Abstract
Objectives:
This open-label 12-week study was conducted to evaluate the efficacy and safety of tofacitinib, a JAK inhibitor, in active, treatment-refractory dermatomyositis.
Methods:
Tofacitinib was given as 11 mg XR daily to 10 subjects. All subjects underwent complete washout of all steroid sparing agents. The primary outcome measure was the IMACS definition of improvement. The response rate was measured by the 2016 ACR/EULAR Myositis Response Criteria using the total improvement score (TIS). The Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI), chemokine levels, skin STAT 1 expression by immunohistochemistry, RNA sequencing analysis, and safety were secondary outcome measures.
Results:
All 10 subjects met the primary outcome at 12 weeks. 5 of 10 (50%) had moderate improvement and the other half had minimal improvement on the TIS. The secondary outcome of the mean change in the CDASI activity score from baseline to 12 weeks was statistically significant (28 ± 15.4 vs. 9.5 ± 8.5, p=0.0005). Serum chemokine data (CXCL 9/10) showed a statistically significant change from baseline. Three of the 9 subjects who had a skin biopsy demonstrated a marked decrease in STAT1 signaling in association with suppression of IFN target gene expression. The mean creatine kinase was 82 ± 34.8 highlighting that those treated had skin predominant disease.
Conclusions:
This is the first prospective, open-label clinical trial of tofacitinib in dermatomyositis that demonstrates strong clinical efficacy for a pan JAK inhibitor as measured by validated myositis response criteria. Future randomized controlled trials using JAK-inhibitors should be considered for treating dermatomyositis.
INTRODUCTION
Dermatomyositis (DM) is an idiopathic inflammatory myopathy that primarily affects the muscle and skin. We previously reported a case of refractory DM and inflammatory arthritis treated with tofacitinib, a pan JAK inhibitor, in which clinical improvement in the skin, muscle and joint disease was observed (1). Other small case reports and series have demonstrated that this class of medications may be helpful in treating refractory DM and polymyositis (2–4). In particular, the first case-report of a JAK inhibitor, ruxolitinib, demonstrating efficacy in recalcitrant dermatomyositis was thought to be mediated by blocking IFN regulated pro-inflammatory cytokines such as CXCL9/10 thereby making JAK inhibitors an attractive therapeutic agent in the treatment of dermatomyositis (3). This open-label, 12-week proof-of-concept study was conducted to evaluate the efficacy and safety of tofacitinib in active, treatment refractory DM. We hypothesized that JAK inhibition would reduce the activation of type I-IFN regulated proteins and key chemokines (CXCL9/10) that would correspond with biological activity before and after treatment.
PATIENTS AND METHODS
Study population
This study was conducted at the Johns Hopkins Myositis Center. Thirteen subjects were screened, and 10 were enrolled. Written informed consent was obtained from each study subject. Eligible subjects included adults ≥ 18 years of age with a diagnosis of definite or probable DM by Bohan and Peter Criteria (5,6). Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI) score (7) of at least 5, a prior skin or muscle biopsy diagnostic for DM or prior positive test for at least one myositis specific antibody (MSA) was also required. Although not mandatory, subjects with muscle weakness were eligible for enrollment and required an MMT-8 score ≤ 142 out of 150.
Refractory disease was defined as active disease despite a 12-week trial of steroids and with failure of response to at least prednisone and one other first line immunosuppressive agent such as methotrexate (MTX), mycophenolate mofetil (MMF), or azathioprine (AZA). Maximum prednisone dose allowed at time of entry was 20mg daily, and the dose had to be stable for >2 weeks prior to baseline visit. Steroids were not required before study initiation but only used if the subjects were not tolerating the washout.
To minimize confounding, subjects with the following conditions were excluded: juvenile DM, myositis in overlap with other autoimmune diseases, history of hypersensitivity to any study drugs, cancer-associated myositis, or other types of myositis or myopathies. Subjects with hypersensitivity to study drug, pregnant or lactating women, and any subject with concomitant illnesses including severe cardiopulmonary disease, active infections, inflammatory bowel disease or history of bowel rupture were excluded. Subjects with a history of any malignancy (other than localized basal cell carcinoma of the skin), treated or untreated, within the past 5 years were excluded. Late-stage DM where muscle weakness, according to the investigator (JJP), could be attributable to muscle damage rather than myositis disease activity was also excluded.
Study Design:
This was a proof-of-concept study to evaluate the efficacy and safety of tofacitinib in refractory DM using a prospective, open-label design for 12 weeks. Washout of immunosuppressive or immunomodulatory agents was required as follows: MTX, AZA, MMF, tacrolimus, hydroxychloroquine: 12–16 weeks prior to first dose of study drug; rituximab, cyclophosphamide: 12 months; Intravenous Immunoglobulin (IVIG): 3 months. Study subjects self-administered tofacitinib 11mg extended release by mouth daily for 12 weeks. A forced prednisone taper was instituted at 8 weeks if subjects were on prednisone at study entry. Rescue prednisone was allowed at the discretion of the investigator if there was any disease flare, not to exceed prednisone 20mg daily. Clinical assessments and safety laboratory data were performed every four weeks for 12 weeks, with an optional four-week extension. Skin biopsies (4mm punch) were performed by JJP of unaffected and affected skin at baseline and 12 weeks, needle muscle biopsies using a Pro-Mag Ultra Automatic Biopsy Instrument (Argon Medical Devices) were performed by DGL at baseline and 12 weeks as previously described (8), and Magnetic Resonance Imaging (MRI) of the bilateral thigh muscles were obtained at baseline and at 12 weeks.
Adverse events
Adverse event (AEs) and serious adverse events (SAEs) were monitored and reported in a standardized manner using the Common Terminology Criteria of the National Cancer Institute V4.03, with the investigator (JJP) determining their relatedness to the drug.
Primary and secondary end points
The primary end point for the trial was the IMACS definition of improvement (DOI): 3 of any of the 6 core set measures (CSMs) improved by ≥ 20%, with no more than 2 CSMs worsening by ≥ 25% (worsening measure cannot include the MMT) (9). The response rate was determined by the Total Improvement Score (TIS) using the 2016 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) myositis response criteria (10).
Secondary outcomes included safety and change from baseline of the following measures (assays used are described below): CDASI, STAT 1 expression in skin, CXCL-9 and CXCL-10 levels, MRI of the thighs, and RNA sequencing of blood, skin and muscle biopsies.
Immunohistochemistry :
Skin paraffin sections from uninvolved and involved (baseline and 12-weeks after starting treatment) skin biopsies were immunostained using an anti-STAT1 mouse monoclonal antibody (6.7 μg/ml, 4°C overnight, Novus Biologicals) followed by a horseradish peroxidase-conjugated anti-mouse secondary antibody (1:500, Dako) as described (11). Control staining of sections was performed with an equivalent concentration of isotype-matched mouse IgG, or in the absence of primary antibody (see Supplemental Figure 1). Staining was visualized using the diaminobenzidine (DAB) substrate chromagen system (Dako), with the reaction time being held constant for all. Nuclei were stained using Mayer’s hematoxylin solution (Dako). Light microscopy images were obtained using a Zeiss Axioskop 50 with a Zeiss AxioCam HRC camera and AxioVision 4.9.1 software. The camera settings were held constant for all images, enabling a semi-quantitative comparison of staining intensity to be assessed across sections. Two investigators (LC-R and LG-A) independently examined the staining. Nine subjects had skin biopsies performed pre and post treatment. One subject declined the week 12 skin biopsy.
CXCL 9 and 10 levels:
Serum from all of the study subjects was obtained at 4-week intervals, aliquoted and banked at −80°C. Levels of CXCL9 and CXCL10 in these banked sera were assayed using commercially available ELISA kits, per the manufacturer’s protocol (R&D Systems).
Autoantibody assays:
Autoantibodies were assayed on banked sera using a commercially available line immunoblot platform (EuroImmun; Myositis profile).
RNASeq Library Preparation and Sequencing:
RNA sequencing was performed in the blood, skin, and muscle. NEBNext Poly(A) Magnetic Isolation Module (NEB #E7490) and NEBNext Ultra II Directional RNA Library Prep Kit for Illumina (Cat# E7765) were used to generate libraries. The procedure included Poly A RNA isolation and fragmentation, cDNA synthesis and end repair, sequencing adapter ligation and PCR amplification to prepare the ‘libraries’. The resulting library insert size was 200bp-500bp peaked around 300bp. Libraries were uniquely barcoded and pooled for NovaSeq6000 sequencing. Pooled libraries were sequenced on an Illumina® NovaSeq6000 instrument using standard protocols for S2 50bp Paired end sequencing. 112 samples were multiplexed for an estimated 75 million reads per sample.
Bioinformatics analysis –
RNA sequencing (RNA-seq) data was analyzed to determine the genes present in each subject, their expression levels, and the differences between expression levels at different time points during treatment. Genes with a p-value <=0.05 and a log2 fold change in expression >=1.5 were considered significant. In all available samples, the downregulated genes were compared from 12 weeks (primary outcome measure) to baseline (an overview of the numbers of downregulated and upregulated genes for each comparison is presented in Supplemental Table 4). Samples were analyzed separately by tissue (blood, skin, muscle). Following quality checking, sequencing reads were aligned to the human genome GRCh38 with the software Tophat2 v.2.1.0 (12), and then assembled into genes and transcripts using the program CLASS2 v2.1.7) (13). Transcripts from all samples were further merged and mapped to the GENCODE v27 gene models to create a unified set of gene annotations for gene quantification and differential analyses. Differentially expressed genes (DEG) were determined with the tool DESeq2 (14), and used for subsequent pathway analysis with Ingenuity Pathway Analysis (Qiagen). Lastly, interferon (IFNGS) scores as previously reported (15) were computed separately in blood and muscle by calculating the median of the log fold change scores at 13 designated genes (EPSTI1, HERC5, IFI27, IFI44, IFI44L, IFI6, IFIT1, IFIT3, ISG15, MX1, OAS1, OAS3 and RSAD2) between subject samples at 12 weeks or baseline, respectively, and a normal sample (SRR607219 for blood and ERR030876 for muscle). A median log fold change of at least ≥1.5 was defined as significant when comparing IFN scores in the muscle from baseline to 12 weeks.
Statistical Analyses:
Baseline demographic, clinical, and laboratory variables were evaluated with descriptive statistics. Continuous variables were summarized as mean ± SDs and categorical variables were summarized as proportions falling in each category. The median Total Improvement Score were compared with the baseline values using the Wilcoxon rank sum test. The CDASI values were compared with the baseline values using paired t-test and Wilcoxon rank sum. The change in chemokine levels over time was compared with the paired Wilcoxon rank sum. To test the correlation between the changes in expression of marker genes identified by RNA-seq differential analysis, for each gene we calculated Pearson Correlation coefficients between TIS or CDASI scores and the changes (log 2-fold change) in expression levels between week 12 and baseline across all subjects.
RESULTS
Ten subjects completed the study without any serious adverse events. Table 1 summarizes the baseline clinical features of all 10 enrolled subjects. The mean age was 45.6 ±10.6 years; 70% were female, and 90% Caucasian, with a mean disease duration of 6.5 ± 4.6 years. The skin disease activity as measured by a mean CDASI at entry was 28 ±15.4, without significant overall muscle involvement at study entry. All subjects were refractory and failed two or more steroid sparing agents for either skin or muscle disease. At entry into the study, all 10 subjects had refractory skin disease with only 1 of the 10 subjects having moderate muscle weakness. Other than rash and muscle weakness, the additional clinical features included calcinosis (3 subjects) and none had ILD.
Table 1.
Baseline characteristics of study cohort (N=10)
| Baseline Characteristics | Value (N (%) or mean ± SD |
|---|---|
| Age ± SD | 45.6 ± 10.6 years |
| Female (%) | 7 (70%) |
| Caucasian | 9 (90%) |
| African American | 1 (10%) |
| Disease duration | 6.45 ± 4.55 years |
| Prednisone 20mg/daily at entry | 4 (40%) |
| Mean CDASI ± SD | 28 ± 15.4 |
| Median CDASI [IQR] | 21 [18, 30] |
| Mean MMT ± SD | 147 ± 5.06 |
| Median MMT [IQR] | 150 [145, 150] |
| Mean CK ± SD | 82 ± 34.8 |
| Median CK [IQR] | 97 [53, 116] |
| Myositis autoantibody | |
| TIF-1 gamma | 7 (70%) |
| Mi-2 | 1(10%) |
| NXP-2 | 2(20%) |
| Prior Immunosuppressive Treatment* | |
| Methotrexate | 9 (90%) |
| Azathioprine | 2 (20%) |
| Mycophenolate mofetil | 5 (50%) |
| Intravenous Immunoglobulin | 6 (60%) |
| Rituximab | 1 (10%) |
SD: standard deviation, CDASI: Cutaneous Dermatomyositis Disease Area and Severity Index, MMT: Manual Muscle Testing, CK: creatine kinase. Maximum dose of prednisone allowed at study entry was 20mg/daily.
Not mutually exclusive
Primary Outcome
All 10 subjects completing the study met the primary outcome of interest, the DOI at 12 weeks. These subjects also met the outcome measure of minimal improvement using the 2016 ACR-EULAR myositis response criteria. The median total improvement score (TIS) was 40 [IQR 32.5, 47.5] (Figure 1a). Seven of the 10 subjects (70%) had at least minimal improvement on the TIS at 4 weeks which was sustained until the primary endpoint at 12 weeks.
Figure 1. Key Outcome Measures including the Total Improvement Score and CDASI.
(a) The median improvement score of all 10 subjects demonstrates that the TIS improved over the 12 week study period; (b) Graphical representation of the TIS of each individual subject in the study and (c) Boxplot demonstrates total CDASI activity scores from baseline to week 12 with the median shown as the line in the box. The whiskers of each boxplot represent the spread of the scores.
Secondary Outcomes
Improvement in Cutaneous Disease Activity as measured by the validated CDASI
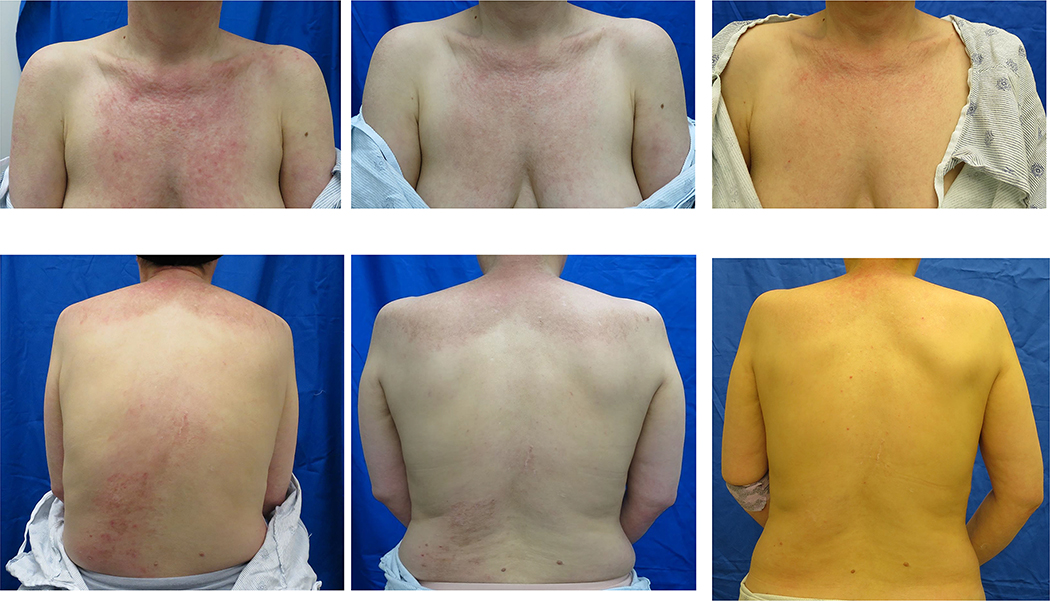
All 10 subjects had at least moderate skin disease activity as validated by the CDASI with a mean CDASI of 28 ±15 at entry that improved to 9.5 ± 8.5 by 12 weeks; this change was statistically significant (p=0·0005) (Figures 1c & 2). Furthermore, the mean change in the CDASI was 18.5, which is a 66% decrease from baseline. A > 40% change in the CDASI has been reported to indicate a meaningful change in quality of life (16). Based on the definition of a CDASI score of ≤ 14 being mild skin disease (7), 7 of 10 (70%) of the subjects improved from moderate to severe skin disease activity to mild disease activity.
Figure 2. Cutaneous improvement after treatment with tofacitinib.
Improvement in Skin Disease with Treatment at Baseline, 4 weeks and 12 weeks in study subjects enrolled in the study.
Chemokines and Immunohistochemistry data
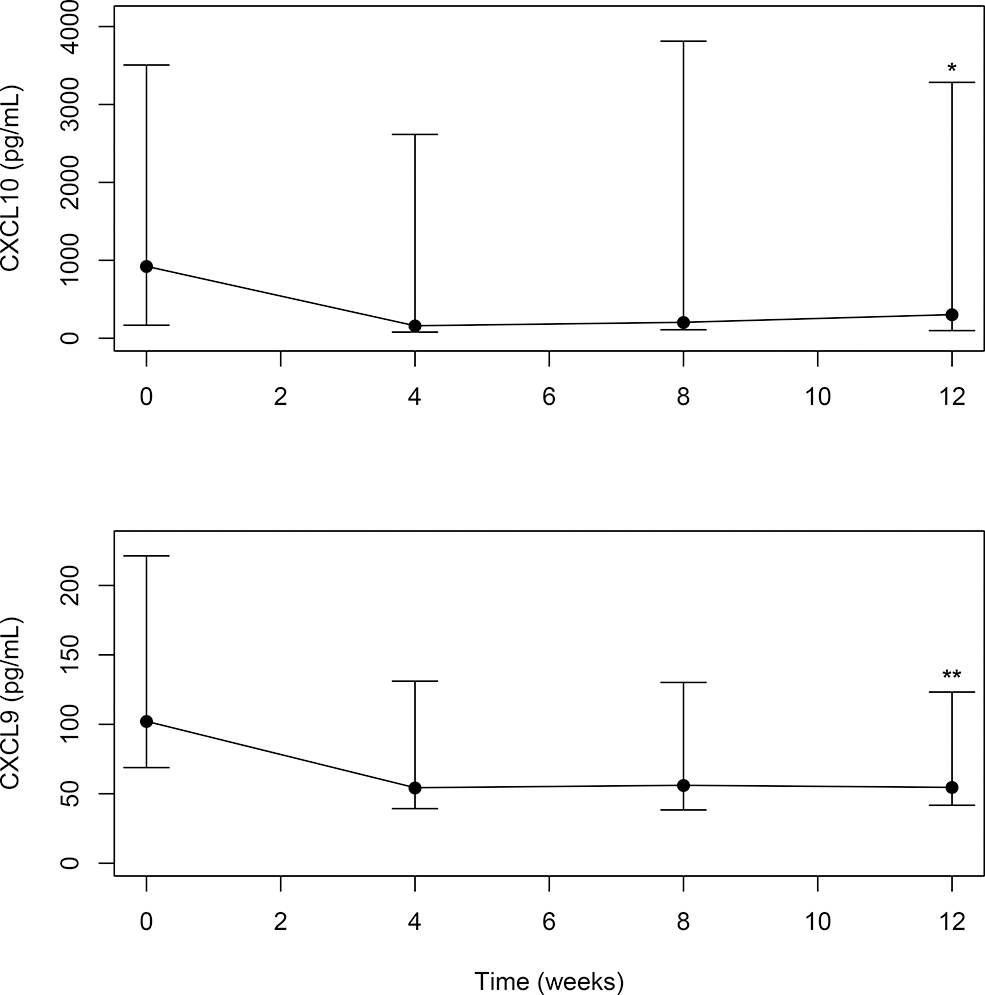
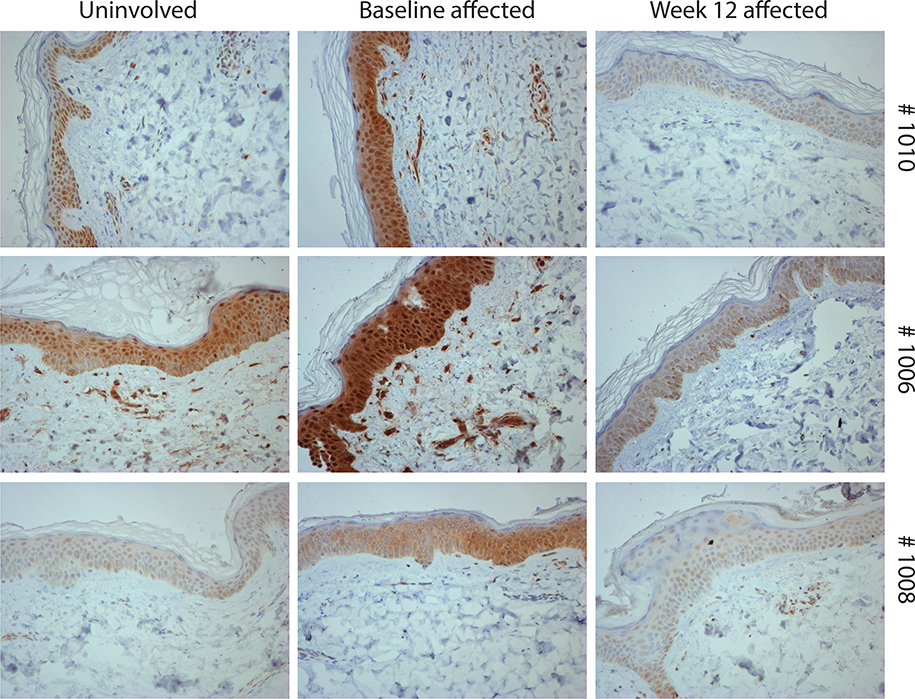
Median serum CXCL9/10 levels of all subjects demonstrated a statistically significant change from baseline to 12 weeks with treatment (CXCL9, p=0.049; CXCL10, p=0.013) (Figure 3). To explore the efficacy of tofacitinib in inhibiting STAT 1 expression in the skin, a semi-quantitative approach was used to assess change in STAT 1 staining. STAT1 immunostaining performed on these skin paraffin sections showed that STAT1 levels were lower in affected skin biopsies from baseline to week 12 in 3 of the 9 subjects (Figure 4). Control immunostaining performed on these skin sections confirmed specificity of the STAT1 staining (see Supplemental Fig 1). Interestingly, of these 3 subjects, 2 of 3 (67%) were moderate responders on the TIS and the mean change in the CDASI of all 3 subjects was 14 ± 3.6. In the other 6 subjects, the following STAT1 staining patterns were observed: no STAT1 staining was observed both at baseline and week 12 (in 4 subjects); high STAT1 levels at baseline and at week 12 (1 subject) and no STAT1 baseline staining but variable levels at week 12 (1 subject). Of note, minimal staining was observed in unaffected skin at baseline (Supplemental Figure 6).
Figure 3. Median Serum Chemokine (CXCL 9 and 10) from baseline (0 weeks) to 12 weeks with 95% confidence intervals for the median.
Levels of both are in pg/mL (Y-axis). *CXCL10: Wilcoxon rank sum test for difference between median at 12 weeks and median at baseline, p = 0.013; ** (CXCL9 (pg/mL)) Wilcoxon rank sum test for difference between median at 12 weeks and median at baseline, p=0.049
Figure 4. STAT1 immunostaining staining is downregulated in skin biopsies from baseline to 12 weeks.
Skin paraffin sections from affected skin biopsied at baseline and 12 weeks after starting treatment were stained for STAT1.
Muscle involvement
The mean manual muscle testing of all 10 subjects was 147 ± 5.06 indicative of only very mild muscle weakness at entry. Two subjects had an MMT<140 at entry, with one subject having an MMT of 127. The latter subject demonstrated a 9-point improvement in the MMT-8 score at 12 weeks. This subject also had objective evidence of edema on muscle MRI which improved from baseline to 12 weeks (Supplemental Figure 2).
RNA sequencing analysis in blood, skin, and muscle
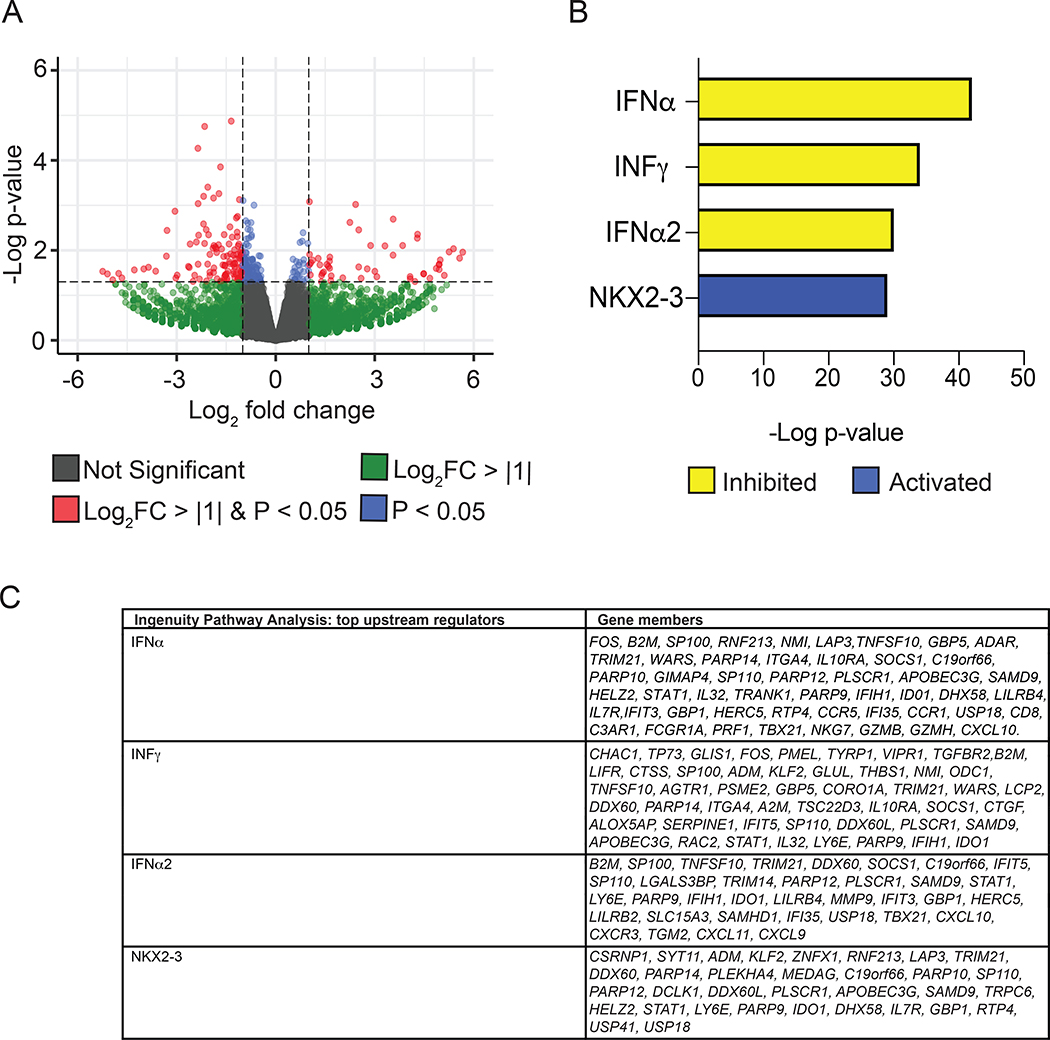
In all available samples, the down-regulated genes were compared from 12 weeks (primary outcome measure) to baseline. In the blood, the genes of interest that were down-regulated were C1QB and C1QC, which are both involved in the complement pathway. In the skin, the predominant gene of interest that was down-regulated was CXCL9. Notably, RNA sequencing analysis of the 3 subjects who also had STAT1 inhibition on immunohistochemistry revealed that skin biopsies taken at week 12 exhibited significant gene expression differences in comparison to baseline, with enrichment for suppression of IFN target gene expression (Figure 5). Lastly, in the muscle, the genes of interest that were down-regulated were C1QB and C1QC, CXCL9, and type I IFN related genes such as IFI27, MX-2, ISG 15, and IFI 16. The interferon score was down-regulated in the muscle in 5 of 8 the subjects with an average change of 6.86 ± 4.32. Overall, the CXCL9 and 10 and complement pathway were highly down-regulated across all tissue types (blood, skin, and muscle) (Supplemental Figure 3). In correlation studies with our clinical outcome measures, the TIS was not correlated with any gene expression in the blood, skin, or muscle. However, there was a moderation correlation between the MX2 gene in the muscle and CDASI (r2=0.56), and CXCL 10 in the blood and the CDASI (r2=0.53) (Supplemental Table 5).
Figure 5. Tofacitinib suppresses IFN target gene expression in skin biopsies.
(A) Volcano plots of differentially expressed genes (dots) between baseline and 12 weeks of tofacitinib treatment. (B) Top Ingenuity Pathway Analysis predictions of upstream regulators of differentially expressed genes assessed by RNA-seq. Adjusted p value < 0.05. Upstream regulators were predicted as inhibited (yellow) or activated (blue). (C) Table shows differentially expressed genes targeted by each upstream regulator.
Steroid Taper
Four of 10 (40%) were on prednisone 20mg/daily at entry with 3 of the 4 (75%) requiring prednisone for active skin disease and 1 of the 4 (25%) requiring prednisone for active skin and muscle disease. Three of 4 (75%) were able to completely taper off all steroids.
Safety and tolerability
Tofacitinib XR 11mg daily was well-tolerated and safe over the 12-week study period. There were no SAEs. There was one recurrent AE, a urinary tract infection (UTI) that required two courses of antibiotics. This subject also had a history of recurrent UTIs prior to entry into the study but at screening did not have a UTI. There were no significant changes in white blood cell count, hemoglobin, platelet count, creatine kinase, or serum creatine over the 12-week trial period. There were also no deep vein thromboses (DVTs) or venous thromboembolisms (VTEs).
DISCUSSION
This is the first proof of concept, open-label, prospective clinical trial of tofacitinib, a pan JAK inhibitor, in DM that has demonstrated strong clinical efficacy as measured by a validated myositis response criteria. Enrolled subjects were those with refractory disease, who had failed at least two steroid-sparing agents, and were not permitted concurrent steroid-sparing immunosuppressant therapies, thereby demonstrating the efficacy of tofacitinib monotherapy. Tofacitinib was well tolerated with no major adverse events requiring discontinuation. All subjects in the study met the ACR/EULAR myositis response criteria at the primary endpoint of 12 weeks, with 50% having met a moderate improvement and 50% meeting at least minimal improvement.
The overall clinical phenotype of the 10 subjects were skin-predominant refractory DM with only one subject with significant muscle weakness. Demonstrated improvement in the TIS was largely driven by the subject and physician global activity and the HAQ-DI since many subjects did not have a low MMT or high CK values at entry. Improvement in the skin disease was quite striking and evident as early as four weeks (Figure 2 and 3). Since there was only one subject with moderate muscle weakness, meaningful conclusions cannot be made regarding muscle strength improvement; however, this subject did have an improvement in strength with concurrent improvement in muscle edema on muscle MRI.
The a priori hypothesis was that the key chemokines such as CXCL9 and CXCL10 would be inhibited via the JAK/STAT pathway and that type I IFN related pathways would also be down-regulated in skin, muscle, and blood. There have been multiple studies demonstrating that CXCL9 and 10 are strong biomarkers in DM (17,18). Similarly, prior studies have established that there is preferential activation of the IFN1 pathway in DM (19–21). Interestingly, our studies demonstrate that CXCL9 and CXCL10 were both down-regulated in blood, skin, and muscle. In particular, biochemical assays of both CXCL9 and CXCL10 showed a statistically significant decrease when compared from baseline to 12 weeks. The importance of the key chemokines being down-regulated with treatment was also further supported by the RNA sequencing results in the skin, blood, and muscle. However, when evaluating the IFN signature of all 10 study subjects, only the muscle tissue demonstrated significant inhibition of specific IFN related genes. Previous studies have shown that the IFN signature is much higher in diseased muscle than blood in DM and may provide an explanation for this finding (19). Nonetheless, the fact that the CXCL9 and CXCL10 were down-regulated is both clinically and mechanistically meaningful given that these chemokines may be strong biomarkers of activity in adult DM. Thus, type II or IFN-γ, in conjunction with type I IFN related pathways, may be playing an important role in the pathogenesis of DM.
The remarkable benefit of tofacitinib in skin disease activity was measured clinically by an improvement in the validated CDASI activity score. Of the 9 subjects who had skin biopsies pre and post treatment, 3 had immunohistochemical results that demonstrated decreased staining of STAT-1 when compared to baseline at 12 weeks. This was coupled with a marked improvement in their CDASI activity score (mean improvement of 72.3%), thereby highlighting that there was evidence of efficacy mediated through the JAK/STAT pathway. RNA sequencing analysis of skin biopsies from these three subjects also showed marked inhibition of IFN target genes, further supporting the a priori hypothesis that tofacitinib would be effective by blocking this pathway. Of note, NKX2–3 was predicted among the top activated upstream regulators. Although polymorphisms of NKX2–3 have been associated with inflammatory bowel disease (22), the precise functional role in inflammation remains unclear.
Myositis specific autoantibodies were assayed in all 10 subjects using a myositis antibody panel (Euroimmun platform). Seven subjects had anti-TIF1-γ antibodies, 2 were anti-NXP2 positive, and one subject was positive for anti-Mi-2. Interestingly, the antibody titers remained unchanged after treatment for 12 weeks. Of note, 5 of 7 (71%) of the anti-TIF1-γ-positive subjects were moderate responders, with the remainder showing minimal responses.
In our trial, tofacitinib was well tolerated, with no subject discontinuing the therapy. While it has been reported that JAK inhibitors are associated with a higher risk of herpes zoster, this was not seen in any of the 10 subjects. Furthermore, there were no other serious infections that required hospitalization during the trial. There was one subject who required two rounds of antibiotics due to recurrent urinary tract infections. She did not have a urinary tract infection at entry but did have a history of recurrent infections in the past 5 years intermittently.
The limitations of this study include the lack of a control group or randomization. Furthermore, there was no separate arm to study a higher dose for efficacy. Lastly, only one subject had moderate muscle weakness, making it difficult to reach any robust clinical conclusions of the effects on muscle. Therefore, the primary endpoint, the TIS, was not driven by the MMT-8 or CK values. Despite these limitations, this open label, prospective clinical trial is first in its class to show efficacy using validated response criteria in refractory DM. Additionally, the extensive studies done on blood, skin and muscle tissue have allowed us to explore the mechanisms by which tofacitinib may be exerting its therapeutic effect. Tofacitinib was well tolerated with minimal adverse events highlighting the importance of further investigating JAK inhibitors in a larger randomized controlled trial in dermatomyositis.
Supplementary Material
Acknowledgements
Illumina sequencing was conducted at the Genetic Resources Core Facility, Johns Hopkins Institute of Genetic Medicine, Baltimore, MD.
Sources of financial support:
JJP and LCS were supported in part by Pfizer Inc. LCR was supported in part by the Donald B. and Dorothy L. Stabler Foundation. The Rheumatic Diseases Research Core Center, where autoantibodies and chemokines were assayed, and immunohistochemistry and RNAseq library preparation were performed, is supported by NIH P30-AR070254. LF was supported in part by NIH R01GM129085. DGL receives support from a National Institute of Neurological Disorders and Stroke Mentored Patient-Oriented Research Career Development Award (5 K23 NS091379). JA and ET was supported by the Jerome L Greene Foundation. AK was an employee at Pfizer when the study was conducted.
References
- 1.Paik JJ, Christopher-Stine L. A case of refractory dermatomyositis responsive to tofacitinib. Semin Arthritis Rheum 2017;46:e19. [DOI] [PubMed] [Google Scholar]
- 2.Kurtzman DJB, Wright NA, Lin J, Femia AN, Merola JF, Patel M, et al. Tofacitinib Citrate for Refractory Cutaneous Dermatomyositis: An Alternative Treatment. JAMA Dermatol 2016. [DOI] [PubMed] [Google Scholar]
- 3.Hornung T, Janzen V, Heidgen F-J, Wolf D, Bieber T, Wenzel J. Remission of recalcitrant dermatomyositis treated with ruxolitinib. N Engl J Med 2014;371:2537–2538. [DOI] [PubMed] [Google Scholar]
- 4.Ladislau L, Suárez-Calvet X, Toquet S, Landon-Cardinal O, Amelin D, Depp M, et al. JAK inhibitor improves type I interferon induced damage: proof of concept in dermatomyositis. Brain 2018;141:1609–1621. [DOI] [PubMed] [Google Scholar]
- 5.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 1975;292:344–347. [DOI] [PubMed] [Google Scholar]
- 6.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med 1975;292:403–407. [DOI] [PubMed] [Google Scholar]
- 7.Anyanwu CO, Fiorentino DF, Chung L, Dzuong C, Wang Y, Okawa J, et al. Validation of the Cutaneous Dermatomyositis Disease Area and Severity Index: characterizing disease severity and assessing responsiveness to clinical change. Br J Dermatol 2015;173:969–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner KR, Hamed S, Hadley DW, Gropman AL, Burstein AH, Escolar DM, et al. Gentamicin treatment of Duchenne and Becker muscular dystrophy due to nonsense mutations. Ann Neurol 2001;49:706–711. [PubMed] [Google Scholar]
- 9.Rider LG, Giannini EH, Brunner HI, Ruperto N, James-Newton L, Reed AM, et al. International consensus on preliminary definitions of improvement in adult and juvenile myositis. Arthritis Rheum 2004;50:2281–2290. [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal R, Rider LG, Ruperto N, Bayat N, Erman B, Feldman BM, et al. 2016 American College of Rheumatology/European League Against Rheumatism criteria for minimal, moderate, and major clinical response in adult dermatomyositis and polymyositis: An International Myositis Assessment and Clinical Studies Group/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Ann Rheum Dis 2017;76:792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiorentino DF, Presby M, Baer AN, Petri M, Rieger KE, Soloski M, et al. PUF60: a prominent new target of the autoimmune response in dermatomyositis and Sjögren’s syndrome. Ann Rheum Dis 2016;75:1145–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 2013;14:R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song L, Sabunciyan S, Florea L. CLASS2: accurate and efficient splice variant annotation from RNA-seq reads. Nucleic Acids Res 2016;44:e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 2014;15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenberg SA, Higgs BW, Morehouse C, Walsh RJ, Kong SW, Brohawn P, et al. Relationship between disease activity and type 1 interferon- and other cytokine-inducible gene expression in blood in dermatomyositis and polymyositis. Genes Immun 2012;13:207–213. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed S, Chakka S, Concha J, Krain R, Feng R, Werth VP. Evaluating important change in cutaneous disease activity as an efficacy measure for clinical trials in dermatomyositis. Br J Dermatol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wienke J, Bellutti Enders F, Lim J, Mertens JS, Hoogen LL van den, Wijngaarde CA, et al. Galectin-9 and CXCL10 as Biomarkers for Disease Activity in Juvenile Dermatomyositis: A Longitudinal Cohort Study and Multicohort Validation. Arthritis & Rheumatology (Hoboken, NJ) 2019;71:1377–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen M, Quan C, Diao L, Xue F, Xue K, Wang B, et al. Measurement of cytokines and chemokines and association with clinical severity of dermatomyositis and clinically amyopathic dermatomyositis. Br J Dermatol 2018;179:1334–1341. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg SA, Pinkus JL, Pinkus GS, Burleson T, Sanoudou D, Tawil R, et al. Interferon-alpha/beta-mediated innate immune mechanisms in dermatomyositis. Ann Neurol 2005;57:664–678. [DOI] [PubMed] [Google Scholar]
- 20.Baechler EC, Bauer JW, Slattery CA, Ortmann WA, Espe KJ, Novitzke J, et al. An interferon signature in the peripheral blood of dermatomyositis patients is associated with disease activity. Mol Med 2007;13:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinal-Fernandez I, Casal-Dominguez M, Derfoul A, Pak K, Plotz P, Miller FW, et al. Identification of distinctive interferon gene signatures in different types of myositis. Neurology 2019;93:e1193–e1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu X, Tang L, Li K, Zheng J, Zhao P, Tao Y, et al. Contribution of NKX2–3 polymorphisms to inflammatory bowel diseases: a meta-analysis of 35358 subjects. Sci Rep 2014;4:3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.