Abstract
Objective
To evaluate the prevalence and associations between structural MRI (sMRI) injury/abnormality at term equivalent age and absent fidgety General Movements Assessment (GMA) and abnormal Hammersmith Infant Neurological Examination (HINE) scores among very preterm infants at 3–4 months corrected age.
Study design
This prospective cohort study enrolled 392 infants born ≤32 weeks’ gestation from five neonatal intensive care units in the greater Cincinnati area between September 2016 and October 2019. Infants completed sMRI at term-equivalent age and GMA and HINE at 3–4 months corrected age. All assessors were blinded.
Results
Of 392 infants, 375 (96%) had complete data. Of these, 44 (12%) exhibited moderate or severe brain abnormalities, 17 (4.5%) had abnormal GMA, and 77 (20.3%) had abnormal HINE. Global and regional abnormality scores on sMRI were significantly correlated with GMA (R2 range: 0.05–0.17) and HINE at 3–4 months corrected age (R2 range: 0.01–0.17). These associations remained significant in multivariable analyses after adjusting for gestational age and sex. There was a significant but low correlation (R2 0.14) between GMA and HINE.
Conclusion
We observed a low prevalence of moderate or severe brain abnormalities in very preterm survivors in this geographically-defined cohort. The much higher prevalence of abnormal motor exam on the HINE compared with GMA and their low correlation suggests that these tests evaluate different constructs, and thus, should be used in combination with sMRI rather than interchangeably.
Keywords: MRI, General Movements Assessment, Hammersmith Infant Neurological Exam, Cerebral Palsy
Cerebral palsy (CP) is the most common cause of childhood physical disability. Although CP originates from a brain abnormality or injury occurring during the prenatal, perinatal, or postnatal period, this motor disability is not commonly diagnosed until 12–24 months of age when clinical deficits are apparent.1–3 However, recent advances in neuroimaging and early prognostic testing are expected to result in more accurate identification of infants at risk of CP by 3–4 months corrected age.1, 4, 5 Early detection and diagnosis are high priorities because neuroplasticity and opportunity for intervention are highest in infants and diminish over time.6 Early intervention is critical to reduce the progression of motor deficits, prevent secondary impairments, and improve independence.
The tools with the best predictive validity to detect CP under five months corrected age include structural magnetic resonance imaging (sMRI) at term, Prechtl Qualitative Assessment of General Movements (GMA) during fidgety period, and Hammersmith Infant Neurological Examination (HINE) at 3–4 months corrected age.1, 7–9 However, the sensitivity and specificity for these assessments vary vastly and are insufficient in isolation for diagnosing CP. Additionally, how these tests complement each other is unclear.10 The recommendation from an international panel of experts for early CP diagnosis is to use sMRI at term in combination with fidgety GMA and/or HINE.1 However, there is little evidence regarding how these three assessments are associated to predict CP in combination. A few studies have examined associations between two of the three assessment prospectively 11–14 and one study examined the tests retrospectively using a case-control study.15 To our knowledge there are no prospective, population-based cohort studies using sMRI, GMA and HINE to examine the associations between these structural and functional assessments. Understanding this relationship is critical to establishing the combined prognostic properties of these assessments to predict CP accurately. The objectives of this study were to evaluate 1) the prevalence of sMRI injury/abnormality at term-equivalent age and absent fidgety GMA and abnormal HINE at 3–4 months corrected age and 2) how these tests correlate with one another in a large, geographically-based prospective cohort of very preterm infants.
Methods
Participants
We conducted a prospective longitudinal cohort study in very preterm infants recruited from five level III/IV neonatal intensive care units (NICUs) in the greater Cincinnati area. We prospectively enrolled 392 infants between September 2016 and October 2019 (Figure 1; available at www.jpeds.com). Inclusion criteria included gestational age ≤ 32 weeks and hospital care at a greater Cincinnati NICU. We excluded infants with known chromosomal or congenital anomalies affecting the central nervous system or cyanotic heart disease. The study was approved by all five hospital Institutional Review Boards. All families consented to the study prior to any study procedures. Maternal and infant clinical variables were obtained by trained research coordinators via chart review using standardized definitions (Table 1).
Figure 1:
Study flow chart
Table 1.
Cohort Maternal and Infant Baseline Characteristics.
| Very preterm infants (N=392) | |
|---|---|
| Maternal Age, mean years (SD) | 29.2 (5.4) |
| Maternal hypertension, n (%) | 154 (39.3%) |
| Maternal diabetes, n (%) | 22 (5.6%) |
| Antenatal steroids, n (%) | 361 (92.1%) |
| Magnesium, n (%) | 329 (83.9%) |
| Infant Hispanic/Latino, n (%)* | 36 (7.2%) |
| Infant race, n (%)** | |
| Black | 104 (26.5%) |
| White | 255 (65.1%) |
| Asian | 8 (2.0%) |
| Other | 20 (5.1%) |
| Singleton, n (%) | 252 (64.3%) |
| Male sex, n (%) | 210 (53.6%) |
| Apgar score at 5 min <5, n(%) | 48 (12.4%) |
| Gestational age, mean weeks (SD) | 29.3 (2.5) |
| Birth weight, mean g (SD) | 1293.0 (448.9) |
| Patent ductus arteriosus, n (%) | 101 (25.8%) |
| Rates of culture positive sepsis, n (%) | 43 (11.0%) |
| Bronchopulmonary dysplasia, n (%)† | 166 (42.4%) |
| Retinopathy of prematurity, n (%) | 134 (34.2%) |
| Germinal matrix/intraventricular hemorrhage (IVH), n (%) | 68 (17.4%) |
| Postmenstrual age at MRI scan, mean weeks (SD) | 42.7 (1.4) |
29 mothers chose not to respond to ethnicity question
6 mothers did not respond to infant race question
Bronchopulmonary dysplasia was defined as any respiratory support, including nasal cannula or higher, irrespective of oxygen need, at 36 weeks postmenstrual age 25.
Measures
MRI.
Structural MRI scans were obtained strictly between 40 to 44 6/7 weeks postmenstrual age. All infants recruited from sites other than Cincinnati Children’s Hospital Medical Center (CCHMC) were scanned as outpatients. Infants from CCHMC were scanned as outpatients and inpatients with 47 (12%) of scans occurring while inpatient. All infants were scanned on the same 3-Tesla MRI magnet (Philips Ingenia) using a 32-channel head coil, located in the CCHMC Imaging Research Center. Infants were not sedated for the scan. Silicone earplugs, blanket swaddle, vacuum immobilization (MedVac, CFI Medical Solutions, Fenton, MI, USA), and feeding before MRI were strategies to promote natural sleep and successful scanning. High-resolution anatomical T2-weighted images (TR/TE=18567/166 ms, flip angle=90°, in-plane resolution=1×1×1 mm), MPRAGE T1-weighted images (3D FFE, TR/TE/TI=8.5/3/1610 ms, flip angle=13°, in-plane resolution=1×1×1 mm), and SWI images (TE/TR=7.2/29 ms, resolution=0.6×0.6×2mm) were acquired.
A single pediatric neuroradiologist masked to clinical history, performed all qualitative and quantitative assessments of MRI using a standardized assessment (Appendix 1; online). Brain abnormalities were defined using a standardized scoring system (Table 2; available at www.jpeds.com).16 For the quantitative scoring system, we adjusted for the infant’s age at MRI using a linear regression analysis equation: corrected brain measurement=measured brain measurement+slope (40−post-menstrual age at MRI scan).16 The scores were summed to generate separate white matter abnormality, gray matter abnormalities, deep gray matter, and cerebellar abnormality scores, with higher values reflecting more abnormalities; descriptive data reported in Table 3.
Table 2:
Description for Kidokoro global abnormality scoring system; online
| Brain Abnormality variables 11 | Scale | Brain Abnormalities | Total score range |
|---|---|---|---|
| Cerebral white matter abnormality (WMA) | 0–4 | 1. cystic degeneration 2. focal signal abnormalities 3. delayed myelination 4. thinning of the corpus callosum 5. dilated lateral ventricles 6. reduction of white matter volume |
0–24 |
| Cortical gray matter abnormalities | 0–4 | 1. signal abnormality 2. delayed gyration 3. dilated extra-cerebral CSF space |
0–12 |
| Deep gray matter | 0–4 | 1. signal abnormalities 2. reduced volume |
0–8 |
| Cerebellar abnormalities | 0–4 | 1. signal abnormalities 2. reduced volume |
0–8 |
Table 3.
Descriptive Statistics for Brain Abnormalities Variables
| Range | Median | Interquartile Range | Intra-rater reliability ICC (95% CI) | |
|---|---|---|---|---|
| White matter abnormality score | 0–20 | 1.0 | 0–3 | 0.87 (0.67–0.95) |
| Grey matter abnormality score | 0–4 | 0 | 0–3 | 0.90 (0.74–0.96) |
| Cerebellar abnormality score | 0–8 | 0 | 0–7 | 0.76 (0.39–0.90) |
| Global brain abnormality score | 0–36 | 2.0 | 1–5 | 0.89 (0.73–0.96) |
| Pons thickness, mm | 8.8–45.6 | 14.3 | 10.5–16.9 | 0.96 (0.91–0.99) |
| Cerebellar vermis (anterior-posterior), mm | 5.5–28.6 | 16.5 | 9.3–24 | 0.85 (0.64–0.94) |
| Cerebellar vermis (cranio-caudal) mm | 15–34.3 | 26.9 | 17.2–32 | 0.88 (0.70–0.95) |
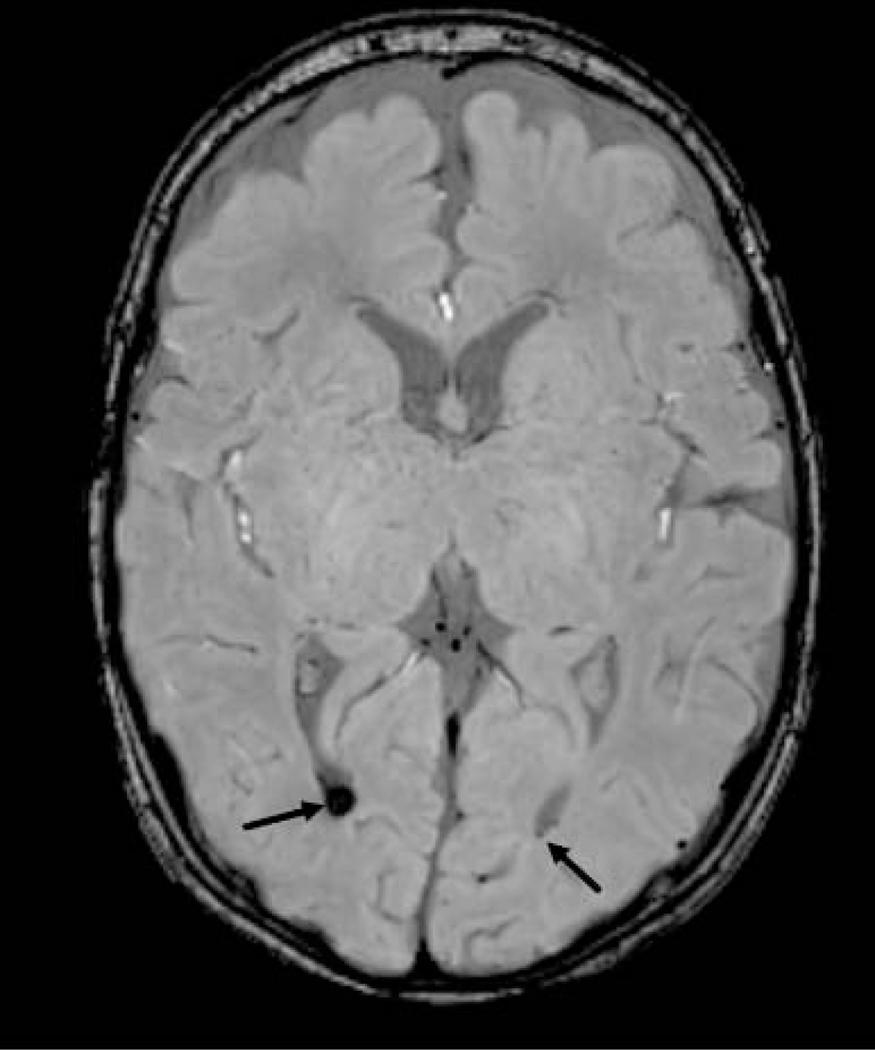
A categorical global brain abnormality score was computed per Kidokoro et al by summing abnormalities, with categories of normal (total score 0–3), mild (total score 4–7), moderate (total score 8–11), and severe (total score ≥ 12).16 In addition, we examined sMRI abnormalities not included in the Kidokoro scores including, presence of germinal matrix hemorrhage and intraventricular hemorrhage and measurement of the pons (anterior-posterior), cerebellar vermis (anterior-posterior and cranio-caudal dimension), and frontal extra-axial space. Structural measurements of the pons and cerebellar vermis were collected as continuous variables and assessments of germinal matrix hemorrhage, intraventricular hemorrhage (present/absent) and frontal extra-axial space (≤5mm/≥5mm) were categorical (Figure 2, A–D; available at www.jpeds.com). A random sample of 20 MRI scans were reread by the same neuroradiologist more than one month later to assess intra-rater reliability.
Figure 2a:
Axial demonstrate artifact at left caudothalamic groove (arrow), consistent with germinal matrix hemorrhage
Figure 2d:
Sagittal T2 image measuring anterior to posterior and craniocaudal dimension of the cerebellar vermis and anterior to posterior dimension of the pons.
Prechtl Qualitative Assessment of General Movements (GMA).
General movements were video recorded as recommended by Prechtl et al for five minutes in a clinic setting with the undressed, calm infant placed supine.17–19 All GMAs were completed within the fidgety period, with 97% between 12–16 weeks corrected age and the remaining 3% at 11–12 or 17–18 weeks. Infants with variable, complex, and multi-planar movements were scored as normal fidgety. Infants with no fidgety movements (absent) or few movements (sporadic) were scored absent. All videos were scored within 48–72 hours following the recording by a single, certified (via General Movements Trust advanced course) examiner (KH) masked to clinical history and MRI findings, but not HINE score. For questionable videos, a second rating was obtained by a General Movements Trust trainer (CP). Thirty random GMA videos were rescored by the same examiner six months later to assess intra-rater reliability.
Hammersmith Infant Neurological Exam (HINE).
The HINE is a standardized neurological examination for infants 2–24 months of age consisting of 26 items (five domains).20 Each item is scored individually (0–3), and items are summed to obtain domain scores and an overall HINE score (range 0–78), with cut-off scores <56 considered abnormal.21 The right and left limbs were scored separately for items testing both sides of the body. All HINEs were performed by a single examiner masked to clinical history and MRI findings, during the GMA visit. Fifteen randomly selected infants were assessed again for intra-rater reliability, within 2–4 weeks of initial examination.
Statistical Analyses
Intra-rater reliability was assessed using kappa statistics for dichotomous variables and intra-class correlation coefficient (ICC) for continuous variables. We performed linear regression analyses to determine correlations (R2) between sMRI global abnormality score, GMA, and HINE. All analyses were adjusted for gestational age at birth and sex. In multivariable linear regression analyses, we assessed the association between global abnormality MRI score (continuous dependent variable)16 and GMA as well as HINE (continuous). Additional regression analyses examined sMRI subscores (eg, total WM score) with GMA and HINE. HINE global score was compared with the domain scores using the Spearman rho (ρ) correlation coefficient. In secondary analyses, we used multivariable logistic and linear regression to examine the relationship between the additional sMRI variables not included as part of the Kidokoro score with GMA and HINE. Two-sided P values <0.05 were used for statistical significance. All three primary analyses were adjusted for multiple comparisons. Analyses were performed using STATA 16.0 (Stata Corp., College Station, TX).
Results
From the original cohort of 392 infants, 375 (96%) had full data and were included in this study. Twelve infants (3%) did not return for follow-up at three months, 3 infants were excluded due to suboptimal sMRI image quality, and 2 infants did not have GMA scores due to suboptimal state. The mean (SD) gestational age at birth was 29.3 (2.5) weeks, and mean birth weight was 1294 (449) grams. The median NICU length of stay was 64.2 (interquartile range (IQR): 18–91) days. Demographic characteristics are reported in Table 1.
Structural MRI data were collected at mean (SD) of 42.3 (1.3) weeks postmenstrual age with no difference detected between age at scan for patients scanned as inpatients 42.58 (1.46) compared with outpatients 42.63 (1.33) (p=0.97). There were 254 infants (67.7%) classified as having no brain abnormality, 82 with mild abnormality (21.9%), 24 with moderate abnormality (6.4%), and 15 with severe abnormality (4.0%). Regional scores, structural measurements, and intra-rater reliabilities are presented in Table 3. At 3 months corrected age, 17 infants had absent fidgety movements (4.5%). Intra-rater reliability for GMA was 96.7% (kappa 0.89). Abnormal HINE scores were detected in 77 infants (20.3%). HINE scores ranged from 30.5–73 (median 60), and an IQR of 7.0. Intra-rater reliability assessed after mean (SD) of 26.8 (5.16) days between assessments for HINE resulted in an ICC of 0.79. The HINE score was highly (p<0.001) correlated with the domain scores; cranial nerve: ρ =0.55, posture: ρ =0.66, movements: ρ =0.31, tone: ρ =0.77, and reflexes/reactions: ρ =0.52. Twenty-seven infants (7.2%) were diagnosed with an abnormality on at least two of the three study tests (moderate-severe sMRI, absent fidgety movements and/or HINE score <56), and 9 (2.4%) exhibited abnormalities on all three tests.
Association between MRI and GMA.
Global and regional abnormality scores on sMRI at term-equivalent age were significantly correlated with GMA at 3 months corrected age (p<0.001 for all analyses; Table 4). In multivariable analyses, adjusting for gestational age and sex, we identified a significant and independent correlation between global and regional brain abnormality scores and absent fidgety GMA (p<0.001; Table 5). In secondary analyses, additional MRI abnormalities/structural measurements not part of the Kidokoro score were associated with GMA in univariable analyses. In multivariable analyses adjusting for gestational age, sex, and global brain abnormality score, two MRI variables were significantly and independently associated with GMA (N=358): increasing thickness of the pons reduced the odds (OR 0.42, 95% CI 0.22–0.81; p<0.01) and increased extra-axial space (greater than 5 mm) (OR 3.54, 95% CI 1.09–11.53; p=0.036) increased the odds of absent fidgety movements.
Table 4.
Correlations (R2) between structural MRI abnormality scores at term-equivalent age and absent fidgety general movements (GMA) and Hammersmith Infant Neurological Examination (HINE) at three months corrected.
| General Movements | HINE Global Score | |||
|---|---|---|---|---|
| R2 | ß (95% CI) | R2 | ß (95% CI) | |
| Global abnormality | 0.155 | 7.67 (5.85, 9.50)** | 0.169 | −.57 (−.70, −.45)** |
| White matter | 0.165 | 5.12 (3.95, 6.28)** | 0.113 | −.83 (−1.07, −.60)** |
| Cerebellum | 0.050 | 1.52 (.84, 2.19)** | 0.082 | −1.32 (−1.76, −.87)** |
| Cortical gray matter | 0.005 | .35 (−.16, .85) | 0.011 | −.67 (−1.30, −.03)*** |
| Deep gray matter | 0.103 | 1.81 (1.27, 2.36)** | 0.169 | −2.30 (−2.81, −1.78)** |
Correlation (R2) between GMA and HINE was 0.142 (p<.001);
p<.001,
p<.05
Table 5.
Multivariable linear regression results of relationships between structural MRI global and regional abnormality scores at term-equivalent age and absent fidgety general movements (GMA) and Hammersmith Infant Neurological Examination (HINE) at three months corrected, after adjustment for gestational age and infant sex.
| Structural MRI Abnormality Scores at Term-Equivalent Age | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tests at 3 Months Corrected Age | Global abnormality | White matter | Cerebellum | Cortical gray matter | Deep gray matter | |||||
| ß | 95% CI | ß | 95% CI | ß | 95% CI | ß | 95% CI | ß | 95% CI | |
| GMA | 8.11 | 6.08, 10.15*** | 4.87 | 3.70, 6.05*** | 1.25 | 0.61, 1.91*** | 0.87 | 0.39, 1.35*** | 1.65 | 1.12, 2.19*** |
| HINE | ||||||||||
| Cranial nerve function | −0.65 | −0.90, −0.41*** | −0.32 | −0.47, −0.18*** | −0.11 | −0.18, −0.03** | −0.04 | −0.1, 0.02 | −0.17 | −0.23, −0.11*** |
| Posture | −0.36 | −0.56, −0.16*** | −0.17 | −0.29, −0.05** | −0.09 | −0.15, −0.02** | −.002 | −0.05, 0.04 | −0.09 | −0.14, −0.04** |
| Movements | −1.61 | −2.12, −1.09*** | −0.83 | −1.13, 0.53*** | −0.32 | −0.48, −0.16*** | −0.13 | −0.26, −0.01* | −0.30 | −0.43, −0.16*** |
| Tone | −0.41 | −0.55, −0.29*** | −0.23 | −0.30, −0.15*** | −0.06 | −0.10, −0.02** | −0.03 | −0.06, .004 | −0.09 | −0.12, −0.06*** |
| Reflexes and reactions | −0.48 | −0.77, −0.18*** | −0.26 | −0.43, −0,09** | −0.08 | −0.17, .005 | .001 | −0.07, 0.07 | −0.11 | −0.19, −−0.04** |
| Global score | −0.26 | −0.33, −0.19*** | −0.12 | −0.16, −0.08*** | −0.05 | −0.07, −0.03*** | −0.02 | −0.04, −0.01*** | −0.07 | −0.08, −0.05*** |
| Combined | ||||||||||
| GMA | 5.99 | 3.88, 8.09*** | 4.03 | 2.80, 5.27*** | 0.78 | 0.09, 1.47* | 0.73 | 0.22, 1.25** | 1.09 | 0.55, 1.62*** |
| HINE (global) | −0.19 | −0.26, −0.12*** | −0.08 | −0.12, −0.04*** | −0.04 | −0.7, −0.02*** | −0.01 | −0.02, 0.01 | −0.06 | −0.07, −0.04*** |
p<0.05,
p<.0.01,
p<.001
Association between MRI and HINE.
Global and regional abnormality scores on sMRI at term-equivalent age were significantly correlated with HINE at 3 months corrected age (p<0.001; Table 4). We identified a significant and independent correlation between global and regional brain abnormality scores and HINE (p<.001; Table 5). All five HINE domains were significantly associated with MRI global score and most MRI regional scores. In secondary analyses, additional MRI abnormalities/structural measurements not part of the Kidokoro score were associated with HINE in univariable analyses. In multivariable analyses controlling for global brain abnormality score, gestational age and sex, size of the cerebellar vermis (in anterior-posterior) was directly associated (ß=0.37, 95% CI: 0.10, 0.65; p=0.007) and increased extra-axial space showed a negative trend (ß=−1.57, 95% CI: −3.19, 0.05; p=0.058) towards association with HINE (N=360).
Association between MRI and GMA plus HINE.
There was a significant but relatively low correlation between GMA and HINE (R2 0.14; Table 4). Both GMA and HINE were independently and significantly correlated with MRI global abnormality scores (P<.001; Table 5) when adjusting for gestational age and sex, further underscoring the independent nature of GMA versus HINE as related to sMRI. White matter, cerebellum, cortical gray matter, and deep gray matter abnormality scores were also significantly and independently associated with GMA and HINE (Table 5).
Discussion
Early detection and diagnosis of CP is a high priority in rehabilitation. There is consensus that early detection of CP should occur before 6 months corrected age using sMRI, GMA, and/or HINE assessments with purported high sensitivity and specificity and complementary in prognostic properties.1 However, none of these tests are sufficiently accurate in isolation, and the accuracy of these assessments performed in combination to predict CP remains uncertain. Although this key question remains unresolved, our study advances our understanding of how these tests are inter-related. We identified significant correlations between structural abnormalities on sMRI and functional deficits on GMA and HINE. Our findings are in line with two prior studies showing that sMRI/cranial ultrasound were highly correlated with GMA.22, 23 These studies examined the individual and combined values of these tests for predicting CP, indicating that the addition of GMA findings to abnormal neuroimaging at term did not improve prognostic accuracy of CP, likely because they were highly correlated.22, 23 The addition of GMA to sMRI injury lowered the specificity and positive likelihood ratio (LR) for CP prediction in one study.22 In contrast, the addition of GMA to cranial ultrasound reduced the sensitivity and negative LR in the other study.23 Our results suggest considerable overlap may exist in what these prognostic tests are measuring, and therefore their performance in combination may not add much to the prognostic precision for early CP diagnosis if the degree of correlation overshadows the unique information sMRI provides independent of GMA and HINE.
Neuroimaging, GMA, and/or HINE are proposed as the most accurate tools to detect CP before five months of age.1 However, prior studies have not used all tests prospectively within the same cohort. Most studies report the prognostic value of one test in isolation, and few have examined two of the assessments together. By examining the association between sMRI, GMA, and HINE within the same cohort, our study lends greater understanding of how these assessments inter-relate and may help determine the best combination of tools for early CP identification. Our study demonstrated that abnormal sMRI findings at term-equivalent age were significantly associated with absent fidgety GMA and abnormal HINE at 3–4 months CA in very preterm infants. Of the four large regions that contribute to the global brain abnormality score, white matter and deep gray matter abnormalities were the most correlated with abnormal GMA and HINE. White matter abnormalities were the most closely associated with abnormal GMA at 3 months corrected age suggesting that white matter may play a greater role in generating normal fidgety movements than the deep gray matter, cortical gray matter, or cerebellum. Conversely, abnormalities in deep gray matter were the most closely associated with abnormal HINE at 3 months corrected age. This significant association was driven largely by the movements and cranial nerve function subscores of the HINE (Table 5). The higher correlations with white matter and deep nuclear gray matter is consistent with prior literature in preterm and term infants that has demonstrated the importance of injury to the periventricular white matter and basal ganglia/thalamus in the development of CP. Our findings also suggest that thickness of the pons, cerebellar vermis size, and enlarged extra-axial space are additional sMRI variables that may improve our ability to predict CP.
In our study, GMA and HINE were both independently associated with sMRI abnormalities. Furthermore, we found a low degree of correlation between GMA and HINE, suggesting that these tests are unlikely to provide similar prognostic information and should not be used interchangeably. Our data support the idea that the GMA and HINE likely evaluate different constructs and, therefore, should be used in combination rather than isolation for early CP detection. This idea is supported by several recent papers. A systematic review found that the fidgety GMA had highest sensitivity (97%) and specificity (89%), but should not be used in isolation because of false positive results for CP.9 A large retrospective case-control study reported the test properties of combined neuroimaging, GMA, and HINE to predict CP. They reported high (>97%) combined sensitivity, specificity, and positive predictive value.15 However, case-control studies are more biased than cohort studies24 in identifying prognostic factors and cannot be used to calculate prognostic test values because the prevalence of the outcome is artificially determined by the study design (ie, 50% in 1:1 case-control matching) rather than natural disease prevalence.25 The prevalence of CP in the Morgan study was 33% (1:2 case-control matching), which is approximately five-fold greater than observed in very preterm infants, thus resulting in overinflated estimates of sensitivity, specificity, and positive predictive value.15 It will be crucial to follow our population-based cohort to determine CP diagnosis at 2 years corrected age to determine the prognostic test properties of each tests individually and combined to verify the strengths and limitations of this approach.
Our study adds a unique contribution to the literature because our work evaluates a cohort of very preterm infants using sMRI, GMA, and HINE from a geographically defined region, thus limiting sampling bias. Our sMRI results indicate that 23% of infants exhibited mild brain injury/abnormality, and 12% had moderate or severe brain injury/abnormality. The prevalence of brain injury measured by sMRI in our sample was less compared with a study from Hintz et al, finding that 58% of infants had mild brain injury, and 19% had moderate or severe brain injury in a cohort of 480 extremely low birth weight infants.26 In addition to the key difference in inclusion criteria (extremely low birth weight vs. very preterm), the choice of recruiting from academic NICUs only compared with our sampling from academic and community NICUs from a geographically defined region likely contributed to these differences. For our sample, 4.5% of infants exhibited absent fidgety GMA. Comparing our findings to another large geographically recruited cohort showed similar findings with 6% of infants having absent fidgety movements.20 In contrast, comparing these data to another large cohort that was not geographically recruited, the presence of absent fidgety movements was much higher at 15%.23
In our study, 17% of infants exhibited mild, 6% moderate, and 6% severe white matter injury on sMRI. White matter injury and intraventricular hemorrhage have been shown by others as strong prognostic factors for CP.27 White matter injury has also been associated with aberrant GMA.13 Understanding the mechanism and severity of injury at term-equivalent age can be helpful in determining the most appropriate early interventions. Our study showed three sMRI findings independently correlated with HINE in addition to two new regional structural measurements that were significantly associated with GMA. These findings could be important in early CP detection. The regional structural measurements included the thickness of the pons and size of extra-axial space. One previous study found an association between a smaller pons in older children and periventricular leukomalacia.28 However, no other studies have identified the pons as a potential predictor for later CP. Our finding associating the GMA with increased extra-axial space is important as several previous studies have shown the association between microcephaly and poorer neurodevelopmental outcome.29, 30 Our data suggest that an assessment battery consisting of sMRI, GMA, and HINE provides a comprehensive, early assessment of neurological development.
Our study has several strengths. We used a longitudinal cohort study design and recruited all eligible very preterm infants from all regional academic and community level III/IV NICUs encompassing a geographically defined region. The race composition of our cohort was similar to recent state of Ohio census data (eg, percentage of Ohioans who self-identified as Black or White in 2018 was 33.3% and 63.5%, respectively; see Table 5 for analogous cohort data).31 This refection of state demographics permitted unbiased inferences about disease prevalence and associations between our prognostic tests. We collected data at term-equivalent age (sMRI) and three months corrected age (GMA and HINE), which represented uniform time points known to be optimal for test performance. All assessments were also performed with high reliability and masked to clinical history. The sample in this cohort study collecting all three assessments in concert is very large. Additionally, using the Kidokoro scoring system, a more semi-quantitative and less subjective approach to evaluating neonatal sMRI, made our results more rigorous and reproducible. Study limitations include wide confidence intervals for the intra-rater reliability for the cerebellar abnormality scores. Although our study identifies important associations between sMRI and GMA and HINE, the correlations were small and it does not yet report long-term data. It will be important to continue to follow this cohort to understand how these significant associations between sMRI, GMA, and HINE will affect their combined ability to accurately predict CP at two years CA, before definitive recommendations can be made.
In conclusion, we observed a low prevalence of moderate or severe brain abnormalities and absent fidgety GMA in very preterm survivors in this geographically defined cohort. The much higher prevalence of abnormal motor examination on the HINE compared with the GMA and their low correlation suggests that these tests evaluate different constructs and thus might need to be used in combination with structural MRI rather than interchangeably for early detection of cerebral palsy. Determining the combined value of these tests in predicting cerebral palsy at 2 years corrected age is an important next step.
Supplementary Material
Figure 2b:
Axial susceptibility weighted image demonstrating susceptibility artifact (arrows) at posterior horns of the lateral ventricles demonstrating evidence for intraventricular hemorrhage.
Figure 2c:
Coronal T2 image measuring large frontal extra-axial space
Acknowledgments
Supported by National Institutes of Health (R01-NS094200-05 and R01 NS096037-03) from the National Institute of Neurological Disorders and Stroke (NINDS) to N.P. The content is solely the responsibility of the authors and does not necessarily represent the official views of NINDS or the National Institutes of Health. The authors declare no conflicts of interest.
Abbreviation List:
- GMA
General Movements Assessment
- HINE
Hammersmith Infant Neurological Exam
- CP
Cerebral Palsy
- sMRI
Structural magnetic resonance imaging
- NICU
Neonatal Intensive Care Unit
- ICC
Intra-class correlation coefficient
- LR
Likelihood ratio
Footnotes
Authors’ disclosure: The authors have one author disclosure to report. Colleen Peyton is on the speaker’s bureau of the Prechtl’s General Movements Trust.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Novak I, Morgan C, Adde L, Blackman J, Boyd RN, Brunstrom-Hernandez J, et al. Early, Accurate Diagnosis and Early Intervention in Cerebral Palsy: Advances in Diagnosis and Treatment. JAMA Pediatr. 2017;171:897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Granild-Jensen JB, Rackauskaite G, Flachs EM, Uldall P. Predictors for early diagnosis of cerebral palsy from national registry data. Dev Med Child Neurol. 2015;57:931–5. [DOI] [PubMed] [Google Scholar]
- [3].Hubermann L, Boychuck Z, Shevell M, Majnemer A. Age at Referral of Children for Initial Diagnosis of Cerebral Palsy and Rehabilitation: Current Practices. Journal of Child Neurology. 2016;31:364–9. [DOI] [PubMed] [Google Scholar]
- [4].Oberg GK, Jacobsen BK, Jorgensen L. Predictive Value of General Movement Assessment for Cerebral Palsy in Routine Clinical Practice. Phys Ther. 2015;95:1489–95. [DOI] [PubMed] [Google Scholar]
- [5].Peyton C, Yang E, Kocherginsky M, Adde L, Fjortoft T, Stoen R, et al. Relationship between white matter pathology and performance on the General Movement Assessment and the Test of Infant Motor Performance in very preterm infants. Early Human Development. 2016;95:23–7. [DOI] [PubMed] [Google Scholar]
- [6].Ulrich BD. Opportunities for early intervention based on theory, basic neuroscience, and clinical science. Phys Ther. 2010;90:1868–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Van’t Hooft J, van der Lee JH, Opmeer BC, Aarnoudse-Moens CS, Leenders AG, Mol BW, et al. Predicting developmental outcomes in premature infants by term equivalent MRI: systematic review and meta-analysis. Syst Rev. 2015;4:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Romeo DM, Ricci D, Brogna C, Mercuri E. Use of the Hammersmith Infant Neurological Examination in infants with cerebral palsy: a critical review of the literature. Developmental Medicine & Child Neurology. 2015. [DOI] [PubMed] [Google Scholar]
- [9].Kwong AKL, Fitzgerald TL, Doyle LW, Cheong JLY, Spittle AJ. Predictive validity of spontaneous early infant movement for later cerebral palsy: a systematic review. Dev Med Child Neurol. 2018;60:480–9. [DOI] [PubMed] [Google Scholar]
- [10].Parikh NA. Are Structural Magnetic Resonance Imaging and General Movements Assessment Sufficient for Early, Accurate Diagnosis of Cerebral Palsy? JAMA Pediatr. 2018;172:198–9. [DOI] [PubMed] [Google Scholar]
- [11].Spittle AJ, Brown NC, Doyle LW, Boyd RN, Hunt RW, Bear M, et al. Quality of general movements is related to white matter pathology in very preterm infants. Pediatrics. 2008;121:E1184–E9. [DOI] [PubMed] [Google Scholar]
- [12].Skiold B, Eriksson C, Eliasson AC, Aden U, Vollmer B. General movements and magnetic resonance imaging in the prediction of neuromotor outcome in children born extremely preterm. Early Hum Dev. 2013;89:467–72. [DOI] [PubMed] [Google Scholar]
- [13].Peyton C, Yang E, Msall ME, Adde L, Stoen R, Fjortoft T, et al. White Matter Injury and General Movements in High-Risk Preterm Infants. AJNR Am J Neuroradiol. 2017;38:162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Peyton C, Einspieler C, Fjortoft T, Adde L, Schreiber MD, Drobyshevsky A, et al. Correlates of Normal and Abnormal General Movements in Infancy and Long-Term Neurodevelopment of Preterm Infants: Insights from Functional Connectivity Studies at Term Equivalence. Journal of Clinical Medicine. 2020;9:834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Morgan C, Romeo DM, Chorna O, Novak I, Galea C, Del Secco S, et al. The Pooled Diagnostic Accuracy of Neuroimaging, General Movements, and Neurological Examination for Diagnosing Cerebral Palsy Early in High-Risk Infants: A Case Control Study. J Clin Med. 2019;8:1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kidokoro H, Neil JJ, Inder TE. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. AJNR Am J Neuroradiol. 2013;34:2208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ferrari F, Einspieler C, Prechtl HF, BOS A, Cioni G. Prechtl’s method on the qualitative assessment of general movements in preterm, term and young infants: Mac Keith Press; 2004. [DOI] [PubMed] [Google Scholar]
- [18].Einspieler C, Prechtl HF. Prechtl’s assessment of general movements: a diagnostic tool for the functional assessment of the young nervous system. Ment Retard Dev Disabil Res Rev. 2005;11:61–7. [DOI] [PubMed] [Google Scholar]
- [19].Prechtl HF, Ferrari F, Cioni G. Predictive value of general movements in asphyxiated fullterm infants. Early Hum Dev. 1993;35:91–120. [DOI] [PubMed] [Google Scholar]
- [20].Romeo DM, Guzzetta A, Scoto M, Cioni M, Patusi P, Mazzone D, et al. Early neurologic assessment in preterm-infants: integration of traditional neurologic examination and observation of general movements. Eur J Paediatr Neurol. 2008;12:183–9. [DOI] [PubMed] [Google Scholar]
- [21].Romeo DM, Cioni M, Palermo F, Cilauro S, Romeo MG. Neurological assessment in infants discharged from a neonatal intensive care unit. Eur J Paediatr Neurol. 2013;17:192–8. [DOI] [PubMed] [Google Scholar]
- [22].Constantinou JC, Adamson-Macedo EN, Mirmiran M, Fleisher BE. Movement, imaging and neurobehavioral assessment as predictors of cerebral palsy in preterm infants. Journal of Perinatology. 2007;27:225–9. [DOI] [PubMed] [Google Scholar]
- [23].Datta AN, Furrer MA, Bernhardt I, Huppi PS, Borradori-Tolsa C, Bucher HU, et al. Fidgety movements in infants born very preterm: predictive value for cerebral palsy in a clinical multicentre setting. Developmental Medicine and Child Neurology. 2017;59:618–24. [DOI] [PubMed] [Google Scholar]
- [24].Laupacis A, Wells G, Richardson WS, Tugwell P, Guyatt GH, Browman G, et al. Users’ guides to the medical literature: V. How to use an article about prognosis. Jama. 1994;272:234–7. [DOI] [PubMed] [Google Scholar]
- [25].Mathes T, Pieper D. An algorithm for the classification of study designs to assess diagnostic, prognostic and predictive test accuracy in systematic reviews. Syst Rev. 2019;8:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hintz SR, Barnes PD, Bulas D, Slovis TL, Finer NN, Wrage LA, et al. Neuroimaging and neurodevelopmental outcome in extremely preterm infants. Pediatrics. 2015;135:e32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Linsell L, Malouf R, Morris J, Kurinczuk JJ, Marlow N. Prognostic factors for cerebral palsy and motor impairment in children born very preterm or very low birthweight: a systematic review. Developmental Medicine and Child Neurology. 2016;58:554–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Argyropoulou MI, Xydis V, Drougia A, Argyropoulou PI, Tzoufi M, Bassounas A, et al. MRI measurements of the pons and cerebellum in children born preterm; associations with the severity of periventricular leukomalacia and perinatal risk factors. Neuroradiology. 2003;45:730–4. [DOI] [PubMed] [Google Scholar]
- [29].Neubauer V, Griesmaier E, Pehbock-Walser N, Pupp-Peglow U, Kiechl-Kohlendorfer U. Poor postnatal head growth in very preterm infants is associated with impaired neurodevelopment outcome. Acta Paediatrica. 2013;102:883–8. [DOI] [PubMed] [Google Scholar]
- [30].Cheong JL, Hunt RW, Anderson PJ, Howard K, Thompson DK, Wang HX, et al. Head growth in preterm infants: correlation with magnetic resonance imaging and neurodevelopmental outcome. Pediatrics. 2008;121:e1534–40. [DOI] [PubMed] [Google Scholar]
- [31].Health ODo. Birth Statistics Census Data. Internet2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.