Abstract
Leukemia inhibitory factor (LIF) is a multi-functional cytokine of the interleukin-6 (IL-6) superfamily. Initially identified as a factor that inhibits the proliferation of murine myeloid leukemia cells, LIF displays a wide variety of important functions in a cell-, tissue- and context-dependent manner in many physiological and pathological processes, including regulating cell proliferation, pluripotent stem cell self-renewal, tissue/organ development and regeneration, neurogenesis and neural regeneration, maternal reproduction, inflammation, infection, immune response, and metabolism. Emerging evidence has shown that LIF plays an important but complex role in human cancers; while LIF displays a tumor suppressive function in some types of cancers, including leukemia, LIF is overexpressed and exerts an oncogenic function in many more types of cancers. Further, targeting LIF has been actively investigated as a novel strategy for cancer therapy. This review summarizes the recent advances in the studies on LIF in human cancers and its potential application in cancer therapy. A better understanding of the role of LIF in different types of cancers and its underlying mechanisms will help to develop more effective strategies for cancer therapy.
Keywords: LIF, cytokine, IL-6 superfamily, cancer, cancer therapy
1. Introduction
LIF is a multi-functional cytokine of the IL-6 superfamily. In addition to LIF, this superfamily also includes IL-6, IL-11, Oncostatin M (OSM), cardiotrophin-1 (CT-1), ciliary neurotrophic factor (CNTF), cardiotrophin-like cytokine (CLC), and IL-27 (Murakami et al., 2019; Rose-John, 2018; Yue et al., 2015). LIF was initially identified as a factor that inhibits the proliferation of murine myeloid leukemia M1 cells and induces their terminal differentiation (Gearing et al., 1987; Hilton et al., 1988; Lowe et al., 1989). That is why LIF was named leukemia inhibitory factor. Interestingly, at almost the same time, LIF was also identified as the differentiation-inhibitory factor that maintains the pluripotency of mouse embryonic stem cells and suppresses their differentiation (Smith et al., 1988; Williams et al., 1988), the hepatocyte-stimulating factor III that induces liver cells to produce acute-phase proteins in cultured rat hepatoma cells (Baumann et al., 1987), the cholinergic neuronal differentiation factor (CNDF) that causes neurotransmitter switching in neurons (Yamamori et al., 1989), and the melanoma-derived lipoprotein lipase inhibitor (MLPLI) that blocks the transportation of lipid to adipocytes (Mori et al., 1989). These early studies demonstrated that LIF is a multi-functional cytokine that plays different roles in different cells, tissues and organs.
LIF protein is a monomeric glycoprotein which is often modified by glycosylation. While the molecular weight of the unglycosylated LIF protein is ~20–25 kDa, the molecular weight of the glycosylated LIF is in the range of 37–63 kDa (Metcalfe, 2011; Simpson et al., 1988; Yue et al., 2015). LIF exists as a compact four-helix bundle topology stabilized by three disulfide bridges, which is important for receptor binding (Boulanger et al., 2003; Robinson et al., 1994). LIF binds to its heterodimer receptor complex on the cell membrane composed of a LIF receptor (LIFR) and a glycoprotein gp130 (Nicola and Babon, 2015). LIF binds to both gp130 and LIFR with high affinity, and the interaction of LIF with LIFR is ~80-fold tighter than with gp130 (Boulanger et al., 2003; Hilton and Nicola, 1992) (Figure 1A–C). The gp130/LIFR complex is also the receptor for several other IL-6 family members, including OSM, CNTF, CT-1 and CLC, all of which signal through the gp130/LIFR heterodimer (Boulanger and Garcia, 2004). The gp130/LIFR complex is constitutively associated with members of the JAK family of tyrosine kinases (Stahl et al., 1994). When LIF binds to its receptor, the JAK family kinases are rapidly activated to initiate the tyrosine phosphorylation cascade of three major signaling pathways, including the JAK/STAT pathway (Stahl et al., 1994), the MAPK pathway (Thoma et al., 1994) and the PI3K pathway (Fahmi et al., 2013; Oh et al., 1998). It has also been reported that LIF can regulate many other signaling pathways, including the mTOR, PTEN, IGF1, TGFβ, FGF, VEGF/HIF-1α, integrin, estrogen receptor, Notch, Toll/NF-κB, Wnt/β-catenin, ephrin and YAP pathways (Chen et al., 2012; Rosario and Stewart, 2016; Wang et al., 2019). Through regulation of these different signaling pathways, LIF is involved in many different physiological and pathological processes, including regulating myeloid leukemia cell differentiation, pluripotent stem cell self-renewal, tissue/organ development and regeneration (e.g. the muscle, kidney, bone, and intestine), neurogenesis and neural regeneration, maternal reproduction, inflammation, infection, immune response, and metabolism (Davis et al., 2019; Nicola and Babon, 2015; Pasquin et al., 2016; Rosario and Stewart, 2016; West, 2019) (Figure 1D). Interestingly, a growing body of studies have reported that LIF also plays an important role in initiation and progression of solid tumors in addition to its role in suppression of leukemia. In this review, we summarize recent advances on the functions of LIF in different physiological processes and diseases, especially in cancer, and the potential application of LIF-related therapies in cancer and other diseases.
Figure 1. LIF is a multi-functional cytokine.
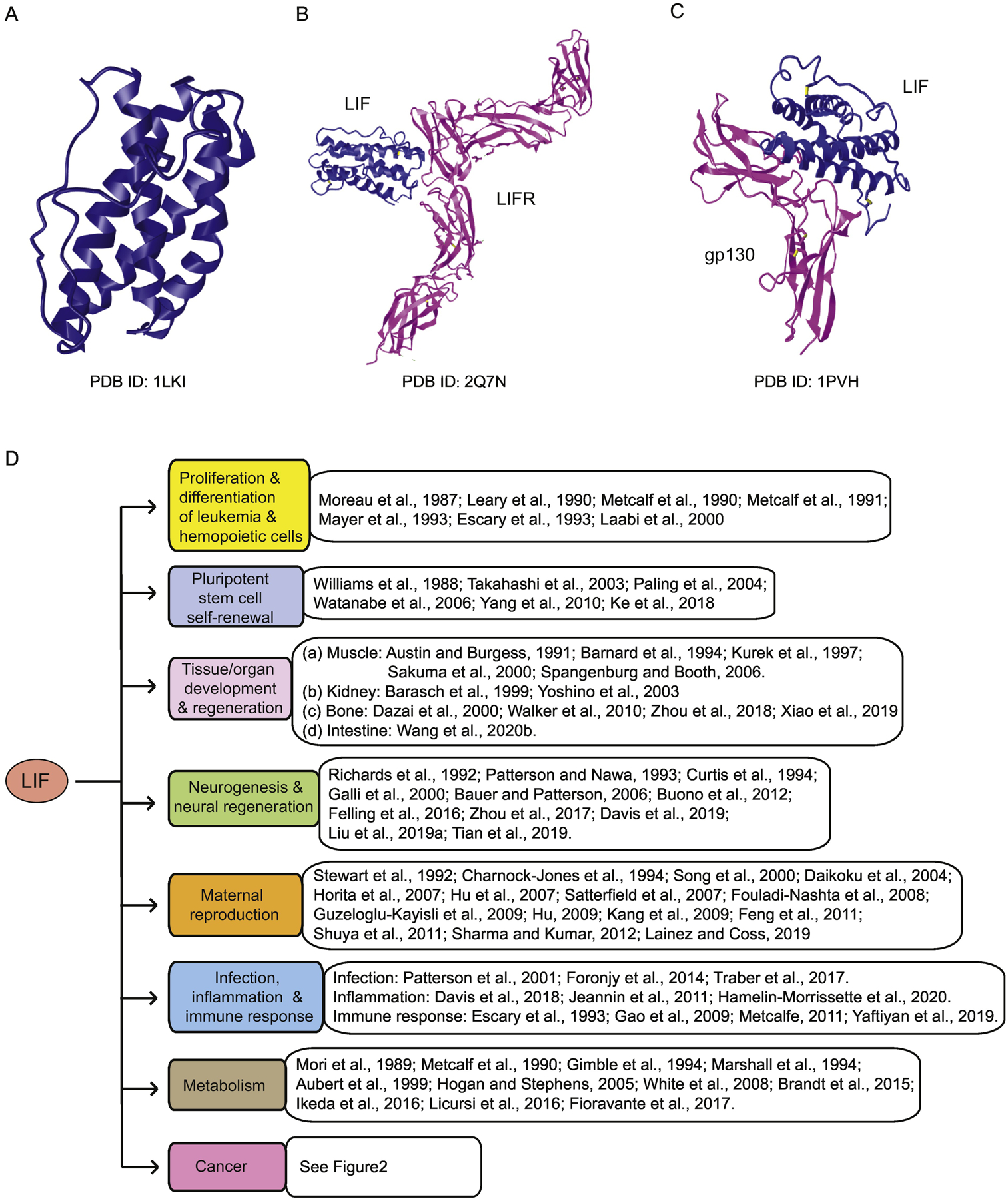
(A) The crystal structure of LIF protein. (B) The crystal structure of LIF-LIFR complex. (C) The crystal structure of LIF-gp130 complex. A-C: from Protein Data Bank (PDB, https://www.ncbi.nlm.nih.gov/Structure/pdb/). (D) As a multi-functional cytokine, LIF regulates many different physiological and pathological biological processes.
2. The multiple functions of LIF in biological processes and diseases
LIF is a multi-functional cytokine that plays a wide variety of important roles in different cells, tissues and organs, including the proliferation and differentiation of leukemia and hemopoietic cells, pluripotent stem cell self-renewal, tissue/organ development and regeneration, neurogenesis and neural regeneration, maternal reproduction, immune response, metabolism, as well as cancer (Figure 1D).
2.1. Proliferation and differentiation of leukemia and hemopoietic cells
Although LIF was initially identified as a factor to inhibit proliferation of myeloid leukemia M1 cells, studies have reported that LIF can promote proliferation of hematopoietic cells. For instance, LIF was reported to potentiate mouse megakaryocyte colony formation stimulated by IL-3 (Metcalf et al., 1991) and stimulate the proliferation of human hematopoietic cell line DA1 and the murine leukemic cell line GB2 (Laabi et al., 2000; Moreau et al., 1987). LIF also enhances the proliferation of hematopoietic stem cells in vitro (Escary et al., 1993; Leary et al., 1990), and increases the number of platelets and megakaryocytes in mice (Mayer et al., 1993; Metcalf et al., 1990).
2.2. Pluripotent stem cell self-renewal
In contrast to its differentiating role in murine myeloid leukemia M1 cells, LIF displays a differentiation-inhibitory effect on normal embryonic stem cells in mice, which maintains the pluripotency of embryonic stem cells and stimulates their self-renewal (Williams et al., 1988). The JAK/STAT and PI3K pathways were reported to mediate the effect of LIF on maintaining the pluripotency of mouse embryonic stem cells (Paling et al., 2004; Takahashi et al., 2003; Watanabe et al., 2006). In addition, LIF is necessary for the induction of pluripotent stem cells from mature somatic cells and their self-renewal (Yang et al., 2010). Recently, it was reported that LIF promotes the proliferation of marmoset induced pluripotent stem cells (iPSCs) in cultures, and maintains the iPSC self-renewal by activation of pluripotency-associated transcription factor T-box 3 (TBX3) via the PI3K/AKT signaling pathway (Ke et al., 2018).
2.3. Tissue/organ development and regeneration
LIF is involved in development of different tissues/organs and their regeneration in response to tissue damage or injury. (a) The muscle: LIF promotes proliferation of myoblasts (the precursors of skeletal muscle) and inhibits their differentiation towards myotubes in vitro (Austin and Burgess, 1991). LIF deficiency reduces muscle regeneration following muscle injury in mice (Kurek et al., 1997), and LIF infusion promotes skeletal muscle regeneration (Barnard et al., 1994). In mouse models, exercise induces LIF expression in the muscle, and LIF-deficient mice fail to show a hypertrophic response to muscle loading (Sakuma et al., 2000; Spangenburg and Booth, 2006). (b) The kidney: LIF is involved in kidney development and regeneration. LIF is secreted by ureteric buds in the kidney and induces conversion of mesenchyme into epithelium (Barasch et al., 1999). ATP depletion in cultured rat renal epithelial cells (an in vitro model of acute renal failure) and ischemia-reperfusion injury in mice both induce LIF expression (Yoshino et al., 2003). Furthermore, inhibition of endogenous LIF by a LIF-neutralizing antibody reduces the cell number and DNA synthesis during the recovery period after ATP depletion in vitro (Yoshino et al., 2003). (c) The bone: LIF also regulates the bone remodeling process to restore damaged regions, maintain calcium levels in blood, and respond to diet and hormones. LIF promotes the differentiation of bone marrow stromal cells to osteoblast lineage and inhibits their differentiation towards adipocytes, which enhances bone formation (Walker et al., 2010). LIF enhances the reconstruction of defected bone by binding to the osteoblast cell surface and induces bone formation both in vitro and in vivo (Dazai et al., 2000). LIF inhibits apoptosis and promotes the expression of extracellular matrix components in degenerated nucleus pulposus cells, and shows a protective role of LIF in intervertebral disc degeneration, a disease characterized by degeneration of one or more of the discs that separate the bones of the spine (Xiao et al., 2019). Treating degenerated nucleus pulposus cells with recombinant LIF promotes the extracellular matrix synthesis through activation of the MAPK-ERK1/2 pathway (Zhou et al., 2018). (d) The intestine: Recently, using LIF knockout mouse models, we found that LIF regulates function of intestinal stem cells in maintaining the intestinal epithelial homeostasis and intestinal regeneration after radiation-induced damage through the AKT/GSK3β/β-catenin signaling in mice (Wang et al., 2020b).
2.4. Neurogenesis and neural regeneration
LIF plays an important role in differentiation and regeneration of the nervous system, including both neurons and glia. LIF was reported to switch the production of neurotransmitters from catecholamine to acetylcholine in cultured rat sympathetic neurons (Patterson and Nawa, 1993). LIF promotes the neuron development in the spinal cord and induces neurogenesis with CNTF in vitro (Galli et al., 2000; Richards et al., 1992). LIF also acts as a neurotrophic factor for motor and sensory neurons, which plays an important role in neuronal maintenance and axonal regeneration after nerve injuries in rats (Curtis et al., 1994). LIF exerts a neuroprotective effect in a rat model of stroke through inducing the expression of transcription factor myeloid zinc finger-1 (MZF-1) and its downstream target superoxide dismutase 3 (Davis et al., 2019). LIF increases neural stem cell (NSC) populations and enhances NSC self-renewal by inhibiting their differentiation (Bauer and Patterson, 2006; Buono et al., 2012), which contributes to neural regeneration after injury (Tian et al., 2019). LIF-transfected NSCs improves glial cell regeneration and ameliorates white matter injury (Tian et al., 2019). Brain injuries, such as intracerebral hemorrhage, were reported to induce LIF, resulting in reactive astrogliosis with increased glial fibrillary acidic protein (GFAP) mRNA expression (a marker in reactive astrocytes) as well as the reduction of intracerebral hemorrhage-induced neurogenesis and angiogenesis (Liu et al., 2019a; Zhou et al., 2017). In addition, cerebral hypoxia-ischemia induces LIF production by astrocytes within the subventricular zone, which in turn triggers the regenerative response to expand the neural stem/progenitors through the Notch signaling pathway (Felling et al., 2016).
2.5. Maternal reproduction
LIF plays an essential role in maternal reproduction. While LIF knockout in male mice does not affect reproduction, LIF knockout in female mice leads to infertility because of blastocyst implantation failure (Stewart et al., 1992). It was later shown that LIF expression is induced in the uterine endometrial cells at the time of blastocyst formation, which is crucial for blastocyst implantation (Charnock-Jones et al., 1994). Further studies showed that LIF has several additional functions that regulate reproduction. For instance, LIF induces the expression of the EGF family of growth factors (e.g. amphiregulin, epiregulin and heparin-binding EGF-like growth factor) (Song et al., 2000), implantation genes (e.g. Msx-1 and Wnt-4) (Daikoku et al., 2004), and junction proteins (e.g. L-selectins, E-cadherins, claudin and occludin) (Satterfield et al., 2007; Sharma and Kumar, 2012), which contributes to the preparation of the endometrium for the blastocyst implantation. LIF is also important for decidualization and leukocyte recruitment during the blastocyst implantation (Guzeloglu-Kayisli et al., 2009; Shuya et al., 2011). LIF promotes the synthesis of prostaglandins, an important mediator of implantation and decidualization, through increasing the expression of IL-1 and cyclooxygenase-2 (Fouladi-Nashta et al., 2008; Horita et al., 2007; Song et al., 2000). Interestingly, our previous studies showed that LIF is a direct target gene of tumor suppressor p53 and mediates the function of p53 in regulation of maternal reproduction in both mice and humans (Hu, 2009; Hu et al., 2007; Kang et al., 2009). p53 plays a central role in tumor suppression mainly through its function as a transcription factor, and p53 is frequently mutated in human cancers (Levine, 2019; Liu et al., 2019b; Zhang et al., 2020). Our previous study showed that p53 knockout female mice display impaired maternal reproduction due to reduced LIF levels during implantation resulted from loss of p53, which can be restored by administering recombinant LIF protein to p53 knockout female mice at the implantation stage (Hu et al., 2007). We further showed that p53 and estrogen receptor α are activated and coordinately induce LIF expression in endometrial tissues during implantation to ensure the proper blastocyst implantation (Feng et al., 2011). p53 codon 72 single nucleotide polymorphism (SNP; R72 or P72) is the most common SNP in the p53 gene that influences p53 activity and is associated with the cancer risk (Barnoud et al., 2019; Zhao et al., 2018). Analysis of a list of SNPs in the p53 pathway and the LIF gene in the in vitro fertilization (IVF) patients, we found that the p53 P72 allele and some SNPs in the LIF gene are enriched in IVF patients as risk factors for recurrent implantation failure, supporting the role of p53 and LIF in human reproduction (Kang et al., 2009). A recent report shows that LIF also regulates male reproductive ability; LIF induces cFOS and represses the gonadotropin-releasing hormone (GnRH) gene in neurons, leading to reduction of GnRH neuron spine density specific for male mice and sex-specific impairment in reproductive function (Lainez and Coss, 2019).
2.6. Infection, inflammation and immune response
(a) Immune response: Many studies have shown that LIF regulates immune response. An early study showed that LIF is essential for normal thymic architecture and LIF knockout mice display reduced responsiveness of their thymocytes to the mitogen concanavalin A (Escary et al., 1993). It was later reported that regulatory T cells (Tregs) produce high levels of LIF, which in turn induces the production of Tregs by increasing the expression of the Treg lineage transcription factor Foxp3 and decreasing the expression of T helper type 17 cell lineage transcription factor RORγt (Gao et al., 2009; Metcalfe, 2011). Thus, LIF appears to be tolerogenic by promoting Treg differentiation and inhibiting T helper type 17 cell differentiation (Gao et al., 2009; Metcalfe, 2011). LIF also modulates the maturation of dendritic cells (DCs), leading to the development of semi-mature and tolerogenic DCs, which helps establish the immunosuppressive environment (Yaftiyan et al., 2019). (b) Inflammation: LIF is also induced in response to inflammatory stimulation in macrophages and switches macrophages from a pro-inflammatory to an anti-inflammatory phenotype (Davis et al., 2018; Jeannin et al., 2011). A recent study further showed that in response to pro-inflammatory stress factors, LIF inhibits IFNγ and granulocyte-macrophage colony-stimulating factor (GM-CSF)-induced activation of STAT1 and STAT5 in macrophages, and inhibits macrophage cell motility via STAT3 and matrix metalloproteinase 9 (MMP-9) (Hamelin-Morrissette et al., 2020). (c) Infection: LIF contributes to immune response to viral and bacterial infections. LIF is upregulated in the placenta from HIV-1-infected women and inhibits HIV-1 replication in placenta and thymus tissues, suggesting a role of LIF in innate immunity against viral infections (Patterson et al., 2001). In response to respiratory syncytial virus infection, LIF is induced to protect the lung from lung injury and enhanced pathology during the viral infection (Foronjy et al., 2014). LIF also plays a critical role in lung tissue protection in bacterial pneumonia; LIF is induced in lung epithelial cells via the NF-κB pathway to improve tissue resilience during pneumonia (Traber et al., 2017).
2.7. Metabolism
LIF has been reported to regulate metabolism. Earlier studies identified LIF as a lipoprotein lipase inhibitor, suggesting a role of LIF in metabolism, especially in lipid metabolism (Marshall et al., 1994; Mori et al., 1989). Injection of recombinant LIF in mice causes significant weight loss (Metcalf et al., 1990). In cultured adipocytes, LIF treatment promotes lipolysis by inhibiting lipoprotein lipase activity, suggesting that LIF may induce weight loss through its catabolic effects (Marshall et al., 1994). Furthermore, LIF induces fatty acid oxidation and glucose uptake via the PI3K pathway and mTORC2 in the skeletal muscle, which may contribute to the fat loss effect of LIF (Brandt et al., 2015). High-fat diet induces the downregulation of LIF expression in the brain stem (Licursi et al., 2016), and hypothalamic expression of LIF protects mice from the development of diet-induced obesity (Fioravante et al., 2017). LIF also regulates the differentiation of adipocytes. Interestingly, different effects of LIF on the differentiation of adipocytes have been reported in different cell lines. For instance, LIF was reported to promote adipocyte differentiation in the mouse preadipocytes Ob1771 and 3T3-F442A cell lines (Aubert et al., 1999), but show minimal effects on adipocyte differentiation in the mouse 3T3-L1 cell line (Hogan and Stephens, 2005; White et al., 2008), and even prevent adipogenesis in bone marrow stromal cells (Gimble et al., 1994). Interestingly, a recent study reported that LIF can differentially regulate the early and late stages of adipocyte differentiation through its differential regulation of the Wnt signaling pathway; while LIF promotes proliferation and recruitment of preadipocytes from multipotent mesenchymal stem cells at the early stage of adipocyte differentiation, it promotes differentiation of preadipocytes into mature adipocytes at the late stage of adipocyte differentiation (Ikeda et al., 2016).
As summarized above, LIF plays important roles in a variety of physiological and pathology processes, and exerts its functions in a highly cell type-, tissue type-, and development stage-dependent manner through regulation of different signaling pathways (Figure 1D).
3. LIF and cancer
3.1. The role of LIF in cancer
A growing body of studies have shown a complex role of LIF in cancer. Although LIF was initially identified to inhibit the proliferation of leukemia cells, studies have indicated an oncogenic function of LIF in many different types of solid tumors. LIF was found to be frequently overexpressed in many types of solid tumors, including colorectal cancer (Yu et al., 2014), breast cancer (Li et al., 2014; Quaglino et al., 2007), pancreatic cancer (Peng et al., 2014; Shi et al., 2019; Wang et al., 2020a), melanoma (Kuphal et al., 2013; Maruta et al., 2009), osteosarcoma (Liu et al., 2015a), nasopharyngeal carcinoma (Liu et al., 2013a), prostate cancer (Liu et al., 2019c), cholangiocarcinoma (Wang et al., 2016), oral squamous cell carcinoma (OSCC) (Lin et al., 2020), and endometrial carcinoma (Xiao et al., 2015). Furthermore, high LIF expression has been reported to correlate with poor prognosis in patients with different types of cancers, including pancreatic adenocarcinoma, nasopharyngeal carcinoma, prostate adenocarcinomas, cholangiocarcinoma, OSCC, colorectal cancer, and breast cancer (Li et al., 2014; Lin et al., 2020; Liu et al., 2013a; Liu et al., 2019c; Wang et al., 2020a; Wang et al., 2016; Yu et al., 2014). Many studies using in vitro cell culture systems and animal models have reported that LIF overexpression promotes cancer cell proliferation, metastasis, immune evasion, stemness, resistance to therapy, and cachexia in different types of solid tumors (Figure 2).
Figure 2. The complex role of LIF in human cancers.
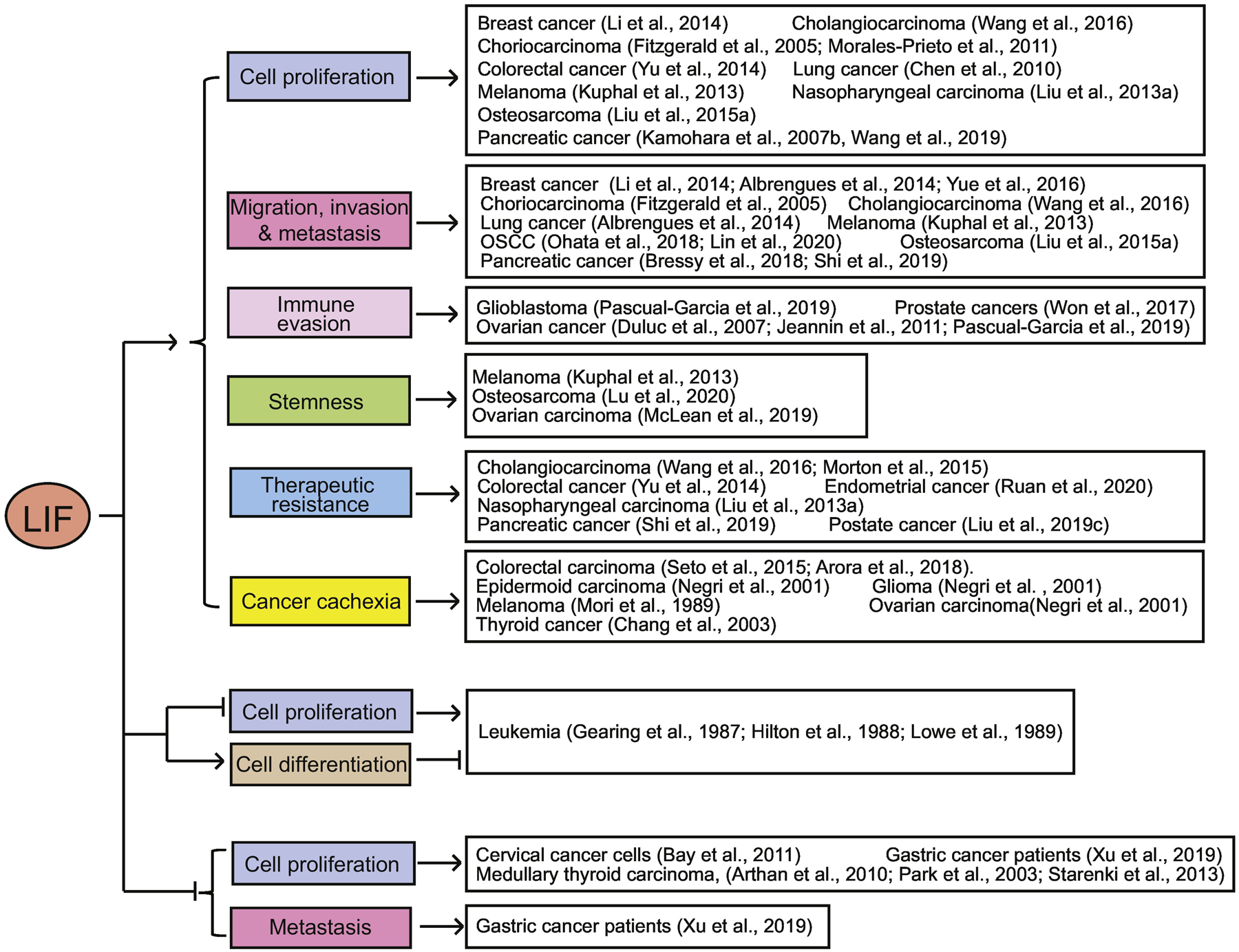
LIF promotes proliferation, metastasis, immune evasion, stemness, and therapeutic resistance of cancer cells, as well as cancer cachexia in many different types of solid tumors. On the contrary, LIF inhibits tumorigenesis in leukemia and some special types of solid tumors, such as gastric cancer, medullary thyroid carcinoma, and cervical cancer.
(1). Cell proliferation
Overexpression of LIF in cancers has been reported to promote proliferation of cancer cells through different mechanisms and signaling pathways. (a) The STAT3 pathway: LIF promotes proliferation of choriocarcinoma cells through activation of the STAT3 signaling (Fitzgerald et al., 2005). LIF promotes the growth of lung tumors through activation of STAT3 signaling in the Gprc5a−/− mouse lung tumor model (Chen et al., 2010). LIF was also reported to promote tumor growth in human osteosarcoma via activating the STAT3 signaling (Liu et al., 2015a). (b) The p53/MDM2 pathway: We found that LIF promotes proliferation and chemoresistance of colorectal cancer cells using both in vitro cell culture systems and xenograft tumor models through inhibition of tumor suppressor p53 (Yu et al., 2014). Mechanistically, LIF activates STAT3 to transcriptionally induce expression of ID1, a helix-loop-helix protein inhibitor of differentiation and DNA binding, to transcriptionally upregulate MDM2, the most critical negative regulator of p53, to ubiquitinate and degrade p53 (Yu et al., 2014). (c) The mTOR pathway: Our previous study also revealed that LIF promotes growth and metastasis of xenograft breast tumors through activating the AKT/mTOR pathway (Li et al., 2014). LIF was also found to activate the mTORC1/S6K signaling pathway to promote tumor growth, inhibit DNA damage responses and enhance radioresistance in Epstein-Barr virus (EBV)-associated nasopharyngeal carcinoma (Liu et al., 2013a). (d) IL-8: LIF promotes proliferation of pancreatic cancer cells through the induction of IL-8 (also called CXCL-8), a cytokine that has been shown to play an important role in promoting tumor progression in a variety of human cancers (Kamohara et al., 2007b). (e) BMP4/7: In malignant melanoma, LIF promotes cell proliferation and colony formation mainly through inducing the expression of bone morphogenetic proteins 4 and 7 (BMP4 and BMP7), two secreted ligand proteins of the TGF-β superfamily which play important roles in bone formation and promoting the progression of various types of cancers (Kuphal et al., 2013). (f) YAP: Recently, LIF was reported to promote proliferation of pancreatic cancer cells through activation of the YAP signaling pathway (Wang et al., 2019). (g) MicroRNAs (miRNAs): miRNAs play an important role in tumorigenesis through regulation of gene expression (Hayes et al., 2014; Liu et al., 2017). LIF has been reported to regulate miRNAs to promote proliferation of cancer cells. For instance, LIF represses the expression of miR-141, a miRNA that attenuates embryonic stem cell differentiation and epithelial-mesenchymal transition (EMT) of breast cancer cells, to promote proliferation of choriocarcinoma JEG-3 cells (Morales-Prieto et al., 2011). LIF treatment induces miR-181c, which downregulates the levels of N-Myc downstream-regulated gene 2 (NDRG2), to promote proliferation, chemoresistance, and metastasis of cholangiocarcinoma cells (Wang et al., 2016). Interestingly, NDRG2 can inhibit LIF transcription through disrupting the binding of Smad complex to the LIF promoter (Wang et al., 2016).
(2). Migration, invasion and metastasis
LIF has been shown to play an important role in promoting migration, invasion and metastasis in different types of cancers. (a) STAT3, AKT and mTOR pathways: As we mentioned above, LIF can activate different pathways, including STAT3, AKT and mTOR pathways, which mediate the promoting effect of LIF on migration, invasion and metastasis of cancer cells. For instance, LIF can promote migration, invasion and metastasis of cancer cells through activating STAT3 signaling in choriocarcinoma and osteosarcoma, activating the AKT/mTOR signaling in breast cancer, inducing expression of BMP4 and BMP7 in malignant melanoma, and inducing miR-181c to downregulate NDRG2 expression in cholangiocarcinoma (Fitzgerald et al., 2005; Kuphal et al., 2013; Li et al., 2014; Liu et al., 2015a; Wang et al., 2016). (b) Cancer-associated fibroblasts (CAFs): In addition to being secreted by cancer cells to regulate cancer cells in an autocrine fashion, LIF is also secreted by CAFs, the activated fibroblasts present in the cancer stroma that play a crucial role in cancer progression and metastasis, and regulates cancer cells in a paracrine fashion (Yoshida, 2020). It was reported that LIF produced by CAFs promotes cancer cell migration and invasion in a paracrine fashion in OSCC (Ohata et al., 2018). (c) The TGF-β signaling: A recent study identified that the Inhibin Subunit Beta A (INHBA), a modulator of TGF-β signaling pathway, is a crucial downstream effector of LIF that mediates the function of LIF in promoting metastasis of OSCC (Lin et al., 2020). In addition, LIF can be induced by TGF-β in both fibroblasts and cancer cells to mediate TGF-β-dependent actomyosin contractility and extracellular matrix remodeling in fibroblasts, resulting in pro-invasion microenvironment to promote cancer metastasis in vitro and in vivo (Albrengues et al., 2014). (d) Epithelial-mesenchymal transition (EMT): LIF can regulate the EMT to promote metastasis. Previously, we found that LIF induces the expression of oncomiR miR-21 through activation of the STAT3 signaling to promote EMT in breast cancer cells, and blocking miR-21 function greatly reduces the promoting effect of LIF overexpression on EMT and the migration ability of breast cancer cells (Yue et al., 2016). Recently, it was reported that LIF secreted from pancreatic stellate cells (PSCs) promotes the EMT of pancreatic cancer cells in pancreatic ductal adenocarcinoma (PDAC), contributing to metastasis of PDAC (Shi et al., 2019). (e) Neural remodeling: LIF was also reported to promote tumor metastasis through regulating neural remodeling in tumors (Bressy et al., 2018). Emerging evidence has shown that intratumoral nerves promote tumor progression; tumor cells promote the growth of nerves in the tumor microenvironment, and in return nerves release neurotransmitters to promote tumor proliferation and metastasis (Venkatesh and Monje, 2017). LIF secreted from stromal cells in PDAC promotes neural remodeling, which is characterized by high nerve densities in PDAC due to peripheral nerve fiber infiltration and axonogenesis. Mechanistically, LIF induces the differentiation and migration of glial nerve sheath Schwann cells via the JAK/STAT3/AKT signaling and promotes neuronal plasticity of ganglia neurons in the dorsal root in PDAC, leading to the increase of nerve density and neural remodeling in PDAC to promote cancer proliferation and metastasis (Bressy et al., 2018). (f) Hippo-YAP signaling pathway: LIF can inhibit the activity of the Hippo pathway by reducing the phosphorylation of YAP and increasing YAP nuclear translocation, resulting in the increased metastasis in gastric cancer (Bian et al., 2020). In addition, LIF was also reported to promote cancer vascular dissemination and local invasion through modulation of YAP1-FAK/PXN signaling in nasopharyngeal carcinoma (Liu et al., 2018).
(3). Immune evasion
Recent studies have revealed an important role of LIF in immune evasion of cancer. The immune system has the potential to recognize and eliminate cancer cells, and evasion of immune surveillance contributes to cancer development (Waldman et al., 2020). (a) Macrophage colony-stimulating factor: It was reported that ovarian cancer ascites contains high concentrations of LIF and IL-6 that can switch monocyte differentiation into tumor-associated macrophage (TAM)-like cells by increasing macrophage colony-stimulating factor consumption (Duluc et al., 2007; Jeannin et al., 2011). (b) Polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs): LIF induces the expansion and activation of PMN-MDSCs in Toll-like receptor 9 (TLR9) positive prostate cancers to promote immune evasion (Won et al., 2017). It was reported that blood samples from patients with prostate cancer display increased expression levels of LIF and LIFR in circulating PMN-MDSCs, and furthermore, the LIF expression is positively correlated with TLR9 in prostate cancer specimens (Won et al., 2017). (c) CCL2/CXCL9: In addition, LIF increases CCL2 expression to promote the recruitment of TAMs, and reduces CXCL9 expression to inhibit CD8+ T cell tumor infiltration, leading to immune evasion (Pascual-Garcia et al., 2019).
(4). Stemness
LIF can modulate stemness of cancer stem cells to promote tumor progression via different mechanisms. (a) BMP4/7: In malignant melanoma, LIF induces the expression of BMP4 and BMP7, as well as the classical stem cell proteins, such as SOX2, NANOG, OCT3/4 and GBX2, to enhance cancer stem cell-like behavior, which in turn promotes melanoma progression (Kuphal et al., 2013). (b) STAT3: Ovarian carcinoma-associated mesenchymal stem cells produce high levels of LIF, resulting in the elevated the percentage of ovarian cancer stem-like cells and cancer cell stemness through activating the STAT3 signaling, which promotes tumor growth (McLean et al., 2019). Notably, IL-6 plays a redundant role with LIF in regulating ovarian carcinoma-associated mesenchymal stem cells, and therefore, blocking LIF and IL-6 signaling simultaneously shows a stronger inhibitory effect on the pro-tumorigenic activity of ovarian carcinoma-associated mesenchymal stem cells than blocking LIF only (McLean et al., 2019). (c) Notch1: In osteosarcoma, LIF promotes activation of stemness-related genes through the Notch1 signaling pathway, thereby conferring tumor cells with stem cell-like characteristics, such as the increased sphere-forming potential, stimulated self-renewal, and enhanced metastasis ability (Lu et al., 2020).
(5). Therapeutic resistance
(a) mTOR, p53 and miR-181c: As mentioned above, LIF has been reported to promote resistance of cancer cells towards cancer therapies in different types of cancers with different mechanisms, including activation of the mTORC1/S6K signaling pathway in nasopharyngeal carcinoma to enhance radioresistance, inhibition of p53 tumor suppressive function in colorectal cancer to promote chemoresistance, and induction of miR-181c expression in cholangiocarcinoma to promote chemoresistance (Liu et al., 2013a; Wang et al., 2016; Yu et al., 2014). (b) Mcl-1: LIF protects cholangiocarcinoma cells from chemotherapeutic agents-induced apoptosis through the PI3K/AKT-dependent myeloid cell leukemia 1 (Mcl-1) activation (Morton et al., 2015). (c) EMT: The EMT of pancreatic cancer cells induced by LIF from pancreatic stellate cells (PSCs) may also contribute to the impaired efficacy of chemotherapy in PDAC (Shi et al., 2019). (d) Others: In addition, it was reported that LIF increases resistance of endometrial cancer cells to cisplatin and paclitaxel, two widely-used cancer chemotherapeutic agents (Ruan et al., 2020). LIF can also promote resistance of prostate cancer cells to enzalutamide, an androgen receptor inhibitor often used for the androgen deprivation therapy (ADT) in prostate cancer (Liu et al., 2019c). However, their underlying mechanisms are not clear yet.
(6). Cancer cachexia
LIF has been reported to play an important role in cancer cachexia. Cancer cachexia is a multifactorial syndrome with systematic loss of body weight mainly caused by reduction of adipose and muscle mass, as well as chronic inflammation, anorexia, and fatigue (Baracos et al., 2018). Cachexia occurs in ~50% of cancer patients, and cancer patients with cachexia often display poor response to cancer therapies, including chemotherapy and radiotherapy, and have increased treatment toxicity (Baracos et al., 2018). (a) Lipoprotein lipase: In an early study, LIF was identified as a lipoprotein lipase inhibitor from the melanoma cell line SEKI that reduces the intake of fatty acids by adipocytes to promote lipid catabolism in adipose tissues, leading to cancer cachexia in mice (Mori et al., 1989). In line with this finding, later studies reported that intracerebral injection of human epidermoid carcinoma A431 cells, ovarian carcinoma OVCAR3 cells, and glioma GBLF cells (Negri et al., 2001) and subcutaneous injection of thyroid cancer Thena cells in mice (Chang et al., 2003) result in high levels of LIF in serum, which leads to cachexia. (b) JAK2/STAT3: A recent study using C26 mouse colorectal carcinoma cells showed that LIF is elevated in the C26 conditioned medium in vitro and the serum of mice bearing C26 xenograft tumors, which induces atrophy in the cultured myotubes and in the muscle of mice (Seto et al., 2015). Furthermore, treatments with the LIF neutralizing antibody or JAK2 inhibitors, or overexpression of a dominant negative STAT3, can block the atrophy in myotubes caused by C26 conditioned medium or the atrophy in the mouse muscle caused by C26 tumors, which indicates that the LIF-JAK2-STAT3 signaling is important for cachexia induced by colon carcinoma (Seto et al., 2015). (c) Adipose triglyceride lipase (ATGL): Recently, it was also reported that LIF induces ATGL-mediated lipolysis through activation of the JAK/STAT signaling in adipocytes and leads to loss of the adipose tissue and body weight, which contributes to cachexia and can be counterbalanced by downregulating the leptin signaling (Arora et al., 2018).
(6). Tumor suppression function of LIF
While LIF displays an oncogenic effect in most types of solid tumors, LIF was also reported to show a tumor suppressive effect in some special tumor types in addition to its well-known function in inhibiting the proliferation of murine myeloid leukemia M1 cells and inducing their terminal differentiation (Gearing et al., 1987; Hilton et al., 1988; Lowe et al., 1989). (a) Gastric cancer: Although LIF is overexpressed in many types of solid tumors as mentioned above, interestingly, LIF was reported to be downregulated in some gastric cancers, and furthermore, its downregulation is associated with advanced clinical stage, lymph node metastasis, and poor overall survival in gastric cancer patients (Xu et al., 2019). LIF also displays anti-cancer stem cell properties in gastric cancer through activation of LATS1/2 Hippo kinases, thereby decreasing downstream YAP/TAZ nuclear accumulation and TEAD transcriptional activity (Seeneevassen et al., 2020). (b) Medullary thyroid carcinoma: While activation of Ras/Raf/MEK/ERK pathway often promotes cell proliferation, sustained activation of this pathway has also been reported to lead to cell cycle arrest (Park et al., 2003). In medullary thyroid carcinoma, activation of Ras/Raf/MEK/ERK pathway induces LIF expression and secretion, which in turn leads to growth arrest and differentiation through activation of the JAK/STAT3 pathway in both autocrine and paracrine manners (Park et al., 2003). Studies also showed that LIF can suppress progression of medullary thyroid carcinoma through inducing cell cycle arrest in G0/G1 phase and reducing RET and E2F1 expression in both cultured cells and xenograft tumor models (Arthan et al., 2010; Park et al., 2003; Starenki et al., 2013). (C) Cervical cancer: Additionally, in cervical cancer cells, LIF treatment decreases human papillomavirus (HPV) long control region activation and reduces HPV E6 and E7 mRNA levels, which in turn suppresses proliferation of cervical cancer cells (Bay et al., 2011).
Taken together, these results indicate that LIF can impact tumorigenesis in a highly tumor type-specific manner. While LIF displays an oncogenic effect in most types of solid tumors by promoting proliferation, metastasis, immune evasion, stemness, cachexia, and therapeutic resistance, LIF can also show a tumor suppressive effect in some special tumor types (Figure 2).
3.2. LIF regulation in cancer
LIF overexpression has been observed in many types of solid tumors, and different mechanisms have been reported to contribute to LIF overexpression.
(1). Epigenetics
It was reported that LIF expression is epigenetically upregulated via DNA demethylation and changes in histone methylation status within the promoter region of the LIF gene (Shin et al., 2011). While the CpG sites in the LIF promoter region are hypermethylated in normal breast epithelial cells, they are often extensively demethylated during breast tumorigenesis (Shin et al., 2011). The levels of methyl-CpG-binding protein (MeCP2) occupancy and histone H3-Lys9-dimethylation (hallmarks for transcriptional repression) are significantly decreased in the LIF promoter region during breast tumorigenesis, while the level of H3-Lys4-dimethylation (a hallmark for transcriptional activation) is increased within the LIF promoter region at the same time (Shin et al., 2011). Recently, it was reported that the histone H3-Lys27-trimethylation demethylase UTX, which sustains the proliferation of cancer cells, impairs histone H3-Lys27-trimethylation accumulation and induces histone H3-Lys27-acetylation at the LIF gene locus, leading to increased LIF expression and LIF-mediated expression of the stemness-related genes (Lu et al., 2020).
(2). Stresses and oncogenes
(a) Hypoxia: Our study found that LIF expression can be induced in colorectal cancer by hypoxia, a condition that often exists in solid tumors (Wu et al., 2015). Hypoxia in colorectal cancer stabilizes hypoxia inducible factor 2α (HIF-2α), leading to the binding of HIF-2 to the two hypoxia-responsive elements in the LIF promoter region to enhance the transcription of LIF (Wu et al., 2015). (b) TGF-β: TGF-β also induces LIF expression and activates the JAK/STAT pathway in a Smad-dependent manner in glioma-initiating cells, which is responsible for the initiation and recurrence of glioma (Penuelas et al., 2009). Furthermore, TGF-β induces the production and secretion of LIF in many different types of cancer cells and fibroblasts (Albrengues et al., 2014). (c) STAT5: LIF was also reported to be directly transcriptionally regulated by STAT5 in myeloid cell lines; STAT5 binds to the LIF promoter to induce LIF expression following the activation of the JAK2/STAT5 pathway (Salas et al., 2011). (d) Oncogenic proteins: LIF expression can also be regulated by some oncogenic proteins. For example, LIF expression is induced by oncogenic KRAS (e.g. KRAS G12D) through the MEK/ERK signaling pathway in PDAC (Wang et al., 2019). ZBTB46, a lineage-specific transcription factor that is crucial for EMT and metastasis of prostate cancer, binds to the promoter region of the LIF gene and induces LIF expression to promote tumor growth and therapeutic resistance in prostate cancer (Liu et al., 2019c). Interestingly, androgen deprivation therapy induces LIF expression through upregulation of ZBTB46 in ADT-resistant prostate cancer (Liu et al., 2019c), suggesting that some cancer therapies can induce LIF expression in cancers and in turn promote cancer progression (e.g. therapeutic resistance). Zinc Finger E-Box Binding Homeobox 1 (ZEB1) was reported to promote EMT and display an oncogenic effect in many cancers, including colorectal cancer, breast cancer, pancreatic cancer, osteosarcoma, lung cancer, etc. (Drapela et al., 2020; Liu et al., 2015b). Interestingly, a recent study reported that ZEB1 deletions were observed in more than 50% of glioblastomas and 15% in low grade gliomas (grade II and grade III) with frequent loss of heterozygosity (Edwards et al., 2017). Furthermore, ZEB1 binds to the promoter region of LIF and represses LIF expression in glioma cancer stem cells, which in turn inhibits glioma cancer stem cell self-renewal and promotes their differentiation (Edwards et al., 2017). It is unclear whether ZEB1 can also repress LIF expression in other types of tumors in addition to glioblastomas and low-grade gliomas.
(3). Inflammation and infection
It was reported that in pancreatic carcinoma cells pro-inflammatory cytokines, such as LIF itself, TNF-α, IL-1β, IL-6, and IL-8, induce LIF mRNA. The induction of LIF mRNA by LIF treatment can be inhibited by inhibitors of protein kinase C (PKC), protein tyrosine kinase (PTK) and Ca2+/Calmodulin. While the precise mechanisms underlying these regulations are still unclear, these observations suggest that inflammatory cytokine network may alter the expression of LIF through these pathways in the tumor microenvironment (Kamohara et al., 2007a). Additionally, in EBV-associated nasopharyngeal carcinoma, EBV-encoded protein latent membrane protein 1 (LMP1) can induce LIF production through activation of the NF-κB pathway by its C terminus–activating regions to promote tumorigenesis (Liu et al., 2013a).
(4). Tumor suppressors
Our previous study showed that LIF is a direct transcriptional target of tumor suppressor p53 (Hu et al., 2007). p53 binds to the p53-binding element in the first intron of the LIF gene to upregulate the basal LIF expression levels in various tissues of mice, including the uterine. p53 deficiency reduces the uterine LIF levels at the blastocyst implantation stage, resulting in impaired implantation and infertility in the p53 deficient female mice (Hu et al., 2007). It was also reported that p53 induces LIF expression in medulloblastoma (Baxter and Milner, 2010). Knockdown of WIP1 or SIRT1, which stabilizes and activates p53, was reported to enhance LIF expression and induce apoptosis in medulloblastoma (Baxter and Milner, 2010).
In sum, the regulation of LIF in cells appears to be complicated; LIF is regulated by epigenetic modifications, different stress signals and cytokines in the microenvironment, and some oncogenic transcription factors, as well as the tumor suppressors (e.g. p53). The accurate regulation of LIF by different mechanisms in different cells and tissues in a highly context-dependent manner contributes to the complex functions of LIF in both physiological and pathological processes.
4. The therapeutic role of LIF in cancer and other diseases
4.1. The therapeutic application of LIF in cancer
(1). Neutralizing antibodies
Given the important role of LIF in cancer progression in many solid tumors, LIF has the direct potential to be developed as a therapy target for cancers (Figure 3A). As a cytokine that functions through binding to the gp130/LIFR on cell membrane, LIF signaling has been shown to be blocked by LIF-neutralizing antibodies that efficiently antagonize many of LIF’s functions in many different studies (Chen et al., 2013; Fischer et al., 2014; Mao et al., 2016; Nogueira-Silva et al., 2012; Terakawa et al., 2011). The LIF neutralizing antibody has been shown to suppress the proliferation of different cancer cell lines in cultures (Kellokumpu-Lehtinen et al., 1996). The LIF-neutralizing antibody also inhibits the osteoclast recruitment by the mouse tumor cells, which is important for bone metastasis of tumors, to suppress tumor metastasis (Akatsu et al., 1998). Our previous study also showed that the LIF-neutralizing antibody can block the promoting effect of LIF on migration and invasion of breast cancer cells (Li et al., 2014). Moreover, it was reported that the LIF-neutralizing antibody blocks the STAT3 activation and proinvasive extracellular matrix remodeling in stroma fibroblasts, which leads to the inhibition of cancer cell invasion (Albrengues et al., 2014). Similarly, the LIF neutralizing antibody was shown to inhibit OSCC migration and invasion induced by LIF produced by normal human dermal fibroblasts (Ohata et al., 2018). The LIF-neutralizing antibody was also reported to reduce the percentage of tumor-infiltrating PMN-MDSCs and inhibit prostate tumor growth in mice (Won et al., 2017). Injection of the LIF-neutralizing antibody into PDAC-bearing mice was reported to reduce the intratumoral nerve density in PDAC and limit the tumor-promoting effect of the neural remodeling on PDAC progression (Bressy et al., 2018). In addition, the LIF-neutralizing antibody was shown to abolish C26 conditioned media-induced STAT3 activation and myotube atrophy, suggesting the potential role of the LIF-neutralizing antibody in treating cancer cachexia (Seto et al., 2015).
Figure 3. The potential therapeutic application of LIF in human cancers and other diseases.
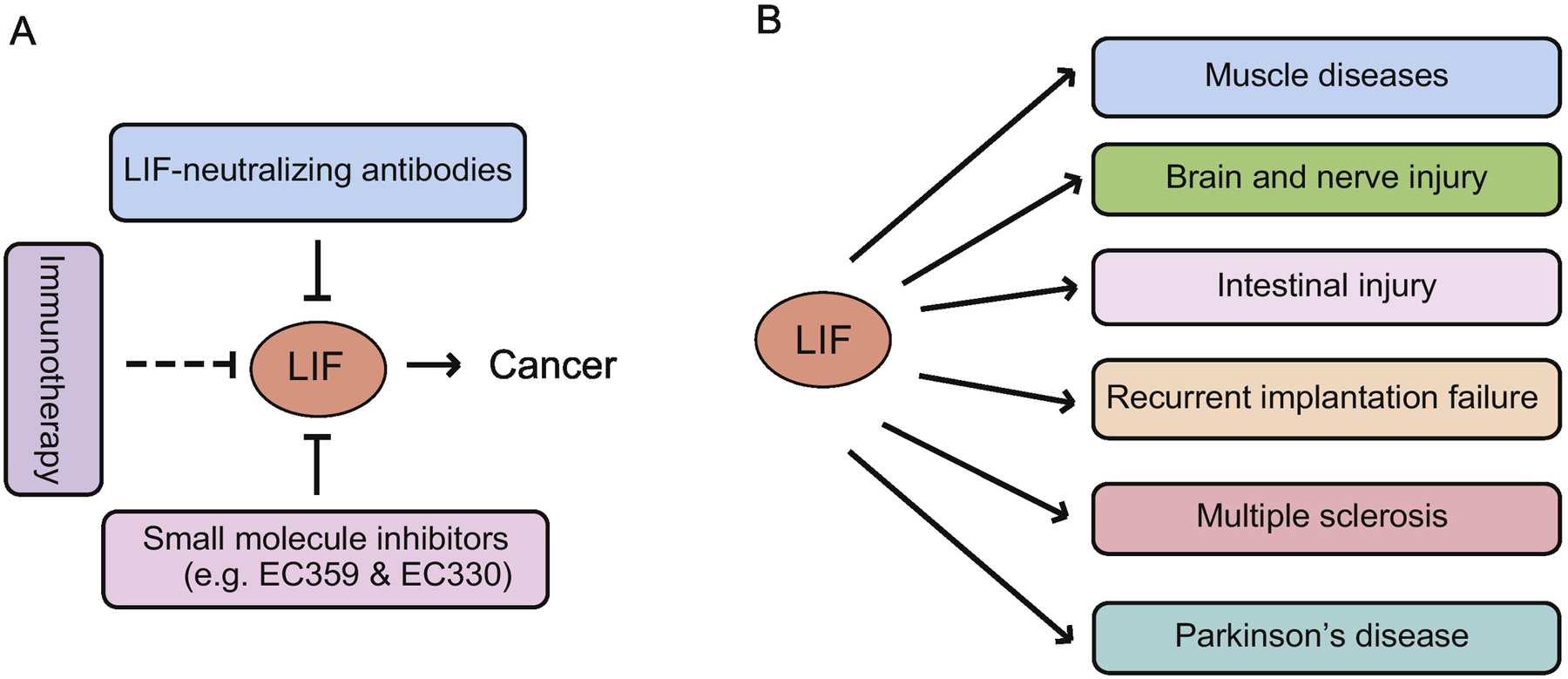
(A) The therapeutic strategies targeting LIF in cancers include LIF neutralizing antibodies, small molecule inhibitors, and immunotherapy. (B) In addition to cancer, LIF can also be used to treat muscle diseases, brain and nerve injury, intestinal injury, recurrent implantation failure, multiple sclerosis, and Parkinson’s disease.
Since LIF promotes the therapeutic resistance in cancer, the combination of LIF-neutralizing antibodies with other treatments for cancer has been reported to display a more effective therapeutic effect in some cancers. For instance, in the PDAC mouse model, the LIF-neutralizing antibody treatment slows the tumor progression, augments chemotherapy efficacy of gemcitabine, and prolongs the survival of mice (Shi et al., 2019). Similarly, the LIF-neutralizing antibody synergizes with gemcitabine to repress the pancreatic tumor progression in a pancreatic xenograft model, a syngeneic KRAS G12D-driven PDAC mouse model, and in patient-derived xenografts as well (Wang et al., 2019). The LIF-neutralizing antibody also increases CXCL9 expression in TAMs and induces CD8+ T cell tumor infiltration in immunocompetent mouse models of glioblastoma and ovarian cancer, which in turn suppresses tumor growth and improves survival of mice (Pascual-Garcia et al., 2019). Further, treating glioblastoma with the LIF-neutralizing antibody and the PD1 immune checkpoint antibody leads to tumor regression, immunological memory and increased overall survival of mice (Pascual-Garcia et al., 2019). A phase I clinical trial was initiated in patients with advanced solid tumors, include NSCLC, ovarian cancer, and pancreatic cancer using a humanized anti-LIF monoclonal antibody MSC-1 (Hyman et al., 2018).
(2). Small molecule inhibitors and other strategies to targeting LIF
In addition to the LIF-neutralizing antibody, LIF inhibitors are being developed to target LIF in cancers. Two first-in-class inhibitors, EC359 and EC330, have been shown to competitively inhibit LIFR complex by occupying LIF-binding site (Viswanadhapalli et al., 2019; Yue et al., 2020). EC359 and EC330 treatment attenuates the activation of the STAT3, mTOR, and AKT pathways by LIF, leading to the inhibited proliferation, increased apoptosis and reduced invasiveness and stemness of breast cancer cells, and inhibition of growth of breast cancer xenografts and patient-derived breast xenografts (Viswanadhapalli et al., 2019; Yue et al., 2020). EC359 was also reported to inhibit the activation of pancreatic cancer stroma in combination with gemcitabine in an organoid model generated by murine pancreatic cancer FC1245 cells and pancreatic stellate ImPaSC cells (Hall et al., 2019). EC330 was also reported to inhibit the development of ADT-resistant prostate cancers and is associated with neuroendocrine differentiation after the ADT, which induces LIF expression (Liu et al., 2019c). It is noted that while EC359 blocks LIF/LIFR signaling, it can also block the signaling of other LIFR ligands (e.g. CTF1, CNTF, and OSM) that interact at LIF/LIFR interface (Viswanadhapalli et al., 2019). Interestingly, a recent study showed that immunizing BALB/c mice with truncated mouse LIF and LIFR proteins as antigens can suppress tumor formation of breast cancer initiating cells derived from mouse MC4-L2 breast cancer cells in mice, with LIFR immunization showing a more superior effect than LIF immunization, suggesting that LIF and LIFR might be effective targets for immunotherapy of the tumors with overexpression of LIF and LIFR (Ghanei et al., 2020).
(3). Recombinant LIF
As mentioned above, LIF shows a growth inhibitory effect on medullary thyroid carcinoma (Arthan et al., 2010; Park et al., 2003; Starenki et al., 2013). Administration of the recombinant LIF locally or systemically was shown to effectively suppress grow of xenografts established by medullary thyroid carcinoma cells in mice, which suggests that LIF can be used as potential anti-cancer agent for medullary thyroid carcinoma (Starenki et al., 2013). It was also shown that the recombinant LIF downregulates p21 and cyclin D1 in gastric cancer cells, and overexpression of LIF delays the progress of gastric cancer xenografts, suggesting that administration of recombinant LIF might be a potential strategy to treat gastric cancers with decreased LIF expression (Xu et al., 2019).
4.2. LIF as a potential cancer biomarker
LIF is overexpressed in many types of solid tumors, and LIF is also secreted into the serum, which make LIF a potential biomarker for some types of tumors. For example, LIF serum levels were found to be elevated in nasopharyngeal carcinoma patients, and the high LIF levels are correlated with a high risk of local tumor recurrence and radioresistance of tumors, suggesting that serum LIF levels can be used as a biomarker to predict local recurrence and radioresistance of nasopharyngeal carcinoma (Liu et al., 2013a). In the serum from human PDAC patients and PDAC mice, LIF levels are positively correlated with intratumoral nerve density, which suggests that LIF is a candidate serum biomarker and diagnostic tool for PDAC (Bressy et al., 2018). In line with this observation, another study reported that LIF levels in the pancreas correlate with PDAC differentiation status as well as overall survival of PDAC patients, and furthermore, the changes in the levels of circulating LIF correlate with tumor response to therapy (Shi et al., 2019). A recent study also reported that higher tumor grade and metastatic prostate cancers exhibit higher LIF levels in patient serum, which suggests that LIF can be used as a serum biomarker for advanced prostate cancer (Liu et al., 2019c). However, the elevation of serum LIF levels can be caused not only by cancer cells and/or cancer surrounding tissues but also by other factors, such as infection and inflammation that are frequently observed in other diseases in addition to cancers, which may limit the application of the levels of serum LIF in early tumor diagnosis and tumor outcome prediction. Therefore, many more studies, especially clinical studies, are needed to further test the potential application of the levels of serum LIF as a biomarker for cancer.
4.3. The therapeutic application of LIF in other diseases
As a multi-functional cytokine involved in many fundamental physiological and biological processes and diseases, therapeutic application of LIF has also been suggested for many diseases in addition to cancer (Figure 3B). For example, satellite cells are stem cells in the skeletal muscle that can be used for transplantation therapy. Recombinant LIF treatment was reported to promote the proliferation of muscle satellite cells in vitro, maintain their undifferentiated states, and enhance their transplantation efficiency, which contributes to the optimization of culture conditions for cell transplantation therapy to several muscle diseases, including Duchenne muscular dystrophy (Ito et al., 2016; Spangenburg and Booth, 2002). In mouse models of spinal cord disease (motor neuron disease) and spinal cord injury, systemic administration of LIF was found to increase the number of neural precursor cells (Azari et al., 2005). LIF-transfected NSCs improve glial cell regeneration and ameliorate white matter injury in rats with middle cerebral artery occlusion, which suggests that LIF-transfected NSCs could be a potential treatment for cerebral infarction (Tian et al., 2019). Our recent study showed that LIF promotes regeneration of the intestinal epithelium in response to radiation and protects against radiation-induced gastrointestinal syndrome in mice (Wang et al., 2020b).
LIF plays an essential role in implantation and reproduction, which has suggested the potential application of LIF in the prediction and treatment for impaired reproduction. We have shown that the SNPs of the LIF gene and genes in p53 pathway that regulate LIF can help the prediction of the success of in vitro fertilization with recurrent implantation failure in patients (Kang et al., 2009). Similar associations were found in patients with recurrent pregnancy loss (Fraga et al., 2014). A recent study also reported the possibility to predict recurrent implantation failure by polymorphisms of the LIF and estrogen receptor-1 genes (Vagnini et al., 2019). LIF is downregulated in recurrent implantation failure patients with the abnormally increased endometrial KLF12, and exogenous LIF reverses the KLF12-mediated repression of BeWo spheroid adhesion, which suggests that LIF administration is a potential therapeutic strategy for recurrent implantation failure (Huang et al., 2018).
Multiple sclerosis, a non-curable demyelinating disease, is characterized by focal areas of inflammation with defacement of myelin and myelin-producing cells (oligodendrocytes and astrocytes) (Metcalfe, 2018). TNF-α was reported to stimulate astrocytes to secret LIF, which in turn induces production and maturation of oligodendrocytes from precursor cells and enhances oligodendrocyte remyelination of axons in the mouse hippocampus after cuprizone-induced demyelination (Deverman and Patterson, 2012; Fischer et al., 2014). In a multiple sclerosis mouse model, treating mice with LIF-overexpressing Wharton’s jelly stem cells leads to the reduction of brain lesions and brain cellular infiltration (Hosseini et al., 2017). Parkinson’s disease, a neurodegenerative disease, is characterized by the progressive loss of dopaminergic neurons in the substantia nigra. Cell-based neurogenic therapeutic strategies using endogenous NSCs to repair damaged neurons are potential treatments for Parkinson’s disease (Katz and Skalka, 1990). In line with the function of LIF in enhancing self-renewal of NSCs, LIF treatment improves the motor functions in the Parkinson’s disease mouse model induced by 6-hydroxydopamine, which suggests a potential therapeutic role of LIF in Parkinson’s disease (Liu et al., 2013b).
5. Summary and perspectives
As summarized above, LIF displays different functions in different cells, tissues, and organs under different conditions and at different development stages through different mechanisms. While LIF displays an oncogenic effect in many solid tumors, LIF displays a tumor suppressive effect in leukemia and some special types of solid tumors. Further, different signaling pathways are involved in mediating the complex role of LIF in tumorigenesis. The different biological outcomes of LIF might be caused by many different reasons, including different levels and status of the gp130/LIFR complex, different levels of other IL-6 family cytokines especially those sharing the same receptor complex with LIF, different status of downstream signaling pathways that mediate LIF functions, and different levels and status of the proteins and enzymes that are regulated by LIF and carry out the functions of LIF in different types of cells and tissues and under different circumstances. To make the situation more complicated, LIF is not only produced by cancer cells and functions in an autocrine manner, but also produced by the tumor microenvironment and functions in a paracrine manner.
With more and more studies showing the important role of LIF in tumorigenesis, targeting LIF has become a promising therapeutic strategy for many cancers with LIF overexpression. LIF-neutralizing antibodies as a single agent or in combination with other treatments has been shown as a potential new strategy for cancer treatment. Currently, majority of these studies are still at the pre-clinical stage, and further studies to optimize the condition of LIF-neutralizing antibodies for clinical use are necessary. Small molecule inhibitors present another potential strategy to target LIF in cancers. However, the specificity and potency of these small molecule inhibitors need to be improved for clinical use. The other potential strategies to target LIF-overexpressing cancers include blocking LIF overexpression and/or blocking the critical signaling pathways that mediate the oncogenic effects of LIF in cancers. Given the complex role and mechanism of LIF in cancers, a better understanding of the mechanism underlying LIF overexpression and the downstream signaling pathways of LIF in different cancers is necessary for developing more effective strategies targeting LIF in cancers. As indicated by many studies, LIF itself (recombinant LIF) has the direct potential to be used for treating some special tumor types in which LIF displays a tumor suppressive function as well as some other diseases (e.g. recurrent implantation failure, the brain and nerve injury, multiple sclerosis and Parkinson’s disease, etc.). However, majority of these studies are based on cell culture systems and animal models, and there are only a limited number of clinical trials that are mainly focused on fertility. More studies on animal models and clinical trials are required to test the potential application of recombinant LIF in these afore-mentioned diseases. Given that LIF can have different effects on different types of cells and tissues, it is important to develop specific strategies for targeted delivery of recombinant LIF or LIF inhibitors to specific cells and tissues for clinical treatments.
In summary, while accumulating studies have indicated that LIF plays an important but complex role in many physiological and pathological processes, the precise role and mechanism of LIF in these processes are far from clear. A better and more sophisticated understanding of the role and mechanism of LIF in these processes will be crucial for future application of targeting LIF in treating cancers and other diseases.
Acknowledgement
This work was supported in part by grants from National Institutes of Health (NIH; R01CA227912 and R01CA214746 to Z.F., and R01CA203965 to W.H.) and Congressionally Directed Medical Research Programs (CDMRP; W81XWH-16-1-0358 to W.H.).
Abbreviations List
- ADT
androgen deprivation therapy
- BMP
bone morphogenetic proteins
- CAF
cancer-associated fibroblast
- CLC
cardiotrophin-like cytokine
- CNTF
ciliary neurotrophic factor
- CT-1
cardiotrophin-1
- EMT
epithelial-mesenchymal transition
- ERK
extracellular signal related kinase
- gp130
glycoprotein 130
- HIF
hypoxia inducible factor
- IFN
interferon
- IL
interleukin
- iPSC
induced pluripotent stem cell
- JAK
Janus kinase
- LIF
Leukemia inhibitory factor
- LIFR
LIF receptor
- MAPK
mitogen-activated protein kinase
- MEK
MAPK/ERK kinase
- mTOR
mechanistic target of rapamycin
- mTORC
mTOR complex
- NDRG2
N-myc downstream-regulated gene 2
- NSC
neural stem cell
- OSCC
oral squamous cell carcinoma
- OSM
Oncostatin M
- PDAC
pancreatic ductal adenocarcinoma
- PMN-MDSC
polymorphonuclear myeloid-derived suppressor cell
- α-SMA
alpha-smooth muscle actin
- SNP
single nucleotide polymorphism
- SOCS3
suppressor of cytokine signaling 3
- STAT
signal transducer and activator of transcription
- TAM
tumor-associated macrophage
- TGF
transforming growth factor
- TLR
Toll-like receptor
- Treg
regulatory T cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement:
Authors declare no conflict of interest.
Submission Declaration
This paper has not been published and is not under consideration for publication elsewhere.
References
- Akatsu T, Ono K, Katayama Y, Tamura T, Nishikawa M, Kugai N, Yamamoto M, and Nagata N (1998). The mouse mammary tumor cell line, MMT060562, produces prostaglandin E2 and leukemia inhibitory factor and supports osteoclast formation in vitro via a stromal cell-dependent pathway. J Bone Miner Res 13, 400–408. [DOI] [PubMed] [Google Scholar]
- Albrengues J, Bourget I, Pons C, Butet V, Hofman P, Tartare-Deckert S, Feral CC, Meneguzzi G, and Gaggioli C (2014). LIF mediates proinvasive activation of stromal fibroblasts in cancer. Cell Rep 7, 1664–1678. [DOI] [PubMed] [Google Scholar]
- Arora GK, Gupta A, Narayanan S, Guo T, Iyengar P, and Infante RE (2018). Cachexia-associated adipose loss induced by tumor-secreted leukemia inhibitory factor is counterbalanced by decreased leptin. JCI Insight 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthan D, Hong SK, and Park JI (2010). Leukemia inhibitory factor can mediate Ras/Raf/MEK/ERK-induced growth inhibitory signaling in medullary thyroid cancer cells. Cancer Lett 297, 31–41. [DOI] [PubMed] [Google Scholar]
- Aubert J, Dessolin S, Belmonte N, Li M, McKenzie FR, Staccini L, Villageois P, Barhanin B, Vernallis A, Smith AG, et al. (1999). Leukemia inhibitory factor and its receptor promote adipocyte differentiation via the mitogen-activated protein kinase cascade. J Biol Chem 274, 24965–24972. [DOI] [PubMed] [Google Scholar]
- Austin L, and Burgess AW (1991). Stimulation of myoblast proliferation in culture by leukaemia inhibitory factor and other cytokines. J Neurol Sci 101, 193–197. [DOI] [PubMed] [Google Scholar]
- Azari MF, Profyris C, Zang DW, Petratos S, and Cheema SS (2005). Induction of endogenous neural precursors in mouse models of spinal cord injury and disease. Eur J Neurol 12, 638–648. [DOI] [PubMed] [Google Scholar]
- Baracos VE, Martin L, Korc M, Guttridge DC, and Fearon KCH (2018). Cancer-associated cachexia. Nat Rev Dis Primers 4, 17105. [DOI] [PubMed] [Google Scholar]
- Barasch J, Yang J, Ware CB, Taga T, Yoshida K, Erdjument-Bromage H, Tempst P, Parravicini E, Malach S, Aranoff T, et al. (1999). Mesenchymal to epithelial conversion in rat metanephros is induced by LIF. Cell 99, 377–386. [DOI] [PubMed] [Google Scholar]
- Barnard W, Bower J, Brown MA, Murphy M, and Austin L (1994). Leukemia inhibitory factor (LIF) infusion stimulates skeletal muscle regeneration after injury: injured muscle expresses lif mRNA. J Neurol Sci 123, 108–113. [DOI] [PubMed] [Google Scholar]
- Barnoud T, Parris JLD, and Murphy ME (2019). Common genetic variants in the TP53 pathway and their impact on cancer. Journal of molecular cell biology 11, 578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer S, and Patterson PH (2006). Leukemia inhibitory factor promotes neural stem cell self-renewal in the adult brain. J Neurosci 26, 12089–12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H, Onorato V, Gauldie J, and Jahreis GP (1987). Distinct sets of acute phase plasma proteins are stimulated by separate human hepatocyte-stimulating factors and monokines in rat hepatoma cells. J Biol Chem 262, 9756–9768. [PubMed] [Google Scholar]
- Baxter EW, and Milner J (2010). p53 Regulates LIF expression in human medulloblastoma cells. J Neurooncol 97, 373–382. [DOI] [PubMed] [Google Scholar]
- Bay JM, Patterson BK, and Teng NN (2011). Leukemia inhibitory factor downregulates human papillomavirus-16 oncogene expression and inhibits the proliferation of cervical carcinoma cells. Infect Dis Obstet Gynecol 2011, 463081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian SB, Yang Y, Liang WQ, Zhang KC, Chen L, and Zhang ZT (2020). Leukemia inhibitory factor promotes gastric cancer cell proliferation, migration, and invasion via the LIFR-Hippo-YAP pathway. Ann N Y Acad Sci., online ahead of print. [DOI] [PubMed] [Google Scholar]
- Boulanger MJ, Bankovich AJ, Kortemme T, Baker D, and Garcia KC (2003). Convergent mechanisms for recognition of divergent cytokines by the shared signaling receptor gp130. Mol Cell 12, 577–589. [DOI] [PubMed] [Google Scholar]
- Boulanger MJ, and Garcia KC (2004). Shared cytokine signaling receptors: structural insights from the gp130 system. Adv Protein Chem 68, 107–146. [DOI] [PubMed] [Google Scholar]
- Brandt N, O’Neill HM, Kleinert M, Schjerling P, Vernet E, Steinberg GR, Richter EA, and Jorgensen SB (2015). Leukemia inhibitory factor increases glucose uptake in mouse skeletal muscle. Am J Physiol Endocrinol Metab 309, E142–153. [DOI] [PubMed] [Google Scholar]
- Bressy C, Lac S, Nigri J, Leca J, Roques J, Lavaut MN, Secq V, Guillaumond F, Bui TT, Pietrasz D, et al. (2018). LIF Drives Neural Remodeling in Pancreatic Cancer and Offers a New Candidate Biomarker. Cancer Res 78, 909–921. [DOI] [PubMed] [Google Scholar]
- Buono KD, Vadlamuri D, Gan Q, and Levison SW (2012). Leukemia inhibitory factor is essential for subventricular zone neural stem cell and progenitor homeostasis as revealed by a novel flow cytometric analysis. Dev Neurosci 34, 449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JW, Yeh KY, Shen YC, Hsieh JJ, Chuang CK, Liao SK, Tsai LH, and Wang CH (2003). Production of multiple cytokines and induction of cachexia in athymic nude mice by a new anaplastic thyroid carcinoma cell line. J Endocrinol 179, 387–394. [DOI] [PubMed] [Google Scholar]
- Charnock-Jones DS, Sharkey AM, Fenwick P, and Smith SK (1994). Leukaemia inhibitory factor mRNA concentration peaks in human endometrium at the time of implantation and the blastocyst contains mRNA for the receptor at this time. J Reprod Fertil 101, 421–426. [DOI] [PubMed] [Google Scholar]
- Chen D, Sun Y, Wei Y, Zhang P, Rezaeian AH, Teruya-Feldstein J, Gupta S, Liang H, Lin HK, Hung MC, et al. (2012). LIFR is a breast cancer metastasis suppressor upstream of the Hippo-YAP pathway and a prognostic marker. Nat Med 18, 1511–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Hausman BS, Luo G, Zhou G, Murakami S, Rubin J, and Greenfield EM (2013). Protein kinase inhibitor gamma reciprocally regulates osteoblast and adipocyte differentiation by downregulating leukemia inhibitory factor. Stem Cells 31, 2789–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Deng J, Fujimoto J, Kadara H, Men T, Lotan D, and Lotan R (2010). Gprc5a deletion enhances the transformed phenotype in normal and malignant lung epithelial cells by eliciting persistent Stat3 signaling induced by autocrine leukemia inhibitory factor. Cancer Res 70, 8917–8926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis R, Scherer SS, Somogyi R, Adryan KM, Ip NY, Zhu Y, Lindsay RM, and DiStefano PS (1994). Retrograde axonal transport of LIF is increased by peripheral nerve injury: correlation with increased LIF expression in distal nerve. Neuron 12, 191–204. [DOI] [PubMed] [Google Scholar]
- Daikoku T, Song H, Guo Y, Riesewijk A, Mosselman S, Das SK, and Dey SK (2004). Uterine Msx-1 and Wnt4 signaling becomes aberrant in mice with the loss of leukemia inhibitory factor or Hoxa-10: evidence for a novel cytokine-homeobox-Wnt signaling in implantation. Mol Endocrinol 18, 1238–1250. [DOI] [PubMed] [Google Scholar]
- Davis SM, Collier LA, Foran EA, Leonardo CC, Ajmo CT Jr., and Pennypacker KR (2019). Neuroprotective activity of leukemia inhibitory factor is relayed through myeloid zinc finger-1 in a rat model of stroke. Metab Brain Dis 34, 631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SM, Collier LA, Winford ED, Leonardo CC, Ajmo CT Jr., Foran EA, Kopper TJ, Gensel JC, and Pennypacker KR (2018). Leukemia inhibitory factor modulates the peripheral immune response in a rat model of emergent large vessel occlusion. J Neuroinflammation 15, 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazai S, Akita S, Hirano A, Rashid MA, Naito S, Akino K, and Fujii T (2000). Leukemia inhibitory factor enhances bone formation in calvarial bone defect. J Craniofac Surg 11, 513–520. [DOI] [PubMed] [Google Scholar]
- Deverman BE, and Patterson PH (2012). Exogenous leukemia inhibitory factor stimulates oligodendrocyte progenitor cell proliferation and enhances hippocampal remyelination. J Neurosci 32, 2100–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapela S, Bouchal J, Jolly MK, Culig Z, and Soucek K (2020). ZEB1: A Critical Regulator of Cell Plasticity, DNA Damage Response, and Therapy Resistance. Front Mol Biosci 7, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duluc D, Delneste Y, Tan F, Moles MP, Grimaud L, Lenoir J, Preisser L, Anegon I, Catala L, Ifrah N, et al. (2007). Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood 110, 4319–4330. [DOI] [PubMed] [Google Scholar]
- Edwards LA, Li A, Berel D, Madany M, Kim NH, Liu M, Hymowitz M, Uy B, Jung R, Xu M, et al. (2017). ZEB1 regulates glioma stemness through LIF repression. Sci Rep 7, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escary JL, Perreau J, Dumenil D, Ezine S, and Brulet P (1993). Leukaemia inhibitory factor is necessary for maintenance of haematopoietic stem cells and thymocyte stimulation. Nature 363, 361–364. [DOI] [PubMed] [Google Scholar]
- Fahmi A, Smart N, Punn A, Jabr R, Marber M, and Heads R (2013). p42/p44-MAPK and PI3K are sufficient for IL-6 family cytokines/gp130 to signal to hypertrophy and survival in cardiomyocytes in the absence of JAK/STAT activation. Cell Signal 25, 898–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felling RJ, Covey MV, Wolujewicz P, Batish M, and Levison SW (2016). Astrocyte-produced leukemia inhibitory factor expands the neural stem/progenitor pool following perinatal hypoxia-ischemia. J Neurosci Res 94, 1531–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Zhang C, Kang HJ, Sun Y, Wang H, Naqvi A, Frank AK, Rosenwaks Z, Murphy ME, Levine AJ, et al. (2011). Regulation of female reproduction by p53 and its family members. FASEB J 25, 2245–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioravante M, Bombassaro B, Ramalho AF, Dragano NR, Morari J, Solon C, Tobar N, Ramos CD, and Velloso LA (2017). Inhibition of hypothalamic leukemia inhibitory factor exacerbates diet-induced obesity phenotype. J Neuroinflammation 14, 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer R, Wajant H, Kontermann R, Pfizenmaier K, and Maier O (2014). Astrocyte-specific activation of TNFR2 promotes oligodendrocyte maturation by secretion of leukemia inhibitory factor. Glia 62, 272–283. [DOI] [PubMed] [Google Scholar]
- Fitzgerald JS, Tsareva SA, Poehlmann TG, Berod L, Meissner A, Corvinus FM, Wiederanders B, Pfitzner E, Markert UR, and Friedrich K (2005). Leukemia inhibitory factor triggers activation of signal transducer and activator of transcription 3, proliferation, invasiveness, and altered protease expression in choriocarcinoma cells. Int J Biochem Cell Biol 37, 2284–2296. [DOI] [PubMed] [Google Scholar]
- Foronjy RF, Dabo AJ, Cummins N, and Geraghty P (2014). Leukemia inhibitory factor protects the lung during respiratory syncytial viral infection. BMC Immunol 15, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouladi-Nashta AA, Mohamet L, Heath JK, and Kimber SJ (2008). Interleukin 1 signaling is regulated by leukemia inhibitory factor (LIF) and is aberrant in Lif−/− mouse uterus. Biol Reprod 79, 142–153. [DOI] [PubMed] [Google Scholar]
- Fraga LR, Dutra CG, Boquett JA, Vianna FS, Goncalves RO, Paskulin DD, Costa OL, Ashton-Prolla P, Sanseverino MT, and Schuler-Faccini L (2014). p53 signaling pathway polymorphisms associated to recurrent pregnancy loss. Mol Biol Rep 41, 1871–1877. [DOI] [PubMed] [Google Scholar]
- Galli R, Pagano SF, Gritti A, and Vescovi AL (2000). Regulation of neuronal differentiation in human CNS stem cell progeny by leukemia inhibitory factor. Dev Neurosci 22, 86–95. [DOI] [PubMed] [Google Scholar]
- Gao W, Thompson L, Zhou Q, Putheti P, Fahmy TM, Strom TB, and Metcalfe SM (2009). Treg versus Th17 lymphocyte lineages are cross-regulated by LIF versus IL-6. Cell Cycle 8, 1444–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearing DP, Gough NM, King JA, Hilton DJ, Nicola NA, Simpson RJ, Nice EC, Kelso A, and Metcalf D (1987). Molecular cloning and expression of cDNA encoding a murine myeloid leukaemia inhibitory factor (LIF). EMBO J 6, 3995–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanei Z, Mehri N, Jamshidizad A, Joupari MD, and Shamsara M (2020). Immunization against leukemia inhibitory factor and its receptor suppresses tumor formation of breast cancer initiating cells in BALB/c mouse. Sci Rep 10, 11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimble JM, Wanker F, Wang CS, Bass H, Wu X, Kelly K, Yancopoulos GD, and Hill MR (1994). Regulation of bone marrow stromal cell differentiation by cytokines whose receptors share the gp130 protein. J Cell Biochem 54, 122–133. [DOI] [PubMed] [Google Scholar]
- Guzeloglu-Kayisli O, Kayisli UA, and Taylor HS (2009). The role of growth factors and cytokines during implantation: endocrine and paracrine interactions. Semin Reprod Med 27, 62–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BR, Cannon A, Thompson C, Santhamma B, Chavez-Riveros A, Bhatia R, Nair HB, Nickisch K, Batra SK, and Kumar S (2019). Utilizing cell line-derived organoids to evaluate the efficacy of a novel LIFR-inhibitor, EC359 in targeting pancreatic tumor stroma. Genes Cancer 10, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelin-Morrissette J, Dallagi A, Girouard J, Ravelojaona M, Oufqir Y, Vaillancourt C, Van Themsche C, Carrier C, and Reyes-Moreno C (2020). Leukemia inhibitory factor regulates the activation of inflammatory signals in macrophages and trophoblast cells. Mol Immunol 120, 32–42. [DOI] [PubMed] [Google Scholar]
- Hayes J, Peruzzi PP, and Lawler S (2014). MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med 20, 460–469. [DOI] [PubMed] [Google Scholar]
- Hilton DJ, and Nicola NA (1992). Kinetic analyses of the binding of leukemia inhibitory factor to receptor on cells and membranes and in detergent solution. J Biol Chem 267, 10238–10247. [PubMed] [Google Scholar]
- Hilton DJ, Nicola NA, and Metcalf D (1988). Purification of a murine leukemia inhibitory factor from Krebs ascites cells. Anal Biochem 173, 359–367. [DOI] [PubMed] [Google Scholar]
- Hogan JC, and Stephens JM (2005). Effects of leukemia inhibitory factor on 3T3-L1 adipocytes. J Endocrinol 185, 485–496. [DOI] [PubMed] [Google Scholar]
- Horita H, Kuroda E, Hachisuga T, Kashimura M, and Yamashita U (2007). Induction of prostaglandin E2 production by leukemia inhibitory factor promotes migration of first trimester extravillous trophoblast cell line, HTR-8/SVneo. Hum Reprod 22, 1801–1809. [DOI] [PubMed] [Google Scholar]
- Hosseini A, Estiri H, Akhavan Niaki H, Alizadeh A, Abdolhossein Zadeh B, Ghaderian SMH, Farjadfar A, and Fallah A (2017). Multiple Sclerosis Gene Therapy with Recombinant Viral Vectors: Overexpression of IL-4, Leukemia Inhibitory Factor, and IL-10 in Wharton’s Jelly Stem Cells Used in EAE Mice Model. Cell J 19, 361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W (2009). The role of p53 gene family in reproduction. Cold Spring Harb Perspect Biol 1, a001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Feng Z, Teresky AK, and Levine AJ (2007). p53 regulates maternal reproduction through LIF. Nature 450, 721–724. [DOI] [PubMed] [Google Scholar]
- Huang C, Sun H, Wang Z, Liu Y, Cheng X, Liu J, Jiang R, Zhang X, Zhen X, Zhou J, et al. (2018). Increased Kruppel-like factor 12 impairs embryo attachment via downregulation of leukemia inhibitory factor in women with recurrent implantation failure. Cell Death Discov 4, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman DM, Brana I, Spreafico A, Schram AM, Pandya NB, Hoffman K, Hallet R, Giblin P, Anido J, Ruano I, et al. (2018). A phase 1 study of MSC-1, a humanized anti-LIF monoclonal antibody, in patients with advanced solid tumors. J Clin Oncol 36, 15 suppl, TPS2602. [Google Scholar]
- Ikeda S, Itoh S, Yamamoto Y, Yamauchi Y, Matsushita K, Naruse H, and Hayashi M (2016). Developmental Stage-Dependent Effects of Leukemia Inhibitory Factor on Adipocyte Differentiation of Murine Bone Marrow Stromal Cells. Cell Biochem Biophys 74, 11–17. [DOI] [PubMed] [Google Scholar]
- Ito N, Shimizu N, Tanaka H, and Takeda S (2016). Enhancement of Satellite Cell Transplantation Efficiency by Leukemia Inhibitory Factor. J Neuromuscul Dis 3, 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannin P, Duluc D, and Delneste Y (2011). IL-6 and leukemia-inhibitory factor are involved in the generation of tumor-associated macrophage: regulation by IFN-gamma. Immunotherapy 3, 23–26. [DOI] [PubMed] [Google Scholar]
- Kamohara H, Ogawa M, Ishiko T, Sakamoto K, and Baba H (2007a). Leukemia inhibitory factor functions as a growth factor in pancreas carcinoma cells: Involvement of regulation of LIF and its receptor expression. Int J Oncol 30, 977–983. [PubMed] [Google Scholar]
- Kamohara H, Takahashi M, Ishiko T, Ogawa M, and Baba H (2007b). Induction of interleukin-8 (CXCL-8) by tumor necrosis factor-alpha and leukemia inhibitory factor in pancreatic carcinoma cells: Impact of CXCL-8 as an autocrine growth factor. Int J Oncol 31, 627–632. [PubMed] [Google Scholar]
- Kang HJ, Feng Z, Sun Y, Atwal G, Murphy ME, Rebbeck TR, Rosenwaks Z, Levine AJ, and Hu W (2009). Single-nucleotide polymorphisms in the p53 pathway regulate fertility in humans. Proc Natl Acad Sci U S A 106, 9761–9766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz RA, and Skalka AM (1990). Generation of diversity in retroviruses. Annu Rev Genet 24, 409–445. [DOI] [PubMed] [Google Scholar]
- Ke M, He Q, Hong D, Li O, Zhu M, Ou WB, He Y, and Wu Y (2018). Leukemia inhibitory factor regulates marmoset induced pluripotent stem cell proliferation via a PI3K/Aktdependent Tbx3 activation pathway. Int J Mol Med 42, 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellokumpu-Lehtinen P, Talpaz M, Harris D, Van Q, Kurzrock R, and Estrov Z (1996). Leukemia-inhibitory factor stimulates breast, kidney and prostate cancer cell proliferation by paracrine and autocrine pathways. Int J Cancer 66, 515–519. [DOI] [PubMed] [Google Scholar]
- Kuphal S, Wallner S, and Bosserhoff AK (2013). Impact of LIF (leukemia inhibitory factor) expression in malignant melanoma. Exp Mol Pathol 95, 156–165. [DOI] [PubMed] [Google Scholar]
- Kurek JB, Bower JJ, Romanella M, Koentgen F, Murphy M, and Austin L (1997). The role of leukemia inhibitory factor in skeletal muscle regeneration. Muscle Nerve 20, 815–822. [DOI] [PubMed] [Google Scholar]
- Laabi Y, Metcalf D, Mifsud S, and Di Rago L (2000). Differentiation commitment and regulator-specific granulocyte-macrophage maturation in a novel pro-B murine leukemic cell line. Leukemia 14, 1785–1795. [DOI] [PubMed] [Google Scholar]
- Lainez NM, and Coss D (2019). Leukemia Inhibitory Factor Represses GnRH Gene Expression via cFOS during Inflammation in Male Mice. Neuroendocrinology 108, 291–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary AG, Wong GG, Clark SC, Smith AG, and Ogawa M (1990). Leukemia inhibitory factor differentiation-inhibiting activity/human interleukin for DA cells augments proliferation of human hematopoietic stem cells. Blood 75, 1960–1964. [PubMed] [Google Scholar]
- Levine AJ (2019). The many faces of p53: something for everyone. Journal of molecular cell biology 11, 524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Yang Q, Yu H, Wu L, Zhao Y, Zhang C, Yue X, Liu Z, Wu H, Haffty BG, et al. (2014). LIF promotes tumorigenesis and metastasis of breast cancer through the AKT-mTOR pathway. Oncotarget 5, 788–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licursi M, Alberto CO, Dias A, Hirasawa K, and Hirasawa M (2016). High-fat diet-induced downregulation of anorexic leukemia inhibitory factor in the brain stem. Obesity (Silver Spring) 24, 2361–2367. [DOI] [PubMed] [Google Scholar]
- Lin TA, Wu TS, Li YJ, Yang CN, Illescas Ralda MM, and Chang HH (2020). Role and Mechanism of LIF in Oral Squamous Cell Carcinoma Progression. J Clin Med 9, 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Lu Y, Li J, Liu Y, Liu J, and Wang W (2015a). Leukemia inhibitory factor promotes tumor growth and metastasis in human osteosarcoma via activating STAT3. APMIS 123, 837–846. [DOI] [PubMed] [Google Scholar]
- Liu CZ, Zhou HJ, Zhong JH, Tang T, Cui HJ, Zhou JH, Zhang Q, and Mei ZG (2019a). Leukemia Inhibitory Factor Decreases Neurogenesis and Angiogenesis in a Rat Model of Intracerebral Hemorrhage. Curr Med Sci 39, 298–304. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhang C, Hu W, and Feng Z (2015b). Tumor suppressor p53 and its mutants in cancer metabolism. Cancer Lett 356, 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhang C, Hu W, and Feng Z (2019b). Tumor suppressor p53 and metabolism. J Mol Cell Biol 11, 284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhang C, Zhao Y, and Feng Z (2017). MicroRNA Control of p53. J Cell Biochem 118, 7–14. [DOI] [PubMed] [Google Scholar]
- Liu SC, Hsu T, Chang YS, Chung AK, Jiang SS, OuYang CN, Yuh CH, Hsueh C, Liu YP, and Tsang NM (2018). Cytoplasmic LIF reprograms invasive mode to enhance NPC dissemination through modulating YAP1-FAK/PXN signaling. Nat Commun 9, 5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SC, Tsang NM, Chiang WC, Chang KP, Hsueh C, Liang Y, Juang JL, Chow KP, and Chang YS (2013a). Leukemia inhibitory factor promotes nasopharyngeal carcinoma progression and radioresistance. J Clin Invest 123, 5269–5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Peng M, Zang D, and Zhang B (2013b). Leukemia inhibitory factor promotes nestin-positive cells, and increases gp130 levels in the Parkinson disease mouse model of 6-hydroxydopamine. Neurosciences (Riyadh) 18, 363–370. [PubMed] [Google Scholar]
- Liu YN, Niu S, Chen WY, Zhang Q, Tao Y, Chen WH, Jiang KC, Chen X, Shi H, Liu A, et al. (2019c). Leukemia Inhibitory Factor Promotes Castration-resistant Prostate Cancer and Neuroendocrine Differentiation by Activated ZBTB46. Clin Cancer Res 25, 4128–4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe DG, Nunes W, Bombara M, McCabe S, Ranges GE, Henzel W, Tomida M, Yamamoto-Yamaguchi Y, Hozumi M, and Goeddel DV (1989). Genomic cloning and heterologous expression of human differentiation-stimulating factor. DNA 8, 351–359. [DOI] [PubMed] [Google Scholar]
- Lu B, He Y, He J, Wang L, Liu Z, Yang J, Gao Z, Lu G, Zou C, and Zhao W (2020). Epigenetic Profiling Identifies LIF as a Super-enhancer-Controlled Regulator of Stem Cell-like Properties in Osteosarcoma. Mol Cancer Res 18, 57–67. [DOI] [PubMed] [Google Scholar]
- Mao S, Li X, Wang J, Ding X, Zhang C, and Li L (2016). miR-17–92 facilitates neuronal differentiation of transplanted neural stem/precursor cells under neuroinflammatory conditions. J Neuroinflammation 13, 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall MK, Doerrler W, Feingold KR, and Grunfeld C (1994). Leukemia inhibitory factor induces changes in lipid metabolism in cultured adipocytes. Endocrinology 135, 141–147. [DOI] [PubMed] [Google Scholar]
- Maruta S, Takiguchi S, Ueyama M, Kataoka Y, Oda Y, Tsuneyoshi M, and Iguchi H (2009). A role for leukemia inhibitory factor in melanoma-induced bone metastasis. Clin Exp Metastasis 26, 133–141. [DOI] [PubMed] [Google Scholar]
- Mayer P, Geissler K, Ward M, and Metcalf D (1993). Recombinant human leukemia inhibitory factor induces acute phase proteins and raises the blood platelet counts in nonhuman primates. Blood 81, 3226–3233. [PubMed] [Google Scholar]
- McLean K, Tan L, Bolland DE, Coffman LG, Peterson LF, Talpaz M, Neamati N, and Buckanovich RJ (2019). Leukemia inhibitory factor functions in parallel with interleukin-6 to promote ovarian cancer growth. Oncogene 38, 1576–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D, Hilton D, and Nicola NA (1991). Leukemia inhibitory factor can potentiate murine megakaryocyte production in vitro. Blood 77, 2150–2153. [PubMed] [Google Scholar]
- Metcalf D, Nicola NA, and Gearing DP (1990). Effects of injected leukemia inhibitory factor on hematopoietic and other tissues in mice. Blood 76, 50–56. [PubMed] [Google Scholar]
- Metcalfe SM (2011). LIF in the regulation of T-cell fate and as a potential therapeutic. Genes Immun 12, 157–168. [DOI] [PubMed] [Google Scholar]
- Metcalfe SM (2018). LIF and multiple sclerosis: One protein with two healing properties. Mult Scler Relat Disord 20, 223–227. [DOI] [PubMed] [Google Scholar]
- Morales-Prieto DM, Schleussner E, and Markert UR (2011). Reduction in miR-141 is induced by leukemia inhibitory factor and inhibits proliferation in choriocarcinoma cell line JEG-3. Am J Reprod Immunol 66 Suppl 1, 57–62. [DOI] [PubMed] [Google Scholar]
- Moreau JF, Bonneville M, Godard A, Gascan H, Gruart V, Moore MA, and Soulillou JP (1987). Characterization of a factor produced by human T cell clones exhibiting eosinophil-activating and burst-promoting activities. J Immunol 138, 3844–3849. [PubMed] [Google Scholar]
- Mori M, Yamaguchi K, and Abe K (1989). Purification of a lipoprotein lipase-inhibiting protein produced by a melanoma cell line associated with cancer cachexia. Biochem Biophys Res Commun 160, 1085–1092. [DOI] [PubMed] [Google Scholar]
- Morton SD, Cadamuro M, Brivio S, Vismara M, Stecca T, Massani M, Bassi N, Furlanetto A, Joplin RE, Floreani A, et al. (2015). Leukemia inhibitory factor protects cholangiocarcinoma cells from drug-induced apoptosis via a PI3K/AKT-dependent Mcl-1 activation. Oncotarget 6, 26052–26064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Kamimura D, and Hirano T (2019). Pleiotropy and Specificity: Insights from the Interleukin 6 Family of Cytokines. Immunity 50, 812–831. [DOI] [PubMed] [Google Scholar]
- Negri DR, Mezzanzanica D, Sacco S, Gadina M, Benigni F, Cajola L, Finocchiaro G, Ghezzi P, and Canevari S (2001). Role of cytokines in cancer cachexia in a murine model of intracerebral injection of human tumours. Cytokine 15, 27–38. [DOI] [PubMed] [Google Scholar]
- Nicola NA, and Babon JJ (2015). Leukemia inhibitory factor (LIF). Cytokine Growth Factor Rev 26, 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira-Silva C, Piairo P, Carvalho-Dias E, Peixoto FO, Moura RS, and Correia-Pinto J (2012). Leukemia inhibitory factor in rat fetal lung development: expression and functional studies. PLoS One 7, e30517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Fujio Y, Kunisada K, Hirota H, Matsui H, Kishimoto T, and Yamauchi-Takihara K (1998). Activation of phosphatidylinositol 3-kinase through glycoprotein 130 induces protein kinase B and p70 S6 kinase phosphorylation in cardiac myocytes. J Biol Chem 273, 9703–9710. [DOI] [PubMed] [Google Scholar]
- Ohata Y, Tsuchiya M, Hirai H, Yamaguchi S, Akashi T, Sakamoto K, Yamaguchi A, Ikeda T, and Kayamori K (2018). Leukemia inhibitory factor produced by fibroblasts within tumor stroma participates in invasion of oral squamous cell carcinoma. PLoS One 13, e0191865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paling NR, Wheadon H, Bone HK, and Welham MJ (2004). Regulation of embryonic stem cell self-renewal by phosphoinositide 3-kinase-dependent signaling. J Biol Chem 279, 48063–48070. [DOI] [PubMed] [Google Scholar]
- Park JI, Strock CJ, Ball DW, and Nelkin BD (2003). The Ras/Raf/MEK/extracellular signal-regulated kinase pathway induces autocrine-paracrine growth inhibition via the leukemia inhibitory factor/JAK/STAT pathway. Mol Cell Biol 23, 543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Garcia M, Bonfill-Teixidor E, Planas-Rigol E, Rubio-Perez C, Iurlaro R, Arias A, Cuartas I, Sala-Hojman A, Escudero L, Martinez-Ricarte F, et al. (2019). LIF regulates CXCL9 in tumor-associated macrophages and prevents CD8(+) T cell tumor-infiltration impairing anti-PD1 therapy. Nat Commun 10, 2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquin S, Sharma M, and Gauchat JF (2016). Cytokines of the LIF/CNTF family and metabolism. Cytokine 82, 122–124. [DOI] [PubMed] [Google Scholar]
- Patterson BK, Behbahani H, Kabat WJ, Sullivan Y, O’Gorman MR, Landay A, Flener Z, Khan N, Yogev R, and Andersson J (2001). Leukemia inhibitory factor inhibits HIV-1 replication and is upregulated in placentae from nontransmitting women. J Clin Invest 107, 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson PH, and Nawa H (1993). Neuronal differentiation factors/cytokines and synaptic plasticity. Cell 72 Suppl, 123–137. [DOI] [PubMed] [Google Scholar]
- Peng F, Zhou J, Sheng W, Zhang D, and Dong M (2014). [Expression and significance of leukemia inhibitory factor in human pancreatic cancer]. Zhonghua Yi Xue Za Zhi 94, 90–95. [PubMed] [Google Scholar]
- Penuelas S, Anido J, Prieto-Sanchez RM, Folch G, Barba I, Cuartas I, Garcia-Dorado D, Poca MA, Sahuquillo J, Baselga J, et al. (2009). TGF-beta increases glioma-initiating cell self-renewal through the induction of LIF in human glioblastoma. Cancer Cell 15, 315–327. [DOI] [PubMed] [Google Scholar]
- Quaglino A, Schere-Levy C, Romorini L, Meiss RP, and Kordon EC (2007). Mouse mammary tumors display Stat3 activation dependent on leukemia inhibitory factor signaling. Breast Cancer Res 9, R69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards LJ, Kilpatrick TJ, Bartlett PF, and Murphy M (1992). Leukemia inhibitory factor promotes the neuronal development of spinal cord precursors from the neural tube. J Neurosci Res 33, 476–484. [DOI] [PubMed] [Google Scholar]
- Robinson RC, Grey LM, Staunton D, Vankelecom H, Vernallis AB, Moreau JF, Stuart DI, Heath JK, and Jones EY (1994). The crystal structure and biological function of leukemia inhibitory factor: implications for receptor binding. Cell 77, 1101–1116. [DOI] [PubMed] [Google Scholar]
- Rosario GX, and Stewart CL (2016). The Multifaceted Actions of Leukaemia Inhibitory Factor in Mediating Uterine Receptivity and Embryo Implantation. Am J Reprod Immunol 75, 246–255. [DOI] [PubMed] [Google Scholar]
- Rose-John S (2018). Interleukin-6 Family Cytokines. Cold Spring Harb Perspect Biol 10, a028415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan X, Zhong M, Liu W, Liu Q, Lu W, Zheng Y, and Zhang X (2020). [Overexpression of leukemia inhibitory factor enhances chemotherapy tolerance of endometrial cancer cells in vitro]. Nan Fang Yi Ke Da Xue Xue Bao 40, 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma K, Watanabe K, Sano M, Uramoto I, and Totsuka T (2000). Differential adaptation of growth and differentiation factor 8/myostatin, fibroblast growth factor 6 and leukemia inhibitory factor in overloaded, regenerating and denervated rat muscles. Biochim Biophys Acta 1497, 77–88. [DOI] [PubMed] [Google Scholar]
- Salas EM, Garcia-Barchino MJ, Labiano S, Shugay M, Perez-Encinas M, Quinteiro C, Garcia-Delgado M, Vizmanos JL, and Novo FJ (2011). LIF, a Novel STAT5-Regulated Gene, Is Aberrantly Expressed in Myeloproliferative Neoplasms. Genes Cancer 2, 593–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterfield MC, Dunlap KA, Hayashi K, Burghardt RC, Spencer TE, and Bazer FW (2007). Tight and adherens junctions in the ovine uterus: differential regulation by pregnancy and progesterone. Endocrinology 148, 3922–3931. [DOI] [PubMed] [Google Scholar]
- Seeneevassen L, Giraud J, Molina-Castro S, Sifre E, Tiffon C, Beauvoit C, Staedel C, Megraud F, Lehours P, Martin OCB, et al. (2020). Leukaemia Inhibitory Factor (LIF) Inhibits Cancer Stem Cells Tumorigenic Properties through Hippo Kinases Activation in Gastric Cancer. Cancers (Basel) 12, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto DN, Kandarian SC, and Jackman RW (2015). A Key Role for Leukemia Inhibitory Factor in C26 Cancer Cachexia. J Biol Chem 290, 19976–19986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, and Kumar P (2012). Understanding implantation window, a crucial phenomenon. J Hum Reprod Sci 5, 2–6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Shi Y, Gao W, Lytle NK, Huang P, Yuan X, Dann AM, Ridinger-Saison M, DelGiorno KE, Antal CE, Liang G, et al. (2019). Targeting LIF-mediated paracrine interaction for pancreatic cancer therapy and monitoring. Nature 569, 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JE, Park SH, and Jang YK (2011). Epigenetic up-regulation of leukemia inhibitory factor (LIF) gene during the progression to breast cancer. Mol Cells 31, 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuya LL, Menkhorst EM, Yap J, Li P, Lane N, and Dimitriadis E (2011). Leukemia inhibitory factor enhances endometrial stromal cell decidualization in humans and mice. PLoS One 6, e25288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson RJ, Hilton DJ, Nice EC, Rubira MR, Metcalf D, Gearing DP, Gough NM, and Nicola NA (1988). Structural characterization of a murine myeloid leukaemia inhibitory factor. Eur J Biochem 175, 541–547. [DOI] [PubMed] [Google Scholar]
- Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M, and Rogers D (1988). Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature 336, 688–690. [DOI] [PubMed] [Google Scholar]
- Song H, Lim H, Das SK, Paria BC, and Dey SK (2000). Dysregulation of EGF family of growth factors and COX-2 in the uterus during the preattachment and attachment reactions of the blastocyst with the luminal epithelium correlates with implantation failure in LIF-deficient mice. Mol Endocrinol 14, 1147–1161. [DOI] [PubMed] [Google Scholar]
- Spangenburg EE, and Booth FW (2002). Multiple signaling pathways mediate LIF-induced skeletal muscle satellite cell proliferation. Am J Physiol Cell Physiol 283, C204–211. [DOI] [PubMed] [Google Scholar]
- Spangenburg EE, and Booth FW (2006). Leukemia inhibitory factor restores the hypertrophic response to increased loading in the LIF(−/−) mouse. Cytokine 34, 125–130. [DOI] [PubMed] [Google Scholar]
- Stahl N, Boulton TG, Farruggella T, Ip NY, Davis S, Witthuhn BA, Quelle FW, Silvennoinen O, Barbieri G, Pellegrini S, et al. (1994). Association and activation of Jak-Tyk kinases by CNTF-LIF-OSM-IL-6 beta receptor components. Science 263, 92–95. [DOI] [PubMed] [Google Scholar]
- Starenki D, Singh NK, Jensen DR, Peterson FC, and Park JI (2013). Recombinant leukemia inhibitory factor suppresses human medullary thyroid carcinoma cell line xenografts in mice. Cancer Lett 339, 144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, and Abbondanzo SJ (1992). Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 359, 76–79. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Carpino N, Cross JC, Torres M, Parganas E, and Ihle JN (2003). SOCS3: an essential regulator of LIF receptor signaling in trophoblast giant cell differentiation. EMBO J 22, 372–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terakawa J, Wakitani S, Sugiyama M, Inoue N, Ohmori Y, Kiso Y, Hosaka YZ, and Hondo E (2011). Embryo implantation is blocked by intraperitoneal injection with anti-LIF antibody in mice. J Reprod Dev 57, 700–707. [DOI] [PubMed] [Google Scholar]
- Thoma B, Bird TA, Friend DJ, Gearing DP, and Dower SK (1994). Oncostatin M and leukemia inhibitory factor trigger overlapping and different signals through partially shared receptor complexes. J Biol Chem 269, 6215–6222. [PubMed] [Google Scholar]
- Tian L, Zhu W, Liu Y, Gong Y, Lv A, Wang Z, Ding X, Li S, Fu Y, Lin Y, et al. (2019). Neural Stem Cells Transfected with Leukemia Inhibitory Factor Promote Neuroprotection in a Rat Model of Cerebral Ischemia. Neurosci Bull 35, 901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traber KE, Symer EM, Allen E, Kim Y, Hilliard KL, Wasserman GA, Stewart CL, Jones MR, Mizgerd JP, and Quinton LJ (2017). Myeloid-epithelial cross talk coordinates synthesis of the tissue-protective cytokine leukemia inhibitory factor during pneumonia. Am J Physiol Lung Cell Mol Physiol 313, L548–L558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagnini LD, Renzi A, Petersen B, Canas M, Petersen CG, Mauri AL, Mattila MC, Ricci J, Dieamant F, Oliveira JBA, et al. (2019). Association between estrogen receptor 1 (ESR1) and leukemia inhibitory factor (LIF) polymorphisms can help in the prediction of recurrent implantation failure. Fertil Steril 111, 527–534. [DOI] [PubMed] [Google Scholar]
- Venkatesh H, and Monje M (2017). Neuronal Activity in Ontogeny and Oncology. Trends Cancer 3, 89–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanadhapalli S, Luo Y, Sareddy GR, Santhamma B, Zhou M, Li M, Ma S, Sonavane R, Pratap UP, Altwegg KA, et al. (2019). EC359: A First-in-Class Small-Molecule Inhibitor for Targeting Oncogenic LIFR Signaling in Triple-Negative Breast Cancer. Mol Cancer Ther 18, 1341–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman AD, Fritz JM, and Lenardo MJ (2020). A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol 20, 651–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EC, McGregor NE, Poulton IJ, Solano M, Pompolo S, Fernandes TJ, Constable MJ, Nicholson GC, Zhang JG, Nicola NA, et al. (2010). Oncostatin M promotes bone formation independently of resorption when signaling through leukemia inhibitory factor receptor in mice. J Clin Invest 120, 582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Liu K, Yang Y, Wang T, Rao Q, Guo W, and Zhang Z (2020a). Prognostic value of leukemia inhibitory factor and its receptor in pancreatic adenocarcinoma. Future Oncol 16, 4461–4473. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang J, Zhao Y, Zhang X, Liu J, Zhang C, Haffty B, Verzi M, Zhang L, Gao N, et al. (2020b). LIF is essential for ISC function and protects against radiation-induced gastrointestinal syndrome. Cell death & disease 11, 588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Xie C, Pan S, Liang Y, Han J, Lan Y, Sun J, Li K, Sun B, Yang G, et al. (2016). N-myc downstream-regulated gene 2 inhibits human cholangiocarcinoma progression and is regulated by leukemia inhibitory factor/MicroRNA-181c negative feedback pathway. Hepatology 64, 1606–1622. [DOI] [PubMed] [Google Scholar]
- Wang MT, Fer N, Galeas J, Collisson EA, Kim SE, Sharib J, and McCormick F (2019). Blockade of leukemia inhibitory factor as a therapeutic approach to KRAS driven pancreatic cancer. Nat Commun 10, 3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Umehara H, Murayama K, Okabe M, Kimura T, and Nakano T (2006). Activation of Akt signaling is sufficient to maintain pluripotency in mouse and primate embryonic stem cells. Oncogene 25, 2697–2707. [DOI] [PubMed] [Google Scholar]
- West NR (2019). Coordination of Immune-Stroma Crosstalk by IL-6 Family Cytokines. Front Immunol 10, 1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White UA, Stewart WC, Mynatt RL, and Stephens JM (2008). Neuropoietin attenuates adipogenesis and induces insulin resistance in adipocytes. J Biol Chem 283, 22505–22512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, Gearing DP, Wagner EF, Metcalf D, Nicola NA, and Gough NM (1988). Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature 336, 684–687. [DOI] [PubMed] [Google Scholar]
- Won H, Moreira D, Gao C, Duttagupta P, Zhao X, Manuel E, Diamond D, Yuan YC, Liu Z, Jones J, et al. (2017). TLR9 expression and secretion of LIF by prostate cancer cells stimulates accumulation and activity of polymorphonuclear MDSCs. J Leukoc Biol 102, 423–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Yu H, Zhao Y, Zhang C, Wang J, Yue X, Yang Q, and Hu W (2015). HIF-2alpha mediates hypoxia-induced LIF expression in human colorectal cancer cells. Oncotarget 6, 4406–4417.25726527 [Google Scholar]
- Xiao Q, Zeng JH, Zhou H, Qiu QH, Ke B, Deng L, Hu ZM, Roh J, and Dai M (2019). Expression and effects of leukemia inhibitory factor on nucleus pulposus degeneration. Mol Med Rep 19, 2377–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Jin O, Han S, Nie R, Zhu L, Gao X, and Li L (2015). Correlations of leukemia inhibitory factor and macrophage migration inhibitory factor with endometrial carcinoma. Eur J Gynaecol Oncol 36, 146–149. [PubMed] [Google Scholar]
- Xu G, Wang H, Li W, Xue Z, and Luo Q (2019). Leukemia inhibitory factor inhibits the proliferation of gastric cancer by inducing G1-phase arrest. J Cell Physiol 234, 3613–3620. [DOI] [PubMed] [Google Scholar]
- Yaftiyan A, Eskandarian M, Jahangiri AH, Kazemi Sefat NA, and Moazzeni SM (2019). Leukemia inhibitory factor (LIF) modulates the development of dendritic cells in a dual manner. Immunopharmacol Immunotoxicol 41, 455–462. [DOI] [PubMed] [Google Scholar]
- Yamamori T, Fukada K, Aebersold R, Korsching S, Fann MJ, and Patterson PH (1989). The cholinergic neuronal differentiation factor from heart cells is identical to leukemia inhibitory factor. Science 246, 1412–1416. [DOI] [PubMed] [Google Scholar]
- Yang J, van Oosten AL, Theunissen TW, Guo G, Silva JC, and Smith A (2010). Stat3 activation is limiting for reprogramming to ground state pluripotency. Cell Stem Cell 7, 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida GJ (2020). Regulation of heterogeneous cancer-associated fibroblasts: the molecular pathology of activated signaling pathways. J Exp Clin Cancer Res 39, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino J, Monkawa T, Tsuji M, Hayashi M, and Saruta T (2003). Leukemia inhibitory factor is involved in tubular regeneration after experimental acute renal failure. J Am Soc Nephrol 14, 3090–3101. [DOI] [PubMed] [Google Scholar]
- Yu H, Yue X, Zhao Y, Li X, Wu L, Zhang C, Liu Z, Lin K, Xu-Monette ZY, Young KH, et al. (2014). LIF negatively regulates tumour-suppressor p53 through Stat3/ID1/MDM2 in colorectal cancers. Nat Commun 5, 5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue X, Wu F, Wang J, Kim K, Santhamma B, Dileep KV, Zhang KYJ, Viswanadhapalli S, Vadlamudi RK, Ahmed G, et al. (2020). EC330, a small-molecule compound, is a potential novel inhibitor of LIF signaling. J Mol Cell Biol 12, 477–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue X, Wu L, and Hu W (2015). The regulation of leukemia inhibitory factor. Cancer Cell Microenviron 2, e877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue X, Zhao Y, Zhang C, Li J, Liu Z, Liu J, and Hu W (2016). Leukemia inhibitory factor promotes EMT through STAT3-dependent miR-21 induction. Oncotarget 7, 3777–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Liu J, Xu D, Zhang T, Hu W, and Feng Z (2020). Gain-of-function mutant p53 in cancer progression and therapy. J Mol Cell Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Wu L, Yue X, Zhang C, Wang J, Li J, Sun X, Zhu Y, Feng Z, and Hu W (2018). A polymorphism in the tumor suppressor p53 affects aging and longevity in mouse models. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Shen J, Hu Z, and Zhong X (2018). Leukemia inhibitory factor promotes extracellular matrix synthesis in degenerative nucleus pulposus cells via MAPK-ERK1/2 signaling pathway. Biochem Biophys Res Commun 507, 253–259. [DOI] [PubMed] [Google Scholar]
- Zhou HJ, Yang X, Cui HJ, Tang T, Zhong JH, Luo JK, Yang AL, Zhang QM, Zhou JH, and Zhang Q (2017). Leukemia Inhibitory Factor Contributes to Reactive Astrogliosis via Activation of Signal Transducer and Activator of Transcription 3 Signaling after Intracerebral Hemorrhage in Rats. J Neurotrauma 34, 1658–1665. [DOI] [PubMed] [Google Scholar]


