Abstract
Background
Postural control is impaired in older adults, as evidenced from greater variability of the center of pressure (COP) during postural tasks. Although COP variability associates with low-frequency COP oscillations (<1 Hz) in young adults, it remains unknown if the age-associated increase in COP variability relates to greater low-frequency COP oscillations.
Research Question
Do low-frequency oscillations contribute to greater postural sway (center of pressure (COP) variability) in older adults when attempting to voluntarily maintain posture in a forward leaning position compared to young adults?
Methods
Seven young (25.7 ± 4.8) and seven older (71.0 ± 7.0) adults performed a postural lean forward task and attempted to match a COP target in the anterior-posterior direction as steady as possible. We quantified the COP variability as the standard deviation (SD) of COP displacements in the anterior-posterior and medial-lateral directions and quantified the frequency modulation of COP as the power in COP displacement spectra from 0–1 Hz.
Results
We found that older adults had significantly greater anterior-posterior SD of COP (p = 0.027) and power below 0.5 Hz (p = 0.048) than young adults, but power from 0.5–1 Hz was similar (p = 0.083). In contrast, the medial-lateral SD of COP (p = 0.5) and power from 0–1 Hz (p=0.228) was similar for the two age groups. For both the anterior-posterior and medial-lateral direction, the SD of COP was related to low frequency oscillations below 0.5 Hz.
Significance
For the first time, we show that the age-associated increase in postural variability relates to greater COP oscillations below 0.5 Hz.
Keywords: COP, Variability, anterior-posterior, medial-lateral, low-frequency oscillations
Introduction
An established finding in the aging literature is the amplification of motor fluctuations with age. The evidence comes from single-joint tasks where young and older adults attempt to trace a steady[1]–[4] or oscillating[5] force target. The greater force variability in older adults during these tasks relates to greater force oscillations below 1 Hz, with most of the age-associated differences occurring below 0.5 Hz[1], [4], [5]. Low-frequency oscillations (<1 Hz) in the motor output are also present in the center of pressure (COP) and affect postural control. Recently, we found that increased low-frequency oscillations in the COP strongly associate with COP variability in young adults[6]. Given that older adults exhibit greater COP variability during postural tasks[7], [8], we test the hypothesis that increased postural variability in older adults relates to greater low-frequency COP oscillations.
Older adults exhibit greater motor variability than young adults in a variety of tasks[3], [9]–[12]. During motor tasks that require single-joint steady contractions, older adults are more variable than young adults regardless of limb[13]–[15]. For example, when maintaining a constant force with visual feedback older adults exhibit greater force variability with abduction of the index finger[1], [2], [13], knee extension[15], and elbow flexion[14], [15]. Increased motor variability during single-joint force tasks is associated with impaired manual dexterity[16] and the ability to react precisely during a simulated driving task[17]. Similarly, older adults exhibit greater motor variability during multi-joint tasks. For example, in a balance task, older adults exhibited greater postural sway during quiet standing[7]. There is evidence that increased postural variability is associated with increased risk of falls in older adults[8], [9]. Although there is strong evidence that greater motor variability during single-joint motor tasks in older adults is associated with low frequency oscillations, the association between COP variability and COP oscillations remains unknown.
Conventionally, motor variability is viewed to represent noise in the central nervous system[18]–[20] based on the workings of information theory[18]. However, emerging evidence over the last 40 years suggests that the variability in the motor output is not random but strongly rhythmical(<1Hz)[1], [2], [21]–[24]. This is significant because there is a parallel between oscillations in the motor output and oscillations of neuronal activity which are fundamental to the communication between different parts of the CNS[25]. Furthermore, there is evidence that these oscillations can be manipulated to improve motor variability[26]. For example, given visual guidelines restricting the amplitude of fluctuations during a steady force task, participants were able to voluntarily decrease the amplitude of low frequency oscillations that subsequently decreased their motor variability[26]. Therefore, testing the above hypothesis is critical to the future application of rehabilitation strategies that can potentially target the origin of COP oscillations in older adults to improve their postural control.
We expect the low frequency oscillations in COP to be greater in older adults and associate with greater postural variability. Indirect support comes from, a 20-day bed-rest study, which found that older adults increased postural variability during quiet standing following bed rest and this increase in variability associated with greater COP oscillations below 1 Hz[27]. Additional evidence comes from neurophysiological experiments showing that aging increases the low-frequency oscillations (<1 Hz) in the neural drive to the motor unit pool. For example, Vaillancourt and colleagues (2002) first showed that both the force and single motor unit activity of the first dorsal interosseous muscle is primarily modulated below 1 Hz[28]. Our lab has published a series of papers [4], [5], [24], [26] where we show that the greater motor output oscillations <1 Hz strongly relate to frequency modulation of whole muscle activity at similar frequencies. Recent work from Farina’s group confirms that greater <1 Hz motor output oscillations in older adults associate with a stronger modulation of the motor unit pool from 0–1 Hz[29]. It is possible, therefore, that a stronger <1 Hz modulation of the motor neuron pool in older adults alters the control of muscles that are directly involved with the control of postural tasks.
Here, we test the hypothesis that greater COP variability in older adults relates to greater low frequency oscillations in the COP. To test this hypothesis, we asked young and older participants to maintain a forward lean posture and quantified their COP variability and COP oscillations. We chose to study this task because it necessitates the control of voluntary contractions while maintaining upright balance, which is often the case during numerous activities of daily living, such as walking or ascending and descending stairs. If we find that low-frequency oscillations affect the COP variability, then future studies can focus on identifying rehabilitation protocols that mitigate low-frequency oscillations during tasks that require both voluntary contractions and upright balance. This could be clinically impactful because it will reduce the risk of falls in older adults.
METHODS
Participants
Seven young adults (25.7 ± 4.8, 5 women) and seven older adults (71.0 ± 7.0, 3 women) volunteered to participate in this study. All participants reported being healthy without any known neurological or orthopedic disorders. Young and older participants had similar MOCA[30] scores (28.6 ± 2.0 and 28.0 ± 2.4), leg length (0.82 ± 0.07 vs. 0.85 ± 0.04 m), and body weight (142.7 ± 31.9 vs. 156.6 ± 21.4lbs). All participants had normal or corrected-to-normal vision and were right handed and right footed, except one old adult, as assessed with the Edinburgh Handedness Inventory[31] and the Waterloo Footedness Questionnaire[32]. The Institutional Review Board at the University of Florida approved the procedures, and participants signed a written informed consent before participating in the study.
Experimental approach
Participants performed one testing session that lasted 30 min. This session involved performing a forward postural leaning task. Participants performed the following: 1) a maximal forward leaning task to determine maximal lean distance; 2) familiarization of the experimental procedure that included a verbal explanation and practice trials of the forward postural leaning task; and 3) three trials of the forward postural leaning task. Participants positioned their feet on a force plate with a stance width of 20% of their leg length. To maintain the same feet position throughout the experiment, we marked their feet position on the force plate. Participants kept both hands at their side and leaned using only their ankles. The trunk and hips remained straight in line with the legs during leaning.
Experimental arrangement
Maximal lean task.
We identified the maximal forward lean distance before the postural lean task. Participants leaned forward as far as possible while keeping both feet on the force plate. Once they reached their maximal distance, they returned to a neutral upright position. We quantified the maximum lean distance as the maximum anterior-posterior COP position during the lean subtracted from the neutral standing position. They performed three trials and we used the greatest lean distance out of the three as the maximum forward postural lean distance.
Forward lean task.
We asked participants to lean forward using only their ankles and match a red horizontal target line. We used a customized Matlab® (Math WorksTM Inc., Natick, MA, USA) program to display the target line and anterior-posterior COP line in the middle of a 32-inch monitor (SyncMasterTM 320MP-2, Samsung Electronics America, Ridgefield Park, NJ, USA). The monitor was located 1 m away at eye level. The anterior-posterior COP produced by the participant was displayed as a blue line progressing with time from left to right (Figure 1). We kept the visual gain constant at 3° (visual angle). The target line was 80% of the maximum forward postural lean distance. The lean distance was determined so to obtain a significant amount of activation in the plantar flexor muscles and to make the task challenging to perform. We instructed participants to voluntarily control their postural sway and keep the anterior-posterior COP on the target line as accurately and consistently as they could. The task duration was 15s. Each participant performed two practice trials and three test trials with a break of at least 1 min between the trials to avoid fatigue.
Figure 1.
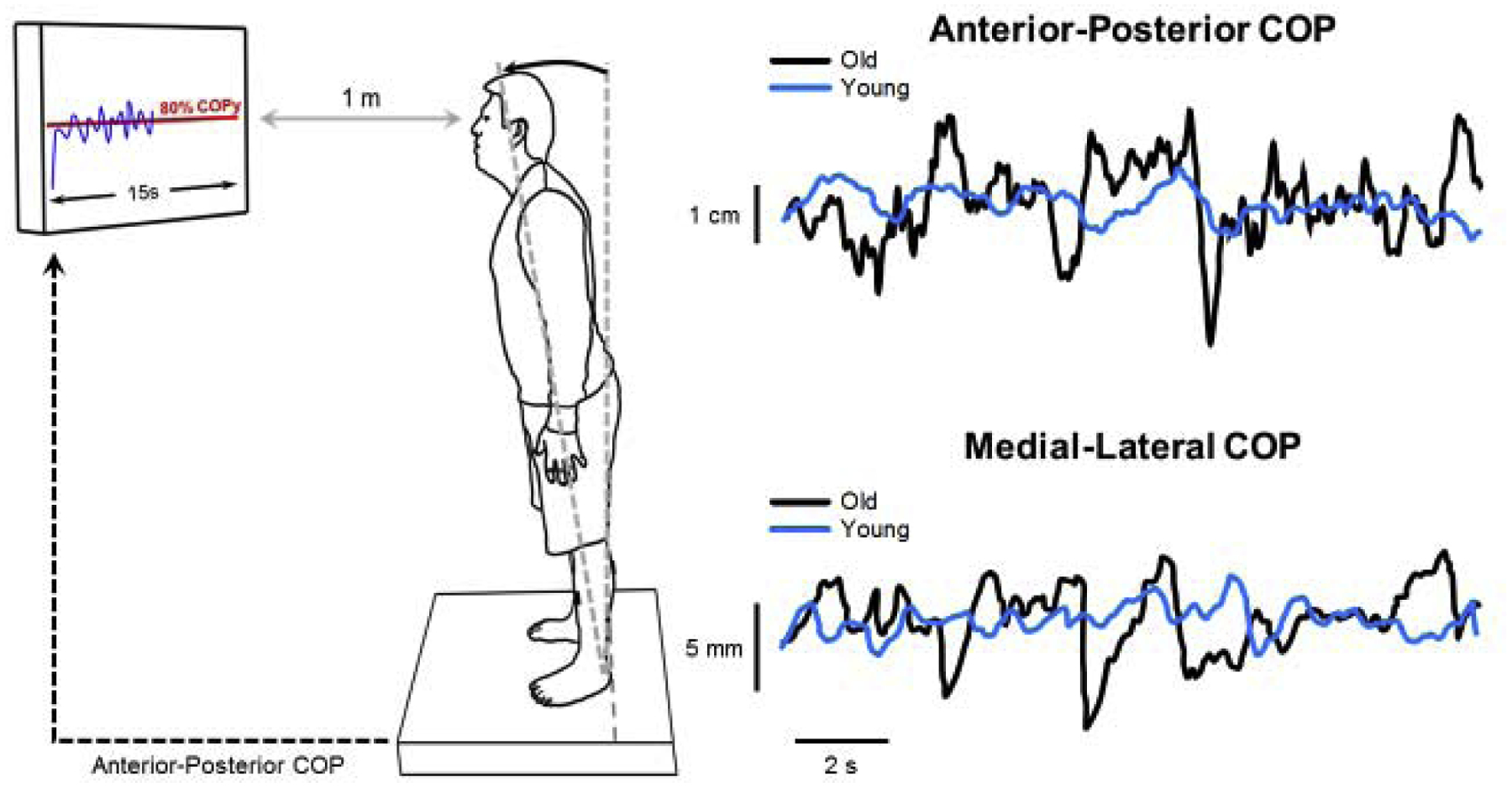
Schematic of the experimental setup for the lean task. The participant stood on a force plate and we instructed them to lean forward and voluntarily control their postural sway to match their anterior-posterior COP (blue line) to the target line (red line) as accurately and consistently as possible. Representative trace of anterior-posterior and medial-lateral COP for both young (light blue) and older (black) adults.
Center of pressure (COP) measurements.
We quantified the anterior-posterior and medial-lateral COP from the force plate signals during the maximal and forward leaning task. We sampled anterior-posterior and medial-lateral COP at 1,000 Hz with a NI-DAQ card (model USB6218, National Instruments, Austin, TX, USA) and stored on a personal computer.
Data analysis
Using a custom-written program in Matlab®, we analyzed the 10 seconds of data prior to one second from the end of the trial. This allowed us to avoid fluctuations in the motor output due to an early relaxation at the end of the trial. We chose an analysis time window of 10 seconds, as we have done before[1], [4], [5], [33], because this allows sufficient time to quantify variability and enough resolution to accurately characterize frequencies below 1 Hz. We included only test trials and ignored practice trials for analysis. Prior to data analysis, the program low-pass filtered the raw COP signal at 20 Hz with a fourth-order (bi-directional) Butterworth filter.
COP variability and oscillations.
We quantified COP variability as the standard deviation (SD) of the anterior-posterior and medial-lateral COP displacements. We quantified COP oscillations using a Fourier analysis and the resulting power spectrum density (PSD)[34]. For the Fourier analysis, we detrended the anterior-posterior and medial-lateral COP displacements. The window size was set at 10s, to maximize the PSD resolution (0.1 Hz) for our data (Figure 2). For statistical comparisons, we divided the PSD of COP into two frequency bins (0–0.5 Hz, 0.5–1.0 Hz)[26], [6]. The dependent variable was the absolute power in each frequency bin.
Figure 2.
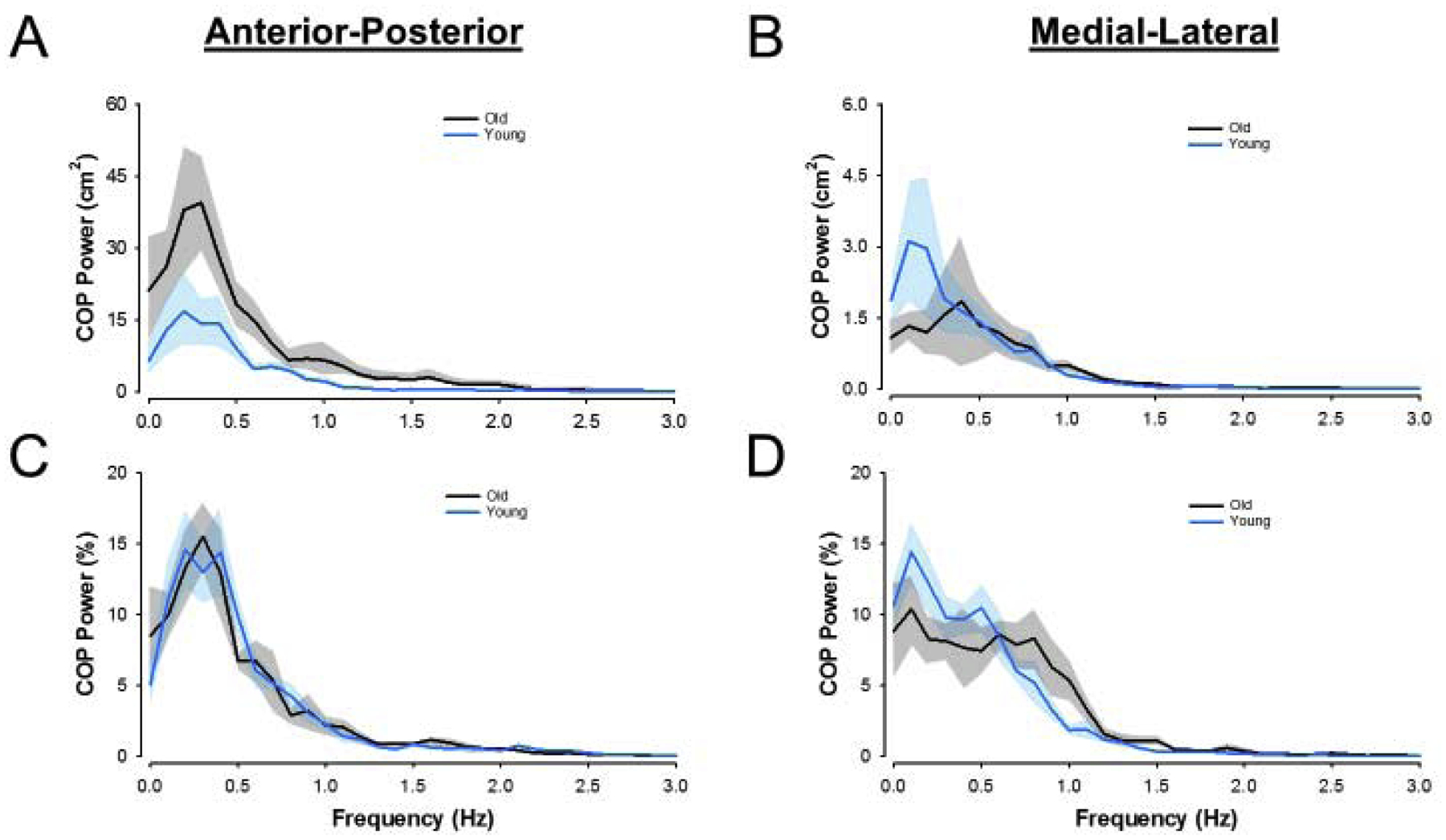
PSD Plots. Power spectrum density data for young (light blue) and older (black) adults. The top row shows raw PSD plots for the anterior-posterior direction (a) and medial-lateral direction (b). The bottom row shows normalized PSD plots for the anterior-posterior direction (c) and medial-lateral direction (d). Solid lines represent group averages with shaded regions representing standard error of the mean of the group data.
Statistical Analysis
Due to our small sample size, we used nonparametric tests to understand differences between young and older adults. We used the independent samples Mann-Whitney U Test to determine whether the following variables were different: 1) COP variability, 2) COP oscillations 0–0.5 Hz, 3) COP oscillations 0.5–1 Hz, 4) COP peak frequency, and 5) COP peak power. We also used stepwise multiple linear regression analysis to establish statistical models that predict the COP variability from COP oscillations. The goodness-of-fit of each regression was given by the squared correlation (R2; Green & Salkind, 2011). We used the Durbin-Watson (DW) statistic to detect the presence of autocorrelation. The alpha level for all statistical tests was 0.05. We performed all statistical analyses with the IBM statistics 24.0 statistical package (IBM Inc., New York). Data are reported as mean ± SD within the text and as mean ± standard error of the mean (SEM) in the figures.
RESULTS
COP variability and COP oscillations.
We compared the SD of COP for young and older adults to test the hypothesis that older adults have greater COP variability than young adults. We found that older adults exhibited greater anterior-posterior variability than young adults (old: 0.52 ± 0.14 cm, young: 0.36 ± 0.15 cm, Mann-Whitney U = 9, one-tailed p = 0.027, η2 = 0.3; Figure 3A). However, young and older adults exhibited similar variability in the medial-lateral direction (old: 0.12 ± 0.04 cm, young: 0.13 ± 0.05 cm, Mann-Whitney U = 25, one-tailed p = 0.5, η2 < 0.1; Figure 3B).
Figure 3.
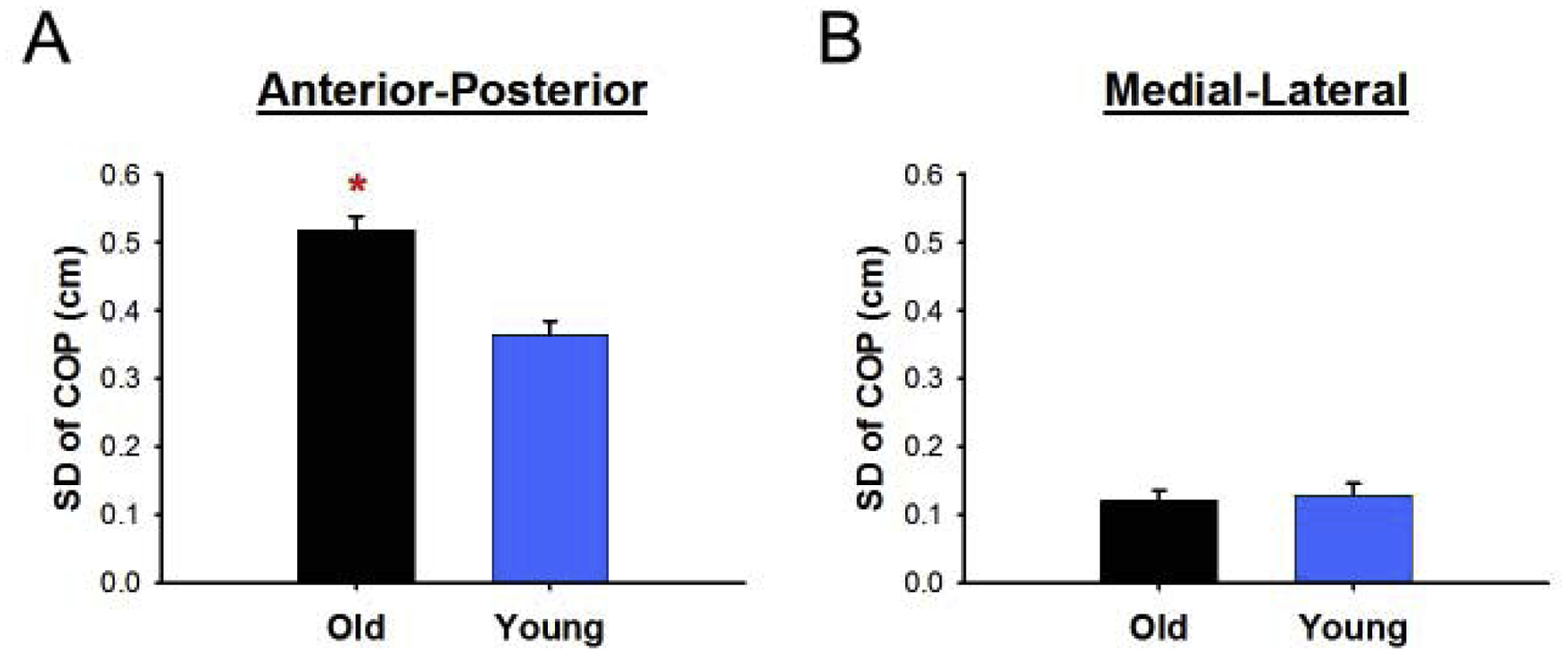
Variability differences between young and older adults. (a) Older adults had greater anterior-posterior COP variability than young adults. (b) Older adults had similar medial-lateral COP variability than young adults. *Significant differences between young and older adults.
We compared the power in COP from 0–0.5 and 0.5–1 Hz for young and older adults to test the hypothesis that older adults will have greater power than young adults in those frequency bands. We found that older adults exhibited greater anterior-posterior COP power from 0–0.5 Hz (old: 150.6 ± 98.3 cm2, young: 64.8 ± 55.9 cm2, Mann-Whitney U = 11, one-tailed p = 0.048, η2 = 0.23; Figure 4A) but similar anterior-posterior COP power from 0.5–1 Hz (old: 56.4 ± 41.3 cm2, young: 26.6 ± 10.8 cm2, Mann-Whitney U = 13, one-tailed p = 0.083, η2 = 0.16; Figure 4B) compared with young adults. We also found that normalized anterior-posterior COP power from 0–0.5 Hz (old: 60.2 ± 19.8 %, young: 58.1 ± 11.1 %, Mann-Whitney U = 21, one-tailed p = 0.355, η2 < 0.1) and 0.5–1 Hz (old: 25.0 ± 11.5 %, young: 28.5 ± 6.96 %, Mann-Whitney U = 31, one-tailed p = 0.228, η2 < 0.1) was similar between young and older adults. In addition, we compared the peak frequency power below 1 Hz. We found that anterior-posterior COP peak frequency was similar between young and older adults (old: 0.39 ± 0.23 Hz, young: 0.31 ± 0.13 Hz, Mann-Whitney U = 19, one-tailed p = 0.268, η2 < 0.1). However, older adults exhibited greater anterior-posterior COP peak power relative to young adults (old: 68.9 ± 45.7 cm2, young: 27.9 ± 20.6 cm2, Mann-Whitney U = 10, one-tailed p = 0.037, η2 = 0.26).
Figure 4.
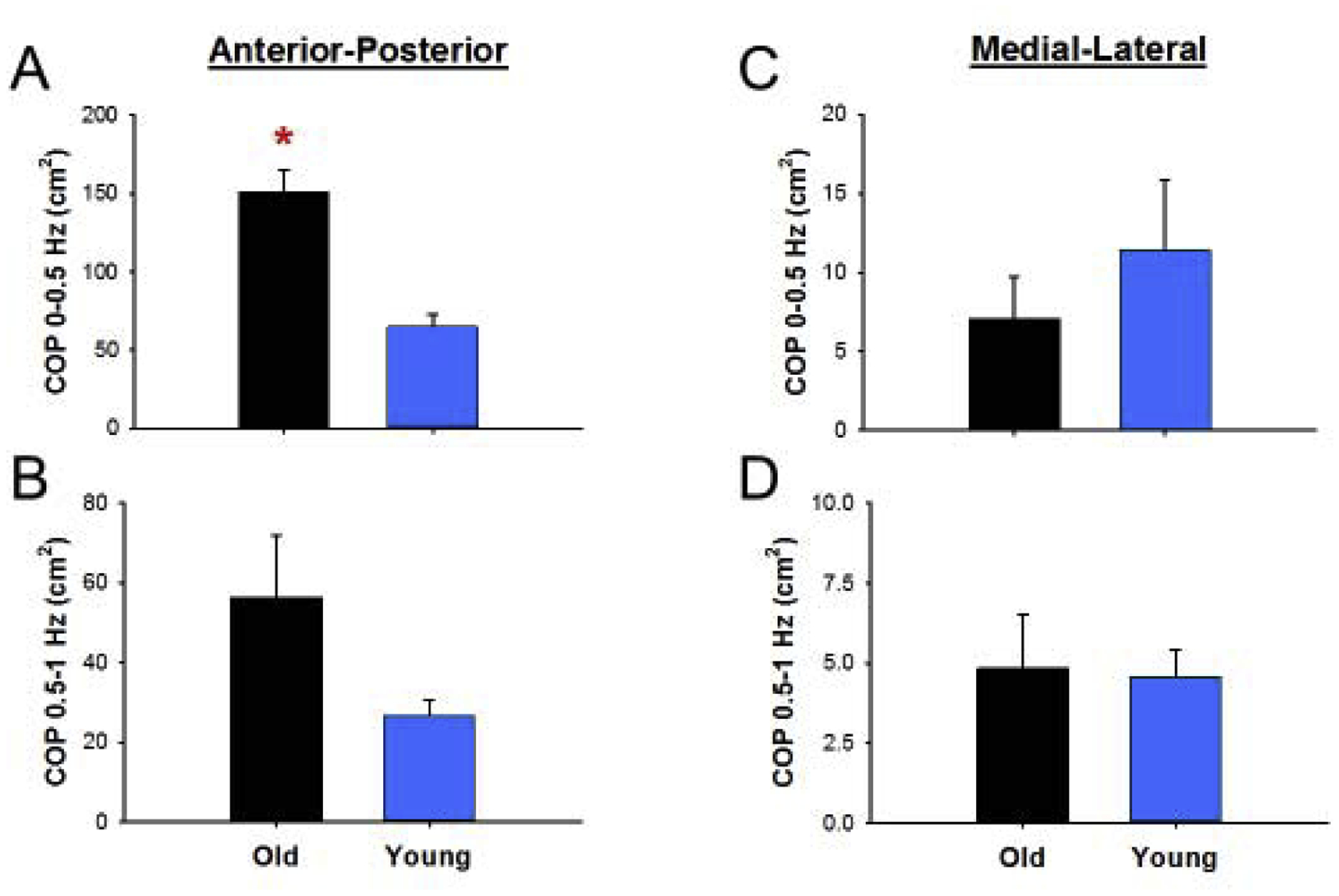
COP oscillation differences between young and older adults. (a) Older adults had greater absolute anterior-posterior COP power from 0–0.5 Hz and (b) similar COP power from 0.5–1 Hz relative to young adults. (c) Older adults had similar absolute medial-lateral COP power from 0–0.5 Hz and 0.5–1 Hz relative to young adults. *Significant differences between young and older adults.
Along the medial-lateral direction, young and older adults exhibited similar COP power from 0–0.5 (old: 7.06 ± 7.11 cm2, young: 11.4 ± 11.6 cm2, Mann-Whitney U = 31, one-tailed p = 0.228, η2 < 0.1; Figure 4C) and 0.5–1 Hz (old: 4.84 ± 4.41 cm2, young: 4.59 ± 2.19 cm2, Mann-Whitney U = 31, one-tailed p = 0.228, η2 < 0.1; Figure 4D). Additionally, young and older adults exhibited similar normalized COP power from 0–0.5 Hz (old: 43.7 ± 15.8 %, young: 56.4 ± 15.3 %, Mann-Whitney U = 36, one-tailed p = 0.083, η2 = 0.17) and 0.5–1 Hz (old: 38.2 ± 13.5 %, young: 33.6 ± 11.1 %, Mann-Whitney U = 21, one-tailed p = 0.355, η2 < 0.1). Thus, older adults appear to exhibit greater COP variability and COP oscillations from 0–0.5 Hz in the anterior-posterior direction.
COP RMSE.
We compared the RMSE of COP for young and older adults to determine whether the performance of the task was comparable between young and old adults. We found that older adults exhibited similar RMSE relative to the young adults (old: 0.60 ± 0.15 cm, young: 0.46 ± 0.19 cm, Mann-Whitney U = 13, one-tailed p = 0.083, η2 = 0.16). Thus, older adults performed the task equally well to young adults.
Association between COP variability and oscillations.
To predict the COP variability from the COP oscillations, we used a stepwise regression analysis. We found that the anterior-posterior COP 0–0.5 Hz oscillations was the single significant predictor of the anterior-posterior COP variability (R2 = 0.75, DW = 2.63, p < 0.01; Figure 5A). On the medial-lateral direction, we found that the COP oscillations from 0–0.5 (r=0.45 part correlation) and 0.5–1 Hz (r=0.34 part correlation) were both significant predictors of COP variability (R2 = 0.92, DW = 1.29, p < 0.01; figure 5B). These findings suggest that power from 0–0.5 Hz is the strongest predictor of anterior-posterior and medial-lateral COP variability.
Figure 5.
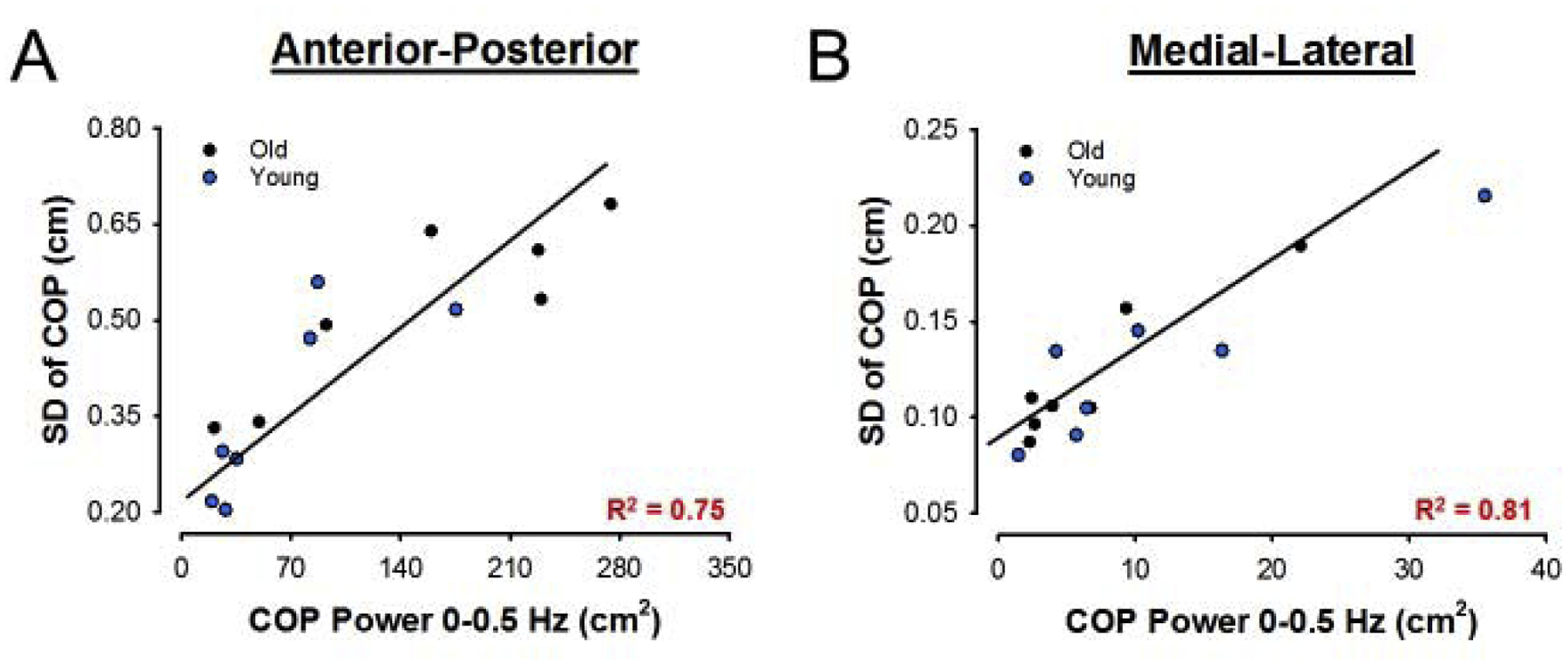
Anterior-posterior (a) and medial-lateral (b) association between COP SD and COP oscillations from 0–0.5 Hz with young (blue dots) and old (black dots) together.
DISCUSION
In this experiment, we aimed to determine if increased postural variability in older adults relates to amplified COP oscillations below 1 Hz. To accomplish this, we asked young and older participants to maintain a forward postural lean and quantified their COP variability and COP oscillations in both the anterior-posterior and medial-lateral direction. A forward postural lean paradigm provides an easy and controlled model to examine postural control requiring continuous voluntary effort. We found that older adults had greater anterior-posterior COP variability and power below 0.5 Hz relative to young adults, but power from 0.5–1 Hz was similar. In contrast, the medial-lateral COP variability and power from 0–1 Hz was similar for both age groups. Additionally, for both the anterior-posterior and medial-lateral direction, COP variability was related to COP oscillations below 0.5 Hz. Therefore, these findings provide novel evidence that increased postural variability during a voluntary task in older adults associates with greater COP oscillations below 0.5 Hz.
One of the consequences of healthy aging is impaired motor control, specifically increased motor variability. Different experiments manipulating the limb being used[13]–[15] or the task performed[3], [9]–[12] provide consistent evidence of age-related increases in variability. From single-joint experiments, it is hypothesized that older adults exhibit increased variability because their voluntary activation of the motor neuron pool and muscle contains greater low-frequency oscillations that correspond to amplified oscillations in the motor output[1]–[5], [9]–[12], [24], [26]. For example, recent work from Farina’s group provide strong evidence that greater 0–1 Hz motor output oscillations in older adults associate with a stronger modulation of the motor unit pool from 0–1 Hz[29]. Additionally, our lab has shown that when older adults perform a steady contraction with finger abduction, they exhibited greater amplitude of force oscillations < 0.5 Hz[1] that is related to similar frequency modulation of whole muscle activity[4], [5], [24], [26]. Therefore, our findings provide support that a stronger <1 Hz modulation of the motor neuron pool in older adults alters the control of muscles that directly influence postural stability.
Contrasting the anterior-posterior and medial-lateral findings raise two important questions. The first question is: Why are COP oscillations greater in the anterior-posterior direction but not in the medial-lateral direction for older adults? We asked participants to voluntarily stabilize their body at a target by leaning forward and provided visual feedback of their COP displacement in the anterior-posterior direction. Due to the instructions and feedback provided, it is reasonable that participants directed their voluntary control along the anterior-posterior direction. Previous studies provide evidence that voluntary activation plays an important role in low-frequency modulation to the motor output. For example, participants exhibited amplified low-frequency oscillations in force while performing a steady contraction at 30%, compared to one at 5% MVC[24]. Furthermore, when given visual guidelines, participants were able to voluntarily decrease low-frequency oscillations in force that consequently decreased force variability[26]. Therefore, it is possible that voluntary effort is more pronounced and consequently magnifies low-frequency modulation in the anterior-posterior rather than the medial-lateral direction.
The second question is: Why do we observe effects primarily from 0–0.5 Hz rather than 0.5–1 Hz? Previous studies demonstrate that most of the fluctuations in the motor output occur below 1 Hz. More specifically, oscillations from 0–0.5 Hz contribute more than 60% to motor output variability as evidenced from single-joint[1], [2], [5], [24], [26], [28] and postural control[6] experiments. Additionally, oscillations 0–0.5 Hz, but not 0.5–1 Hz, modulate muscle activity[24], [6], [36] and relates to force fluctuations in the same frequency range[24]. On the other hand, there is also evidence that oscillations from 0.5–1 Hz contribute to variability in older adults but only with manipulation of visual feedback. For example, Fox and colleagues (2013) demonstrated that when older adults perform a steady contraction, magnification of visual feedback shifted the power from 0–0.5 Hz to 0.5–1 Hz, and related to greater variability[1]. These findings suggest that oscillations from 0.5–1 Hz play a more significant role in modulating the motor output with utilization of magnified visual feedback. In our experiment, visual gain was kept constant for both young and older adults. Furthermore, despite only being provided visual feedback about the COP in the anterior-posterior direction, we observed the same frequency modulation of the COP in the medial-lateral direction. Thus, our findings suggest that the motor output is primarily modulated from 0–0.5 Hz rather than 0.5–1 Hz, and this modulation is likely not related to the use of visual feedback.
Here, we chose to study a task that necessitates the control of voluntary contractions while maintaining upright balance. We used such a task for the following two reasons: 1) Motor output variability is defined as the unintended fluctuations in the output of voluntary contractions [37]. Thus, in order to understand the effects of motor variability on postural control we asked participants to perform a postural balance task while maintaining continuous voluntary control of muscles. 2) The selected task is clinically relevant because numerous activities of daily living, such as walking or ascending and descending stairs, require the control of voluntary contractions while maintaining upright balance. We find that the greater COP variability in older adults relates to greater low-frequency oscillations in the COP. This is clinically important because it links the exacerbated low-frequency oscillations in the COP with COP variability, a strong predictor of the risk of falls [38]. Future studies can focus on identifying rehabilitation protocols that mitigate low-frequency oscillations during tasks that require both voluntary contractions and upright balance. Indeed, our previous work on single joint movements suggests that low-frequency oscillations, and consequently motor variability, can be mitigated with the use of specific visual feedback [26]. Thus, our results provide the basis for expanding this type of work to improve balance in older adults.
Limitations
This study is limited by a small sample size of both young and older adults, and our results would be more generalizable with a larger sample size. Although we did not quantify whether older adults incorporate a different strategy than young adults during this task (e.g. more hip strategy), the findings suggest that they don’t. This is shown from a similar structure in the COP power spectrum (Figure 2C,D) and observational records that both groups used an ankle strategy during this relatively easy leaning forward task. Furthermore, it is unclear if gender differences could contribute to group differences. To our knowledge, no studies have examined gender differences during a forward postural lean task. Evidence is mixed during quiet standing suggesting no difference between men and women[39], [40], worse postural balance for women [41], [42] and worse postural balance for men. Finally, whether the <0.5 Hz COP oscillations relate to an altered voluntary drive to the motor neuron pool and consequently altered activation of the postural muscles remains unclear. Future studies should quantify the modulation of multiple motor units at the soleus, gastrocnemius, hamstrings, quadriceps, and lower back muscles during quiet standing and postural leaning forward tasks.
CONCLUSION
The findings of this small study suggest that increased low-frequency oscillations in the COP associate with more postural variability during a voluntary leaning forward task in older adults. To our knowledge, this is the first study to show that greater COP oscillations associate with the greater COP variability in older adults relative to young adults. These findings are clinically important because they suggest that rehabilitation interventions mitigating low-frequency oscillations in COP could improve postural control in older adults and minimize the risk of falls.
Highlights.
Older adults show greater COP SD than young adults during a forward postural lean.
Older adults show greater COP oscillations <0.5 Hz relative to young adults.
Greater COP oscillations < 0.5 Hz associate with greater COP variability.
Funding:
T32HD043730
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors state no conflict of interest.
References
- [1].Fox EJ, Baweja HS, Kim C, Kennedy DM, Vaillancourt DE, and Christou EA, “Modulation of Force below 1 Hz: Age-Associated Differences and the Effect of Magnified Visual Feedback,” PLoS One, vol. 8, no. 2, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Vaillancourt DE and Newell KM, “Aging and the time and frequency structure of force output variability,” J. Appl. Physiol, vol. 94, no. 3, pp. 903–912, 2003. [DOI] [PubMed] [Google Scholar]
- [3].Christou EA, “Aging and variability of voluntary contractions,” Exercise and Sport Sciences Reviews, vol. 39, no. 2. pp. 77–84, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Park SH, Kwon M, and Christou EA, “Motor output oscillations with magnification of visual feedback in older adults,” Neurosci. Lett, vol. 647, pp. 8–13, April. 2017. [DOI] [PubMed] [Google Scholar]
- [5].Park SH, Kim C, Yacoubi B, and Christou EA, “Control of oscillatory force tasks: Low-frequency oscillations in force and muscle activity,” Hum. Mov. Sci, vol. 64, pp. 89–100, April. 2019. [DOI] [PubMed] [Google Scholar]
- [6].Watanabe T, Nojima I, Sugiura H, Yacoubi B, and Christou EA, “Voluntary control of forward leaning posture relates to low-frequency neural inputs to the medial gastrocnemius muscle,” Gait Posture, vol. 68, pp. 187–192, February. 2019. [DOI] [PubMed] [Google Scholar]
- [7].Prado JM, Stoffregen TA, and Duarte M, “Postural Sway during Dual Tasks in Young and Elderly Adults,” J. Gerontol, no. 53, pp. 274–281, 2007. [DOI] [PubMed] [Google Scholar]
- [8].Oliveira MR et al. , “One-legged stance sway of older adults with and without falls.,” PLoS One, vol. 13, no. 9, p. e0203887, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Carville SF, Perry MC, Rutherford OM, Smith ICH, and Newham DJ, “Steadiness of quadriceps contractions in young and older adults with and without a history of falling,” Eur. J. Appl. Physiol, vol. 100, no. 5, pp. 527–533, June. 2007. [DOI] [PubMed] [Google Scholar]
- [10].Semmler JG, Kornatz KW, V Dinenno D, Zhou S, and Enoka RM, “Motor unit synchronisation is enhanced during slow lengthening contractions of a hand muscle.,” J. Physiol, vol. 545, no. 2, pp. 681–95, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ranganathan VK, Siemionow V, Sahgal V, and Yue GH, “Effects of Aging on Hand Function,” J. Am. Geriatr. Soc, vol. 49, no. 11, pp. 1478–1484, November. 2001. [DOI] [PubMed] [Google Scholar]
- [12].Vaillancourt DE, Larsson L, and Newell KM, “Effects of aging on force variability, single motor unit discharge patterns, and the structure of 10, 20, and 40 Hz EMG activity,” Neurobiol. Aging, vol. 24, no. 1, pp. 25–35, January. 2003. [DOI] [PubMed] [Google Scholar]
- [13].Galganski ME, Fuglevand AJ, and Enoka RM, “Reduced control of motor output in a human hand muscle of elderly subjects during submaximal contractions.,” J. Neurophysiol, vol. 69, no. 6, pp. 2108–15, June. 1993. [DOI] [PubMed] [Google Scholar]
- [14].Graves AE, Kornatz KW, and Enoka RM, “Older Adults Use a Unique Strategy to Lift Inertial Loads With the Elbow Flexor Muscles,” J. Neurophysiol, vol. 83, no. 4, pp. 2030–2039, April. 2000. [DOI] [PubMed] [Google Scholar]
- [15].Tracy BL, V Dinenno D, Jorgensen B, and Welsh SJ, “Aging, Visuomotor Correction, and Force Fluctuations in Large Muscles,” Large Muscles. Med. Sci. Sport. Exerc, vol. 39, no. 3, pp. 469–479, 2007. [DOI] [PubMed] [Google Scholar]
- [16].Kornatz KW, Christou EA, and Enoka RM, “Practice reduces motor unit discharge variability in a hand muscle and improves manual dexterity in old adults,” J. Appl. Physiol, vol. 98, no. 6, pp. 2072–2080, June. 2005. [DOI] [PubMed] [Google Scholar]
- [17].Lodha N, Moon H, Kim C, Onushko T, Christou EA, and Kritchevsky S, “Motor Output Variability Impairs Driving Ability in Older Adults.,” J. Gerontol. A. Biol. Sci. Med. Sci, vol. 71, no. 12, p. glw013, December. 2016. [DOI] [PubMed] [Google Scholar]
- [18].Schmidt RA, Zelaznik H, Hawkins B, Frank JS, and Quinn JT, “Motor-output variability: a theory for the accuracy of rapid motor acts.,” Psychol. Rev, vol. 47, no. 5, pp. 415–51, September. 1979. [PubMed] [Google Scholar]
- [19].Harris CM and Wolpert DM, “Signal-dependent noise determines motor planning,” Nature, vol. 394, no. 6695, pp. 780–784, August. 1998. [DOI] [PubMed] [Google Scholar]
- [20].Faisal AA, Selen LPJ, and Wolpert DM, “Noise in the nervous system.,” Nat. Rev. Neurosci, vol. 9, no. april, pp. 292–303, April. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Slifkin AB and Newell KM, “Noise, information transmission, and force variability.,” J. Exp. Psychol. Hum. Percept. Perform, vol. 25, no. 3, pp. 837–851, 1999. [DOI] [PubMed] [Google Scholar]
- [22].Slifkin AB and Newell KM, “Variability and Noise in Continuous Force Production,” J. Mot. Behav, vol. 32, no. 2, pp. 141–150, June. 2000. [DOI] [PubMed] [Google Scholar]
- [23].Lodha N, Misra G, Coombes SA, Christou EA, Cauraugh JH, and Draganski B, “Increased Force Variability in Chronic Stroke: Contributions of Force Modulation below 1 Hz,” PLoS One, vol. 8, no. 12, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Moon H et al. , “Force control is related to low-frequency oscillations in force and surface EMG.,” PLoS One, vol. 9, no. 11, p. e109202, January. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Engel AK and Fries P, “Beta-band oscillations-signalling the status quo?,” Current Opinion in Neurobiology, vol. 20, no. 2. pp. 156–165, 2010. [DOI] [PubMed] [Google Scholar]
- [26].Park SH, Casamento-Moran A, Yacoubi B, and Christou EA, “Voluntary reduction of force variability via modulation of low-frequency oscillations,” Exp. Brain Res, vol. 235, no. 9, pp. 2717–2727, 2017. [DOI] [PubMed] [Google Scholar]
- [27].Kouzaki M et al. , “Effects of 20-day bed rest with and without strength training on postural sway during quiet standing,” Acta Physiol, vol. 189, no. 3, pp. 279–292, March. 2007. [DOI] [PubMed] [Google Scholar]
- [28].Vaillancourt DE, Larsson L, and Newell KM, “Time-dependent structure in the discharge rate of human motor units,” Clin. Neurophysiol, vol. 113, no. 8, pp. 1325–1338, August. 2002. [DOI] [PubMed] [Google Scholar]
- [29].Castronovo AM, Mrachacz-Kersting N, Stevenson AJT, Holobar A, Enoka RM, and Farina D, “Decrease in force steadiness with aging is associated with increased power of the common but not independent input to motor neurons,” J. Neurophysiol, vol. 120, no. 4, pp. 1616–1624, October. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bourgeois-Marcotte J, Flamand-Roze C, Denier C, and Monetta L, “LAST-Q: adaptation et normalisation franco-québécoises du Language Screening Test,” Rev. Neurol. (Paris), vol. 171, no. 5, pp. 433–436, April. 2015. [DOI] [PubMed] [Google Scholar]
- [31].Oldfield RC, “The assessment and analysis of handedness: the Edinburgh inventory.,” Neuropsychologia, vol. 9, no. 1, pp. 97–113, March. 1971. [DOI] [PubMed] [Google Scholar]
- [32].Elias LJ and Bryden MP, “Footedness is a better predictor of language lateralisation than handedness.,” Laterality, vol. 3, no. 1, pp. 41–51, January. 1998. [DOI] [PubMed] [Google Scholar]
- [33].Lodha N and Christou EA, “Low-frequency oscillations and control of the motor output,” Frontiers in Physiology, vol. 8, no. FEB. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Christou EA, “Visual feedback attenuates force fluctuations induced by a stressor,” in Medicine and Science in Sports and Exercise, 2005, vol. 37, no. 12, pp. 2126–2133. [DOI] [PubMed] [Google Scholar]
- [35].Green SB and Salkind NJ, Using SPSS for Windows and Macintosh : analyzing and understanding data. Pearson/Prentice Hall, 2011. [Google Scholar]
- [36].Yoshitake Y and Shinohara M, “Oscillations in motor unit discharge are reflected in the low-frequency component of rectified surface EMG and the rate of change in force,” Exp. Brain Res, vol. 231, no. 3, pp. 267–276, November. 2013. [DOI] [PubMed] [Google Scholar]
- [37].Christou EA, “Aging and variability of voluntary contractions,” Exercise and Sport Sciences Reviews, vol. 39, no. 2. NIH Public Access, pp. 77–84, April-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pizzigalli L, Micheletti Cremasco M, Mulasso A, and Rainoldi A, “The contribution of postural balance analysis in older adult fallers: A narrative review,” J. Bodyw. Mov. Ther, vol. 20, no. 2, pp. 409–417, April. 2016. [DOI] [PubMed] [Google Scholar]
- [39].Bryant EC, Trew ME, Bruce AM, Kuisma RME, and Smith AW, “Gender differences in balance performance at the time of retirement,” Clin. Biomech, vol. 20, no. 3, pp. 330–335, March. 2005. [DOI] [PubMed] [Google Scholar]
- [40].Hageman PA, Leibowitz JM, and Blanke D, “Age and gender effects on postural control measures,” Arch. Phys. Med. Rehabil, vol. 76, no. 10, pp. 961–965, October. 1995. [DOI] [PubMed] [Google Scholar]
- [41].Kim J-W et al. , “Sex differences in the postural sway characteristics of young and elderly subjects during quiet natural standing,” Geriatr. Gerontol. Int, vol. 10, no. 2, pp. 191–198, February. 2010. [DOI] [PubMed] [Google Scholar]
- [42].Panzer VP, Bandinelli S, and Hallett M, “Biomechanical assessment of quiet standing and changes associated with aging,” Arch. Phys. Med. Rehabil, vol. 76, no. 2, pp. 151–157, February. 1995. [DOI] [PubMed] [Google Scholar]


