Abstract
OBJECTIVE:
Gaps in access to appropriate cancer care, and associated cancer mortality, have widened across socioeconomic groups. We examined whether demographic and socioeconomic factors influenced receipt of adjuvant radiation therapy (RT) in patients with high-risk, early-stage endometrial cancer.
METHODS:
A retrospective study cohort was selected from 349,404 endometrial carcinoma patients from the National Cancer Database in whom adjuvant RT would be recommended per national guidelines. The study included surgically treated patients with endometrioid endometrial cancer with one of the following criteria: 1) FIGO 2009 stage IB, grade 1/2 disease, age ≥60 years; 2) stage IB, grade 3 disease; or 3) stage II disease. Logistic regression analysis was performed to identify factors associated with omission of adjuvant RT. Association between adjuvant RT, covariables, and overall survival (OS) was assessed with multivariable Cox proportional hazards models.
RESULTS:
19,594 patients were eligible for analysis; 47% did not receive adjuvant RT. Omission of adjuvant RT was more prevalent among African-American, Hispanic, and Asian compared to non-Hispanic white patients (OR 0.79, 95%CI: 0.69–0.91; OR 0.75, 95%CI: 0.64–0.87; OR 0.75, 95%CI: 0.60–0.94, respectively). Lower median household income of patient’s area of residence, lack of health insurance, treatment at non-academic hospitals, farther distance to treatment facilities, and residence in metropolitan counties were associated with omission of adjuvant RT (OR≤0.81, p≤0.01). Such omission was independently associated with worse OS (HR1.43, p<0.001).
CONCLUSION:
Adjuvant RT is omitted in 47% of patients with early-stage, high-risk endometrial cancer, which is associated with poor access to appropriate, high-quality care and worse outcome.
INTRODUCTION
Although the overall cancer death rate has dropped continuously over the last 2 decades, the gap of socioeconomic inequality in cancer mortality continues to widen. Within the United States, the cancer mortality rate is approximately 20% higher in the poorest counties compared with the more affluent ones (1). It is further estimated that one third of cancer deaths in American adults can be prevented with the elimination of socioeconomic disparities (2). An important contributor to the difference in cancer mortality is barriers to high-quality cancer treatment. These barriers to cancer care are multifaceted and often originate from poverty, inadequate insurance coverage, lack of access to high-level care facilities, and geographic isolation (3–7).
While the overall cancer mortality rate has declined in many types of cancers, endometrial cancer is an exception. From 1999–2016, deaths from endometrial cancer increased 1.1% per year, for an overall total of 21% (8). Most patients with early-stage disease do not require adjuvant treatment; however, national guidelines and major societies, including the American Society of Clinical Oncology (ASCO), the European Society for Medical Oncology (ESMO), and the American Society for Radiation Oncology (ASTRO), recommend adjuvant radiation therapy (RT) for patients considered high risk for recurrence (9–11). The recommendation is based on high-level evidence from multiple randomized trials demonstrating improved pelvic disease control with adjuvant RT in high-risk patients (12–14).
The care disparities in radiation oncology have been linked to racial and ethnic differences and inequalities in income, geographic location, insurance coverage, and access to high-volume facilities (15–20). The aim of this study was threefold: to determine the adherence rate of national guidelines recommending adjuvant RT, to evaluate potential barriers to the receipt of adjuvant RT, and to assess the impact of omission of adjuvant RT on outcome.
METHODS
Data source
The study population was derived from the National Cancer Database (NCDB), established by the Commission on Cancer of the American College of Surgeons and the American Cancer Society as a nationwide, comprehensive oncology registry that captures approximately 70% of all diagnosed malignancies in the United States (21). The database contains 349,404 endometrial carcinoma patients diagnosed in the U.S. between 2004 and 2012.
Study population
We selected a cohort of patients with early-stage endometrial cancer and high-risk features in whom adjuvant RT was warranted based on national cancer treatment guidelines and consensus committees from major cancer societies (9, 10, 22). The study population included surgically treated patients with endometrial cancer of endometrioid histology who satisfied one of the following criteria: 1) International Federation of Gynecology and Obstetrics (FIGO) 2009 stage IB, grade 1 or 2 disease, and age 60 years or older; 2) stage IB, grade 3 disease, and any age; and 3) stage II, any grade disease, and any age. Patients were excluded for non-endometrioid histology, endocervical gland involvement only, undetermined cell type or histology, death within 30 days after surgery, unknown follow-up time or living status, and missing values in collected variables. Since the role of adjuvant chemotherapy in patients with early-stage endometrioid endometrial cancer remains unclear, patients who received adjuvant chemotherapy were excluded. Patients were also excluded if they received adjuvant RT with unconventional isotopes (I-131, P-32, Sr-89/90).
Statistical analyses
Patient clinical, demographic, and socioeconomic variables were selected for analyses based on the consensus of contributing authors. Analyzed variables included age, FIGO stage, grade, lymph node assessment during surgery, race, median income, insurance type, facility type, distance to facility, and residence location. Income was defined by median household income of each patient’s area of residence. Facility type was divided into academic versus non-academic types. Academic facilities were defined as Commission on Cancer-accredited hospitals that see 500 or more new cases per year and participate in postgraduate medical education in at least four areas. All other facilities were categorized as non-academic facilities. Residence in metropolitan or non-metropolitan counties was classified based on United States Department of Agriculture rural-urban continuum codes (23).
Univariate and multivariate logistic regression analyses were performed for variables associated with receipt of adjuvant treatment. The covariates in the analysis included age (<60, 60–69, and ≥70 years), stage (IB, II), surgical lymph node assessment (examined vs. not examined), race and ethnicity (non-Hispanic white, African-American, Hispanic, Asian, and other), median household income (<$38,000, $38,000-$47,999, $48,000-$62,999, and ≥$63,000), insurance status (private, Medicare, Medicaid, and none), facility type (academic vs. non-academic), distance to treatment facility (<10 miles, 10–30 miles, and >30 miles), and county of residence (metropolitan vs. non-metropolitan). Distance categories were chosen based on median (11 miles) and 75% percentile (30 miles) travel distances. Travel distance to treatment facility was plotted against the probability of receiving adjuvant RT or the probability of receiving a specific modality of RT (e.g., external-beam radiation, brachytherapy). Multivariate Cox proportional hazards regression model was performed to identify factors associated with survival outcomes. A two-sided p value of less than 0.05 was considered significant. All analyses were performed using R software 3.5.2 package.
RESULTS
Patient characteristics
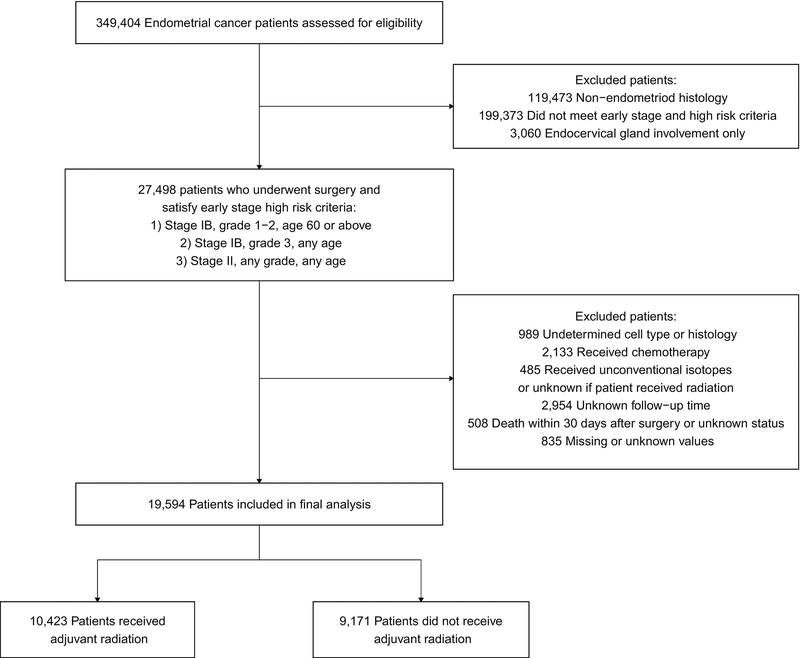
A total of 27,498 patients met the inclusion criteria for the study (Figure 1). Patients were further excluded from this cohort for undetermined cell/histology type (n=989), receiving adjuvant chemotherapy (n=2,133), use of unconventional radioisotopes (I-131, P-32, or Sr-89/90) or unknown if received adjuvant RT (n=485), unknown follow-up time (n=2,954), death within 30 days after surgery or unknown status (n=508), and missing or unknown values (n=835). The final analysis included 19,594 patients.
Figure 1.
CONSORT diagram
The median age of the cohort was 68 years; 14% of patients were younger than 60 years of age and 44% were 70 years of age or older (Table 1). The majority of the patients had stage IB disease (74%); the remaining patients had stage II disease (26%). Surgical lymph node assessment was performed in 82% of the patients. Patients in this cohort exhibited diverse socioeconomic backgrounds. Annual household income levels ranged from less than $38,000 for 16% of patients to more than $63,000 for 32% of patients. The majority of patients (59%) had Medicare insurance; 35% had private insurance and 3% were uninsured. Thirty-eight percent of patients were treated at academic hospitals, and 62% were treated at non-academic hospitals.
Table 1.
Comparison of baseline variables between patients who did not receive radiation therapy (No RT) and those who received adjuvant radiation (RT)
| RT (N=10423) | No RT (N=9171) | p value | Total (N=19594) | |
|---|---|---|---|---|
| Age | <0.001 | |||
| <60 | 1900 (18.2%) | 870 (9.5%) | 2770 (14.1%) | |
| 60–69 | 4449 (42.7%) | 3732 (40.7%) | 8181 (41.8%) | |
| >70 | 4074 (39.1%) | 4569 (49.8%) | 8643 (44.1%) | |
| Charlson Deyo Score | <0.001 | |||
| 0 | 7855 (75.4%) | 6506 (70.9%) | 14361 (73.3%) | |
| 1 | 2135 (20.5%) | 2085 (22.7%) | 4220 (21.5%) | |
| 2+ | 433 (4.2%) | 580 (6.3%) | 1013 (5.2%) | |
| Disease Stage | <0.001 | |||
| IB | 7023 (67.4%) | 7495 (81.7%) | 14518 (74.1%) | |
| II | 3400 (32.6%) | 1676 (18.3%) | 5076 (25.9%) | |
| Grade | <0.001 | |||
| 1 | 3381 (32.4%) | 3753 (40.9%) | 7134 (36.4%) | |
| 2 | 4562 (43.8%) | 3752 (40.9%) | 8314 (42.4%) | |
| 3 | 2480 (23.8%) | 1666 (18.2%) | 4146 (21.2%) | |
| LN Assessment | <0.001 | |||
| LN Examined | 8628 (82.8%) | 7389 (80.6%) | 16017 (81.7%) | |
| LN Not Examined | 1795 (17.2%) | 1782 (19.4%) | 3577 (18.3%) | |
| Race and Ethnicity | 0.113 | |||
| Non-Hispanic White | 9108 (87.4%) | 7955 (86.7%) | 17063 (87.1%) | |
| African-American | 566 (5.4%) | 481 (5.2%) | 1047 (5.3%) | |
| Hispanic | 397 (3.8%) | 378 (4.1%) | 775 (4.0%) | |
| Asian | 172 (1.7%) | 155 (1.7%) | 327 (1.7%) | |
| Others | 180 (1.7%) | 202 (2.2%) | 382 (1.9%) | |
| Distance (miles) | <0.001 | |||
| <10 | 5217 (50.1%) | 3830 (41.8%) | 9047 (46.2%) | |
| 10–30 | 3124 (30.0%) | 2624 (28.6%) | 5748 (29.3%) | |
| >30 | 2082 (20.0%) | 2717 (29.6%) | 4799 (24.5%) | |
| Insurance Type | <0.001 | |||
| Private | 4041 (38.8%) | 2854 (31.1%) | 6895 (35.2%) | |
| Medicare | 5719 (54.9%) | 5828 (63.5%) | 11547 (58.9%) | |
| Medicaid | 372 (3.6%) | 245 (2.7%) | 617 (3.1%) | |
| No insurance | 291 (2.8%) | 244 (2.7%) | 535 (2.7%) | |
| Facility | <0.001 | |||
| Academic | 4119 (39.5%) | 3299 (36.0%) | 7418 (37.9%) | |
| Non-academic | 6304 (60.5%) | 5872 (64.0%) | 12176 (62.1%) | |
| Median Household Income | <0.001 | |||
| >$63,000 | 3550 (34.1%) | 2766 (30.2%) | 6316 (32.2%) | |
| $48,000–$62,999 | 2903 (27.9%) | 2521 (27.5%) | 5424 (27.7%) | |
| $38,000–$47,999 | 2470 (23.7%) | 2342 (25.5%) | 4812 (24.6%) | |
| <$38,000 | 1500 (14.4%) | 1542 (16.8%) | 3042 (15.5%) | |
| Area of Residence | <0.001 | |||
| Non-Metro | 1919 (18.4%) | 1971 (21.5%) | 3890 (19.9%) | |
| Metropolitan | 8504 (81.6%) | 7200 (78.5%) | 15704 (80.1%) | |
p value represents univariate logistic regression analysis of each variable and its association with receipt of radiation. RT: radiation therapy. LN: lymph node.
Receipt of adjuvant RT
Of the 19,594 patients in the analysis, 9,171 (47%) did not receive any adjuvant RT and 10,423 (53%) did. Among patients who received adjuvant RT, 5,081 (49%) received brachytherapy alone and 5,342 (51%) received external-beam radiation.
Factors associated with omission of adjuvant therapy
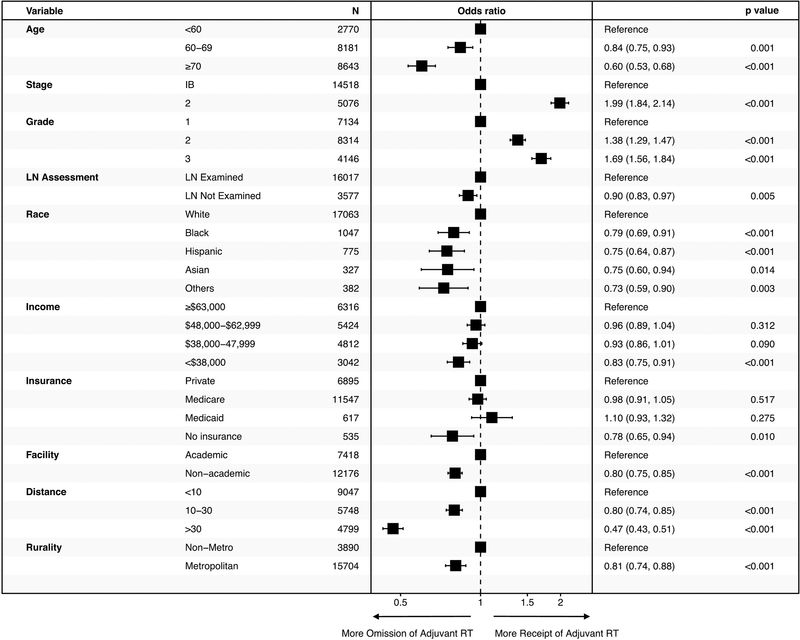
Multivariate analysis of racial/ethnic, demographic, and socioeconomic factors revealed disparities in the care of patients with early-stage, high-risk endometrial cancer (Figure 2). Omission of adjuvant RT was more prevalent among African-American, Hispanic, and Asian patients compared to non-Hispanic white patients (OR 0.79, 95% CI: 0.69–0.91; OR 0.75, 95% CI: 0.64–0.87, and OR 0.75, 95% CI: 0.60–0.94, respectively). Similarly, adjuvant RT was more likely to be omitted for patients with the lowest household income level (<$38,000) compared to the highest income level (≥$63,000) (OR 0.83, 95% CI: 0.76–0.91) and for those with no insurance compared to those with private insurance (OR 0.79, 95% CI: 0.65–0.94). Treatment at a non-academic center compared to academic center also correlated with a higher rate of adjuvant RT omission (OR 0.80, 95% CI: 0.75–0.85).
Figure 2.
Multivariable logistic regression analysis of factors associated with the receipt of adjuvant radiation. Odds ratios with 95% confidence intervals were plotted with the reference groups listed at the top of each variable category. LN: lymph node.
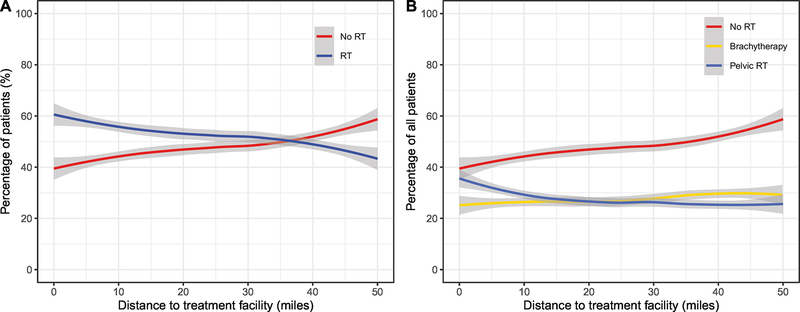
Distance from treatment facility was inversely correlated with the likelihood of receiving adjuvant RT. Median travel distance to a treatment facility was 11 miles, and 75% of patients lived within 30 miles from a treatment facility. Patients who lived more than 30 miles away from a treatment facility were significantly less likely to receive adjuvant RT than patients who lived less than 10 miles from a treatment facility (OR 0.47, 95% CI: 0.43–0.51). On further analysis, the majority (>50%) of patients received adjuvant RT if their travel distance to a treatment facility was less than 37 miles (Figure 3A). The decline in the receipt of adjuvant RT based on distance was only seen in those receiving external-beam radiation and not those receiving brachytherapy (Figure 3B). Residence in a metropolitan county compared to a non-metropolitan county was associated with a higher likelihood of adjuvant RT omission (OR 0.81, 95% CI: 0.74–0.88 ).
Figure 3.
Association of distance to facility and percentage of patients receiving adjuvant radiation (A) and percentage of patients receiving either brachytherapy or external-beam radiation (pelvic RT) (B). RT: radiation therapy. The 95% confidence intervals of fitted curves are represented by the shaded areas.
When the multivariate analysis was limited to patients who underwent surgical lymph node assessment (83% of the cohort), similar results were observed (Supplement Figure 1). When the multivariate analysis was limited to patients who had no comorbid conditions (Charlson Deyo score of 0), we observed similar significance in associations between existing variables and omission of radiation (Supplemental Figure 2).
Factors associated with overall survival
Five-year and ten-year overall survival (OS) rates for the entire cohort were 79.6% and 55.8%, respectively. The median OS for the entire cohort was not reached.
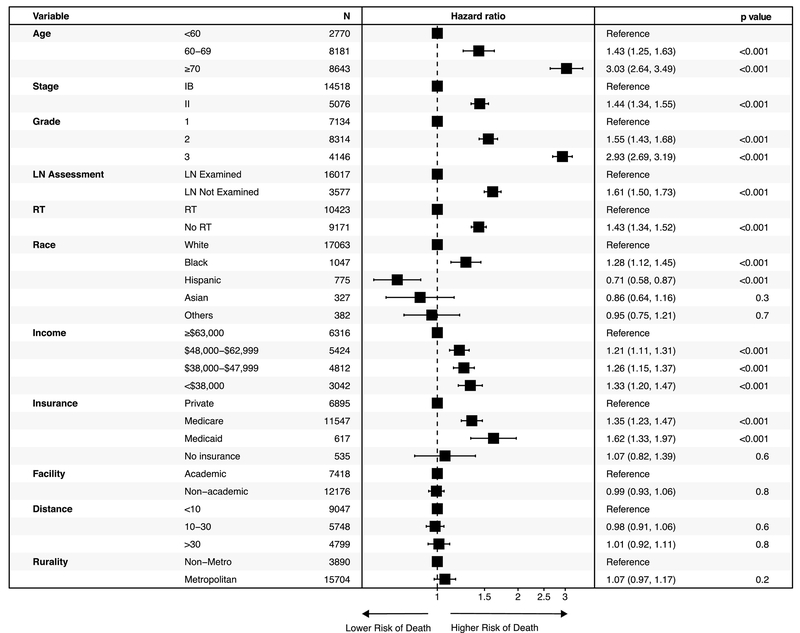
In the Cox proportional hazards model, African-American race was independently associated with worse survival (HR 1.28, 95% CI: 1.12–1.45, p<0.001). Patients with a household income <$38,000 had worse survival than those with higher income levels (HR 1.33, 95% CI: 1.2–1.47, p<0.001). Type of insurance also correlated with outcome. Patients with Medicare (HR 1.35, 95% CI: 1.23–1.47, p<0.001) or Medicaid insurance (HR 1.62, 95% CI: 1.33–1.97 p<0.001) had worse survival than those with private insurance. The omission of adjuvant RT adversely influenced survival on univariate analysis (5-year OS rate: 76.5% vs. 82.1%, p<0.001) as well as multivariate analysis (HR 1.43, 95% CI: 1.34–1.52, p<0.001) (Figure 4). When the analysis was limited to patients who underwent surgical lymph node assessment (83% of the population), similar results were observed (Supplement Figure 3). When the study cohort was restricted to patients without comorbidity (Charlson Deyo score of 0), omission of adjuvant radiation remained associated with worse OS (HR 1.39, 95% CI: 1.28–1.50, p<0.001) (Supplemental Figure 4).
Figure 4.
Cox proportional hazards ratio of mortality according to baseline covariates. LN: lymph node. RT: radiation therapy.
DISCUSSION
ASCO and ASTRO evidence-based guidelines include strong recommendations for adjuvant RT in patients with early-stage endometroid endometrial cancer with high-risk features (10, 11). In this study of early-stage, high-risk endometrioid endometrial cancer, in which we included patients at the highest risk of recurrence (stage IB, grade 1, 2 disease and age ≥60 years; stage IB, grade 3 disease; and stage II disease), we found that a significant proportion (47%) of patients did not receive RT. This study also unveiled large disparities in access to adjuvant RT based on race and ethnicity, income, insurance status, type of medical practice, distance to treatment facilities, and location of residence.
Our study found African-American patients were 21% less likely, and Hispanic and Asian patients were 25% less likely, to receive adjuvant RT compared to non-Hispanic white patients. Racial disparities in receipt of care have been well documented in endometrial cancer (24–30). However, it was often explained away by the observation that African-American women compared to white women were more likely to present with advanced disease, have disease of aggressive histology such as serous or carcinosarcoma, and were less likely to undergo surgical treatment (24, 31). In the current study, we limited our analysis to early-stage disease, excluding serous, carcinosarcoma, and clear cell histologies, and all patients underwent hysterectomy. In contrast, when patients are enrolled in a prospective observational study such as NRG Oncology/GOG 210, for which adherence to guidelines is required, disparities in the receipt of adjuvant RT by race vanish (27). A patient’s socioeconomic status was another factor associated with receipt of RT. In our study, patients with a household income of less than $38,000 were 17% less likely to receive adjuvant RT than patients with an annual income of more than $63,000. In addition, uninsured patients were 28% less likely to receive adjuvant RT than patients with private insurance.
We also found travel distance to treatment facilities and lack of access to an academic institution as strong predictors of omission of adjuvant RT. Patients who had received their treatment at non-academic facilities were 20% less likely to receive RT than patients with access to academic facilities. Patients traveling farther than 30 miles to treatment were 53% less likely to receive adjuvant RT compared to patients traveling less than 10 miles. The association between lack of access to academic facilities or longer travel distance and decreased likelihood of receiving RT treatment has been shown in other types of cancer, including breast and prostate cancers (20, 32, 33). It is worth noting that while the average travel distance to a treatment facility for metropolitan residents in our study was 9 miles compared to 44 miles for non-metropolitan residents, metropolitan residents were 19% less likely to receive adjuvant RT. Such a paradoxical observation, where urban patients report distance as a barrier to care more often than rural patients (34), can be explained by transportation mode. Urban residents must rely on public transportation while rural residents have increased access to private cars (4).
The omission of adjuvant RT was independently associated with worse OS (HR 1.43, p<0.001). While the aim of this study was not to revisit the question of whether or not adjuvant RT impacts OS in endometrial cancer, it is worth noting that the patients included in this study were those at highest risk of relapse for early-stage endometrioid adenocarcinoma of the uterus. Furthermore, there have not been any randomized trials comparing surgery alone to surgery plus adjuvant RT in such a high-risk patient population. African-American race was also independently associated with worse survival (HR 1.28, p<0.001). Investigators have pointed out that African-American patients with endometrial cancer are more likely to develop recurrence irrespective of treatments received, pointing towards the need for a better understanding of the biology of endometrial cancer in African-American patients (35). While this is true, it does not negate the fact that these same patients should be receiving more adjuvant therapy, not less. In addition, income <$38,000 (HR 1.33, p<0.001) and Medicare (HR 1.35, p<0.001) or Medicaid insurance (HR 1.62, p<0.001) were associated with worse survival. Some of the factors associated with adjuvant RT omission did not independently influence OS, most likely because socioeconomic status is intimately intertwined with race and ethnicity and a major barrier of access to appropriate care (24, 36).
In this study cohort, a subset of patients (17%) did not undergo surgical lymph node assessment. These patients were included in this study because they reflect the enrollment criteria of published randomized trials that looked at the efficacy of adjuvant RT (12, 13) and practice patterns in the general community. To limit any potential confounding influence of lack of surgical lymph node assessment on our results, we performed the same multivariate logistic regression analysis and Cox proportional hazards ratio analysis on patients who underwent surgical lymph nodal assessment (83% of the cohort). This yielded the same results in terms of factors associated with the omission of adjuvant RT and factors associated with worse OS.
This study is not without its limitations. It is retrospective in nature and subject to patient selection bias. We attempted to account for as many relevant variables as possible in our multivariable analysis. However, studies evaluating adherence to national guidelines can only be gleaned from data collected from entities such as the NCDB. Also, given NCDB is a hospital-based registry and may not completely capture outpatient radiation records, it may be subject to ascertainment bias. Prior reports have estimated 10–30% under-ascertainment of radiation receipt in Surveillance, Epidemiology, and End Results (SEER) registries (37, 38). To our knowledge, the extent of ascertainment bias in NCDB studies is unknown. A potential source of underreporting could be miscoding of radiotherapy excluded in our study, such as unconventional isotopes and chemotherapy. And with an adjuvant RT omission rate as high as 47%, a 10–30% rate of underreporting would not fully explain the high rate of omission noted, nor would it account for the significant correlation with socioeconomic factors and worse survival.
CONCLUSION
Adjuvant RT was omitted in a significant proportion of patients with high-risk, early-stage endometrial cancer. Such omission was associated with racial and ethnic minorities, lower income, poor access to academic facilities, and farther distance to treatment facilities. Omission of adjuvant RT was associated with worse survival, which underscores the importance of identifying the barriers to care access and improve adherence to national guidelines.
Supplementary Material
Supplemental Figure 1 (online only). Multivariable logistic regression analysis of factors associated with the receipt of adjuvant radiation after excluding patients who did not have lymph node assessment. Odds ratios with 95% confidence intervals were plotted with the reference groups listed at the top of each variable category.
Supplemental Figure 2 (online only). Multivariable logistic regression analysis of factors associated with the receipt of adjuvant radiation after excluding patients with Charlson Deyo score of 1 or above. Odds ratios with 95% confidence intervals were plotted with the reference groups listed at the top of each variable category.
Supplemental Figure 3 (online only). Cox proportional hazards ratio of mortality according to baseline covariates after excluding patients who did not have lymph node assessment. RT: radiation therapy.
Supplemental Figure 4 (online only). Cox proportional hazards ratio of mortality according to baseline covariates after excluding patients with Charlson Deyo score of 1 or above. RT: radiation therapy.
Highlights.
Socioeconomic inequalities are consistent barriers to appropriate, high-quality cancer care
47% of those with high-risk, early-stage endometrial cancer did not receive guideline-indicated adjuvant radiation therapy
Factors associated with this omission include low income, lack of health insurance, and area of residence
Acknowledgments
Funding: Funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Conflict of Interest Statement
C. Jillian Tsai reports consulting for Varian outside the submitted work. Dr. Abu-Rustum reports grants paid to his institution from Stryker/Novadaq, Olympus, and GRAIL outside the submitted work. The other authors have no conflict of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(l):7–34. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Jemal A, Wender RC, Gansler T, Ma J, Brawley OW. An assessment of progress in cancer control. CA Cancer J Clin. 2018;68(5):329–39. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg ML. Inequity in cancer care: explanations and solutions for disparity. Semin Radiat Oncol. 2008;18(3):161–7. [DOI] [PubMed] [Google Scholar]
- 4.Spees LP, Wheeler SB, Varia M, Weinberger M, Baggett CD, Zhou X, et al. Evaluating the urban-rural paradox: The complicated relationship between distance and the receipt of guideline-concordant care among cervical cancer patients. Gynecol Oncol. 2019;152(1):112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson A. Unequal treatment: confronting racial and ethnic disparities in health care. J Natl Med Assoc. 2002;94(8):666–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman HP, Payne R. Racial injustice in health care. N Engl J Med. 2000;342(14):1045–7. [DOI] [PubMed] [Google Scholar]
- 7.Freeman HP. Poverty, culture, and social injustice: determinants of cancer disparities. CA Cancer J Clin. 2004;54(2):72–7. [DOI] [PubMed] [Google Scholar]
- 8.Henley SJ, Miller JW, Dowling NF, Benard VB, Richardson LC. Uterine Cancer Incidence and Mortality - United States, 1999–2016. MMWR Morb Mortal Wkly Rep. 2018;67(48):1333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colombo N, Creutzberg C, Amant F, Bosse T, Gonzalez-Martin A, Ledermann J, et al. ESMOESGO-ESTRO Consensus Conference on Endometrial Cancer: diagnosis, treatment and follow-up. Ann Oncol. 2016;27(1):16–41. [DOI] [PubMed] [Google Scholar]
- 10.Klopp A, Smith BD, Alektiar K, Cabrera A, Damato AL, Erickson B, et al. The role of postoperative radiation therapy for endometrial cancer: Executive summary of an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2014;4(3):137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer LA, Bohlke K, Powell MA, Fader AN, Franklin GE, Lee LJ, et al. Postoperative Radiation Therapy for Endometrial Cancer: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation Oncology Evidence-Based Guideline. J Clin Oncol. 2015;33(26):2908–13. [DOI] [PubMed] [Google Scholar]
- 12.Creutzberg CL, Nout RA, Lybeert ML, Warlam-Rodenhuis CC, Jobsen JJ, Mens JW, et al. Fifteen-year radiotherapy outcomes of the randomized PORTEC-1 trial for endometrial carcinoma. Int J Radiat Oncol Biol Phys. 2011;81(4):e631–8. [DOI] [PubMed] [Google Scholar]
- 13.Group AES, Blake P, Swart AM, Orton J, Kitchener H, Whelan T, et al. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): pooled trial results, systematic review, and meta-analysis. Lancet. 2009;373(9658):137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, et al. Aphase III trialof surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92(3):744–51. [DOI] [PubMed] [Google Scholar]
- 15.Grant SR, Walker GV, Koshy M, Shaitelman SF, Klopp AH, Frank SJ, et al. Impact of Insurance Status on Radiation Treatment Modality Selection Among Potential Candidates for Prostate, Breast, or Gynecologic Brachytherapy. Int J Radiat Oncol Biol Phys. 2015;93(5):968–75. [DOI] [PubMed] [Google Scholar]
- 16.Kaleem T, Smith GL, Miller RC. Impact of care disparities in radiation oncology. Adv Radiat Oncol. 2018;3(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin CC, Bruinooge SS, Kirkwood MK, Hershman DL, Jemal A, Guadagnolo BA, et al. Association Between Geographic Access to Cancer Care and Receipt of Radiation Therapy for Rectal Cancer. Int J Radiat Oncol Biol Phys. 2016;94(4):719–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin JF, Berger JL, Krivak TC, Beriwal S, Chan JK, Sukumvanich P, et al. Impact of facility volume on therapy and survival for locally advanced cervical cancer. Gynecol Oncol. 2014;132(2):416–22.. [DOI] [PubMed] [Google Scholar]
- 19.McClelland S 3rd, Page BR, Jaboin JJ, Chapman CH, Deville C Jr, Thomas CR Jr. The pervasive crisis of diminishing radiation therapy access for vulnerable populations in the United States, part 1: African-American patients. Adv Radiat Oncol. 2017;2(4):523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang DD, Muralidhar V, Mahal BA, Beard CJ, Mouw KW, Martin NE, et al. Travel Distance as a Barrier to Receipt of Adjuvant Radiation Therapy After Radical Prostatectomy. Am J Clin Oncol. 2018;41(10):953–9. [DOI] [PubMed] [Google Scholar]
- 21.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oncology NCCNCGi. Uterine Neoplasms. 2019(Version 2.2019).
- 23.US Department of Agriculture ERS. Rural-urban continuum codes. 2013.
- 24.Bregar AJ, Alejandro Rauh-Hain J,Spencer R, Clemmer JT, Schorge JO, Rice LW, et al. Disparities in receipt of care for high-grade endometrial cancer: A National Cancer Data Base analysis. Gynecol Oncol. 2017;145(1):114–21. [DOI] [PubMed] [Google Scholar]
- 25.Clarke MA, Devesa SS, Harvey SV, Wentzensen N. Hysterectomy-Corrected Uterine Corpus Cancer Incidence Trends and Differences in Relative Survival Reveal Racial Disparities and Rising Rates of Nonendometrioid Cancers. J Clin Oncol. 2019;37(22):1895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fader AN, Habermann EB, Hanson KT, Lin JF, Grendys EC, Dowdy SC. Disparities in treatment and survival for women with endometrial cancer: A contemporary national cancer database registry analysis. Gynecol Oncol. 2016;143(1):98–104. [DOI] [PubMed] [Google Scholar]
- 27.Felix AS, Cohn DE, Brasky TM, Zaino R, Park K, Mutch DG, et al. Receipt of adjuvant endometrial cancer treatment according to race: an NRG Oncology/Gynecologic Oncology Group 210 Study. Am J Obstet Gynecol. 2018;219(5):459e1–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang AB, Huang Y, Hur C, Tergas AI, Khoury-Collado F, Melamed A, et al. Impact of quality of care on racial disparities in survival for endometrial cancer. Am J Obstet Gynecol. 2020;223(3):396e1–e13.. [DOI] [PubMed] [Google Scholar]
- 29.Kaspers M, Llamocca E, Quick A, Dholakia J, Salani R, Felix AS. Black and Hispanic women are less likely than white women to receive guideline-concordant endometrial cancer treatment. Am J Obstet Gynecol. 2020;223(3):398e1–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rauh-Hain JA, Melamed A, Schaps D, Bregar AJ, Spencer R, Schorge JO, et al. Racial and ethnic disparities over time in the treatment and mortality of women with gynecological malignancies. Gynecol Oncol. 2018;149(1):4–11. [DOI] [PubMed] [Google Scholar]
- 31.Rauh-Hain JA, Pepin KJ, Meyer LA, Clemmer JT, Lu KH, Rice LW, et al. Management for Elderly Women With Advanced-Stage, High-Grade Endometrial Cancer. Obstet Gynecol. 2015;126(6):1198–206. [DOI] [PubMed] [Google Scholar]
- 32.Goyal S, Chandwani S, Haffty BG, Demissie K. Effect of travel distance and time to radiotherapy on likelihood of receiving mastectomy. Ann Surg Oncol. 2015;22(4):1095–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Onega T, Cook A, Kirlin B, Shi X, Alford-Teaster J, Tuzzio L, et al. The influence of travel time on breast cancer characteristics, receipt of primary therapy, and surveillance mammography. Breast Cancer Res Treat. 2011;129(1):269–75.21553117 [Google Scholar]
- 34.Haggerty JL, Roberge D, Levesque JF, Gauthier J, Loignon C. An exploration of rural-urban differences in healthcare-seeking trajectories: implications for measures of accessibility. Health Place. 2014;28:92–8. [DOI] [PubMed] [Google Scholar]
- 35.Felix AS, Brasky TM, Cohn DE, Mutch DG, Creasman WT, Thaker PH, et al. Endometrial carcinoma recurrence according to race and ethnicity: An NRG Oncology/Gynecologic Oncology Group 210 Study. Int J Cancer. 2018;142(6):1102–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fedewa SA, Lerro C, Chase D, Ward EM. Insurance status and racial differences in uterine cancer survival: a study of patients in the National Cancer Database. Gynecol Oncol. 2011;122(1):63–8. [DOI] [PubMed] [Google Scholar]
- 37.Jagsi R, Abrahamse P, Hawley ST, Graff JJ, Hamilton AS, Katz SJ. Underascertainment of radiotherapy receipt in Surveillance, Epidemiology, and End Results registry data. Cancer. 2012;118(2):333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker GV, Giordano SH, Williams M, Jiang J, Niu J, MacKinnon J, et al. Muddy water? Variation in reporting receipt of breast cancer radiation therapy by population-based tumor registries. Int J Radiat Oncol Biol Phys. 2013;86(4):686–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 (online only). Multivariable logistic regression analysis of factors associated with the receipt of adjuvant radiation after excluding patients who did not have lymph node assessment. Odds ratios with 95% confidence intervals were plotted with the reference groups listed at the top of each variable category.
Supplemental Figure 2 (online only). Multivariable logistic regression analysis of factors associated with the receipt of adjuvant radiation after excluding patients with Charlson Deyo score of 1 or above. Odds ratios with 95% confidence intervals were plotted with the reference groups listed at the top of each variable category.
Supplemental Figure 3 (online only). Cox proportional hazards ratio of mortality according to baseline covariates after excluding patients who did not have lymph node assessment. RT: radiation therapy.
Supplemental Figure 4 (online only). Cox proportional hazards ratio of mortality according to baseline covariates after excluding patients with Charlson Deyo score of 1 or above. RT: radiation therapy.