkesh, Farahmandfar, & Khodagholi, 2019).
In summary, a large amount of evidence shows that natural products are able to promote mitochondrial biogenesis in a variety of cellular and animal models of neurological disorders (a complete compound list is found in Table 1). Most of these compounds belong to the classes of plant polyphenols, terpenoids, and alkaloids, suggesting their therapeutic potential for modulating age-associated mitochondrial disorders.
2.2. Modulation of mitochondrial fusion and fission dynamics
Mitochondria form a dynamic network with the ability to constantly elongate (fusion) and fragment (fission) within eukaryotic cells. Mitochondrial fusion/fission dynamics are crucial for the maintenance of mitochondrial homeostasis and resilience against cellular stresses. While mitochondrial fusion accelerates the exchange of mitochondrial materials (e.g., mtDNA, proteins, lipids) and assists in the repair of defective mitochondria, mitochondrial fission is important for the removal of damaged mitochondria through mitophagy (Figure 2) (Youle & van der Bliek, 2012). In mammalian cells, mitochondrial fusion is mainly controlled by proteins such as optic atrophy protein 1 (OPA1) and mitofusins (MFN1 and MFN2). In contrast, fission depends mainly on proteins like dynamin-related protein 1 (DRP1), mitochondrial fission 1 protein (FIS1), and mitochondrial fission factor (MFF). Defects in mitochondrial dynamics by disruption of either fusion or fission are known to occur with aging as well as in many neuropathological conditions. Specifically, decreased mitochondrial fusion and increased fission have been associated with the progression of neurodegenerative diseases (Itoh, Nakamura, Iijima, & Sesaki, 2013). Therefore, pharmacological interventions that maintain mitochondrial dynamics could be an approach for treating neurological disorders.
Figure 2.
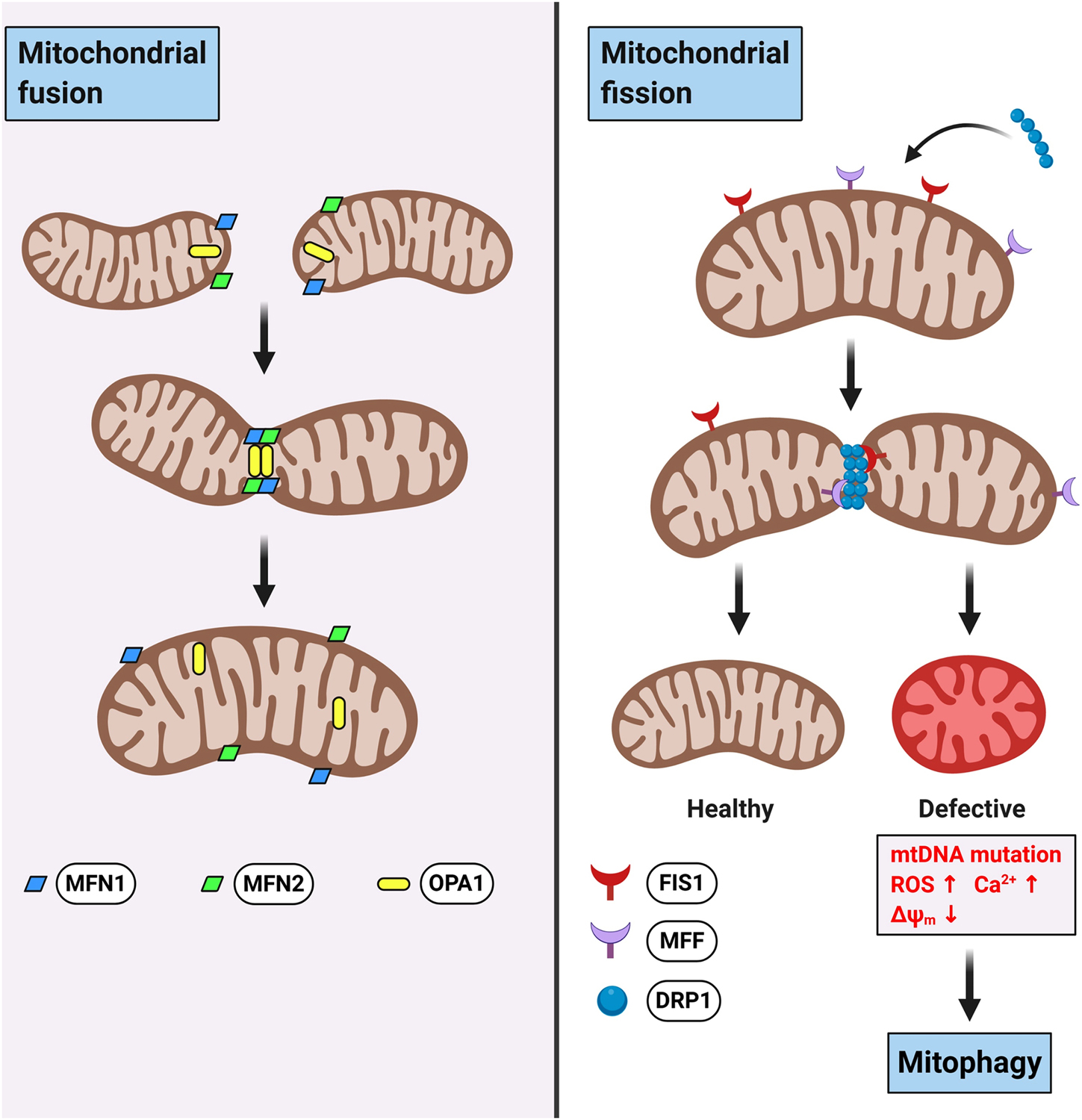
Schematic representation of mitochondrial fusion and fission dynamics.
Over the past few years, several natural products have been reported to modulate mitochondrial dynamics (Table 1). Resveratrol was shown to regulate mitochondrial fusion/fission dynamics through increasing the expression of MFN2 and OPA1 while decreasing the expression of DRP1 and FIS1 in the PD model of rotenone-induced dopamine neurotoxicity in rat brains (Peng, Tao, et al., 2016). Resveratrol also increased OPA1 and MFN2 expression to promote mitochondrial fusion in the hippocampus of SAMP8 mice, a model of dementia (Palomera-Avalos, et al., 2017).
Curcumin and its derivatives can also modulate mitochondrial dynamics for neuroprotection. It was found that curcumin can reduce mitochondrial fission by decreasing the expression of DRP1 and FIS1, and enhance fusion by increasing the expression of OPA1, MFN1 and MFN2 in the brains of SAMP8 mice (Eckert, et al., 2013). Tetrahydrocurcumin, a natural derivative of curcumin, was shown to regulate mitochondrial fusion/fission dynamics in mouse brain endothelial cells while protecting against homocysteine-induced oxidative stress and cell death (Vacek, et al., 2018).
Regarding flavonoids, the flavonol quercetin was found to regulate mitochondrial dynamics by inhibiting the expression of DRP1 and FIS1 and at the same time increasing the expression of MFN1 and MFN2 in the rat hippocampus, thereby improving hypoxia-induced memory deficits (Liu, et al., 2015). The flavanone liquiritigenin from Glycyrrhiza uralensis promoted mitochondrial fusion and prevented Aβ-induced neurotoxicity in human SK-N-MC nerve cells (Jo, et al., 2016). Xanthohumol is a prenylated chalconoid from the hop Humulus lupulus and a common ingredient in beers. Studies showed that xanthohumol alleviated kainic acid-induced excitotoxicity in the rat brain, a model of HD, in part by up-regulating MFN2 expression to promote mitochondrial fusion and prevent mitochondrial dysfunction (Wang, Ho, Hung, Kuo, & Wang, 2020). In a mouse model of subarachnoid hemorrhage, EGCG was found to protect mitochondrial function by down-regulating the expression of DRP1 and FIS1 in the brain (Chen, et al., 2018).
Ferulic acid, a hydroxycinnamic acid from Ferula foetida, was reported to reduce neuronal death in the striata of the 6-OHDA-lesioned rat model of PD, while inhibiting DRP1 and increasing MFN2 expression (Anis, et al., 2020). α-Tocopherol (vitamin E) is a terpenoid quinone rich in many vegetable oils that was found to preserve mitochondrial dynamics (increased mitochondrial OPA1) and protect against glutamate toxicity in the mouse HT22 hippocampal nerve cells (Sanderson, Raghunayakula, & Kumar, 2015).
Regarding natural products from animals, ursodeoxycholic acid, a bioactive steroid in bear bile, has been reported to modulate DRP1 and improve mitochondrial dynamics in fibroblasts from patients with sporadic and familial AD (Bell, et al., 2018). 15-Deoxy- ∆12,14-prostaglandin J2 is an endogenous fatty acid metabolite in humans that was found to stimulate mitochondrial remodeling against oxygen-glucose deprivation by regulation of DRP1 and OPA1 in primary neurons (Wappler, Institoris, Dutta, Katakam, & Busija, 2013). Melatonin was found to prevent DRP1-dependent mitochondrial fission induced by MPP+ in rat primary cortical neurons (Chuang, et al., 2016).
As for microbial sources, santacruzamate A, a peptide-like metabolite from the marine cyanobacterium Symploca sp., attenuated Aβ toxicity in PC12 cells and rescued cognitive impairment in APP/PS1 mice. Its neuroprotective mechanisms were shown to be in part due to inhibition of mitochondrial fission (decreases in DRP1, FIS1, and MFF) (Chen, et al., 2019).
Together, certain natural polyphenols, quinones, steroids, and peptidic metabolites have been shown to modulate mitochondrial fusion/fission dynamics while protecting brain cells in various models of neurological disorders (a complete compound list is found in Table 1).
2.3. Modulation of mitochondrial bioenergetics
Mitochondrial bioenergetics refers to the enzymatic and metabolic processes involved in the biochemical and molecular pathways of energy production and transformation within the mitochondrion. Bioenergetics and metabolic regulation are the primary functions of mitochondria. In mammalian cells, the metabolite pyruvate derived from glycolysis is oxidized to acetyl-coenzyme A (acetyl-CoA) that fuels the Krebs cycle in the mitochondrial matrix, powering up cellular respiration (Figure 1). NADH and FADH2 from the Krebs cycle are then oxidized to NAD and FAD, respectively, which are then used as substrates of the ETC to produce ATP via OXPHOS (Figure 3). The mitochondrial OXPHOS system comprises protein complexes I to IV and ATP synthase (complex V), where the electron movement is driven by the redox potential and the proton gradient across the mitochondrial inner membrane. The ATP molecules generated from mitochondrial respiration by ATP synthase are then exported to the cytosol to fuel a variety of vital cellular functions. During mitochondrial electron transfer, oxygen consumption yields inevitable byproducts such as the ROS superoxide radical (O2−) that is generated predominantly at the mitochondrial complexes I and III (Sipos, Tretter, & Adam-Vizi, 2003). As such, disruptions of the finely tuned process of mitochondrial respiration can cause an abnormal production of ROS thus contributing to multiple neuropathologies (Morán, et al., 2012).
Figure 3. Schematic representation of mitochondrial respiration, mitochondrial membrane potential, calcium uptake, ROS production, and related pathological pathways.
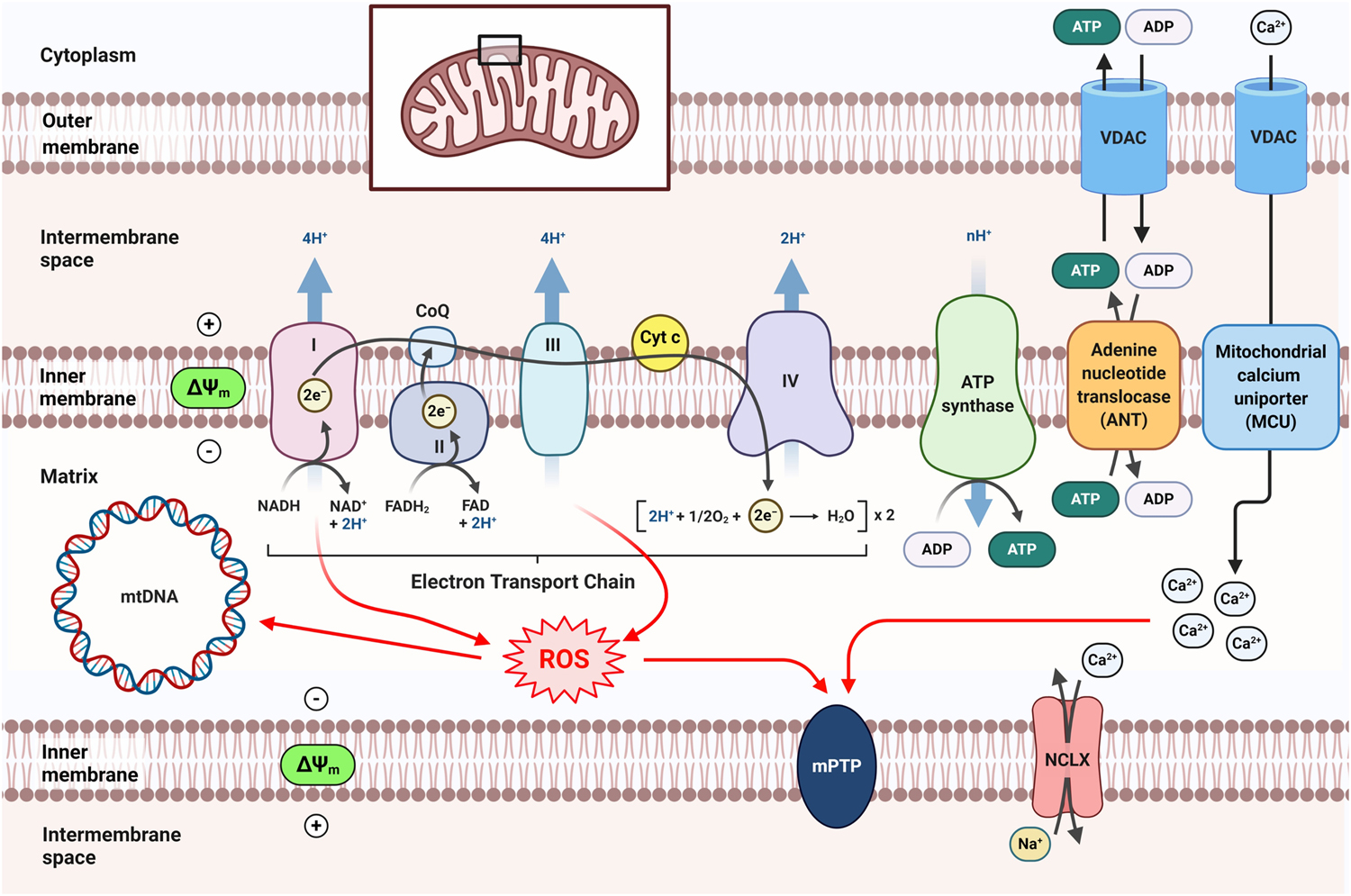
CoQ, coenzyme Q (ubiquinone); Cyt c, cytochrome c; NCLX, Na+/Ca2+ exchanger.
In recent years, many bioactive natural products have been reported to modulate mitochondrial bioenergetics and related pathways (Table 1). These effects were often associated with neuroprotection in multiple disease models. For example of flavonoids, fisetin, a flavonol rich in strawberries and other fruits, showed improvement of mitochondrial complex I activity in the brains of rotenone-treated rats, a model of PD (Alikatte, Palle, Rajendra Kumar, & Pathakala, 2020). The isoflavones genistein and daidzein, commonly found in soybeans, maintained mitochondrial respiration efficiency to prevent potassium deprivation-induced cell death in cerebellar granule cells (Atlante, Bobba, Paventi, Pizzuto, & Passarella, 2010). The flavone baicalein prevented rotenone-induced mitochondrial ATP deficiency in both PC12 cells and brain tissue (Li, et al., 2012). The citrus flavanone naringenin protected from H2O2-induced cell death in SH-SY5Y cells where it restored the activities of complexes I and V and increased ATP production (de Oliveira, Brasil, & Andrade, 2017). Silibinin, a flavonolignan from Silybum marianum L., modulated OXPHOS complex enzyme activity to maintain mitochondrial bioenergetics against MPP+ induced dopaminergic neurotoxicity in the rat brain (Geed, Garabadu, Ahmad, & Krishnamurthy, 2014).
Flavonoids with glycosidic groups are also known to promote mitochondrial bioenergetics under stress. Naringin, a flavanone glycoside in Citrus plants, showed neuroprotective effects against rotenone-induced neurotoxicity in a rat model of PD where it increased the activity of mitochondrial ETC complexes in the rat substantia nigra (Garabadu & Agrawal, 2020). The anthocyanidin glucoside chrysanthemin, from bilberry, was shown to restore the mitochondrial respiration rate in the presence of H2O2-induced oxidative stress in rat C6 glial cells (Ereminas, et al., 2017). Gastrodin, a simple phenolic glycoside in Gastrodia elata Blume, showed neuroprotection against H2O2-induced oxidative stress in SH-SY5Y cells where it increased mitochondrial respiration and ATP generation (de Oliveira, Brasil, & Fürstenau, 2018).
Some polyphenols like cannabidiol (CBD), derived from Cannabis sativa L., improved mitochondrial respiration and glucose metabolism thereby protecting against OGD/R damage in HT22 neural cells (Sun, Hu, Wu, & Zhang, 2017). 5-Heptadecylresorcinol, commonly found in cereals, was protective against H2O2-induced mitochondrial dysfunction in PC12 cells, where it enhanced mitochondrial respiration and ATP production (Liu, et al., 2020). Auraptene, a prenyloxycoumarin from Citrus spp., increased the oxygen consumption rate in response to MPP+ treatment in substantia nigra-derived SN4741 dopaminergic neuronal cells (Jang, et al., 2019). Hydroxytyrosol, a simple catechol derivative abundant in olive oil, showed beneficial effects by increasing the activities of mitochondrial OXPHOS complexes and ATP production in the brains of APP/PS1 transgenic mice (Peng, Hou, et al., 2016).
With respect to plant-derived terpenoids, the monoterpene linalool, commonly found in botanical essential oils, showed protective effects against glutamate toxicity, where it increased mitochondrial respiration in HT22 cells (Sabogal-Guáqueta, et al., 2019). Bicelaphanol A, a dimeric trinorditerpene from Celastrus orbiculatus, increased ATP production in mitochondria and protected from H2O2-induced mitochondrial stress in PC12 cells (Wang, et al., 2013). The diterpene quinones, tanshinone I and tanshinone IIa from Salvia miltiorrhiza Bunge (Danshen), protected mitochondrial function from paraquat and glutamate toxicity in human SH-SY5Y neural cells, where they preserved the activity of ETC complexes and ATP production (de Oliveira, Schuck, & Bosco, 2017; Li, et al., 2017).
With respect to alkaloids, huperzine A is a sesquiterpene alkaloid from Huperzia serrata that has been approved as a potent acetylcholinesterase (AChE) inhibitor for the treatment of AD in China. A study has shown that huperzine A, independent of its AChE inhibitory effect, ameliorated Aβ-induced impairments in ATP production and ETC complex enzyme activity in isolated cortical mitochondria from APP/PS1 transgenic mice. (Yang, Ye, Huang, Tang, & Zhang, 2012). Neferine, a bisbenzylisoquinoline alkaloid from Nelumbo nucifera, increased mitochondrial respiration and protected brain mitochondria in a rat model of ischemic stroke (Wu, et al., 2019). The indole hormone melatonin was shown to protect brain mitochondria from aging in SAMP8 mice by increasing the activities of ETC complexes and ATP production (Carretero, et al., 2009).
In addition to phytochemicals, animal-derived natural products, particularly steroids, were noted for their abilities to maintain mitochondrial bioenergetics in neuronal cells. Several human endogenous neurosteroids (i.e., progesterone, estradiol, allopregnanolone, estrone, testosterone, 3α-androstanediol, and dehydroepiandrosterone) have been shown to improve mitochondrial respiration and ATP production in SH-SY5Y cells as well as in primary cortical neurons (Grimm, Schmitt, Lang, Mensah-Nyagan, & Eckert, 2014), while restoring brain bioenergetic deficits in the 3xTg mouse model of AD (Wang, Yao, Chen, Mao, & Brinton, 2020). The bile acid steroids ursodeoxycholic acid and ursocholanic acid were found to improve mitochondrial respiration and ATP generation in fibroblasts from AD and PD patients (Bell, et al., 2018; Mortiboys, Aasly, & Bandmann, 2013). α-Lipoic acid, an organosulfur compound derived from fatty acids in animals and humans, was found to increase the activities of ETC complexes in the striatum of the 3-nitropropionic acid-induced HD rat model and ameliorate HD-like behavioral deficits (Mehrotra, Kanwal, Banerjee, & Sandhir, 2015).
Taken together, as summarized in Table 1, many plant-derived polyphenols, terpenoids and alkaloids appear to be promising modulators of mitochondrial respiration thereby contributing to neuroprotection. In addition, a subset of steroids from animal sources also have shown similar beneficial effects on mitochondria.
2.4. Modulation of mitochondrial calcium (Ca2+) homeostasis
Mitochondria, in coordination with the endoplasmic reticulum (ER), are crucial for the buffering and regulation of cellular calcium by sequestering and releasing the ions into the cytosol. Therefore, mitochondrial Ca2+ homeostasis is vital to a variety of cellular functions (Zündorf & Reiser, 2011). Driven by the mitochondrial membrane potential and transported by the mitochondrial ion channels (e.g., voltage-dependent anion channel, mitochondrial calcium uniporter, etc.) (Figure 3), Ca2+ ions enter the mitochondrial matrix, influencing processes such as respiration, ATP production, mitochondrial dynamics, and cell fate (Pathak & Trebak, 2018). Prolonged mitochondrial Ca2+ overload leads to increased ROS generation, ΔΨm dissipation, metabolic dysfunction, and induction of apoptosis/necrosis through the opening of the mitochondrial permeability transition pore (mPTP). It is evident that excessive mitochondrial Ca2+ influx is detrimental to the neuron and has been implicated in many pathophysiological processes such as those observed in neurodegeneration and ischemia (Maher, et al., 2018). As such, modulating mitochondrial Ca2+ uptake represents an emerging therapeutic strategy for several age-associated neurological disorders.
A handful of polyphenolic natural products have been reported to maintain mitochondrial Ca2+ homeostasis while conferring neuroprotection (Table 1). For instance, it was shown that resveratrol prevented Ca2+-induced mitochondrial swelling after hypoxic injury in rat brain neurons (Kesherwani, Atif, Yousuf, & Agrawal, 2013). Curcumin prevented okadaic acid-induced memory impairment in mice, where it reduced mitochondrial Ca2+ uptake in the hippocampus and cerebral cortex (Rajasekar, et al., 2013). Baicalein was shown to inhibit 6-OHDA/ascorbic acid induced mitochondrial Ca2+ accumulation and cell death in SH-SY5Y cells (Wang, et al., 2017). The hexamethoxyflavone nobiletin from the peel of Citrus sunki attenuated mitochondrial Ca2+ overload induced by glutamate in rat primary cortical neurons (Lee, Amarsanaa, et al., 2018). Fumarprotocetraric acid from the lichen fungus Cetraria islandica protected against H2O2-induced toxic mitochondrial Ca2+ uptake in both human SH-SY5Y neuroblastoma and U373-MG astrocytoma cells (Fernández-Moriano, Divakar, Crespo, & Gómez-Serranillos, 2017).
Terpenoids are also known to modulate mitochondrial Ca2+. The kaurane diterpenes linearol and sidol from Sideritis spp. prevented both mitochondrial and cytosolic Ca2+ overload induced by H2O2 in rat PC12 cells as well as human U373-MG cells (González-Burgos, Duarte, Carretero, Moreira, & Gómez-Serranillos, 2016). The triterpenoid ginsenosides Rb1 and Rg1 protected against rotenone-induced mitochondrial Ca2+ influx in SH-SY5Y cells (Fernández-Moriano, González-Burgos, Iglesias, Lozano, & Gómez-Serranillos, 2017). Hyperforin and its derivative from Hypericum perforatum are polyketidic terpenoids and were reported to be neuroprotective in AD models. They prevented memory loss and Aβ neurotoxicity in APP/PS1 mice, and counteracted mitochondrial Ca2+ overload in rat hippocampal neurons exposed to Aβ (Zolezzi, et al., 2013).
Overall, a limited number of natural products (Table 1) have been reported to specifically ameliorate mitochondrial Ca2+ overload in neurons and/or astrocytes as well as in brain tissue of disease models. Noticeably, a majority of them are highly lipophilic compounds, which may help them penetrate the mitochondrion to buffer Ca2+ intake.
2.5. Modulation of mitochondrial membrane potential (∆Ψm)
The mitochondrial membrane potential (ΔΨm) is generated by the OXPHOS proton pumps (complexes I, III and IV) across the mitochondrial inner membrane, thereby powering ATP production and ion transport (i.e., Ca2+) (Figure 3). Under physiological conditions, the levels of ΔΨm are relatively stable and play a critical role in mitochondrial homeostasis. However, dramatic or prolonged changes of ΔΨm are deleterious and could trigger mitochondrial damage and eventually cell death (Ward, et al., 2007; Zorova, et al., 2018). It has been observed that the ΔΨm levels decrease in many cellular and animal models of age-associated diseases (Nicholls, 2004), and researchers have identified neuroprotective natural products to counteract the loss of ΔΨm (Table 1).
Because of its mechanistic relevance, pharmacological modulation of ΔΨm usually affects other mitochondrial functions such as biogenesis, dynamics, bioenergetics, and Ca2+ flux, and vice versa (Figures 1–3). In many cases, changes in ΔΨm appear to be a pharmacological consequence of the compound actions and may or may not be necessarily responsible for their protective effects. Nonetheless, measurement of ΔΨm change is a useful mitochondrial parameter for characterizing the neuroprotective effects of a compound, particularly in phenotypic screening systems. To avoid redundancy in this section, we have chosen to focus on the natural products that have not been previously discussed and where the literature has shown that the restoration of ΔΨm is correlated to their protective effects. A complete summary of the natural products that were reported to modulate ΔΨm can be found in Table 1.
Flavonoids have been reported to be able to maintain ΔΨm against a variety of neuronal stressors. For example, the flavonol myricetin from many fruits and vegetables attenuated ΔΨm loss and cell death induced by MPP+ in the MES23.5 dopaminergic cell model (Zhang, Ma, Wang, Xie, & Xie, 2011). The flavanol theaflavic acid from black tea prevented ΔΨm loss and cell damage from OGD/R in PC12 cells (Li, Shi, et al., 2020). Rutin, a flavonol glycoside, ameliorated ΔΨm loss and amylin-induced neurotoxicity in SH-SY5Y cells, which is relevant to type 2 diabetes associated with AD (Yu, Li, et al., 2015). Orientin, an 8-C-glycosylflavone commonly found in the bamboo Phyllostachys nigra attenuated ΔΨm depolarization in the hippocampus of Aβ-induced mice while improving cognitive function (Yu, Wang, et al., 2015).
Other polyphenolic compounds such as rasidasin II, a lignan from red raspberry, attenuated H2O2-induced ΔΨm loss in SH-SY5Y cells (Zhou, Yao, et al., 2018). α-Arbutin, a phenolic glycoside from bearberry, was found to be protective against rotenone-induced ΔΨm impairment in SH-SY5Y cells as well as in a fruit fry model of PD (Ding, et al., 2020). Coniferyl ferulate from the root of Angelica sinensis inhibited ΔΨm depolarization during glutamate-induced toxicity in PC12 cells (Gong, Zhou, Gong, & Qin, 2020).
A number of terpenoids have been reported to be effective modulators of ΔΨm. For example, the sesquiterpene artemisinin, a well-known antimalarial drug isolated from the sweet wormwood Artemisia annua, prevented H2O2-induced ΔΨm loss in a cellular model of age-related neurodegeneration using RGC-5 cells (Yan, Wang, Gao, Xu, & Zheng, 2017). Geniposide, an iridoid monoterpene glycoside from Gardenia jasminoides, prevented ΔΨm loss in isolated brain mitochondria from APP/PS1 mice (Lv, Liu, Liu, Chen, & Zhang, 2014). The triterpenoid celastrol from Tripterygium wilfordii was reported to alleviate ΔΨm depolarization caused by rotenone-induced mitochondrial dysfunction in SH-SY5Y cells (Deng, Shi, Liu, & Qu, 2013). Gracilin A is a unique norditerpenoid from the marine sponge Spongionella sp., and a study has shown that it could prevent H2O2-induced oxidative stress and restore ΔΨm in cortical neurons (Leirós, Sánchez, et al., 2014). Astaxanthin, a keto-carotenoid (known as a tetraterpenoid) from the freshwater microalgae Haematococcus pluvialis, was able to prevent MPP+-induced ΔΨm loss and cytotoxicity in SH-SY5Y cells (Lee, Kim, & Lee, 2011).
Some natural quinones and alkaloids are also known to preserve ΔΨm in neurons. For instance, the diterpenoid quinone cryptotanshinone restored ΔΨm in human-induced neuronal progenitor cells derived from fibroblasts of familial PD patients (Lee, et al., 2020). A nitrogen-containing 3-alkyl-1,4-benzoquinone from the plant Embelia ribes maintained ΔΨm and mitochondrial function against severe oxidative stress in primary FRDA fibroblasts (Madathil, Khdour, Jaruvangsanti, & Hecht, 2012). The naphthoquinone anhydroexfoliamycin from Streptomyces spp. also protected against H2O2-induced ΔΨm loss in mouse primary cortical neurons (Leirós, Alonso, et al., 2014). The pyrroloiminoquinone alkaloid makaluvamine J from marine Zyzzya sponges showed protective effects against H2O2-induced ΔΨm loss in SH-SY5Y cells (Alonso, et al., 2016). 3,3’-Diindolylmethane is an indole alkaloid found in many cruciferous vegetables and it has been shown to counteract the ΔΨm loss and neuronal death induced by MPP+ in SH-SY5Y cells (Ito, et al., 2017). The steroidal alkaloid tomatidine from the tomato plant Lycopersicon esculentum preserved ΔΨm against glutamate-induced toxicity in SH-SY5Y cells (Taveira, et al., 2014).
In summary, flavonoids, terpenoids, quinones, and alkaloids from various sources are neuroprotective and have shown their potential to maintain ΔΨm in neuronal models of disease.
2.6. Neuroprotection against mitochondrial oxidative stress in oxytosis/ferroptosis
Given the postmitotic characteristic of neurons in the adult brain, neurodegeneration with aging leads to a progressive course of neuronal cell death in a way that can hardly be regenerated. Although brain atrophy is a hallmark of many CNS disorders, it is known that many forms of neuronal death are non-apoptotic as they do not involve DNA fragmentation and activation of the caspase cascade in neurons (Berghe, Linkermann, Jouan-Lanhouet, Walczak, & Vandenabeele, 2014). The molecular mechanisms executing regulated cell death that differ from apoptosis are still poorly defined (Fricker, Tolkovsky, Borutaite, Coleman, & Brown, 2018).
Over 20 years ago, our laboratory identified oxytosis, a non-apoptotic, regulated cell death pathway that is due to glutathione (GSH) depletion triggered by inhibition of the cystine/glutamate antiporter, system Xc−, and mediated by the dysregulated production of ROS from mitochondria that results in lethal lipid peroxidation and calcium influx (Figure 4) (Li, Maher, & Schubert, 1997; Tan, Sagara, Liu, Maher, & Schubert, 1998; Tan, Schubert, & Maher, 2001). Importantly, we have also shown that the oxytosis pathway is essentially identical to the more recently named ferroptosis pathway (Dixon, et al., 2012) that is triggered by inhibition of either system Xc− or glutathione peroxidase 4 (GPX4) and is associated with accumulation of intracellular iron (Fe2+) (Figure 4) (Lewerenz, Ates, Methner, Conrad, & Maher, 2018; Stockwell, et al., 2017). GSH is the major endogenous antioxidant, and a reduction in GSH is observed in the aging brain and is accelerated in neurodegenerative diseases including AD and PD (Currais & Maher, 2013; Mandal, Saharan, Tripathi, & Murari, 2015; Smeyne & Smeyne, 2013). GSH dysregulation in mitochondria is also observed in many metabolic and neurological disorders (Gao, et al., 2019; Ribas, García-Ruiz, & Fernández-Checa, 2014; Stockwell, et al., 2017). Therefore, oxytosis/ferroptosis is a unique regulated cell death pathway with characteristics of mitochondrial oxidative stress and dysfunction (Maher, Currais, & Schubert, 2020). In the past decade, our group has identified specific inhibitors that block the oxytosis/ferroptosis pathway, which is an evolving therapeutic strategy for age-associated neurological diseases (Maher, Currais, et al., 2020).
Figure 4. Schematic representation of the oxytosis/ferroptosis pathway.
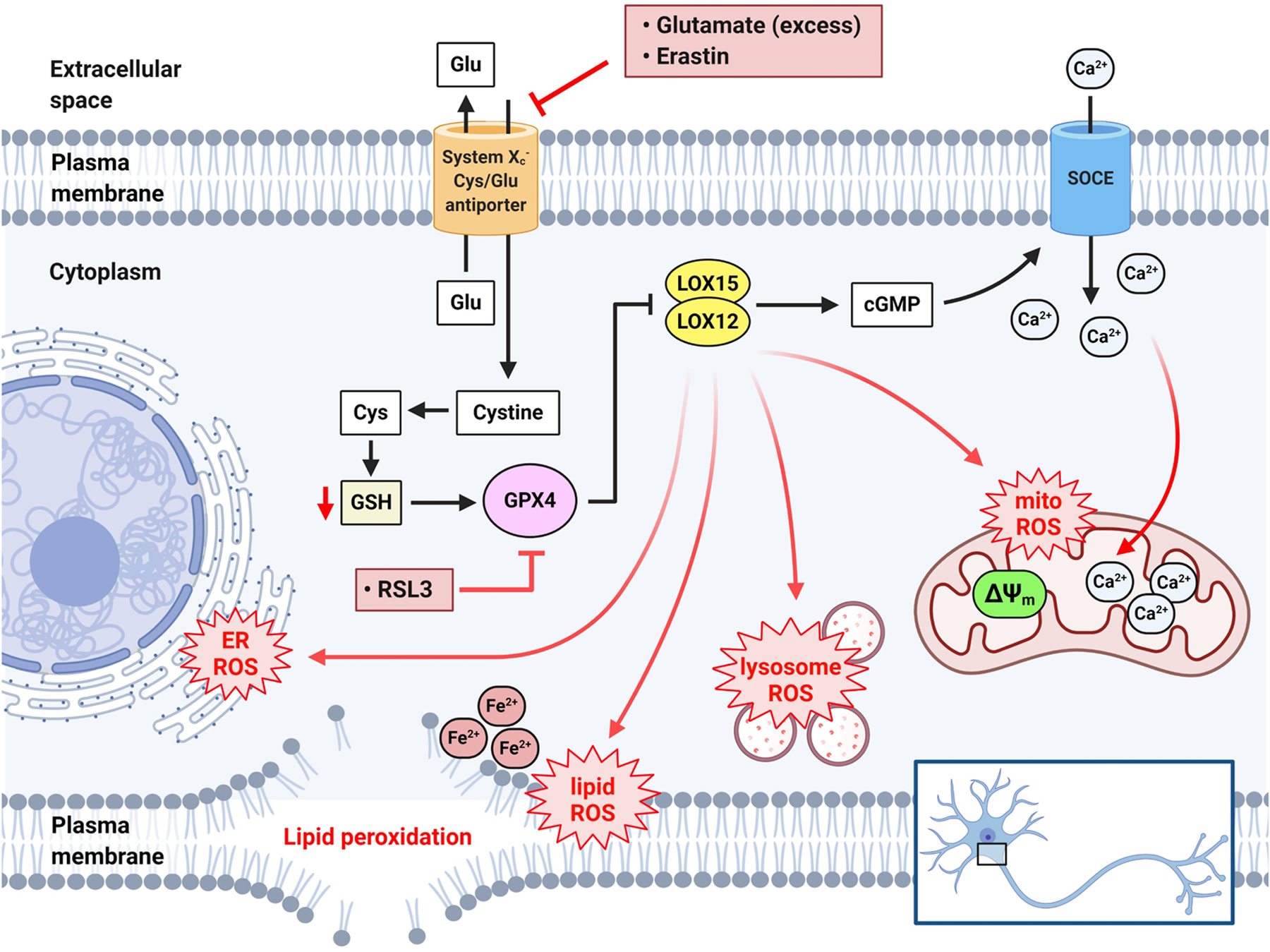
Cystine uptake by system Xc−, associated with the counter-transport of glutamate (Glu), is inhibited by excess Glu or erastin. This leads to depletion of the endogenous antioxidant GSH and subsequent inhibition of the GSH-dependent enzyme GPX4. GPX4 can also be directly inhibited by RSL3. GPX4 inhibition leads to activation of lipoxygenase (LOX) to initiate ROS production and increase cGMP. cGMP then activates SOCE on the plasma membrane allowing Ca2+ influx into the cytosol and subsequent accumulation in mitochondria. The additive effect of mitochondrial ROS and Ca2+ overload exacerbates mitochondrial oxidative stress and dysfunction. GPX4 inhibition and LOX activation, in conjunction with intracellular Fe2+, also lead to lipid peroxidation (lipid ROS) in different cellular compartments such as mitochondria, ER, lysosome, and plasma membrane, thereby augmenting the overall ROS in the cell.
In early studies using glutamate-induced toxicity in mouse HT22 hippocampal neurons and primary cortical neurons as models of oxytosis/ferroptosis, we screened and identified several promising natural products including curcumin, resveratrol, α-tocopherol (vitamin E), and specific flavonoids (i.e., galangin, baicalein, kaempferol, luteolin, fisetin, quercetin, morin) that are highly neuroprotective (Ishige, Schubert, & Sagara, 2001; Schubert, Kimura, & Maher, 1992). As described above, curcumin from the curry spice turmeric is a multifunctional natural product that modulates various aspects of mitochondrial function (Table 1). Curcumin protected against glutamate-induced toxicity with an EC50 value of 6 μM (Liu, Dargusch, Maher, & Schubert, 2008). Further optimization of curcumin through structure-activity relationship driven medicinal chemistry led to development of J147 (EC50 = 11 nM), a compound that enhances memory and is highly neuroprotective in cell and mouse models of AD (Chen, Prior, et al., 2011; Currais, et al., 2015; Prior, Dargusch, Ehren, Chiruta, & Schubert, 2013). Importantly, we have recently demonstrated that J147 targets the mitochondrial ATP synthase and modulates its activity (Goldberg, et al., 2018). J147 is currently in Phase 1 clinical trials for AD (NCT03838185). Another example is fisetin, which was identified in our screens as a protective flavonol against oxytosis/ferroptosis with an EC50 value of 3 μM (Ishige, et al., 2001; Maher, Akaishi, & Abe, 2006). Pharmacological studies of fisetin showed its therapeutic benefits in preclinical models of AD, PD, HD, traumatic brain injury, stroke, as well as aging (Maher, 2020). We later developed CMS121, a synthetic derivative of fisetin, with improved potency (EC50 = 200 nM) against oxytosis/ferroptosis as well as other improved pharmacological properties for the treatment of AD (Ates, Goldberg, Currais, & Maher, 2020; Chiruta, Schubert, Dargusch, & Maher, 2012). CMS121 is currently in Investigational New Drug (IND) studies. Interestingly, both J147 and CMS121 protect cultured nerve cells from oxytosis/ferroptosis induced by either glutamate (an inhibitor of system Xc−) or RSL3 (an inhibitor of GPX4), and they both have an overlapping mechanism of action that is associated with strong anti-aging effects by increasing mitochondrial acetyl-CoA and activating AMPK in the brains of SAMP8 mice (Currais, et al., 2019).
More recently, additional anti-oxytotic/ferroptotic natural products have been identified by our group. The flavanone sterubin from the plant Yerba santa (Eriodictyon californicum) showed effective neuroprotection against oxytosis/ferroptosis (EC50 = 0.8 μM) (Fischer, Currais, Liang, Pinto, & Maher, 2019; Maher, Fischer, et al., 2020). The flavonol isoquercitrin showed neuroprotection against glutamate toxicity with an EC50 value of 25 μM as well as anti-amyloidogenic effects (Carmona, Martín-Aragón, Goldberg, Schubert, & Bermejo-Bescós, 2020). The dimeric indole alkaloid voacamine from Voacanga africana protected HT22 cells against glutamate toxicity with an EC50 value of 0.7 μM (Currais, et al., 2014). Several phytocannabinoids such as tetrahydrocannabinol (THC), cannabidiol (CBD), cannabinol (CBN), and cannabichromene (CBC) from the Cannabis plant also showed promising protective effects against oxytosis, ATP depletion, and Aβ toxicity with potencies in the sub-micromolar range (EC50 < 1 μM) (Schubert, et al., 2019).
Other research laboratories have also reported on a number of natural products with diverse chemical structures that prevent neuronal death in cellular models using system Xc− inhibitors (i.e., glutamate and erastin) as inducers of oxytosis/ferroptosis (Figure 4). For instance, flavonoids such as 7,8-dihydroxyflavone from Tridax procumbens (Chen, Chua, et al., 2011), cudraflavone B from Cudrania tricuspidata (Lee, Ko, Kim, Kim, & Jeong, 2014), liquiritigenin from Glycyrrhiza uralensis (Yang, Park, & Song, 2013), morachalcones from mulberry leaves (Wen, et al., 2020), EGCG from green tea (He, Xu, Yang, & Sun, 2019), and procyanidins from grape seeds (Song, Lee, & Kang, 2019), have all been shown to protect against glutamate/erastin-induced mitochondrial oxidative injury and nerve cell death in HT22 cells. In addition, puerarin, an 8-C-glycosylisoflavone from Pueraria lobate, protected against glutamate toxicity in SH-SY5Y cells (Zhu, et al., 2016).
Other plant-derived polyphenols with simple or complex structures have also been found to be protective against glutamate-induced oxytosis/ferroptosis in HT22 cells. These include the stilbenoids pterostilbene (Wang, Liu, et al., 2016) and artoindonesianin O (Seo, et al., 2014), the diarylheptanoids juglanin C (Yang, Sung, Kim, & Kim, 2011) and acerogenin A (Lee, Cha, Woo, Kim, & Jang, 2015), the lignans mulberrofuran K (Xia, et al., 2019) and pinoresinol (In, et al., 2015), the neolignans obovatol and honokiol (Yang, Lee, Park, Lee, & Song, 2013), the anthraquinones emodin (Ahn, et al., 2016) and chrysophanol (Chae, et al., 2017), the prenylcinnamate artepillin C (Takashima, Ichihara, & Hirata, 2019), the xanthone γ-mangostin (Wang, Li, et al., 2016), the coumarin daphnetin (Du, et al., 2014), and the ellagitannin casuarinin (Song, Kang, & Choi, 2017).
Plant-derived terpenoids such as the monoterpenoid oleuropein (Kim, et al., 2018), the sesquiterpene artemisinin (Lin, Li, Winters, Liu, & Yang, 2018), and the diterpenoid andrographolide (Yang & Song, 2014) were reported to show neuroprotective effects against glutamate-induced oxytosis in HT22 cells. The triterpenoids protopanaxadiol (Bak, et al., 2016) and asiatic acid (Xu, et al., 2012) protected against glutamate-induced cytotoxicity in PC12 and SH-SY5Y cells.
Moreover, several plant-derived alkaloids such as huperzine A (Mao, Zhou, Li, & Liu, 2016), geissoschizine methyl ether (Sun, Ren, Qi, Yuan, & Simpkins, 2016), sanguinarine (Park, et al., 2014), and fangchinoline (Bao, Tao, & Zhang, 2019) also protected against glutamate-induced mitochondrial dysfunction in HT22 cells.
Besides, certain fungus-derived polyketidic metabolites were reported to be anti-oxytotic/ferroptotic in HT22 cells. These include polyozellin from the mushroom Polyozellus multiplex (Yang & Song, 2015), evariquinone from Colletotrichum sp. (Song, et al., 2018), and fusarubin from Fusarium solani (Choi, et al., 2020). In addition, the phenazine alkaloid pontemazine B from the bacterium Streptomyces sp. also protected from glutamate toxicity in HT22 cells (Cha, et al., 2015).
As summarized in Table 1, in recent years a growing number of natural products have been reported to prevent nerve cell death caused by oxytosis/ferroptosis, a process that is associated with mitochondrial oxidative stress and dysfunction. Most of them belong to the polyphenols, terpenoids, alkaloids, and polyketidic quinones. Impressively, some of these anti-oxytotic/ferroptotic natural products are either the same or appear to share common structural characteristics with the aforementioned neuroprotective compounds that modulate mitochondrial biogenesis, fusion/fission dynamics, bioenergetics, calcium uptake, etc.
3. Conclusions and perspectives
Human neurological disorders arise from complex and multifaceted pathological mechanisms. Although the etiology of the individual disorders may differ, there is a consensus towards mitochondrial inefficiency or dysfunction in the brain with aging as being a key pathological process shared by these disorders (Currais, 2015; Grimm & Eckert, 2017). In recent years, novel therapeutic approaches to the use of small molecule drugs to target mitochondria in the CNS have emerged (Andreux, et al., 2013; Cunnane, et al., 2020; Schubert, et al., 2018).
Natural products are a valuable source of drug candidates (Newman & Cragg, 2020). As summarized in Table 1, a total of 127 natural products covering a diverse array of structural classes have been reported over the past ten years to be efficacious in mitigating mitochondrial dysfunction and promoting cell survival in different in vitro and in vivo models of neurological disorders. More importantly, a number of these compounds, as exemplified by the cases of resveratrol, curcumin, baicalein, quercetin, EGCG and melatonin, are able to modulate at least four out of five mitochondrial functions (biogenesis, fusion/fission dynamics, bioenergetics, calcium homeostasis, and membrane potential), as well as prevent mitochondrial oxidative stress-induced nerve cell death in the form of oxytosis/ferroptosis (Figure 5). Interestingly, if one looks at the biological sources of these compounds in Figure 6A, 104 out of 127 compounds (about 82%) are derived from plants, while only 14 (11%) and 9 (7%) compounds are derived from animals and microbes, respectively, suggesting an increased potential of natural phytochemicals to target mitochondria for CNS drug discovery.
Figure 5.
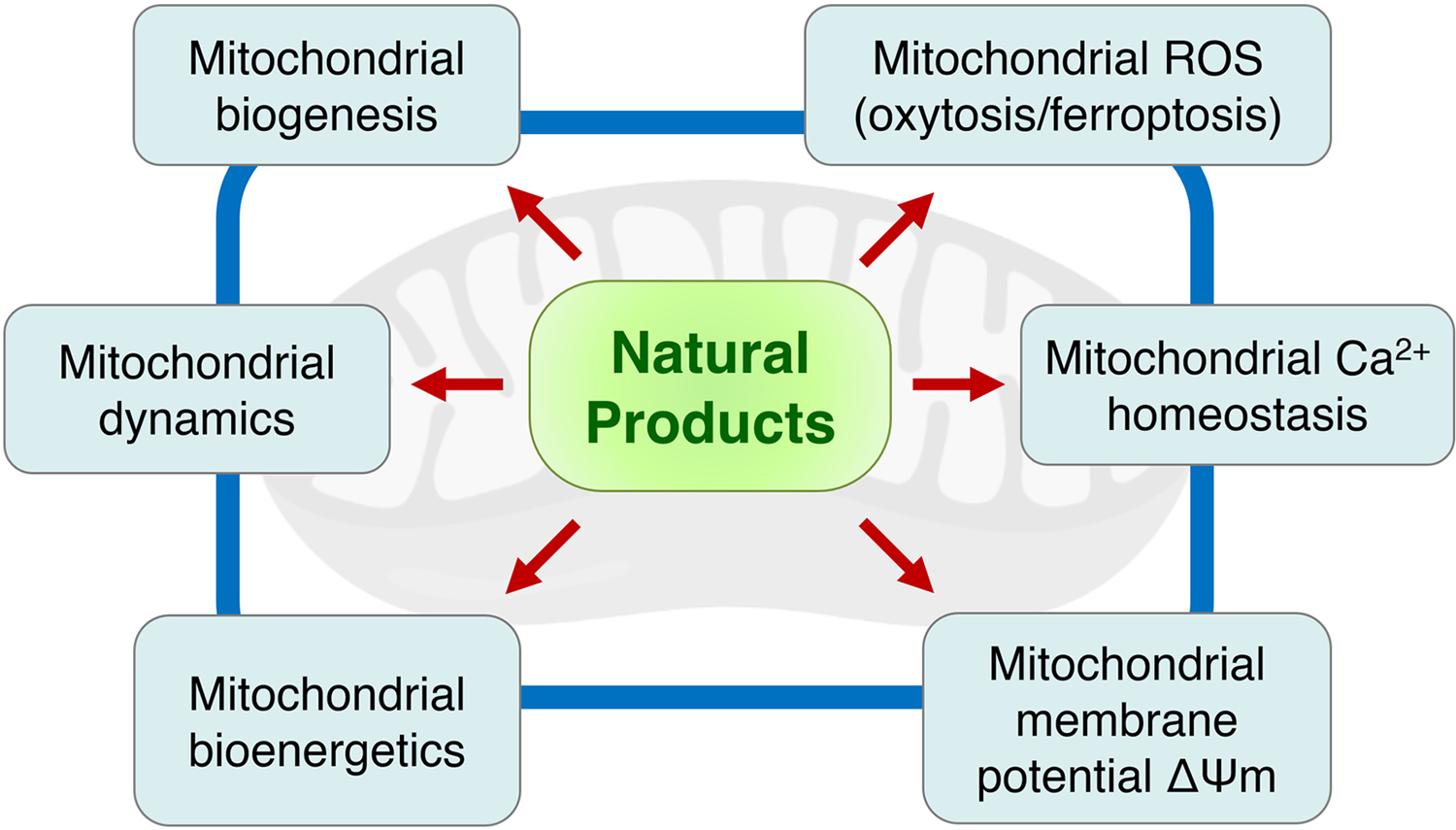
The effects of natural products on multiple mitochondrial functions associated with neuroprotection.
Figure 6. Natural products targeting mitochondrial dysfunction reported from January 2010 to May 2020, n = 127.
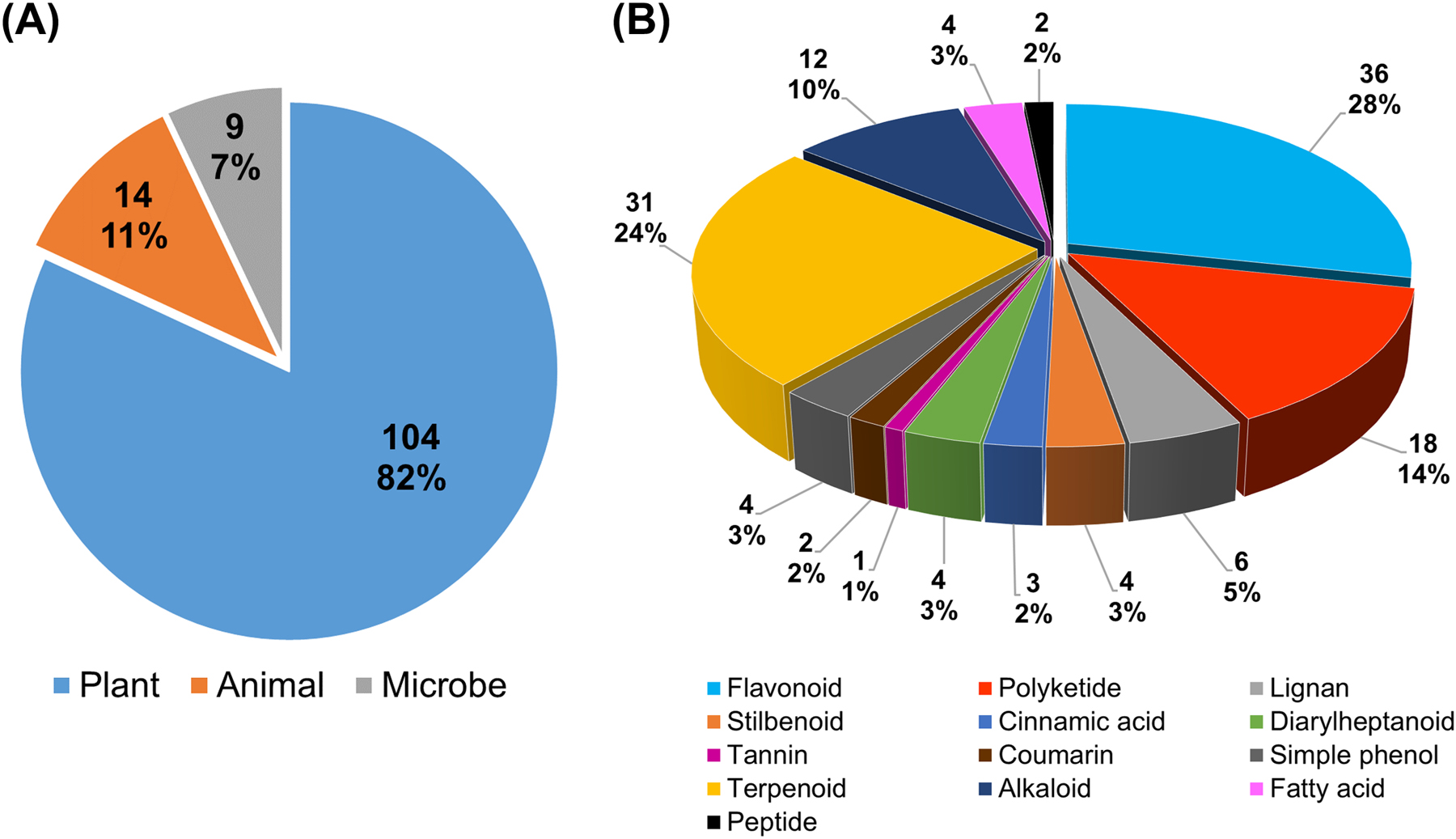
(A) Pie chart of natural products by biological source. (B) Pie chart of natural products by structural class.
Regarding the structural classification of these natural products, the breakdown shown in the pie chart in Figure 6B indicates that flavonoids (i.e., flavone, flavonol, flavanol, flavanone, flavanonol, anthocyanidin, isoflavone, isoflavane, and chalcone as flavonoid precursor) are a predominant group, accounting for 36 compounds (28%). The second largest group is terpenoids (i.e., monoterpene, sesquiterpene, diterpene, triterpene, trinorditerpenoid, steroid, and carotenoid) that account for 31 compounds (24%). It is likely that the inherent lipophilic nature of certain flavonoids and terpenoids makes them more able to cross the cell and mitochondrial membranes and subsequently modulate mitochondrial function to achieve neuroprotection. Polyketides and alkaloids are the third and fourth groups that account for 14% and 10%, respectively. The remaining groups belong to stilbenoids (e.g., resveratrol) (3%), diarylheptanoids (e.g., curcumin) (3%), cinnamic acids (2%), lignans (5%), tannins (1%), coumarins (2%), simple phenols (3%), fatty acids (3%), and peptides (2%).
Noticeably, 90 out of the 127 natural products (71%) are polyphenolic and simple phenolic compounds (a sum of flavonoids, polyketides, stilbenoids, diarylheptanoids, cinnamic acids, lignans, tannins, coumarins, simple phenols, and certain terpenoids/alkaloids with phenolic groups). This is not surprising because phenolic natural products are well-known antioxidants for ROS scavenging and exert at least part of their function by blocking and alleviating the surge of cytotoxic byproducts from cellular respiration and ATP generation in the aging mitochondria. In addition, these natural antioxidants are effective and pleiotropic modulators of mitochondrial biogenesis and dynamics, calcium homeostasis and membrane potential, as well as inhibitors of oxytosis/ferroptosis (Table 1 and Figure 5). Notably, certain bioactivities of the phenolic compounds, such as mitochondrial biogenesis and dynamics, are not directly associated with antioxidant capacity. These modes of action could play critical roles in neuroprotection apart from antioxidant activity. Therefore, our analyses offer curated literature evidence to support the use of phenolic natural products to modulate mitochondrial pathology in age-associated neurological disorders. One point worthy of discussion is that most of the bioassays on mitochondrial function discussed in this review are based on cell culture and resulted in the identification of neuroprotective phenolic compounds. Thus, it underscores the merits of phenotypic screening that can offer an opportunity to better realize the therapeutic value of phenolic natural products (Prior, et al., 2014).
Our observations also provide input to the controversial debate of whether polyphenolic natural products should be regarded as worthwhile for drug discovery and development, given that they are considered as pan assay interference compounds (PAINS) in single protein-based assays (Baell, 2016). With respect to the view from medicinal chemists, the main criticisms of PAINS (e.g., curcumin, resveratrol, flavonoids, quinones, and other polyphenols) are due to their reactive and promiscuous binding properties against a wide variety of proteins during high-throughput screening, thereby generating artifacts and false positives (Baell & Walters, 2014). Because one of the objectives of such target-based approaches is to achieve high specificity/selectivity of drug action against a single target of relevance, the PAINS appear to mislead, waste research efforts and increase costs for lead optimization in downstream drug discovery. However, from the standpoint of biologists, this does not apply in phenotypic drug screening using cellular and animal models that primarily focus on drug efficacy in living biological systems and deal with “unknown/uncertain protein targets” (Prior, et al., 2014; Schubert & Maher, 2012). This is particularly true for CNS drug discovery for which our knowledge of basic biology in neurosciences is still very limited. In fact, to date, all AD drug candidates based on presumed protein targets relevant to the disease (i.e., amyloid, tau, etc.) have failed in human clinical trials (Cummings, Lee, Ritter, Sabbagh, & Zhong, 2020). Paradoxically, the targets and modes of action of some commonly used CNS drugs (e.g., lithium, modafinil, and valproate) remain poorly defined (Lewis, 2016). Owing to an urgent need for effective drugs to treat neurodegenerative and other neurological disorders, the priority in CNS drug discovery should be to identify drug candidates that show pharmacological efficacy in model organisms and later to test them rigorously in human patients in clinical settings. Therefore, the antagonism towards PAINS may be unjustified. In fact, the majority of the natural products discussed throughout this review show positive effects on mitochondria and are PAINS. Moreover, the three mitochondria-targeted agents, MitoQ (NCT03514875), S-equol (NCT02142777) and J147 (NCT03838185) currently in clinical trials for mild cognitive impairment (MCI) and AD, are derivatives of a quinone, an isoflavonoid, and curcumin, respectively. The intrinsic biophysics and biochemistry of mitochondria such as their double membrane system, ion/proton gradients, ETC, redox cycles, etc. might actually favor therapeutic interventions with polyphenolic natural products for the treatment of neurological disorders. In principle, a balanced approach to a CNS drug pipeline that incorporates both phenotypic- and target-based strategies should be embraced (Schubert & Maher, 2012; Swinney & Anthony, 2011), and diverse chemotypes including PAINS that show neuroprotective effects in drug screening should be investigated (Rodrigues, Reker, Schneider, & Schneider, 2016).
Although much progress has been made towards identifying novel agents/drug leads from natural sources to target mitochondria, there are still significant challenges towards developing effective neurotherapeutics. Lead compounds derived from natural products usually contain a greater number of stereogenic centers, steric effects, heteroatoms, and polycyclic systems compared to synthetic molecules, which are deemed as liabilities because they are hard to modify and usually contradict the Lipinski’s “rule-of-five” dogma describing druglikeness for small molecules (Lipinski, 2004). Other major concerns regarding polyphenolic natural products for CNS therapeutic application include their poor oral bioavailability and lower extent of blood-brain barrier (BBB) permeability in vivo (Hu, Wu, & Liu, 2017). It hence raises a question about whether the concentrations of polyphenolic natural products eliciting their in vitro effects could be achievable in vivo. Indeed, pharmacokinetic studies have shown that polyphenolics are metabolized easily after oral administration and the concentrations of their intact form in serum and in brain (from nanomolar to low micromolar range) often do not meet the requisite for in vitro effects observed in cell culture (from sub-micromolar to micromolar range). However, an apparent in vivo efficacy of the compounds is still observed in animal models of CNS disorders (Ines, Regina, Diana, Ines, & Claudia Nunes dos, 2017). It is known that the first-pass metabolism involving phase II conjugation (i.e., glucuronidation and sulfation) of polyphenolics with concerted actions of efflux transporters (i.e., permeability glycoprotein) causes their poor oral bioavailability and BBB penetration, and facilitates metabolic clearance (Shuai, et al., 2016; Wu, Kulkarni, Basu, Zhang, & Hu, 2011). Thus, it reduces the concentrations of polyphenolic compounds in the CNS for target engagement. However, glucuronidation and sulfation are known to be reversible in vivo, resulting in an equilibrium between the parent molecule and its conjugated metabolites distributed throughout the vascular and nervous systems (Andrade, Grosso, Valentao, & Bernardo, 2016). And the levels of individual conjugated metabolites (e.g., resveratrol metabolites) could be over ten times higher than those of the parent molecule in serum (Baur & Sinclair, 2006). In fact, increasing evidence indicates that metabolic conjugates along with the parent polyphenolics can be detected in the brain and that these conjugates are still bioactive (Ishisaka, Mukai, Terao, Shibata, & Kawai, 2014; Pogačnik, et al., 2016). Moreover, some glycosidic and glucuronidated phenolics bind to glucose transporters (GLUTs), sodium glucose co-transporters (SGLT1/2) and other carrier-mediated transporters, which enables their distribution across the intestinal barrier via transepithelial transport (Jesus, et al., 2017; Lies, Martens, Schmidt, Boll, & Wenzel, 2012), and across the BBB via secondary active transport (Andrade, et al., 2016; Patching, 2017). These data suggest that polyphenolic natural products in intact and/or metabolically modified forms may still be able to enter into the brain and exert therapeutic actions on mitochondria in the CNS.
Notwithstanding, future studies must emphasize lead optimization for potency/selectivity, physicochemical properties, ADME-Tox properties, bioavailability and BBB penetration of candidate neuroprotective natural compounds towards a better CNS druglikeness (Wager, Hou, Verhoest, & Villalobos, 2016). In addition, rational and strategic drug design by integrating chemical scaffolds and pharmacophores from polyphenols, terpenoids and alkaloids might add precision medicine to specifically target aspects of mitochondrial physiology in the CNS (Rodrigues, et al., 2016). This will require the collaboration of natural product/medicinal chemists, mitochondrial biologists, neuroscientists, and pharmacologists in drug discovery programs. Furthermore, a better understanding of the different molecular pathways underlying neurological disorders relevant to mitochondrial dysfunction will help scientists to develop novel classes of natural product-inspired therapeutics for healthy brain aging and other age-associated disorders.
In conclusion, over the past ten years there has been an increase in the development of new strategies based on natural products to target mitochondrial dysfunction in neurological disorders. We hope that this review will lead readers to appreciate the importance of neuroprotective agents from Nature and offer new insights into mitochondrial pharmacology and CNS drug discovery towards the improvement of human health.
Acknowledgments
The work was supported in part by the Paul F. Glenn Center for Biology of Aging Research at the Salk Institute Fellowship Award (ZL), an Innovation Award from the Salk Institute (AC), the Shiley-Marcos Alzheimer’s Disease Research Center at the University of California, San Diego (AC), the Edward N. & Della Thome Memorial Foundation (PM), and the National Institutes of Health grants R01AG046153, RF1AG054714, R21AG064287, and R41AI104034 (PM and DS).
Abbreviations:
- AD
Alzheimer’s disease
- PD
Parkinson’s disease
- HD
Huntington’s disease
- FDA
Food and Drug Administration
- CNS
central nervous system
- mtDNA
mitochondrial deoxyribonucleic acid
- ETC
electron transport chain
- OXPHOS
oxidative phosphorylation
- ROS
reactive oxygen species
- ΔΨm
mitochondrial membrane potential
- [Ca2+]m
mitochondrial calcium
- mPTP
mitochondrial permeability transition pore
- PGC-1α
peroxisome proliferator-activated receptor- γ coactivator 1α
- PPARγ
peroxisome proliferator-activated receptor- γ
- AMPK
adenosine monophosphate-activated protein kinase
- SIRT
sirtuin
- NRF
nuclear respiratory factor
- TFAM
mitochondrial transcription factor A
- OPA1
optic atrophy protein 1
- MFN
mitofusin
- DRP1
dynamin-related protein 1
- FIS1
mitochondrial fission 1 protein
- MFF
mitochondrial fission factor
- LOX
lipoxygenase
- GSH
glutathione
- GPX4
glutathione peroxidase 4
- VDAC
voltage-dependent anion channel
- SOCE
store-operated calcium entry
- NAD
nicotinamide adenine dinucleotide
- FAD
flavin adenine dinucleotide
- Glu
glutamate
- Cys
cysteine
- AMP
adenosine monophosphate
- ADP
adenosine diphosphate
- ATP
adenosine triphosphate
- cGMP
cyclic guanosine monophosphate
- Aβ
β-amyloid
- 6-OHDA
6-hydroxydopamine
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MPP+
1-methyl-4-phenylpyridinium
- OGD/R
oxygen–glucose-deprivation/reperfusion
- EGCG
epigallocatechin-3-gallate
- SAMP8
senescence accelerated mice prone 8
- MOA
mode of action
- PAINS
pan assay interference compounds
- ADME-Tox
absorption, distribution, metabolism, excretion and toxicity
- BBB
blood-brain barrier
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The Salk Institute holds the patents for J147 and CMS121. The authors declare that there are no other conflicts of interest.
References
- Ahn SM, Kim HN, Kim YR, Choi YW, Kim CM, Shin HK, & Choi BT (2016). Emodin from Polygonum multiflorum ameliorates oxidative toxicity in HT22 cells and deficits in photothrombotic ischemia. Journal of Ethnopharmacology, 188, 13–20. [DOI] [PubMed] [Google Scholar]
- Alikatte K, Palle S, Rajendra Kumar J, & Pathakala N (2020). Fisetin improved rotenone-induced behavioral deficits, oxidative changes, and mitochondrial dysfunctions in rat model of Parkinson’s disease. Journal of Dietary Supplements, 1–15. [DOI] [PubMed]
- Alonso E, Alvariño R, Leirós M, Tabudravu JN, Feussner K, Dam MA, Rateb ME, Jaspars M, & Botana LM (2016). Evaluation of the antioxidant activity of the marine pyrroloiminoquinone makaluvamines. Marine Drugs, 14, 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade PB, Grosso C, Valentao P, & Bernardo J (2016). Flavonoids in neurodegeneration: Limitations and strategies to cross CNS barriers. Current Medicinal Chemistry, 23, 4151–4174. [DOI] [PubMed] [Google Scholar]
- Andreux PA, Houtkooper RH, & Auwerx J (2013). Pharmacological approaches to restore mitochondrial function. Nature Reviews Drug Discovery, 12, 465–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anis E, Zafeer MF, Firdaus F, Islam SN, Anees Khan A, Ali A, & Hossain MM (2020). Ferulic acid reinstates mitochondrial dynamics through PGC1α expression modulation in 6-hydroxydopamine lesioned rats. Phytotherapy Research, 34, 214–226. [DOI] [PubMed] [Google Scholar]
- Ansari Dezfouli M, Zahmatkesh M, Farahmandfar M, & Khodagholi F (2019). Melatonin protective effect against amyloid β-induced neurotoxicity mediated by mitochondrial biogenesis; involvement of hippocampal Sirtuin-1 signaling pathway. Physiology & Behavior, 204, 65–75. [DOI] [PubMed] [Google Scholar]
- Ates G, Goldberg J, Currais A, & Maher P (2020). CMS121, a fatty acid synthase inhibitor, protects against excess lipid peroxidation and inflammation and alleviates cognitive loss in a transgenic mouse model of Alzheimer’s disease. Redox Biology, 36, 101648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlante A, Bobba A, Paventi G, Pizzuto R, & Passarella S (2010). Genistein and daidzein prevent low potassium-dependent apoptosis of cerebellar granule cells. Biochemical Pharmacology, 79, 758–767. [DOI] [PubMed] [Google Scholar]
- Ay M, Luo J, Langley M, Jin H, Anantharam V, Kanthasamy A, & Kanthasamy AG (2017). Molecular mechanisms underlying protective effects of quercetin against mitochondrial dysfunction and progressive dopaminergic neurodegeneration in cell culture and MitoPark transgenic mouse models of Parkinson’s Disease. Journal of Neurochemistry, 141, 766–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baell J, & Walters MA (2014). Chemistry: Chemical con artists foil drug discovery. Nature, 513, 481–483. [DOI] [PubMed] [Google Scholar]
- Baell JB (2016). Feeling nature’s PAINS: Natural products, natural product drugs, and pan assay interference compounds (PAINS). Journal of Natural Products, 79, 616–628. [DOI] [PubMed] [Google Scholar]
- Bak D-H, Kim HD, Kim YO, Park CG, Han S-Y, & Kim J-J (2016). Neuroprotective effects of 20(S)-protopanaxadiol against glutamate-induced mitochondrial dysfunction in PC12 cells. International Journal of Molecular Medicine, 37, 378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang S, Song JH, Lee D, Lee C, Kim S, Kang KS, Lee JH, & Shim SH (2019). Neuroprotective secondary metabolite produced by an endophytic fungus, Neosartorya fischeri JS0553, isolated from Glehnia littoralis. Journal of Agricultural and Food Chemistry, 67, 1831–1838. [DOI] [PubMed] [Google Scholar]
- Bao F, Tao L, & Zhang H (2019). Neuroprotective effect of natural alkaloid fangchinoline against oxidative glutamate toxicity: Involvement of Keap1-Nrf2 axis regulation. Cellular and Molecular Neurobiology, 39, 1177–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barhwal K, Das SK, Kumar A, Hota SK, & Srivastava RB (2015). Insulin receptor A and Sirtuin 1 synergistically improve learning and spatial memory following chronic salidroside treatment during hypoxia. Journal of Neurochemistry, 135, 332–346. [DOI] [PubMed] [Google Scholar]
- Baur JA, & Sinclair DA (2006). Therapeutic potential of resveratrol: The in vivo evidence. Nature Reviews Drug Discovery, 5, 493–506. [DOI] [PubMed] [Google Scholar]
- Behl C, Davis JB, Lesley R, & Schubert D (1994). Hydrogen peroxide mediates amyloid beta protein toxicity. Cell, 77, 817–827. [DOI] [PubMed] [Google Scholar]
- Bélanger M, Allaman I, & Magistretti, Pierre J (2011). Brain energy metabolism: Focus on astrocyte-neuron metabolic cooperation. Cell Metabolism, 14, 724–738. [DOI] [PubMed] [Google Scholar]
- Bell SM, Barnes K, Clemmens H, Al-Rafiah AR, Al-ofi EA, Leech V, Bandmann O, Shaw PJ, Blackburn DJ, Ferraiuolo L, & Mortiboys H (2018). Ursodeoxycholic acid improves mitochondrial function and redistributes Drp1 in fibroblasts from patients with either sporadic or familial Alzheimer’s disease. Journal of Molecular Biology, 430, 3942–3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellantuono I (2018). Find drugs that delay many diseases of old age. Nature, 554, 293–295. [DOI] [PubMed] [Google Scholar]
- Berghe TV, Linkermann A, Jouan-Lanhouet S, Walczak H, & Vandenabeele P (2014). Regulated necrosis: The expanding network of non-apoptotic cell death pathways. Nature Reviews Molecular Cell Biology, 15, 135–147. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, & Greenamyre JT (2000). Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nature Neuroscience, 3, 1301–1306. [DOI] [PubMed] [Google Scholar]
- Biasutto L, Szabo’ I, & Zoratti M (2011). Mitochondrial effects of plant-made compounds. Antioxidants & Redox Signaling, 15, 3039–3059. [DOI] [PubMed] [Google Scholar]
- Blandini F, & Armentero M-T (2012). Animal models of Parkinson’s disease. The FEBS Journal, 279, 1156–1166. [DOI] [PubMed] [Google Scholar]
- Blum D, Torch S, Lambeng N, Nissou M-F, Benabid A-L, Sadoul R, & Verna J-M (2001). Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: Contribution to the apoptotic theory in Parkinson’s disease. Progress in Neurobiology, 65, 135–172. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, & Halliwell B (2019). Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nature Reviews Neuroscience, 20, 148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell BCV, De Silva DA, Macleod MR, Coutts SB, Schwamm LH, Davis SM, & Donnan GA (2019). Ischaemic stroke. Nature Reviews Disease Primers, 5, 70. [DOI] [PubMed] [Google Scholar]
- Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, & Auwerx J (2009). AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature, 458, 1056–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona V, Martín-Aragón S, Goldberg J, Schubert D, & Bermejo-Bescós P (2020). Several targets involved in Alzheimer’s disease amyloidogenesis are affected by morin and isoquercitrin. Nutritional Neuroscience, 23, 575–590. [DOI] [PubMed] [Google Scholar]
- Carretero M, Escames G, López LC, Venegas C, Dayoub JC, García L, & Acuña-Castroviejo D (2009). Long-term melatonin administration protects brain mitochondria from aging. Journal of Pineal Research, 47, 192–200. [DOI] [PubMed] [Google Scholar]
- Cha JW, Lee SI, Kim MC, Thida M, Lee JW, Park J-S, & Kwon HC (2015). Pontemazines A and B, phenazine derivatives containing a methylamine linkage from Streptomyces sp. UT1123 and their protective effect to HT-22 neuronal cells. Bioorganic & Medicinal Chemistry Letters, 25, 5083–5086. [DOI] [PubMed] [Google Scholar]
- Chae U, Min JS, Leem HH, Lee HS, Lee HJ, Lee SR, & Lee DS (2017). Chrysophanol suppressed glutamate-induced hippocampal neuronal cell death via regulation of dynamin-related protein 1-dependent mitochondrial fission. Pharmacology, 100, 153–160. [DOI] [PubMed] [Google Scholar]
- Chan DC (2006). Mitochondria: Dynamic organelles in disease, aging, and development. Cell, 125, 1241–1252. [DOI] [PubMed] [Google Scholar]
- Chen J, Chua K-W, Chua CC, Yu H, Pei A, Chua BHL, Hamdy RC, Xu X, & Liu C-F (2011). Antioxidant activity of 7,8-dihydroxyflavone provides neuroprotection against glutamate-induced toxicity. Neuroscience Letters, 499, 181–185. [DOI] [PubMed] [Google Scholar]
- Chen L, Liu Y. c., Tan H, Zhang Y, Xu J, Liu W. l., Li Z. y., & Li W. p. (2019). Santacruzamate A ameliorates AD-like pathology by enhancing ER stress tolerance through regulating the functions of KDELR and Mia40-ALR in vivo and in vitro. Frontiers in Cellular Neuroscience, 13, 61. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chen Q, Prior M, Dargusch R, Roberts A, Riek R, Eichmann C, Chiruta C, Akaishi T, Abe K, Maher P, & Schubert D (2011). A novel neurotrophic drug for cognitive enhancement and Alzheimer’s disease. PLoS One, 6, e27865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen J, Sun X, Shi X, Wang L, Huang L, & Zhou W (2018). Evaluation of the neuroprotective effect of EGCG: a potential mechanism of mitochondrial dysfunction and mitochondrial dynamics after subarachnoid hemorrhage. Food & Function, 9, 6349–6359. [DOI] [PubMed] [Google Scholar]
- Chen Y, Huang L, Zhang H, Diao X, Zhao S, & Zhou W (2017). Reduction in autophagy by (−)-epigallocatechin-3-gallate (EGCG): a potential mechanism of prevention of mitochondrial dysfunction after subarachnoid hemorrhage. Molecular Neurobiology, 54, 392–405. [DOI] [PubMed] [Google Scholar]
- Chiruta C, Schubert D, Dargusch R, & Maher P (2012). Chemical modification of the multitarget neuroprotective compound fisetin. Journal of Medicinal Chemistry, 55, 378–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HG, Song JH, Park M, Kim S, Kim C-E, Kang KS, & Shim SH (2020). Neuroprotective γ-pyrones from Fusarium Solani JS-0169: cell-based identification of active compounds and an informatics approach to predict the mechanism of action. Biomolecules, 10, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang J-I, Pan I-L, Hsieh C-Y, Huang C-Y, Chen P-C, & Shin JW (2016). Melatonin prevents the dynamin-related protein 1-dependent mitochondrial fission and oxidative insult in the cortical neurons after 1-methyl-4-phenylpyridinium treatment. Journal of Pineal Research, 61, 230–240. [DOI] [PubMed] [Google Scholar]
- Cochemé HM, & Murphy MP (2008). Complex I is the major site of mitochondrial superoxide production by paraquat. Journal of Biological Chemistry, 283, 1786–1798. [DOI] [PubMed] [Google Scholar]
- Costa V, Giacomello M, Hudec R, Lopreiato R, Ermak G, Lim D, Malorni W, Davies KJA, Carafoli E, & Scorrano L (2010). Mitochondrial fission and cristae disruption increase the response of cell models of Huntington’s disease to apoptotic stimuli. EMBO Molecular Medicine, 2, 490–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa V, & Scorrano L (2012). Shaping the role of mitochondria in the pathogenesis of Huntington’s disease. The EMBO Journal, 31, 1853–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, & Krainc D (2006). Transcriptional repression of PGC-1α by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell, 127, 59–69. [DOI] [PubMed] [Google Scholar]
- Cummings J, Lee G, Ritter A, Sabbagh M, & Zhong K (2020). Alzheimer’s disease drug development pipeline: 2020. Alzheimer’s & Dementia: Translational Research & Clinical Interventions, 6, e12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnane SC, Trushina E, Morland C, Prigione A, Casadesus G, Andrews ZB, Beal MF, Bergersen LH, Brinton RD, de la Monte S, Eckert A, Harvey J, Jeggo R, Jhamandas JH, Kann O, la Cour CM, Martin WF, Mithieux G, Moreira PI, Murphy MP, Nave K-A, Nuriel T, Oliet SHR, Saudou F, Mattson MP, Swerdlow RH, & Millan MJ (2020). Brain energy rescue: An emerging therapeutic concept for neurodegenerative disorders of ageing. Nature Reviews Drug Discovery, 19, 609–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currais A (2015). Ageing and inflammation – A central role for mitochondria in brain health and disease. Ageing Research Reviews, 21, 30–42. [DOI] [PubMed] [Google Scholar]
- Currais A, Chiruta C, Goujon-Svrzic M, Costa G, Santos T, Batista MT, Paiva J, Céu Madureira M. d., & Maher P (2014). Screening and identification of neuroprotective compounds relevant to Alzheimer׳s disease from medicinal plants of S. Tomé e Príncipe. Journal of Ethnopharmacology, 155, 830–840. [DOI] [PubMed] [Google Scholar]
- Currais A, Goldberg J, Farrokhi C, Chang M, Prior M, Dargusch R, Daugherty D, Armando A, Quehenberger O, Maher P, & Schubert D (2015). A comprehensive multiomics approach toward understanding the relationship between aging and dementia. Aging, 7, 937–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currais A, Huang L, Goldberg J, Petrascheck M, Ates G, Pinto-Duarte A, Shokhirev MN, Schubert D, & Maher P (2019). Elevating acetyl-CoA levels reduces aspects of brain aging. eLife, 8, e47866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currais A, & Maher P (2013). Functional consequences of age-dependent changes in glutathione status in the brain. Antioxidants & Redox Signaling, 19, 813–822. [DOI] [PubMed] [Google Scholar]
- Dauer W, & Przedborski S (2003). Parkinson’s disease: Mechanisms and models. Neuron, 39, 889–909. [DOI] [PubMed] [Google Scholar]
- de Oliveira MR, Brasil FB, & Andrade CMB (2017). Naringenin attenuates H2O2-induced mitochondrial dysfunction by an Nrf2-dependent mechanism in SH-SY5Y cells. Neurochemical Research, 42, 3341–3350. [DOI] [PubMed] [Google Scholar]
- de Oliveira MR, Brasil FB, & Fürstenau CR (2018). Evaluation of the mitochondria-related redox and bioenergetics effects of gastrodin in SH-SY5Y cells exposed to hydrogen peroxide. Journal of Molecular Neuroscience, 64, 242–251. [DOI] [PubMed] [Google Scholar]
- de Oliveira MR, Schuck PF, & Bosco SMD (2017). Tanshinone I induces mitochondrial protection through an Nrf2-dependent mechanism in paraquat-treated human neuroblastoma SH-SY5Y cells. Molecular Neurobiology, 54, 4597–4608. [DOI] [PubMed] [Google Scholar]
- Deng Y-N, Shi J, Liu J, & Qu Q-M (2013). Celastrol protects human neuroblastoma SH-SY5Y cells from rotenone-induced injury through induction of autophagy. Neurochemistry International, 63, 1–9. [DOI] [PubMed] [Google Scholar]
- Devi L, Prabhu BM, Galati DF, Avadhani NG, & Anandatheerthavarada HK (2006). Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain is associated with mitochondrial dysfunction. The Journal of Neuroscience, 26, 9057–9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Xiong Y, Sun J, Chen C, Gao J, & Xu H (2018). Asiatic acid prevents oxidative stress and apoptosis by inhibiting the translocation of α-synuclein into mitochondria. Frontiers in Neuroscience, 12, 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Kong D, Zhou T, Yang N. d., Xin C, Xu J, Wang Q, Zhang H, Wu Q, Lu X, Lim K, Ma B, Zhang C, Li L, & Huang W (2020). α-Arbutin protects against Parkinson’s disease-associated mitochondrial dysfunction in vitro and in vivo. Neuromolecular Medicine, 22, 56–67. [DOI] [PubMed] [Google Scholar]
- Dixon Scott J., Lemberg Kathryn M., Lamprecht Michael R., Skouta R, Zaitsev Eleina M., Gleason Caroline E., Patel Darpan N., Bauer Andras J., Cantley Alexandra M., Yang Wan S., Morrison B, & Stockwell Brent R. (2012). Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell, 149, 1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G, Tu H, Li X, Pei A, Chen J, Miao Z, Li J, Wang C, Xie H, Xu X, & Zhao H (2014). Daphnetin, a natural coumarin derivative, provides the neuroprotection against glutamate-induced toxicity in HT22 cells and ischemic brain injury. Neurochemical Research, 39, 269–275. [DOI] [PubMed] [Google Scholar]
- Eckert GP, Schiborr C, Hagl S, Abdel-Kader R, Müller WE, Rimbach G, & Frank J (2013). Curcumin prevents mitochondrial dysfunction in the brain of the senescence-accelerated mouse-prone 8. Neurochemistry International, 62, 595–602. [DOI] [PubMed] [Google Scholar]
- Ekstrand MI, & Galter D (2009). The MitoPark mouse - An animal model of Parkinson’s disease with impaired respiratory chain function in dopamine neurons. Parkinsonism & Related Disorders, 15, S185–S188. [DOI] [PubMed] [Google Scholar]
- Ekstrand MI, Terzioglu M, Galter D, Zhu S, Hofstetter C, Lindqvist E, Thams S, Bergstrand A, Hansson FS, Trifunovic A, Hoffer B, Cullheim S, Mohammed AH, Olson L, & Larsson N-G (2007). Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proceedings of the National Academy of Sciences of the United States of America, 104, 1325–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ereminas G, Majiene D, Sidlauskas K, Jakstas V, Ivanauskas L, Vaitiekaitis G, & Liobikas J (2017). Neuroprotective properties of anthocyanidin glycosides against H2O2-induced glial cell death are modulated by their different stability and antioxidant activity in vitro. Biomedicine & Pharmacotherapy, 94, 188–196. [DOI] [PubMed] [Google Scholar]
- Fernández-Moriano C, Divakar PK, Crespo A, & Gómez-Serranillos MP (2017). In vitro neuroprotective potential of lichen metabolite fumarprotocetraric acid via intracellular redox modulation. Toxicology and Applied Pharmacology, 316, 83–94. [DOI] [PubMed] [Google Scholar]
- Fernández-Moriano C, González-Burgos E, Iglesias I, Lozano R, & Gómez-Serranillos MP (2017). Evaluation of the adaptogenic potential exerted by ginsenosides Rb1 and Rg1 against oxidative stress-mediated neurotoxicity in an in vitro neuronal model. PLoS One, 12, e0182933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretta A, Gaballo A, Tanzarella P, Piccoli C, Capitanio N, Nico B, Annese T, Di Paola M, Dell’Aquila C, De Mari M, Ferranini E, Bonifati V, Pacelli C, & Cocco T (2014). Effect of resveratrol on mitochondrial function: Implications in parkin-associated familiar Parkinson’s disease. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1842, 902–915. [DOI] [PubMed] [Google Scholar]
- Fischer W, Currais A, Liang Z, Pinto A, & Maher P (2019). Old age-associated phenotypic screening for Alzheimer’s disease drug candidates identifies sterubin as a potent neuroprotective compound from Yerba santa. Redox Biology, 21, 101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Iborra S, Plaza-Zabala A, Montpeyo M, Sebastian D, Vila M, & Martinez-Vicente M (2020). Mutant HTT (huntingtin) impairs mitophagy in a cellular model of Huntington disease. Autophagy, 1–18. [DOI] [PMC free article] [PubMed]
- Fricker M, Tolkovsky AM, Borutaite V, Coleman M, & Brown GC (2018). Neuronal cell death. Physiological Reviews, 98, 813–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Jin J, Cichewicz RH, Hageman SA, Ellis TK, Xiang L, Peng Q, Jiang M, Arbez N, Hotaling K, Ross CA, & Duan W (2012). trans-(−)-ϵ-Viniferin increases mitochondrial sirtuin 3 (SIRT3), activates AMP-activated protein kinase (AMPK), and protects cells in models of Huntington disease. Journal of Biological Chemistry, 287, 24460–24472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Yi J, Zhu J, Minikes AM, Monian P, Thompson CB, & Jiang X (2019). Role of mitochondria in ferroptosis. Molecular Cell, 73, 354–363.e353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garabadu D, & Agrawal N (2020). Naringin exhibits neuroprotection against rotenone-induced neurotoxicity in experimental rodents. Neuromolecular Medicine, 22, 314–330. [DOI] [PubMed] [Google Scholar]
- Gauthier S, Albert M, Fox N, Goedert M, Kivipelto M, Mestre-Ferrandiz J, & Middleton LT (2016). Why has therapy development for dementia failed in the last two decades? Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 12, 60–64. [DOI] [PubMed] [Google Scholar]
- Geed M, Garabadu D, Ahmad A, & Krishnamurthy S (2014). Silibinin pretreatment attenuates biochemical and behavioral changes induced by intrastriatal MPP+ injection in rats. Pharmacology Biochemistry and Behavior, 117, 92–103. [DOI] [PubMed] [Google Scholar]
- Goldberg J, Currais A, Prior M, Fischer W, Chiruta C, Ratliff E, Daugherty D, Dargusch R, Finley K, Esparza-Moltó PB, Cuezva JM, Maher P, Petrascheck M, & Schubert D (2018). The mitochondrial ATP synthase is a shared drug target for aging and dementia. Aging Cell, 46, e12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong W, Zhou Y, Gong W, & Qin X (2020). Coniferyl ferulate exerts antidepressant effect via inhibiting the activation of NMDAR-CaMKII-MAPKs and mitochondrial apoptotic pathways. Journal of Ethnopharmacology, 251, 112533. [DOI] [PubMed] [Google Scholar]
- González-Burgos E, Duarte AI, Carretero ME, Moreira PI, & Gómez-Serranillos MP (2016). Kaurane diterpenes as mitochondrial alterations preventive agents under experimental oxidative stress conditions. Pharmaceutical Biology, 54, 705–711. [DOI] [PubMed] [Google Scholar]
- Grimm A, & Eckert A (2017). Brain aging and neurodegeneration: From a mitochondrial point of view. Journal of Neurochemistry, 143, 418–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm A, Schmitt K, Lang UE, Mensah-Nyagan AG, & Eckert A (2014). Improvement of neuronal bioenergetics by neurosteroids: Implications for age-related neurodegenerative disorders. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1842, 2427–2438. [DOI] [PubMed] [Google Scholar]
- Guo X, Disatnik M-H, Monbureau M, Shamloo M, Mochly-Rosen D, & Qi X (2013). Inhibition of mitochondrial fragmentation diminishes Huntington’s disease–associated neurodegeneration. The Journal of Clinical Investigation, 123, 5371–5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Sun X, Hu D, Wang Y-J, Fujioka H, Vyas R, Chakrapani S, Joshi AU, Luo Y, Mochly-Rosen D, & Qi X (2016). VCP recruitment to mitochondria causes mitophagy impairment and neurodegeneration in models of Huntington’s disease. Nature Communications, 7, 12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris Julia J., Jolivet R, & Attwell D (2012). Synaptic energy use and supply. Neuron, 75, 762–777. [DOI] [PubMed] [Google Scholar]
- He J, Xu L, Yang L, & Sun C (2019). Anti-oxidative effects of catechins and theaflavins on glutamate-induced HT22 cell damage. RSC Advances, 9, 21418–21428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera F, Martin V, García-Santos G, Rodriguez-Blanco J, Antolín I, & Rodriguez C (2007). Melatonin prevents glutamate-induced oxytosis in the HT22 mouse hippocampal cell line through an antioxidant effect specifically targeting mitochondria. Journal of Neurochemistry, 100, 736–746. [DOI] [PubMed] [Google Scholar]
- Herzig S, & Shaw RJ (2018). AMPK: guardian of metabolism and mitochondrial homeostasis. Nature Reviews Molecular Cell Biology, 19, 121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, & Bohr VA (2019). Ageing as a risk factor for neurodegenerative disease. Nature Reviews Neurology, 15, 565–581. [DOI] [PubMed] [Google Scholar]
- Hu M, Wu B, & Liu Z (2017). Bioavailability of polyphenols and flavonoids in the era of precision medicine. Molecular Pharmaceutics, 14, 2861–2863. [DOI] [PubMed] [Google Scholar]
- Huang L, McClatchy DB, Maher P, Liang Z, Diedrich JK, Soriano-Castell D, Goldberg J, Shokhirev M, Yates JR, Schubert D, & Currais A (2020). Intracellular amyloid toxicity induces oxytosis/ferroptosis regulated cell death. Cell Death & Disease, 11, 828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- In S-J, Seo K-H, Song N-Y, Lee D-S, Kim Y-C, & Baek N-I (2015). Lignans and neolignans from the stems of Vibrunum erosum and their neuroprotective and anti-inflammatory activity. Archives of Pharmacal Research, 38, 26–34. [DOI] [PubMed] [Google Scholar]
- Ines F, Regina M, Diana M, Ines C, & Claudia Nunes dos S (2017). Polyphenols beyond barriers: A glimpse into the brain. Current Neuropharmacology, 15, 562–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intihar TA, Martinez EA, & Gomez-Pastor R (2019). Mitochondrial dysfunction in Huntington’s disease; Interplay between HSF1, p53 and PGC-1α transcription factors. Frontiers in Cellular Neuroscience, 13, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishige K, Schubert D, & Sagara Y (2001). Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radical Biology and Medicine, 30, 433–446. [DOI] [PubMed] [Google Scholar]
- Ishisaka A, Mukai R, Terao J, Shibata N, & Kawai Y (2014). Specific localization of quercetin-3-O-glucuronide in human brain. Archives of Biochemistry and Biophysics, 557, 11–17. [DOI] [PubMed] [Google Scholar]
- Ito K, Eguchi Y, Imagawa Y, Akai S, Mochizuki H, & Tsujimoto Y (2017). MPP+ induces necrostatin-1- and ferrostatin-1-sensitive necrotic death of neuronal SH-SY5Y cells. Cell Death Discovery, 3, 17013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Nakamura K, Iijima M, & Sesaki H (2013). Mitochondrial dynamics in neurodegeneration. Trends in Cell Biology, 23, 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang Y, Choo H, Lee MJ, Han J, Kim SJ, Ju X, Cui J, Lee YL, Ryu MJ, Oh ES, Choi S-Y, Chung W, Kweon GR, & Heo JY (2019). Auraptene mitigates Parkinson’s disease-like behavior by protecting inhibition of mitochondrial respiration and scavenging reactive oxygen species. International Journal of Molecular Sciences, 20, 3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesus AR, Vila-Viçosa D, Machuqueiro M, Marques AP, Dore TM, & Rauter AP (2017). Targeting type 2 diabetes with C-glucosyl dihydrochalcones as selective sodium glucose co-transporter 2 (SGLT2) inhibitors: Synthesis and biological evaluation. Journal of Medicinal Chemistry, 60, 568–579. [DOI] [PubMed] [Google Scholar]
- Jiang T, Yin F, Yao J, Brinton RD, & Cadenas E (2013). Lipoic acid restores age-associated impairment of brain energy metabolism through the modulation of Akt/JNK signaling and PGC1α transcriptional pathway. Aging Cell, 12, 1021–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo DS, Shin DW, Park SJ, Bae J-E, Kim JB, Park NY, Kim J-S, Oh JS, Shin J-W, & Cho D-H (2016). Attenuation of Aβ toxicity by promotion of mitochondrial fusion in neuroblastoma cells by liquiritigenin. Archives of Pharmacal Research, 39, 1137–1143. [DOI] [PubMed] [Google Scholar]
- Johri A, Chandra A, & Flint Beal M (2013). PGC-1α, mitochondrial dysfunction, and Huntington’s disease. Free Radical Biology and Medicine, 62, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou M-J, Peng T-I, Hsu L-F, Jou S-B, Reiter RJ, Yang C-M, Chiao C-C, Lin Y-F, & Chen C-C (2010). Visualization of melatonin’s multiple mitochondrial levels of protection against mitochondrial Ca2+-mediated permeability transition and beyond in rat brain astrocytes. Journal of Pineal Research, 48, 20–38. [DOI] [PubMed] [Google Scholar]
- Kapogiannis D, & Mattson MP (2011). Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. The Lancet Neurology, 10, 187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesherwani V, Atif F, Yousuf S, & Agrawal SK (2013). Resveratrol protects spinal cord dorsal column from hypoxic injury by activating Nrf-2. Neuroscience, 241, 80–88. [DOI] [PubMed] [Google Scholar]
- Kim MH, Min J-S, Lee JY, Chae U, Yang E-J, Song K-S, Lee H-S, Lee HJ, Lee S-R, & Lee D-S (2018). Oleuropein isolated from Fraxinus rhynchophylla inhibits glutamate-induced neuronal cell death by attenuating mitochondrial dysfunction. Nutritional Neuroscience, 21, 520–528. [DOI] [PubMed] [Google Scholar]
- Langston J, Ballard P, Tetrud J, & Irwin I (1983). Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science, 219, 979–980. [DOI] [PubMed] [Google Scholar]
- Larsson N-G, Wang J, Wilhelmsson H, Oldfors A, Rustin P, Lewandoski M, Barsh GS, & Clayton DA (1998). Mitochondrial transcription factor A is necessary for mtDNA maintance and embryogenesis in mice. Nature Genetics, 18, 231–236. [DOI] [PubMed] [Google Scholar]
- Lee D-H, Kim C-S, & Lee YJ (2011). Astaxanthin protects against MPTP/MPP+-induced mitochondrial dysfunction and ROS production in vivo and in vitro. Food and Chemical Toxicology, 49, 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D-S, Cha B-Y, Woo J-T, Kim Y-C, & Jang J-H (2015). Acerogenin A from Acer nikoense Maxim prevents oxidative stress-induced neuronal cell death through Nrf2-mediated heme oxygenase-1 expression in mouse hippocampal HT22 cell line. Molecules, 20, 12545–12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D-S, Ko W, Kim D-C, Kim Y-C, & Jeong G-S (2014). Cudarflavone B provides neuroprotection against glutamate-induced mouse hippocampal HT22 cell damage through the Nrf2 and PI3K/Akt signaling pathways. Molecules, 19, 10818–10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-E, Sim H, Yoo HM, Lee M, Baek A, Jeon Y-J, Seo K-S, Son M-Y, Yoon JS, & Kim J (2020). Neuroprotective effects of cryptotanshinone in a direct reprogramming model of Parkinson’s disease. Molecules, 25, 3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Amarsanaa K, Wu J, Jeon S-C, Cui Y, Jung S-C, Park D-B, Kim S-J, Han S-H, Kim H-W, Rhyu IJ, & Eun S-Y (2018). Nobiletin attenuates neurotoxic mitochondrial calcium overload through K+ influx and ΔΨm across mitochondrial inner membrane. Korean Journal of Physiology & Pharmacology, 22, 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Bae O-N, Serfozo K, Hejabian S, Moussa A, Reeves M, Rumbeiha W, Fitzgerald SD, Stein G, Baek S-H, Goudreau J, Kassab M, & Majid A (2012). Asiatic acid attenuates infarct volume, mitochondrial dysfunction, and matrix metalloproteinase-9 induction after focal cerebral ischemia. Stroke, 43, 1632–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Ban J-J, Chung J-Y, Im W, & Kim M (2018). Amelioration of Huntington’s disease phenotypes by Beta-Lapachone is associated with increases in Sirt1 expression, CREB phosphorylation and PGC-1α deacetylation. PLoS One, 13, e0195968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leirós M, Alonso E, Sanchez JA, Rateb ME, Ebel R, Houssen WE, Jaspars M, Alfonso A, & Botana LM (2014). Mitigation of ROS insults by Streptomyces secondary metabolites in primary cortical neurons. ACS Chemical Neuroscience, 5, 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leirós M, Sánchez JA, Alonso E, Rateb ME, Houssen WE, Ebel R, Jaspars M, Alfonso A, & Botana LM (2014). Spongionella secondary metabolites protect mitochondrial function in cortical neurons against oxidative stress. Marine Drugs, 12, 700–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewerenz J, Ates G, Methner A, Conrad M, & Maher P (2018). Oxytosis/ferroptosis— (Re-) emerging roles for oxidative stress-dependent non-apoptotic cell death in diseases of the central nervous system. Frontiers in Neuroscience, 12, 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis T (2016). Mystery mechanisms. In The Scientist. New York City: LabX Media Group. [Google Scholar]
- Li H, Han W, Wang H, Ding F, Xiao L, Shi R, Ai L, & Huang Z (2017). Tanshinone IIa inhibits glutamate-induced oxidative toxicity through prevention of mitochondrial dysfunction and suppression of MAPK activation in SH-SY5Y human neuroblastoma cells. Oxidative Medicine and Cellular Longevity, 2017, 4517486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Tan L-H, Zou H, Zou Z-X, Long H-P, Wang W-X, Xu P-S, Liu L-F, Xu K-P, & Tan G-S (2020). Palhinosides A–H: Flavone glucosidic truxinate esters with neuroprotective activities from Palhinhaea cernua. Journal of Natural Products, 83, 216–222. [DOI] [PubMed] [Google Scholar]
- Li X-X, He G-R, Mu X, Xu B, Tian S, Yu X, Meng F-R, Xuan Z-H, & Du G-H (2012). Protective effects of baicalein against rotenone-induced neurotoxicity in PC12 cells and isolated rat brain mitochondria. European Journal of Pharmacology, 674, 227–233. [DOI] [PubMed] [Google Scholar]
- Li Y, Maher P, & Schubert D (1997). A role for 12-lipoxygenase in nerve cell death caused by glutathione depletion. Neuron, 19, 453–463. [DOI] [PubMed] [Google Scholar]
- Li Y, Shi J, Sun X, Li Y, Duan Y, & Yao H (2020). Theaflavic acid from black tea protects PC12 cells against ROS-mediated mitochondrial apoptosis induced by OGD/R via activating Nrf2/ARE signaling pathway. Journal of Natural Medicines, 74, 238–246. [DOI] [PubMed] [Google Scholar]
- Liang Z, & Li QX (2018). Discovery of selective, substrate-competitive, and passive membrane permeable glycogen synthase kinase-3β inhibitors: Synthesis, biological evaluation, and molecular modeling of new C-glycosylflavones. ACS Chemical Neuroscience, 9, 1166–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, Shi F, Wang Y, Lu L, Zhang Z, Wang X, & Wang X (2011). Neuroprotective effects of tenuigenin in a SH-SY5Y cell model with 6-OHDA-induced injury. Neuroscience Letters, 497, 104–109. [DOI] [PubMed] [Google Scholar]
- Liang Z, Zhang B, Su WW, Williams PG, & Li QX (2016). C-Glycosylflavones alleviate tau phosphorylation and amyloid neurotoxicity through GSK3β inhibition. ACS Chemical Neuroscience, 7, 912–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lies B, Martens S, Schmidt S, Boll M, & Wenzel U (2012). Flavone potently stimulates an apical transporter for flavonoids in human intestinal Caco-2 cells. Molecular Nutrition & Food Research, 56, 1627–1635. [DOI] [PubMed] [Google Scholar]
- Lin J, Handschin C, & Spiegelman BM (2005). Metabolic control through the PGC-1 family of transcription coactivators. Cell Metabolism, 1, 361–370. [DOI] [PubMed] [Google Scholar]
- Lin MT, & Beal MF (2006). Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature, 443, 787–795. [DOI] [PubMed] [Google Scholar]
- Lin S-P, Li W, Winters A, Liu R, & Yang S-H (2018). Artemisinin prevents glutamate-induced neuronal cell death via Akt pathway activation. Frontiers in Cellular Neuroscience, 12, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski CA (2004). Lead- and drug-like compounds: The rule-of-five revolution. Drug Discovery Today: Technologies, 1, 337–341. [DOI] [PubMed] [Google Scholar]
- Liu G-S, Zhang Z-S, Yang B, & He W (2012). Resveratrol attenuates oxidative damage and ameliorates cognitive impairment in the brain of senescence-accelerated mice. Life Sciences, 91, 872–877. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang Y, Hao Y, Wang Z, Yang Z, Wang Z, & Wang J (2020). 5-Heptadecylresorcinol attenuates oxidative damage and mitochondria-mediated apoptosis through activation of the SIRT3/FOXO3a signaling pathway in neurocytes. Food & Function, 11, 2535–2542. [DOI] [PubMed] [Google Scholar]
- Liu L, Zhang W, Wang L, Li Y, Tan B, Lu X, Deng Y, Zhang Y, Guo X, Mu J, & Yu G (2014). Curcumin prevents cerebral ischemia reperfusion injury via increase of mitochondrial biogenesis. Neurochemical Research, 39, 1322–1331. [DOI] [PubMed] [Google Scholar]
- Liu P, Zou D, Chen K, Zhou Q, Gao Y, Huang Y, Zhu J, Zhang Q, & Mi M (2016). Dihydromyricetin improves hypobaric hypoxia-induced memory impairment via modulation of SIRT3 signaling. Molecular Neurobiology, 53, 7200–7212. [DOI] [PubMed] [Google Scholar]
- Liu P, Zou D, Yi L, Chen M, Gao Y, Zhou R, Zhang Q, Zhou Y, Zhu J, Chen K, & Mi M (2015). Quercetin ameliorates hypobaric hypoxia-induced memory impairment through mitochondrial and neuron function adaptation via the PGC-1α pathway. Restorative Neurology and Neuroscience, 33, 143–157. [DOI] [PubMed] [Google Scholar]
- Liu Y, Dargusch R, Maher P, & Schubert D (2008). A broadly neuroprotective derivative of curcumin. Journal of Neurochemistry, 105, 1336–1345. [DOI] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, & Kroemer G (2013). The hallmarks of aging. Cell, 153, 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv C, Liu X, Liu H, Chen T, & Zhang W (2014). Geniposide attenuates mitochondrial dysfunction and memory deficits in APP/PS1 transgenic mice. Current Alzheimer Research, 11, 580–587. [DOI] [PubMed] [Google Scholar]
- Madathil MM, Khdour OM, Jaruvangsanti J, & Hecht SM (2012). Synthesis and biological activities of N-(3-carboxylpropyl)-5-amino-2-hydroxy-3-tridecyl-1,4-benzoquinone and analogues. Journal of Natural Products, 75, 2209–2215. [DOI] [PubMed] [Google Scholar]
- Maher P (2020). Preventing and treating neurological disorders with the flavonol fisetin. Brain Plasticity, In press, 10.3233/BPL-200104. [DOI] [PMC free article] [PubMed]
- Maher P, Akaishi T, & Abe K (2006). Flavonoid fisetin promotes ERK-dependent long-term potentiation and enhances memory. Proceedings of the National Academy of Sciences of the United States of America, 103, 16568–16573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher P, Currais A, & Schubert D (2020). Using the oxytosis/ferroptosis pathway to understand and treat age-associated neurodegenerative diseases. Cell Chemical Biology, In press, 10.1016/j.chembiol.2020.10.010. [DOI] [PMC free article] [PubMed]
- Maher P, Fischer W, Liang Z, Soriano-Castell D, Pinto AFM, Rebman J, & Currais A (2020). The value of herbarium collections to the discovery of novel treatments for Alzheimer’s disease, a case made with the genus Eriodictyon. Frontiers in Pharmacology, 11, 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher P, van Leyen K, Dey PN, Honrath B, Dolga A, & Methner A (2018). The role of Ca2+ in cell death caused by oxidative glutamate toxicity and ferroptosis. Cell Calcium, 70, 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal PK, Saharan S, Tripathi M, & Murari G (2015). Brain glutathione levels – a novel biomarker for mild cognitive impairment and Alzheimer’s disease. Biological Psychiatry, 78, 702–710. [DOI] [PubMed] [Google Scholar]
- Mao X-Y, Zhou H-H, Li X, & Liu Z-Q (2016). Huperzine A alleviates oxidative glutamate toxicity in hippocampal HT22 cells via activating BDNF/TrkB-dependent PI3K/Akt/mTOR signaling pathway. Cellular and Molecular Neurobiology, 36, 915–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrotra A, Kanwal A, Banerjee SK, & Sandhir R (2015). Mitochondrial modulators in experimental Huntington’s disease: Reversal of mitochondrial dysfunctions and cognitive deficits. Neurobiology of Aging, 36, 2186–2200. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Sone N, & Saitoh T (1987). Effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 1-methyl-4-phenylpyridinium ion on activities of the enzymes in the electron transport system in mouse brain. Journal of Neurochemistry, 48, 1787–1793. [DOI] [PubMed] [Google Scholar]
- Morán M, Moreno-Lastres D, Marín-Buera L, Arenas J, Martín MA, & Ugalde C (2012). Mitochondrial respiratory chain dysfunction: Implications in neurodegeneration. Free Radical Biology and Medicine, 53, 595–609. [DOI] [PubMed] [Google Scholar]
- Moreno-Ulloa A, Nogueira L, Rodriguez A, Barboza J, Hogan MC, Ceballos G, Villarreal F, & Ramirez-Sanchez I (2014). Recovery of indicators of mitochondrial biogenesis, oxidative stress, and aging with (−)-epicatechin in senile mice. The Journals of Gerontology: Series A, Biological Sciences and Medical Sciences, 70, 1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortiboys H, Aasly J, & Bandmann O (2013). Ursocholanic acid rescues mitochondrial function in common forms of familial Parkinson’s disease. Brain, 136, 3038–3050. [DOI] [PubMed] [Google Scholar]
- Murphy MP, & Hartley RC (2018). Mitochondria as a therapeutic target for common pathologies. Nature Reviews Drug Discovery, 17, 865–886. [DOI] [PubMed] [Google Scholar]
- Nadal X, del Río C, Casano S, Palomares B, Ferreiro-Vera C, Navarrete C, Sánchez-Carnerero C, Cantarero I, Bellido ML, Meyer S, Morello G, Appendino G, & Muñoz E (2017). Tetrahydrocannabinolic acid is a potent PPARγ agonist with neuroprotective activity. British Journal of Pharmacology, 174, 4263–4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan SV, Dave KR, Saul I, & Perez-Pinzon MA (2015). Resveratrol preconditioning protects against cerebral ischemic injury via nuclear erythroid 2-related factor 2. Stroke, 46, 1626–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman DJ, & Cragg GM (2020). Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. Journal of Natural Products, 83, 770–803. [DOI] [PubMed] [Google Scholar]
- Nicholls DG (2004). Mitochondrial membrane potential and aging. Aging Cell, 3, 35–40. [DOI] [PubMed] [Google Scholar]
- Nirmaladevi D, Venkataramana M, Chandranayaka S, Ramesha A, Jameel NM, & Srinivas C (2014). Neuroprotective effects of bikaverin on H2O2-induced oxidative stress mediated neuronal damage in SH-SY5Y cell line. Cellular and Molecular Neurobiology, 34, 973–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunnari J, & Suomalainen A (2012). Mitochondria: In sickness and in health. Cell, 148, 1145–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomera-Avalos V, Griñán-Ferré C, Puigoriol-Ilamola D, Camins A, Sanfeliu C, Canudas AM, & Pallàs M (2017). Resveratrol protects SAMP8 brain under metabolic stress: Focus on mitochondrial function and Wnt pathway. Molecular Neurobiology, 54, 1661–1676. [DOI] [PubMed] [Google Scholar]
- Park J, Min J-S, Chae U, Lee JY, Song K-S, Lee H-S, Lee HJ, Lee S-R, & Lee D-S (2017). Anti-inflammatory effect of oleuropein on microglia through regulation of Drp1-dependent mitochondrial fission. Journal of Neuroimmunology, 306, 46–52. [DOI] [PubMed] [Google Scholar]
- Park SY, Jin ML, Kim YH, Kim C-M, Lee SJ, & Park G (2014). Involvement of heme oxygenase-1 in neuroprotection by sanguinarine against glutamate-triggered apoptosis in HT22 neuronal cells. Environmental Toxicology and Pharmacology, 38, 701–710. [DOI] [PubMed] [Google Scholar]
- Patching SG (2017). Glucose transporters at the blood-brain barrier: Function, regulation and gateways for drug delivery. Molecular Neurobiology, 54, 1046–1077. [DOI] [PubMed] [Google Scholar]
- Pathak T, & Trebak M (2018). Mitochondrial Ca2+ signaling. Pharmacology & Therapeutics, 192, 112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng K, Tao Y, Zhang J, Wang J, Ye F, Dan G, Zhao Y, Cai Y, Zhao J, Wu Q, Zou Z, Cao J, & Sai Y (2016). Resveratrol regulates mitochondrial biogenesis and fission/fusion to attenuate rotenone-induced neurotoxicity. Oxidative Medicine and Cellular Longevity, 2016, 6705621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Hou C, Yang Z, Li C, Jia L, Liu J, Tang Y, Shi L, Li Y, Long J, & Liu J (2016). Hydroxytyrosol mildly improve cognitive function independent of APP processing in APP/PS1 mice. Molecular Nutrition & Food Research, 60, 2331–2342. [DOI] [PubMed] [Google Scholar]
- Pérez-Gracia E, Torrejón-Escribano B, & Ferrer I (2008). Dystrophic neurites of senile plaques in Alzheimer’s disease are deficient in cytochrome c oxidase. Acta Neuropathologica, 116, 261–268. [DOI] [PubMed] [Google Scholar]
- Perez Ortiz JM, & Swerdlow RH (2019). Mitochondrial dysfunction in Alzheimer’s disease: Role in pathogenesis and novel therapeutic opportunities. British Journal of Pharmacology, 176, 3489–3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pienaar IS, & Chinnery PF (2013). Existing and emerging mitochondrial-targeting therapies for altering Parkinson’s disease severity and progression. Pharmacology & Therapeutics, 137, 1–21. [DOI] [PubMed] [Google Scholar]
- Pogačnik L, Pirc K, Palmela I, Skrt M, Kim KS, Brites D, Brito MA, Ulrih NP, & Silva RFM (2016). Potential for brain accessibility and analysis of stability of selected flavonoids in relation to neuroprotection in vitro. Brain Research, 1651, 17–26. [DOI] [PubMed] [Google Scholar]
- Porquet D, Griñán-Ferré C, Ferrer I, Camins A, Sanfeliu C, del Valle J, & Pallàs M (2014). Neuroprotective role of trans-resveratrol in a murine model of familial Alzheimer’s disease. Journal of Alzheimer’s Disease, 42, 1209–1220. [DOI] [PubMed] [Google Scholar]
- Prior M, Chiruta C, Currais A, Goldberg J, Ramsey J, Dargusch R, Maher PA, & Schubert D (2014). Back to the future with phenotypic screening. ACS Chemical Neuroscience, 5, 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior M, Dargusch R, Ehren JL, Chiruta C, & Schubert D (2013). The neurotrophic compound J147 reverses cognitive impairment in aged Alzheimer’s disease mice. Alzheimer’s Research and Therapy, 5, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu J, Chen W, Hu R, & Feng H (2016). The injury and therapy of reactive oxygen species in intracerebral hemorrhage looking at mitochondria. Oxidative Medicine and Cellular Longevity, 2016, 2592935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekar N, Dwivedi S, Tota S. k., Kamat PK, Hanif K, Nath C, & Shukla R (2013). Neuroprotective effect of curcumin on okadaic acid induced memory impairment in mice. European Journal of Pharmacology, 715, 381–394. [DOI] [PubMed] [Google Scholar]
- Rao SP, Sharma N, & Kalivendi SV (2020). Embelin averts MPTP-induced dysfunction in mitochondrial bioenergetics and biogenesis via activation of SIRT1. Biochimica et Biophysica Acta (BBA) - Bioenergetics, 1861, 148157. [DOI] [PubMed] [Google Scholar]
- Rasbach KA, & Schnellmann RG (2008). Isoflavones promote mitochondrial biogenesis. Journal of Pharmacology and Experimental Therapeutics, 325, 536–543. [DOI] [PubMed] [Google Scholar]
- Rashedinia M, Saberzadeh J, Khosravi Bakhtiari T, Hozhabri S, & Arabsolghar R (2019). Glycyrrhizic acid ameliorates mitochondrial function and biogenesis against aluminum toxicity in PC12 cells. Neurotoxicity Research, 35, 584–593. [DOI] [PubMed] [Google Scholar]
- Reddy PH, Manczak M, Yin X, Grady MC, Mitchell A, Kandimalla R, & Kuruva CS (2016). Protective effects of a natural product, curcumin, against amyloid β induced mitochondrial and synaptic toxicities in Alzheimer’s disease. Journal of Investigative Medicine, 64, 1220–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann H, Fhirke S, Hebenstreit G, Schrubar H, & Riederer P (1993). Analyses of energy metabolism and mitochondrial genome in post-mortem brain from patients with Alzheimer’s disease. Journal of Neurology, 240, 377–380. [DOI] [PubMed] [Google Scholar]
- Ren K-D, Liu W-N, Tian J, Zhang Y-Y, Peng J-J, Zhang D, Li N-S, Yang J, Peng J, & Luo X-J (2019). Mitochondrial E3 ubiquitin ligase 1 promotes brain injury by disturbing mitochondrial dynamics in a rat model of ischemic stroke. European Journal of Pharmacology, 861, 172617. [DOI] [PubMed] [Google Scholar]
- Ribas V, García-Ruiz C, & Fernández-Checa JC (2014). Glutathione and mitochondria. Frontiers in Pharmacology, 5, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues T, Reker D, Schneider P, & Schneider G (2016). Counting on natural products for drug design. Nature Chemistry, 8, 531–541. [DOI] [PubMed] [Google Scholar]
- Sabogal-Guáqueta AM, Hobbie F, Keerthi A, Oun A, Kortholt A, Boddeke E, & Dolga A (2019). Linalool attenuates oxidative stress and mitochondrial dysfunction mediated by glutamate and NMDA toxicity. Biomedicine & Pharmacotherapy, 118, 109295. [DOI] [PubMed] [Google Scholar]
- Sanderson TH, Raghunayakula S, & Kumar R (2015). Release of mitochondrial Opa1 following oxidative stress in HT22 cells. Molecular and Cellular Neuroscience, 64, 116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibye-Knudsen M, Fang EF, Croteau DL, Wilson DM III, & Bohr VA (2015). Protecting the mitochondrial powerhouse. Trends in Cell Biology, 25, 158–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm S, Huang G, Gunesch S, Lang F, Roa J, Högger P, Sabaté R, Maher P, & Decker M (2018). Regioselective synthesis of 7-O-esters of the flavonolignan silibinin and SARs lead to compounds with overadditive neuroprotective effects. European Journal of Medicinal Chemistry, 146, 93–107. [DOI] [PubMed] [Google Scholar]
- Schubert D (2005). Glucose metabolism and Alzheimer’s disease. Ageing Research Reviews, 4, 240–257. [DOI] [PubMed] [Google Scholar]
- Schubert D, Currais A, Goldberg J, Finley K, Petrascheck M, & Maher P (2018). Geroneuroprotectors: Effective geroprotectors for the brain. Trends in Pharmacological Sciences, 39, 1004–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D, Kepchia D, Liang Z, Dargusch R, Goldberg J, & Maher P (2019). Efficacy of cannabinoids in a pre-clinical drug-screening platform for Alzheimer’s disease. Molecular Neurobiology, 56, 7719–7730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D, Kimura H, & Maher P (1992). Growth factors and vitamin E modify neuronal glutamate toxicity. Proceedings of the National Academy of Sciences of the United States of America, 89, 8264–8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D, & Maher P (2012). An alternative approach to drug discovery for Alzheimer’s disease dementia. Future Medicinal Chemistry, 4, 1681–1688. [DOI] [PubMed] [Google Scholar]
- Seo J-S, Leem Y-H, Lee K-W, Kim S-W, Lee J-K, & Han P-L (2010). Severe motor neuron degeneration in the spinal cord of the Tg2576 mouse model of Alzheimer disease. Journal of Alzheimer’s Disease, 21, 263–276. [DOI] [PubMed] [Google Scholar]
- Seo K-H, Lee D-Y, Jeong R-H, Lee D-S, Kim Y-E, Hong E-K, Kim Y-C, & Baek N-I (2014). Neuroprotective effect of prenylated arylbenzofuran and flavonoids from Morus alba fruits on glutamate-induced oxidative injury in HT22 hippocampal cells. Journal of Medicinal Food, 18, 403–408. [DOI] [PubMed] [Google Scholar]
- Shahmoradian SH, Lewis AJ, Genoud C, Hench J, Moors TE, Navarro PP, Castaño-Díez D, Schweighauser G, Graff-Meyer A, Goldie KN, Sütterlin R, Huisman E, Ingrassia A, Gier Y. d., Rozemuller AJM, Wang J, Paepe AD, Erny J, Staempfli A, Hoernschemeyer J, Großerüschkamp F, Niedieker D, El-Mashtoly SF, Quadri M, Van Ijcken WFJ, Bonifati V, Gerwert K, Bohrmann B, Frank S, Britschgi M, Stahlberg H, Van de Berg WDJ, & Lauer ME (2019). Lewy pathology in Parkinson’s disease consists of crowded organelles and lipid membranes. Nature Neuroscience, 22, 1099–1109. [DOI] [PubMed] [Google Scholar]
- Shirendeb UP, Calkins MJ, Manczak M, Anekonda V, Dufour B, McBride JL, Mao P, & Reddy PH (2011). Mutant huntingtin’s interaction with mitochondrial protein Drp1 impairs mitochondrial biogenesis and causes defective axonal transport and synaptic degeneration in Huntington’s disease. Human Molecular Genetics, 21, 406–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai W, Huijie X, Mengjing Z, Danyi L, Zhijie L, Dong D, & Baojian W (2016). Recent advances in understanding of kinetic interplay between phase II metabolism and efflux transport. Current Drug Metabolism, 17, 922–929. [DOI] [PubMed] [Google Scholar]
- Sipos I, Tretter L, & Adam-Vizi V (2003). Quantitative relationship between inhibition of respiratory complexes and formation of reactive oxygen species in isolated nerve terminals. Journal of Neurochemistry, 84, 112–118. [DOI] [PubMed] [Google Scholar]
- Smeyne M, & Smeyne RJ (2013). Glutathione metabolism and Parkinson’s disease. Free Radical Biology and Medicine, 62, 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH, Kang KS, & Choi Y-K (2017). Protective effect of casuarinin against glutamate-induced apoptosis in HT22 cells through inhibition of oxidative stress-mediated MAPK phosphorylation. Bioorganic & Medicinal Chemistry Letters, 27, 5109–5113. [DOI] [PubMed] [Google Scholar]
- Song JH, Kim S, Yu JS, Park DH, Kim S-Y, Kang KS, Lee S, & Kim KH (2019). Procyanidin B2 3″-O-gallate isolated from Reynoutria elliptica prevents glutamate-induced HT22 cell death by blocking the accumulation of intracellular reactive oxygen species. Biomolecules, 9, 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH, Lee C, Lee D, Kim S, Bang S, Shin M-S, Lee J, Kang KS, & Shim SH (2018). Neuroprotective compound from an endophytic fungus, Colletotrichum sp. JS-0367. Journal of Natural Products, 81, 1411–1416. [DOI] [PubMed] [Google Scholar]
- Song JH, Lee H-J, & Kang KS (2019). Procyanidin C1 activates the Nrf2/HO-1 signaling pathway to prevent glutamate-induced apoptotic HT22 cell death. International Journal of Molecular Sciences, 20, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Chen J, Petrilli A, Liot G, Klinglmayr E, Zhou Y, Poquiz P, Tjong J, Pouladi MA, Hayden MR, Masliah E, Ellisman M, Rouiller I, Schwarzenbacher R, Bossy B, Perkins G, & Bossy-Wetzel E (2011). Mutant huntingtin binds the mitochondrial fission GTPase dynamin-related protein-1 and increases its enzymatic activity. Nature Medicine, 17, 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK, Kagan VE, Noel K, Jiang X, Linkermann A, Murphy ME, Overholtzer M, Oyagi A, Pagnussat GC, Park J, Ran Q, Rosenfeld CS, Salnikow K, Tang D, Torti FM, Torti SV, Toyokuni S, Woerpel KA, & Zhang DD (2017). Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell, 171, 273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam SR, & Chesselet M-F (2013). Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Progress in Neurobiology, 106–107, 17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Ren X, Qi W, Yuan D, & Simpkins JW (2016). Geissoschizine methyl ether protects oxidative stress-mediated cytotoxicity in neurons through the ‘neuronal Warburg effect’. Journal of Ethnopharmacology, 187, 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Hu F, Wu J, & Zhang S (2017). Cannabidiol attenuates OGD/R-induced damage by enhancing mitochondrial bioenergetics and modulating glucose metabolism via pentose-phosphate pathway in hippocampal neurons. Redox Biology, 11, 577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinney DC, & Anthony J (2011). How were new medicines discovered? Nature Reviews Drug Discovery, 10, 507–519. [DOI] [PubMed] [Google Scholar]
- Takashima M, Ichihara K, & Hirata Y (2019). Neuroprotective effects of Brazilian green propolis on oxytosis/ferroptosis in mouse hippocampal HT22 cells. Food and Chemical Toxicology, 132, 110669. [DOI] [PubMed] [Google Scholar]
- Tan S, Sagara Y, Liu Y, Maher P, & Schubert D (1998). The regulation of reactive oxygen species production during programmed cell death. Journal of Cell Biology, 141, 1423–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S, Schubert D, & Maher P (2001). Oxytosis: A novel form of programmed cell death. Current Topics in Medicinal Chemistry, 1, 497–506. [DOI] [PubMed] [Google Scholar]
- Taveira M, Sousa C, Valentão P, Ferreres F, Teixeira JP, & Andrade PB (2014). Neuroprotective effect of steroidal alkaloids on glutamate-induced toxicity by preserving mitochondrial membrane potential and reducing oxidative stress. The Journal of Steroid Biochemistry and Molecular Biology, 140, 106–115. [DOI] [PubMed] [Google Scholar]
- Terman A, Kurz T, Navratil M, Arriaga EA, & Brunk UT (2010). Mitochondrial turnover and aging of long-lived postmitotic cells: The mitochondrial–lysosomal axis theory of aging. Antioxidants & Redox Signaling, 12, 503–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacek JC, Behera J, George AK, Kamat PK, Kalani A, & Tyagi N (2018). Tetrahydrocurcumin ameliorates homocysteine-mediated mitochondrial remodeling in brain endothelial cells. Journal of Cellular Physiology, 233, 3080–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti D, de Bari L, de Rasmo D, Signorile A, Henrion-Caude A, Contestabile A, & Vacca RA (2016). The polyphenols resveratrol and epigallocatechin-3-gallate restore the severe impairment of mitochondria in hippocampal progenitor cells from a Down syndrome mouse model. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1862, 1093–1104. [DOI] [PubMed] [Google Scholar]
- Valenti D, De Rasmo D, Signorile A, Rossi L, de Bari L, Scala I, Granese B, Papa S, & Vacca RA (2013). Epigallocatechin-3-gallate prevents oxidative phosphorylation deficit and promotes mitochondrial biogenesis in human cells from subjects with Down’s syndrome. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1832, 542–552. [DOI] [PubMed] [Google Scholar]
- van der Merwe C, van Dyk HC, Engelbrecht L, van der Westhuizen FH, Kinnear C, Loos B, & Bardien S (2017). Curcumin rescues a PINK1 knock down SH-SY5Y cellular model of Parkinson’s disease from mitochondrial dysfunction and cell death. Molecular Neurobiology, 54, 2752–2762. [DOI] [PubMed] [Google Scholar]
- Wager TT, Hou X, Verhoest PR, & Villalobos A (2016). Central nervous system multiparameter optimization desirability: Application in drug discovery. ACS Chemical Neuroscience, 7, 767–775. [DOI] [PubMed] [Google Scholar]
- Wang B, Liu H, Yue L, Li X, Zhao L, Yang X, Wang X, Yang Y, & Qu Y (2016). Neuroprotective effects of pterostilbene against oxidative stress injury: Involvement of nuclear factor erythroid 2-related factor 2 pathway. Brain Research, 1643, 70–79. [DOI] [PubMed] [Google Scholar]
- Wang CC, Ho YH, Hung CF, Kuo JR, & Wang SJ (2020). Xanthohumol, an active constituent from hope, affords protection against kainic acid-induced excitotoxicity in rats. Neurochemistry International, 133, 104629. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu Q-B, Hou Z-L, Shi S-C, Ren H, Yao G-D, Lin B, Huang X-X, & Song S-J (2020). Discovery of guaiane-type sesquiterpenoids from the roots of Daphne genkwa with neuroprotective effects. Bioorganic Chemistry, 95, 103545. [DOI] [PubMed] [Google Scholar]
- Wang L-Y, Wu J, Yang Z, Wang X-J, Fu Y, Liu S-Z, Wang H-M, Zhu W-L, Zhang H-Y, & Zhao W-M (2013). (M)- and (P)-Bicelaphanol A, dimeric trinorditerpenes with promising neuroprotective activity from Celastrus orbiculatus. Journal of Natural Products, 76, 745–749. [DOI] [PubMed] [Google Scholar]
- Wang S-F, Liu L-F, Wu M-Y, Cai C-Z, Su H, Tan J, Lu J-H, & Li M (2017). Baicalein prevents 6-OHDA/ascorbic acid-induced calcium-dependent dopaminergic neuronal cell death. Scientific Reports, 7, 8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S-N, Li Q, Jing M-H, Alba E, Yang X-H, Sabaté R, Han Y-F, Pi R-B, Lan W-J, Yang X-B, & Chen J-K (2016). Natural xanthones from Garcinia mangostana with multifunctional activities for the therapy of Alzheimer’s disease. Neurochemical Research, 41, 1806–1817. [DOI] [PubMed] [Google Scholar]
- Wang T, Yao J, Chen S, Mao Z, & Brinton RD (2020). Allopregnanolone reverses bioenergetic deficits in female triple transgenic Alzheimer’s mouse model. Neurotherapeutics, 17, 178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wappler EA, Institoris A, Dutta S, Katakam PVG, & Busija DW (2013). Mitochondrial dynamics associated with oxygen-glucose deprivation in rat primary neuronal cultures. PLoS One, 8, e63206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward MW, Huber HJ, Weisová P, Düssmann H, Nicholls DG, & Prehn JHM (2007). Mitochondrial and plasma membrane potential of cultured cerebellar neurons during glutamate-induced necrosis, apoptosis, and tolerance. The Journal of Neuroscience, 27, 8238–8249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Shi D, Zhou T, Tu J, He M, Jiang Y, & Yang B (2020). Identification of two novel prenylated flavonoids in mulberry leaf and their bioactivities. Food Chemistry, 315, 126236. [DOI] [PubMed] [Google Scholar]
- Whitaker RM, Corum D, Beeson CC, & Schnellmann RG (2016). Mitochondrial biogenesis as a pharmacological target: A new approach to acute and chronic diseases. Annual Review of Pharmacology and Toxicology, 56, 229–249. [DOI] [PubMed] [Google Scholar]
- WHO. (2017). Global action plan on the public health response to dementia 2017–2025. In (pp. 52). Geneva, Switzerland: World Health Organization. [Google Scholar]
- Wu B, Kulkarni K, Basu S, Zhang S, & Hu M (2011). First-pass metabolism via UDP-glucuronosyltransferase: A barrier to oral bioavailability of phenolics. Journal of Pharmaceutical Sciences, 100, 3655–3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Chen J, Yang R, Duan F, Li S, & Chen X (2019). Mitochondrial protective effect of neferine through the modulation of nuclear factor erythroid 2-related factor 2 signalling in ischaemic stroke. British Journal of Pharmacology, 176, 400–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C-L, Tang G-H, Guo Y-Q, Xu Y-K, Huang Z-S, & Yin S (2019). Mulberry Diels-Alder-type adducts from Morus alba as multi-targeted agents for Alzheimer’s disease. Phytochemistry, 157, 82–91. [DOI] [PubMed] [Google Scholar]
- Xiong N, Huang J, Chen C, Zhao Y, Zhang Z, Jia M, Zhang Z, Hou L, Yang H, Cao X, Liang Z, Zhang Y, Sun S, Lin Z, & Wang T (2012). Dl-3-n-butylphthalide, a natural antioxidant, protects dopamine neurons in rotenone models for Parkinson’s disease. Neurobiology of Aging, 33, 1777–1791. [DOI] [PubMed] [Google Scholar]
- Xu M-F, Xiong Y-Y, Liu J-K, Qian J-J, Zhu L, & Gao J (2012). Asiatic acid, a pentacyclic triterpene in Centella asiatica, attenuates glutamate-induced cognitive deficits in mice and apoptosis in SH-SY5Y cells. Acta Pharmacologica Sinica, 33, 578–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Ma Q, Fan C, Chen X, Zhang H, & Tang M (2019). Ginsenosides Rb1 and Rg1 protect primary cultured astrocytes against oxygen-glucose deprivation/reoxygenation-induced injury via improving mitochondrial function. International Journal of Molecular Sciences, 20, 6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan F, Wang H, Gao Y, Xu J, & Zheng W (2017). Artemisinin protects retinal neuronal cells against oxidative stress and restores rat retinal physiological function from light exposed damage. ACS Chemical Neuroscience, 8, 1713–1723. [DOI] [PubMed] [Google Scholar]
- Yang D, Wu W, Gan G, Wang D, Gong J, Fang K, & Lu F (2020). (−)-Syringaresinol-4-O-β-D-glucopyranoside from Cortex Albizziae inhibits corticosterone-induced PC12 cell apoptosis and relieves the associated dysfunction. Food and Chemical Toxicology, 141, 111394. [DOI] [PubMed] [Google Scholar]
- Yang E-J, Lee J-Y, Park S-H, Lee T, & Song K-S (2013). Neuroprotective effects of neolignans isolated from Magnoliae Cortex against glutamate-induced apoptotic stimuli in HT22 cells. Food and Chemical Toxicology, 56, 304–312. [DOI] [PubMed] [Google Scholar]
- Yang E-J, Min JS, Ku H-Y, Choi H-S, Park M-K, Kim MK, Song K-S, & Lee D-S (2012). Isoliquiritigenin isolated from Glycyrrhiza uralensis protects neuronal cells against glutamate-induced mitochondrial dysfunction. Biochemical and Biophysical Research Communications, 421, 658–664. [DOI] [PubMed] [Google Scholar]
- Yang E-J, Park GH, & Song K-S (2013). Neuroprotective effects of liquiritigenin isolated from licorice roots on glutamate-induced apoptosis in hippocampal neuronal cells. Neurotoxicology, 39, 114–123. [DOI] [PubMed] [Google Scholar]
- Yang E-J, & Song K-S (2014). Andrographolide, a major component of Andrographis paniculata leaves, has the neuroprotective effects on glutamate-induced HT22 cell death. Journal of Functional Foods, 9, 162–172. [Google Scholar]
- Yang E-J, & Song K-S (2015). Polyozellin, a key constituent of the edible mushroom Polyozellus multiplex, attenuates glutamate-induced mouse hippocampal neuronal HT22 cell death. Food & Function, 6, 3678–3686. [DOI] [PubMed] [Google Scholar]
- Yang H, Sung SH, Kim J, & Kim YC (2011). Neuroprotective diarylheptanoids from the leaves and twigs of Juglans sinensis against glutamate-induced toxicity in HT22 cells. Planta Medica, 77, 841–845. [DOI] [PubMed] [Google Scholar]
- Yang J-L, Mukda S, & Chen S-D (2018). Diverse roles of mitochondria in ischemic stroke. Redox Biology, 16, 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Ye C-Y, Huang X-T, Tang X-C, & Zhang H-Y (2012). Decreased accumulation of subcellular amyloid-β with improved mitochondrial function mediates the neuroprotective effect of huperzine A. Journal of Alzheimer’s Disease, 31, 131–142. [DOI] [PubMed] [Google Scholar]
- Youle RJ, & van der Bliek AM (2012). Mitochondrial fission, fusion, and stress. Science, 337, 1062–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousufuddin M, & Young N (2019). Aging and ischemic stroke. Aging, 11, 2542–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Wang S, Chen X, Yang H, Li X, Xu Y, & Zhu X (2015). Orientin alleviates cognitive deficits and oxidative stress in Aβ1–42-induced mouse model of Alzheimer’s disease. Life Sciences, 121, 104–109. [DOI] [PubMed] [Google Scholar]
- Yu X-L, Li Y-N, Zhang H, Su Y-J, Zhou W-W, Zhang Z-P, Wang S-W, Xu P-X, Wang Y-J, & Liu R-T (2015). Rutin inhibits amylin-induced neurocytotoxicity and oxidative stress. Food & Function, 6, 3296–3306. [DOI] [PubMed] [Google Scholar]
- Yu Y, Zhao Y, Teng F, Li J, Guan Y, Xu J, Lv X, Guan F, Zhang M, & Chen L (2018). Berberine improves cognitive deficiency and muscular dysfunction via activation of the AMPK/SIRT1/PGC-1a pathway in skeletal muscle from naturally aging rats. The Journal of Nutrition, Health & Aging, 22, 710–717. [DOI] [PubMed] [Google Scholar]
- Zafeer MF, Firdaus F, Anis E, & Mobarak Hossain M (2019). Prolong treatment with trans-ferulic acid mitigates bioenergetics loss and restores mitochondrial dynamics in streptozotocin-induced sporadic dementia of Alzheimer’s type. Neurotoxicology, 73, 246–257. [DOI] [PubMed] [Google Scholar]
- Zeissler M-L, Eastwood J, McCorry K, Hanemann OC, Zajicek JP, & Carroll CB (2016). Delta-9-tetrahydrocannabinol protects against MPP + toxicity in SH-SY5Y cells by restoring proteins involved in mitochondrial biogenesis. Oncotarget, 7, 46603–46614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng R, Wang X, Zhou Q, Fu X, Wu Q, Lu Y, Shi J, Klaunig JE, & Zhou S (2019). Icariin protects rotenone-induced neurotoxicity through induction of SIRT3. Toxicology and Applied Pharmacology, 379, 114639. [DOI] [PubMed] [Google Scholar]
- Zeng R, Zhou Q, Zhang W, Fu X, Wu Q, Lu Y, Shi J, & Zhou S (2019). Icariin-mediated activation of autophagy confers protective effect on rotenone induced neurotoxicity in vivo and in vitro. Toxicology Reports, 6, 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Ma Z, Wang J, Xie A, & Xie J (2011). Myricetin attenuated MPP+-induced cytotoxicity by anti-oxidation and inhibition of MKK4 and JNK activation in MES23.5 cells. Neuropharmacology, 61, 329–335. [DOI] [PubMed] [Google Scholar]
- Zhang X, Du L, Zhang W, Yang Y, Zhou Q, & Du G (2017). Therapeutic effects of baicalein on rotenone-induced Parkinson’s disease through protecting mitochondrial function and biogenesis. Scientific Reports, 7, 9968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Yao-Yue C, Qin G-W, & Guo L-H (2012). Luteolin from Purple Perilla mitigates ROS insult particularly in primary neurons. Neurobiology of Aging, 33, 176–186. [DOI] [PubMed] [Google Scholar]
- Zheng A, Li H, Xu J, Cao K, Li H, Pu W, Yang Z, Peng Y, Long J, Liu J, & Feng Z (2015). Hydroxytyrosol improves mitochondrial function and reduces oxidative stress in the brain of db/db mice: role of AMP-activated protein kinase activation. British Journal of Nutrition, 113, 1667–1676. [DOI] [PubMed] [Google Scholar]
- Zhou L, Yao G-D, Lu L-W, Song X-Y, Lin B, Wang X-B, Huang X-X, & Song S-J (2018). Neolignans from red raspberry (Rubus idaeus L.) exhibit enantioselective neuroprotective effects against H2O2-induced oxidative injury in SH-SY5Y cells. Journal of Agricultural and Food Chemistry, 66, 11390–11397. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Wang S, Li Y, Yu S, & Zhao Y (2018). SIRT1/PGC-1α signaling promotes mitochondrial functional recovery and reduces apoptosis after intracerebral hemorrhage in rats. Frontiers in Molecular Neuroscience, 10, 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Wang K, Zhang K, Lin X, Zhu L, & Zhou F (2016). Puerarin protects human neuroblastoma SH-SY5Y cells against glutamate-induced oxidative stress and mitochondrial dysfunction. Journal of Biochemical and Molecular Toxicology, 30, 22–28. [DOI] [PubMed] [Google Scholar]
- Zille M, Karuppagounder SS, Chen Y, Gough PJ, Bertin J, Finger J, Milner TA, Jonas EA, & Ratan RR (2017). Neuronal death after hemorrhagic stroke in vitro and in vivo shares features of ferroptosis and necroptosis. Stroke, 48, 1033–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziqubu K, Dludla PV, Joubert E, Muller CJF, Louw J, Tiano L, Nkambule BB, Kappo AP, & Mazibuko-Mbeje SE (2020). Isoorientin: A dietary flavone with the potential to ameliorate diverse metabolic complications. Pharmacological Research, 158, 104867. [DOI] [PubMed] [Google Scholar]
- Zolezzi JM, Carvajal FJ, Ríos JA, Ordenes D, Silva-Alvarez C, Godoy JA, & Inestrosa NC (2013). Tetrahydrohyperforin induces mitochondrial dynamics and prevents mitochondrial Ca 2+ overload after Aβ and Aβ-AChE complex challenge in rat hippocampal neurons. Journal of Alzheimer’s Disease, 37, 735–746. [DOI] [PubMed] [Google Scholar]
- Zorova LD, Popkov VA, Plotnikov EY, Silachev DN, Pevzner IB, Jankauskas SS, Babenko VA, Zorov SD, Balakireva AV, Juhaszova M, Sollott SJ, & Zorov DB (2018). Mitochondrial membrane potential. Analytical Biochemistry, 552, 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zündorf G, & Reiser G (2011). Calcium dysregulation and homeostasis of neural calcium in the molecular mechanisms of neurodegenerative diseases provide multiple targets for neuroprotection. Antioxidants & Redox Signaling, 14, 1275–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]


