Abstract
Background:
We recently described the association between periodontal disease (PD) and stroke risk.
Purpose:
The purpose of this study was to test the association between PD, dental care utilization and incident AF, as well as AF as a mediator to PD- stroke association.
Methods:
In dental Atherosclerosis Risk in Communities Study (ARIC), participants without prior AF underwent full-mouth periodontal measurements. PD was defined on an ordinal scale as healthy (referent), mild, moderate and severe. In ARIC main cohort, participants were classified as regular or episodic dental care users. These patients were followed for AF, over 17 years. Cox proportional hazards models adjusted for AF risk factors were used to study relationships between PD severity, dental care utilization and AF. Mediation analysis was used to test if AF mediated the PD- stroke association.
Results:
In dental ARIC cohort, 5,958 were assessed without prior AF, 754 were found to have AF. Severe PD was associated with AF on both univariable (crude HR, 1.54; 95% CI, 1.26-1.87) and multivariable (adjusted HR, 1.31, 95% CI, 1.06-1.62) analyses. Mediation analysis suggested AF mediates the association between PD and stroke. In the main ARIC cohort, 9,666 participants without prior AF were assessed for dental care use, 1558 were found to have AF. Compared with episodic users, regular users had a lower risk for AF on univariable (crude HR, 0.82, 95% CI, 0.74–0.90) and multivariable (adjusted HR, 0.88, 95% CI, 0.78–0.99) analyses.
Conclusions:
PD is associated with AF. The association may explain the PD-stroke risk. Regular users had a lower risk of incident AF compared with episodic users.
Keywords: Periodontal Disease, Gingival Disease, Infection, Atrial Fibrillation, Stroke
Subject Terms: Atrial Fibrillation, Ischemic Stroke, Inflammation
Graphical Abstract
INTRODUCTION
Periodontal disease (PD) is an inflammatory disease caused by immune dysfunction and affects soft and hard structures that support the teeth and is initiated by bacteria settled within the mouth.1 Gingivitis is the mildest form of PD and also initiated by bacterial biofilm that accumulate on the teeth adjacent to the gum, however, is considered a normal, non-destructive and protective response without alveolar bone destruction. (refs the same as above). The prevalence of PD, both gingivitis and periodontitis, is estimated to be as high as 90% of the worldwide population, but the prevalence of severe periodontitis is around 10-15%.2,3
Periodontitis is associated with an increase in systemic inflammatory markers, through chronic low-grade bacteremia. 24,53 PD is associated with higher C-reactive protein (CRP) levels, higher white cell count, interleukin 6 and levels of other acute phase reaction proteins.6-9 These inflammatory markers have been related to the overall origination and progression of atherosclerosis.11-16 Inflammation is also suggested to play a role in the initiation and persistence of atrial fibrillation (AF). 17-21 Lymphomononuclear cell accumulation in the atrium wall has been demonstrated in AF patients, but not in patients in normal sinus rhythm. 22 Systemic inflammation, characterized by inflammatory markers including tumor necrosis factor α, interleukin 6, and CRP, have been associated with a higher AF risk.21, 23-25 Taken together, it is fair to hypothesize that systemic inflammation originating from PD may cause the development of AF. 29
The association between PD in addition to fewer teeth with increased risk of ischemic stroke has been described.26 Several observational studies have reported association between poor periodontal health and increased stroke risk.27-29 We recently reported that PD is associated with increased risks of atherothrombotic as well as cardioembolic ischemic stroke subtypes.30 AF remains the most common cause for ischemic stroke of cardioembolic origin. 31 Recently using a Taiwanese National Population Based Cohort Study, Chen et al, described an increased AF risk in patients with PD. Lack of data on potential confounders were limitations of the study. 32 Thus, in this project, we investigated the association between PD and incident AF as a potential mechanism for explaining the previously reported link between PD and stroke. Specifically, we tested the hypothesis whether PD is associated with increased incidence of AF in the predominantly bi-racial community from the United States. Subsequently we tested whether AF serves as a potential mediator between PD and stroke in the same population.
MATERIALS AND METHODS
Study Population
In the year1987, the Atherosclerosis Risk in Communities (ARIC) Study began its cohort to examine the etiology of atherosclerosis and outcomes.33 Participants for this cohort were enrolled from four separate communities in the US including Forsyth County, North Carolina, Jackson, Mississippi, suburbs of Minneapolis, Minnesota, and Washington County, Maryland. The initial baseline visits were conducted from 1987 to 1989 and follow-up took place on the following timeline: visit 2 (1990-1992), visit 3 (1993-1995), visit 4 (1996-1998), visit 5 (2011-2013) and more recently visit 6 (2016-2017). Vascular risk factors were examined, and hospital medical records were obtained, particularly if hospitalizations occurred. The study team also contacted the ARIC study subjects on a yearly basis to evaluate their well-being and any adverse events that had occurred comprising stroke and AF.33 This study analyzed the outcomes at follow-up through the end of 2013. As of current, AF and ischemic stroke and subtype have not been adjudicated for visit 6. The institutional review board of all participating institutions approved the study and all subjects provided informed consent to participate.
At Visit 4 in the Dental Atherosclerosis Risk in Communities Study (DARIC) study participants underwent full mouth periodontal assessment of six sites per tooth from 5,958 cohort participants without prior AF. These subjects were followed for AF determined using electrocardiograms, hospital discharge diagnosis codes, and death certificates, over an average of approximately 17 years. Dentate participants agreeing to a dental exam were included. Participants with medical contraindication to dental exam, those with dental implants only and individuals not African American or white were excluded (due to limited sample size of other races), were excluded.14 Subjects with prior history of AF were also excluded. The ARIC Investigators are willing to share the data used in this article with a researcher for the purposes of reproducing the results, subject to completion of a data use agreement ensuring appropriate protection of the confidentiality of ARIC participants’ data.
Assessment of PD
PD was classified during visit 4 within the DARIC study. Details on the periodontal profile class (PPC) may be found elsewhere.34 Briefly, participants were grouped into periodontal health (PPC-A), mild PD (PPC-B and C), moderate PD (PPC-D and E) and severe PD (PPC-F and G) groups. Among these groups, periodontal health (PPC-A) served as the comparator group. By definition, they were classified as PPC-A or periodontal health, PPC-B or mild periodontal disease, PPC-C or high gingival index score, PPC-D or tooth loss, PPC-E or posterior periodontal disease, PPC-F or severe tooth loss, and PPC-G or severe periodontal disease. PPC-A through G indicated higher grades of periodontal disease. The PPC is a data-driven agnostic classification system that do not utilize a priori assumptions of disease parameters to create seven different classes of disease that follow a gradient in terms of attachment loss, but not necessarily a gradient in clinical inflammation (ie, gingival index and bleeding scores).34 The addition of the PPC phenotype to traditional vascular risk factors resulted in improved risk models linking PD with diabetes, stroke, coronary heart disease, and systemic inflammatory markers in the ARIC and National Health and Nutrition Examination Survey (NHANES) dataset.35 It was more strongly associated with systemic markers of inflammation than comparison indices namely Centers for Disease Control/American Academy of Periodontology (CDC/AAP) index and the European Periodontal index.35
Assessment of Dental Care Utilization:
Dental care utilization was classified using patient-reported responses to the dental history questionnaire administered at the fourth visit (1996–1998) of the ARIC study . Subjects were classified as regular users (those who sought routine dental care) or episodic users (those who sought dental care only when in discomfort, something needed to be fixed, never, or did not receive regular dental care).
Assessment of AF
Incident AF was assessed by three methods including study EKGs, hospital discharge diagnosis codes, and death certificates.36 Standard, 10-second, 12-lead EKGs were obtained at baseline and at each of the subsequent follow-up examinations. Tracings were performed in the supine position using MAC PC Personal Cardiographs (Marquette Electronics Inc.) and transmitted electronically to the ARIC EKG Reading Center (Epidemiological Cardiology Research Center, Wake Forest School of Medicine, Winston Salem, North Carolina), where they underwent automated reading and coding. Tracings with AF were reviewed by a cardiologist. AF was identified from hospitalizations or death certificates using International Classification of Diseases, Ninth Revisions, Clinical Modification (ICD-9-CM) codes 427.31 or 427.32. These discharge diagnosis codes have been shown to have a positive predictive value of 89% in ARIC for AF diagnosis.36 Similar methodology revealed a sensitivity of 80–85% and specificity of 97-99% in a meta-analysis of published studies.37
Ischemic Stroke
Adjudication regarding stroke within the ARIC Study has been previously described.38 Neuroimaging confirmation of ischemic stroke diagnosis using CT or MRI was obtained from chart-review. Stroke diagnosis was based on computer-derived diagnosis and physician medical record review, with differences adjudicated by a second physician reviewer.
Other Variables of Interest
The other variables were evaluated during our study: age (continuous and categorized as ≥60 and <60 years), sex, race (categorized as white and African American), 3-level educational status (advanced: 17-21 years, intermediate: 12-16 years or basic: ≤11 years), smoking status, alcohol use, body mass index (BMI, continuous as well as categorized as obesity), hypertension, diabetes, hyperlipidemia, low density lipoprotein (LDL) cholesterol (continuous and categorized as ≥100 and <100 mg/dl), prevalent coronary artery disease (CAD), and congestive heart failure (CHF).33 Prevalent CAD was a combination of self-report at baseline plus events hence classified between visit 1 and visit 4. Prevalent CHF was defined by the reported current intake of heart failure medication at visit 4 or evidence of manifest HF with presence of specific cardiac and pulmonary symptoms. Age, sex, and race were reported by participants during baseline. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Obesity was defined as BMI ≥30. Hypertension was defined as systolic blood pressures of 140 mmHg or higher, a diastolic blood pressure higher than 90 mmHg, or use of medications to treat hypertension. Blood samples were obtained after individuals had fasted for 8 hours. Diabetes was determined by self-report of a physician diagnosis of diabetes, non-fasting blood glucose level of 200 mg/dL or higher, fasting blood glucose level of 126 mg/dL or higher or use of insulin or other oral hypoglycemic medications.
Statistical Analysis
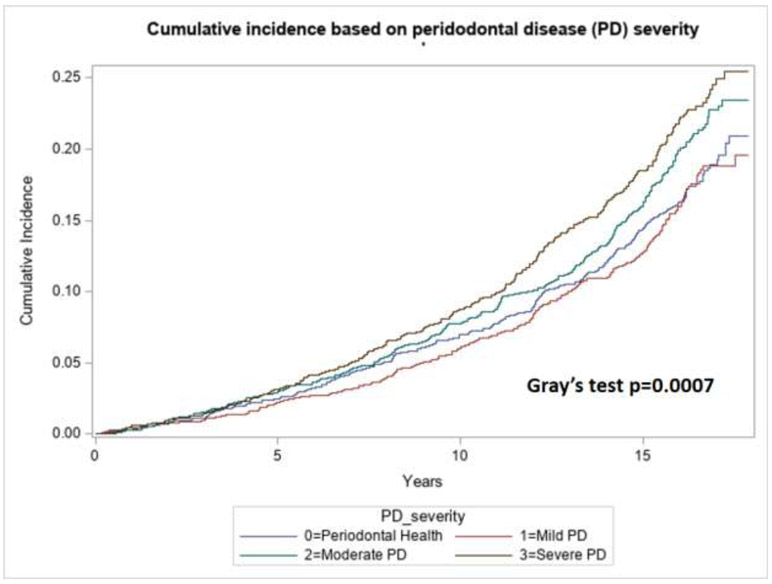
All participants, with or without PD, were assessed for follow-up data on AF. Demographics including age, sex, and race as well as covariates including hypertension, diabetes, hypercholesterolemia, smoking status, current alcohol use, fasting lipid profile, coronary artery disease, and congestive heart failure were assessed. Mean age, BMI, total and LDL cholesterol were compared between groups with periodontal health, mild, moderate and severe PD using analysis of variance (ANOVA). All categorical variables were compared using X2 tests. Crude and adjusted hazards ratio (HR) and 95% confidence intervals of AF by PD severity groups were calculated using Cox proportional hazards models. Initially, the cumulative event rates for the time to incident AF were estimated by cumulative incident function. Differences across periodontal health, mild, moderate and severe PD, were compared using the Gray’s test for equality of cumulative incident function. Subsequently, the HR was used to identify if severe PD is a risk factor for incident AF after adjusting for confounders. We assessed multiplicative interactions between PD severity and incident AF on risk of stroke.
Mediation analysis using the Baron and Kenny causal-steps approach was conducted to test if all four steps were satisfied, suggestive of a mediated model. The four steps included were:
The total effect of PD on cardioembolic stroke (C) was significant.
The effect of PD on AF (A) was significant.
The effect of AF on cardioembolic stroke controlled for PD (B) was significant.
The direct effect of PD on cardioembolic stroke adjusted for AF (C’) was less than C.
Subsequently percent mediation was assessed as the natural indirect effect as a proportion of the total effect adjusting for confounders using general linear modeling.
Finally, the HR was used to determine if regular dental care use reduced the risk for incident AF in univariable and multivariate analyses. All the data analyses for this study were conducted utilizing SAS version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Of 5,958 assessed at Visit 4 without prior AF, 754 were found to have AF over a 17-year follow-up period. Table 1 describes the baseline clinical characteristics of the population with PD broken into periodontal health (PPC-A), mild PD (PPC-B and C), moderate PD (PPC-D and E) and severe PD (PPC-F and G) groups. Periodontal health comprised 28% of the cohort; PPC B-G made up the remaining 72% of this cohort with mild (25%) moderate (27%), and severe (20%) PD. The group of patients with periodontal disease (grouped as mild moderate and severe groups) were significantly older, had higher mean BMI and LDL cholesterol compared to the periodontal health group. They also had significantly higher proportions of hypertension, diabetes, smokers, CAD and CHF. The group of patients with periodontal disease had lower proportion of alcohol users and subjects with advanced level of education compared to the periodontal health group.
Table 1:
Baseline characteristics and risk factors of subjects with no, mild, moderate and severe PD in the Atherosclerosis Risk in Communities (ARIC) Study
| Entire Cohort (N=5958) |
Perio Health (PPC-A) (N=1669) |
Mild PD (PPC-B+C) (N=1466) |
Moderate PD (PPC-D+E) (N=1617) |
Severe PD (PPC-F+ G) (N=1206) |
p value | |
|---|---|---|---|---|---|---|
| Age* (year) | 59.5±5.6 | 58.7 ±5.5 | 59.2± 5.7 | 60.3±5.6 | 59.7±5.6 | <0.0001 |
| Age ≥ 60 years (%) | 2841 (47.7%) | 707 (42.4%) | 661 (45.1%) | 871 (50.3%) | 602 (49.8%) | <0.0001 |
| Male Sex (%) | 2711 (45.5%) | 556 (33.3%) | 739 (50.4%) | 814 (50.3%) | 602 (49.9%) | <0.0001 |
| Race (%) | ||||||
| White | 4927 (82.7%) | 1609 (96.4%) | 1054 (71.9%) | 1479 (91.5%) | 785 (65.1%) | <0.0001 |
| African American | 1031 (17.3%) | 60 (3.6%) | 412 (28.1%) | 138 (8.5%) | 421 (34.9%) | |
| Education level 3 (Advanced: 17-21 y) | 2584(43.5%) | 878 (52.7%) | 667 (45.7%) | 686 (42.5%) | 353 (29.3%) | <0.0001 |
| Education level 2 (Intermediate: 12-16 y) | 2608(43.9%) | 701 (42.1%) | 599 (41.0%) | 757 (46.8%) | 551 (45.8%) | |
| Education level 1 (Basic or 0: ≤11 y) | 752 (12.7%) | 86 (5.2%) | 194 (13.3%) | 173 (10.7%) | 299 (24.9%) | |
| Obesity (BMI† ≥ 25) | 1749 (29%) | 360 (22%) | 486 (33%) | 463 (29%) | 440 (37%) | <0.0001 |
| Hypertension (%) | 2052 (34.5%) | 459 (27.6%) | 564 (38.5%) | 532 (33.0%) | 497 (41.4%) | <0.0001 |
| Diabetes (%) | 400 (6.7%) | 57 (3.4%) | 123(8.4%) | 91 (5.6%) | 129 (10.7%) | <0.0001 |
| Hypercholesterolemia (%) | 1992 (33.7%) | 587 (35.3%) | 468 (32.3%) | 555 (34.5%) | 382 (32.0%) | 0.16 |
|
Total cholesterol* (mg/dL) |
204.9±41.7 | 205.8 ±41.4 | 204.0 ±41.0 | 204.4±42.5 | 205.4±42.0 | 0.59 |
|
LDL cholesterol
* (mg/dL) |
124.3±36.3 | 121.6 ±35.4 | 123.7 ±36.2 | 125.3±37.0 | 127.4±36.5 | 0.0002 |
| LDL ≥ 100 (%) | 4577 (76.8%) | 1239 (72.9%) | 1129 (77.0%) | 1255 (77.6%) | 954 (79.1%) | 0.006 |
| Ever smoked (%) | 3317 (55.7%) | 811 (48.6%) | 729 (49.7%) | 1034 (64.0%) | 743 (61.6%) | <0.0001 |
| Alcohol use (%) | 3428 (57.5%) | 1107 (66.3%) | 770 (52.5%) | 1017 (62.9%) | 534 (44.3%) | <0.0001 |
| CAD (%) | 197 (3.3%) | 30 (1.8%) | 49 (3.4%) | 55 (3.4%) | 63 (5.2%) | <0.0001 |
| CHF (%) | 33 (0.6%) | 1 (0.1%) | 7 (0.5%) | 11 (0.7%) | 14 (1.2%) | 0.001 |
Entries are mean ± SD
The BMI (body mass index) is the weight in kilograms divided by the square of the height in meters
During a 17-year follow-up period, incident AF was noted in 617 (14.4 %) out of 4289 with PD, 197 (11.8%) out of 1669 with periodontal health. Univariable and multivariate Cox regression analyses were performed to calculate the crude and adjusted HR, respectively. Adjusted HR included adjustments for the covariates age, sex, race, hypertension, diabetes, smoking, alcohol use, coronary artery disease (CAD), congestive heart failure and 3-level educational status. The group with moderate and severe PD was associated with incident AF compared with periodontal health group (Figure 1, Gray’s test p value 0.0007). The PD severe group had a significant association with incident AF in both univariable (HR 1.54, 95% CI 1.26-1.87) and multivariate analyses (HR 1.31,95% CI 1.06-1.62) as noted in Table 2. Moderate PD group was associated with higher rates of incident AF only on univariable (HR 1.40, 95% CI 1.16-1.69) and not so on multivariate analyses (HR 1.12,95% CI 0.93-1.36).
Figure 1:
Cumulative incident function of AF according to the level of PD within the patient population with periodontal health (PPC-A) and periodontal disease classified as mild, moderate and severe PD.
Table 2:
HR (95% confidence intervals [CI]) for the association of PD compared with no PD (reference group) and risk of AF with test of PD*AF interaction‡.
| Entire Cohort (N=5958) |
Incident AF 814(13.7%) |
Crude HR |
95% CI | P | Adjusted HR* |
95% CI | P |
|---|---|---|---|---|---|---|---|
| Periodontal health (N=1669) | 197 (11.8%) | 1.0 | Ref. | - | 1.0 | Ref. | - |
| Mild PD (N=1466) | 174 (11.9%) | 1.05 | 0.86-1.29 | 0.64 | 0.94 | 0.76-1.16 | 0.57 |
| Moderate PD (N=1617) | 247 (15.3%) | 1.40 | 1.16-1.69 | <0.0001 | 1.12 | 0.93-1.36 | 0.24 |
| Severe PD (N=1206) | 196(16.3%) | 1.54 | 1.26-1.87 | <0.0001 | 1.31 | 1.06-1.62 | 0.01 |
Adjusted for age≥60, race, sex, hypertension, diabetes, LDL≥100, obesity, smoking, alcohol use, CAD, CHF and education level
Type III p-value for PD*AF interaction was not statistically significant p value 0.11
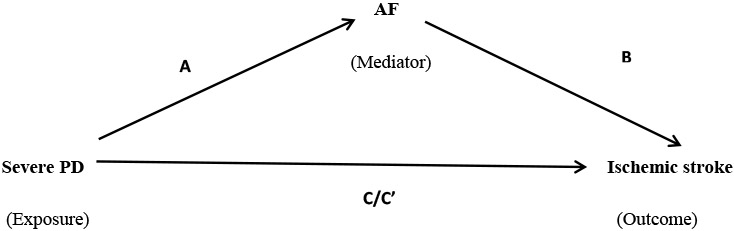
Using Baron and Kenny causal-steps approach of mediation analysis, we found that all four steps were satisfied suggestive of a mediated model (Table 3). A modest 17% (p=0.03) effect of severe PD on stroke was mediated by AF.
Table 3:
Mediation analysis testing the role of AF in mediating the effect of severe PD on ischemic stroke.
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Outcome event | Crude HR* |
95% CI | P | Adjusted HR* |
95% CI | P | ||
| Exposed | Unexposed | |||||||
| A | 196/1206 | 197/1669 | 1.54 | 1.26-1.87 | <0.0001 | 1.31 | 1.06-1.62 | 0.01 |
| B | 92/814 | 233/5144 | 7.76 | 5.95-10.13 | <0.0001 | 5.75 | 4.35-7.61 | <0.0001 |
| C | 91/1206 | 48/1669 | 2.91 | 2.05-4.13 | <0.0001 | 1.93 | 1.33-2.82 | 0.001 |
| C’ | 91/1206 | 48/1669 | 2.71 | 1.91-3.85 | <0.0001 | 1.84 | 1.27-2.69 | 0.001 |
Adjusted for age≥60, race, sex, hypertension, diabetes, LDL≥100, obesity, smoking, alcohol use, CAD, CHF and education level
A: Total effect of severe PD on AF
B: Total effect of AF on ischemic stroke additionally adjusted for severe PD
C: Total effect of severe PD on ischemic stroke
C’: Total effect of severe PD on ischemic stroke additionally adjusted for AF
In the overall ARIC cohort during the fourth ARIC visit (1996– 1998), a total of 9,666 participants without prior history of AF (mean age±standard deviation=62.8±5.6, 56% female, 78% white, and 22% African-American) were assessed for dental care use. Over a 17-year follow-up period, a total of 1,558 participants had incident AF. Regular dental care users had a reduced risk for incident AF (crude HR, 0.82, 95% CI, 0.74–0.90), compared with a reference group of episodic dental care users. After adjustment for vascular risk factors (including age≥60, race, sex, hypertension, diabetes, LDL≥100, obesity, smoking, alcohol use, CAD, CHF and education level), regular dental care use continued to be associated with decreased rates of incident AF (adjusted HR, 0.88, 95% CI, 0.78–0.99).
DISCUSSION
Overall, in a biracial cohort of the dental ARIC study, we report an independent association between severe PD and AF. Mediation analysis suggested AF mediates the association between PD and cardioembolic stroke. In the main ARIC cohort, regular users had a lower risk for AF.. Severe PD includes two groups, PPC-F and G that represent very different situations, although representing the highest severity of PD. Group G (severe PD) may be a reasonable intervention point for periodontal treatment which may reduce AF and associated stroke risk. Group F (severe tooth loss) have an average of 8 teeth left after years of PD progression. The relationship between Group F and AF and stroke likely is due to years of inflammatory disease that resulted in the tooth loss and may not be amenable to periodontal treatment. Further we report that regular dental care users had a reduced risk for incident AF, compared with a control group of episodic dental care users. These results are in line with those reported from a Taiwanese National Health Insurance Research Database. 32 This population-based cohort study was one of the first studies to suggest an association between PD and AF. The risk of new onset AF was attenuated in subjects receiving regular dental scaling. 39 Recently we reported that PD is associated with cardioembolic stroke risk, in addition to atherothrombotic stroke subtype. 30 AF being the most common reason for cardioembolic stroke, it is not surprising that we found that AF could be a possible mediator of PD - cardioembolic stroke risk.
PD is a common chronic inflammatory disease that is associated with systemic inflammation leading to a raised level of CRP and other biomarkers of inflammation. 40-42 These biomarkers of inflammation have been closely linked to the pathogenesis of atherosclerosis leading to cardiovascular events. 43, 44 Therefore, systemic inflammation could portray the underlying mechanism that explains the PD -cardiovascular disease link. Similarly, numerous studies have associated AF to the inflammatory process. 45 High CRP level was associated with a elevated risk of AF development, higher burden of AF and AF recurrence rate after electrical cardioversion and catheter ablation. 46-48A recent animal study demonstrated that periodontitis could induce inflammatory changes in the atrial myocardium, which resulted in structural and electrophysiologic changes of the atrium to facilitate AF development. 49 Therefore, untreated PD may predispose individuals to new AF development due to concomitant inflammatory burden.
In this study, we showed that the new AF risk may be modestly reduced (22% relative risk reduction) through regular dental care. In a prior study which enrolled patients with severe periodontitis, their inflammatory risk category based on CRP measurement was reduced by periodontal therapy. 39 The decrease in CRP level was more prominent among patients with a better response to periodontal treatment. Sun et al, evaluated the effects of periodontal treatment on the levels of CRP, TNF α and IL 6 among fifty patients with impaired fasting glucose and one-hundred six patients with diabetes mellitus. 50 They found that levels of these inflammatory biomarkers significantly reduced after institution of strategies to improve oral hygiene. The findings were replicated in another study performed by Correa et al. 51 Therefore, we presumed that periodontal care through dental scaling and root planning may decrease the burden of inflammation and prevent patients from developing AF. However, a further prospective trial is necessary to confirm this finding.
There were a few limitations within this study that are worthy of note. The ascertainment of AF was using a combination of three methods that included study EKG, hospital discharge diagnosis code, and death certificate. It is possible that without long-term monitoring, that paroxysmal AF may have been undetected in some subjects. The reliance on a single PD assessment, and owing to the observational nature of our investigation, the possibility of unmeasured confounding cannot be eliminated. Furthermore, socioeconomic factors such as access to care, income, and health-care behaviors may be potential confounders. However, we adjusted for educational levels that in these data serve as a surrogate for socioeconomic status and did not find a significant change in the effect measure.
Despite the limitations mentioned previously, the strength of this study includes its large community-based cohort, long follow up duration and assessment of several covariates that may confound the study results. The ascertainment of AF and PD is closely aligned with existing diagnostic criteria. We believe this may be one of the first studies to evaluate the association between PD and AF within the US. Within this large community-based, cohort of both white and African American subjects without prior AF we report an association between AF with severe classes of PD. Hence the results of this study A randomized clinical trial may also help ascertain if severe PD (PPC-F and G) patients may benefit from AF detection and subsequent anticoagulation or antiplatelet therapy as a primary stroke prevention strategy.
Acknowledgments
Sources of funding:
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). The authors thank the staff and participants of the ARIC study for their important contributions.
Souvik Sen MD is the PI on 1 R01 MD009738 PeRiodontal treatment to Eliminate Minority InEquality and Rural disparities in Stroke (PREMIERS) supported by National Institute of Minority Health Disparity.
Alvaro Alonso was supported by American Heart Association grant 16EIA26410001.
Footnotes
Conflict(s)-of-Interest/Disclosure(s):
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Loos BG, Van Dyke TE. The role of inflammation and genetics in periodontal disease. Periodontol 2000. 2020. June;83(1):26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA, Genco RJ. Periodontitis in US adults. National Health andNutrition Examination Survey 2009-2014. J Am Dent Assoc.2018;149(7):576–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of severe periodontitis in1990-2010: a systematic review and meta-regression. J Dent Res.2014;93:1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mustapha IZ, Debrey S, Oladubu M, Ugarte R. Markers of systemic bacterial exposure in periodontal disease and cardiovascular disease risk: A systematic review and meta-analysis. J Periodontol. 2007;78(12):2289–2302. [DOI] [PubMed] [Google Scholar]
- 5.Romagna C, Dufour L, Troisgros O, Lorgis L, Richard C, Buffet P, Soulat G, Casillas JM, Rioufol G, Touzery C, Zeller M, Laurent Y, Cottin Y. Periodontal disease: a new factor associated with the presence of multiple complex coronary lesions. J Clin Periodontol. 2012;39(1):38–44. [DOI] [PubMed] [Google Scholar]
- 6.Loos BG. Systemic markers of inflammation in periodontitis. J Periodontol. 2005;76(11-s):2106–2115. [DOI] [PubMed] [Google Scholar]
- 7.Elkind MS, Cole JW. Do common infections cause stroke? Semin Neurol. 2006;26(01):088–099. [DOI] [PubMed] [Google Scholar]
- 8.Beck JD, Offenbacher S. Systemic effects of periodontitis: Epidemiology of periodontal disease and cardiovascular disease. J Periodontol. 2005;76(11-s):2089–2100. [DOI] [PubMed] [Google Scholar]
- 9.Genco R, Offenbacher S, Beck J. Periodontal disease and cardiovascular disease: epidemiology and possible mechanisms. J Am Dent Assoc. 2002;133 Suppl:14S–22S. [DOI] [PubMed] [Google Scholar]
- 10.Salzberg TN, Overstreet BT, Rogers JD, Califano JV., Best AM, Schenkein HA. C-reactive protein levels in patients with aggressive periodontitis. J Periodontol. 2006;77(6):933–939. [DOI] [PubMed] [Google Scholar]
- 11.You Z, Cushman M, Jenny NS, Howard G, REGARDS. Tooth loss, systemic inflammation, and prevalent stroke among participants in the reasons for geographic and racial difference in stroke (REGARDS) study. Atherosclerosis. 2009;203(2):615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al Rifai M, Schneider AL, Alonso A, Maruthur N, Parrinello CM, Astor BC, Hoogeveen RC, Soliman EZ, Chen LY, Ballantyne CM, Halushka MK, Selvin E. sRAGE, inflammation, and risk of atrial fibrillation: results from the Atherosclerosis Risk in Communities (ARIC) Study. J Diabetes Complications. 2015;29(2):180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beck JD, Eke P, Heiss G, Madianos P, Couper D, Lin D, Moss K, Elter J, Offenbacher S.. Periodontal disease and coronary heart disease. Circulation. 2005;112(1):19–24. [DOI] [PubMed] [Google Scholar]
- 14.Elter JR, Champagne CME, Offenbacher S, Beck JD. Relationship of periodontal disease and tooth loss to prevalence of coronary heart disease. J Periodontol. 2004;75(6):782–790. [DOI] [PubMed] [Google Scholar]
- 15.Sen S, Chung M, Duda V, Giamberardino L, Hinderliter A, Offenbacher S. Periodontal disease associated with aortic arch atheroma in patients with stroke or transient ischemic attack. J Stroke Cerebrovasc Dis. 2017;26(10):2137–2144. [DOI] [PubMed] [Google Scholar]
- 16.Janket S-J, Baird AE, Chuang S-K, Jones JA, Mass B. Meta-analysis of periodontal disease and risk of coronary heart disease and stroke. 2003;95(5): 559–69. [DOI] [PubMed] [Google Scholar]
- 17.Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nature reviews Cardiology. 2015; 12(4):230–43. [DOI] [PubMed] [Google Scholar]
- 18.Al-Zaiti SS. Inflammation-induced atrial fibrillation: pathophysiological perspectives and clinical implications. Heart & lung: the journal of critical care. 2015; 44(1):59–62. [DOI] [PubMed] [Google Scholar]
- 19.Giannopoulos G, Cleman MW, Deftereos S. Inflammation fueling atrial fibrillation substrate: seeking ways to "cool" the heart. Medicinal chemistry. 2014; 10(7):663–71. [DOI] [PubMed] [Google Scholar]
- 20.Galea R, Cardillo MT, Caroli A, Marini MG, Sonnino C, Narducci ML, Biasucci LM.. Inflammation and C-reactive protein in atrial fibrillation: cause or effect? Tex Heart Inst J. 2014. October 1;41(5):461–8.opal Hospital, Texas Children's Hospital. 2014; 41(5):461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Issac TT, Dokainish H, Lakkis NM. Role of inflammation in initiation and perpetuation of atrial fibrillation: a systematic review of the published data. Journal of the American College of Cardiology. 2007; 50(21):2021–8. [DOI] [PubMed] [Google Scholar]
- 22.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997; 96(4):1180–4. [DOI] [PubMed] [Google Scholar]
- 23.Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. Journal of the American College of Cardiology. 2012; 60(22):2263–70. [DOI] [PubMed] [Google Scholar]
- 24.Engelmann MD, Svendsen JH. Inflammation in the genesis and perpetuation of atrial fibrillation. European heart journal. 2005; 26(20):2083–92. [DOI] [PubMed] [Google Scholar]
- 25.Liu T, Li G, Li L, Korantzopoulos P. Association between C-reactive protein and recurrence of atrial fibrillation after successful electrical cardioversion: a meta-analysis. Journal of the American College of Cardiology. 2007; 49(15):1642–8. [DOI] [PubMed] [Google Scholar]
- 26.Joshipura KJ, Hung HC, Rimm EB, Willett WC, Ascherio A. Periodontal disease, tooth loss, and incidence of ischemic stroke. Stroke. 2003; 34(1):47–52. [DOI] [PubMed] [Google Scholar]
- 27.Scannapieco FA, Bush RB, Paju S. Associations Between periodontal disease and risk for atherosclerosis, cardiovascular disease, and stroke. A systematic review. Ann Periodontol. 2003;8(1):38–53. [DOI] [PubMed] [Google Scholar]
- 28.Desvarieux M, Demmer RT, Rundek T, Boden-Albala B, Jacobs DR Jr, Sacco RL, Papapanou PN. Periodontal microbiota and carotid intima-media thickness: the Oral Infections and Vascular Disease Epidemiology Study (INVEST). Circulation. 2005;111(5):576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Syrjänen J, Peltola J, Valtonen V, Iivanainen M, Kaste M, Huttunen JK. Dental infections in association with cerebral infarction in young and middle-aged men. J Intern Med. 1989;225(3): 179–184. [DOI] [PubMed] [Google Scholar]
- 30.Sen S, Giamberardino LD, Moss K, Morelli T, Rosamond WD, Gottesman RF, Beck J, Offenbacher S. Periodontal disease, regular dental care use, and incident ischemic stroke. Stroke. 2018;49(2):355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Topcuoglu AM, Liu L, Kim D-E, Gurol ME. Updates on prevention of cardioembolic strokes. Journal of Stroke 2018;20(2): 180–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen D-Y, Lin C-H, Chen Y-M, Chen H-H. Risk of Atrial fibrillation or flutter associated with periodontitis: A nationwide, population-based, cohort study. PLoS One. 2016;ll(10):e0165601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 34.Morelli T, Moss KL, Beck J, Preisser JS, Wu D, Divaris K, Offenbacher S. Derivation and validation of the periodontal and tooth profile classification system for patient stratification. J Periodontol. 2017;88:153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beck JD, Moss KL, Morelli T, Offenbacher S. Periodontal Profile Class Is Associated With Prevalent Diabetes, Coronary Heart Disease, Stroke, and Systemic Markers of C-reactive Protein and interleukin-6. J Periodontol. 201;89(2):157–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alonso A, Agarwal SK, Soliman EZ, et al. Incidence of AF in whites and African-Americans: The Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158(1):111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying AF using administrative data. Pharmacoepidemiol Drug Saf. 2012;21:141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Androulakis XM, Kodumuri N, Giamberardino L, Rosamond WD, Gottesman RF, Yim E, Sen S. Ischemic stroke subtypes and relationship with migraine with aura in the ARIC study. Neurology 2016;87:2527–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen S-J, Liu C-J, Chao T-F, Wang K-L, Chen T-J, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Wu TJ, Chen TJ, Chen SA. Dental scaling and atrial fibrillation: a nationwide cohort study. Int J Cardiol. 2013;168(2):1360–3. [DOI] [PubMed] [Google Scholar]
- 40.D'Aiuto F, Ready D, Tonetti MS. Periodontal disease and C-reactive protein-associated cardiovascular risk. J Periodontal Res 2004;39:236–41. [DOI] [PubMed] [Google Scholar]
- 41.Slade GD, Ghezzi EM, Heiss G, Beck JD, Riche E, Offenbacher S. Relationship between periodontal disease and C-reactive protein among adults in the Atherosclerosis Risk in Communities study. Arch Intern Med 2003; 163:1172–9. [DOI] [PubMed] [Google Scholar]
- 42.Noack B, Genco RJ, Trevisan M, Grossi S, Zambon JJ, De Nardin E. Periodontal infections contribute to elevated systemic C-reactive protein level. J Periodontol 2001;72:1221–7. [DOI] [PubMed] [Google Scholar]
- 43.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000;342:836–43. [DOI] [PubMed] [Google Scholar]
- 44.Danesh J,Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 2004;350:1387–97. [DOI] [PubMed] [Google Scholar]
- 45.Issac TT, Dokainish H, Lakkis NM. Role of inflammation in initiation and perpetuation of atrial fibrillation: a systematic review of the published data. J Am Coll Cardiol 2007;50:2021–8. [DOI] [PubMed] [Google Scholar]
- 46.Anderson JL, Allen May cock CA, Lappe DL, et al. Frequency of elevation of C-reactive protein in atrial fibrillation. Am J Cardiol 2004;94:1255–9. [DOI] [PubMed] [Google Scholar]
- 47.Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation 2001;104:2886–91. [DOI] [PubMed] [Google Scholar]
- 48.Lin YJ, Tsao HM, Chang SL, et al. Prognostic implications of the high-sensitive C-reactive protein in the catheter ablation of atrial fibrillation. Am J Cardiol 2010; 105:495–501. [DOI] [PubMed] [Google Scholar]
- 49.Yu G, Yu Y, Li YN, Shu R. Effect of periodontitis on susceptibility to atrial fibrillation in an animal model. J Electrocardiol 2010;43:359–66. [DOI] [PubMed] [Google Scholar]
- 50.Sun WL, Chen LL, Zhang SZ, Ren YZ, Qin GM. Changes of adiponectin and inflammatory cytokines after periodontal intervention in type 2 diabetes patients with periodontitis. Arch Oral Biol 2010;55:970–4. [DOI] [PubMed] [Google Scholar]
- 51.Correa FO, Goncalves D, Figueredo CM, Bastos AS, Gustafsson A, Orrico SR. Effect of periodontal treatment onmetabolic control, systemic inflammation and cytokines in patients with type 2 diabetes. J Clin Periodontol 2010;37:53–8. [DOI] [PubMed] [Google Scholar]