Abstract
The purpose of this study was to evaluate the efficacy of the VIP-HANA application (app) for improving symptom burden in a randomized control trial of 100 people living with HIV (PLWH) who have non-AIDS conditions associated with HIV. The intervention group received the VIP-HANA app which allowed them to report their symptoms every week and receive self-management strategies tailored to their symptoms. The control arm received an app to report their symptoms every week but did not receive any strategies. The results of our study suggest that symptom burden improved in the participants of both study arms. Although these findings do not support the efficacy of VIP-HANA in improving symptom burden in PLWH who have HANA conditions, this could be a function of the study design. Findings suggest that PLWH are interested in monitoring their symptoms, which could have implications for the wider use of digital health for patient surveillance.
Resumen
El propósito de este estudio fue evaluar la eficacia de la aplicación VIP-HANA para mejorar la carga de síntomas en una prueba controlada aleatorizada de 100 personas que viven con VIH con condiciones no de SIDA asociadas al VIH. El grupo de intervención recibió la aplicación VIP-HANA que les permitió reportar sus síntomas cada semana y recibir estrategias de autogestión personalizadas. El brazo de control recibió una aplicación para reportar sus síntomas cada semana, pero no recibió ninguna estrategia. Los resultados de nuestro estudio sugieren que la carga general de los síntomas mejoro entre los participantes en ambos brazos del estudio. Aunque estos hallazgos no apoyan la eficacia de la aplicación VIP-HANA para mejorar la carga de síntomas en PVVS con condiciones de HANA, esto puede ser una función del diseño del estudio. Estos hallazgos sugieren que PVVS están interesados en monitorear sus síntomas, lo que puede tener implicaciones para el uso más amplio de salud digital para la vigilancia de pacientes.
Introduction
HIV has evolved from an acute to a chronic illness largely due to antiretroviral therapy (ART).(1) As a result, people living with HIV (PLWH) are living longer, and the average age of PLWH in the US is approximately 50 years old. (2) As PLWH age, they are developing the chronic illnesses and co-morbid conditions that are often seen in older persons who are uninfected. (3, 4) Sixty percent of deaths in PLWH occur from HIV-associated non-AIDS (HANA) conditions (e.g., cardiovascular disease, liver disease, diabetes, and asthma), (3) and people suffering from these conditions are more likely to be affected by bothersome symptoms. (5–7)
HANA conditions are becoming more common as PLWH age. Care of PLWH affected by HANA conditions is a priority area of the Office of AIDS Research HIV and Aging Working Groups. (5) One important area in need of further study is managing symptoms of PLWH with HANA conditions. (5) Since PLWH are living longer and being affected by HANA conditions, they are also experiencing more symptoms that influence functional capacity. (10) An individual’s ability to identify and self-manage symptoms of their HIV illness has been shown to improve patient outcomes and quality of life (8, 9) and functional capacity. (10) This is particularly relevant because reduced functional capacity increases vulnerability to developing co-morbid conditions. Given that symptom management has the potential to help PLWH with HANA conditions improve functional capacity, quality of life, and ART adherence,(11) effective management of symptoms is an important component of healthcare for PLWH. The goal of symptom management is to avert, mitigate or delay a negative symptom that has the potential to compromise functioning through biomedical/professional intervention, or self-management strategies. (12) Yet, there has been little research specific to support self-management of HANA conditions for PLWH.
VIP-HANA App
In response to this knowledge gap, our study team developed the Video Information Provider (VIP)-HANA app to help PLWH self-manage their symptoms. To achieve this, we used an iterative process described elsewhere. (13) In summary, 769 PLWH with HANA conditions completed an anonymous online survey to assess the frequency and severity of HANA-related symptoms and collected information on the self-management strategies PLWH used to ameliorate them. (14) Sample self-care strategies tailored by race and sex can be found in Table 1. These strategies were incorporated into the VIP-HANA app using branching logic. After designing an initial version of the VIP-HANA app, the system underwent a comprehensive usability evaluation(15) to ensure that the app was usable and understandable to our end-users. (16) Following refinements of the app, the study team finalized the VIP-HANA app for further testing in a randomized controlled trial (RCT). The purpose of this study was to assess the efficacy of the VIP-HANA app for improving symptom burden in a RCT of 100 PLWH with HANA conditions.
Table 1.
Sample Self-Care Strategies for Weight Loss, Nausea/ Vomiting, and Difficulty with Urination Symptoms Tailored by HANA Condition, Sex, and Race
| Top 3 Ranked Self-Care Strategies for Symptom of Weight Loss tailored by HANA condition | ||
| HANA Condition | Ranking | Self-Care strategy |
| COPD | 1 | Try changing your diet. Try slowly adding more fresh foods and whole grains to your diet. Try reducing foods with added sugar, processed foods, and fast foods. |
| 2 | Some people find trying to eat smaller quantities of food at one time may help. | |
| 3 | Make sure you stay hydrated and drink lots of fluids during the day. | |
| No COPD | 1 | Try to add more physical activity and exercise into your life. Exercise and regular movement has been shown to help with weight loss. |
| 2 | Try eating more and more often. Focus on nutrient-rich foods, such as fresh fruits, veggies, and nuts. | |
| 3 | Try to reduce the amount of salt you consume. Try to avoid foods that have a lot of salt also such as salad dressings, sauces, and fast food. | |
| Top 3 Ranked Self-Care Strategies for Symptoms of Nausea/Vomiting by HANA condition | ||
|
HANA Condition |
Ranking | Self-Care strategy |
| Diabetes | 1 | While nauseated, you may want to limit your eating. Try the BRAT diet: bread, rice, applesauce, and toast. |
| 2 | Avoid greasy foods, fried foods, and alcohol, and try eating dry foods, such as toast and crackers. | |
| 3 | Take frequent sips of water or suck on ice chips. | |
| No Diabetes | 1 | Make sure you stay hydrated and drink lots of fluids during the day. |
| 2 | Try drinking Ginger Ale to relieve nausea. | |
| 3 | Try eating dry foods, such as toast and crackers. Try to eat when you are not feeling sick. | |
| Top 3 Ranked Self-Care Strategies for Difficulty with Urination Tailored by Sex and Race | ||
| Race | Ranking | Self-Care strategy |
| White | 1 | Make sure you stay hydrated and drink lots of fluids during the day. |
| 2 | Try developing body self-awareness by regularly bringing attention to your body in various circumstances, and by mentally "scanning" the body to "check in" with feelings of comfort and discomfort. | |
| 3 (Male only) | You may find that ejaculating can help alleviate some issues of urination. | |
| 3 | Try exercising the muscles in the area to strengthen them. One way to do this is by trying to stop urinating mid-way. | |
| Non-White | 1 | While you should take care to remain hydrated, you should try to limit the liquid that you drink (especially drinks other than water). |
| 2 | Try to hold your urine longer than usual before using the bathroom, which might strengthen the muscles that control urination. | |
| 3 | Try an over-the-counter supplement, such as cranberry pills, or UTI pills, like AZO. | |
Methods
This RCT was registered at ClinicalTrails.gov, NCT03182738. The Institutional Review Board (IRB) of Columbia University Irving Medical Center reviewed and approved all research activities. The primary outcome of the study was reduction of symptom burden, and the secondary outcomes focused on engagement with healthcare provider, app use and perceived usability.
Sampling and Recruitment
Recruitment for the RCT began in January 2018 and ended in October 2018. Participants were recruited from our study registry of individuals who had participated in prior studies and consented to be contacted for future studies, as well as through study flyers posted at HIV-related community organizations throughout New York City.
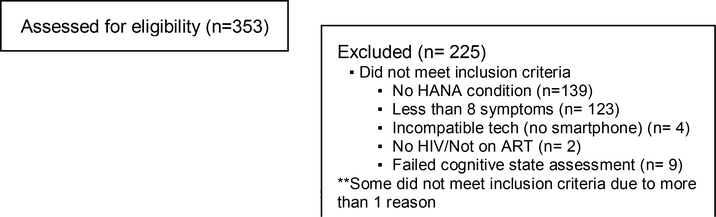
Inclusion criteria were the following: a) 18 years or older; b) having an HIV/AIDS diagnosis; c) ownership of a smartphone or tablet; d) able to communicate and read in English; e) taking ART; f) a diagnosis of at least one of the following HANA conditions: cardiovascular disease, liver disease, asthma, chronic obstructive pulmonary disease (COPD), osteoporosis, diabetes, arthritis, renal failure, and/or chronic diagnosis of bronchitis; and g) having at least eight of the following 29 HANA symptoms listed in Figure 1 in the past seven days. Potential participants were excluded if they were pregnant, currently participating in another mobile app study or text message study for PLWH, or provided an incorrect response to an abridged Mini Mental State Examination. (17)
Figure 1.
List of 29 HANA Related Symptoms
Procedures
Eligible participants provided written informed consent at their initial in-person visit. Afterwards, each participant was randomized to either the intervention or the control arm. Participants were randomized 1:1 to the intervention or the control arm. Both groups were asked to report the frequency and severity of 29 HANA symptoms listed in Figure 1 on their smartphone via the VIP-HANA app. Participants in the intervention arm received tailored self-management strategies based on their symptom reporting, HANA condition, and gender. Participants in the control arm received an app for reporting their symptoms but did not receive self-management strategies.
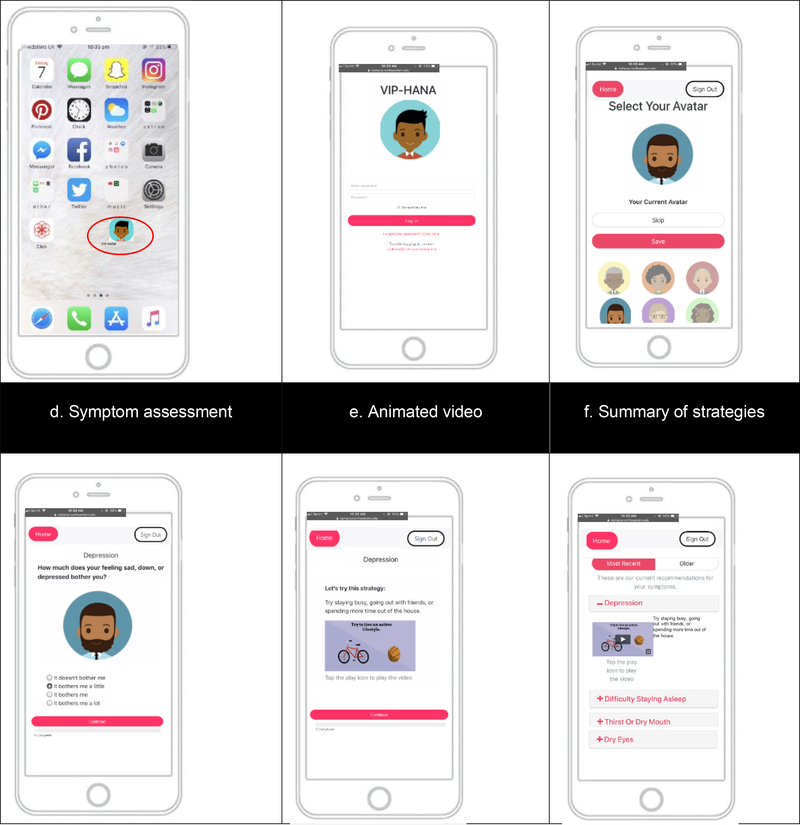
Upon enrollment, study personnel helped participants with downloading the app at the baseline visit. As part of the installation, study staff assisted participants with creating a shortcut to the app on their home screen (Figure 2a). They then clicked on the app icon, logged in to the VIP-HANA app (Figure 2b) and selected an avatar (Figure 2c) who guided them through the VIP-HANA app. Participants were instructed to log in once a week to use the app which allowed them to assess their symptoms and receive self-management strategies. Both study groups completed weekly assessments of their symptoms (Figure 2d). The intervention group participants received self-management strategies tailored to their gender and race/ethnicity for reducing the reported symptoms, whereas the control group did not. In addition to the text of the self-management strategies, the intervention group participants also watched a short-animated video that illustrated the self-management strategy (Figure 2e). At the end of each app session, participants were able to view a graph illustrating their symptoms over time, and the intervention group participants received a summary of the recommended self-management strategies tailored to their symptoms (Figure 2f).
Figure 2:
Sample Screenshots of the VIP-HANA App
All participants were asked to use the app every week for the total duration of the study (6 months) and received a reminder to complete the app weekly. Participants set a reminder based on their day/time preference and the App sent a weekly e-mail reminder to login to the app and complete the survey. Three reminders were sent if a participant had not completed the survey. Participants were compensated with $5 for each session completed. At the end of the initial visit, participants completed an electronic survey comprising a large battery of instruments including those related to demographics and our primary and secondary outcome measures. Participants returned for an in-person office visit at 3 months and at 6 months to complete follow-up electronic surveys. Table 2 provides an overview of the study sample’s demographics characteristics.
Table 2:
Participant characteristics at baseline by group, N=100
| Total, N=100 n (%) | Intervention, N=50 n (%) | Control, N=50 n (%) | pa | |
|---|---|---|---|---|
| Age, Mean (SD) | 52.8 (10.8) | 51.9 (10.4) | 53.7 (11.2) | 0.41 |
| Gender identity | 0.19 | |||
| Male | 52 (52) | 31 (62) | 21 (42) | |
| Female | 44 (44) | 18 (36) | 26 (52) | |
| Transgender female | 3 (3) | 1 (2) | 2 (4) | |
| Genderqueer | 1 (1) | 0 (0) | 1 (2) | |
| Sex at birth | 0.11 | |||
| Male | 56 (56) | 32 (64) | 24 (48) | |
| Female | 44 (44) | 18 (36) | 26 (52) | |
| Race | 0.77 | |||
| White | 10 (10) | 5 (10) | 5 (10) | |
| Black/African American | 65 (65) | 34 (68) | 31 (62) | |
| Native Hawaiian or other Pacific Islander | 1 (1) | 0 (0) | 1 (2) | |
| Multiracial | 3 (3) | 1 (2) | 2 (4) | |
| Other | 21 (21) | 10 (20) | 11 (22) | |
| Latino/ Hispanic Ethnicity | 0.08 | |||
| Yes | 30 (30) | 12 (24) | 18 (36) | |
| No | 70 (70) | 38 (76) | 32 (64) | |
| Employment | 0.78 | |||
| Employed full-time | 2 (2) | 1 (2) | 1 (2) | |
| Employed part-time | 10 (10) | 6 (12) | 4 (8) | |
| Disabled/Student | 4 (4) | 2 (4) | 2 (4) | |
| Employed off the books | 3 (3) | 1 (2) | 2 (4) | |
| Unemployed | 25 (25) | 13 (26) | 12 (24) | |
| Disabled | 41 (41) | 20 (40) | 21 (42) | |
| Retired | 9 (9) | 5 (10) | 4 (8) | |
| Student | 3 (3) | 2 (4) | 1 (2) | |
| Disabled + (Retired, Student) | 3 (3) | 0 (0) | 3 (6) | |
| Education | 0.85 | |||
| None | 3 (3) | 2 (4) | 1 (2) | |
| Elementary school | 2 (2) | 1 (2) | 1 (2) | |
| Some high school | 20 (20) | 9 (18) | 11 (22) | |
| High school diploma (or GED) | 31 (31) | 15 (30) | 16 (32) | |
| Some college | 29 (29) | 15 (30) | 14 (28) | |
| Associate/technical degree | 6 (6) | 2 (4) | 4 (8) | |
| Bachelor/college degree | 5 (5) | 3 (6) | 2 (4) | |
| Professional/graduate degree | 4 (4) | 3 (6) | 1 (2) | |
| Annual Income | 0.04 | |||
| Less than $10,000 | 46 (46) | 29 (58) | 17 (34) | |
| $10,000–$19,999 | 27 (27) | 7 (14) | 20 (40) | |
| $20,000–$39,999 | 13 (13) | 8 (16) | 5 (10) | |
| $40,000 or more | 4 (4) | 3 (6) | 1 (2) | |
| Don’t know | 10 (10) | 3 (6) | 7 (14) | |
| Smartphone type | 0.48 | |||
| Android phone | 71 (71) | 35 (70) | 36 (72) | |
| iPhone | 23 (23) | 12 (24) | 11 (22) | |
| Other (e.g. tablet, netbook, etc) | 6 (6) | 3 (6) | 3 (6) |
Column percentages may not sum to 100% due to rounding error.
p-values by t-test, Wilcox rank sum test, or Chi-square test.
Measures
Symptom Burden
Symptom Burden Score was the primary outcome and was used to assess the frequency and severity of the most common symptoms in PLWH. The score used in this study is an expanded version of the 20-item HIV symptom index (18), with a score calculated for 29 symptoms. Each symptom was given a score ranging from 0 to 4 with scores indicating the following: 0 (not experienced), 1 (experienced – it doesn’t bother me), 2 (experienced – it bothers me a little), 3 (experienced – it bothers me), and 4 (experienced – it bothers me a lot). The higher the score (closer to 4), the great the symptom burden. The score was calculated using responses from each participant’s weekly app session over the duration of 6 months. The 20-item HIV symptom index demonstrated strong construct validity and has shown to have strong associations with physical and mental health summary scores in past validation studies. (18) Measures of secondary outcomes listed below were assessed via the electronic survey administered at the initial baseline visit, 3-month follow-up visit, and 6-month follow-up visit.
Engagement with Healthcare Provider
The Engagement with Healthcare Provider scale was validated for PLWH to rate their interactions with any of their healthcare providers. (19) The scale includes 13 items each with a 4-point Likert scale where 1=always true and 4=never true. A total score was calculated for this scale with a possible range of 13–52. A low score (a score closer to 13) indicates greater engagement between the patient and provider. Psychometric properties of the scale include a one-factor solution emerging from a principal components factor analysis with Varimax rotation with an Eigenvalue of 8.6 and 66.5% of the variance explained. Cronbach’s alpha reliability estimate was 0.96. (19)
App Use
To measure app use, data was exported from the VIP-HANA app to Excel to identify the average number of times participants used the app and the average time spent on individual app sessions, by group. The dataset was cleaned to only include complete app sessions. Extreme outliers were removed using the interquartile range rule to properly calculate the average time spent during app sessions. (20)
Usability
Health Information Technology Usability Evaluation Scale (Health-ITUES)
The Health-ITUES questionnaire was used to identify perceived usability of the VIP-HANA app. The scale is comprised of 20 items rated on a 5-point Likert scale ranging from strongly disagree (1) to strongly agree (5) and has 4 subscales that emphasize quality of work life, usefulness, ease of use, and user control. The Health-ITUES was validated for the evaluation of the usability of mHealth technology and showed high internal consistency reliability (Cronbach alpha=.85-.92). Further, each of the Health-ITUES subscales and the overall scale was moderately to strongly correlated with the PSSUQ scales (r=.46-.70), demonstrating the criterion validity of the Health-ITUES. An overall score for the Health-ITUES was calculated as the average of all 20 items, as it has been validated in PLWH. (21)
Post-Study System Usability Questionnaire (PSSUQ)
The PSSUQ assesses user satisfaction with the VIP-HANA app using 3 subscales: usefulness, information quality, and interface quality. The questionnaire used for the trial included 16 items rated on a 7-point Likert scale ranging from strongly agree (1) to strongly disagree (7) and included a “not applicable” option. (22) The overall score for the PSSUQ was created so that a lower score indicates higher user satisfaction with the app. (23) Factor analysis of the PSSUQ from 5 years of usability studies indicated a 3-factor structure with strong estimated reliabilities (ranging from .83–.96) demonstrating strong psychometric properties for this tool. (23)
Statistical Analysis
All analyses were performed using SAS version 9.4. Descriptive statistics were used to analyze demographic, engagement with healthcare provider, and usability data. T-tests or Wilcoxon rank sum tests and Chi-squared tests were used to examine differences between study groups for each of these variables.
For the primary outcome, symptom burden, we compared mean scores in 3 periods: Weeks 1–5 as period 1, weeks 6–17 as period 2; and weeks 18 to 6 months as period 3. Linear mixed models were used to (1) estimate mean scores and corresponding standard errors of each of 29 symptoms in the 3 periods for each arm; (2) estimate and test for score changes from period 1 to periods 2 and 3 for each arm; and (3) compare difference in score changes between 2 arms. The analysis excluded values when an observation was missing. The maximum likelihood-based method is used to estimate parameters of models. The model parameter estimates will be unbiased if the missing is completely at random (MCAR) or missing at random (MAR). The power calculation for the study was based on one specific symptom, i.e., there is sufficient power (>80%) to test expected difference between 2 arms for a given symptom. However, when we calculated power, we did not consider multiple tests of 28 symptoms. All p-values were adjusted for multiple tests by controlling false discovery rate (FDR) at 0.05.
RESULTS
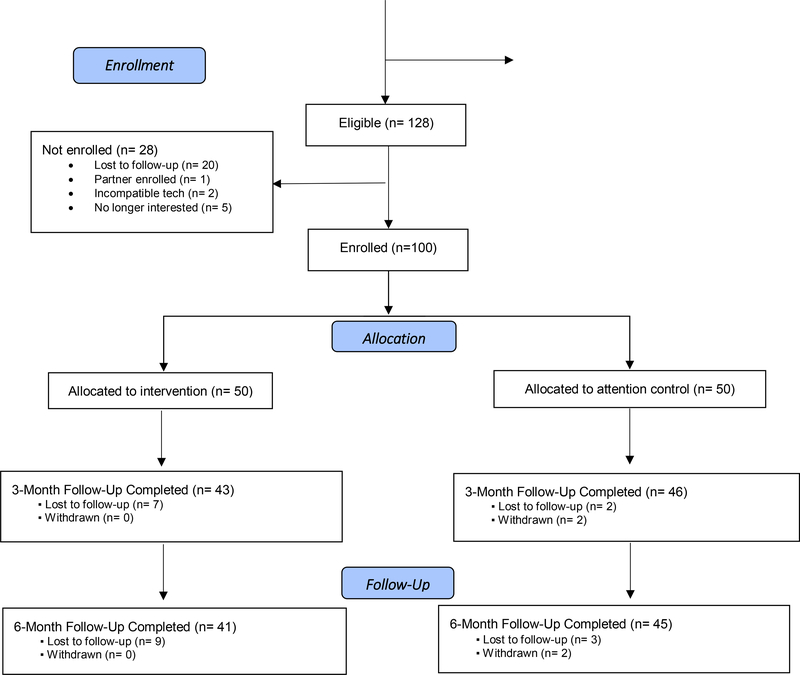
100 participants were enrolled, with 89 subjects retained at 3-months and 86 subjects retained at 6-months (Figure 3). The average age among participants was about 53 years old, with over 50% identifying as male and 65% identifying as African American. Our study outcomes focused on changes in symptom burden, participants’ use of the app, their engagement with their healthcare provider and their perceived usability of the app.
Figure 3.
VIP HANA Trial CONSORT Diagram
Symptom Burden:
During period 1 of the trial, all 100 participants provided a response to all 29 symptoms, but only 88 participants provided a response for symptom burden at period 2 (42 subjects in the intervention and 46 subjects in the control). Table 3 illustrates the differences in symptom burden between groups from period 1 to period 3.
Table 3:
Difference in Symptom Burden Score from period 1 to period 3
| Symptoma | Arm | Estimateb | SE | p-valuec | Compare 2 arms p-valued |
|---|---|---|---|---|---|
| Muscle aches or pain | Intervention | 0.36 | 0.10 | 0.001 | 0.940 |
| Control | 0.34 | 0.09 | 0.001 | ||
| Overall low energy or fatigue | Intervention | 0.58 | 0.09 | <0.001 | 0.951 |
| Control | 0.59 | 0.08 | <0.001 | ||
| Decreased sex drive | Intervention | 0.18 | 0.08 | 0.069 | 0.100 |
| Control | 0.43 | 0.08 | <0.001 | ||
| Depression | Intervention | 0.02 | 0.10 | 0.876 | 0.049 |
| Control | 0.40 | 0.09 | <0.001 | ||
| Anxiety | Intervention | 0.22 | 0.09 | 0.032 | 0.951 |
| Control | 0.23 | 0.08 | 0.013 | ||
| Difficulty staying asleep | Intervention | −0.08 | 0.09 | 0.480 | 0.094 |
| Control | 0.19 | 0.08 | 0.064 | ||
| Difficulty concentrating | Intervention | 0.02 | 0.07 | 0.863 | 0.122 |
| Control | 0.22 | 0.07 | 0.005 | ||
| Difficulty falling asleep | Intervention | −0.07 | 0.09 | 0.560 | 0.295 |
| Control | 0.12 | 0.09 | 0.299 | ||
| Unplanned changes in weight | Intervention | 0.03 | 0.08 | 0.795 | 0.076 |
| Control | 0.29 | 0.07 | 0.001 | ||
| Lightheadedness or dizziness | Intervention | 0.01 | 0.08 | 0.940 | 0.076 |
| Control | 0.27 | 0.07 | 0.001 | ||
| Thirst or dry mouth | Intervention | 0.39 | 0.09 | <0.001 | 0.082 |
| Control | 0.11 | 0.08 | 0.271 | ||
| Shortness of breath | Intervention | 0.18 | 0.08 | 0.069 | 0.935 |
| Control | 0.15 | 0.08 | 0.111 | ||
| Clumsiness or difficulty with balance | Intervention | −0.03 | 0.08 | 0.771 | 0.184 |
| Control | 0.15 | 0.07 | 0.081 | ||
| Fever, night sweats, or chills | Intervention | 0.02 | 0.08 | 0.863 | 0.844 |
| Control | 0.08 | 0.07 | 0.431 | ||
| Constipation, gas, or bloating | Intervention | 0.25 | 0.09 | 0.018 | 0.935 |
| Control | 0.28 | 0.08 | 0.004 | ||
| Diarrhea | Intervention | 0.18 | 0.08 | 0.069 | 0.968 |
| Control | 0.18 | 0.08 | 0.041 | ||
| Cough | Intervention | −0.01 | 0.09 | 0.959 | 0.844 |
| Control | 0.06 | 0.08 | 0.577 | ||
| Speech difficulties | Intervention | −0.16 | 0.05 | 0.010 | 0.013 |
| Control | 0.09 | 0.05 | 0.123 | ||
| Difficulty remembering | Intervention | 0.16 | 0.08 | 0.111 | 0.935 |
| Control | 0.13 | 0.08 | 0.168 | ||
| Heartburn | Intervention | 0.08 | 0.08 | 0.448 | 0.535 |
| Control | 0.19 | 0.07 | 0.023 | ||
| Changes in appetite | Intervention | 0.12 | 0.08 | 0.233 | 0.882 |
| Control | 0.07 | 0.07 | 0.444 | ||
| Dry eyes | Intervention | 0.28 | 0.07 | 0.001 | 0.076 |
| Control | 0.04 | 0.07 | 0.637 | ||
| Problems achieving or maintaining erection | Intervention | 0.15 | 0.10 | 0.258 | 0.712 |
| Control | 0.02 | 0.13 | 0.920 | ||
| Neuropathy | Intervention | 0.14 | 0.09 | 0.233 | 0.844 |
| Control | 0.07 | 0.09 | 0.567 | ||
| Pain or discomfort during sex | Intervention | −0.12 | 0.05 | 0.069 | 0.129 |
| Control | 0.02 | 0.05 | 0.741 | ||
| Ringing in ears or noise intolerance | Intervention | 0.17 | 0.07 | 0.029 | 0.076 |
| Control | −0.07 | 0.06 | 0.431 | ||
| Difficulty with urination | Intervention | 0.02 | 0.07 | 0.876 | 0.712 |
| Control | −0.06 | 0.06 | 0.480 | ||
| Nausea or vomiting | Intervention | 0.15 | 0.07 | 0.069 | 0.414 |
| Control | 0.03 | 0.06 | 0.743 |
The estimates for each variable listed here were not controlled for baseline characteristics. When baseline characteristics (age, sex, race) were controlled for, no changes were observed.
Positive estimate indicates that the symptom score decreased from period 1 to period 3
Test for difference in mean score change from period 1 to period 3 by intervention arms. All p values were adjusted for multiple tests by controlling false discovery rate (FDR) at 0.05.
Test for difference in mean score change from period 1 to period 3 between 2 arms. All p values were adjusted for multiple tests by controlling false discovery rate (FDR) at 0.05.
Lower symptom burden was seen in intervention group participants from period 1 to period 2 who experienced the following symptoms: fatigue (p<0.0001); thirst or dry mouth (p<0.0001); muscle aches (p=0.0013); problems achieving or maintaining an erection (p=0.013); decreased sex drive (p=0.0021); neuropathy (p=0.016); diarrhea (p=0.017); constipation, gas, or bloating (p=0.014); anxiety (p=0.0088); ringing in ears or noise intolerance (p=0.0027); heartburn (p=0.0097); dry eyes (p=0.0044); heart burn (p=0.0088); and nausea or vomiting (p=0.009). Intervention group participants had a greater decrease in symptom burden score than control group participants for following symptoms: neuropathy (p=0.023); ringing in ears or noise intolerance (p=0.0066); thirst or dry mouth (p=0.023); nausea or vomiting (p=0.0066); and dry eyes (0.023).
There was no significant difference in symptom burden change from period 1 to period 3 for the intervention as compared to control group. The control group showed a greater decrease than the intervention group for depression (p=0.049). Similar results were seen in the comparison of symptom burden score change from period 1 to period 3 in both study arms. Intervention group participants had lower symptom burden scores at period 3 compared to period 1 on the following symptoms: fatigue (p<0.0001); dry month (p<0.0001); muscle aches (p=0.0012); dry eyes (p=0.006); constipation, gas, or bloating (p=0.018); anxiety (0.032); and ringing in ears or noise intolerance (p=0.029).
Engagement with Healthcare Provider
The average score on the Engagement with Healthcare Provider Scale was similar for both groups at baseline, where the score for the intervention group was 17.78 (SD=8.07) and for the control group was 17.13 (SD=6.52) with no significant difference (p=0.66). However, at the 3-month follow-up, those in the control group appeared to have a trend towards better engagement with their healthcare provider with a mean score of 17.33 (SD=5.43) compared to the intervention group that had a score of 20.17 (SD=8.68), but with no significant difference (p=0.08). Average scores at the 6-month follow-up were one again very similar in both groups., with the intervention group scoring 18.05 (SD=7.72) and the control group having an average score of 18.70 (SD=8.02) with no significant difference between groups (p=0.71).
VIP-HANA App Use
App use between the study groups was greater among those in the control arm participants with an average of 28.2 times (SD=12.7), whereas participants in the intervention group used the app an average of 25.3 times (SD=13.7) over the 6 month study period. On average, the intervention group participants spent 5.9 (SD=3.9) minutes on the app per app session during the study period, while the control group subjects spent an average of 3.9 (SD=2.1) minutes during each session. There was a significant difference in the total time (in minutes) spent on the VIP-HANA app (p<0.001) between study groups. Table 4 provides an overview on the app use.
Table 4:
Overview of VIP-HANA App Usage Over 6 Months
| VIP-HANA App Usage | N | Mean | SD | P-value | |
|---|---|---|---|---|---|
| Number of app sessions | Intervention | 50 | 25.3 | 13.7 | 0.268 |
| Control | 50 | 28.2 | 12.7 | ||
| Time spent on app session (in minutes) | Intervention | 50 | 5.9 | 3.9 | <0.001 |
| Control | 49 | 3.9 | 2.1 |
Usability
At the 6-month follow-up, 86 participants completed two usability measures: the Health-ITUES and the PSSUQ questionnaires. The mean and standard deviation for all 4 subscales and the overall score for the Health-ITUES are reported in Table 5. Overall, the subjects rated the usability of the VIP-HANA app as high usability with scores >4.2 out of 5. Table 6 illustrates the mean scores and standard deviation for the 3 subscales and the overall PSSUQ score. These scores likewise indicate that participants found the app to be highly usable with an overall mean score of 1.77, with a lower score indicating greater usability.
Table 5:
Health-ITUES Scores (N=86)
| Scale (Range 1–5) | Mean | SD |
|---|---|---|
| Quality of work life | 4.34 | 0.94 |
| Perceived usefulness | 4.22 | 0.96 |
| Perceived ease of use | 4.48 | 0.74 |
| User control | 4.28 | 0.91 |
| Overall Health-ITUES score | 4.32 | 0.80 |
Table 6:
PSSUQ Scores (N=86)
| Scale (Range 1–7) | Mean | SD |
|---|---|---|
| Usefulness | 1.63 | 0.91 |
| Information quality | 1.90 | 1.13 |
| Interface quality | 1.78 | 1.14 |
| Overall PSSUQ score | 1.77 | 0.99 |
Discussion
The goal of the study was to develop a self-management tool for PLWH with HANA conditions to better manage their symptom burden. Research has demonstrated that quality of life and functional capacity decrease as the number and severity of HIV-related symptoms increase. (24, 25) Patients’ symptom experiences and symptom management success are strongly related to HIV disease progression and adverse clinical profiles (26, 27), making the management of HIV and HANA related symptoms particularly important. Our primary study outcomes therefore focused on improvement of 29 symptoms most experienced by PLWH with HANA conditions.
Findings from our study suggest that overall symptom burden improved among participants in both study arms. Although these findings do not support the efficacy of the VIP-HANA app in improving symptom burden in PLWH with HANA conditions, this may be a function of the study design and specifically, the control arm condition. Participants in both study arms tracked their symptoms and were provided with a log of their symptoms over time. While participants in the control arm did not receive self-management strategies through the app, it is possible that these participants consulted outside sources (i.e. Internet, friends and healthcare providers), as we found in our earlier research (28) for strategies to help ameliorate their symptoms. There is also the possibility that assessments alone were sufficient for improving symptom burden which has been demonstrated in other clinical trials. (29)
Engagement with health care provider was very high in both study groups with no significant differences between the study groups. Interestingly, the control group did show slightly greater improvement in engagement with their health care provider at 3-months as compared to the intervention group participants. Given that the VIP-HANA app provided self-care strategies for the intervention group, participants in this group may have been less likely to increase engagement with their health care provider because they were able to rely on the app to self-manage their symptoms. In contrast, control group participants may have had a heightened awareness of their symptoms as they were reflecting on their symptoms every week. Additionally, since the control group participants were not given self-care strategies, these participants may have been more likely to engage with their health care providers to seek care for ameliorating their newly identified symptoms.
Further, as part of the evaluation of the VIP-HANA app, we also conducted follow-up focus groups sessions. The results are reported elsewhere (30) and participants reported that they found the app to be very useful as a tool for communicating with their healthcare providers. Participants used the app between doctor’s visits and shared the log of their symptoms with their healthcare providers suggesting that this can be a useful tool for supporting healthcare engagement, which is salient in PLWH, who have a chronic and infectious illness that requires regular interaction with the healthcare system. Identification of symptoms alone was more powerful than anticipated and possibly motivated both self-management and other health seeking behaviors in participants in both study groups.
Notably, participants in both study groups made regular use of the app and found the app to be highly usable. The technology acceptance literature suggests that usability is a key factor in actual use of the technology. (31) These findings reinforce the need for developing highly usable technology. This is especially important in a population with lower education than the general population, and so developing consumer health technology that is usable for this population has the potential for improving access to healthcare. (32–35)
There are several limitations of this study. First, the sample was limited to New York City which limits the generalizability of these findings. Second, there may be sample bias. People who were more conscientious about their health were more interested in participating in the study. Finally, the 20-item HIV Symptom Index was validated but the expanded 29-item which was used in this study was not validated.
Conclusion
Findings suggest that PLWH are willing and interested in tracking their symptoms which may have implications for the broader use of digital health for patient surveillance. This is especially important in our study population who are almost exclusively racial/ethnic minority persons with very low-income. Given the health disparities that are evident in these groups, developing digital health surveillance tools may have important consequences and have the potential to enable health disparate patients to better self-manage their health as well as be more active in their own healthcare needs.
Clinical Resources
HIV Symptom Management Guidelines: http://hivinsite.ucsf.edu/InSite?page=kb-03-01-06
AHRQ Digital Healthcare Research Program: https://digital.ahrq.gov/
National Institute of Aging – HIV, AIDS and Older People: https://www.nia.nih.gov/health/hiv-aids-and-older-people
Acknowledgements:
Research reported in this publication was supported by the National Institute of Nursing Research of the National Institutes of Health under award numbers: R01NR015737 and K24NR018621. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1).Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382(9903):1525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Fujimoto K, Wang P, Flash CA, Kuhns LM, Zhao Y, Amith M, et al. Network Modeling of PrEP Uptake on Referral Networks and Health Venue Utilization Among Young Men Who Have Sex with Men. AIDS Behav 2019;23(7):1698–707. [DOI] [PubMed] [Google Scholar]
- 3).Neuhaus J, Angus B, Kowalska JD, La Rosa A, Sampson J, Wentworth D, et al. Risk of all-cause mortality associated with nonfatal AIDS and serious non-AIDS events among adults infected with HIV. AIDS. 2010;24(5):697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Justice AC, Braithwaite RS. Lessons learned from the first wave of aging with HIV. AIDS. 2012;26 Suppl 1:S11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).High KP, Brennan-Ing M, Clifford DB, Cohen MH, Currier J, Deeks SG, et al. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr 2012;60 Suppl 1:S1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Phillips AN, Neaton J, Lundgren JD. The role of HIV in serious diseases other than AIDS. AIDS. 2008;22(18):2409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Greene M, Justice AC, Lampiris HW, Valcour V. Management of human immunodeficiency virus infection in advanced age. JAMA. 2013;309(13):1397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Schnall R, Cho H, Mangone A, Pichon A, Jia H. Mobile Health Technology for Improving Symptom Management in Low Income Persons Living with HIV. AIDS Behav 2018;22(10):3373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Nicholas PK, Kirksey KM, Corless IB, Kemppainen J. Lipodystrophy and quality of life in HIV: symptom management issues. Appl Nurs Res 2005;18(1):55–8. [DOI] [PubMed] [Google Scholar]
- 10).Balderson BH, Grothaus L, Harrison RG, McCoy K, Mahoney C, Catz S. Chronic illness burden and quality of life in an aging HIV population. AIDS Care. 2013;25(4):451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Wilson IB, Cleary PD. Clinical predictors of functioning in persons with acquired immunodeficiency syndrome. Med Care. 1996;34(6):610–23. [DOI] [PubMed] [Google Scholar]
- 12).The University of California SFSoNSMFG. A Model for Symptom Management. Image J Nurs Sch 1994;26:272–6. [PubMed] [Google Scholar]
- 13).Schnall R, Liu J, Mohr DC, Bakken S, Hirshfield S, Siegel K, et al. Multi-Modal Methodology for Adapting Digital Health Tools to New Populations: Adaptation of the Video Information Provider (VIP) for Persons Living with HIV with HIV-Associated Non-AIDS (HANA) Conditions. Stud Health Technol Inform 2019;264:1347–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Iribarren S, Siegel K, Hirshfield S, Olender S, Voss J, Krongold J, et al. Self-Management Strategies for Coping with Adverse Symptoms in Persons Living with HIV with HIV Associated Non-AIDS Conditions. AIDS Behav 2018;22(1):297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Stonbraker S, Cho H, Hermosi G, Pichon A, Schnall R. Usability Testing of a mHealth App to Support Self-Management of HIV-Associated Non-AIDS Related Symptoms. Stud Health Technol Inform 2018;250:106–10. [PMC free article] [PubMed] [Google Scholar]
- 16).Stonbraker S, Porras T, Schnall R. Patient preferences for visualization of longitudinal patient-reported outcomes data. Journal of the American Medical Informatics Association. 2019;27(2):212–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Schultz-Larsen K, Lomholt RK, Kreiner SJJoCE. Mini-Mental Status Examination: a short form of MMSE was as accurate as the original MMSE in predicting dementia. 2007;60(3):260–7. [DOI] [PubMed] [Google Scholar]
- 18).Justice AC, Holmes W, Gifford AL, Rabeneck L, Zackin R, Sinclair G, et al. Development and validation of a self-completed HIV symptom index. J Clin Epidemiol 2001;54 Suppl 1:S77–90. [DOI] [PubMed] [Google Scholar]
- 19).Bakken S, Holzemer WL, Brown MA, Powell-Cope GM, Turner JG, Inouye J, et al. Relationships between perception of engagement with health care provider and demographic characteristics, health status, and adherence to therapeutic regimen in persons with HIV/AIDS. AIDS Patient Care STDS. 2000;14(4):189–97. [DOI] [PubMed] [Google Scholar]
- 20).Van den Broeck J, Cunningham SA, Eeckels R, Herbst K. Data cleaning: detecting, diagnosing, and editing data abnormalities. PLoS Med 2005;2(10):e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Schnall R, Cho H, Liu J. Health Information Technology Usability Evaluation Scale (Health-ITUES) for Usability Assessment of Mobile Health Technology: Validation Study. JMIR Mhealth Uhealth. 2018;6(1):e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Lewis JR. Psychometric Evaluation of the PSSUQ Using Data from Five Years of Usability Studies. International Journal of Human-Computer Interaction. 2002;14(3–4):463–388. [Google Scholar]
- 23).Lewis JR. Psychometric evaluation of the PSSUQ using data from five years of usability studies. International Journal of Human-Computer Interaction. 2002;14(3–4):463–88. [Google Scholar]
- 24).Lorenz KA, Cunningham WE, Spritzer KL, Hays RD. Changes in symptoms and health-related quality of life in a nationally representative sample of adults in treatment for HIV. Qual Life Res 2006;15(6):951–8. [DOI] [PubMed] [Google Scholar]
- 25).Siegel K, Schrimshaw EW, Dean L. Symptom interpretation: implications for delay in HIV testing and care among HIV-infected late middle-aged and older adults. AIDS Care. 1999;11(5):525–35. [DOI] [PubMed] [Google Scholar]
- 26).Leserman J, Jackson ED, Petitto JM, Golden RN, Silva SG, Perkins DO, et al. Progression to AIDS: the effects of stress, depressive symptoms, and social support. Psychosom Med 1999;61(3):397–406. [DOI] [PubMed] [Google Scholar]
- 27).Spirig R, Moody K, Battegay M, De Geest S. Symptom management in HIV/AIDS: advancing the conceptualization. ANS Adv Nurs Sci 2005;28(4):333–44. [DOI] [PubMed] [Google Scholar]
- 28).Schnall R, Liu J, Iribarren S. Information sources of self-care strategies for persons living with HIV. Int J Med Inform 2018;111:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Hoekstra J, de Vos R, van Duijn NP, Schadé E, Bindels PJE. Using the Symptom Monitor in a Randomized Controlled Trial: The Effect on Symptom Prevalence and Severity. Journal of Pain and Symptom Management. 2006;31(1):22–30. [DOI] [PubMed] [Google Scholar]
- 30).Cho H, Porras T, Flynn G, Schnall R. Usability of a Consumer Health Informatics Tool Following Completion of a Clinical Trial: Focus Group Study. J Med Internet Res 2020;22(6):17708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Davis F Perceived usefulness, perceived ease of use, and user acceptance of information technology. MIS Quarterly. 1989;13(3):319–40. [Google Scholar]
- 32).Keselman A, Logan R, Smith CA, Leroy G, Zeng-Treitler Q. Developing informatics tools and strategies for consumer-centered health communication. J Am Med Inform Assoc 2008;15(4):473–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Alpay L, Verhoef J, Xie B, Te’eni D, Zwetsloot-Schonk JH. Current Challenge in Consumer Health Informatics: Bridging the Gap between Access to Information and Information Understanding. Biomed Inform Insights. 2009;2(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Christopher Gibbons M Use of health information technology among racial and ethnic underserved communities. Perspect Health Inf Manag 2011;8:1f. [PMC free article] [PubMed] [Google Scholar]
- 35).George S, Moran E, Duran N, Jenders RA. Using animation as an information tool to advance health research literacy among minority participants. AMIA Annu Symp Proc 2013;2013:475–84. [PMC free article] [PubMed] [Google Scholar]