Abstract
Objective:
To evaluate how women with epithelial ovarian cancer (EOC), dichotomized by BRCA status, tolerate intravenous (IV) or intraperitoneal (IP) chemotherapy given with veliparib and bevacizumab (bev) on a GOG phase I study (GOG 9923, NCT00989651).
Methods:
This is an unplanned, post hoc analysis of an IRB approved, multi-institutional, prospective study (GOG 9923). Clinical characteristics and toxicity data based on BRCA status were evaluated and descriptive statistics were used to summarize baseline patient characteristics and toxicities. The Kaplan Meier method was used to generate survival estimates.
Results:
Four hundred twenty-four patients were evaluable. Patients were treated with IV carboplatin, paclitaxel, and bev every 21 days (regimen 1), weekly IV paclitaxel with carboplatin and bev (regimen 2) or IV paclitaxel and bev with IP cisplatin (regimen 3). Bev was continued as maintenance in all arms. Within each of these regimens, veliparib was given either twice daily for the entirety of each cycle (continuous) or on days −2 to 5 (intermittent). Ten percent of patients treated on regimen 1, 12% on regimen 2, and 19.8% on regimen 3 had BRCA-associated tumors. Patients with BRCA-associated tumors, when compared to wild type, experienced similar rates of anemia, febrile neutropenia (, abdominal pain, colonic perforation, nausea, vomiting, and peripheral sensory neuropathy. Median progression free survival (PFS) was not significantly different between BRCA-associated and wild type cancers (HR 0.96, CI 0.65–1.42), though this study’s primary aim was not to evaluate outcomes.
Conclusions:
Germline BRCA mutations positively affect chemosensitivity in EOC, but whether differences in toxicities among BRCA-associated and BRCA wild type tumors existed was previously not reported. In this population with newly diagnosed ovarian cancer no differences in reported toxicity between the two groups was observed.
Keywords: Post Hoc analyses, BRCA, toxicities, epithelial ovarian cancer
INTRODUCTION
Epithelial ovarian cancer (EOC), while accounting for only 1.2% of all new cancer cases, is the 5th leading cause of cancer related deaths in women.1,2 Traditional frontline therapy for patients with advanced EOC includes primary or interval primary cytoreduction3–6 with the addition of a platinum/taxane doublet regimen.7 Various routes of administration of platinum/taxane chemotherapy have yielded progression free (PFS) and overall survival (OS) rates ranging from 10.3 to 23.8 months and 39.3 to 65.6 months, respectively.8,9 The addition of bevacizumab has added, on average, 4 months to PFS, but has not changed OS and currently the 5 year OS in patients with EOC remains suboptimal at 48.6%.2
Recent data, however, show significant improvements in outcomes with the addition of poly (ADP-ribose) polymerase inhibitor (PARPi) maintenance in both germline and somatic BRCA-associated cancers10, though additional data suggest that all homologous recombination deficient (HRD) as well as homologous recombination proficient (HRP) tumors may benefit from PARPi therapy.11,12 As expected, patients with BRCA-associated tumors in particular have demonstrated the greatest response to PARPi secondary to the concept of synthetic lethality.
While they portend better response to therapy with PARPi, BRCA mutations may also predispose patients to increased toxicity with both cytotoxic and targeted therapies. The toxicity of primary concern is bone marrow suppression as BRCA 1 and BRCA 2 are two key proteins in the Fanconi anemia DNA repair pathway which is responsible for the maintenance of hematopoetic stem cell function. Inheritance of BRCA1 in particular is responsible for the FANCS subtype of Fanconi anemia, which, upon loss of heterozygosity, can lead to Fanconi anemia and subsequent bone marrow failure.13,14
Data evaluating the degree of toxicity associated with chemotherapy in patients harboring a BRCA mutation has been mixed, though several studies have demonstrated more frequent adverse events (AEs) in patients with BRCA-associated cancers. Huszano et al. evaluated 41 women with BRCA-associated breast cancer as compared to over 200 patients with sporadic breast cancers, all of whom were treated with an anthracycline based regimen. They detected neutropenia more frequently in patients with a BRCA1/2 mutation (32% vs 10%), though this only held true with cycle 1, but not subsequent cycles of chemotherapy.15 Another retrospective review by Badora-Rybicka et al. demonstrated that women with BRCA1-associated ovarian cancer experienced more grade 3–4 hematological toxicities than women with BRCA wild type tumors when undergoing therapy with a platinum/taxane doublet for newly diagnosed ovarian cancer.16 Additionally, a study by Friedlaender et al evaluated 221 women with breast cancer undergoing treatment with (neo)adjuvant chemotherapy (predominantly with doublet cytotoxic therapy - anthracycline and alkylating agent). They found that BRCA1 carriers had a significantly increased rate of febrile neutropenia following cycle 1 compared to BRCA wild type patients (35% vs 10%, p<0.001)17.
In addition to hematologic toxicities, it has been suggested that deficiencies in DNA repair pathways may lead to increased rates of neuropathy with taxane therapy as in vitro data shows accumulation of DNA breaks in cell lines exposed to paclitaxel18. Sucheston, et al evaluated SNPs in the Fanconi Anemia/BRCA pathway and found that, while there was no association with SNPs in BRCA1 and neurotoxicity, SNPs in FANCD2 were associated with an almost twofold increase in the risk of grade 3–4 neurotoxicity19. In this study we aim to evaluate the differences in toxicities experienced by women with BRCA-associated and wild type tumors treated with platinum/taxane doublet therapy with the addition of veliparib and bevacizumab on NRG/Gynecologic Oncology Group protocol (GOG) 9923.
METHODS
This is an unplanned, ad hoc analysis of GOG-9923, an IRB approved, multi-institutional, phase I prospective study of women with newly diagnosed, stage II-IV EOC treated with IV carboplatin, paclitaxel, and bevacizumab every 21 days (regimen 1), weekly IV paclitaxel with carboplatin and bevacizumab (regimen 2) or IV paclitaxel and bevacizumab with IP cisplatin (regimen 3). Bevacizumab was continued as maintenance in all arms. Within each of these regimens, veliparib was given with cytotoxic chemotherapy either twice daily for the entirety of each cycle (continuous) or on days −2 to 5 (intermittent). Primary endpoints of maximum tolerated dose (MTD) and recommended phase 2 dose (RPh2) were previously published.20 Patient eligibility has been previously published.20 In this study, clinical characteristics and toxicity data based on BRCA status were evaluated. Descriptive statistics and Kaplan Meier methods were utilized for analysis. P-values comparing AE frequencies by BRCA status were estimated by chi-square tests without adjustment for multiple testing, among patients with BRCA status known.
RESULTS
Four hundred twenty-four patients were enrolled and evaluable. Of these, 173 (40.8%) were treated on regimen 1, 128 (30.2%) on regimen 2, and 123 (29%) on regimen 3. Overall, the majority of patients were 50–69 years old (66.9%), Caucasian (90.1%), and had a performance status of 0–1 (99.3%). Serous histology (77.6%) was most frequent, followed by endometrioid histology (7.5%), and clear cell histology (5.9%). These findings were similar when patients were evaluated by treatment regimen. Of patients treated on regimen 1, 10.3% percent had BRCA-associated tumors, 51.7% had BRCA wild type tumors, and BRCA status was unknown in 37.9%. Similarly 12.2% of patients on regimen 2 had BRCA-associated tumors, 46.6% were BRCA wild type, and 41.2% had an unknown BRCA status. Finally, 19.8% of patients on regimen 3 had BRCA-related disease while 46.8% had BRCA wild type tumors and 33.3% had an unknown BRCA status (Table 1).
Table 1:
Patient characteristics by treatment regimen. Population: all enrolled evaluable patients (n=424)
| Characteristic | Regimen 1 N(%) |
Regimen 2 N(%) |
Regimen 3 N(%) |
Total N(%) |
|---|---|---|---|---|
| Age (y) | ||||
| <40 | 9 (5.2) | 7 (5.4) | 6 (4.9) | 22 (5.2) |
| 40–49 | 29 (16.8) | 16 (12.5) | 26 (21.1) | 71 (16.7) |
| 50–59 | 60 (34.7) | 47 (36.7) | 42 (34.1) | 149 (35.1) |
| 60–69 | 54 (31.2) | 41 (32) | 40 (32.5) | 135 (31.8) |
| 70–79 | 21 (12.1) | 16 (12.5) | 9 (7.3) | 46 (10.8) |
| ≥80 | 0 | 1 (0.8) | 0 | 1 (0.2) |
| BRCA 1 or 2 | ||||
| BRCA+ | 18(10.3%) | 16(12.2%) | 25(19.8%) | 59 (13.7%) |
| BRCAwt | 90(51.7%) | 61(46.6%) | 59(46.8%) | 210 (48.7%) |
| Unknown | 66(37.9%) | 54 (41.2%) | 42(33.3%) | 162 (37.6%) |
| Race | ||||
| White | 154 (89) | 113 (88.3) | 115 (93.5) | 382 (90.1) |
| Black | 7 (4.0) | 8 (6.3) | 4 (3.3) | 19 (4.5) |
| Asian | 8 (4.6) | 3 (2.3) | 3 (2.4) | 14 (3.3) |
| Am Indian | 1 (0.6) | 0 | 0 | 1 (0.2) |
| Unknown | 3 (1.7) | 4 (3.1) | 1 (0.8) | 8 (1.9) |
| Performance Status | ||||
| 0 | 118 (68.2) | 83 (64.8) | 87 (70.7) | 288 (67.9) |
| 1 | 54 (31.2) | 43 (33.6) | 36 (29.3) | 133 (31.4) |
| 2 | 1 (0.6) | 2 (1.6) | 0 | 3 (.7) |
| Histology | ||||
| Serous | 128 (74) | 100 (78.1) | 101 (82.1) | 329 (77.6) |
| Endometrioid | 15 (8.7) | 7 (5.5) | 10 (8.1) | 32 (7.5) |
| Clear Cell | 14 (8.1) | 7 (5.5) | 4 (3.3) | 25 (5.9) |
| Adeno (NOS) | 3 (1.7) | 4 (3.1) | 4 (3.3) | 11 (2.6) |
| Mucinous | 2 (1.2) | 0 | 0 | 2 (0.5) |
| Mixed | 5 (2.9) | 4 (3.1) | 3 (2.4) | 12 (2.8) |
| Carcinosarcoma | 5 (2.9) | 5 (3.9) | 1 (0.8) | 11(2.6) |
| Other | 1 (0.6) | 1 (0.8) | 0 | 2 (0.5) |
Grade 1–2 hematologic AEs were experienced by 62.5% (108 women) of patients treated on regimen 1, 51.6% (66 women) of patients treated on regimen 2, and 67.5% (83 women) of patients treated on regimen 3. Grade 1–2 metabolic/nutrition AEs, gastrointestinal AEs, and infectious AEs were experienced by 60.2%, 78.6%, and 34.1% of patients in regimen 1. Patients in regimen 2 were noted to have similar grade 1 and 2 AE rates at 51.6%, 80.5%, and 34.3%, respectively. In regimen 3, 53.6%, 75.6%, and 31.7% of patients developed grade 1–2 metabolic/nutrition, gastrointestinal, and infectious AEs.
Thirty-two percent (56 women) of all patients in regimen 1 had grade 3–5 hematologic AEs while 22.5% (39 women) experienced metabolic/nutrition AEs, 17.3% (30 women) experienced gastrointestinal AEs, and 9.9% (17 women) experienced infectious AEs. In regimen 2, 46.9%, 27.3%, 16.3%, and 14.1% of patients experienced these same toxicities, respectively. 26% of patients in regimen 3 experienced grade 3–5 hematologic toxicities. In regimen 3, metabolic/nutrition AEs were experienced by 33.4% of patients while gastrointestinal and infectious AEs occurred in 21.9% and 10.5% of patients. When evaluated by continuous or intermittent veliparib dosing, grade 3–5 AEs were generally similar. Specifically, grade 3–5 hematologic toxicities were experienced by 35.7% and 34.3% of patients, respectively.
Among BRCA-associated and BRCA wild type cancers (excluding BRCA unknown), 58.6% and 62.4% experienced grade 1–2 hematologic toxicities, respectively. Patients with BRCA-associated cancers had a 58.6% incidence of grade 1–2 metabolic/nutrition AEs, 75.9% incidence of grade 1–2 gastrointestinal AEs, and 37.9% incidence of grade 1–2 infectious AEs. Similarly, 54.3%, 79.5%, and 34.8% of patients with BRCA wild type cancers experienced these same grade 1–2 toxicities.
Between BRCA-associated and BRCA wild type cancers (excluding BRCA unknown), grade 3–5 toxicities were grossly similar. Specifically, BRCA-associated cancers, when compared to wild type cancers, experienced similar rates of anemia (29.3% vs 27.2%, p=0.73), febrile neutropenia (8.8% vs 9.1%, p=0.92), abdominal pain (8.8% vs 4.8%, p=0.26), colonic perforation (1.7% vs 1%, p=0.62), nausea (6.9% vs 6.2%, p=0.85), vomiting (5.2 vs 4.8%, p=0.89), and peripheral sensory neuropathy (0% vs 1.4%, p=0.36) (Table 2). Median PFS was not significantly different between BRCA-associated and wild type cancers (HR 0.96, CI 0.65–1.42) (Figure 1), though this study’s primary aim was not to evaluate outcomes.
Table 2:
Adverse events with maximum grade of 3 or higher by BRCA status. Population: patients with known BRCA status (n=58 BRCA-associated, n=210 BRCA wild type, Overall n=268)
| Toxicity |
BRCA positive N(%) |
BRCA negative N(%) |
p-value |
|---|---|---|---|
| Anemia | 17 (29.3%) | 57 (27.2%) | p=0.73 |
| Febrile Neutropenia | 5 (8.8%) | 19 (9.1%) | p=0.92 |
| Abdominal Pain | 5 (8.8%) | 10 (4.8%) | p=0.26 |
| Colonic Perforation | 1 (1.7%) | 2 (1%) | p=0.62 |
| Nausea | 4 (6.9%) | 13 (6.2%) | p=0.85 |
| Vomiting | 3 (5.2%) | 10 (4.8%) | p=0.89 |
| Peripheral Sensory Neuropathy | 0 (0%) | 3 (1.4%) | p=0.36 |
Figure 1:
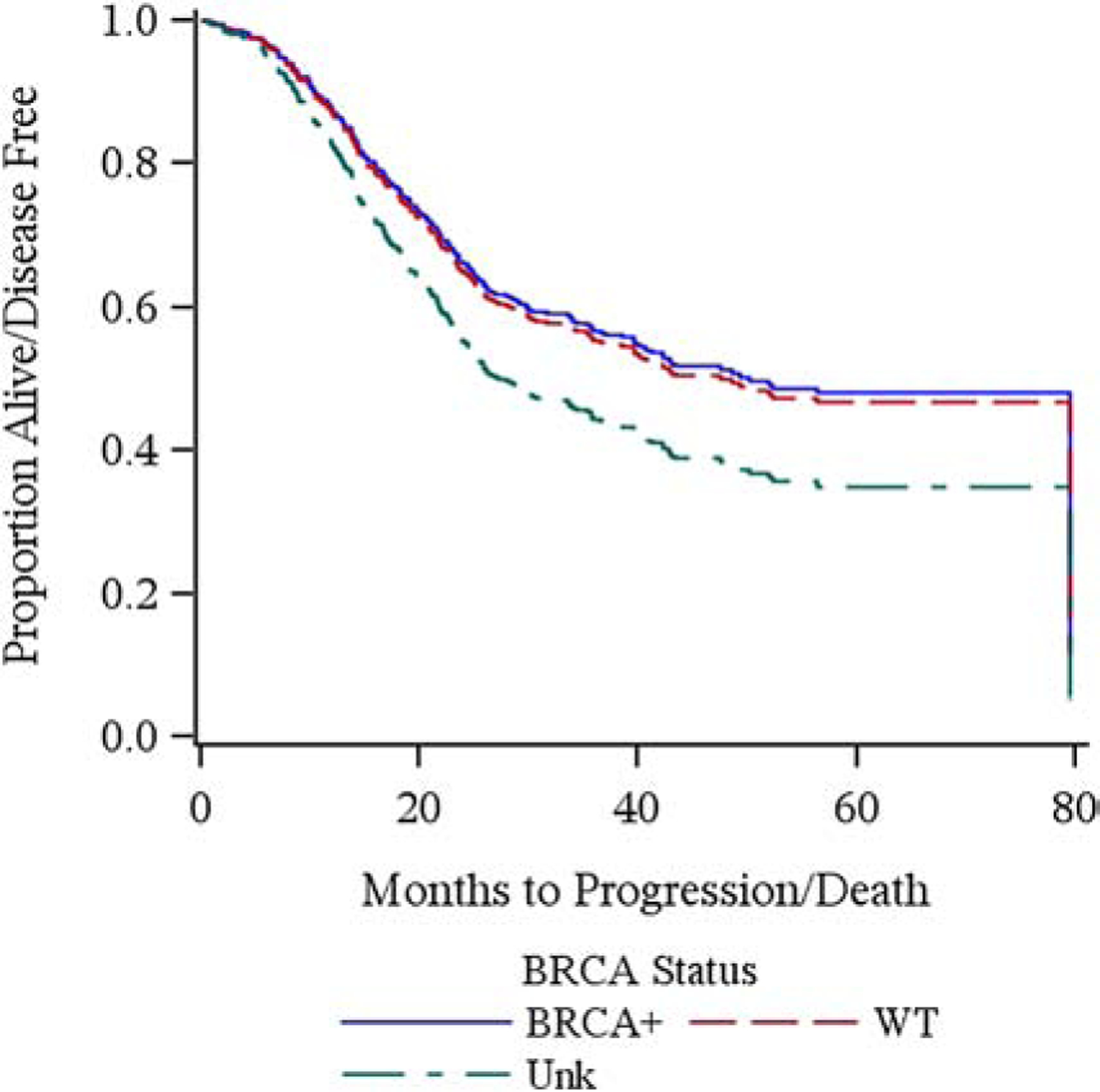
Progression free survival by BRCA status. Population: all patients (n=424).
DISCUSSION
BRCA status, both germline and somatic, is a known biomarker of response to PARPi and incorporation of PARPi into upfront and recurrent therapy for EOC in BRCA-associated ovarian cancer has produced very promising results with regards to PFS and OS.10,21 Given this exciting development, BRCA-associated tumors will increasingly be treated with PARPi, which means understanding the unique toxicity profile experienced by this patient population is of the utmost importance.
Interestingly, while this study was not powered to evaluate differences in PFS or OS among patients with BRCA-associated and BRCA wild type cancers as outcome data was collected retrospectively and did not include all patients treated on this study, we still did not demonstrate any significant signal of improved prognosis among women with BRCA associated cancers. This is likely multifactorial. When considering the previously published data incorporating the prognostic importance of residual disease, we do see a strong signal of improved outcomes among patients with BRCA-associated cancers compared to those with BRCA wild type or genotype unknown cancers (Microscopic disease: PFS not reached, 34.2months, and 24.5 months, respectively).20
Patients with BRCA-associated tumors have demonstrated variable degrees of toxicity when receiving cytotoxic chemotherapy. In a comparison of 41 patients with BRCA related breast cancer to over 200 sporadic breast cancers, all of whom were treated with an anthracycline based regimen, neutropenia was detected more frequently in patients with a BRCA1/2 mutation (32% vs 10%), though this held true only with cycle 1 of chemotherapy.15 In a separate study, febrile neutropenia also occurred more often in women with germline BRCA1-associated breast cancer undergoing cytotoxic chemotherapy compared to BRCA wild type cancers17. Another retrospective review demonstrated that patients with BRCA1-associated ovarian cancer experienced more grade 3–4 hematological toxicities than their BRCA wild type ovarian cancer counterparts when undergoing therapy with a platinum/taxane doublet for newly diagnosed disease.16
Additional data, however, demonstrated that BRCA status has no effect on toxicities experienced. Drooger et al evaluated 701 patients with breast cancer, of which 85 were BRCA related. Women were treated with anthracycline/taxane therapy. No significant difference was noted in rate of febrile neutropenia, delay to next cycle of chemotherapy, or need to switch to an altered chemotherapy regimen.22 Similar results were noted in a retrospective UK study where 62 women with breast cancer were matched to BRCA wild type patients with breast cancer. The most common regimens used were cyclophosphamide/methotrexate/fluorouracil (CMF) and fluorouracil/epirubicin/cyclophosphamide (FCE). Again there was no significant increase in toxicity amongst patients with BRCA-associated disease compared to their matched, sporadic, counterparts and in fact, regimen alterations were more common for control participants than for their BRCA counterparts when receiving CMF therapy.23
In this study evaluating incorporation of veliparib to 3 distinct routes of administration of paclitaxel and carboplatin or cisplatin with bevacizumab, we saw similar rates of toxicities amongst patients with BRCA-associated ovarian cancer and BRCA wild type disease. This data suggests that concomitant administration of platinum/taxane doublet therapy with both bevacizumab and veliparib can be safely administered in both BRCA-associated and BRCA wild type disease without a significant difference in toxicity profile.
RESEARCH HIGHLIGHTS.
BRCA status does not affect hematologic AEs experienced by women receiving cytotoxic therapy with bevacizumab and veliparib
Rates of non-hematologic and hematologic AEs were similar between all 3 regimens
Rates of hematologic AEs were similar between continuous and intermittent veliparib dosing
Median progression free survival was not significantly different between BRCA associated and wild type cancers
Acknowledgments
This study was supported by National Institute of Health grants to NRG Oncology (1 U10 CA180822) and NRG Operations (U10CA180868).
Dr. Miller reports grants from NIH/NCI, grants from NIH/NCI, grants from NIH/NCI, grants from NCI , non-financial support from Argos Therapeutics, Inc, outside the submitted work. Dr. Bell-McGuinn is employed at AbbVie. No other conflicts.
Dr. Russell Schilder received grant funding from the NCI through the GOG. He also received monies for consultancy from Incyte and Flatiron. He serviced on the ad hoc ad board for Clovis. He is the Chair, DSMB at Celsion. Dr. Schilder also received money from Pfizer for a on time lecture honorarium.
Dr. Mathews reports grants from National Cancer Institute, during the conduct of the study; grants from Syros, grants from Deciphera, grants from Astra Zeneca, grants from Astellas Pharma, grants from Tesaro/GSK, grants from Seattle Genetics, grants from Regeneron, grants from Moderna, outside the submitted work.
Dr. Duska reports personal fees from Astra Zeneca, grants, personal fees and other from Genentech/Roche, grants from Cerulean/NextGen/(GOG 3008), grants from AbbVie/(GOG 3005), grants from Tesaro, grants from Pfizer, grants and other from GlaxoSmithKlein/Novartis, grants from Morab, grants and personal fees from MorphoTek, grants, personal fees and other from Merck, grants from Aduro BioTech, grants from Syndax, grants from Ludwig, grants from LEAP Therapeutics, grants from Eisai, grants from Lycera, grants and personal fees from Genentech/Roche, grants and personal fees from Inovio, personal fees from Advance Medical, personal fees from UpToDate, personal fees from Cue Biopharma, personal fees from British Journal of OB/GYN, personal fees from Parexel, personal fees from State of California, personal fees from Elsevier, personal fees from ASCO, personal fees from Expert review, personal fees from ClearView Health Care, personal fees from National Cancer Institute, personal fees from JB Learning, grants from Advaxis, outside the submitted work.
Dr. O’Cearbhaill reports personal fees from Tesaro, personal fees from GlaxoSmithKline, personal fees from Regeneron, other from Genentech USA, personal fees from Genmab Therapeutics, outside the submitted work; and Non-compensated steering committee member for the PRIMA, Moonstone (Tesaro/GSK) and DUO-O (AstraZeneca) studies. My institute receives funding for clinical research from Celgene/Juno, Tesaro/GSK, Ludwig Cancer Institute, Abbvie, Regeneron, TCR2 Therapeutics, Atara Biotherapeutics, MarkerTherapeutics, Syndax Pharmaceuticals, Genmab Therapeutics, Sellas Therapeutics, Genentech, Kite Pharma, Gynecologic Oncology Foundation.
Dr. Hays served on Advisory Boards in consultancy for Clovis, AstraZeneca, Merck, Tesaro, Ipsen and Pfizer.
Dr. Armstrong was an Advisory Board participant for Abbvie.
Dr. Fracasso was an employee of Bristol-Myers Squibb (BMS) from May 1, 2014 until November 30, 2018 and with Adaptimmune LLC from December 1, 2018 until September 4, 2020 and, as such, she has stock with both companies. She is now an independent consultant for Adaptimmune. Prior to her employment with BMS and Adaptimmune and her consultancy, she was a Professor of Medicine and Obstetrics and Gynecology at the University of Virginia (UVA) where she is now affiliated as a Visiting Professor. Her work on this study was done while she was a Professor at UVA and no activities in this submitted work have any relationship to her work at BMS or Adaptimmune LLC.
Dr. Aghajanian reports personal fees from Tesaro, personal fees from Immunogen, grants and personal fees from Clovis, grants from Genentech, grants from AbbVie, grants from Astra Zeneca, grants from Astra Zeneca, personal fees from Eisai/Merck, personal fees from Mersana Therapeutics, personal fees from Roche/Genentech, personal fees from Abbvie, personal fees from AstraZeneca, outside the submitted work.
Dr. Moore reports personal fees and other from Astra Zeneca, grants, personal fees and other from Genentech/Roche, grants, personal fees and other from Immunogen, grants, personal fees and other from Clovis, grants, personal fees and other from Tesaro, personal fees and other from Pfizer, personal fees from Janssen, personal fees from Aravive, personal fees from VBL Therapeutics, personal fees and other from Onco Med, personal fees from Samumed, grants and other from Lilly, personal fees from Eisai, personal fees from Vavotar, personal fees from Abbvie, personal fees from Tarveda, outside the submitted work.
Dr. Moore discloses advisory board participation for Abbvie, Aravive, Astra Zeneca, Eisai, Genentech/Roche, GSK/Tesaro, Immunogen, Merck, Myriad, Mersana, Tarveda, Vavotar. She serves on steering committees for GSK/Tesaro, VBL Therapeutics, Genentech/Roche and IDMC for Incyte.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
All other co-authors have no conflicts of interest to declare.
REFERENCES
- 1.Surveillance, Epidemiology, and End Results Program: Cancer Statistics Facts: Ovarian Cancer, National Cancer Institute, 2019 [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2019. CA Cancer J Clin 69:7–34, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Bristow RE, Tomacruz RS, Armstrong DK, et al. : Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol 20:1248–59, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Winter WE 3rd, Maxwell GL, Tian C, et al. : Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol 25:3621–7, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Wright AA, Bohlke K, Armstrong DK, et al. : Neoadjuvant Chemotherapy for Newly Diagnosed, Advanced Ovarian Cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 34:3460–73, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fagotti A, Ferrandina G, Vizzielli G, et al. : Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): Final analysis of peri-operative outcome. Eur J Cancer 59:22–33, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Ozols RF, Bundy BN, Greer BE, et al. : Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol 21:3194–200, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Armstrong DK, Bundy B, Wenzel L, et al. : Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 354:34–43, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Burger RA, Brady MF, Bookman MA, et al. : Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 365:2473–83, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Moore K, Colombo N, Scambia G, et al. : Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med 379:2495–2505, 2018 [DOI] [PubMed] [Google Scholar]
- 11.González-Martín A, Pothuri B, Vergote I, et al. : Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med 381:2391–2402, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Coleman RL, Fleming GF, Brady MF, et al. : Veliparib with First-Line Chemotherapy and as Maintenance Therapy in Ovarian Cancer. N Engl J Med 381:2403–2415, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.West AH, Knollman H, Dugan J, et al. : Hematologic toxicity in BRCA1 and BRCA2 mutation carriers during chemotherapy: A retrospective matched cohort study. Cancer Med 8:5609–5618, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawyer SL, Tian L, Kähkönen M, et al. : Biallelic mutations in BRCA1 cause a new Fanconi anemia subtype. Cancer Discov 5:135–42, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huszno J, Budryk M, Kołosza Z, et al. : The influence of BRCA1/BRCA2 mutations on toxicity related to chemotherapy and radiotherapy in early breast cancer patients. Oncology 85:278–82, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Badora-Rybicka A, Budryk M, Nowara E, et al. : Treatment related toxicity in BRCA1-associated epithelial ovarian cancer - is DNA repairing impairment associated with more adverse events? Contemp Oncol (Pozn) 20:381–384, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedlaender A, Vuilleumier A, Viassolo V, et al. : BRCA1/BRCA2 germline mutations and chemotherapy-related hematological toxicity in breast cancer patients. Breast Cancer Research and Treatment 174:775–783, 2019 [DOI] [PubMed] [Google Scholar]
- 18.Branham MT, Nadin SB, Vargas-Roig LM, et al. : DNA damage induced by paclitaxel and DNA repair capability of peripheral blood lymphocytes as evaluated by the alkaline comet assay. Mutat Res 560:11–7, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Sucheston LE, Zhao H, Yao S, et al. : Genetic predictors of taxane-induced neurotoxicity in a SWOG phase III intergroup adjuvant breast cancer treatment trial (S0221). Breast Cancer Res Treat 130:993–1002, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore KN, Miller A, Bell-McGuinn KM, et al. : A phase I study of intravenous or intraperitoneal platinum based chemotherapy in combination with veliparib and bevacizumab in newly diagnosed ovarian, primary peritoneal and fallopian tube cancer. Gynecol Oncol 156:13–22, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mirza MR, Monk BJ, Herrstedt J, et al. : Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N Engl J Med 375:2154–2164, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Drooger JC, Heemskerk-Gerritsen BA, Smallenbroek N, et al. : Toxicity of (neo) adjuvant chemotherapy for BRCA1-and BRCA2-associated breast cancer. 156:557–566, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shanley S, McReynolds K, Ardern-Jones A, et al. : Acute chemotherapy-related toxicity is not increased in BRCA1 and BRCA2 mutation carriers treated for breast cancer in the United Kingdom. Clin Cancer Res 12:7033–8, 2006 [DOI] [PubMed] [Google Scholar]


