Abstract
In malaria-endemic regions, people often get exposed to various pathogens simultaneously, generating co-infection scenarios. In such scenarios, overlapping symptoms pose serious diagnostic challenges. The delayed diagnosis may lead to an increase in disease severity and catastrophic events. Recent coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has affected various areas globally, including malaria-endemic regions. The Plasmodium and SARS-CoV-2 co-infection and its effect on health are yet unexplored. We present a case report of a previously healthy, middle-aged individual from the malaria-endemic area who suffered SARS-CoV-2 and Plasmodium falciparum co-infection. The patient developed severe disease indications in a short time period. The patient showed neurological symptoms, altered hematological as well as liver-test parameters, and subsequent death in a narrow time span. We hereby discuss the various aspects of this case regarding treatment and hematological parameters. Further, we have put forward perspectives related to the mechanism behind severity and neurological symptoms in this fatal parasite-virus co-infection case. In malaria-endemic regions, due to overlapping symptoms, suspected COVID-19 patients should also be monitored for diagnosis of malaria without any delay. The SARS-CoV-2 and Plasmodium co-infection could increase the disease severity in a short time span. In treatment, dexamethasone may not help in severe cases having malaria as well as COVID-19 positive status and needs further exploration.
Keywords: Malaria, Plasmodium falciparum, SARS-CoV-2, COVID-19, Co-infection, Cerebral malaria, Neurological manifestation
1. Introduction
Malaria caused by the smart parasite Plasmodium falciparum (P. falciparum) shows frequent outbreaks in endemic regions. The morbidity of malaria is high in endemic regions and can develop severe manifestations if left untreated [1]. The common malaria symptoms include fever, chills, sweating, headaches, tiredness, vomiting, nausea, etc. The severe manifestations include cerebral malaria with abnormal behaviour, impairment of consciousness, focal and generalized convulsions, seizures, coma, or other abnormal neurological signs [2], [3], [4], [5]. Another fatal complication of severe malaria is acute respiratory distress syndrome (ARDS), a condition of inhibition in oxygen exchange due to an inflammatory reaction in the lungs, which may develop even after treatment when there is a decrease in parasitemia [5], [6], [7].
Coronavirus disease-19 (COVID-19) caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has affected the entire world. The virus was first reported in Wuhan, China, and later reported in other countries around the world generating a pandemic situation. [8]. The common symptoms of COVID-19 include fever, dry cough, malaise, while the less common are body pain, headache, irritation or pain in the throat, diarrhea, anosmia, impaired taste, conjunctivitis, etc. In severe cases, struggling breathing or inadequate breath, pain or pressure in the chest, and speechlessness have been observed [9]. Many of these symptoms are overlapping with other diseases like malaria. The pandemic pressure and increasing medical burden propelled clinicians to provide necessary and on-board treatment to many patients in a narrow period [10]. Hence during the medical investigation, the possibility of the presence of the other disease or co-infection might get overlooked and the patient may be misdiagnosed.
Various reports indicate changes in distinct hematological and biochemical parameters in patients suffering from any of the two diseases. These clinical parameters can provide valuable information on how the patient responds to the disease and the treatment. Considering the overlapping symptoms of severe malaria and severe COVID-19, the patients in malaria-endemic regions should always be treated cautiously. The diagnosis and treatment of one may reduce the possibility of detection of another. In co-infection cases, if one of the diseases remains undiagnosed, it can increase the severity of the disease and create catastrophic health effects. Although COVID-19 and other malaria co-infection case has been reported earlier [11], [12], [13], [14], [15], [16], [17], we hereby present the first case of P. falciparum and SARS-CoV-2 co-infection driven neurological manifestation.
2. Case presentation
A 28-year-old man with a condition of body ache, cold, fever, and drowsiness for two days was presented to the hospital in July. The patient was not having any previous chronic medical conditions. The patient was having respiratory distress at the time of admission wherein he had been provided with oxygen support. Following continuous respiratory distress, he was further provided with non-invasive ventilation support. On admission, an intravenous antibiotic course (azithromycin), vitamin-C supplement, and proton-pump inhibitors (pantoprazole) was prescribed for a day. At the time of admission sample for the COVID-19 qRT-PCR test was sent, reports came after two days. The patient was diagnosed as COVID-19 positive. The hematological investigations revealed that person was having leukocytosis (WBCs > 10 × 103/μL), thrombocytopenia (Platelets < 150 × 103/μL), lymphocytopenia (lymphocytes <20%) and reduced eosinophils (<1%). The other hematological components were within normal ranges such as RBC count, hemoglobin, packed cell volume (PCV), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), Red Blood Cell Distribution Width (RDW)-CV, RDW-SD, Mean platelet volume (MPV), neutrophils, lymphocyte, monocyte, basophil, activated partial thromboplastin time (APPT), Prothrombin Time Test and INR (PT/INR) (Table 1 ). The biochemical investigations denoted elevated serum components such as serum alkaline phosphatases (SALP), bilirubin-direct (DBIL), bilirubin-total (TBIL), ferritin, serum gamma-glutamyl transferase (SGGT), alanine aminotransferases (ALT) and aspartate aminotransferases (AST). On the second day of hospitalization, the patient developed altered sensorium and signs of meningoencephalitis. The respective treatment was then initiated which included antibiotics (cephalosporin as well as vancomycin for meningitis, doxycycline for suspected COVID-19), anti-viral (acyclovir), anti-epileptic (levetiracetam), corticosteroid (dexamethasone as anti-inflammatory against suspected COVID-19), proton-pump inhibitors (pantoprazole), vitamin B and C. On the third day of hospitalization, the patient underwent a malaria diagnostic test due to suspicion of an ongoing malaria transmission period in the region. The patient tested positive for P. falciparum malaria by immunochromatography test. The antimalarial (artesunate) treatment was then initiated along with other ongoing treatments. The result of serum ferritin obtained on this day showed elevated ferritin (>250 ng/mL). The reports of investigations of some biochemical parameters obtained on hospitalization Day 4 showed lower albumin (<3.50 mg/dL), hypoproteinemia (<6.40 mg/dL). The other parameters such as SALP, ALT, AST, SGGT, DBIL, TBIL were found to be elevated than the same ones as of Day 2 of hospitalization. On the dawn of hospitalization Day 4, the patient showed severe hypoxia. Therefore, the endotracheal intubation was carried out to the patient. Subsequently, bradycardia was observed in the patient. The resuscitation was tried. However, despite all of the efforts, the patient couldn't be revived and was declared dead.
Table 1.
Hematological and biochemical profile of the patient on respective days of illness/hospitalization. The altered parameter values have been highlighted in bold letters.
| Tests | Results |
Normal range | ||
|---|---|---|---|---|
| Illness Day 4, Hospital Day 2 | Illness Day 5, Hospital Day 3 | Illness Day 6, Hospital Day 4 | ||
| Haematology | ||||
| WBC count | 12.30 × 103 | – | – | 4.00–10.00 × 103/μL |
| RBC count | 4.75 × 106 | – | – | 4.50–5.50 × 106/μL |
| Haemoglobin | 13.80 | – | – | 13.00–17.00 g |
| PCV | 40.50 | – | – | 36.00%–46.00% |
| MCV | 85.20 | – | – | 83.00–101.00 fL/μm3 |
| MCH | 29.10 | – | – | 27.00–32.00 pg |
| MCHC | 34.10 | – | – | 31.50–34.50 g/dL |
| RDW-CV | 14.20 | – | – | 11.60%–14.00% |
| RDW-SD | 42.40 | – | – | 39.00–46.00 fL |
| Platelet count | 40.00 × 103 | – | – | 150.00–410.00 x103/μL |
| MPV | 12.10 | – | – | 7.50–12.00 fL |
| Neutrophils | 80.00% | – | – | 40.00%–80.00% |
| Lymphocyte | 10.00% | – | – | 20.00%–40.00% |
| Monocyte | 10.00% | – | – | 2.00%–10.00% |
| Eosinophil | 0.00% | – | – | 1.00%–6.00% |
| Basophil | 0.00% | – | – | 0–2.00% |
| APTT | 36.10 | 30.00 | – | 30.00–40.00 s |
| PT with INR | 13.10 | 13.60 | – | 11.00–13.50 s (INR 0.8–1.1) |
| Biochemistry | ||||
| Magnesium | 1.90 | – | – | 1.60–2.60 mg/dL |
| Calcium | 7.30 | – | – | 8.60–10.30 mg/dL |
| Chloride | 95.00 | – | – | 95.00–110.00 mmol/L |
| Phosphorus | 2.70 | – | – | 2.50–4.50 mg/dL |
| Potassium | 5.20 | – | – | 3.50–5.50 mmol/L |
| Sodium | 131.00 | – | – | 136.00–145.00 mmol/L |
| Urea | 33.00 | – | – | 12.00–42.00 mg/dL |
| Albumin | 3.60 | – | 2.90 | 3.50–5.20 gm/dL |
| Total protein | 6.30 | – | 5.60 | 6.40–8.30 gm/dL |
| Globulin | 2.70 | – | 2.70 | 2.00–3.50 g/dL |
| SALP | 117.00 | – | 204.00 | 40.00–129.00 U/L |
| SGGT | 184.00 | – | 70.00 | 10.00–60.00 U/L |
| AST | 79.00 | – | 101.00 | 0–40.00 U/L |
| ALT | 100.00 | – | 74.10 | 5.00–40.00 U/L |
| DBIL | 2.94 | – | 8.70 | 0.00–0.30 mg/dL |
| TBIL | 5.18 | – | 10.26 | 0.20–1.20 mg/dL |
| Creatinine | 0.60 | – | – | 0.90–1.30 mg/dL |
| Ferritin | – | 1,000.00 | – | 20.00–250.00 ng/ml |
Packed cell volume (PCV), Mean corpuscular volume (MCV), Mean corpuscular hemoglobin (MCH), Mean corpuscular hemoglobin concentration (MCHC), Red Blood Cell Distribution Width (RDW), Mean platelet volume (MPV), Partial thromboplastin time (APPT), Prothrombin Time Test and INR (PT/INR), Serum alkaline phosphatases (SALP), Bilirubin-direct (DBIL), Bilirubin-total (TBIL), Serum gamma-glutamyl transferase (SGGT), Serum aspartate aminotransferase (AST), Serum alanine aminotransferases (ALT).
3. Discussion and conclusion
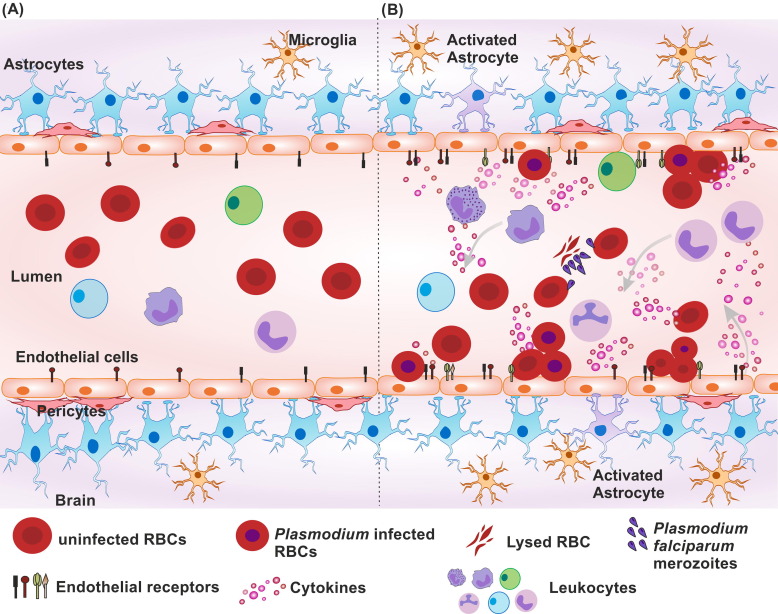
The COVID-19 outbreak has affected the world, including the malaria-endemic areas. To the best of our knowledge, this is the first case report of P. falciparum and SARS-CoV-2 co-infection driven neurological manifestations. The patient belonged to a malaria-endemic state of Odisha from India and was of the middle age group. The state with only 4% land area and 3% of population of the country, accounts for around 40% of the total malaria burden of India [18], [19]. Earlier studies also have reported malaria and viral co-infections in this region [20], [21]. The estimated incubation period of P. falciparum malaria and COVID-19 being around 7–14 days and 2–17 days respectively [22], [23], there is a possibility of co-infection in this endemic zone. There are several types of malaria, of which P. falciparum is the most severe type worldwide, with reported fatality rate being as high as 30% (cerebral malaria) in some areas [24]. The current case of death of a patient in a short time hints towards the unusual complication of parasite-virus co-infection. The parasite and virus co-infection can act as a double burden to the body’s immune system. The immune cells could undergo a dilemma whether to produce a response for clearance of one pathogen or another and ultimately ending up with the exaggerated immune response. The hematological parameters can provide a gist into how the body deals with the co-infection scenario. In the current case, the patient's WBC count was elevated, which could be correlated to the impact of severe COVID-19 [25], [26] instead of malaria. In malaria cases, low to normal WBC counts are generally observed [27], [28], [29]. Thrombocytopenia and lymphocytopenia is also commonly observed in severe COVID-19 as well as severe malaria [30], [31]. All other altered biochemical parameters like elevated bilirubin, AST, ALT, SALP, ferritin, SGGT as well as reduced sodium and calcium levels are observed in the patient are correlative to severe malaria as well as severe COVID-19 cases. Elevated bilirubin can be the result of Plasmodium mediated excessive RBC lysis. Hemolysis can show mild elevation in bilirubin but an extensive increase could be due to hepatic involvement. To the addition of increased bilirubin, the other increased liver function biomarkers like AST, ALT, SGGT, SALP indicate that the co-infection situation has affected the liver function in patients. The increased WBCs and normal RBC related parameters depict that the body's response is inclined more towards COVID-19. In case of severe malaria, generally decline in RBC-related parameters i.e. RBC count, hemoglobin, etc. is observed [28], [32]. These parameters and the patient’s symptoms also indicate that co-infection may have driven hepatic encephalopathy. Nevertheless, due to the presence of malaria parasites in a patient's blood, the neurological consequence in the patient could be an outcome of cerebral malaria. Reports have shown that SARS-CoV-2 has the potential to alter the endothelial cell functioning of the body [8], [33]. During the COVID-driven cytokine storm or due to viral infection to endothelial cells itself, the endothelial activation can occur. Similarly, in malaria cases, endothelial activation and elevated surface adhesion molecules are observed [34], [35]. Post activation, the myriad of elevated endothelial surface markers may help the attachment of RBCs leading to infected RBC sequestration (Fig. 1 ) as seen in the case of Epstein-Barr virus and P. falciparum co-infection [36]. This is one of the important manifestations of cerebral malaria. Moreover, the dual infection scenario and its aftermath could modulate the blood–brain barrier permeability. This further can imbalance the selective transport of biological agents inside the brain and allow the viral entry into CNS through various routes [37]. The disturbed ionic balance like reduced calcium and sodium in the blood can also contribute to this event. Overall situation may aid up to the inflammation at the cerebral site. Ultimately there would be the generation of various meningoencephalitis-like symptoms including altered sensorium. In the current case, the possibility of a similar scenario cannot be ruled out.
Fig. 1.
Schematic representation of possible microenvironment at blood-brain barrier in (A) normal individual, (B) Malaria and COVID-19 positive individual. Individual suffering from COVID-19 and malaria experience double pathogen burden. In severe condition, the cytokine storm is generated from immune cells as an impact of COVID-19 which further causes endothelial activation. Plasmodium infected RBCs can attach to endothelial cells and also cause the endothelial activation. As a consequence of this, and exaggerated host response the cell surface receptor expression of endothelial cells can increase. Ultimately, the excessive numbers of Plasmodium infected and uninfected RBCs get adhered to the endothelial wall for infected RBC sequestration. Endothelial cells further can modulate the nearby cells like astrocytes to cause their activation in response to insult at blood-brain barrier. This could facilitate increased barrier permeability, leukocyte extravasation and severe inflammation at site. The overall scenario may aid up the pathogenesis of cerebral malaria.
In the current case, the patient showed poor response to the treatment as well which can be depicted from continuously worsening hematological parameters on subsequent days. The hospitalization day-2 reports showed the markers denoted in severe COVID-19 cases. The patient was suspected of severe COVID-19 and hence was provided with a corticosteroid- dexamethasone. Previous reports conclude dexamethasone to be deleterious in cerebral malaria [38], [39], [40]. In the current case, the course of dexamethasone did not help in improving the patient’s condition. Hence more study regarding use of dexamethasone in such co-infection cases needs further exploration. The major event in the current case was the diagnosis of malaria as well. It has been delayed to the third day of hospitalization instead of at the time of admission due to symptoms coinciding with that of COVID-19. The patient belonged to a malaria-endemic region and was admitted during the monsoon season when the spread of the mosquito-borne disease is generally at its peak. The diagnostic delay of malaria cases during the COVID-19 pandemic time due to overlapping symptoms has also been reported in various other cases [11], [41]. More light should be shed on this area considering its importance and possibility of spreading any CoV outbreak in the near future in malaria-endemic countries. The co-infections could drive the change in the clinical representation of the disease. Otherwise, without actually changing the clinical representation, it can affect the various systems of the body. In such a case, like the current one, it would be too late till the actual cause is diagnosed. The report will help in considering the chances of co-infection and improvement of clinical management in such cases.
Ethics statement
The protocol for the present study was approved by the ethical committees of the Indian Institute of Technology Indore, Indore (BSBE/IITI/IHEC-05/2020); School of Biotechnology, Kalinga Institute of Industrial Technology, Bhubaneshwar (KIIDU/KSBT/2020/345); and Kalinga Institute of Medical Sciences, Bhubaneshwar (KIIT/KIMS/IEC/372/2020). All procedures were performed by following the revised declaration of Helsinki. Written consent was obtained from patient’s family member.
Acknowledgements
This work was supported by Council for Scientific and Industrial Research [Grant no 37(1693)/17/EMR-II]; Department of Science and Technology as Ramanujan fellowship [Grant no. SB/S2/RJN-132/20/5]. We are thankful to Department of Biotechnology; University Grants Commission, Govt. of India for fellowship to Omkar Indari, Budhadev Baral and Kartik Muduli respectively in the form of research stipend. We gratefully acknowledge the Kalinga Institute of Medical Sciences, Bhubaneshwar; School of Biotechnology, Kalinga Institute of Industrial Technology, Bhubaneshwar; and Indian Institute of Technology Indore for providing facilities and support. We appreciate lab colleagues for insightful discussions and advice.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Author contributions
Omkar Indari: Conceptualization, Writing - Original Draft, Visualization, Validation. Budhadev Baral: Data Curation, Conceptualization, Visualization, Validation. Kartik Muduli: Data Curation, Conceptualization, Visualization, Validation. Ambika Prasad Mohanty: Investigation, Methodology. Natabar Swain: Investigation, Methodology. Nirmal Kumar Mohakud: Conceptualization, Supervision, Project administration, Writing - Review & Editing. Hem Chandra Jha: Conceptualization, Supervision, Project Administration, Writing - Review & Editing.
References
- 1.Del Prado G.R.L., García C.H., Cea L.M., Espinilla V.F., Moreno M.F.M., Márquez A.D., Polo M.J.P., García I.A. Malaria in developing countries. J. Infect. Develop. Countries. 2014;8(01):001–004. doi: 10.3855/jidc.4610. [DOI] [Google Scholar]
- 2.Bartoloni A., Zammarchi L. Clinical aspects of uncomplicated and severe malaria. Mediterranean J. Hematol. Infectious Dis. 2012;4(1) doi: 10.4084/mjhid.2012.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trampuz A., Jereb M., Muzlovic I., Prabhu R.M. Clinical review: severe malaria. Crit. Care. 2003;7(4):1–9. doi: 10.1186/cc2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luzolo A.L., Ngoyi D.M. Cerebral malaria. Brain Res. Bull. 2019;145:53–58. doi: 10.1016/j.brainresbull.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Bruneel F. Human cerebral malaria: 2019 mini review. Revue Neurol. 2019;175(7–8):445–450. doi: 10.1016/j.neurol.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Mohan A., Sharma S.K., Bollineni S. Acute lung injury and acute respiratory distress syndrome in malaria. J. Vector Borne Dis. 2008;45(3):179–193. [PubMed] [Google Scholar]
- 7.Taylor W.R., Hanson J., Turner G.D., White N.J., Dondorp A.M. Respiratory manifestations of malaria. Chest. 2012;142(2):492–505. doi: 10.1378/chest.11-2655. [DOI] [PubMed] [Google Scholar]
- 8.Jakhmola S., Indari O., Kashyap D., Varshney N., Rani A., Sonkar C., Baral B., Chatterjee S., Das A., Kumar R., Jha H.C. Recent updates on COVID-19: a holistic review. Heliyon. 2020;6(12):e05706. doi: 10.1016/j.heliyon.2020.e05706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coronavirus disease (COVID-19), https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-covid-19#, (2020) (accessed 16 December 2020).
- 10.Indari O., Jakhmola S., Elangovan M., Jha H.C. An update on antiviral therapy against SARS-CoV-2: how far have we come? Front. Pharmacol. 2021;12:632677. doi: 10.3389/fphar.2021.632677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lev D., Biber A., Lachish T., Leshem E., Schwartz E. Malaria in travellers in the time of corona. J. Travel Med. 2020;270:taaa067. doi: 10.1093/jtm/taaa067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sardar S., Sharma R., Alyamani T.Y.M., Aboukamar M. COVID-19 and Plasmodium vivax malaria co-infection. IDCases. 2020;21:e00879. doi: 10.1016/j.idcr.2020.e00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahajan N.N., Gajbhiye R.K., Bahirat S., Lokhande P.D., Mathe A., Rathi S., Warty N., Mahajan K.N., Srivastava V., Kuppusamy P., Mohite S.C. Co-infection of malaria and early clearance of SARS-CoV-2 in healthcare workers. J. Med. Virol. 2021;93(4):2431–2438. doi: 10.1002/jmv.v93.410.1002/jmv.26760. [DOI] [PubMed] [Google Scholar]
- 14.Ray M., Vazifdar A., Shivaprakash S. Co-infection with malaria and coronavirus disease-2019. J. Glob. Infect. Dis. 2020;12(3):162–163. doi: 10.4103/jgid.jgid_160_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muhammad Y., Aminu Y.K., Ahmad A.E., Iliya S., Muhd N., Yahaya M., Mustapha A.S., Tahiru A., Abdulkadir S.S., Ibrahim J.S., Ahmad A.B., Muhammad I.Y., Shehu Z., Yakubu A., Muhd B.K., Ahmed A., Faruk U.A. An elevated 8-isoprostaglandin F2 alpha (8-iso-PGF2α) in COVID-19 subjects co-infected with malaria. Pan Afr. med. 2020;37:78. doi: 10.11604/pamj.2020.37.78.25100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kishore R., Dhakad S., Arif N., Dar L., Mirdha B., Aggarwal R., Kabra S. COVID-19: possible cause of induction of relapse of Plasmodium vivax infection. Indian J. Pediatr. 2020;87(9):751–752. doi: 10.1007/s12098-020-03441-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Onosakponome E.O., Wogu M.N., López-Arellano M.E. The role of sex in malaria-COVID19 coinfection and some associated factors in Rivers State, Nigeria. J. Parasitol. Res. 2020;2020:8829848. doi: 10.1155/2020/8829848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pradhan M.M., Meherda P.K. Malaria elimination drive in Odisha: hope for halting the transmission. J. Vector Borne Dis. 2019;56:53–55. doi: 10.4103/0972-9062.257775. [DOI] [PubMed] [Google Scholar]
- 19.Pradhan A., Anasuya A., Pradhan M.M., Kavitha A.K., Kar P., Sahoo K.C., Panigrahi P., Dutta A. Trends in Malaria in Odisha, India—an analysis of the 2003–2013 time-series data from the national vector borne disease control program. PLoS One. 2016;11:e0149126. doi: 10.1371/journal.pone.0149126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saswat T., Sahoo N., Muduli S., Debata N.K., Chattopadhyay S., Chattopadhyay S. Epidemiological trends and molecular dynamics of dengue, chikungunya virus infection, coinfection, and other undifferentiated fever during 2015–2016 in Odisha, India. J. Med. Virol. 2019;91(2):163–170. doi: 10.1002/jmv.v91.210.1002/jmv.25307. [DOI] [PubMed] [Google Scholar]
- 21.Prevalence of dengue viral and malaria parasitic co-infections in an epidemic district, Angul of Odisha, India: An eco-epidemiological and cross-sectional study for the prospective aspects of public health, J. Infect. Public Health. 9 (2016) 421–428, https://doi.org/10.1016/j.jiph.2015.10.019. [DOI] [PubMed]
- 22.CDC-Centers for Disease Control, Prevention, CDC - Malaria - About Malaria - Disease. https://www.cdc.gov/malaria/about/disease.html#incubation, 2009 (accessed 16 April 2021).
- 23.Quesada J.A., López-Pineda A., Gil-Guillén V.F., Arriero-Marín J.M., Gutiérrez F., Carratala-Munuera C. Incubation period of COVID-19: a systematic review and meta-analysis. Rev. Clin. Esp. 2021;221:109–117. doi: 10.1016/j.rce.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiley J. Severe malaria. Trop. Med. Int. Health. 2014;19:7–131. doi: 10.1111/tmi.12313_1. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Y., Nie H.-X., Hu K., Wu X.-J., Zhang Y.-T., Wang M.-M., Wang T., Zheng Z.-S., Li X.-C., Zeng S.-L. Abnormal immunity of non-survivors with COVID-19: predictors for mortality. Infect. Dis. Poverty. 2020;9(1):108. doi: 10.1186/s40249-020-00723-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang G., Kovalic A.J., Graber C.J. Prognostic value of leukocytosis and lymphopenia for coronavirus disease severity. Emerg. Infect. Dis. 2020;26(8):1839–1841. doi: 10.3201/eid2608.201160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Omalu I.C., Oguche S., Gyang V., Akindigh T., Egah D., Gokop B. Standard white blood cell count for malaria density estimation: a need for review? Ann. Trop. Med. PH. 2008;1(1):29. doi: 10.4103/1755-6783.43075. [DOI] [Google Scholar]
- 28.Kotepui M., Phunphuech B., Phiwklam N., Chupeerach C., Duangmano S. Effect of malarial infection on haematological parameters in population near Thailand-Myanmar border. Malar. J. 2014;13(1):218. doi: 10.1186/1475-2875-13-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKenzie F.E., Prudhomme W.A., Magill A.J., Forney J.R., Permpanich B., Lucas C., Gasser R.A., Jr, Wongsrichanalai C. White blood cell counts and malaria. J. Infect. Dis. 2005;192(2):323–330. doi: 10.1086/431152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wool G.D., Miller J.L. The impact of COVID-19 disease on platelets and coagulation. Pathobiology. 2021;88(1):14–26. doi: 10.1159/000512007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrell C.N. Understanding platelets in malaria infection. Curr. Opin. Hematol. 2014;21(5):445–449. doi: 10.1097/MOH.0000000000000073. [DOI] [PubMed] [Google Scholar]
- 32.M. Kotepui, D. Piwkham, B. PhunPhuech, N. Phiwklam, C. Chupeerach, S. Duangmano, Effects of malaria parasite density on blood cell parameters, PLoS One 10(3) (2015), e0121057. https://doi.org/10.1371/journal.pone.0121057. [DOI] [PMC free article] [PubMed]
- 33.Sardu C., Gambardella J., Morelli M.B., Wang X., Marfella R., Santulli G. Hypertension, thrombosis, kidney failure, and diabetes: is COVID-19 an endothelial disease? a comprehensive evaluation of clinical and basic evidence. J. Clin. Med. 2020;9(5):1417. doi: 10.3390/jcm9051417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jakobsen P., Morris-Jones S., Rønn A., Hviid L., Theander T., Elhassan I., Bygbjerg I., Greenwood B. Increased plasma concentrations of sICAM-1, sVCAM-1 and sELAM-1 in patients with Plasmodium falciparum or P. vivax malaria and association with disease severity. Immunology. 1994;83(4):665. [PMC free article] [PubMed] [Google Scholar]
- 35.Adukpo S., Kusi K.A., Ofori M.F., Tetteh J.K., Amoako-Sakyi D., Goka B.Q., Adjei G.O., Edoh D.A., Akanmori B.D., Gyan B.A. High plasma levels of soluble intercellular adhesion molecule (ICAM)-1 are associated with cerebral malaria. PLoS One. 2013;8(12):e84181. doi: 10.1371/journal.pone.0084181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Indari O., Chandramohanadas R., Jha H.C. Epstein-Barr virus infection modulates blood–brain barrier cells and its co-infection with Plasmodium falciparum induces RBC adhesion. Pathog. Dis. 2021;79(1):ftaa080. doi: 10.1093/femspd/ftaa080. [DOI] [PubMed] [Google Scholar]
- 37.Jakhmola S., Indari O., Chatterjee S., Jha H.C. SARS-CoV-2, an underestimated pathogen of the nervous system. SN Comprehensive Clin. Med. 2020;2(11):2137–2146. doi: 10.1007/s42399-020-00522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warrell D.A., Looareesuwan S., Warrell M.J., Kasemsarn P., Intaraprasert R., Bunnag D., Harinasuta T. Dexamethasone proves deleterious in cerebral malaria: a double-blind trial in 100 comatose patients. N. Engl. J. Med. 1982;306(6):313–319. doi: 10.1056/NEJM198202113060601. [DOI] [PubMed] [Google Scholar]
- 39.Hoffman S.L., Rustama D., Punjabi N.H., Surampaet B., Sanjaya B., Dimpudus A.J., McKee K.T., Paleologo F.P., Campbell J.R., Marwoto H., Laughlin L. High-dose dexamethasone in quinine-treated patients with cerebral malaria: a double-blind, placebo-controlled trial. J. Infect. Dis. 1988;158(2):325–331. doi: 10.1093/infdis/158.2.325. [DOI] [PubMed] [Google Scholar]
- 40.Gay F., Zougbédé S., N’Dilimabaka N., Rebollo A., Mazier D., Moreno A. Cerebral malaria: what is known and what is on research. Rev Neurol. 2012;168(3):239–256. doi: 10.1016/j.neurol.2012.01.582. [DOI] [PubMed] [Google Scholar]
- 41.Mahajan N.N., Kesarwani S.N., Shinde S.S., Nayak A., Modi D.N., Mahale S.D., Gajbhiye R.K. Co-infection of malaria and dengue in pregnant women with SARS-CoV-2. Int. J. Gynecol. Obstet. 2020;151(3):459–462. doi: 10.1016/j.neurol.2012.01.582. [DOI] [PMC free article] [PubMed] [Google Scholar]