Abstract
Forming eye contact is important in dog–human communication. In this study we measured what factors affect dogs’ propensity for forming eye contact with an experimenter. We investigated the effect of [1] cephalic index (head shape’s metric, indicator of higher visual acuity at the centre of the visual field), [2] breed function (visual cooperativeness), [3] age and [4] playfulness with strangers in 125 companion dogs. Cephalic index was measured individually and analysed as a continuous variable. Results showed that [1] dogs with a higher cephalic index (shorter head) established eye contact faster. Since cephalic index is highly variable even within a breed, using artificial head shape groups or breed average cephalic index values is not recommended. [2] Breed function also affected dogs’ performance: cooperative breeds and mongrels established eye contact faster than dogs from non-cooperative breeds. [3] Younger dogs formed eye contact faster than older ones. [4] More playful dogs formed eye contact faster. Our results suggest that several factors affect dogs’ interspecific attention, and therefore their visual communication ability.
Subject terms: Zoology, Animal behaviour
Introduction
Dogs are well adapted to living with humans, partly due to their effective use of human communicative signals. Humans predominantly use eye contact to establish communication1, and dogs are sensitive to this cue (e.g. they follow human pointing2 and gaze3 more successfully if eye contact is established prior to the presentation of the cue). Dogs’ increased attention to humans enhances the effectiveness of dog–human communication and thus cooperation. Gaze direction can moreover be considered as an indicator of attentional focus4. Mutual gaze also plays a role in dog–human bonding. Its duration is associated with increased oxytocin levels in both dogs and their human partners5. As eye contact plays a fundamental role in dog–human relationships, it is important to know which factors influence it.
The amount of variation in the head shape of modern dog breeds is unique6. Relationships have been found between dogs’ head morphology, brain organization and sensory abilities7–11. For instance, there is an indirect connection between head shape and dogs’ visual acuity10. Head shape can be measured objectively with its common metric, the cephalic index12, a ratio of the width and the length of the head. The cephalic index is correlated with the distribution of the eyes’ retinal ganglion cells. These cells are responsible for the initial pre-processing of visual information from retinal photoreceptors. There is a difference between long-headed (dolichocephalic; low cephalic index value) and short-headed (brachycephalic; high cephalic index value) dogs with respect to the retinal ganglion cells’ distribution. In the case of dolichocephalic dogs, these cells form a horizontally aligned visual streak, while in brachycephalic dogs the cells have a higher density at the centre of the field of vision and lower in the periphery10. As a likely consequence, brachycephalic dogs may be better able to focus their attention to stimuli at the centre of their visual field, where their communication partner is situated, because they are less disturbed by other visual stimuli coming from the periphery. As a result, they may display a better visual communication ability. Gácsi et al.13 found that brachycephalic dogs are more successful at following humans’ visual gestures than dolichocephalic dogs. To detect these cues, the animals need to look at the human’s upper body, thus brachycephalic dogs may be also more prone to form eye contact with humans. In line with this, Bognár et al.14 showed that brachycephalic dogs watch motionless, projected faces of both dogs and humans over a longer time than dolichocephalic dogs. Taken together, these above-mentioned differences between brachycephalic and dolichocephalic dogs suggest that cephalic index may be linked with changes in the way dogs perceive stimuli and possibly process information, and hence with differences in canine behaviour and social cognition.
The typical classification of dogs’ head shape based on cut-off values (Fig. 1) has been criticized by Georgevsky et al.15 as arbitrary. The cephalic index is a continuous variable with no sharply separable thresholds, and the value can vary over a wide range even within a breed (see Stone et al.16). Therefore, we decided to study the effect of head shape using continuous cephalic index values instead of threshold-based grouping, and at an individual level instead of using breed averages. This way we do not have to exclude mongrels, for which a breed average cannot be calculated.
Figure 1.
The typical classification of dogs’ head shape based on the cephalic index value. Cephalic index (CI) is the ratio of the maximum width of the head (A) multiplied by 100 divided by the head’s maximum length (B). The shorter a dog’s head is, the higher the cephalic index.
As morphological characteristics may not be independent of a breed’s function, it should also be taken into account. During breeding, dogs were selected for different types of work, which may have modified their communication skills. Consequently, not all breeds pay attention equally to human visual cues17–21. One possible way to group dog breeds is based on the role of vision in their cooperation with a human partner. Visually cooperative breeds have been selected to work in continuous visual contact and interaction (e.g. herding dogs), thus they are expected to be attentive to humans. On the contrary, visually non-cooperative breeds are not in visual contact with the human during their work (for example sled dogs and hounds), so they are not expected to pay as close attention to a human as cooperative ones. Previous research found that cooperative breeds are more successful at following human pointing gestures than non-cooperative breeds and mixed breed dogs13. On the other hand, we found no difference between breed types in their attention to motionless, projected faces of both dogs and humans14. Selection for different functions and head shape are not independent, but the relationship between them is not exclusive15. Dogs with low and high cephalic index value can be found among both cooperative and non-cooperative breeds. Based on this, breed function and head shape can cumulatively affect the visual communication ability of dogs.
Aging also significantly impacts visual attention22–31. A generalised slowing of information processing provides an explanation for an overall age-related decline in cognition25. Both an age-related reduction in visual processing speed26 and a decrease in visual contrast sensitivity have been reported in the human literature32–34, which can affect face perception32,35,36. Reduced visual processing speed could also result in a decrease in aged dogs’ social attention, which could hinder cooperation and communication between dogs and humans. Several previous studies showed an age-related decrease in visual24,31 and social attention27–30 (e.g. reported behavioural signs in older dogs included a reduction in attention towards the owner, and declines in play related activities etc.). Wallis et al.27 examined the effect of aging on Border collies’ (aged from 6 months to 14 years) propensity to form eye contact with an unfamiliar human. They found a quadratic relationship between propensity and age, and the performance peaked in middle aged dogs (3–6 years old animals established eye contact the fastest). Later Chapagain et al.24 expanded this to other dog breeds in a sample of dogs aged above 6 years, and found no effect of aging. As head shape and breed function may affect visual communication ability in dogs, to study the effect of aging, these factors should be accounted for.
Additionally, dogs’ propensity to form eye contact with an unfamiliar human can also be influenced by sociability (friendliness toward strangers16). Jakovcevic et al.37 reported that more sociable dogs gaze longer at a human face, than less sociable ones.
Since previous studies compared arbitrary head shape groups and/or the cephalic index was averaged by breed, currently, it is not possible to determine whether the differences in association with head shape that were found are due to breed differences or if they can be detected also independent of breed (e.g., by comparing individuals with different CI within breeds). Since cephalic index varies in a high range even within a breed16, it would be worthwhile to measure it individually, and also analyse its connections individually. The aim of this study was to consider the possible influencing factors that affect dogs’ human-directed attention and the visual communication ability in order to better understand their complex interplay, and to investigate whether head shape, breed function, age and/or sociability show any association with the individual’s propensity to form eye contact with humans.
In this study, we replicated the test of Wallis et al.27 and Chapagain et al.24, to measure pet dogs’ propensity to form eye contact with an unfamiliar experimenter, who rewarded the dog for repeatedly making eye contact with her. We investigated whether cephalic index is connected with this propensity, also taking into account other important influencing factors, such as breed function, age and sociability with humans. In order to gather data on the sociability of dogs, we tested their interest in a stranger. We examined the extent of greeting behaviour towards an unfamiliar human, and, as dogs' playfulness with humans and their interest in strangers are closely connected38, we also tested their playing behaviour towards the experimenter. We hypothesized that:
The cephalic index is positively correlated with dogs’ propensity to make eye contact with a human, so that shorter headed dogs (with a higher cephalic index) would form eye contact faster.
Visually cooperative breeds would form eye contact faster than non-cooperative breeds and mixed breed dogs.
Older dogs would form eye contact slower than younger dogs.
More social dogs would form eye contact faster with the unfamiliar person than less social ones.
Methods
Ethical statement
The Animal Welfare Committee of Eötvös Loránd University approved and accepted the experimental protocol (Ref. no.: PE/EA/2019-5/2017) and the tests were performed in accordance with the Hungarian regulations on animal experimentation and the Guidelines for the use of animals in research described by the Association for the Study Animal Behaviour (ASAB) and ARRIVE.
Subjects
In this study 130 pet dogs were tested, from which five had to be excluded: (1) because of problems with the video (N = 1), (2) visibility of dog’s eyes (due to coat N = 1), (3) problems with eating the food from the ground because of mouth morphology (N = 2) and (4) insufficient food motivation (N = 1). Wallis et al.27 found that the peak of dogs’ performance in eye contact forming with humans is at middle age, and we were interested in aging, not maturation, hence we only tested dogs older than 2.5 years. Thus, 125 dogs (male = 62) were included in the analysis (cephalic index value: 43.5–74.7 (median = 53.2); age: 31.4–174.5 months (median = 106.5 months).
The grouping of the dogs into breed functions was based on Gácsi et al.13 and the dogs’ breed history. The Cooperative breed group (N = 42) contained breeds which have been selected to work in continuous visual contact and interaction with a human partner (e.g. sheepdogs, gundogs), in contrast to the Non-cooperative breed group (N = 27; e.g. hounds, sled dogs, guard dogs, earthdogs). The Mixed breed group (N = 56) consisted of non-purebred dogs with unknown ancestors. Owners provided information about how did they get their dog; 68% of mixed breed dogs in our sample (38/56) were adopted from an animal shelter or found on the street, while only 7% of them (4/56) were adopted from a previous owner, and 16% of them (9/56) were gifted to the actual owner (with no information on whether they were found on the street or rescued from a shelter). The origin of 9% of mixed breeds (5/56) was totally unknown. It is unlikely, that the mixed breed dogs in our sample were first-generation mixtures, but testing the mixed breed dogs' lineages goes beyond the aims of the present study.
Neither the distribution of cephalic index nor the distribution of age differed between the breed groups (see Table 1). Cephalic index value and age did not correlate (R = 0.137; p = 0.129). All subjects’ demographic data are presented in Supplementary Table S1.
Table 1.
Cephalic index and age distribution of the sample among the different breed function groups.
| Cooperative | Non-cooperative | Mixed | Statistics | |
|---|---|---|---|---|
| Cephalic index (mean ± SD) | 53.28 ± 5.28 | 53.84 ± 8.27 | 53.57 ± 5.14 | F2,122 = 0.075; p = 0.928 |
| Age (month, mean ± SD) | 107.70 ± 38.10 | 89.27 ± 37.90 | 101.30 ± 39.87 | F2,122 = 1.850; p = 0.162 |
Dogs can be taught to form eye contact with the owner. In several dog schools, one of the first tasks is to teach the dog to make eye contact and thereby increase attention to the owner. We have data on the previous dog school experience (yes/no) from only 113 dogs. 73 dogs attended dog school, while 40 dogs did not. Dog school attendance had no connection to dogs’ performance in our test (hazard ratio = 1.049; p = 0.847).
Cephalic index coding
As mentioned in the introduction, the typical classification of dogs’ head shape based on cut-off values (Fig. 1) has been criticized by Georgevsky et al.15 due the arbitrary nature of the grouping, thus we studied the effect of head shape with actual cephalic index values. We measured each dog’s cephalic index from photographs. The method of measuring cephalic index from photographs was suggested by previous studies11,16,39. The cephalic index value was measured from photographs with the GIMP image editing program 2.2.13. (http://www.gimp.org/). The index was calculated as the ratio of the maximum width of the head multiplied by 100 divided by the head’s maximum length (Fig. 1). Skull width was measured from one zygomatic arch to the other and skull length was measured from the nose to the occipital protuberance. Each picture was taken from the same angle (perpendicular to the top of the skull; see examples in Fig. 1). The distance of the camera (Samsung T710 Galaxy Tab S2) to the top of the dogs’ head was not uniform (as each dog was a different height, and the camera was not fixed), however, this did not affect the measurement, as cephalic index is a ratio. To check the reliability of measuring the cephalic index from photographs, a second coder, naïve to the hypotheses of the study, measured a random sample of subjects (~ 20% of dogs; ICC: 0.91, p < 0.001), and in addition, the heads of ~ 20% of the dogs were also measured with a calliper (ICC: 0.98, p < 0.001). In the case of a Puli, measuring the cephalic index from a photograph was not possible because of its hair, thus it was measured only with a calliper (the dog’s hair was tied up during the test, so its eyes were visible).
Behaviour tests
All dogs participated in the “Canine Cognitive Battery” (Kubinyi et al., in prep.), which consists of 12 subtests. The prerequisite of participation was to meet the requirements of a sensory examination40. The same experimenter performed all subtests for an individual dog; however, the experimenter identity could differ between dogs. The first test where the dog met with the unfamiliar experimenter was the Greeting test, which was immediately followed by the Human-directed play test. The Eye contact establishment test was the tenth subtest. Thus, before the Eye contact establishment subtest, all dogs had prior experience with the experimenter, who positively interacted with the dog (stroking, playing, speaking, and feeding them). As the participating dogs were enrolled in our longitudinal research project and it was important for the experimenter to be unfamiliar to the dog at the beginning of the Greeting subtest, 8 experimenters took part in testing to comply with this requirement (the experimenter’s ID can be found in Supplementary Table S1). All experimenters were young women (age: 20–27 years). All three tests were carried out during one test session in the same laboratory room (6.27 m * 5.4 m).
Eye contact establishment test
In this test, which was based on Wallis et al.27 and Chapagain et al.24, dogs were rewarded for repeatedly forming eye contact with the experimenter. During the test, the experimenter stood in the centre of the laboratory room, while the owner sat on a chair (Fig. 2A). The experimenter held a clicker-like device (which made a “boing” sound, different from the usual clicker sound) in one of her hands, while the other hand was free. During the test, both hands were in a relaxed position by her side. She also had a food pouch at her back on her belt containing pieces of sausage as a food reward.
Figure 2.
Test setup. Eye contact establishment test (A), Greeting test (B) and Human-directed play test (C).
At the beginning of the test, the experimenter called the dog to her, and threw a piece of sausage from her pouch on the ground. Then she remained motionless until the dog formed eye contact with her. She marked the correct behaviour using the clicker-like device before again throwing a piece of sausage. Unlike Wallis et al.27 and Chapagain et al.24, the experimenter did not rustle her pouch when the dog no longer showed interest. The test ended after 15 eye contacts or after 300 s elapsed.
Sociability tests
Greeting test
Before this test, the dog had the opportunity to explore the laboratory room for 2 min in the presence of the owner. At the beginning of the Greeting test, the owner stood in the middle of the room and leashed the dog. The experimenter entered the room for the first time, approached the dog-owner pair and said “Hello” to the owner and the dog. She stopped for 1 s in front of the dog, outside the reach of the leash. If the dog approached the experimenter and showed “friendly” or neutral behaviour, she stepped towards the dog, and petted it while continuously speaking to the dog in a friendly tone (Fig. 2B). If the dog showed fearful behaviour, she ignored the dog and talked to the owner for approx. 30 s. After this test the owner unleashed the dog, and the experimenter left the room.
Human-directed play test
To measure the playfulness of dogs, we tested them in a situation where they could freely interact with the experimenter. At the beginning of the test, the owner and the dog (off-leash) were in the room, and the experimenter entered with toys (ball and rope) in her hand (Fig. 2C). She offered the toys to the dog, and the dog was free to choose between them. Then, they played with the chosen toy until the first minute elapsed. If the dog was not interested in toys, she tried to initiate social play for 1 min.
Data collection
We recorded the tests with video cameras, which were connected to computers outside of the testing room. The Eye contact establishment test was coded from videos by using Solomon Coder beta 19.08.02 (copyright 2006–2019 by András Péter). The latency to form each eye contact with the experimenter was measured from the moment the dog took the food into its mouth until it formed eye contact with the experimenter again. We defined eye contact as the situation in which the dog oriented towards the front of the experimenter and looked up with both eyes into the experimenter's eyes. Eye contact occurrences were indicated by the experimenter with an auditory marker (clicker-like device), thus eye contact was coded from the videos’ audio spectrograms. The videos were coded in 0.1 s time frames. We analysed the first 15 eye contacts of each dog. If a dog went over the allotted 300 s, but formed less than 15 eye contacts, we gave the maximum latency to each remaining trial (e.g. if the dog formed eye contact 13-times within 300 s, in the 14th and 15th trials the latency to form eye contact was set to 300 s and marked as a censored event). In this way, we did not have to exclude those dogs from the analysis which did not form eye contact 15 times within the allotted time period.
The Greeting test and Human-directed play test were live coded using a binary variable, based on the following definitions: (A) Greeting test: (1) greet immediately in a friendly way (score = 1; N = 73): the dog approached the experimenter immediately when she entered the room and she could pet it; (2) no greeting behaviour (score = 0; N = 52): the dog did not approach the experimenter without calling or she could not pet it; (B) Human-directed play test: (1) high playfulness (score = 1; N = 60): the dog played enthusiastically with the experimenter, it brought back the ball at least once to her or tugged the rope; (2) low playfulness (score = 0; N = 65): the dog did not touch the toys, or it ran after the ball, but did not bring it back to the experimenter, or it took the rope into its mouth a bit, but did not tug it.
A second coder, naïve to the hypotheses of the study, coded a random sample of subjects (~ 20% of dogs). This sample was analysed using intra-class correlations to check the interrater reliability. We found robust reliability for latency to form eye contact (ICC: 0.82–1.00, median: 1.00, p < 0.001), greeting behaviour (ICC: 0.81, p < 0.001) and playfulness with a human (ICC: 0.91, p < 0.001).
Statistical analysis
We analysed the results using R statistical software (version 3.6.3)41 in Rstudio42. We used survival analysis, as suggested for latency outcomes in behavioural experiments by Jahn-Eimermacher et al.43, as it can handle events which have not occurred within a specified time. We examined each latency per dog (i.e. 15 latencies belong to each dog), thus Mixed Effects Cox Regression Models (“coxme” function of “coxme”44 package) were used to analyse the effect of cephalic index value, breed function, age and sociability (greeting behaviour score and playfulness score) on the latency to form eye contact, with subject ID as a random factor. We also included trial numbers and experimenter identity in the analysis as confounding variables.
Binomial Generalized Linear Models with logit link (“glm” function of “stats”41 package) were used to check the possible relationship between the demographic and morphological factors (cephalic index value, breed function, age) and the sociability binary scores (greeting behaviour and playfulness with a human). We also included experimenter identity in the analysis as a confounding variable. To test the independence of the two subtests of sociability, we used a Pearson's Chi-squared test with Yates' continuity correction (“chisq.test” function of “stats”41 package).
For the Mixed Effects Cox Regression Models, bottom-up model selection was used (“anova” function of “stats”41 package), where the inclusion criteria were a significant likelihood ratio test for each tested variable. The most parsimonious model contained breed function, playfulness with a human and trial number as factors, and cephalic index value and age as covariates (for more details see Supplementary Table S3). A Tukey post-hoc test was used for comparisons between the three breed function groups (“emmeans” function of “emmeans”45 package). For the Binomial Generalized Linear Models, AIC based model selection was applied to find the most parsimonious model using “dredge” function of “MuMIn”46 package. According to the model selection, it contained only age as a covariate for both Sociability subtests, and no other factors (for more details see Supplementary Table S4–S5).
We used the “vif” function of the “car”47 package to check the possible multicollinearity among the independent variables. The variance inflation factor (VIF) measures how much the variance of any one of the variables is inflated due to multicollinearity in the overall model. If the VIF score is over 5, there is a problem with multicollinearity.
We used the “survfit” function of the “survival”48 package and the “ggsurvplot” function of the “survminer”49 package to create survival plots and the “ggplot” function of the “ggplot2”50 package to produce probability plots.
Results
All VIF scores were under 1.6, revealing no multicollinearity among the independent variables (for more information, check Supplementary Table S2).
Trial number had a significant effect on latency to form eye contact, indicating that dogs learnt about the task during the test (p < 0.001). Post-hoc test showed that dogs became faster during the trials, e.g. in the 15th trial, dogs were quicker to establish eye contact with the experimenter than in the 1st trial with a hazard ratio of 2.064 (95% CI = (1.575–2.705), Z = 5.25, p < 0.001). We did not find a significant interaction between cephalic index and trial number; breed function and trial number; age and trial number or playfulness with a human and trial number.
Cephalic index had a significant positive association with latency to form eye contact with a hazard ratio of 1.055 (95% CI = (1.018–1.094), Z = 2.94, p = 0.003; Fig. 3). Dogs with a higher cephalic index (shorter headed dogs) formed eye contact faster than dogs with a lower cephalic index.
Figure 3.
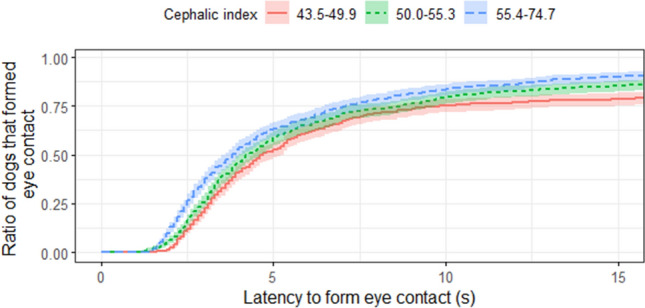
Survival plot for 125 dogs in three head shape groups. Cephalic index was a continuous value in the analysis, but the sample was divided into three groups at the 0.33 and 0.67 quartiles for visualisation purposes only. On the X axis the latency to form eye contact can be read (for visualisation purposes, data are censored at 15 s), while on the Y axis the ratio of dogs that formed eye contact with the experimenter is displayed.
Breed function also had a significant effect on latency to form eye contact (p < 0.001; Fig. 4). A Tukey post-hoc test showed that cooperative breeds were more prone to establish eye contact more quickly with the experimenter than non-cooperative breeds with a hazard ratio of 2.965 (95% CI = (1.653–5.317), Z = 3.64, p < 0.001). Mixed breed dogs were also more inclined to form eye contact earlier than non-cooperative breeds with a hazard ratio of 2.935 (95%CI = (1.719–5.011), Z = 3.95, p < 0.001), while we found no difference between cooperative breeds and mixed breed dogs (hazard ratio of 1.010 (95% CI = (0.618–1.652), Z = 0.04, p = 0.999)).
Figure 4.
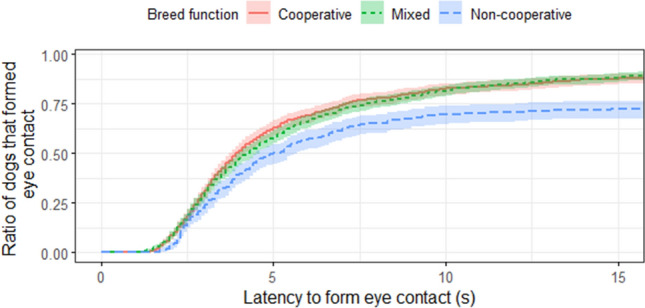
Survival plot for 125 pet dogs in the three breed function groups. E.g. after 15 s elapsed, usually ~ 90% of cooperative breeds and mixed breed dogs have already formed eye contact with the experimenter, while at the same time only ~ 75% of non-cooperative breeds had.
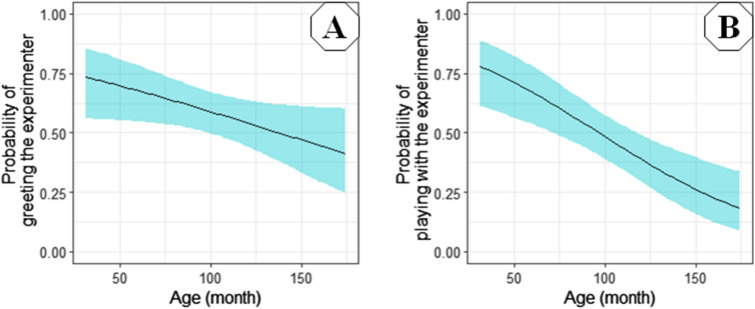
Age had a significant negative effect on latency to form eye contact with a hazard ratio of 0.986 (95% CI = (0.981–0.991), Z = − 5.27, p < 0.001; Fig. 5), on greeting an unfamiliar human with a hazard ratio of 0.991 (95% CI = (0.981–1.000), Z = − 1.97, p = 0.049; Fig. 6A) and on playfulness with a human with a hazard ratio of 0.981 (95% CI = (0.971–0.991), Z = − 3.73, p < 0.001; Fig. 6B).
Figure 5.
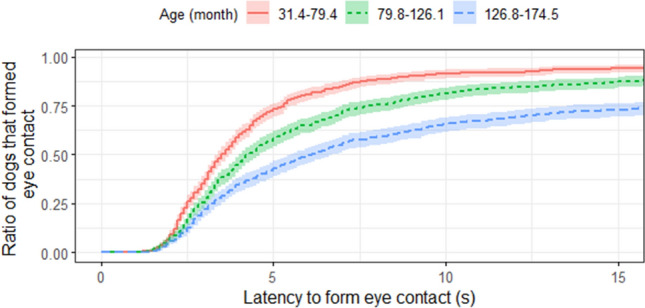
Survival plot for the 125 pet dogs divided into three age groups. Age was a continuous value in the analysis, but the sample was divided into three groups at the 0.33 and 0.67 quartiles for visualisation purposes only. For example, after 10 s elapsed, almost all dogs younger than 6 years (31.4–79.4 months) formed eye contact with the experimenter, while only circa 65% of the dogs older than 10 years (126.8–174.5 months) did so.
Figure 6.
Probability of greeting the experimenter without her needing to call the dog (A) and playing more enthusiastically with her (B), according to age in months. Younger dogs were more likely to greet the experimenter and had higher playfulness, than the older ones.
Finally, among the Sociability tests, only playfulness with a human had a significant effect on latency to form eye contact. Dogs with high playfulness were quicker to establish eye contact with the experimenter than dogs with low playfulness with a hazard ratio of 1.673 (95% CI = (1.074–2.608), Z = 2.28, p = 0.023; Fig. 7). Dogs’ greeting behaviour did not predict their performance in the Eye contact establishment test, although the sociability scores (greeting behaviour and playfulness with a human) were not independent (χ2(1) = 14.435, p < 0.001). Seventy-three percent of dogs which did not approach the experimenter immediately in the Greeting test, also did not play with her in the Human-directed play test, while only thirty-seven percent of dogs which approached her without calling, did not play with her.
Figure 7.
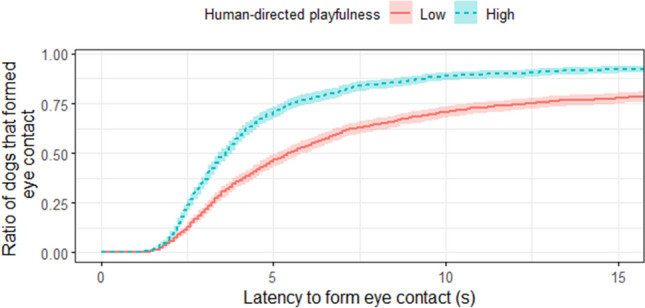
Survival plot for 125 dogs divided into high and low playfulness with a human. E.g. after 10 s elapsed, usually ~ 90% of dogs with high playfulness had already formed eye contact with the experimenter, while at the same time only ~ 75% of dogs with low playfulness did so.
We found no effect of experimenter identity on any of the three tests, and no effect of cephalic index value and breed function on the sociability scores.
Discussion
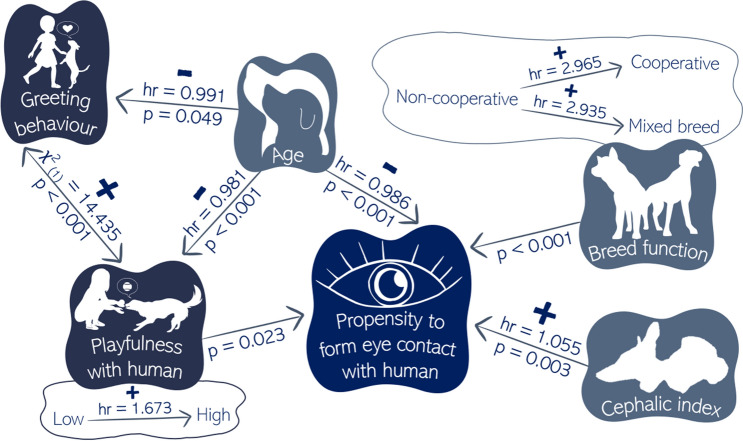
In this study, we examined the effect of four influencing factors cephalic index, breed function, age, and sociability on the propensity of pet dogs to form eye contact with a human. Based on the results, there are stable traits that affect dogs’ performance throughout their lifetime, such as head morphology and breed function. In addition, individual characteristics that can change over time, such as age and sociability, also modify the visual communication ability of dogs. The main results are summarized in Fig. 8.
Figure 8.
Summary diagram of the main results (created by using drawings designed by Freepik51). Each effects’ hazard ratios (hr) and p values (p) are presented. The levels of categorical variables are presented separately, and the direction of the effects are shown (“ + ” positive and “ − ” negative).
An important finding of this study is the effect of head shape (notably its metric, the cephalic index) on dogs’ latency to form eye contact. Dogs with a higher cephalic index value (shorter headed dogs) were quicker to make eye contact with the human than dogs with a lower cephalic index value (longer headed dogs). This finding is consistent with those of Gácsi et al.13 and Bognár et al.14 who compared the typical dog head shape groups (see Fig. 1) and found that brachycephalic (short-headed) dogs are more successful at following human pointing gestures13 and pay more attention to projected faces14 than dolichocephalic (long-headed) dogs. This may be due to differences in the retinal ganglion cell distribution and thus shorter headed dogs’ better visual acuity10. Consequently, shorter headed dogs are more attentive to people, which may make them appear more social and easier to interact with. This might in turn explain the explosion in the popularity of these breeds (such as the Pug52 and French bulldog53 etc.). The findings of McGreevy et al.39 and Stone et al.16 are in line with this idea, as they reported that brachycephalic dogs are more friendly, interactive and cooperative with unfamiliar humans and stranger-directed fear is most common in dolichocephalic dogs. Another factor which can explain the increasing popularity of brachycephalic breeds is the “baby schema effect”, as mammals have a preference for paedomorphic faces54–56. The term “baby schema” refers to a set of facial features (i.e. large head and a round face, large eyes etc.), which elicits the so-called “cute response”, an increased attention and willingness to care for individuals with infantile features56. The characteristics of brachycephalic dogs’ heads are in accordance with the baby schema features, thus the owners of these dogs may pay more attention towards them and are more likely to engage in mutual gaze with their animals. Therefore, these dogs may have more opportunity to learn to engage with humans and make eye-contact with them. It is also possible that selection for brachycephaly has been accompanied by selection for seeking eye-contact with humans. Therefore, the underlying causes of the association between the propensity to form eye contact with humans and the head shape of dogs needs to be further tested. It is worth noting that head shape is also linked to brain size, namely that cephalic index negatively correlates with estimated brain weight57, which might also influence the behaviour and cognition of these breeds. Further studies regarding the relationship between cephalic index, brain size and dog cognition would be desirable. An important question for further research is whether shorter headed dogs' greater visual acuity is correlated with any differences in the brain processes underlying attention.
This study also supports our assumption, that the cephalic index scale can be examined individually and as a continuous variable, without the need to form arbitrary head shape groups. Thus, we suggest that further research on the effect of dogs' head shape consider cephalic index as a continuous variable and measure it at the individual level, since this could prove more informative.
We also found that breed function affects dogs’ latency to form eye contact. As expected, and supporting previous findings13,18, non-cooperative breeds were slower in the test, and less prone to form eye contact with the experimenter. This suggests that selective breeding for working in visual contact with humans still has a measurable effect on dog behaviour. Surprisingly, there were no differences between cooperative breeds and mixed breed dogs. We expected that the performance of mixed breed dogs would be similar to non-cooperative breeds. It is possible that we have tested more mixed breed dogs which were mixes of cooperative breeds than non-cooperative breeds. However, in the current study we could not test this, as the breed makeup of the mixed breed dogs in our sample was unknown. Alternatively, as a consequence of domestication, sensitivity to human visual communication was typical in ancient dogs, since early humans likely bred only those individuals which showed more human-like skills58, and during modern purebred dogs' intensive selective breeding process—which started 200–300 years ago59–62—the non-cooperative breeds lost their propensity as they were bred for working independently from the human partner. However, this hypothesis is in conflict with the observation that ancient breeds (i.e. dogs with similar genetic signatures to wolves) were reported to be less prone to form eye contact with humans19. In contrast, previous studies suggested that there might be a different, indirect selection force on mixed breed dogs compared to purebreds13,63, as they mostly live and reproduce on their own, without an owner, but still have to rely on humans for resources. Mixed breed dogs living on street with a propensity to form eye contact with humans might have higher fitness, as people may favour such dogs and give them food more readily, which can be crucial to survive on streets. Comparing mixed breed dogs with different experience with humans, study found that street dogs did not differ from mixed breed pet dogs in the propensity to look at an unfamiliar human64. Although we have no information about the life experiences or lineages of the mixed breed dogs in our sample, at least 68% of them were adopted from an animal shelter or found on the street. Shelter dogs may have lived for years as pets, but most of the dogs in animal shelters come from the streets. They have therefore adjusted to eating what they find or what they can beg from humans.
Age had a negative effect on performance in all three tests. These results are consistent with other studies in dogs reporting an age-related decrease in dogs’ social attention27,31, higher touch sensitivity, and lower companionability and playfulness65–67. Our study supports the age-dependent decline in attention and sociability among healthy pet dogs in general, even when considering the dogs’ breed history and head morphology. There are, however, other possible explanations: Older dogs play less, because they may have joint pain or other physical pain which makes playing uncomfortable for them. Owners tend to stop playing with their older dogs68, which could also cause decreased playfulness. Older dogs may have a smaller learning ability than younger dogs. Although the differences in learning speed is unlikely to cause the differences found in latency to form eye contact, because dogs were able to improve their performance over the test regardless their age. Older dogs may have deficiencies in attentional control and to ignore distracting information (food on the ground or the experimenter's hand, which giving the food), as suggested by previous research27,31,69.
From our Sociability tests, only playfulness showed a connection with dogs' propensity to form eye contact with the experimenter. We found that the more playful dogs formed eye contact faster with an unfamiliar human, which is consistent with the findings of Jakovcevic et al.37. Their sociability test was performed in the absence of the owner and they differentiated more sociable dogs from less sociable ones by measuring the amount of time the dog spent close to the experimenter. The only measured interactions between the dog and the experimenter were petting and talking. In spite of these differences, our findings are very similar to Jakovcevic et al.'s, suggesting that we observed different expressions of the same relationship in dogs—a positive association between sociability and visual communication with humans.
One major limitation in this study is that we had no data about the animals’ prior experience with the task-relevant behaviour; whether and to what extent the owner trained the dog to form and maintain eye contact with him/her. We only had data on dogs’ previous dog school attendance (yes/no), which had no significant effect on the latency to form eye contact with the experimenter in our test. In previous studies, where dogs' full training history was considered, different results were found. They calculated each dog’s training score from a full training history reported by the owner24,27. Training score had no significant effect on latency to form eye contact with the experimenter in Border collies27. When testing in a sample with more breeds, it was found that dogs with a higher training score established eye contact sooner24, but the latter study did not consider the dogs' breed function or head shape. To develop a full picture of the connection between head shape, breed function and dogs' propensity to form eye contact with humans, additional studies will be needed that also take into account the dogs' full training history.
This study did not investigate whether differences between individuals in forming eye contact with an unfamiliar person are also predictive of interactions with the owner. Dogs pay more attention to their owner than to unfamiliar individuals69,70. But see Kubinyi et al. (submitted) for an opposite result. Familiarity can affect different dog breeds’ attention to humans in varied ways20. In Maglieri et al.20 German shepherds looked longer at their owner, Czechoslovakian wolfdogs looked longer at the experimenter, while Labrador retrievers paid equal attention to familiar and unfamiliar persons. Kubinyi et al. (submitted) found that dogs can be assigned to four groups based on the frequency of looking at the owner and looking at the experimenter in ambiguous situations. The frequency of looking at the owner and the experimenter correlated (positively) only in one group. Therefore, dogs that are quick to look at an unfamiliar person, may not necessarily respond in the same way to their owner, and vice versa.
In sum, our study suggests that selective breeding for head morphology and different functions impacted dogs’ attention to humans, and consequently their visual communicative abilities. Besides these major effects, individual characteristics, including age and playfulness also influenced the propensity of dogs to form eye contact with an unfamiliar human. We can expect the greatest propensity from short headed dogs, which belong to a visually cooperative breed, that are also young and act playfully with strangers. Since the multicollinearity for the examined predictors was low, the sample groups must be balanced for all of these factors in future studies which investigate dogs’ attention and visual communication abilities.
Supplementary Information
Acknowledgements
This project has received funding from the ÚNKP-19-2 New National Excellence Program of the Ministry for Innovation and Technology and the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (Grant Agreement No. 680040). First of all, we would like to thank the 130 dog-owner dyads for their participation in our research. Second, we thank all our colleagues for their work, namely: Bianka Stiegmann, Anna Egerer, Sarolta Marosi, Vivien Hemző, and Renáta Böröczki for their help with the data collection; Lisa J. Wallis for her help in the development of the tests and for English proofreading; Tamás Faragó for his help in statistical analysis; Eda Köseli for her help with cephalic index’s interobserver coding; Felícia Erdélyi for her help with playfulness’ interobserver coding; Andrea Sommese, Attila Salamon, Ivaylo B. Iotchev, Vivien Reicher, Lisa J. Wallis, Borbála Turcsán and Tamás Faragó for their comments on a previous version of this manuscript.
Author contributions
Z.B., D.S., E.K. designed the study; Z.B., D.S., A.D. collected the data; Z.B. coded the videos, analysed the data, and wrote the first draft and all authors participated in editing the manuscript.
Data availability
All raw data are available as Supplementary material.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-88702-w.
References
- 1.Gomez, J. C. Ostensive behavior in great apes: The role of eye contact. in Reaching into thought: The minds of the great apes 131–151 (1996).
- 2.Kaminski J, Schulz L, Tomasello M. How dogs know when communication is intended for them. Dev. Sci. 2012;15:222–232. doi: 10.1111/j.1467-7687.2011.01120.x. [DOI] [PubMed] [Google Scholar]
- 3.Téglás E, Gergely A, Kupán K, Miklósi Á, Topál J. Dogs’ gaze following is tuned to human communicative signals. Curr. Biol. 2012;22:209–212. doi: 10.1016/j.cub.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 4.Emery NJ. The eyes have it: The neuroethology, function and evolution of social gaze. Neurosci. Biobehav. Rev. 2000 doi: 10.1016/S0149-7634(00)00025-7. [DOI] [PubMed] [Google Scholar]
- 5.Nagasawa M, et al. Oxytocin-gaze positive loop and the coevolution of human-dog bonds. Science (80-) 2015;348:333–336. doi: 10.1126/science.1261022. [DOI] [PubMed] [Google Scholar]
- 6.Drake AG, Klingenberg CP. Large-scale diversification of skull shape in domestic dogs: Disparity and modularity. Am. Nat. 2010;175:289–301. doi: 10.1086/650372. [DOI] [PubMed] [Google Scholar]
- 7.Hussein AK, Sullivan M, Penderis J. Effect of brachycephalic, mesaticephalic, and dolichocephalic head conformations on olfactory bulb angle and orientation in dogs as determined by use of in vivo magnetic resonance imaging. Am. J. Vet. Res. 2012 doi: 10.2460/ajvr.73.7.946. [DOI] [PubMed] [Google Scholar]
- 8.Polgár Z, Kinnunen M, Újváry D, Miklósi Á, Gácsi M. A test of canine olfactory capacity: Comparing various dog breeds and wolves in a natural detection task. PLoS ONE. 2016 doi: 10.1371/journal.pone.0154087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czeibert K, Sommese A, Petneházy Ö, Csörgő T, Kubinyi E. Digital endocasting in comparative canine brain morphology. Front. Vet. Sci. 2020;7:749. doi: 10.3389/fvets.2020.565315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGreevy P, Grassi TD, Harman AM. A strong correlation exists between the distribution of retinal ganglion cells and nose length in the dog. Brain. Behav. Evol. 2004;63:13–22. doi: 10.1159/000073756. [DOI] [PubMed] [Google Scholar]
- 11.Roberts T, McGreevy P, Valenzuela M. Human induced rotation and reorganization of the brain of domestic dogs. PLoS ONE. 2010;5:e11946. doi: 10.1371/journal.pone.0011946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans HE, De Lahunta A. Miller’s Anatomy of the Dog. Elsevier; 2013. [Google Scholar]
- 13.Gácsi M, McGreevy P, Kara E, Miklósi Á. Effects of selection for cooperation and attention in dogs. Behav. Brain Funct. 2009;5:31. doi: 10.1186/1744-9081-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bognár Z, Iotchev IB, Kubinyi E. Sex, skull length, breed, and age predict how dogs look at faces of humans and conspecifics. Anim. Cogn. 2018;21:447–456. doi: 10.1007/s10071-018-1180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Georgevsky D, Carrasco JJ, Valenzuela M, McGreevy P. Domestic dog skull diversity across breeds, breed groupings, and genetic clusters. J. Vet. Behav. 2014;9:228–234. doi: 10.1016/j.jveb.2014.04.007. [DOI] [Google Scholar]
- 16.Stone HR, McGreevy P, Starling MJ, Forkman B. Associations between domestic-dog morphology and behaviour scores in the dog mentality assessment. PLoS ONE. 2016;11:e0149403. doi: 10.1371/journal.pone.0149403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jakovcevic A, Elgier AM, Mustaca AE, Bentosela M. Breed differences in dogs’ (Canis familiaris) gaze to the human face. Behav. Processes. 2010;84:602–607. doi: 10.1016/j.beproc.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Passalacqua C, et al. Human-directed gazing behaviour in puppies and adult dogs Canis lupus familiaris. Anim. Behav. 2011;82:1043–1050. doi: 10.1016/j.anbehav.2011.07.039. [DOI] [Google Scholar]
- 19.Konno A, Romero T, Inoue-Murayama M, Saito A, Hasegawa T. Dog breed differences in visual communication with humans. PLoS ONE. 2016;11:e0164760. doi: 10.1371/journal.pone.0164760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maglieri V, Prato-Previde E, Tommasi E, Palagi E. Wolf-like or dog-like? A comparison of gazing behaviour across three dog breeds tested in their familiar environments. R. Soc. Open Sci. 2019;6:190946. doi: 10.1098/rsos.190946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wobber V, Hare B, Koler-Matznick J, Tomasello M, Wrangham R. Breed differences in domestic dogs’ (Canis familiaris) comprehension of human communicative signals. Interact. Stud. 2009;10:206–224. doi: 10.1075/is.10.2.06wob. [DOI] [Google Scholar]
- 22.Madden DJ. Aging and visual attention. Curr. Dir. Psychol. Sci. 2007;16:70–74. doi: 10.1111/j.1467-8721.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erel H, Levy DA. Orienting of visual attention in aging. Neurosci. Biobehav. Rev. 2016;69:357–380. doi: 10.1016/j.neubiorev.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Chapagain D, et al. Aging of attentiveness in border collies and other pet dog breeds: the protective benefits of lifelong training. Front. Aging Neurosci. 2017;9:100. doi: 10.3389/fnagi.2017.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol. Rev. 1996;103:403–428. doi: 10.1037/0033-295X.103.3.403. [DOI] [PubMed] [Google Scholar]
- 26.Habekost T, et al. Visual processing speed in old age. Scand. J. Psychol. 2013;54:89–94. doi: 10.1111/sjop.12008. [DOI] [PubMed] [Google Scholar]
- 27.Wallis LJ, et al. Lifespan development of attentiveness in domestic dogs: Drawing parallels with humans. Front. Psychol. 2014;5:1–13. doi: 10.3389/fpsyg.2014.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azkona G, et al. Prevalence and risk factors of behavioural changes associated with age-related cognitive impairment in geriatric dogs: PAPER. J. Small Anim. Pract. 2009 doi: 10.1111/j.1748-5827.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 29.Rosado B, et al. Effect of age and severity of cognitive dysfunction on spontaneous activity in pet dogs—Part 2: Social responsiveness. Vet. J. 2012;194:196–201. doi: 10.1016/j.tvjl.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 30.Neilson JC, Hart BL, Cliff KD, Ruehl WW. Prevalence of behavioral changes associated with age-related cognitive impairment in dogs. J. Am. Vet. Med. Assoc. 2001;218:1787–1791. doi: 10.2460/javma.2001.218.1787. [DOI] [PubMed] [Google Scholar]
- 31.Snigdha S, et al. Age and distraction are determinants of performance on a novel visual search task in aged Beagle dogs. Age (Omaha) 2012 doi: 10.1007/s11357-011-9219-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Owsley C, Sekuler R, Boldt C. Aging and low-contrast vision: Face perception. Investig. Ophthalmol. Vis. Sci. 1981;21:362–365. [PubMed] [Google Scholar]
- 33.Chan YM, Pianta MJ, McKendrick AM. Older age results in difficulties separating auditory and visual signals in time. J. Vis. 2014;14:13–13. doi: 10.1167/14.11.13. [DOI] [PubMed] [Google Scholar]
- 34.Chan YM, Battista J, McKendrick AM. Aging effects on collinear facilitation. J. Vis. 2012;12:21–21. doi: 10.1167/12.6.21. [DOI] [PubMed] [Google Scholar]
- 35.Thomas C, et al. Reduction in white matter connectivity, revealed by diffusion tensor imaging, may account for age-related changes in face perception. J. Cogn. Neurosci. 2008;20:268–284. doi: 10.1162/jocn.2008.20025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boutet I, Faubert J. Recognition of faces and complex objects in younger and older adults. Mem. Cognit. 2006;34:854–864. doi: 10.3758/BF03193432. [DOI] [PubMed] [Google Scholar]
- 37.Jakovcevic A, Mustaca AE, Bentosela M. Do more sociable dogs gaze longer to the human face than less sociable ones? Behav. Process. 2012 doi: 10.1016/j.beproc.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 38.Svartberg K, Forkman B. Personality traits in the domestic dog (Canis familiaris) Appl. Anim. Behav. Sci. 2002 doi: 10.1016/S0168-1591(02)00121-1. [DOI] [Google Scholar]
- 39.McGreevy P, et al. Dog behavior co-varies with height, bodyweight and skull shape. PLoS ONE. 2013;8:e80529. doi: 10.1371/journal.pone.0080529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bognár Z, Piotti P, Szabó D, Le Nézet L, Kubinyi E. A novel behavioural approach to assess responsiveness to auditory and visual stimuli before cognitive testing in family dogs. Appl. Anim. Behav. Sci. 2020 doi: 10.1016/j.applanim.2020.105016. [DOI] [Google Scholar]
- 41.R Core Team. R: A Language and Environment for Statistical Computing. (2020).
- 42.RStudio Team. RStudio: Integrated Development for R. RStudio. (2018).
- 43.Jahn-Eimermacher A, Lasarzik I, Raber J. Statistical analysis of latency outcomes in behavioral experiments. Behav. Brain Res. 2011;221:271–275. doi: 10.1016/j.bbr.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Therneau, T. M. coxme: Mixed Effects Cox Models. (2018).
- 45.Lenth, R. emmeans: Estimated Marginal Means, aka Least-Squares Means. (2019).
- 46.Barton, K. MuMIn: Multi-MODEL INFErence. (2019).
- 47.Fox, J. & Weisberg, S. An {R} Companion to Applied Regression. (2019).
- 48.Therneau, T. M. A Package for Survival Analysis in S. Version 2.38. CRAN website—https://cran.r-project.org/package=survival. (2015).
- 49.Kassambara, A., Kosinski, M., Biecek, P. & Fabian, S. survminer: Drawing Survival Curves using ‘ggplot2’ (R package). version 0.4.3 (2018).
- 50.Wickham, H. ggplot2: Elegant Graphics for Data Analysis. (2016).
- 51.Freepik. https://www.freepik.com/.
- 52.The Kennel Club. 10-yearly breed statistics (Toy group). https://www.thekennelclub.org.uk/media/2399/10yrstatstoy.pdf.
- 53.The Kennel Club. 10-yearly breed statistics (Utility group). https://www.thekennelclub.org.uk/media/2400/10yrstatsutility.pdf.
- 54.Sternglanz SH, Gray JL, Murakami M. Adult preferences for infantile facial features: An ethological approach. Anim. Behav. 1977;25:108–115. doi: 10.1016/0003-3472(77)90072-0. [DOI] [PubMed] [Google Scholar]
- 55.Hecht J, Horowitz A. Seeing dogs: Human preferences for dog physical attributes. Anthrozoos. 2015;28:153–163. doi: 10.2752/089279315X14129350722217. [DOI] [Google Scholar]
- 56.Lorenz K. Die angeborenen Formen möglicher Erfahrung. Z. Tierpsychol. 1943;5:235–409. doi: 10.1111/j.1439-0310.1943.tb00655.x. [DOI] [Google Scholar]
- 57.Horschler DJ, et al. Absolute brain size predicts dog breed differences in executive function. Anim. Cogn. 2019;22:187–198. doi: 10.1007/s10071-018-01234-1. [DOI] [PubMed] [Google Scholar]
- 58.Miklósi Á, Topál J. What does it take to become ‘best friends’? Evolutionary changes in canine social competence. Trends Cogn. Sci. 2013 doi: 10.1016/j.tics.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 59.Parker HG, et al. Genetic structure of the purebred domestic domestic dog. Science (80-) 2004;304:1160–1164. doi: 10.1126/science.1097406. [DOI] [PubMed] [Google Scholar]
- 60.Pollinger JP. Selective sweep mapping of genes with large phenotypic effects. Genome Res. 2005;15:1809–1819. doi: 10.1101/gr.4374505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith BP, et al. Taxonomic status of the Australian dingo: The case for Canis dingo Meyer, 1793. Zootaxa. 2019;4564:173. doi: 10.11646/zootaxa.4564.1.6. [DOI] [PubMed] [Google Scholar]
- 62.vonHoldt BM, et al. Genome-wide SNP and haplotype analyses reveal a rich history underlying dog domestication. Nature. 2010;464:898–902. doi: 10.1038/nature08837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Turcsán B, Miklósi Á, Kubinyi E. Owner perceived differences between mixed-breed and purebred dogs. PLoS ONE. 2017;12:e0172720. doi: 10.1371/journal.pone.0172720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marshall-Pescini S, Rao A, Virányi Z, Range F. The role of domestication and experience in ‘looking back’ towards humans in an unsolvable task. Sci. Rep. 2017;7:46636. doi: 10.1038/srep46636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Henriksson J. Scores on dog personality are dependent on questionnaire: A comparison of three questionnaires. Linköping University; 2016. [Google Scholar]
- 66.Wallis LJ, Szabó D, Kubinyi E. Cross-sectional age differences in canine personality traits; influence of breed, sex, previous trauma, and dog obedience tasks. Front. Vet. Sci. 2020;6:493. doi: 10.3389/fvets.2019.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garamszegi LZ, Temrin H, Kubinyi E, Miklósi Á, Kolm N. The role of common ancestry and gene flow in the evolution of human-directed play behaviour in dogs. J. Evol. Biol. 2019 doi: 10.1111/jeb.13567. [DOI] [PubMed] [Google Scholar]
- 68.Wallis LJ, Szabó D, Erdélyi-Belle B, Kubinyi E. Demographic change across the lifespan of pet dogs and their impact on health status. Front. Vet. Sci. 2018;5:200. doi: 10.3389/fvets.2018.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mongillo P, Bono G, Regolin L, Marinelli L. Selective attention to humans in companion dogs Canis familiaris. Anim. Behav. 2010;80:1057–1063. doi: 10.1016/j.anbehav.2010.09.014. [DOI] [Google Scholar]
- 70.Horn L, Range F, Huber L. Dogs’ attention towards humans depends on their relationship, not only on social familiarity. Anim. Cogn. 2013;16:435–443. doi: 10.1007/s10071-012-0584-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw data are available as Supplementary material.