Abstract
Objective:
Depleting pathogenic B cells could treat SLE. However, depleting B cells in the presence of an inflammatory setting such as lupus is difficult. We hypothesized that a Type II anti-CD20 mAb with a different mechanism–obinutuzumab (GA101)—could be more effective in depletion in lupus and that efficient B cell depletion would result in amelioration of disease.
Methods:
We treated lupus-prone MRL/lpr mice expressing human CD20 on B cells (hCD20 MRL/lpr) with either rituximab (RTX) or GA101 and measured depletion under various conditions as well as multiple clinical endpoints.
Results:
A single dose of GA101 was markedly more effective than RTX in depleting B cells in diseased MRL/lpr mice. RTX overcame resistance to B cell depletion in diseased MRL/lpr mice with continuous treatments. GA101 was more effective in treating hCD20 MRL/lpr mice with early disease, as treated mice had reduced glomerulonephritis, lower anti-RNA autoantibody titers and fewer activated CD4 T cells compared to RTX treated mice. GA101 could also treat advanced disease and continual treatment prolonged survival. Using variants of GA101 we also elucidated depletion mechanisms in vivo in mice with lupus.
Conclusions:
Albeit both anti-CD20 antibodies ameliorated disease in an early disease setting, GA101 was more effective than RTX in important parameters, such as glomerulonephritis score. GA101 proved beneficial in an advanced disease model, where it prolonged survival. These data support clinical testing of GA101 in SLE and lupus nephritis.
Keywords: Obinutuzumab (GA101), Rituximab (RTX), B cells, SLE, kidney disease
Introduction
Systemic Lupus Erythematosus (SLE) is a systemic autoimmune disease characterized by activated autoreactive B and T cells, which leads to the production of autoantibodies, immune complexes, infiltration of lymphocytes into target tissues, and production of inflammatory cytokines. (1). Whereas patients typically present with skin and oral lesions, the autoimmune process can damage various organs such as kidney, skin, lungs, brain, and heart (2). A serious and common complication of SLE is glomerulonephritis.
B cells play a key role and serve multiple functions in the mediation and progression of autoimmune diseases, including SLE in mice and humans, by both producing autoantibodies against nuclear antigens and acting as APC to activate pathogenic T cells (3). We initially created B-cell deficient lupus-prone mouse strains, which failed to develop activated CD8 and CD4 T cells, and dermatitis and nephritis were abrogated (4, 5). Similar findings have been obtained in multiple murine models of lupus (6–8).
B cell-targeted therapy for the treatment of SLE has been extensively investigated. Indeed, belimumab, which targets B-cell activating factor (BAFF), showed clinical efficacy in patients with SLE, leading to its FDA approval in 2011. However, two trials of RTX in patients with SLE and lupus nephritis, a well-established anti-CD20 chimeric monoclonal antibody that depletes B cells, failed to meet their respective primary endpoints (9–11). This led to multiple theories to explain the failure, ranging from suboptimal trial design, to the notion that B cells were not in fact critical for lupus pathogenesis.
Prior to these studies, we had created a murine model of anti-CD20 treatment of lupus by introducing a bacterial artificial chromosome transgene that expresses human CD20 into the mouse germline and then fully backcrossing it to both normal and autoimmune-prone genetic backgrounds. We then used conventional murine anti-CD20 antibodies to deplete B cells. These agents successfully depleted B cells in the spleens of BALB/c mice, but were strikingly ineffective in the lymphoid tissues of MRL/lpr mice (12). Nonetheless, we found that by delivering very high doses of anti-CD20 for multiple weeks, we could eventually deplete B cells and mitigate disease (13). Similar findings were obtained by others and us in additional murine lupus strains, using either anti-hCD20 Abs or murine anti-mouse CD20 (8). We went on to show that IgG immune complexes in the serum of autoimmune mice was sufficient to block IgG-anti-CD20-mediated B cell depletion in vivo (12); further, lupus patients are known to be unable to clear IgG-opsonized RBC (14), presumably for similar reasons. Since only limited doses, sufficient to deplete B cell lymphoma in non-autoimmune individuals, were used in the clinical studies (9, 11), we thus proposed that insufficient tissue B cell depletion in at least a subset of lupus patients could explain why RTX failed to reach clinical endpoints (12).
We hypothesized that a second class of anti-CD20 Ab, called a “type II anti-CD20”, which was postulated to have a different mechanism of action in depletion, could be more efficacious. Compared with type I anti-CD20 antibodies like RTX, type II antibodies like GA101 are reported to exhibit more direct B-cell–killing effects, and rely less on complement-dependent cytotoxicity (15). Furthermore, the Fc-part of GA101 is glycoengineered, enhancing its affinity for FcgRIIIa on NK cells and macrophages/monocytes and as a consequence antibody-dependent cellular cytotoxicity and antibody-dependent cellular phagocytosis (15, 16). GA101 is only one of two such antibodies in common use (17, 18) and has been developed and approved under the tradename GAZYVA/GAZYVARO for the treatment of chronic lymphocytic leukemia (19, 20) and follicular lymphoma (21, 22).
In this study, we used our hCD20-Tg murine lupus mouse model to compare the B cell depletion efficiency of GA101 and RTX in MRL/lpr mice. We then further evaluated GA101 B cell depletion efficiency and therapeutic effect in MRL/lpr mice when treatment was given at disease onset, after disease establishment, and with continuous treatment. We also used a mutant of GA101 with abolished effector functions (23) along with a non-glycoengineered version of GA101 (wtGA101) to help elucidate the context of lupus. The overall conclusion of this work is that indeed GA101 is a more potent B cell depleting agent in the face of lupus even though its mechanism of action at least partly overlaps with that of Type I Abs in vivo, and moreover, that GA101 is an effective treatment for murine SLE, more effective than RTX at acute depletion, which leads to better clinical response in some contexts.
Methods
Mice
A BAC transgenic mouse expressing human CD20 on B cells was generated (13) and backcrossed >10 generations onto the MRL/lpr background to make MRL/lpr mice expressing human CD20 (hCD20 MRL/lpr). Mice were euthanized by CO2 inhalation. All mice were housed under specific pathogen–free conditions, and all experiments were performed with the approval of the University of Pittsburgh Institutional Animal Care and Use Committees.
Antibody reagents for in vivo studies
Antibodies used are described in Supplemental Table 1. To reduce immunogenicity in long term experiments murinized GA101 bearing a human Fab region of obinutuzumab and a muIgG2a Fc-portion, and murine rituximab (RTX) based on 2B8 were generated according to standard procedures as glycoengineered (GA101) or wildtype non-glycongineered (wtGA101) antibodies. GA101 P329G LALA mutant (Mutant GA101) was used to investigate the role of Fc-mediated effector function (23, 24). Isotype control IgG2a was purchased from BioXCell (Clone C1.18.4, Catalog# BE0085).
B cell depletion
B cells were depleted by intraperitoneal injection of female hCD20 MRL/lpr mice with indicated amounts of antibody. Unless otherwise specified, for long-term treatment experiments mice were initially given 2mg i.p. of RTX, GA101, or isotype control (mouse IgG2a), then treated twice weekly with 1mg for indicated time periods.
Flow Cytometry Analysis
Preparation of splenocytes has been described (25). The following Abs were produced in the laboratory or purchased as indicated: anti-mouse CD11c PE/Cy7 (N418; Biolegend); anti-mouse TCRβ PerCP-Cy5.5 (H57–597; BioLegend); anti-mouse CD19 BUV395 (1D3; Becton Dickinson), anti-mouse CD19 AL488 (1D3.2); anti-mouse CD4 BV605 (RM4; Becton Dickinson), anti-mouse CD4 PE (GK1.5 Biolegend); anti-mouse CD62L PE/Cy7 (MEL-14; Biolegend); anti-mouse CD44 BV605 (1M7; Becton Dickinson), anti-mouse CD44 APC/Cy7 (IM7; Biolegend); anti-mouse I-A/I-E APC/Cy7 (M5/114.15.2; BioLegend); anti-mouse CD138 BV605 (281–2; Becton Dickinson), anti-mouse CD138 PE (281–2; Biolegend); anti-mouse CD8 PacBlue (TIB-105); anti-mouse CD38 AL680 (90), anti-mouse CD38 PacBlue (90); PNA AL488; anti-mouse kappa PacBlue (187.1); anti-mouse CD11b PE (M1/70; Becton Dickinson); anti-mouse CD35 AL647 (8C12); anti-mouse CD23 PE/Cy7 (B3B4; Biolegend); anti-mouse IgM AL647 (B7–6); anti-mouse IgG2a PE (Goat polyclonal; Southern Biotech). Flow cytometry data were collected on a BD LSR II or BD LSRFortessa and analyzed using FlowJo software (TreeStar).
Evaluation of Disease
Skin disease was scored for dorsal lesions on a scale of 0–5 as described (5), based on lesion area, with up to an additional 0.5 points for facial rash/loss of whiskers and 0.25 points for dermatitis of each ear. Spleens and axillary lymph nodes were weighed. For analysis of renal disease, kidneys were bisected and fixed in 10% formalin. Kidneys were embedded in paraffin, sectioned, and stained with H&E by Histo-Scientific Research Laboratory (Mount Jackson, VA). H&E stained sections were scored for interstitial nephritis (IN) on a scale from 0 – 4 and glomerulonephritis (GN) on a scale from 0–6 as described (26, 27). Proteinuria was assayed using Albustix (Siemens).
ELISAs
ELISAs were performed to measure anti-nucleosome, anti-RNA, and anti-Sm antibodies in the serum. Anti-nucleosome and anti-Sm Ab ELISAs were performed as previously described (28). Anti-RNA Ab ELISAs were performed as described (29). The lower limits of detection were 0.02 ug/ml, 0.02 ug/ml, and 0.1 ug/ml respectively.
Serum rituximab and GA101 concentrations were measured by C2D1 sandwich enzyme linked immunoassay (ELISA). An Anti-Rabbit IgG (Fc specific)-Biotin antibody produced in goat (SAB3700856–2MG Sigma-Aldrich) was used as first capture in streptavidin pre-coated 96-well plates and incubated for 1 hour at room temperature. Coated plates were washed 3 times with 200μL wash buffer (10% of FCS in PBS) and incubated for one hour at room temperature with the second capture antibody, an anti-rituximab (anti-idiotype) antibody (MAB9630 R&D system) or an MonoRabᵀᴹ anti-obinutuzumab antibody (169F10) rabbit mAb (A01967 GenScript).. Serum samples were collected pre-injection (control), and at 1 hour, 6 hours, 24 hours, and 48 hours post-antibody treatment. Serum samples were then diluted in wash buffer at a concentration of 1:40,000. Coated plates were washed 3 times with 200μL wash buffer and incubated for one hour at room temperature with the sera samples. Known concentrations of rituximab or obinutuzumab (ranging from 10ng/mL to 0.15ng/mL) were included to generate a standard curve. The plates were then washed 3 times with 200μL wash buffer and incubated for one hour at room temperature with a goat polyclonal Ab to mouse IgG (HRP), (Abcam, ab97040). Plates were then washed 3 times with 200μL wash buffer and developed for 15 min at room temperature with ABTS solution (Roche ID 11684302001) and signal was detected in an ELISA plate reader (Infinite® M Plex from Tecan Trading AG) using wavelength 405nm and a reference wavelength of 490nm.
Statistical Analysis
Statistical analysis was performed using 2-tailed Mann-Whitney. P values less than 0.05 were considered significant. For survival analysis, the Kaplan-Meier method was used to determine statistical significance. Data were analyzed using GraphPad version 7.0a.
Results
In MRL/lpr mice, GA101 depletes B cells more efficiently than RTX in hCD20 MRL/lpr mice
MRL/lpr mice in our colony start to develop disease at 10-weeks old and by 16-weeks have late stage disease with high titers of circulating autoantibodies and immune-complexes in the serum ((30, 31) and unpublished observations). To determine how, in the setting of established disease, GA101 depletes B cells compared to RTX, 16 week-old transgenic MRL/lpr mice expressing human CD20 on B cells (hCD20 MRL/lpr) were given a single 1mg dose of GA101, Rituximab (RTX), or isotype control (mouse IgG2a) i.p. Two days after treatment, splenic CD19+ B cell populations were enumerated. GA101 more efficiently depleted splenic B cells in 16-week old MRL/lpr mice compared to RTX and control. (Figure 1A).
Figure 1. GA101 depletes MRL/lpr B cells more efficiently than RTX and depletion is not entirely dependent on the Fc portion of GA101.
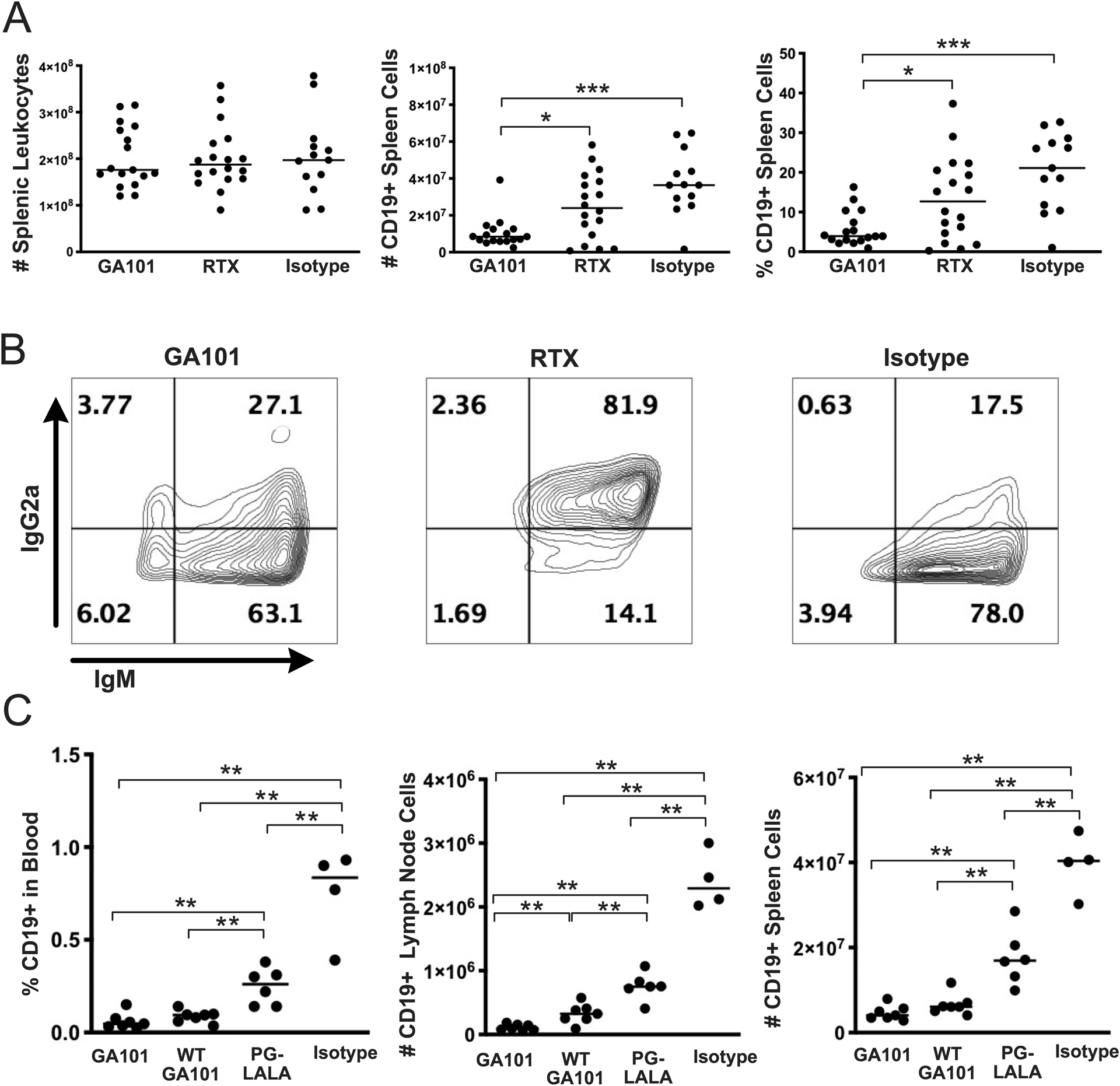
(A) 1mg of GA101, RTX, or control antibody (mouse IgG2a) was i.p. injected into hCD20 MRL/lpr mice. Two days after injection, B cell depletion in the spleen was measured by flow cytometry. Data are pooled from 5 independent experiments. (B) Residual B cells were stained with anti-IgG2a and anti-IgM antibodies to determine if cells were coated with the injected anti-CD20. (C) Depletion of B cells using GA101 variants: Wild type GA101 (WT GA101) is not glyco-engineered; the Fc mutation (PG-LALA) disrupts antibody-mediated phagocytosis. Mice were injected (i.p.) with 2 mg of GA101, WT GA101, PG-LALA, or isotype control (mouse IgG2a) on day 0, then 1mg at days 2 and 4, followed by sacrifice on day 7. Each symbol is an individual mouse and bars represent medians; flow cytometric analysis is representative of 4 mice (GA101), 3 mice (RTX), and 2 mice (Isotype). Statistics were calculated by two-tailed Mann-Whitney test. *p < 0.05, **p < 0.01, ***p < 0.001.
The reduced efficiency of RTX to deplete splenic B cells could have been due to failure of the antibody to bind to B cells or due to poor clearance of anti-CD20-bound cells. To determine if residual B cells were bound by RTX or GA101, we stained cells with anti-mouse IgG2a. Residual B cells were highly antibody positive in RTX-treated but not GA101-treated mice (Figure 1B). This indicates that the RTX antibodies bound to the B cells, but the RTX-opsonized cells were not being phagocytized by macrophages, whereas the GA101 coated B cells were being more rapidly removed.
We also determined the pharmacokinetics of both murinized molecules in a dose-response single administration into both 6 and 13 week old MRL/lpr mice. Both RTX and GA101 had similar kinetics over 48hr, and a dose-dependent peak serum concentration (Supplemental Figure 1). Notably, therapeutic exposure in the mouse was in the range of several hundred ug/ml as is observed with GA101 in the clinic (32). Thus, we conclude that differences in in vivo exposure to drug cannot account for differences in ability of RTX vs GA101 to deplete B cells.
GA101 depletion is not entirely dependent on the Fc effector function of the antibody in hCD20 MRL/lpr mice
We further wanted to gain insight into the role of the glycoengineering of GA101 and whether the Fc portion of the GA101 antibody plays a role in B cell depletion (Figure 1C). Optimal B cell depletion occurred with an intact Fc and glycoengineered GA101. Depletion with the P329GLALA mutant that lacks Fc-mediated effector functions left three-times as many residual B cells in the spleen compared to native glycoengineered GA101. Yet, it still reduced splenic B cells by 55 % compared to the isotype control. The effect of the non-glycoengineered WT version of GA101 was more subtle, as it caused comparable depletion to the glycoengineered GA101 in most settings. Nonetheless, it was significantly inferior to GA101 in depleting LN B cells, and there was a similar trend in blood and spleen. Therefore, GA101 has multiple mechanisms for depletion in vivo in the context of lupus, which include FcR-independent mechanisms, as has been postulated for Type II antibodies, based on in vitro studies (33).
GA101 is modestly more effective than RTX for longer-term therapy of early disease
Although GA101 depleted B cells more efficiently than RTX after a one-time treatment in older hCD20 MRL/lpr mice, we wanted to determine if RTX, compared to GA101, would be able to overcome inhibition of B cell depletion with continued treatment over a 6-week period when started in younger mice, and if so, if this would modify disease. To this end we began treating 10-week old hCD20 MRL/lpr mice, a time at which disease is initiating (early onset, Supplementary Figure 2). After 6 weeks of treatment, proteinuria and skin disease were measured; spleen and axillary lymph nodes were weighed; serum was obtained for anti-nuclear, anti-RNA, and anti-Sm ELISAs; splenocytes were stained for B cells, plasmablasts, and T cell activation markers; and the kidneys were processed and histologic sections scored for kidney infiltrates and glomerulonephritis.
GA101 treatment slightly reduced spleen cell numbers (Figure 2A), an effect attributable to B cell depletion. Notably, in spleen, GA101 depleted B cells more efficiently than RTX (Figure 2B). Both RTX and GA101 treatment depleted plasmablasts (Figure 2C), an effect likely due to depletion of the CD20+ B cell precursors of short-lived plasmablasts (13). Treatment with both Abs resulted in lower spleen and LN weights (Figures 2D,E) and disease improvement in several surrogate markers of autoimmunity, compared to controls. Both groups demonstrated lower anti-RNA, anti-nucleosome, and anti-Sm autoantibodies (Figures 2F–H). With both treatments T cell activation, a B cell-driven phenomenon (34), was ameliorated, with fewer activated and more naive T cells (Figure 2I) compared to controls. Critically, both RTX and GA101 treatment resulted in reduced proteinuria (Figure 3A), and lower interstitial nephritis (Figure 3B); only GA101 treatment resulted in reduced glomerulonephritis scores compared to controls (Figure 3C). Skin disease was not affected (Figure 3D), possibly due to the early initiation of treatment and the relatively younger age at which the mice were analyzed, as skin disease is a late manifestation; more than half of control-treated mice had no detectable skin disease. Despite that both treatments showed an effect in several pharmacodynamic readouts, GA101 treatment led to an even lower glomerulonephritis score than did RTX treatment (Figure 3C). In addition, compared to RTX-treated mice, GA101 treated mice had fewer activated CD4 T cells (Figure 2I), with a greater proportion of phenotypically naive T cells. Finally, anti-RNA antibodies were significantly reduced in GA101-treated compared to RTX-treated mice (Figure 2F).
Figure 2. Comparison of GA101 and RTX-mediated B cell depletion in early disease.
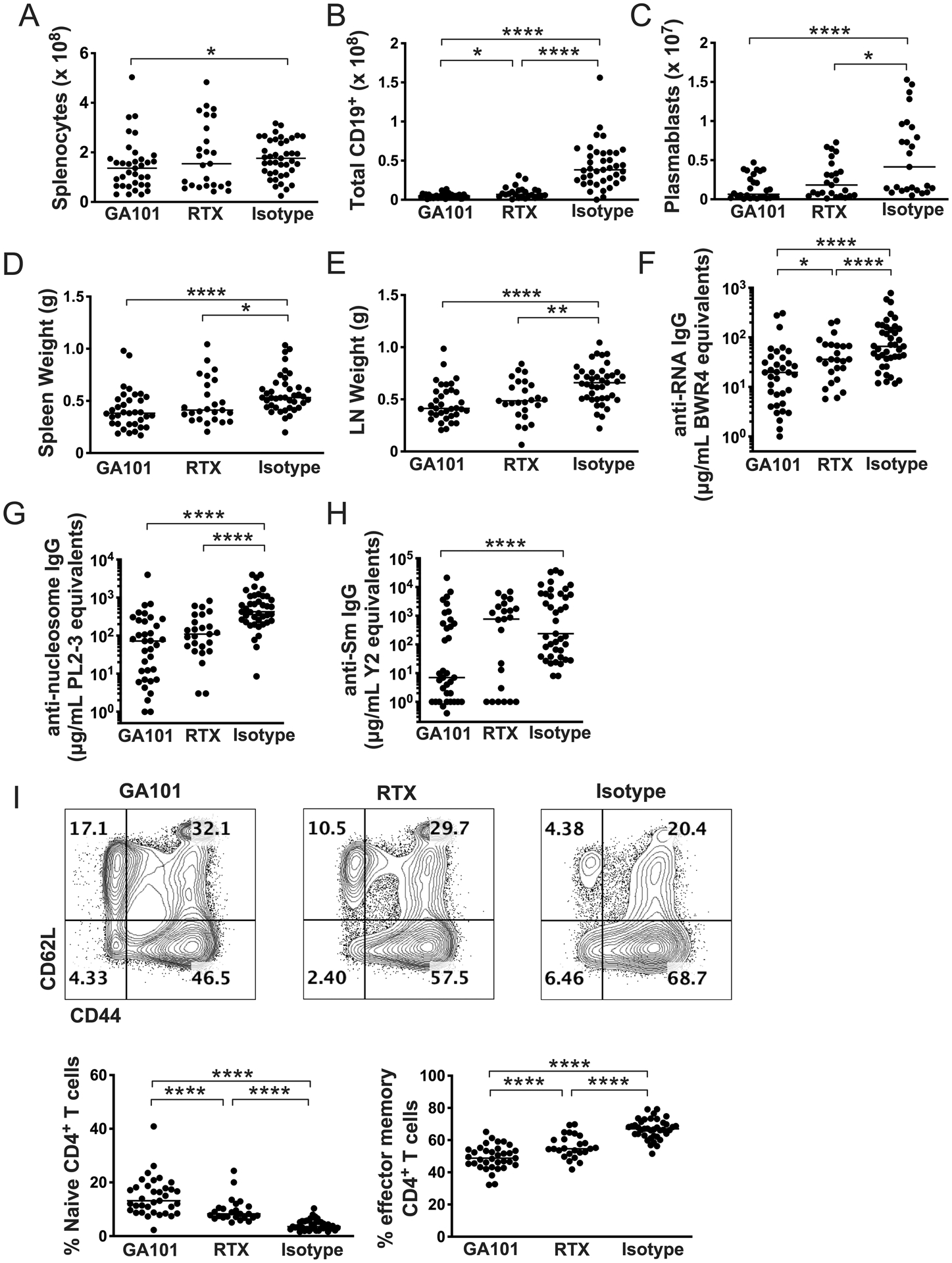
hCD20 MRL/lpr at early onset of disease (10 weeks old) were treated with GA101, RTX, or isotype control for 6 weeks. (A-C) B cell depletion in spleen as determined by flow cytometry. (D & E) Spleens and axillary lymph node weights. (F-H) Serum autoantibody measurement by ELISA. (I) Flow cytometry representative gating and quantitation of percent of effector memory (CD44hi/CD62Llo) CD4+ T cells. Data are combined from 3 independent experiments. Each dot represents an individual mouse and horizontal lines represent the median. Statistics were calculated by two-tailed Mann-Whitney test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Figure 3. Effects of GA101 and RTX-mediated B cell depletion on target organ disease in early autoimmunity.
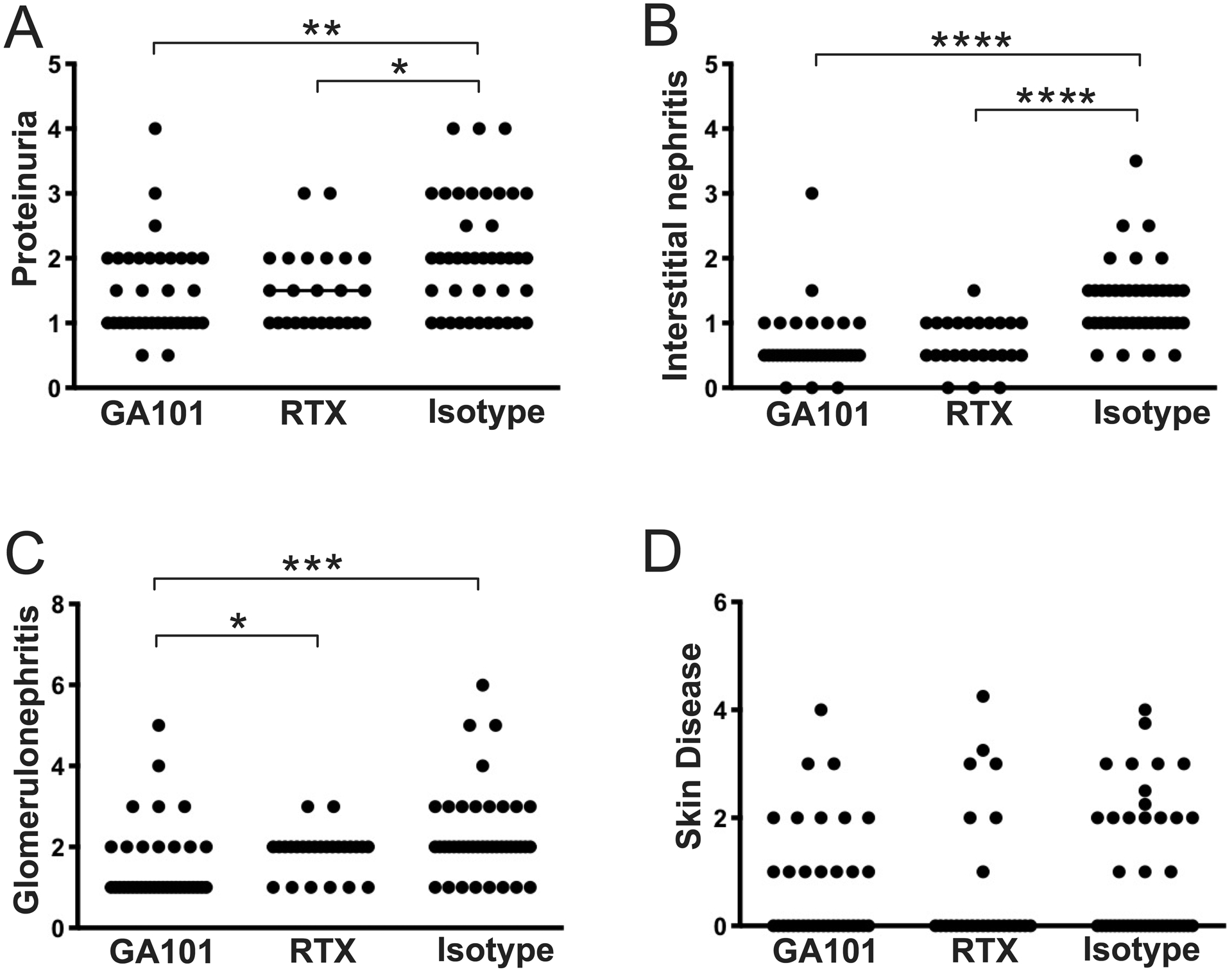
10-week old hCD20 MRL/lpr mice were treated with GA101, RTX, or isotype control for 6 weeks. (A) Proteinuria was evaluated by dipstick assay the day prior to sacrifice. (B & C) H&E stained kidneys sections were scored for interstitial nephritis and glomerulonephritis. (D) Skin disease scores. Data are combined from 3 independent experiments. Each symbol represents an individual mouse and bars represent medians. Statistics were calculated by two-tailed Mann-Whitney test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
GA101 can treat established disease in hCD20 MRL/lpr mice
Given that extended treatment by both GA101 and RTX ameliorated disease when started early in disease, we next wanted to determine if GA101 would successfully deplete B cells and ameliorate disease when treatment started during the established disease state, a setting that had not been tested in this model using any other B cell depletion reagent. We thus began treatment of additional cohorts of 13-week old (established disease, Supplementary Figure 2) hCD20 MRL/lpr mice. After 6 weeks, mice were sacrificed and tissues were processed as before. Even though there was more advanced disease at the start of therapy, B cells were successfully depleted by GA101 (Figures 4A,B). As in the early disease treatment cohorts, plasmablasts were reduced, as was spleen weight (Figures 4C,D), while LN weight was unaffected in this protocol (Figure 4E). All measured autoantibodies were also significantly reduced by GA101 treatment (Figures 4F–H). Again, similar to the early disease cohort, GA101 treatment reduced T cell activation (Figure 4I). Most importantly, GA101 treatment of mice with established disease could still ameliorate many aspects of clinical disease, including proteinuria, interstitial nephritis and skin disease (Figure 5); the latter was not affected in the early disease cohort, probably because there was little disease in the control group (Figure 3D). Only glomerulonephritis was not significantly reduced in the treatment group, although there was a trend in this direction.
Figure 4. Effects of GA101 treatment on immunologic parameter when initiated during established autoimmunity.
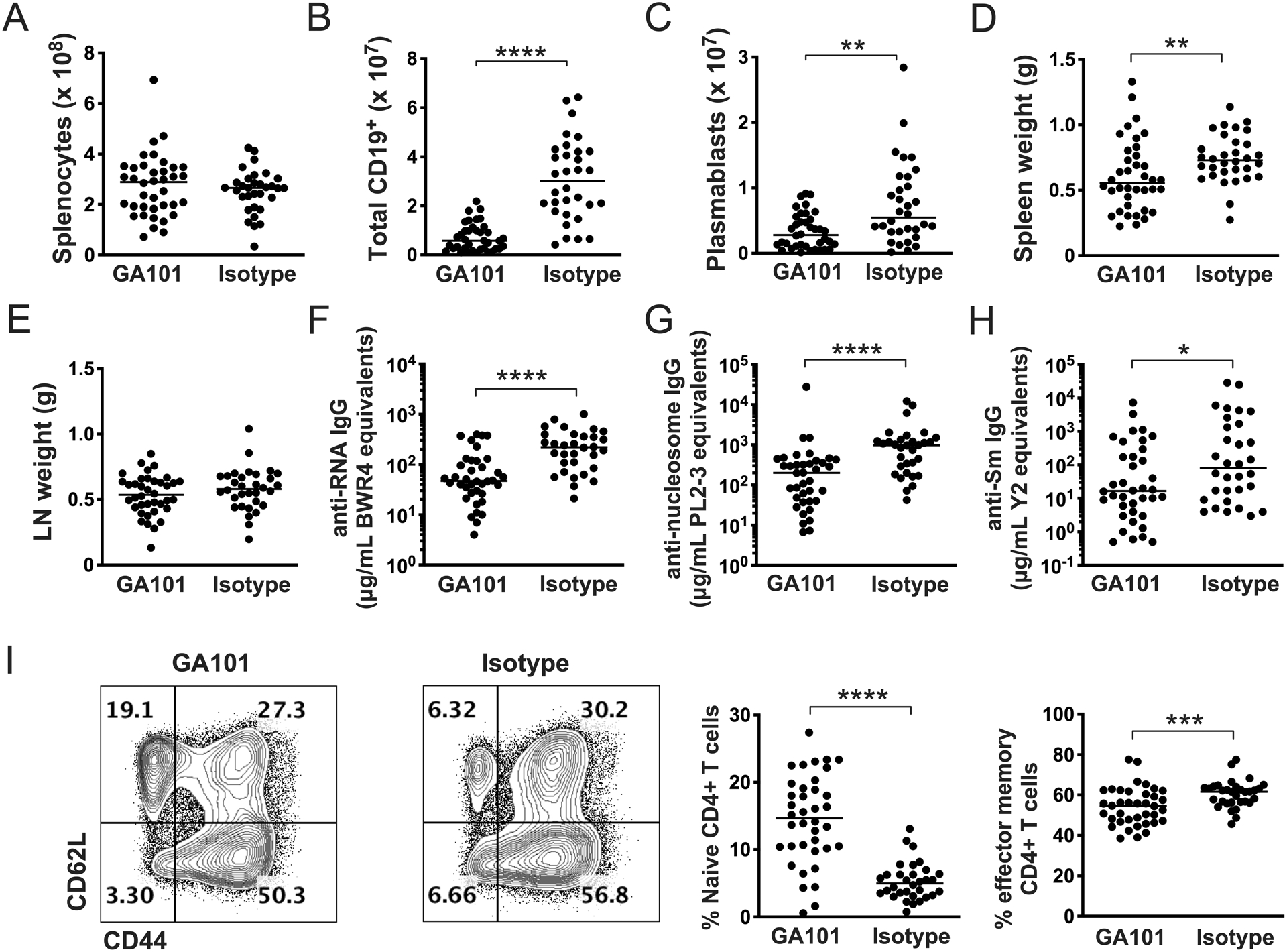
Thirteen-week old hCD20 MRL/lpr mice with established disease were treated with GA101 or isotype control for 6 weeks. (A-C) Effects of treatment on total spleen cell numbers (A), numbers of splenic CD19+ cells (B) and plasmablasts (C) as determined by flow cytometry and cell counting. (D & E) Spleen and axillary lymph node weights. (F-H) Serum autoantibody measurements for the indicated targets by ELISA. (I) Flow cytometry representative gating and quantitation of percent of effector memory (CD44hi/CD62Llo) CD4+ T cells. Data are combined from 3 independent experiments. Each dot represents an individual mouse and horizontal lines represent the median. Statistics were calculated by two-tailed Mann-Whitney test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Figure 5. Effects of GA101 treatment on clinical disease when initiated during established autoimmunity.
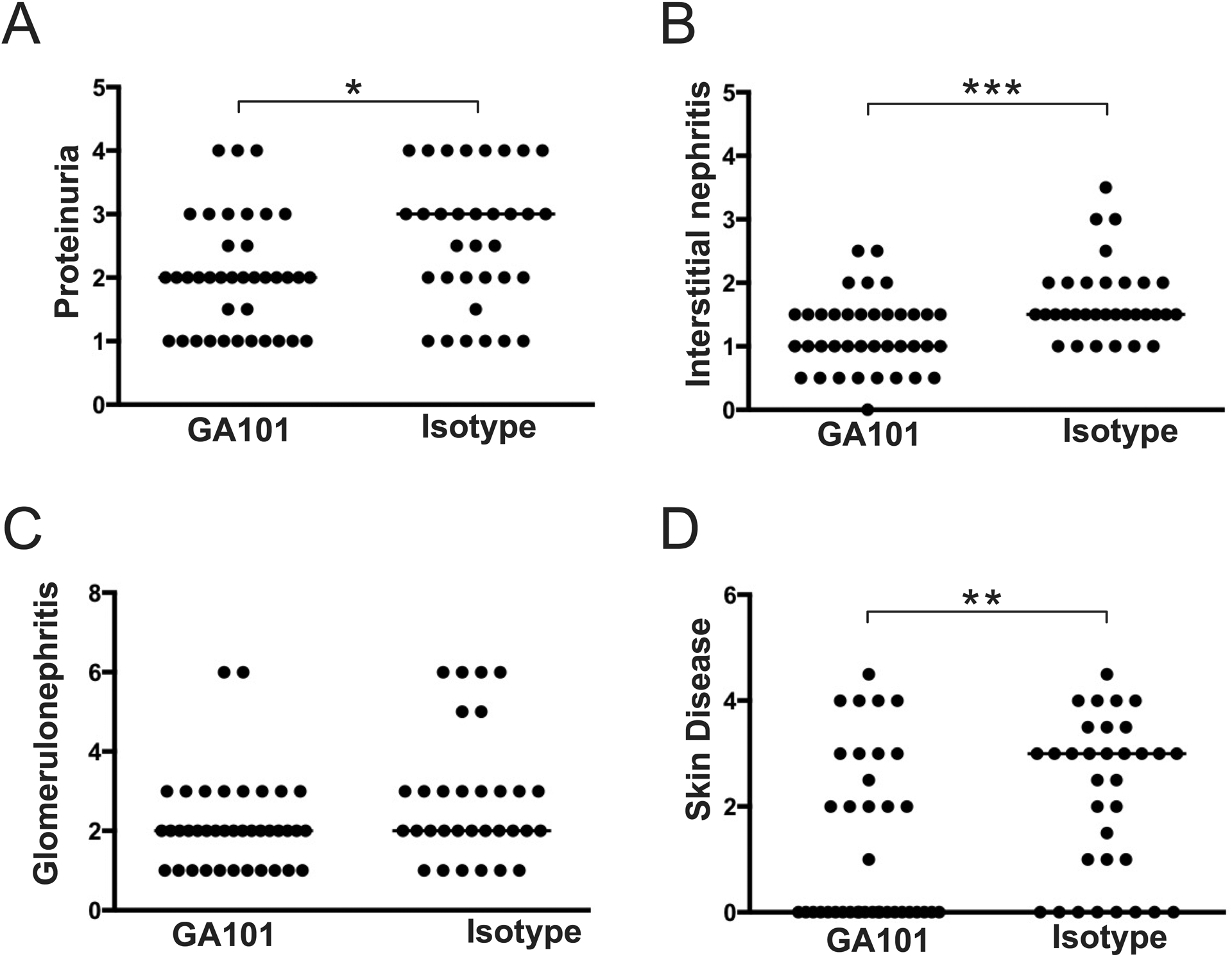
Thirteen-week old hCD20 MRL/lpr mice with established disease were treated with GA101 or isotype control for 6 weeks. (A) Proteinuria was evaluated by dipstick assay the day prior to sacrifice. (B & C) H&E stained kidneys sections were scored for interstitial nephritis and glomerulonephritis. (D) Skin disease scores. Data are combined from 3 independent experiments. Each dot represents an individual mouse and horizontal lines represent the median. Statistics were calculated by two-tailed Mann-Whitney test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Sustained B cell depletion is necessary and sufficient to prolong survival in hCD20 MRL/lpr mice
Given the efficacy of GA101 in treating both early and established disease, we wanted to determine if a 6-week treatment that was initiated early would be sufficient to alter the course of disease, potentially conferring a longer-term benefit. Alternatively, it could have been that sustained GA101 treatment would be needed for prolonged benefit. We therefore designed an experiment to compare one 6-week course with continuing treatment. Ten-week old hCD20 MRL/lpr mice were treated with GA101 or isotype control either for 6-weeks then in one group treatment was discontinued while in another treatment was continued until the mice reached 31 weeks of age. Survival was the endpoint.
Continual depletion resulted in significantly extended survival, as assessed at 20 weeks of age, compared to controls, while treatment for only 6 weeks was did not significantly prolong survival compared to controls (Figure 6). Though there was a trend towards improved survival, treatment for only 6 weeks followed by discontinuation did not significantly increase survival compared to controls. This suggests that while there may be some benefit of transient treatment, depletion likely would need to be sustained for long-term survival benefit in this lupus mouse model.
Figure 6. Effect of treatment with GA101 on survival.
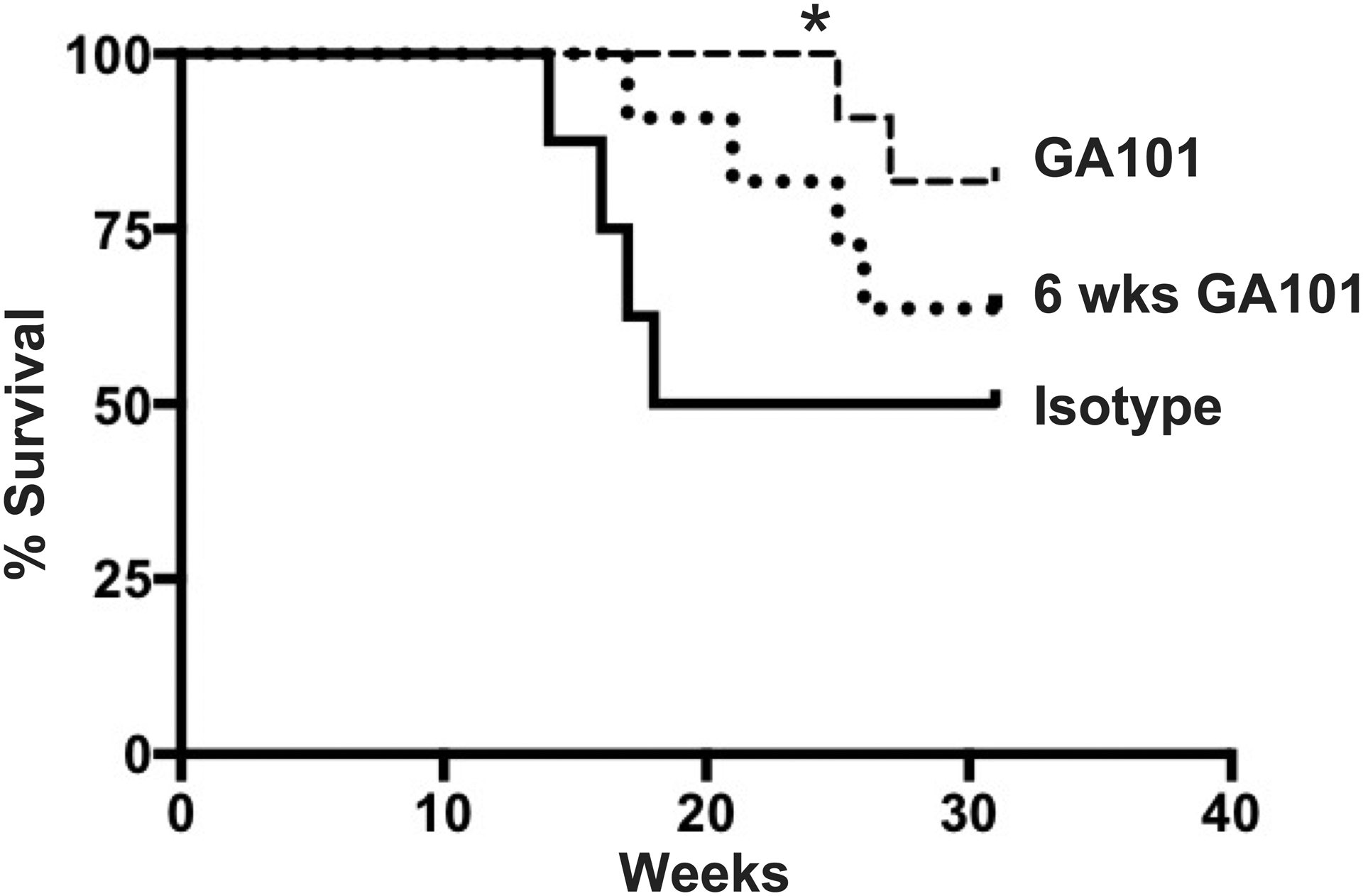
hCD20 MRL/lpr mice were treated continuously with GA101 (n=11) or isotype control (n=8) starting at 10 weeks old. Another group of mice was treated for 6 weeks starting at 10 weeks old, then treatment was discontinued (n=11). Censoring the data at 20 weeks of age, at which point 50% of the control animals had died, showed that GA101 continuous treatment had significantly improved survival compared to isotype-control treated animals (* p=0.018, Fisher’s Exact Test).
Discussion
Despite clear biological evidence that B cells are centrally involved in lupus pathogenesis, depletion of B cells using the chimeric type I anti-CD20 mAb, rituximab, failed to meet endpoints in two randomized clinical trials (9, 11). We have proposed that a major reason for this failure, despite that B cells are a valid target in SLE, is that depletion of B cells is relatively ineffective when using standard agents and treatment protocols (12, 13). We hypothesized that type II anti-CD20 Abs might be more effective in depletion, and that they could be a more efficacious means to achieve clinical responses. Here we have tested these ideas in a relevant preclinical model of lupus.
We found that GA101 is superior to RTX in depleting B cells in lupus-prone mice. This was most evident in short-term depletion experiments, which can reveal inherent differences in depletion efficacy. Over time, RTX was able to deplete B cells, as predicted from earlier studies (12, 13), although it never achieved the same extent of depletion as GA101. The superior depleting ability of GA101 is in line with findings in patients with CLL resulting in improved progression free survival in a head to head comparison with RTX (20) and could be important when translated to other clinical settings, when cost and feasibility will limit amounts and duration of treatment or where type I anti-CD20 antibodies show limited efficacy.
Both RTX and GA101 could treat early disease in hCD20 MRL/lpr mice, however, GA101 was more effective in certain readouts, suggesting overall better efficacy. Earlier studies in this model had shown that conventional type I anti-CD20 mAbs could treat early disease if applied continuously for weeks (13). Similar data were obtained in NZB/W mice by Bekar et al. (8).
More importantly, we found that GA101 could treat more established disease. Although we did not directly compare GA101 to RTX in this model due to logistical reasons (long term experiments requiring many mice in each group and biological replicates to ensure reproducibility), we would predict that GA101 would again be more effective than RTX under these even more challenging conditions based on its differentiated mechanism resulting in superiority in acute depletion, as well as in early disease cohorts.
These findings extend our previous murine model work and that of others in several ways. Treatment of truly established disease is possible. We previously only treated early disease in the MRL/lpr model, while Bekar et al. also treated mice that had just obtained 2+ but did not achieve 3+ proteinuria (8). Though this report mentioned “established disease” in their text, in the B/W model first detection of 2+ proteinuria is best considered new onset disease, as can be discerned from the incidence of proteinuria as reported by Bekar et al.
By comparing GA101 and RTX, we also learned that small differences in depletion, when measured in tissues, can be associated with meaningful differences in preclinical endpoints in hCD20 MRL/lpr mice. There was significantly less glomerulonephritis and less T cell activation in the GA101 vs RTX early disease groups and this was associated with fewer total CD19+ cells in the spleen in the GA101 vs RTX groups, even though depletion was extensive with both treatments.
Finally, from these studies we learned that effective B cell depletion in murine models can extend survival, which had not been tested previously. Nonetheless, this single course of treatment did not induce a state of immune tolerance or remission, suggesting a clinical approach of sustained treatment with GA101. While the current studies are not definitive in this regard, they do provide evidence that continued suppression of B cells can treat disease for extended periods of time in preclinical models.
It is not clear which of the several engineered features of GA101 give it unique depleting properties, but our data supports the hypothesis that it is a combination of several properties. Based on a series of in vitro studies, the depletion mechanisms of anti-CD20 could include complement fixation, antibody-dependent cellular cytotoxicity, opsonization for FcR-mediated clearance by phagocytes such as macrophages, and direct apoptosis-inducing effects on target B cells (33). Classical type I Abs, such as RTX, were thought to be superior at complement-mediated lysis while type II Abs were uniquely effective at inducing cell death. However, in vivo, in hCD20 Tg mice or using murine anti-CD20 (35), depletion by Type I Abs was attributed chiefly to FcR-mediated cell clearance, independent of complement fixation or potential direct effects on CD20+ B cells (36). While a Type II Ab (B1, tositumomab) was reported to be more efficacious than isotype-matched Type I Abs in depleting B cells in normal mice after a single low dose injection, the mechanism was not determined (18).
Here we have investigated the in vivo mechanisms of depletion by GA101 in the challenging setting of lupus, using mutant versions of the antibody. Results of depletion experiments using the “P329G-LALA” version of GA101, which is completely inactivated with respect to FcR-mediated effector function (37), indicate that these FcR-mediated functions do contribute to B cell depletion in the lupus setting in vivo. However, as this mutant Ab still mediated some B cell depletion, FcR-mediated clearance is not the only important mechanism. The non-glycoengineered version of GA101 showed only subtly less efficacy compared to GA101 itself in this model, suggesting only a small contribution of glycoengineering to B cell depletion in hCD20 MRL/lpr mice. The mechanisms by which glycoengineered production, resulting in enhanced affinity for the human FcgRIII, enhances in vivo efficacy e.g. through ADCC or ADCP is not entirely clear. B cell depletion may vary in different compartments (15) and may differ between mouse and human. Increased efficacy based on this may be limited in mouse, due to differences in FcR’s between species, or the in vivo setting of lupus may provide unique barriers to depletion. GA101 has shown superior efficacy in vivo in humans but so far only in the setting of B cell malignancy, particularly with malignancies such as CLL that have relatively low CD20 expression, and in follicular NHL (20, 21). It is tempting to speculate that GA101 is particularly efficacious in more challenging depletion settings due at least in part to its higher affinity for FcRs, mediated by both glycoengineering and its enhanced capability to elicit direct cell death, in part as a consequence of being a Type II CD20 antibody, due to its unique hinge region mutation. The reduced dependency on complement of GA101 may be advantageous in the human autoimmune diseases where depletion by type 1 antibodies may be limited due to hypocomplementemia. Conversely, the enhanced direct cell death could be beneficial in diseases which are driven by pathogenic tissue resident B cell.
Insights from this work into the efficacy and mechanisms of GA101 in a lupus pre-clinical model as well as the use of CD19 CAR-T cells for enhanced B cell depletion in lupus models {Kansal, 2019 #63} could have implications for clinical translation. With a more efficacious depletion tool, B cells as a target might be revisited in SLE patients. Indeed, the initial data that was generated for this manuscript helped to motivate the NOBILITY clinical trial of GA101 in patients (NCT02550652) (38) as well as the pivotal REGENCY study (NCT NCT04221477).
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Jeremy Tilstra for insights and useful discussions. The DLAR staff at the University of Pittsburgh for excellent mouse husbandry.
Support: NIH grant R01AR044077 and an SRA from Roche to the University of Pittsburgh. This work benefitted from NIH 1S10OD011925-01.
Footnotes
Conflicts of Interest: CK: Roche employment, stock, patents; EvP, TS, DS, MP: Roche employment, stock. All other authors declare no conflict.
EvP, DS, MP and CK declare employment with Roche, CK declares ownership of stock and patents with Roche.
References
- 1.Shlomchik MJ. Activating systemic autoimmunity: B’s, T’s, and tolls. Curr Opin Immunol. 2009;21(6):626–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365(22):2110–21. [DOI] [PubMed] [Google Scholar]
- 3.Foster MH. T cells and B cells in lupus nephritis. Semin Nephrol. 2007;27(1):47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan OT, Madaio MP, Shlomchik MJ. The central and multiple roles of B cells in lupus pathogenesis. Immunol Rev. 1999;169:107–21. [DOI] [PubMed] [Google Scholar]
- 5.Chan OT, Madaio MP, Shlomchik MJ. B cells are required for lupus nephritis in the polygenic, Fas-intact MRL model of systemic autoimmunity. J Immunol. 1999;163(7):3592–6. [PubMed] [Google Scholar]
- 6.Kansal R, Richardson N, Neeli I, Khawaja S, Chamberlain D, Ghani M, et al. Sustained B cell depletion by CD19-targeted CAR T cells is a highly effective treatment for murine lupus. Sci Transl Med. 2019;11(482). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Chen F, Putt M, Koo YK, Madaio M, Cambier JC, et al. B cell depletion with anti-CD79 mAbs ameliorates autoimmune disease in MRL/lpr mice. J Immunol. 2008;181(5):2961–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bekar KW, Owen T, Dunn R, Ichikawa T, Wang W, Wang R, et al. Prolonged effects of short-term anti-CD20 B cell depletion therapy in murine systemic lupus erythematosus. Arthritis & Rheumatism. 2010;62(8):2443–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merrill J, Buyon J, Furie R, Latinis K, Gordon C, Hsieh HJ, et al. Assessment of flares in lupus patients enrolled in a phase II/III study of rituximab (EXPLORER). Lupus. 2011;20(7):709–16. [DOI] [PubMed] [Google Scholar]
- 10.Sanz I, Lee FE. B cells as therapeutic targets in SLE. Nat Rev Rheumatol. 2010;6(6):326–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rovin BH, Furie R, Latinis K, Looney RJ, Fervenza FC, Sanchez-Guerrero J, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum. 2012;64(4):1215–26. [DOI] [PubMed] [Google Scholar]
- 12.Ahuja A, Teichmann LL, Wang H, Dunn R, Kehry MR, Shlomchik MJ. An acquired defect in IgG-dependent phagocytosis explains the impairment in antibody-mediated cellular depletion in Lupus. J Immunol. 2011;187(7):3888–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahuja A, Shupe J, Dunn R, Kashgarian M, Kehry MR, Shlomchik MJ. Depletion of B Cells in Murine Lupus: Efficacy and Resistance. The Journal of Immunology. 2007;179(5):3351–61. [DOI] [PubMed] [Google Scholar]
- 14.Frank MM, Hamburger MI, Lawley TJ, Kimberly RP, Plotz PH. Defective reticuloendothelial system Fc-receptor function in systemic lupus erythematosus. N Engl J Med. 1979;300(10):518–23. [DOI] [PubMed] [Google Scholar]
- 15.Mossner E, Brunker P, Moser S, Puntener U, Schmidt C, Herter S, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115(22):4393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrara C, Brünker P, Suter T, Moser S, Püntener U, Umaña P. Modulation of therapeutic antibody effector functions by glycosylation engineering: Influence of Golgi enzyme localization domain and co-expression of heterologous β1, 4-N-acetylglucosaminyltransferase III and Golgi α-mannosidase II. Biotechnology and Bioengineering. 2006;93(5):851–61. [DOI] [PubMed] [Google Scholar]
- 17.Tobinai K, Klein C, Oya N, Fingerle-Rowson G. A Review of Obinutuzumab (GA101), a Novel Type II Anti-CD20 Monoclonal Antibody, for the Treatment of Patients with B-Cell Malignancies. Adv Ther. 2017;34(2):324–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beers SA, Chan CH, James S, French RR, Attfield KE, Brennan CM, et al. Type II (tositumomab) anti-CD20 monoclonal antibody out performs type I (rituximab-like) reagents in B-cell depletion regardless of complement activation. Blood. 2008;112(10):4170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer K, Al-Sawaf O, Bahlo J, Fink A-M, Tandon M, Dixon M, et al. Venetoclax and Obinutuzumab in Patients with CLL and Coexisting Conditions. New England Journal of Medicine. 2019;380(23):2225–36. [DOI] [PubMed] [Google Scholar]
- 20.Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, et al. Obinutuzumab plus Chlorambucil in Patients with CLL and Coexisting Conditions. New England Journal of Medicine. 2014;370(12):1101–10. [DOI] [PubMed] [Google Scholar]
- 21.Marcus R, Davies A, Ando K, Klapper W, Opat S, Owen C, et al. Obinutuzumab for the First-Line Treatment of Follicular Lymphoma. New England Journal of Medicine. 2017;377(14):1331–44. [DOI] [PubMed] [Google Scholar]
- 22.Cheson BD, Chua N, Mayer J, Dueck G, Trněný M, Bouabdallah K, et al. Overall Survival Benefit in Patients With Rituximab-Refractory Indolent Non-Hodgkin Lymphoma Who Received Obinutuzumab Plus Bendamustine Induction and Obinutuzumab Maintenance in the GADOLIN Study. Journal of Clinical Oncology. 2018;36(22):2259–66. [DOI] [PubMed] [Google Scholar]
- 23.Herter S, Herting F, Muth G, van Puijenbroek E, Schlothauer T, Ferrara C, et al. GA101 P329GLALA, a variant of obinutuzumab with abolished ADCC, ADCP and CDC function but retained cell death induction, is as efficient as rituximab in B-cell depletion and antitumor activity. Haematologica. 2018;103(2):e78–e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlothauer T, Herter S, Koller CF, Grau-Richards S, Steinhart V, Spick C, et al. Novel human IgG1 and IgG4 Fc-engineered antibodies with completely abolished immune effector functions. Protein Engineering, Design and Selection. 2016;29(10):457–66. [DOI] [PubMed] [Google Scholar]
- 25.Shlomchik MJ, Zharhary D, Saunders T, Camper SA, Weigert MG. A rheumatoid factor transgenic mouse model of autoantibody regulation. Int Immunol. 1993;5(10):1329–41. [DOI] [PubMed] [Google Scholar]
- 26.Nickerson KM, Cullen JL, Kashgarian M, Shlomchik MJ. Exacerbated autoimmunity in the absence of TLR9 in MRL.Fas(lpr) mice depends on Ifnar1. J Immunol. 2013;190(8):3889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell A, Kashgarian M, Shlomchik M. NADPH oxidase inhibits the pathogenesis of systemic lupus erythematosus. Science Translational Medicine. 2012;4(157):157ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nickerson KM, Christensen SR, Shupe J, Kashgarian M, Kim D, Elkon K, et al. TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J Immunol. 2010;184(4):1840–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blanco F, Kalsi J, Isenberg DA. Analysis of antibodies to RNA in patients with systemic lupus erythematosus and other autoimmune rheumatic diseases. Clin Exp Immunol. 1991;86(1):66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tilstra JS, Avery L, Menk AV, Gordon RA, Smita S, Kane LP, et al. Kidney-infiltrating T cells in murine lupus nephritis are metabolically and functionally exhausted. J Clin Invest. 2018;128(11):4884–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon RA, Herter JM, Rosetti F, Campbell AM, Nishi H, Kashgarian M, et al. Lupus and proliferative nephritis are PAD4 independent in murine models. JCI Insight. 2017;2(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salles GA, Morschhauser F, Solal-Céligny P, Thieblemont C, Lamy T, Tilly H, et al. Obinutuzumab (GA101) in Patients With Relapsed/Refractory Indolent Non-Hodgkin Lymphoma: Results From the Phase II GAUGUIN Study. Journal of Clinical Oncology. 2013;31(23):2920–6. [DOI] [PubMed] [Google Scholar]
- 33.Glennie MJ, French RR, Cragg MS, Taylor RP. Mechanisms of killing by anti-CD20 monoclonal antibodies. Molecular Immunology. 2007;44(16):3823–37. [DOI] [PubMed] [Google Scholar]
- 34.Chan O, Shlomchik MJ. A new role for B cells in systemic autoimmunity: B cells promote spontaneous T cell activation in MRL-lpr/lpr mice. J Immunol. 1998;160:51–9. [PubMed] [Google Scholar]
- 35.Uchida J, Hamaguchi Y, Oliver JA, Ravetch JV, Poe JC, Haas KM, et al. The Innate Mononuclear Phagocyte Network Depletes B Lymphocytes through Fc Receptor-dependent Mechanisms during Anti-CD20 Antibody Immunotherapy. J Exp Med. 2004;199(12):1659–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gong Q, Ou Q, Ye S, Lee WP, Cornelius J, Diehl L, et al. Importance of Cellular Microenvironment and Circulatory Dynamics in B Cell Immunotherapy. The Journal of Immunology. 2005;174(2):817–26. [DOI] [PubMed] [Google Scholar]
- 37.Herter S, Herting F, Muth G, van Puijenbroek E, Schlothauer T, Ferrara C, et al. GA101 P329GLALA, a variant of obinutuzumab with abolished ADCC, ADCP and CDC function but retained cell death induction, is as efficient as rituximab in B-cell depletion and antitumor activity. Haematologica. 2018;103(2):e78–e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furie R, Aroca G, Alvarez A, Fragoso-Loyo H, Zuta Santillan E, Rovin B, et al. A Phase II Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Obinutuzumab or Placebo in Combination with Mycophenolate Mofetil in Patients with Active Class III or IV Lupus Nephritis Arthritis Rheumatol. 2019;7:71. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


