Abstract
Background
Clindamycin is strongly recommended as an adjunctive treatment to β-lactam antibiotics in patients with severe invasive group A β-haemolytic streptococcal (iGAS) infections. However, there is little evidence of a benefit in the use of clindamycin in humans, and its role, if any, in treating patients with invasive non-group A/B β-haemolytic streptococcal (iNABS) infections is unclear.
Methods
For this retrospective multicentre cohort study, we used a dataset from patients in the Cerner Health Facts database, which contains electronic health-based data from 233 US hospitals. We queried the Cerner Health Facts database for inpatients (no age restriction) admitted to hospital in 2000–15, with any clinical cultures positive for β-haemolytic streptococcal taxa of interest, and who had received β-lactam antibiotics within 3 days either side of culture sampling. This group of patients was then queried for those who had also received intravenous or oral clindamycin within 3 days either side of culture sampling. Patients were excluded if they had polymicrobial growth or clindamycin non-susceptible isolates, received linezolid, or had missing variable data needed for analysis. Patients were categorised by Lancefield group (iGAS or iNABS); β-lactam antibiotic-treated patients who had received clindamycin were propensity-matched (1:2) to those who did not receive clindamycin separately for iGAS and iNABS cohorts, and logistic regression was then used to account for residual confounding factors. The primary outcome was the adjusted odds ratio (aOR) of in-hospital mortality in propensity-matched patients treated with adjunctive clindamycin versus those not treated with clindamycin in the iGAS and iNABS infection cohorts.
Findings
We identified 1956 inpatients with invasive β-haemolytic streptococcal infection who had been treated with β-lactam antibiotics across 118 hospitals (1079 with iGAS infections and 877 with iNABS infections). 459 (23·4%) of these patients had received adjunctive clindamycin treatment (343 [31·7%] patients with iGAS infections and 116 [13·2%] patients with iNABS infections). The effect of adjunctive clindamycin therapy on in-hospital mortality differed significantly and showed the opposite trend in iGAS and iNABS infection cohorts (p=0·013 for an interaction). In the iGAS cohort, in-hospital mortality in propensity-matched patients who received adjunctive clindamycin (18 [6·5%] of 277 patients) was significantly lower than in those who did not (55 [11·0%] of 500 patients; aOR 0·44 [95% CI 0·23–0·81]). This survival benefit was maintained even in patients without shock or necrotising fasciitis (six [2·6%] of 239 patients treated with adjunctive clindamycin vs 27 [6·1%] of 422 patients not treated with adjunctive clindamycin; aOR 0·40 [0·15–0·91]). By contrast, in the iNABS infection cohort, in-hospital mortality in propensity-matched patients who received adjunctive clindamycin (ten [9·8%] of 102) was higher than in those who did not (nine [4·6%] of 193), but this difference was not significant (aOR 2·60 [0·94–7·52]). Several subset analyses found qualitatively similar results.
Interpretation
Real-world data suggest that increased use of adjunctive clindamycin for invasive iGAS infections, but not iNABS infections, could improve outcomes, even in patients without shock or necrotising fasciitis.
Funding
Intramural Research Program of the National Institutes of Health Clinical Center and the National Institute of Allergy and Infectious Disease.
Introduction
Group A streptococcus is a leading cause of invasive bacterial disease worldwide, with over half a million cases occurring each year.1 Despite advances in supportive care, source control, and antibiotics, mortality from invasive group A streptococcal infections remains high (ie, mortality in people with streptococcal toxic shock syndrome is up to 59%2), prompting interest in adjunctive interventions. Although intravenous immunoglobulin has been shown to neutralise superantigens and enhance bacterial clearance,3 this treatment has not been shown to be convincingly beneficial as an adjunct, and its widespread use has been limited by cost, batch-to-batch variability in neutralising potency, and accessibility.4,5
Clindamycin, a protein synthesis inhibitor with activity during the stationary phase of bacterial growth, has been shown to decrease the expression and production of group A streptococcal virulence factors and exotoxins.6 However, despite the unique anti-streptococcal properties of clindamycin shown in in vitro and animal models,6 proof of its effectiveness in humans has been hampered by small sample sizes and low quality clinical evidence. Observational studies that have suggested benefit in patients with necrotising fasciitis,7 streptococcal toxic shock syndrome,8 and any invasive group A streptococcal infections9 have not accounted for confounding by indication (ie, the selective use of clindamycin).8 In other observational studies, clindamycin was either not retained in multivariable models,10–12 or when it was retained, either failed to show a significant survival benefit13 or was not assessed separately from other protein-synthesis inhibitors.14 Nonetheless, guidelines currently recommend adding clindamycin to β-lactam antibiotic therapy in patients with invasive group A streptococcal infection, but our understanding of the real-world use and effectiveness of clindamycin for this indication is unclear.15,16
Although long considered components of normal commensal flora,17 non-group A β-haemolytic streptococci—namely, Lancefield groups B, C, and G, are increasingly recognised as a cause of invasive β-haemolytic streptococcal infections,9,18,19 even surpassing group A streptococci as a leading cause in some locations,18 with a large proportion speciating to Streptococcus dysgalactiae subspecies equisimilis.20 Despite some overlap in the clinical disease spectrum, compared with group A streptococci, non-group A streptococci typically do not possess a full repertoire of group A streptococcal virulence genes, including superanti gens,21,22 and are therefore less closely associated with streptococcal toxic shock syndrome and necrotising fasciitis, and also have lower mortality rates.9,19 Although the use of adjunctive clindamycin in invasive non-group A β-haemolytic streptococcal infections has been extrapolated from experience with group A streptococcal infections, this practice has neither been systemically examined9 nor recommended in guidelines. In this study, we used a large US-based electronic health record data repository to examine real-world use patterns of adjunctive clindamycin, and the association of this treatment with in-hospital mortality and hospital length of stay among patients with invasive group A β-haemolytic streptococcal (iGAS) infections and invasive non-group A /B β-haemolytic streptococcal (iNABS) infections, who had already received β-lactam antibiotics.
Methods
Data source and study population
For this retrospective multicentre cohort study, we used a dataset from patients in the Cerner Health Facts database (operated by Cerner Corporation, Kansas City, MO, USA), which contains de-identified electronic health record-based data from 233 US hospitals.
We queried the Cerner Health Facts database for inpatients (no age restriction) admitted to hospital in 2000–15 with any clinical cultures (ie, not surveillance cultures, such as nasal or rectal swabs) positive for β-haemolytic streptococci taxa of interest (appendix p 3) and who had received one or more doses of β-lactam antibiotic within 3 days either side of culture sampling, thus increasing the likelihood of capturing true infections (appendix p 12). By use of a specified random generator seed, one randomly selected encounter per patient was included. The group of patients identified was then queried for those who had also received intravenous or oral clindamycin within 3 days either side of culture sampling. Patients were excluded if they had polymicrobial growth, had clindamycin non-susceptible isolates, had received linezolid (another protein synthesis inhibitor with potential toxin-inhibiting pro perties),23 or had missing variable data needed for analysis (figure 1). Preliminary exploration of the Cerner Health Facts database revealed that the use of adjunctive clindamycin in patients with group B β-haemolytic streptococcal infections (544 [6·6%] of 8270) was relatively low when compared with those who had iGAS infections (643 [28·6%] of 2251) and iNABS infections (310 [12·2%] of 2537), which led us to exclude patients with invasive group B β-haemolytic streptococcal infections from analyses of clindamycin efficacy, as has been done previously.9 As such, our study focused on iGAS and iNABS infections.
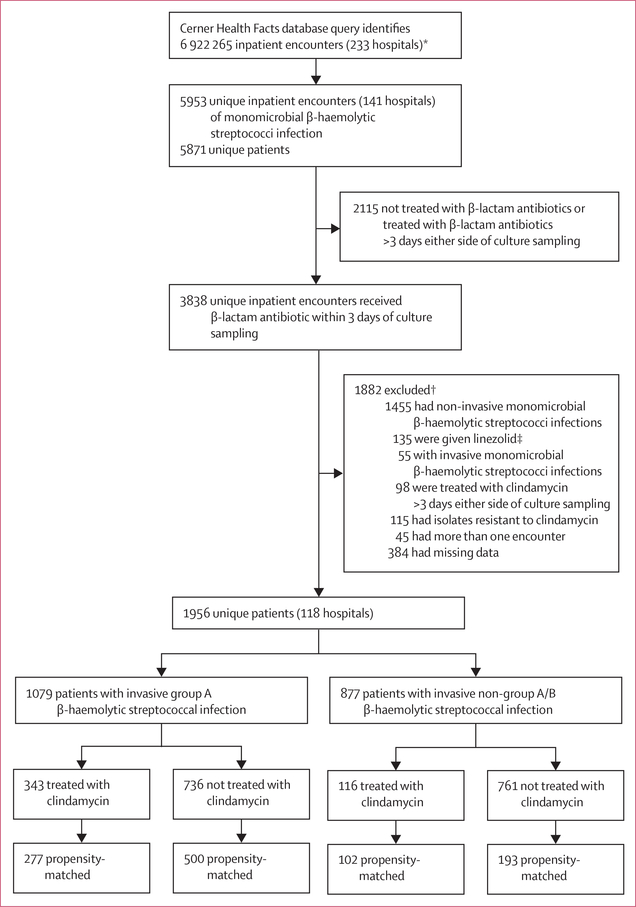
Figure 1. Selection of patients with invasive β-haemolytic streptococcal infections.
*The database was queried for inpatents (aged ≥18 years) with any clinical culture samples displaying monomicrobial growth of select β-haemolytic species (appendix p 3), filtered on the basis of receiving β-lactam antibiotics within 3 days either side of culture sampling with or without clindamycin treatment. †Patients could have met more than one exclusion criterion. ‡No patients were treated with tedizolid.
The National Institute of Health Office of Human Subjects Research waived the need for institutional review board evaluation for this study.
Procedures
Data from centres that reported on billing codes, laboratory and medication orders, and microbiology records were used for analysis. The Cerner Health Facts dataset was leased by the authors following a data use agreement with the Cerner Corporation.
Proven invasive β-haemolytic streptococcal infection was defined as the isolation of β-haemolytic streptococcus from normally sterile body sites, or, as per adaption of US Centers of Diseases Control and Prevention definitions for iGAS,24 isolation of β-haemolytic strepto coccus from a deep wound in patients with International Classification of Diseases version 9 (ICD-9)-coded streptococcal toxic shock syndrome (040.82) or necrotising fasciitis (728·86 or 0·40).24 Probable invasive β-haemolytic streptococcal infection was defined as the isolation of β-haemolytic streptococcus from a non-sterile site in patients with ICD-9-coded lower respiratory, skin, soft tissue and musculoskeletal, or other deep-seated infections.
Statistical analysis
Given differences in virulence factors, the propensity for invasive disease, and associated mortality, iGAS and iNABS infection cohorts were analysed separately. Additionally, preliminary data analyses revealed a significant interaction between Lancefield group status and the effect of adjunctive clindamycin on mortality (p=0·0127), further supporting the decision to analyse these two cohorts separately. β-lactam antibiotic-treated patients who received adjunctive clindamycin were propensity-matched (1:2) to those who did not receive adjunctive clindamycin by propensity of receiving clindamycin by use of the nearest-neighbour method and a 20% caliper for the standard deviation of the logit of the observed propensity score. All variables presented in the table, except for specific β-lactam antibiotic treatment received, were included in the model. Age, Sequential Organ Failure Assessment (SOFA), and Elixhauser Comorbidity Index scores were included as continuous variables. To ensure covariate balance on variables that were considered highly predictive of mortality, patients were exact-matched on proven invasive β-haemolytic streptococcal infection status, vasopressor use, intensive care unit (ICU) status, and presence of necrotising fasciitis. Good covariate balance was verified by assessment of absolute standardised mean differences, with a value of less than 0·1 used as an indicator of reasonable balance for all the post-matching variables.26 Q–Q plots of each covariate from the adjunctive clindamycin-treated and adjunctive clindamycin untreated groups, before and after propensity score matching, were assessed for visual evidence of a reduction in imbalance. To mitigate possible residual confounding factors, logistic regression was done downstream of propensity matching to control for proven invasive disease, vasopressor use, and ICU status; model fit precluded inclusion of additional variables in the downstream regression model. For the baseline characteristics of patients, the Wilcoxon rank-sum test was used to compare continuous variables and the χ2 test was used to compare categorical variables. For the propensity-matched data, Friedman’s test was used to compare continuous variables and the Cochran-Mantel-Haenszel test was used to compare categorical variables among treatment groups.
Table:
Baseline characteristics of all patients with invasive β-haemolytic streptococcal infections treated with β-lactam antibiotics
| Unmatched cohort |
Propensity-matched cohort |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Invasive group A β-haemolytic streptococcal infection (n=1079) |
Invasive non-group A/B β-haemolytic streptococcal infection (n=877) |
Invasive group A β-haemolytic streptococcal infection (n=777) |
Invasive non-group A/B β-haemolytic streptococcal infection (n=295) |
|||||||||
| No clindamycin treatment (n=736) | Clindamycin treatment (n=343) | p value | No clindamycin treatment (n=761) | Clindamycin treatment (n=116) | p value | No clindamycin treatment (n=500) | Clindamycin treatment (n=277) | p value | No clindamycin treatment (n=193) | Clindamycin treatment (n=102) | p value | |
| Patient-related | ||||||||||||
| Age, years | 51 (34–67) | 46 (27–62) | 0.0008 | 60 (46–74) | 54 (42–68) | 0.023 | 48 (30–63) | 47 (26–64) | 0.91 | 55 (41–67) | 59 (43–69) | 0.32 |
| Sex | ||||||||||||
| Male | 423 (575%) | 200 (58.3%) | 0.85 | 518 (68.1%) | 65 (56.0%) | 0.014 | 292 (58.4%) | 160 (57.8%) | 0.81 | 124 (64.2%) | 60 (58.8%) | 0.54 |
| Female | 313 (42.5%) | 143 (42.7%) | 0.85 | 243 (32.9%) | 51 (44.0%) | 0.014 | 208 (42.6%) | 117 (42.2%) | 0.81 | 69 (36.8%) | 42 (41.2%) | 0.54 |
| White | 553 (757%) | 233 (67.9%) | 0.016 | 590 (77.5) | 86 (74.1%) | 0.50 | 359 (778) | 183 (66.1%) | 0.31 | 150 (77.7%) | 75 (73.5%) | 0.37 |
| Elixhauser Comorbidity Index* | 2.0 (0.0–3.0) | 2.0 (0.0–3.0) | 0.18 | 3.0 (1.0–4.0) | 2.5 (1.0–4.0) | 0.40 | 2.0 (0.0–3.0) | 1.0 (0.0–3.0) | 0.50 | 2.0 (1.0–4.0) | 3.0 (1.0–4.0) | 0.55 |
| Immunocompromised† | 58 (7.9%) | 27 (7.9%) | 1.00 | 60 (7.9%) | 12 (10.3%) | 0.47 | 39 (78%) | 22 (7.9%) | 0.93 | 13 (6.7%) | 7 (6.9%) | 1.00 |
| Obese (body-mass index >30 kg/m2)‡ | 60 (8.2%) | 32 (9.3%) | 0.60 | 117 (15.4%) | 27 (23.3%) | 0.045 | 41 (8.2%) | 24 (87%) | 0.89 | 45 (23.3%) | 25 (24.5%) | 0.96 |
| Baseline Sequential Organ Failure Assessment score§ | 1.0 (0.0–3.0) | 1.0 (0.0–3.0) | 0.0090 | 1.0 (0.0–3.0) | 1.0 (0.0–2.0) | 0.42 | 1.0 (0.0–3.0) | 1.0 (0.0–3.0) | 0.29 | 1.0 (0.0–3.0) | 1.0 (0.0–2.0) | 0.89 |
| Infection-related | ||||||||||||
| Proven invasive β-haemolytic streptococcal disease | 398 (54.1%) | 191 (55.7%) | 0.67 | 430 (56.5%) | 66 (56.9%) | 1.00 | 282 (56.4%) | 153 (557%) | 1.00 | 106 (54.9%) | 57 (55.9%) | 1.00 |
| Community-onset | 707 (96.1%) | 329 (95.9%) | 1.00 | 704 (92.5%) | 108 (93.1%) | 0.97 | 483 (96.6%) | 267 (96.4%) | 0.84 | 180 (93.3%) | 96 (94.1%) | 0.87 |
| Site | .. | .. | 0.0020 | .. | .. | 0.27 | .. | .. | 0.69 | .. | .. | 0.69 |
| Musculoskeletal, skin, or soft tissue | 275 (37.4%) | 141(41.1%) | .. | 225 (29.6%) | 36 (31.0%) | .. | 202 (40.4%) | 115 (41.5%) | .. | 60 (31.1%) | 32 (31.4%) | .. |
| Respiratory | 82 (11.1%) | 15 (4.4%) | .. | 130 (17.1%) | 17 (14.7%) | .. | 24 (4.8%) | 12 (4.3%) | .. | 33 (17.1%) | 15 (14.7%) | .. |
| Other deep-seated site¶ | 22 (3.0%) | 11 (3.2%) | .. | 28 (37%) | 5 (4.3%) | .. | 17 (3.4%) | 9 (3.2%) | .. | 9 (4.7%) | 5 (4.9%) | .. |
| Primary bacteraemia | 102 (13.9%) | 36 (10.5%) | .. | 102 (13.4%) | 8 (6.9%) | .. | 68 (13.6%) | 32 (11.6%) | .. | 20 (10.4%) | 7 (6.9%) | .. |
| Secondary bacteraemia | 255 (34.6%) | 140 (40.8%) | .. | 276 (36.3%) | 50 (43.1%) | .. | 189 (378%) | 109 (39.4%) | .. | 71 (36.8%) | 43 (42.2%) | .. |
| Necrotising fasciitis | 12 (1.6%) | 41 (12.0%) | <0.0001 | 2 (0.3%) | 5 (4.3%) | 0.0006 | 5 (1.0%) | 5 (1.8%) | 1.00 | 0 | 0 | NA |
| Year | .. | .. | 0.83 | .. | .. | 0.019 | .. | .. | 0.31 | .. | .. | 0.91 |
| 2000–04 | 77 (10.5%) | 32 (9.3%) | .. | 64 (8.4%) | 7 (6.0%) | .. | 48 (9.6%) | 28 (10.1%) | .. | 10 (5.2%) | 17 (6.9%) | .. |
| 2005–09 | 256 (34.8%) | 123 (35.9%) | .. | 283 (372%) | 59 (50.9%) | .. | 173 (34.6%) | 104 (37.5%) | .. | 89 (46.1%) | 48 (47.1%) | .. |
| 2010–15 | 403 (54.8%) | 188 (54.8%) | .. | 414 (54.4%) | 50 (43.1%) | .. | 279 (55.8%) | 145 (527%) | .. | 94 (48.7%) | 47 (46.1%) | .. |
| Treatment-related | ||||||||||||
| Intensive care unit stay | 128 (17.4%) | 95(277%) | 0.0001 | 123 (16.2%) | 26 (22.4%) | 0.12 | 90 (18.0%) | 55 (19.9%) | 1.00 | 36 (18.7%) | 19 (18.6%) | 1.00 |
| Intravenous immunoglobulin | 7 (1%) | 27 (8%) | <00001 | 1 (<1%) | 2 (2%) | 0.06 | 4 (1%) | 4 (1%) | 0.63 | 0 | 0 | NA |
| Vasopressor use|| | 90 (12.2%) | 83 (24.2%) | <00001 | 72 (9.5%) | 11 (9.5%) | 1.00 | 57 (11.4%) | 37 (13.4%) | 1.00 | 14 (73%) | 7 (6.9%) | 1.00 |
| Debridement within 3 days of infection | 137 (18.6%) | 99 (29) | 0.0002 | 103 (13.5%) | 28 (24.1%) | 0.0044 | 106 (21.2%) | 70 (253%) | 0.33 | 38 (19.7%) | 20 (19.6%) | 1.00 |
| β-lactam antibiotic treatment** | ||||||||||||
| Penicillin | 51 (6.9%) | 83 (24.2%) | <0.0001 | 39 (5.1%) | 9 (7.8%) | 0.35 | 38 (7.6%) | 55 (19.9%) | <0.0001 | 9 (4.7%) | 8 (78%) | 0.39 |
| Ampicillin-sulbactam | 111 (15,1%) | 56 (16.3%) | 0.66 | 86 (11.3%) | 20 (17.2%) | 0.23 | 85 (17.0%) | 44 (15.9%) | 0.76 | 24 (12.4%) | 18 (17.6%) | 0.30 |
| Ampicillin | 133 (18.1%) | 69 (20.1%) | 0.47 | 98 (12.9%) | 30 (25.9%) | 0.0004 | 102 (20.4%) | 56 (20.2%) | 1.00 | 28 (14.5%) | 26 (255%) | 0.03 |
| Cefazolin | 168 (22.8%) | 66 (19.2%) | 0.21 | 159 (20.9%) | 21 (18.1%) | 0.57 | 123 (24.6%) | 60 (21.7%) | 0.40 | 43 (22.3%) | 26 (255%) | 0.56 |
| Piperacillin-tazobactam | 221 (30.0%) | 98 (28.6%) | 0.67 | 257 (33.8%) | 39 (33 6%) | 1.00 | 145 (29.0%) | 69 (24.9%) | 0.26 | 68 (357%) | 32 (31.4%) | 0.59 |
| Ceftriaxone | 276 (37.5%) | 140 (40.8%) | 0.33 | 296 (39) | 52 (44.8%) | 0.27 | 179 (35.8%) | 110 (39.7%) | 0.32 | 67 (34.7%) | 46 (45.1%) | 0.11 |
| Center-related | ||||||||||||
| Academic | 486 (660%) | 229 (66.8%) | 0.87 | 508 (66.8%) | 78 (67.2%) | 1.00 | 335 (67.0%) | 182 (65.7%) | 0.76 | 132 (68.4%) | 68 (67%) | 0.69 |
| Urban | 612 (83 2%) | 277 (80.8%) | 0.38 | 613 (80.6%) | 109 (94.0%) | 0.0007 | 420 (84.0%) | 230 (83.0%) | 0.84 | 181 (93.8%) | 95 (93.1%) | 0.72 |
| Geographic region | .. | .. | <0.001 | .. | .. | 0.80 | .. | .. | 0.99 | .. | .. | 0.59 |
| Midwest | 176 (23.9%) | 49 (14.3%) | .. | 180 (237%) | 32 (27.6%) | .. | 81 (16.2%) | 42 (15.2%) | .. | 44 (22.8%) | 28 (275%) | .. |
| Northeast | 244 (33.2%) | 107 (31.2%) | .. | 262 (34.4%) | 39 (31.2%) | .. | 173 (34.6%) | 88 (31.8%) | .. | 68 (357%) | 33 (32.4%) | .. |
| South | 212 (28.8%) | 111 (32.4%) | .. | 200 (26.3%) | 27 (23.3%) | .. | 153 (30.6%) | 90 (32.5%) | .. | 50 (25.9%) | 25 (25) | .. |
| West | 104 (14.1%) | 76 (227%) | .. | 119 (15.6%) | 18 (15.5%) | .. | 93 (18.6%) | 57 (20.6%) | .. | 31 (16.1%) | 16 (15.7%) | .. |
| Bed capacity | .. | .. | 0.71 | .. | .. | 0.13 | .. | .. | 1.00 | .. | .. | 0.89 |
| <200 | 178 (24.2%) | 78 (227%) | .. | 169 (227%) | 27 (23.3%) | .. | 114 (22.8%) | 64 (23.1%) | .. | 44 (22.8%) | 23 (22.5%) | .. |
| 200–500 | 414 (56.2%) | 191 (55 7%) | .. | 433 (56.9%) | 74 (63.8%) | .. | 281 (56.2%) | 156 (56.3%) | .. | 124 (64.2%) | 65 (63.7%) | .. |
| >500 | 144 (19.6%) | 74 (21.6%) | .. | 159 (20.9%) | 15 (12.9%) | .. | 105 (21.0%) | 57 (20.6%) | .. | 25 (13.0%) | 14 (13.7%) | .. |
Data are median (IQR) or n (%). NA=not applicable. ICD-9=International Classification of Diseases version 9.
This score was calculated by use of ICD-9-clinical modification codes (appendix pp 6–8) and adapted from the methods used by Quan and colleagues.25
Defined by use of ICD-9 codes (see appendix pp 9–10 for the algorithm used).
Defined by use of ICD-9-clinical modification code 278 for obesity.
Calculated by use of an electronic health record-based adaption of the original Sequential Organ Failure Assessment (appendix p 14).
Includes mediastinitis, orchitis or epididymitis, parapharyngeal abscess, periapical abscess, peritonsillar abscess, retropharyngeal abscess, abscess of the mediastinum, and abscess of the salivary gland.
Refers to norepinephrine, epinephrine, phenylephrine, and dopamine administered within a 24-h period either side of culture sampling.
Groups are not mutually exclusive; this variable was not included in the propensity score model.
The primary outcome was the adjusted (for proven invasive diseases, vasopressor use, and ICU status) odds ratio (aOR) of in-hospital mortality, including discharge to hospice, in the propensity-matched iGAS and iNABS cohorts, which included patients with proven and probable invasive infections. Subgroup analyses in both cohorts were done in patients who had: (1) proven infections alone; (2) probable infections alone; (3) skin, soft tissue, or musculoskeletal infections (adjusted for source control or debridement); (4) stayed in the ICU; (5) vasopressor use within 1 day of index culture sampling; (6) necrotising fasciitis; (7) neither vasopressor-dependent shock nor necrotising fasciitis; (8) early clindamycin use (ie, within 1 day either side of culture sampling); and (9) received adjunctive clindamycin for more than 1 day, more than 2 days, and more than 3 days. The secondary outcome was hospital length of stay among survivors. The number needed to treat was calculated as one divided by the difference in mortality rate between recipients and non-recipients of adjunctive clindamycin separately by use of unadjusted and adjusted mortality rates. Follow-up data were present for the duration of hospitalisation.
Primarily, the propensity-matched data were analysed by use of logistic regression models without adjusting for the matched nature of the data.27,28 However, matched approaches, such as conditional logistic regression or generalised estimating equations,29 are also proposed in the literature to account for the dependence among the individuals within each propensity score-matched block. Therefore, we did a further sensitivity analysis using the generalised estimating equations approach, which has been shown to efficiently handle data with incomplete matching blocks,30 as was the case for our data.
Propensity score matching was done via the MatchIt package in R, version 3.5.0. The number needed to treat was estimated with the R package sdtReg. All statistical analyses were done using RStudio, version 1.2.1335–1 (see appendix pp 14–15 for analytical details).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. SSK and AB had full access to all the data in the study. The corresponding author had final responsibility for the decision to submit for publication.
Results
We identified 5953 unique adult inpatient encounters with positive monomicrobial β-haemolytic streptococcal clinical cultures, of whom 3838 (64·5%) had received a β-lactam antibiotic within 3 days either side of culture sampling (figure 1). After excluding patients with clindamycin non-susceptible isolates, who had received linezolid, or who had missing variable data needed for the analysis, 1956 unique patients who had received β-lactam antibiotics from 118 hospitals remained (1079 with iGAS infections and 877 with iNABS infections). Of these patients, 459 (23·4%) had received adjunctive clindamycin within 3 days either side of commencing β-lactam antibiotic treatment (figures 1, 2).
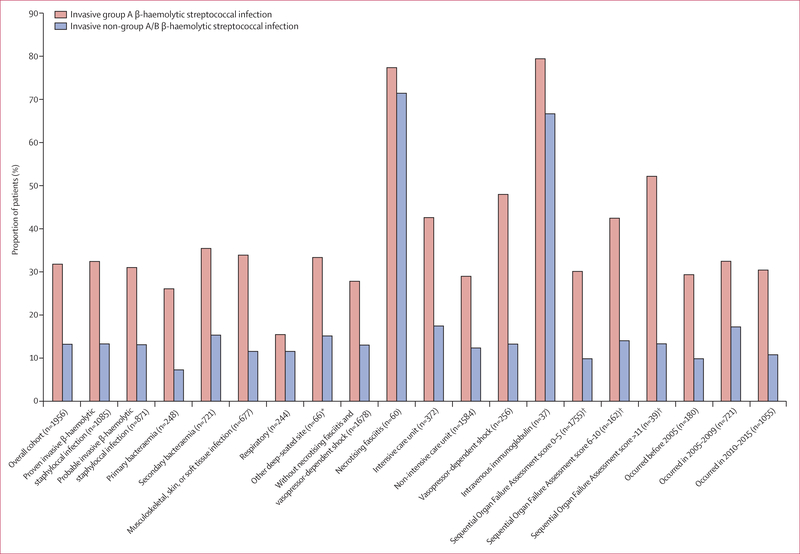
Figure 2. Clindamycin use among patients with invasive β-haemolytic streptococcal infections.
*Includes mediastinitis, orchitis or epididymitis, parapharyngeal abscess, periapical abscess, peritonsillar abscess, retropharyngeal abscess, abscess of the mediastinum, and abscess of the salivary gland. Calculated by use of an electronic health record-based adaption of the original Sequential Organ Failure Assessment (appendix p14).
In the iGAS and iNABS infection cohorts combined, patients who received adjunctive clindamycin compared with those who did not were younger (median age 49 years [IQR 31–64] vs 56 years [40–71], p<0·0001), had a lower median Elixhauser Comorbidity Index score (2·0 [0·0–3·0] vs 2·0 [1·0–4·0], p=0·0021), had more ICU stays (121 [26·4%] of 459 patients vs 251 [16·8%] of 1497 patients, p<0·0001), had a greater dependence on vasopressor therapies (94 [20·5%] patients vs 251 [10·8%] patients, p<0·0001), and had greater intravenous immunoglobulin use (29 [6·3%] patients vs eight [0·5%] patients, p<0·0001). A greater proportion of patients who received adjunctive clindamycin had iGAS infections (343 [74·7%] of 459 patients) compared with those who did not receive adjunctive clindamycin (736 [49·2%] of 1497 patients, p<0·0001), and a greater proportion of those who received adjunctive clindamycin had necrotising fasciitis (40 [12·0%] patients) than those who did not (12 [1·6%] patients, p<0·0001; appendix, pp 16–18). The specific β-lactam antibiotics used among all patients who received adjunctive clindamycin and those who did not were similar (table). Exceptions included more frequent penicillin administration among patients with iGAS infections (83 [24·2%] of 343 patients who received adjunctive clindamycin vs 51 [6·9%] of 736 patients who did not, p<0·0001) and more frequent ampicillin administration among patients with iNABS infections (30 [25·9%] of 116 patients who received adjunctive clindamycin vs 98 [12·9%] of 761 patients who did not, p=0·0004) when compared with those who did not receive adjunctive clindamycin (table).
Of 55 patients who were excluded but who met our criteria for invasive β-haemolytic streptococcal infection and received a β-lactam antibiotic and linezolid within 3 days of a positive β-haemolytic streptococcus culture, 15 (27·3%) had clindamycin susceptibility results. Only one isolate showed intermediate resistance, and the remaining isolates showed no resistance. It therefore appears that documented resistance to clinda mycin was not the major driver for adjunctive use of linezolid instead of clindamycin. However, SOFA scores were higher in patients with invasive β-haemolytic streptococcal infections who received adjunctive clindamycin (2·00 [IQR 0–4]) compared with those who received adjunctive linezolid (1·00 [0–3], p=0·178). Additionally, a higher proportion of patients who received adjunctive clindamycin had proven invasive disease (34 [61·8%] of 55 patients) compared with those who received adjunctive linezolid (257 [56·0%] of 459 patients, p=0·50), and a higher proportion of patients who received adjunctive clindamycin had vasopressor-dependent shock (16 [29·1%]) compared with those who received adjunctive linezolid (94 [20·5%], p=0·19); however, these differences were not significant.
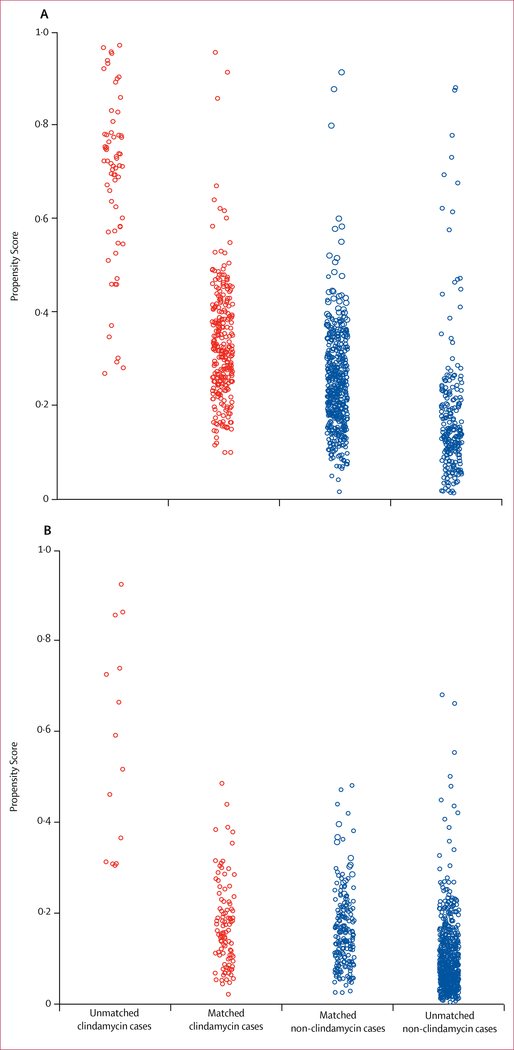
Of the 1956 patients with invasive β-haemolytic streptococcal infections who had received β-lactam antibiotics, 1079 (55·2%) patients had iGAS infections. 343 (31·8%) of these patients had received adjunctive clindamycin, and the median duration of treatment was 4 days (IQR 2–7). 277 patients who received adjunctive clindamycin were propensity matched to 500 patients who did not, with good covariate balance (figure 3A, table).
Figure 3. Distribution of propensity scores (2:1 match) in the invasive group A β-haemolytic streptococcal infection cohort (A) and the invasive non-group A/B β-haemolytic streptococcal infection cohort (B).
Propensity scores were calculated from a logistic regression associated with receipt of clindamycin as a binary outcome to the matching variables (shown in the table) used as predictors for 1956 individuals. From the model, a fitted probability (propensity score) for each patient was calculated to estimate the likelihood of receiving adjunctive clindamycin based on their covariate profile of matching variable values. The propensity scores are visualised on the logit scale.
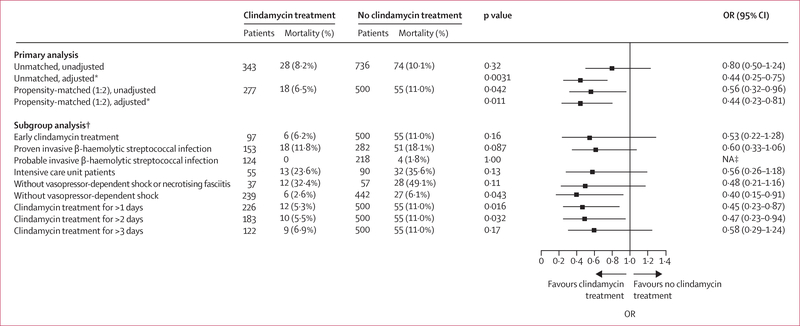
In the unmatched iGAS infection cohort (n=1079), 28 (2·6%) of 343 patients who received adjunctive clindamycin died compared with 74 (10·1%) of 736 patients who did not (p=0·32; aOR 0·80 [95% CI 0·50–1·24]; figure 4). In the propensity-matched iGAS infection cohort (n=777), crude in-hospital mortality was significantly lower among patients who received adjunctive clindamycin (18 [6·5%] of 277 patients) compared with those who did not (55 [11·0%] of 500 patients, p=0·04). After adjusting for residual confounding factors, in-hospital mortality remained lower in patients who had received adjunctive clindamycin compared with those who did not (aOR 0·44 [95% CI 0·23–0·81]; figure 4). The beneficial effect of clindamycin on in-hospital mortality was similar in patients who had received adjunctive clindamycin within the first 24 h of index culture (0·53 [0·22–1·28]) and in those who had received adjunctive clindamycin for more than 1 day (0·45 [0·23–0·87]; figure 4). Similarly, adjunctive clindamycin treatment was associated with decreased mortality, even among patients without vasopressor-dependent shock or necrotising fasciitis (six [2·6%] of 39 patients who received adjunctive clindamycin vs 27 [6·1%] of 442 who did not, p=0·04; aOR 0·40 [95% CI 0·15–0·91]). A non-significant decrease in in-hospital mortality with adjunctive clindamycin treatment compared with no adjunctive clindamycin treatment was also observed in the following analyses of other propensity-matched subgroups: proven iGAS infection (0·60 [0·33–1·06]), ICU stay (0·56 [0·26–1·18]), and administration of vasopressor therapy (0·48 [0·21–1·16]; figure 4). The low number or absence of in-hospital deaths among patients with probable iGAS infection (four [1·8%] of 218 patients who received clindamycin), musculoskeletal, skin, or soft tissue infections (one [0·5%] of 202 patients who did not receive clindamycin), and necrotising fasciitis (no patients), precluded reliable assessment of clindamycin effect on mortality within these subgroups. Sensitivity analyses using a generalised estimating equations approach showed similar results (appendix pp 19–20).
Figure 4. OR of in-hospital mortality in patients with invasive group A β-haemolytic streptococcal infection treated with versus without adjunctive clindamycin.
The ORs (95% CIs) of in-hospital mortality in the primary analysis, by propensity matching and adjustment status, and in subgroup analyses of propensity-matched patients in the invasive group A β-haemolytic streptococcal infection cohort. OR=odds ratio. NA=not applicable. *Adjusted for proven invasive β-haemolytic streptococcal infection, vasopressor-dependent shock, and intensive care unit status. †All subgroup analyses were propensity-matched. ‡Too few deaths in patients with probable invasive β-haemolytic streptococcal infection alone precluded reliable assessment of the effect of clindamycin on mortality.
The number needed to treat for one patient to benefit from adjunctive clindamycin was 22 (95% CI 9–54). By use of the primary model, adjusted for covariates, the number needed to treat was 20 (10–41).
Median hospital length of stay among 259 patients who received adjunctive clindamycin was 7 days (IQR 5–11) compared with 6 days (4–8) among 445 patients who did not (p<0·0001).
Of the 1956 patients with invasive β-haemolytic streptococcal infection who had received β-lactam antibiotics, 877 (44·8%) had iNABS infections. 116 (13·2%) of these patients received adjunctive clindamycin for a median duration of 3 days (IQR 2–5). Of these, 102 (87·9%) patients were propensity matched to 193 patients who did not receive adjunctive clindamycin, with good covariate balance (figure 3B, table).
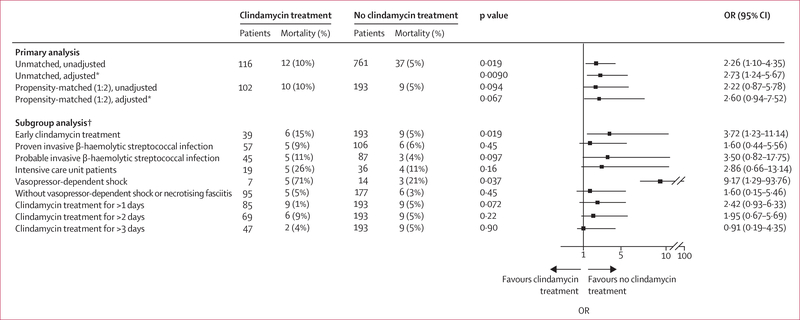
In the unmatched iNABS infection cohort (n=877), 12 (10·3%) of 116 patients who received adjunctive clindamycin died compared with 37 (5·1%) of 761 patients who did not (p=0·0090; aOR 2·73 [95% CI 1·24–5·67]; figure 5). In the propensity-matched iNABS infection cohort (n=295), crude in-hospital mortality was higher among patients who received adjunctive clindamycin (ten [9·8%] of 102 patients) compared with those who did not (nine [4·6%] of 193 patients, p=0·094). After adjusting for residual confounding factors, adjunctive clindamycin treatment was associated with a non-significant increase in in-hospital mortality when compared with no adjunctive clindamycin treatment (aOR 2·60 [95% CI 0·94–7·52]). Early adjunctive clindamycin treatment among 39 (38·2%) of 102 propensity-matched patients was associated with increased in-hospital mortality compared with the 193 patients who did not receive adjunctive clindamycin (3·72 [1·23–11·14]; figure 5). By contrast, receiving adjunctive clindamycin for more than 1 day (2·42 [0·93–6·33]) or for more than 2 days (1·95 [0·67–5·69]) had no significant effect on in-hospital mortality when compared with patients who did not receive adjunctive clindamycin. A similar, but non-significant increase in in-hospital mortality in patients who received adjunctive clindamycin compared with those who did not was also observed in the following subgroup analyses: proven iNABS infections only (1·60 [0·44–5·56]), probable iNABS infections only (3·5 [0·82–17·75]), and the absence of vasopressor-dependent shock or necrotising fasciitis (1·60 [0·15–5·46]; figure 5). The low number of patients, in-hospital deaths, or both, precluded reliable assessment of the effect of adjunctive clindamycin treatment on in-hospital mortality within the remaining subgroups. Sensitivity analyses done by use of generalised estimating equations methods showed similar results (appendix pp 21–22).
Figure 5. OR of in-hospital mortality in patients with invasive non-group A/B β-haemolytic streptococcal infection treated with versus without adjunctive clindamycin.
The ORs (95% CIs) of in-hospital mortality in the primary analysis, by propensity matching and adjustment status, and in subgroup analyses of propensity-matched patients in the invasive group A β-haemolytic streptococcal infection cohort. OR=odds ratio. *Adjusted for proven invasive β-haemolytic streptococcal infection, vasopressor-dependent shock, and intensive care unit status. †All subgroup analyses were propensity-matched.
Median hospital length of stay was similar among 92 patients with iNABS infections who received adjunctive clindamycin treatment (8 days [IQR 5–12]) and the 184 patients who did not (7 days [5–10]) in the propensity-matched cohort (p=0·61).
Discussion
To our knowledge, this retrospective multicentre cohort study is the largest observational study of patients with invasive β-haemolytic streptococcal infections, in whom the real-world use and clinical effectiveness of adjunctive clindamycin has been evaluated to date. Among 118 US hospitals, adjunctive clindamycin was administered, on average, to one in every four patients with invasive β-haemolytic streptococcal infections, and was administered more frequently to patients with severe forms of the disease (ie, those with vasopressor-dependent shock and necrotising fasciitis) compared with those who had non-severe forms, and to those with iGAS infections compared with those who had iNABS infections. In a propensity score-matched analysis, adjunctive clindamycin use in patients with invasive iGAS infections already receiving β-lactam antibiotics was associated with improved shortterm survival compared with that observed in patients receiving β-lactam antibiotic treatment alone. Importantly, the survival benefit associated with adjunctive clindamycin use was observed even among patients with invasive iGAS infection who did not present with vasopressor-dependent shock, necrotising fasciitis, or both. By contrast, the use of adjunctive clindamycin was not associated with a survival benefit among patients with invasive iNABS infections, and was associated with significantly worse survival among patient subgroups requiring vasopressor therapy and those who received early adjunctive clindamycin. Similar to the primary outcome, most other iNABS subgroup analyses showed that adjunctive clindamycin treatment was associated with a non-significant increase in mortality compared with no adjunctive clindamycin treatment.
Despite prompt β-lactam antibiotic therapy, aggressive source control, and advancements in supportive care, mortality among patients with invasive β-haemolytic streptococcal infections remains high, particularly among those with streptococcal toxic shock syndrome and necrotising fasciitis.2 In our propensity-matched cohorts, 54 (27·0%) of 200 patients admitted to the ICU (45 [31·0%] of 145 patients with iGAS infections and nine [16·4%] of 55 patients with iNABS), and 48 (41·7%) of 115 patients who received vasopressor therapy (40 [42·6%] of 94 patients with iGAS and eight [31·1%] of 21 patients with iNABS) had died. In patients presenting with such severe invasive β-haemolytic streptococcal infections, adjunctive clindamycin and intravenous immunoglobulin treatments are often administered, and could have additional benefits.5,7,8 In a meta-analysis published in 2018, intravenous immunoglobulin treatment was associated with a reduction in mortality in a pooled analysis of patients treated with clindamycin, compared with those who did not receive intravenous immunoglobulin.5 Despite the pooling of patients, the small net sample size diminished the precision of the effect size estimates, thus weakening support for a true benefit attributable to intravenous immunoglobulin.5 Additionally, as intra venous immunoglobulin is often administered in combi nation with clindamycin, assessing the effect of intrave nous immunoglobulin on survival independently of clindamycin has been difficult.10,13 Compared with intravenous immunoglobulin, clinda mycin is a more accessible and affordable adjunct. Therefore, confirming clinical effectiveness independent of other therapeutic options, and increasing its use has the potential to significantly affect patient outcomes. The survival benefits of adjunctive clindamycin treatment have been observed in observational studies of adjunctive intravenous immunoglobulin therapy in patients with iGAS infections,10,13 and in those who go on to develop necrotising fasciitis7 or streptococcal toxic shock syndrome.8 However, other observational studies of iGAS infections have failed to show survival benefit of clindamycin in multivariate models.10,11 In addition, a small observational study and a randomised multicentre study examining the effect of adjunctive clindamycin in patients with cellulitis also did not identify an association between adjunctive clindamycin use and either clinical improvement or survival.12,31 The largest body of evidence published thus far was a retrospective cohort study by Couture-Cossette and colleagues9 involving 249 patients with iGAS in f ections and 188 patients with group G and group C β-haemolytic streptococcal infections; 144 (32·9%) of these 437 patients had received adjunctive clindamycin treatment. The results showed a survival benefit among patients with iGAS who had received adjunctive clindamycin within 24 h of index culture compared with those who had not received adjunctive clindamycin. With a cohort that is larger than all previous studies combined (appendix pp 25–27), and using propensity-matched groups and granular electronic health record data to adjust for the severity of acute illness, we found that adjunctive clindamycin treatment in patients with iGAS infections improves survival when compared with no adjunctive clindamycin treatment. Notably, our results suggest that adjunctive clindamycin treatment might be beneficial even in patients with iGAS infections who do not present with vasopressor-dependent shock or necrotising fasciitis, which is a population in whom clindamycin use is currently low.
By contrast with the observed effect of adjunctive clindamycin treatment on survival in patients with iGAS infections, the addition of clindamycin to β-lactam antibiotic treatment in patients with iNABS infections was not beneficial and showed a concerning trend towards worse survival. One contributing factor to this observation could be the difference in the proportion of patients with clindamycin-resistant isolates between those with iGAS and iNABS infections.32 Even though we excluded patients with documented clindamycin resistance (figure 1), susceptibility testing in patients with invasive β-haemolytic streptococcal infections might not have been routinely done, particularly in samples from sites outside of the bloodstream and central nervous system. Among 5953 unique monomicrobial β-haemolytic streptococcal cultures that had been initially screened, only 1172 (19·7%) patients had undergone clindamycin susceptibility testing. Initial data exploration revealed 156 patients (110 treated with β-lactam antibiotics) with clindamycin-resistant cultures who were ultimately excluded (figure 1). A significantly higher proportion of these patients with iNABS infections had clindamycin-resistant cultures (80 [16·2%] of 492) compared with those who had iGAS infections (76 [11·4%] of 669, p=0·020). Assuming that similar patterns of clindamycin resistance existed among patients with missing susceptibility data, then it is possible that clindamycin resistance could have contributed to the observed absence of benefit of adjunctive clindamycin in patients with iNABS infections. However, prospective studies with more complete data on clindamycin susceptibility testing will be needed to provide a more definitive answer to this question.
Additionally, testing for antagonism between β-lactam antibiotics and clindamycin in both susceptible and non-susceptible isolates from patients with iNABS infections has not been explored, and our findings highlight this as an area in need of further investigation. Whole-genome sequencing of prototype species (S dysgalactiae subspecies equisimilis for group C33 and group G21), which are responsible for most invasive iNABS infections (appendix p 3),20 has revealed notable differences in their virulence profiles and pathogenesis (eg, the importance of sugar-metabolising enzymes in group C streptococcus33) when compared with group A streptococci. The presence of virulence factors, such as streptolysin S (associated with necrotising skin infections34), the species-specific streptococcal superantigen gene sepG, and the occasional recombinant event from group A streptococci leading to acquisition of group A superantigen genes sepA/B, have been documented among non-group A/B streptococci.22,35 However, the occurrence of streptococcal toxic shock syndrome, necrotising infection, and other severe forms of infection, are considerably lower in patients with iNABS infections compared with those who have iGAS infections, even in the presence of such genes.19,35 Notably, we found that, compared with those who had iGAS infections, a lower proportion of patients with iNABS infections had necrotising fasciitis (53 [4·9%] of 1079 vs seven [0·8%] of 877) and vasopressor dependence (173 [16·0%] of 1079 vs 83 [9·4%] of 877), and in-hospital mortality was lower in those with iNABS infections (49 [5·6%] of 877) compared with those who had iGAS infections (102 [9·5%] of 1079). Furthermore, patients with streptococcal toxic shock syndrome caused by iNABS infection are frequently older and have more comorbidities than patients with iGAS infections, as was observed in our study (table 1) and in previous studies.20
Before our study, only Couture-Cossette and colleagues had examined the association between adjunctive clindamycin treatment and survival in patients with iNABS infections.9 Among only 24 patients with iNABS infections who received adjunctive clindamycin, an unadjusted analysis showed no association between adjunctive clindamycin treatment and survival. Of note, our findings suggest that it might be naive to assume that adjunctive clindamycin will be effective on the basis of similarities in clinical presentation. However, species information might not be readily available early on in the course (ie, within the first few days) of an infection, thus the risk-benefit balance of early clindamycin use based on clinical presentation alone needs to be evaluated further in prospective randomised studies. Until such data become available, it would seem reasonable to initiate early adjunctive clindamycin, at least in patients with severe clinical presentations, such as necrotising fasciitis and suspected streptococcal toxic shock syndrome. Given that severe clinical presentations are more likely to be caused by iGAS infections rather than iNABS infections, taken together, more patients with iGAS infections are likely to appropriately receive early adjunctive clindamycin treatment than those with iNABS infections, potentially resulting in net benefit at the population-level.
Important limitations to our study should be mentioned. Although propensity-score matching can minimise confounding by indication, residual confounding could still remain. Adjunctive clindamycin was used less frequently in patients with iNABS infections compared with those who had iGAS infections, and despite propensity matching and downstream adjustment, residual confounding factors could have contributed to the concerning trend toward harm in in the iNABS infection cohort. The effect of specific β-lactam antibiotics on in-hospital mortality could not be assessed and might have also contributed to residual unmeasured confounding factors between groups. However, the large number of patients enabled us to generate well balanced propensity-matched patient pairs. The different and opposite effect of clindamycin on survival in patients with iGAS infections compared with those who had iNABS infections was highly significant, as evidenced from the interaction between Lancefield group status and the effect of adjunctive clindamycin on mortality. However, a limitation that we must acknowledge is the absence of data on resistance to clindamycin; even though we excluded all patients with clindamycin-resistant isolates, the true burden of clindamycin resistance might not have been captured due to the low frequency of routine testing. The ICD code-based disease definitions might not have had adequate specificity; however, an algorithm that combines within-window culture data and antimicrobial administration can mitigate this risk.36 Additionally, ICD version 10 codes were not used, as this set of codes was implemented late in our study period; therefore we limited our analysis to patients who were exclusively coded by use of ICD-9 codes. A major limitation of our study was the small number of patients with necrotising fasciitis in our propensity-matched cohort. Therefore, the generalisation of our results to patients with necrotising fasciitis, despite exact matching on this variable, should be done with caution. The characteristics of participating hospitals within the Cerner Health Facts database have been shown to be similar to those of non-participating US hospitals, by comparison to well established population demographic estimates. However, some differences might exist (eg, whether hospitals report microbiology data or the distributions of the socioeconomic status of patients) that could limit the generalisability of our results.37 The toxic effects of clindamycin should be balanced against the potential benefits that this drug might confer. Unfortunately, we were unable to ascertain the proportion of patients who had an adverse drug effect or Clostridioides difficile-associated disease between those who received adjunctive clindamycin and those who did not, because data on these symptoms and signs are unavailable in the Cerner Health Facts database. Given the association between adjunctive clindamycin treatment and C difficile infection, this is an important adverse effect that should be assessed in future studies.38 Apart from linezolid, the use of other protein synthesis inhibitor antibiotics that can decrease exotoxin production was not accounted for.39 However, compared with clindamycin, these drugs are used sparingly for invasive β-haemolytic staphylococcal infections.14 Finally, we were unable to analyse the effectiveness of clindamycin in patients with invasive disease caused by group B streptococcal infection due to the low use of this agent among these patients in our cohort, and we encourage future studies on this topic.
Our findings of improved survival with adjunctive clindamycin treatment in patients with both severe and non-severe presentations of iGAS infection, coupled with the relatively low proportion of patients with iGAS infections who were treated with adjunctive clindamycin, suggest that this adjunctive treatment might be indicated in a wider range of patients than it is currently used in. Based on the non-significant trend towards harm associated with clindamycin use in patients with iNABS infections, adjunctive clindamycin treatment should be avoided in patients with confirmed iNABS infection in the absence of other clinical indications, such as necrotising fasciitis or suspected streptococcal toxic shock syndrome.
In conclusion, clindamycin treatment as an adjunct to β-lactam antibiotics improved survival in patients with iGAS infections when compared with those who did not receive adjunctive clindamycin treatment. However, this survival benefit of adjunctive clindamycin was not observed in patients with iNABS infections who had received β-lactam antibiotics. Our real-world data supports the use of adjunctive clindamycin in patients with iGAS infections. These data could help inform the design and conduct of further trials, and underscore the importance of doing translational studies to explore the potential mechanisms of harm or the absence of benefit of clindamycin in patients with iNABS infections.
Supplementary Material
Research in context
Evidence before this study
Invasive β-haemolytic streptococcal infections, particularly necrotising fasciitis and streptococcal toxic shock syndrome are most often caused by group A streptococcal infections and are associated with a high risk of mortality. Clindamycin is recommended as an adjunctive treatment to β-lactam antibiotics in patients with severe group A streptococcal infections to inhibit toxin production and overcome large inoculums in the stationary phase of bacterial growth. This treatment strategy is largely based on evidence from in vitro and animal models, as robust clinical data are scarce. Additionally, the use of clindamycin in patients with invasive non-group A/B β-haemolytic streptococcal (iNABS) infections is not well defined. We searched PubMed on Oct 30, 2018 using the search string “(clindamycin[tiab] OR clindamycin[mesh])” AND “(streptococcus[mesh])” OR (“invasive streptococcal”[tiab]). We searched for primary research and reviews published from database inception up to Oct 30, 2018, with no language restrictions. The search yielded 1552 studies. Clinical data about adjunctive clindamycin therapy in patients with invasive group A β-haemolytic streptococcal infections (iGAS) were limited to one retrospective case series, one retrospective cohort study, and six population surveillance studies of iGAS, with only one study evaluating the efficacy of adjunctive clindamycin in iNABS infections. Most of these studies reported a trend towards survival benefit of adjunctive clindamycin therapy for invasive iGAS infections. In a small, unadjusted analysis, no difference in mortality was observed between patients with iNABS infection (n=24) who were given adjunctive clindamycin compared with those who were not. By contrast to these studies, a prospective surveillance study of 62 critically ill patients with iGAS infection found no association between clindamycin treatment and survival (appendix pp 25–27).
Added value of this study
Our retrospective study of 1956 patients admitted to 118 hospitals over a 15-year period represents the largest cohort of patients with invasive β-haemolytic streptococcal infections in which the use of adjunctive clindamycin and its effect on survival has been evaluated to date. Propensity matching and adjusting for the severity of acute illness by use of organ failure scores strengthened the validity of our results. The large study size allowed us to assess the effect of adjunctive clindamycin therapy on the outcome of different types of invasive β-haemolytic streptococcal infections (iGAS vs iNABS), and to confirm the survival benefit of adjunctive clindamycin in patients with iGAS across a range of illness severities.
Implications of all the available evidence
The observed benefit of adjunctive clindamycin on survival in patients with iGAS infections already receiving β-lactam antibiotics supports current recommendations by professional societies. Adjunctive clindamycin also improved survival in patients with iGAS infections who did not have shock or necrotising fasciitis, suggesting that wider use of this therapy in less severely affected patients could be warranted. Given the absence of benefit and possible harm observed from adjunctive clindamycin in patients with iNABS, this therapy appears to have no beneficial role in the treatment of these infections.
Acknowledgments
This study was funded by the Intramural Research Program of the US National Institutes of Health (NIH) Clinical Center and the National Institute of Allergy and Infectious Disease. We thank Chris A Van Beneden and Jennifer Onukwube from the US Centers for Disease Control and Prevention for their guidance on Active Bacterial Core Surveillance System-based classification of sterile site cultures from real-world data. We also thank David Fram and Huai Chen of Commonwealth Informatics for their assistance with data mapping and curation, and Kelly Byrne for her assistance with formatting the manuscript text, figures, and tables. The preliminary findings of this study were presented at the annual conference of the Infectious Diseases Society of America on Oct 4, 2017, in San Diego, and on Oct 2–6, 2019, in Washington. The opinions expressed in this article are those of the authors and do not represent any position or policy of the NIH, the US Department of Health and Human Services, or the US Government.
Footnotes
Declaration of interests
We declare no competing interests.
Contributor Information
Ahmed Babiker, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, GA, USA.
Xiaobai Li, Department of Biostatistics, National Institutes of Health Clinical Center, Bethesda, MD, USA.
Yi Ling Lai, Epidemiology Unit, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Jeffrey R Strich, Clinical Epidemiology Section, Critical Care Medicine Department, National Institutes of Health Clinical Center, Bethesda, MD, USA.
Sarah Warner, Clinical Epidemiology Section, Critical Care Medicine Department, National Institutes of Health Clinical Center, Bethesda, MD, USA.
Sadia Sarzynski, Clinical Epidemiology Section, Critical Care Medicine Department, National Institutes of Health Clinical Center, Bethesda, MD, USA.
John P Dekker, Bacterial Pathogenesis and Antimicrobial Resistance Unit, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Robert L Danner, Clinical Epidemiology Section, Critical Care Medicine Department, National Institutes of Health Clinical Center, Bethesda, MD, USA.
Sameer S Kadri, Department of Medicine, Uniformed Services University of the Health Sciences, Bethesda, MD, USA; Clinical Epidemiology Section, Critical Care Medicine Department, National Institutes of Health Clinical Center, Bethesda, MD, USA.
References
- 1.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis 2005; 5: 685–94. [DOI] [PubMed] [Google Scholar]
- 2.O’Loughlin RE, Roberson A, Cieslalc PR, et al. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000–2004. Clin Infect Dis 2007; 45: 853–62. [DOI] [PubMed] [Google Scholar]
- 3.Srislcandan S, Ferguson M, Elliot V, Faulkner L, Cohen J. Human intravenous immunoglobulin for experimental streptococcal toxic shock: bacterial clearance and modulation of inflammation. J Antimicrob Chemother 2006; 58: 117–24. [DOI] [PubMed] [Google Scholar]
- 4.Kadri SS, Swihart BJ, Bonne SL, et al. Impact of intravenous immunoglobulin on survival in necrotizing fasciitis with vasopressor-dependent shock: a propensity score-matched analysis from 130 US hospitals. Clin Infect Dis 2017; 64: 877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parks T, Wilson C, Srislcandan S, Curtis N, Norrby-Teglund A. Polyspecific intravenous immunoglobulin in clindamycin-treated patients with streptococcal toxic shock syndrome: a systematic review and meta-analysis. Clin Infect Dis 2018; 67: 1434–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andreoni F, Zurcher C, Tarnutzer A, et al. Clindamycin affects group A streptococcus virulence factors and improves clinical outcome. J Infect Dis 2017; 215: 269–77. [DOI] [PubMed] [Google Scholar]
- 7.Mulla ZD, Leaverton PE, Wiersma ST. Invasive group A streptococcal infections in Florida. South Med J 2003; 96: 968–73. [DOI] [PubMed] [Google Scholar]
- 8.Linner A, Darenberg J, Sjolin J, Henriques-Normarlc B, Norrby-Teglund A. Clinical efficacy of polyspecific intravenous immunoglobulin therapy in patients with streptococcal toxic shock syndrome: a comparative observational study. Clin Infect Dis 2014; 59: 851–57 [DOI] [PubMed] [Google Scholar]
- 9.Couture-Cossette A, Carignan A, Mercier A, Desruisseaux C, Valiquette L, Pépin J. Secular trends in incidence of invasive beta-hemolytic streptococci and efficacy of adjunctive therapy in Quebec, Canada, 1996–2016. PLoS One 2018; 13: e0206289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaul R, McGeer A, Norrby-Teglund A, et al. Intravenous immunoglobulin therapy for streptococcal toxic shock syndrome—a comparative observational study. The Canadian Streptococcal Study Group. Clin Infect Dis 1999; 28: 800–07 [DOI] [PubMed] [Google Scholar]
- 11.Kaul R, McGeer A, Low DE, Green K, Schwartz B. Population-based surveillance for group A streptococcal necrotizing fasciitis: clinical features, prognostic indicators, and microbiologic analysis of seventy-seven cases. Ontario group A streptococcal study. Am J Med 1997; 103: 18–24. [DOI] [PubMed] [Google Scholar]
- 12.Mehta S, McGeer A, Low DE, et al. Morbidity and mortality of patients with invasive group A streptococcal infections admitted to the ICU. Chest 2006; 130: 1679–86. [DOI] [PubMed] [Google Scholar]
- 13.Carapetis JR, Jacoby P, Carville K, Ang SJ, Curtis N, Andrews R. Effectiveness of clindamycin and intravenous immunoglobulin, and risk of disease in contacts, in invasive group a streptococcal infections. Clin Infect Dis 2014; 59: 358–65. [DOI] [PubMed] [Google Scholar]
- 14.Zimbelman J, Palmer A, Todd J. Improved outcome of clindamycin compared with beta-lactam antibiotic treatment for invasive Streptococcus pyogenes infection. Pediatr Infect Dis J 1999; 18: 1096–100. [DOI] [PubMed] [Google Scholar]
- 15.Stevens DL, Bisno AL, Chambers HF, et al. Executive summary: practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014; 59: 147–59. [DOI] [PubMed] [Google Scholar]
- 16.Sartelli M, Malangoni MA, May AK, et al. World Society of Emergency Surgery (WSES) guidelines for management of skin and soft tissue infections. World J Emerg Surg 2014; 9: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunha CB. Viridans streptococci, nutritionally variant streptococci, and groups C and G streptococci. In: Mandell G, Bennett J, Dolin R, eds. Principles and practice of infectious diseases. Ninth edition. Philadelphia: Elsevier, 2020: 2513–20. [Google Scholar]
- 18.Sylvetslcy N, Raveh D, Schlesinger Y, Rudensky B, Yinnon AM. Bacteremia due to beta-hemolytic streptococcus group G: increasing incidence and clinical characteristics of patients. Am J Med 2002; 112: 622–26. [DOI] [PubMed] [Google Scholar]
- 19.Ekelund K, Skinhøj P, Madsen J, Konradsen HB. Invasive group A, B, C and G streptococcal infections in Denmark 1999–2002: epidemiological and clinical aspects. Clin Microbiol Infect 2005; 11: 569–76. [DOI] [PubMed] [Google Scholar]
- 20.Broyles LN, Van Beneden C, Beall B, et al. Population-based study of invasive disease due to beta-hemolytic streptococci of groups other than A and B. Clin Infect Dis 2009; 48: 706–12. [DOI] [PubMed] [Google Scholar]
- 21.Shimomura Y, Okumura K, Murayama SY, et al. Complete genome sequencing and analysis of a Lancefield group G Streptococcus dysgalactiae subsp. equisimilis strain causing streptococcal toxic shock syndrome (STSS). BMC Genomics 2011; 12: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashikawa S, Iinuma Y, Furushita M, et al. Characterization of group C and G streptococcal strains that cause streptococcal toxic shock syndrome. J Clin Microbiol 2004; 42: 186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diep BA, Equils O, Huang DB, Gladue R. Linezolid effects on bacterial toxin production and host immune response: review of the evidence. Curr Ther Res Clin Exp 2012; 73: 86–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CDC. Case definitions for infectious conditions under public health surveillance. MMWR Recomm Rep 1997; 46: 1–55. [PubMed] [Google Scholar]
- 25.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43: 1130–39. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Kim HJ, Lonjon G, Zhu Y. Balance diagnostics after propensity score matching. Ann Transl Med 2019; 7: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci 2010; 25: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wan F. Matched or unmatched analyses with propensity-score-matched data? Stat Med 2019; 38: 289–300. [DOI] [PubMed] [Google Scholar]
- 29.Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med 2008; 27: 2037–49. [DOI] [PubMed] [Google Scholar]
- 30.Lin IF, Lai MY, Chuang PH. Analysis of matched case-control data with incomplete strata: applying longitudinal approaches. Epidemiology 2007; 18: 446–52. [DOI] [PubMed] [Google Scholar]
- 31.Brindle R, Williams OM, Davies P, et al. Adjunctive clindamycin for cellulitis: a clinical trial comparing flucloxacillin with or without clindamycin for the treatment of limb cellulitis. BMJ Open 2017; 7: e013260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merino Díaz L, Torres Sánchez MJ, Aznar Martín J. Prevalence and mechanisms of erythromycin and clindamycin resistance in clinical isolates of β-haemolytic streptococci of Lancefield groups A, B, C and G in Seville, Spain. Clin Microbiol Infect 2008; 14: 85–87. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe S, Kirikae T, Miyoshi-Akiyama T. Complete genome sequence of Streptococcus dysgalactiae subsp. equisimilis 167 carrying Lancefield group C antigen and comparative genomics of S. dysgalactiae subsp. equisimilis strains. Genome Biol Evol 2013; 5: 1644–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Humar D, Datta V, Bast DJ, Beall B, De Azavedo JC, Nizet V. Streptolysin S and necrotising infections produced by group G streptococcus. Lancet 2002; 359: 124–29. [DOI] [PubMed] [Google Scholar]
- 35.Lother SA, Demczuk W, Martin I, et al. Clonal clusters and virulence factors of group C and G streptococcus causing severe infections, Manitoba, Canada, 2012–2014. Emerg Infect Dis 2017; 23: 1079–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Branch-Elliman W, Strymish J, Gupta K. Development and validation of a simple and easy-to-employ electronic algorithm for identifying clinical methicillin-resistant Staphylococcus aureus infection. Infect Control Hosp Epidemiol 2014; 35: 692–98. [DOI] [PubMed] [Google Scholar]
- 37.DeShazo JP, Hoffman MA. A comparison of a multistate inpatient EHR database to the HCUP nationwide inpatient sample. BMC Health Serv Res 2015; 15: 384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deshpande A, Pasupuleti V, Thota P, et al. Community-associated Clostridium difficile infection and antibiotics: a meta-analysis. J Antimicrob Chemother 2013; 68: 1951–61. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka M, Hasegawa T, Okamoto A, Torii K, Ohta M. Effect of antibiotics on group A streptococcus exoprotein production analyzed by two-dimensional gel electrophoresis. Antimicrob Agents Chemother 2005; 49: 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.